Synthesis of Diazacyclic and Triazacyclic Small-Molecule Libraries Using Vicinal Chiral Diamines Generated from Modified Short Peptides and Their Application for Drug Discovery
Abstract
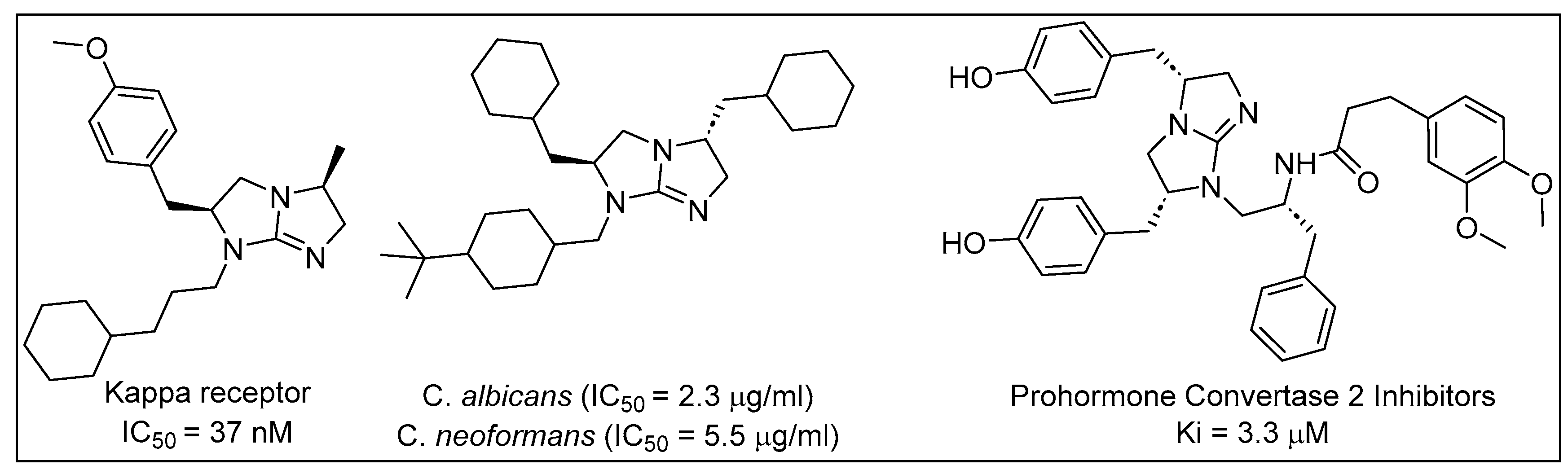
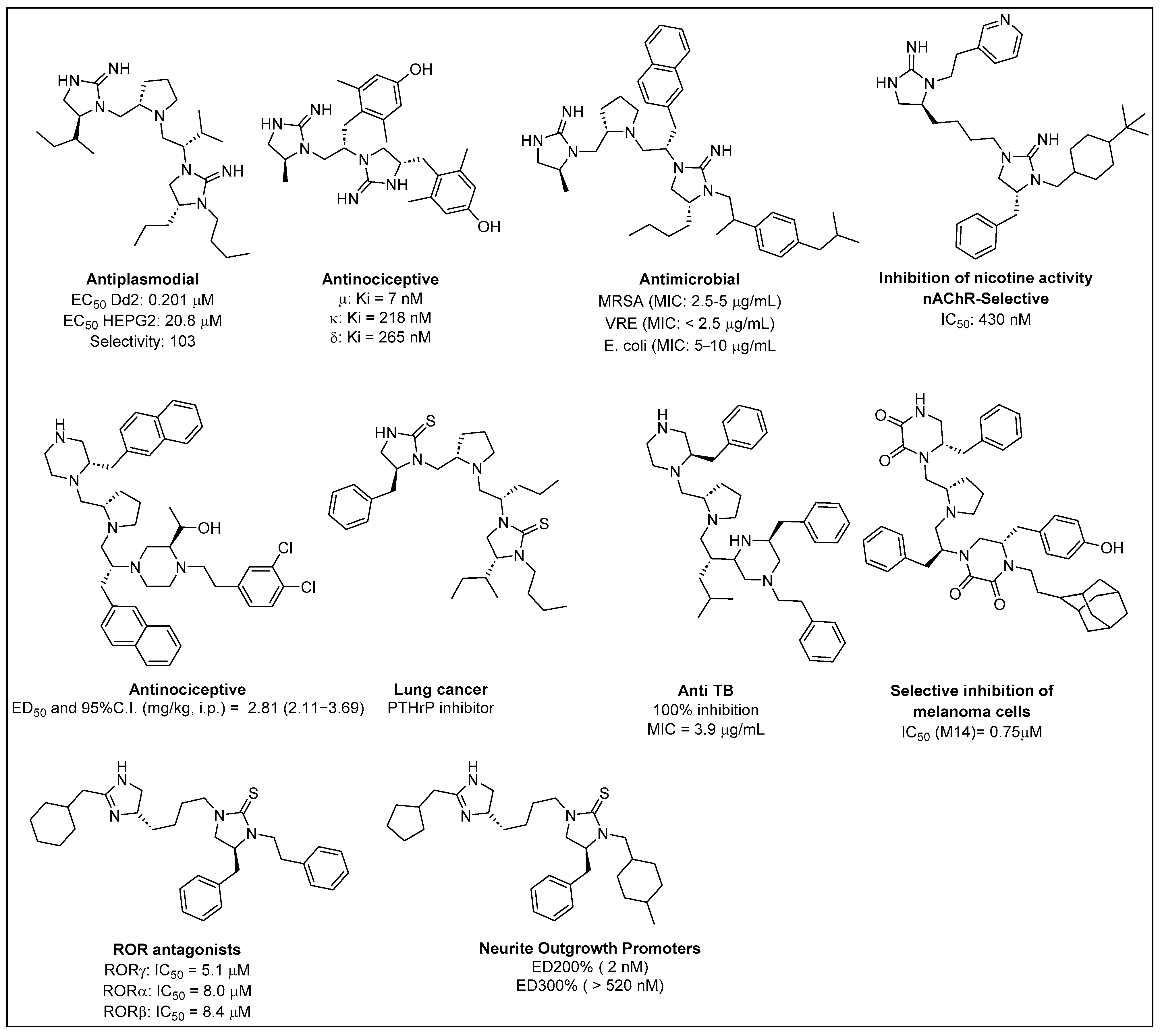
1. Introduction
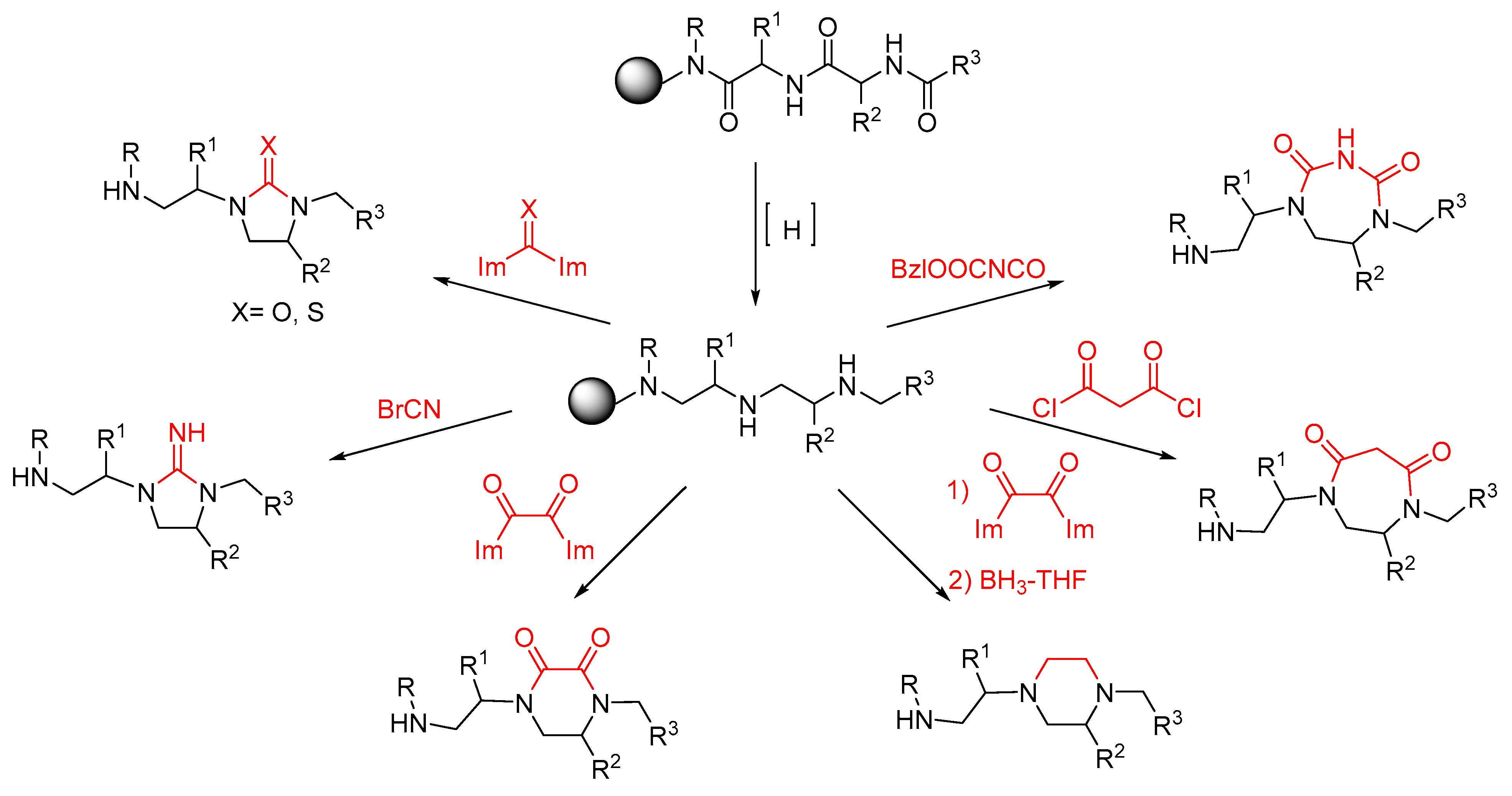
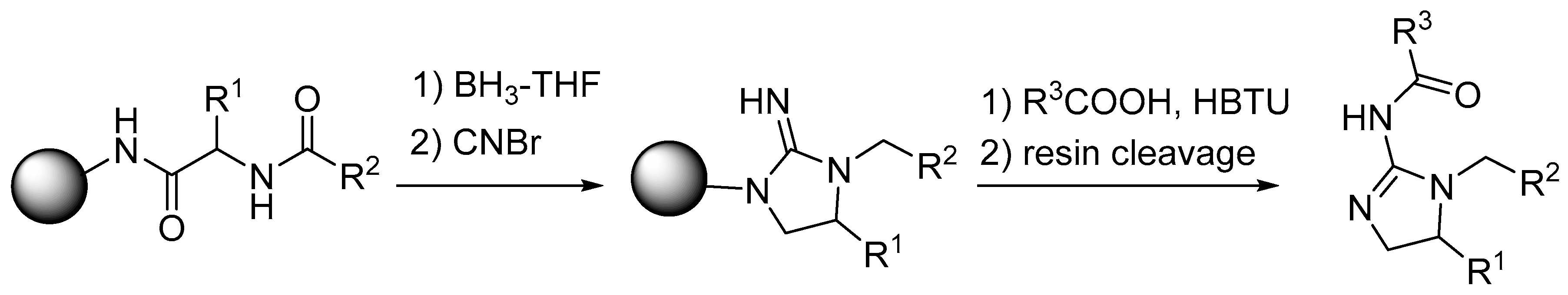
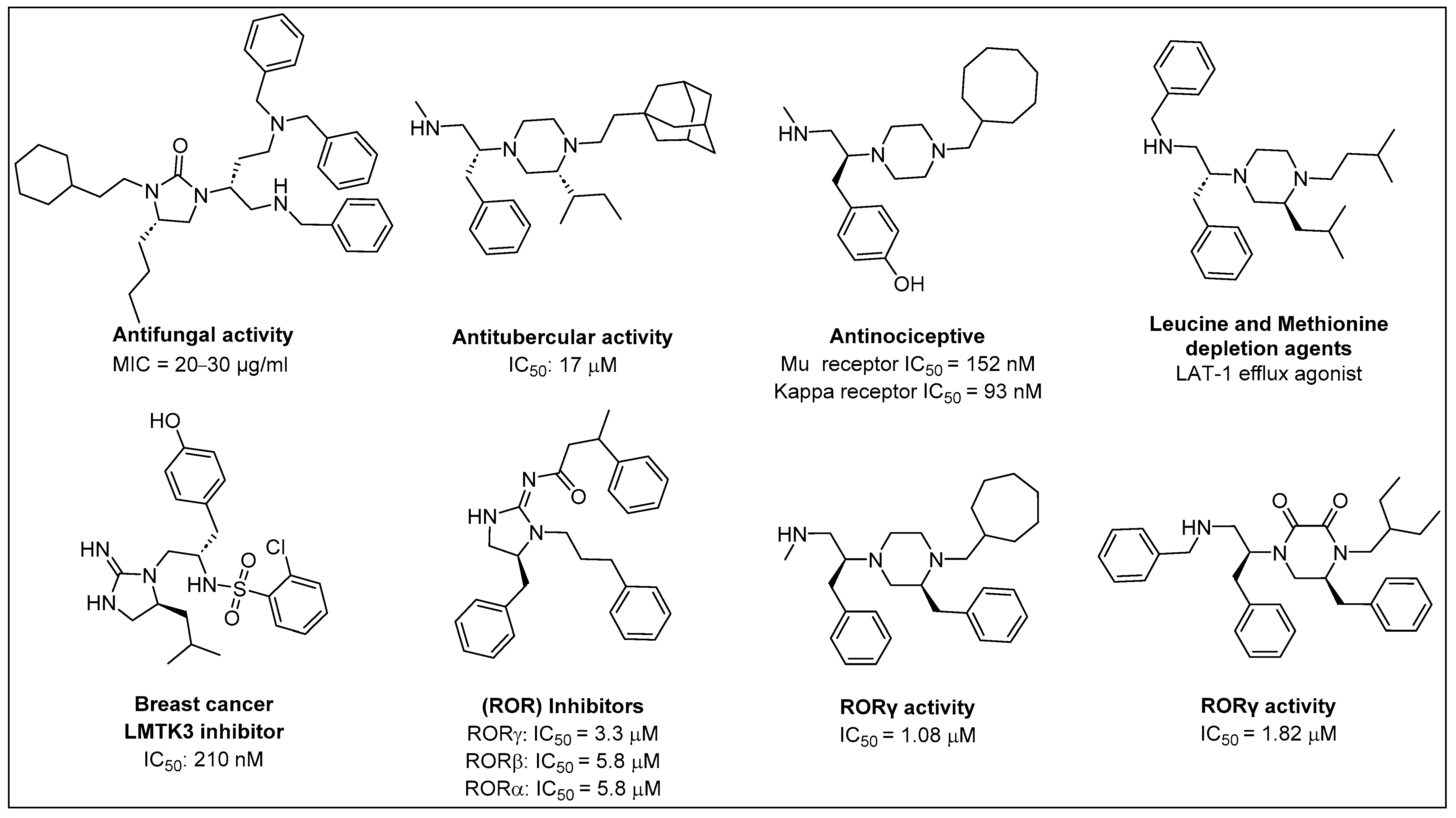
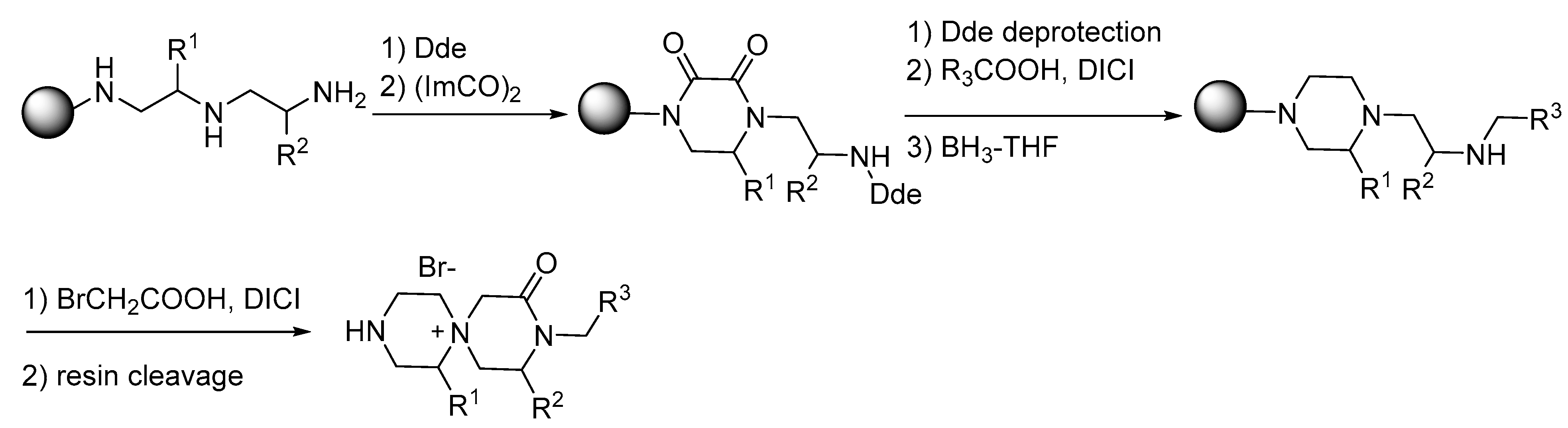
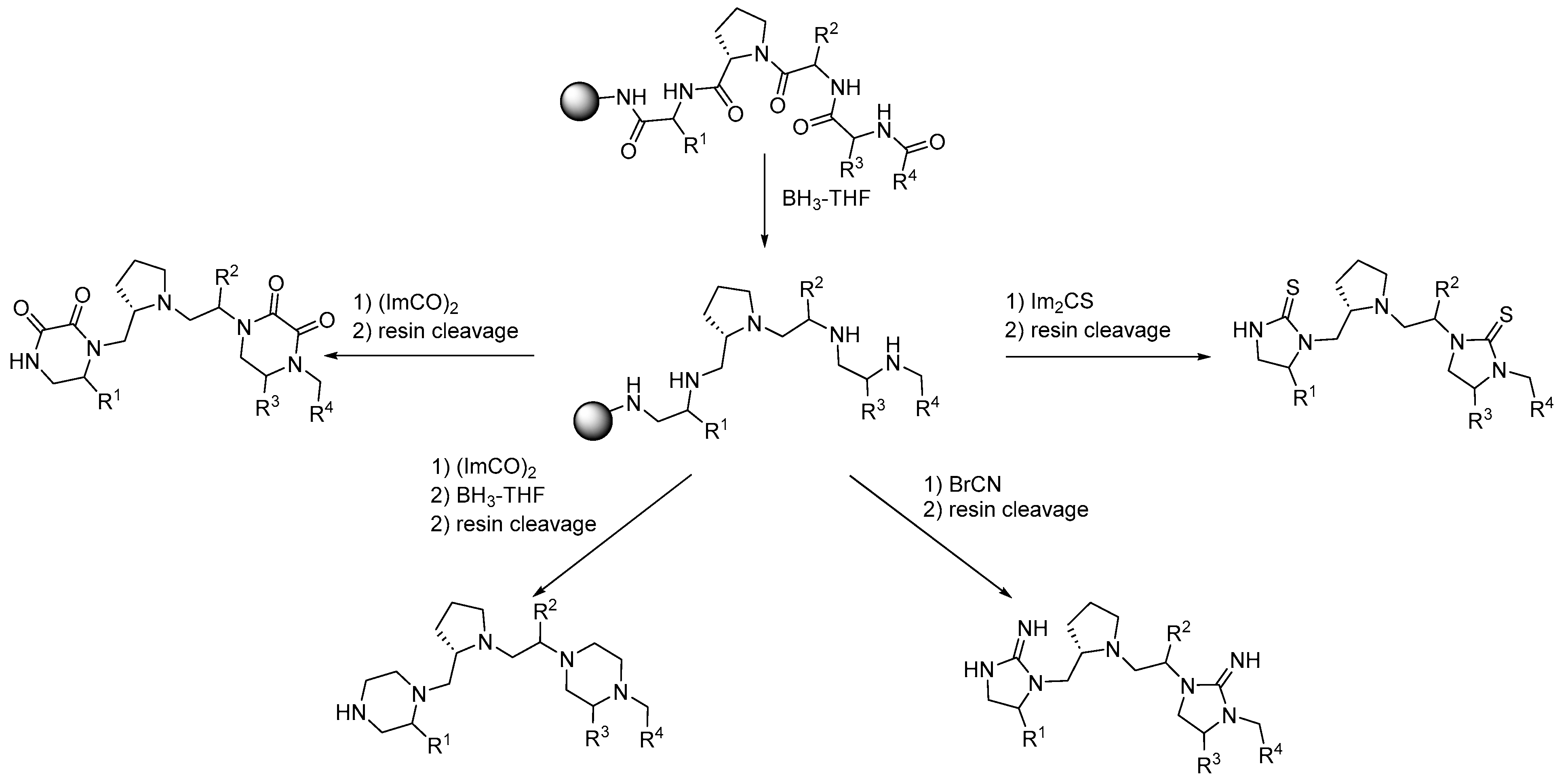
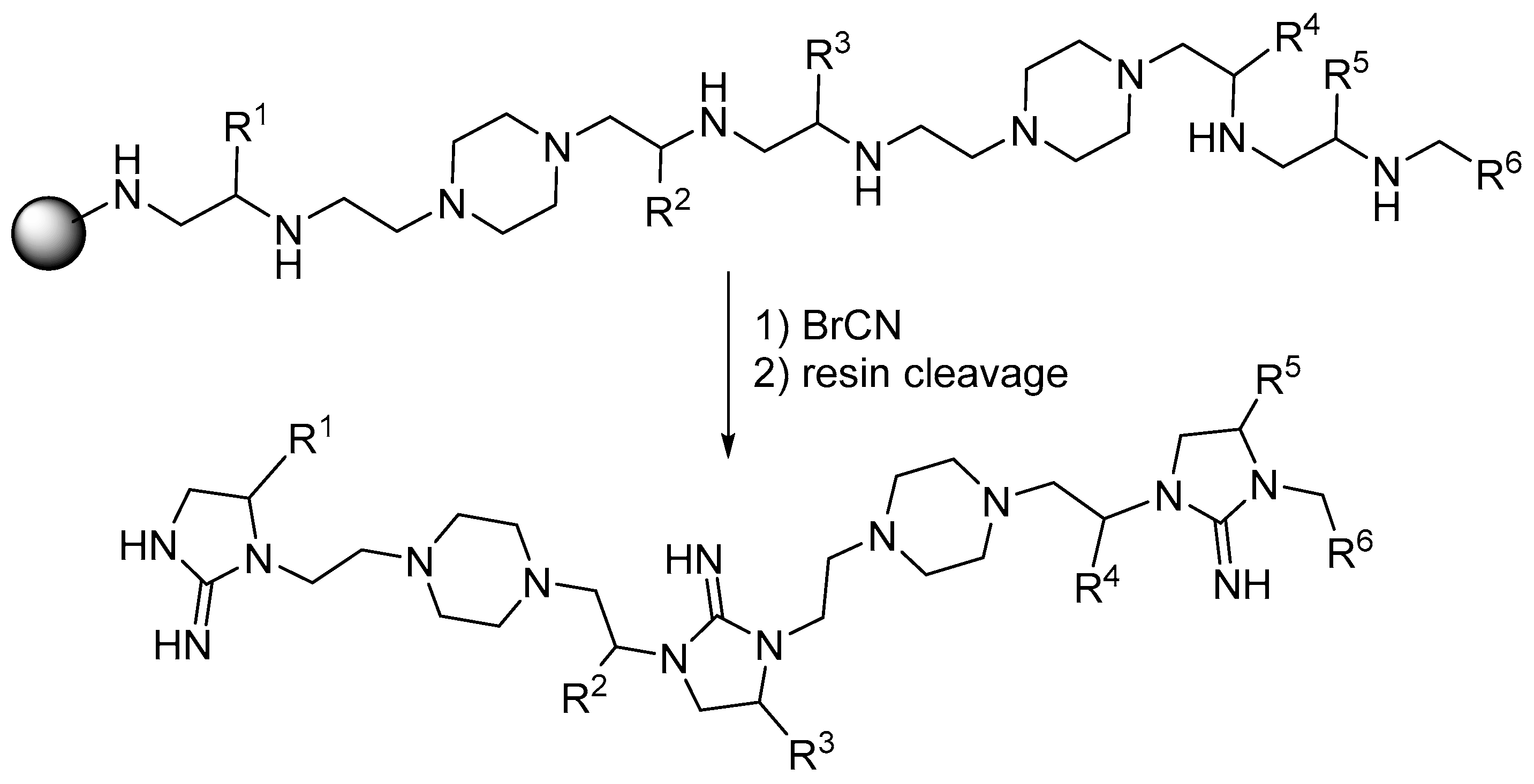
2. Synthesis of Diazacyclic and Triazacyclic Compounds from Vicinal Chiral Diamines
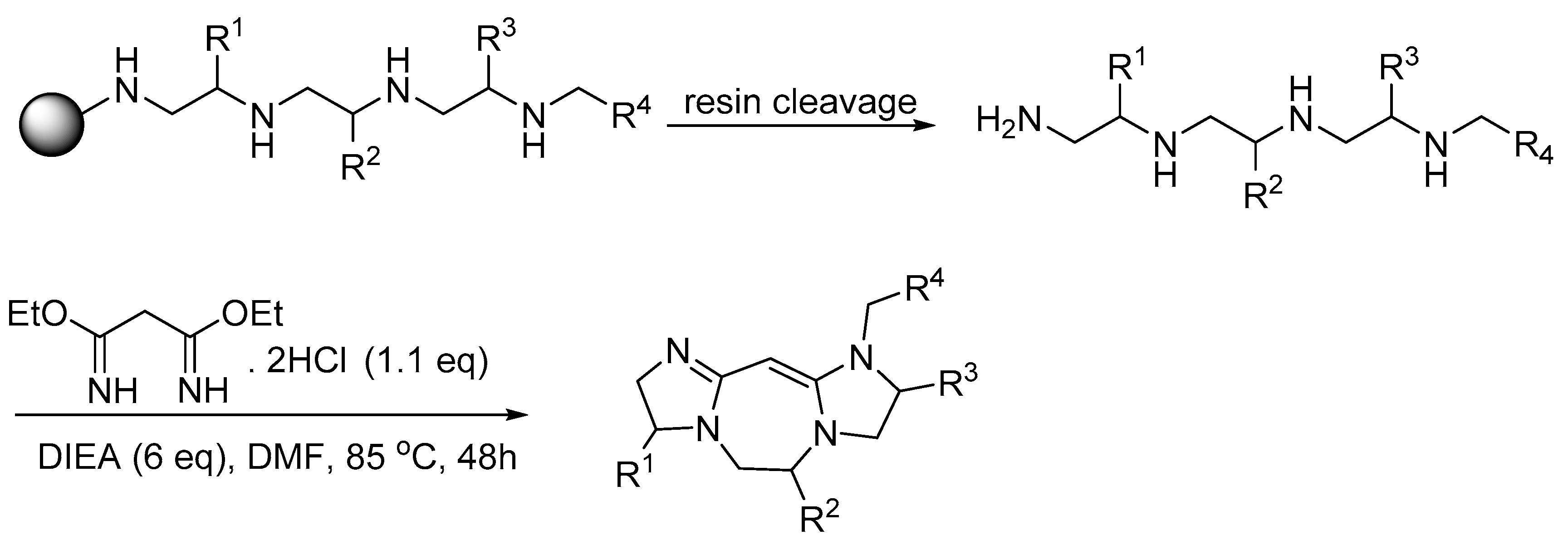
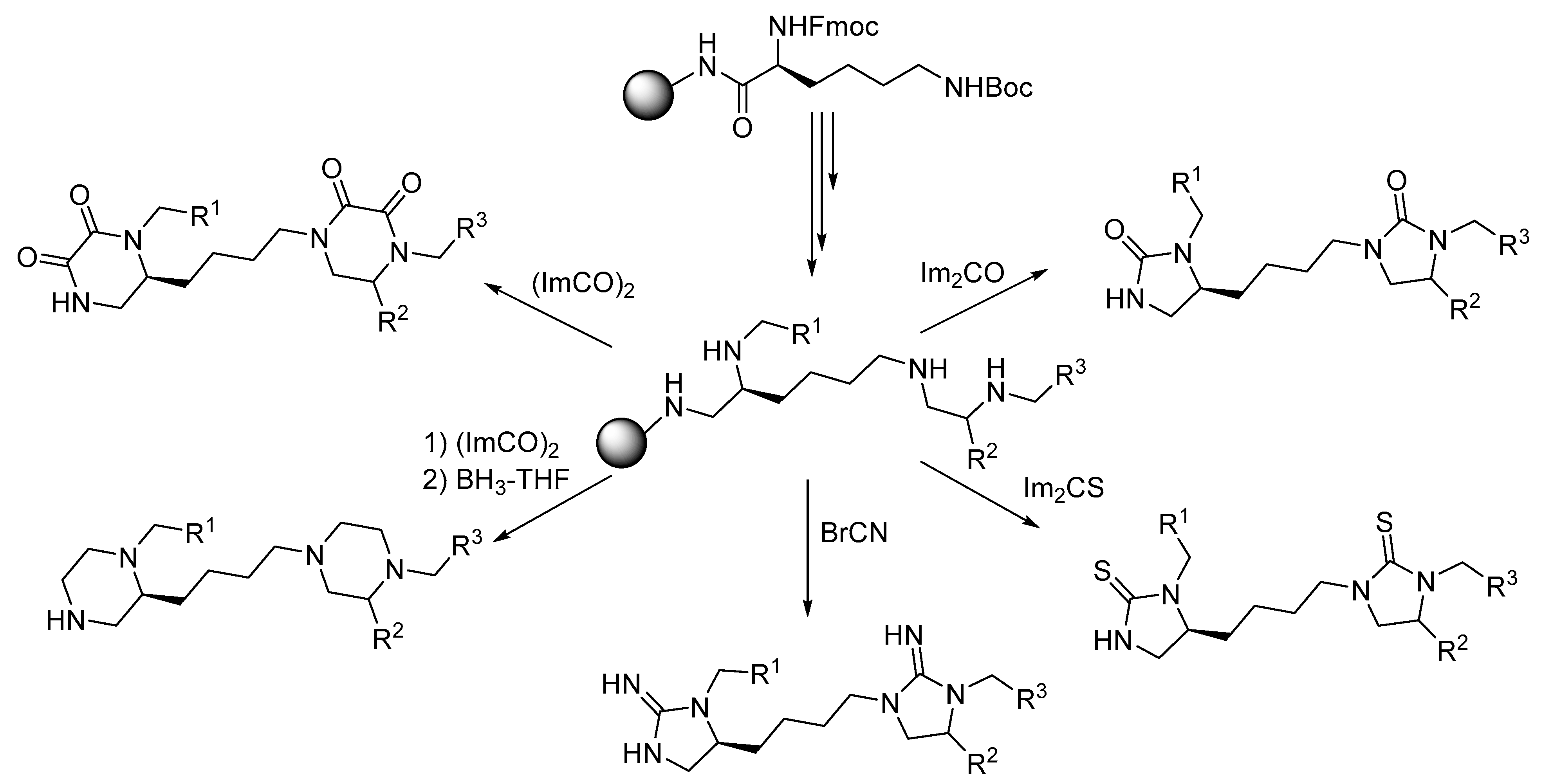
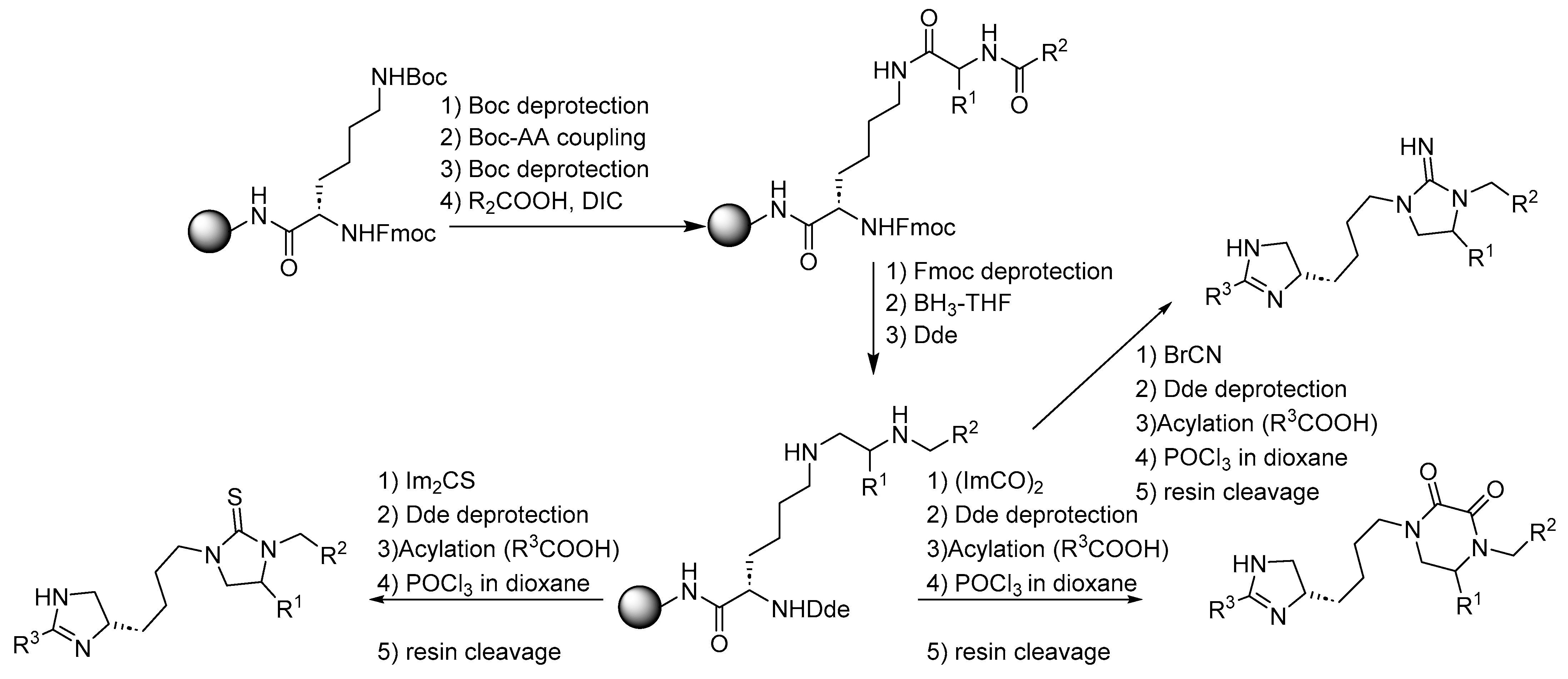
3. Synthesis of Fused Diazacyclic Compounds from Vicinal Polyamines
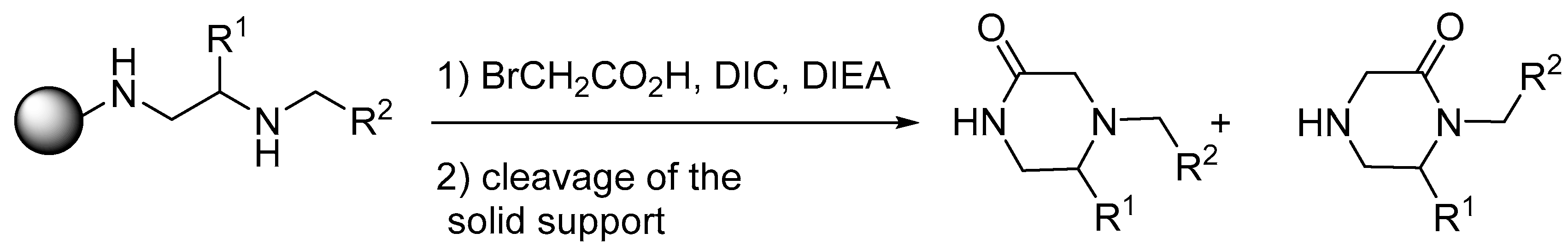
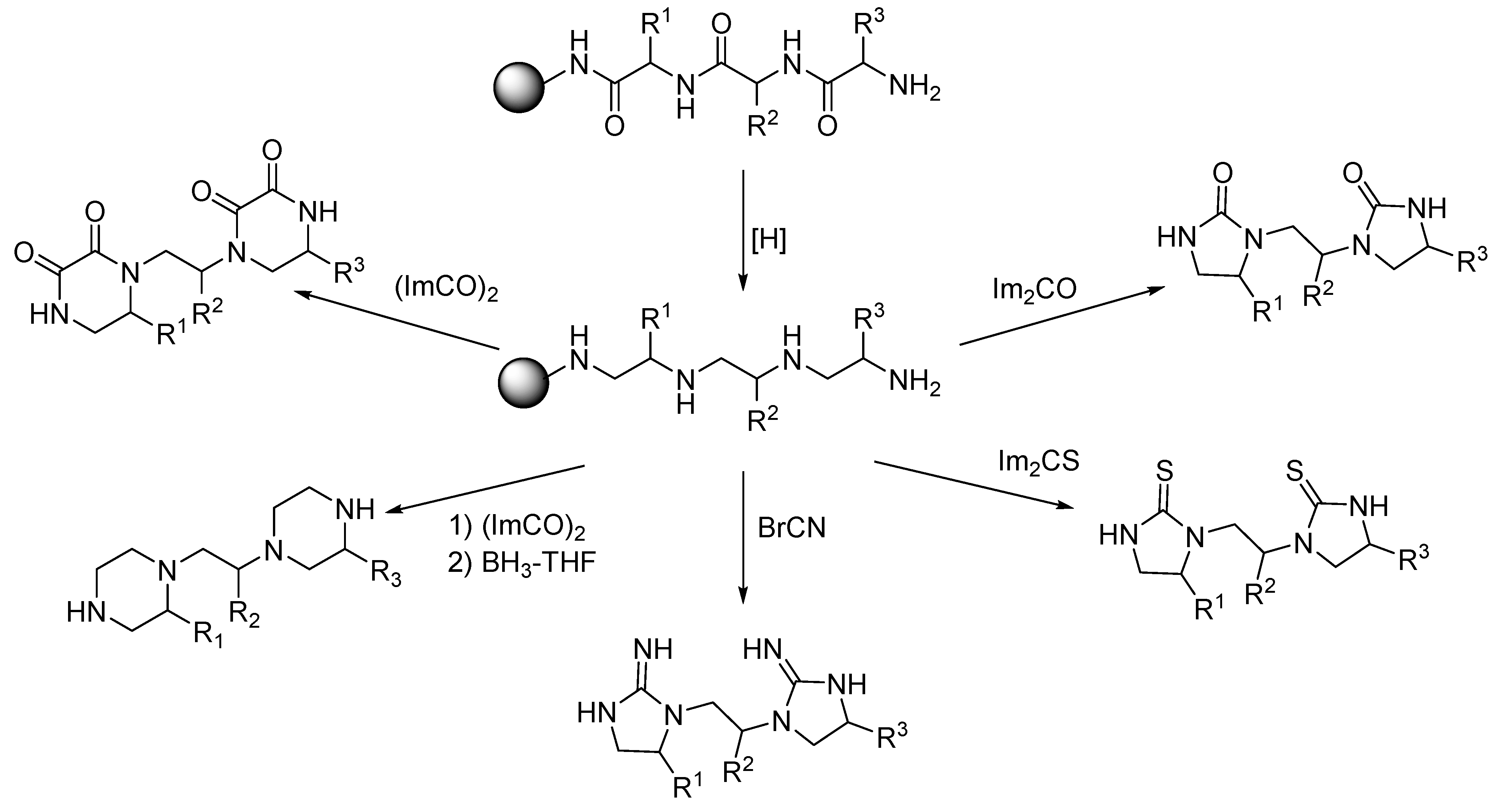
4. Synthesis of Bis-Diazacyclic Compounds from Alternated Vicinal Diamines
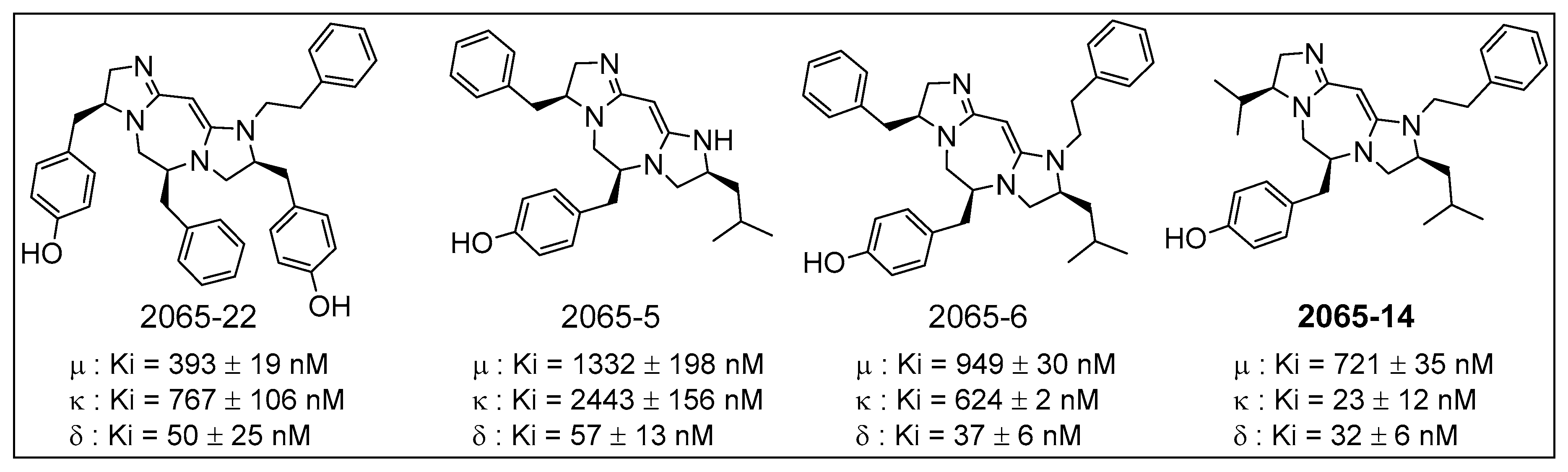
5. Synthesis of Oligodiazacyclic Compounds
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mermer, A.; Keles, T.; Sirin, Y. Recent studies of nitrogen containing heterocyclic compounds as novel antiviral agents: A review. Bioorg. Chem. 2021, 114, 105076. [Google Scholar] [CrossRef]
- Aatif, M.; Raza, M.A.; Javed, K.; Nashre-ul-Islam, S.M.; Farhan, M.; Alam, M.W. Potential Nitrogen-Based Heterocyclic Compounds for Treating Infectious Diseases: A Literature Review. Antibiotics 2022, 11, 1750. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, Z.; Ramazani, A.; Razzaghi-Asl, N. Anti-cancer Nitrogen-Containing Heterocyclic Compounds. Curr. Org. Chem. 2018, 22, 2256–2279. [Google Scholar] [CrossRef]
- Chugh, A.; Kumar, A.; Verma, A.; Kumar, S.; Kumar, P. A review of antimalarial activity of two or three nitrogen atoms containing heterocyclic compounds. Med. Chem. Res. 2020, 29, 1723–1750. [Google Scholar] [CrossRef]
- Santana, A.C.; Silva Filho, R.C.; Menezes, J.C.J.M.D.S.; Allonso, D.; Campos, V.R. Nitrogen-Based Heterocyclic Compounds: A Promising Class of Antiviral Agents against Chikungunya Virus. Life 2021, 11, 16. [Google Scholar] [CrossRef]
- Reymond, J.-L. The Chemical Space Project. Acc. Chem. Res. 2015, 48, 722–730. [Google Scholar] [CrossRef]
- Medina-Franco, J.L.; Chávez-Hernández, A.L.; López-López, E.; Saldívar-González, F.I. Chemical Multiverse: An Expanded View of Chemical Space. Mol. Inform. 2022, 41, e2200116. [Google Scholar] [CrossRef]
- Coley, C.W. Defining and Exploring Chemical Spaces. Trends Chem. 2021, 3, 133–145. [Google Scholar] [CrossRef]
- Lu, C.; Liu, S.; Shi, W.; Yu, J.; Zhou, Z.; Zhang, X.; Lu, X.; Cai, F.; Xia, N.; Wang, Y. Systemic evolutionary chemical space exploration for drug discovery. J. Cheminfor. 2022, 14, 19. [Google Scholar] [CrossRef]
- Lucet, D.; Le Gall, T.; Mioskowski, C. The Chemistry of Vicinal Diamines. Angew. Chem. Int. Ed. Engl. 1998, 37, 2580–2627. [Google Scholar] [CrossRef]
- Agut, J.; Vidal, A.; Rodríguez, S.; González, F.V. Dynamic Kinetic Asymmetric Ring-Opening/Reductive Amination Sequence of Racemic Nitroepoxides with Chiral Amines: Enantioselective Synthesis of Chiral Vicinal Diamines. J. Org. Chem. 2013, 78, 5717–5722. [Google Scholar] [CrossRef] [PubMed]
- Saibabu Kotti, S.R.; Timmons, C.; Li, G. Vicinal diamino functionalities as privileged structural elements in biologically active compounds and exploitation of their synthetic chemistry. Chem. Biol. Drug Des. 2006, 67, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Laktsevich-Iskryk, M.; Krech, A.; Fokin, M.; Kimm, M.; Jarg, T.; Noël, T.; Ošeka, M. Telescoped synthesis of vicinal diamines via ring-opening of electrochemically generated aziridines in flow. J. Flow Chem. 2024, 14, 139–147. [Google Scholar] [CrossRef]
- Michalson, E.T.; Szmuszkovicz, J. Medicinal agents incorporating the 1,2-diamine functionality. In Progress in Drug Research; Jucker, E., Ed.; Birkhäuser: Basel, Switzerland, 1989; pp. 135–149. [Google Scholar] [CrossRef]
- Lee, B.J.; Ickes, A.R.; Gupta, A.K.; Ensign, S.C.; Ho, T.D.; Tarasewicz, A.; Vanable, E.P.; Kortman, G.D.; Hull, K.L. Synthesis of Unsymmetrical Vicinal Diamines via Directed Hydroamination. Org. Lett. 2022, 24, 5513–5518. [Google Scholar] [CrossRef]
- Ostresh, J.M.; Schoner, C.C.; Hamashin, V.T.; Nefzi, A.; Meyer, J.-P.; Houghten, R.A. Solid-Phase Synthesis of Trisubstituted Bicyclic Guanidines via Cyclization of Reduced N-Acylated Dipeptides. J. Org. Chem. 1998, 63, 8622–8623. [Google Scholar] [CrossRef]
- Arutyunyan, S.; Nefzi, A. Synthesis of chiral polyaminothiazoles. J. Comb. Chem. 2010, 12, 315–317. [Google Scholar] [CrossRef][Green Version]
- Nefzi, A.; Appel, J.; Arutyunyan, S.; Houghten, R.A. Parallel synthesis of chiral pentaamines and pyrrolidine containing bis-heterocyclic libraries. Multiple scaffolds with multiple building blocks: A double diversity for the identification of new antitubercular compounds. Bioorg. Med. Chem. Lett. 2009, 19, 5169–5175. [Google Scholar] [CrossRef]
- Nefzi, A.; Giulianotti, M.A.; Ong, N.A.; Houghten, R.A. Solid-phase synthesis of bis-2-imidazolidinethiones from resin-bound tripeptides. Org. Lett. 2000, 2, 3349–3350. [Google Scholar] [CrossRef]
- Nefzi, A.; Ostresh, J.M.; Appel, J.R.; Bidlack, J.; Dooley, C.T.; Houghten, R.A. Identification of potent and highly selective chiral tri-amine and tetra-amine mu opioid receptors ligands: An example of lead optimization using mixture-based libraries. Bioorg. Med. Chem. Lett. 2006, 16, 4331–4338. [Google Scholar] [CrossRef]
- Cabrero-Antonino, J.R.; Adam, R.; Papa, V.; Beller, M. Homogeneous and heterogeneous catalytic reduction of amides and related compounds using molecular hydrogen. Nat. Commun. 2020, 11, 3893. [Google Scholar] [CrossRef]
- Bozovičar, K.; Bratkovič, T. Evolving a Peptide: Library Platforms and Diversification Strategies. Int. J. Mol. Sci. 2019, 21, 215. [Google Scholar] [CrossRef] [PubMed]
- Houghten, R.A.; Appel, J.R.; Blondelle, S.E.; Cuervo, J.H.; Dooley, C.T.; Pinilla, C. The use of synthetic peptide combinatorial libraries for the identification of bioactive peptides. Biotechniques 1992, 13, 412–421. [Google Scholar] [PubMed]
- Houghten, R.A.; Pinilla, C.; Appel, J.R.; Blondelle, S.E.; Dooley, C.T.; Eichler, J.; Nefzi, A.; Ostresh, J.M. Mixture-based synthetic combinatorial libraries. J. Med. Chem. 1999, 42, 3743–3778. [Google Scholar] [CrossRef]
- Lam, K.S.; Salmon, S.E.; Hersh, E.M.; Hruby, V.J.; Kazmierski, W.M.; Knapp, R.J. A new type of synthetic peptide library for identifying ligand-binding activity. Nature 1991, 354, 82–84. [Google Scholar] [CrossRef]
- Nefzi, A.; Dooley, C.; Ostresh, J.M.; Houghten, R.A. Combinatorial chemistry: From peptides and peptidomimetics to small organic and heterocyclic compounds. Bioorg. Med. Chem. Lett. 1998, 8, 2273–2278. [Google Scholar] [CrossRef]
- Nefzi, A.; Ostresh, J.M.; Houghten, R.A. Solid phase synthesis of mixture-based acyclic and heterocyclic small molecule combinatorial libraries from resin-bound polyamides. Biopolymers 2001, 60, 212–219. [Google Scholar] [CrossRef]
- Hoesl, C.E.; Nefzi, A.; Ostresh, J.M.; Yu, Y.; Houghten, R.A. Mixture-based combinatorial libraries: From peptides and peptidomimetics to small molecule acyclic and heterocyclic compounds. Methods Enzym. 2003, 369, 496–517. [Google Scholar] [CrossRef]
- Nefzi, A.; Ostresh, J.M.; Yu, Y.; Houghten, R.A. Combinatorial chemistry: Libraries from libraries, the art of the diversity-oriented transformation of resin-bound peptides and chiral polyamides to low molecular weight acyclic and heterocyclic compounds. J. Org. Chem. 2004, 69, 3603–3609. [Google Scholar] [CrossRef]
- Ostresh, J.M.; Husar, G.M.; Blondelle, S.E.; Dörner, B.; Weber, P.A.; Houghten, R.A. “Libraries from libraries” Chemical transformation of combinatorial libraries to extend the range and repertoire of chemical diversity. Proc. Natl. Acad. Sci. USA 1994, 91, 11138–11142. [Google Scholar] [CrossRef]
- Abdildinova, A.; Kurth, M.J.; Gong, Y.D. Heterocycles as a Peptidomimetic Scaffold: Solid-Phase Synthesis Strategies. Pharmaceuticals 2021, 14, 449. [Google Scholar] [CrossRef]
- Lenci, E.; Trabocchi, A. Peptidomimetic toolbox for drug discovery. Chem. Soc. Rev. 2020, 49, 3262–3277. [Google Scholar] [CrossRef] [PubMed]
- Vagner, J.; Qu, H.; Hruby, V.J. Peptidomimetics, a synthetic tool of drug discovery. Curr. Opin. Chem. Biol. 2008, 12, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Qvit, N.; Rubin, S.J.S.; Urban, T.J.; Mochly-Rosen, D.; Gross, E.R. Peptidomimetic therapeutics: Scientific approaches and opportunities. Drug Discov. Today 2017, 22, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Nefzi, A.; Ostresh, J.M.; Houghten, R.A. Combinatorial chemistry: Mixture-based combinatorial libraries of acyclic and heterocyclic compounds from amino acids and short peptides. In Modern Methods of Drug Discovery; Birkhäuser: Basel, Switzerland, 2003; pp. 109–123. [Google Scholar] [CrossRef]
- Lipinski, C.A. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: Current applications and future directions. Signal Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef]
- Otvos, L.; Wade, J.D. Current challenges in peptide-based drug discovery. Front. Chem. 2014, 2, 62. [Google Scholar] [CrossRef]
- Rossino, G.; Marchese, E.; Galli, G.; Verde, F.; Finizio, M.; Serra, M.; Linciano, P.; Collina, S. Peptides as Therapeutic Agents: Challenges and Opportunities in the Green Transition Era. Molecules 2023, 28, 7165. [Google Scholar] [CrossRef]
- Luo, X.; Chen, H.; Song, Y.; Qin, Z.; Xu, L.; He, N.; Tan, Y.; Dessie, W. Advancements, challenges and future perspectives on peptide-based drugs: Focus on antimicrobial peptides. Eur. J. Pharm. Sci. 2023, 181, 106363. [Google Scholar] [CrossRef]
- Nefzi, A.; Ostresh, J.M.; Houghten, R.A. Parallel solid phase synthesis of tetrasubstituted diethylenetriamines via selective amide alkylation and exhaustive reduction of N-acylated dipeptides. Tetrahedron 1999, 55, 335–344. [Google Scholar] [CrossRef]
- Hall, D.G.; Laplante, C.; Manku, S.; Nagendran, J. Mild Oxidative Cleavage of Borane-Amine Adducts from Amide Reductions: Efficient Solution- and Solid-Phase Synthesis of N-Alkylamino Acids and Chiral Oligoamines. J. Org. Chem. 1999, 64, 698–699. [Google Scholar] [CrossRef]
- Nefzi, A.; Ostresh, J.M.; Giulianotti, M.; Houghten, R.A. Solid-Phase Synthesis of Trisubstituted 2-Imidazolidones and 2-Imidazolidinethiones. ACS Comb. Sci. 1999, 1, 195–198. [Google Scholar] [CrossRef]
- Nefzi, A.; Mimna, R.A.; Houghten, R.A. Parallel solid-phase synthesis of disubstituted 1,6-piperazine-2-ones. J. Comb. Chem. 2002, 4, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Nefzi, A.; Giulianotti, M.A.; Houghten, R.A. Solid phase synthesis of 1,6-disubstituted 2,3-diketopiperazines and 1,2-disubstituted piperazines from N-acylated amino acids. Tetrahedron Lett. 1999, 40, 8539–8542. [Google Scholar] [CrossRef]
- Houghten, R.A. General method for the rapid solid-phase synthesis of large numbers of peptides: Specificity of antigen-antibody interaction at the level of individual amino acids. Proc. Natl. Acad. Sci. USA 1985, 82, 5131–5135. [Google Scholar] [CrossRef] [PubMed]
- Nefzi, A.; Ostresh, J.M.; Meyer, J.-P.; Houghten, R.A. Solid phase synthesis of heterocyclic compounds from linear peptides: Cyclic ureas and thioureas. Tetrahedron Lett. 1997, 38, 931–934. [Google Scholar] [CrossRef]
- Pinilla, C.; Appel, J.R.; Blanc, P.; Houghten, R.A. Rapid identification of high affinity peptide ligands using positional scanning synthetic peptide combinatorial libraries. Biotechniques 1992, 13, 901–905. [Google Scholar]
- Dooley, C.T.; Chung, N.N.; Wilkes, B.C.; Schiller, P.W.; Bidlack, J.M.; Pasternak, G.W.; Houghten, R.A. An all D-amino acid opioid peptide with central analgesic activity from a combinatorial library. Science 1994, 266, 2019–2022. [Google Scholar] [CrossRef]
- Fishbane, S.; Jamal, A.; Munera, C.; Wen, W.; Menzaghi, F. A Phase 3 Trial of Difelikefalin in Hemodialysis Patients with Pruritus. N. Engl. J. Med. 2020, 382, 222–232. [Google Scholar] [CrossRef]
- Fugal, J.; Serpa, S.M. Difelikefalin: A New κ-Opioid Receptor Agonist for the Treatment of Hemodialysis-Dependent Chronic Kidney Disease-Associated Pruritus. Ann. Pharmacother. 2023, 57, 480–488. [Google Scholar] [CrossRef]
- Dooley, C.T.; Ny, P.; Bidlack, J.M.; Houghten, R.A. Selective ligands for the mu, delta, and kappa opioid receptors identified from a single mixture based tetrapeptide positional scanning combinatorial library. J. Biol. Chem. 1998, 273, 18848–18856. [Google Scholar] [CrossRef]
- Dooley, C.T.; Houghten, R.A. Novel Kappa Receptor Selective Opioid Peptides. U.S. Patent WO9640206A1, 1996. [Google Scholar]
- Blondelle, S.E.; Nefzi, A.; Ostresh, J.M.; Houghten, R.A. Novel antifungal compounds derived from heterocyclic positional scanning combinatorial libraries. Pure Appl. Chem. 1998, 70, 2141. [Google Scholar]
- Rideout, M.C.; Boldt, J.L.; Vahi-Ferguson, G.; Salamon, P.; Nefzi, A.; Ostresh, J.M.; Giulianotti, M.; Pinilla, C.; Segall, A.M. Potent antimicrobial small molecules screened as inhibitors of tyrosine recombinases and Holliday junction-resolving enzymes. Mol. Divers. 2011, 15, 989–1005. [Google Scholar] [CrossRef] [PubMed]
- Ranjit, D.K.; Rideout, M.C.; Nefzi, A.; Ostresh, J.M.; Pinilla, C.; Segall, A.M. Small molecule functional analogs of peptides that inhibit lambda site-specific recombination and bind Holliday junctions. Bioorg. Med. Chem. Lett. 2010, 20, 4531–4534. [Google Scholar] [CrossRef] [PubMed]
- Yongye, A.B.; Appel, J.R.; Giulianotti, M.A.; Dooley, C.T.; Medina-Franco, J.L.; Nefzi, A.; Houghten, R.A.; Martínez-Mayorga, K. Identification, structure–activity relationships and molecular modeling of potent triamine and piperazine opioid ligands. Bioorg. Med. Chem. 2009, 17, 5583–5597. [Google Scholar] [CrossRef]
- Massaro, C.; Thomas, J.; Ikhlef, H.; Dinara, S.; Cronk, S.; Moots, H.; Phanstiel, O.I.V. Serendipitous Discovery of Leucine and Methionine Depletion Agents during the Search for Polyamine Transport Inhibitors. J. Med. Chem. 2020, 63, 2814–2832. [Google Scholar] [CrossRef]
- Ortiz, M.A.; Michaels, H.; Molina, B.; Toenjes, S.; Davis, J.; Marconi, G.D.; Hecht, D.; Gustafson, J.L.; Piedrafita, F.J.; Nefzi, A. Discovery of cyclic guanidine-linked sulfonamides as inhibitors of LMTK3 kinase. Bioorg. Med. Chem. Lett. 2020, 30, 127108. [Google Scholar] [CrossRef]
- Ortiz, A.M.; Piedrafita, J.F.; Bunnell, A.; Nefzi, A. Screening, Deconvolution and Parallel Synthesis of Trisubstituted Piperazine and Trisubstituted 2,3-diketopierazine Libraries for the Rapid Identification of Antagonists of the Nuclear Retinoic Acid Receptor-related Orphan Receptor Gamma (RORγ). Lett. Drug Des. Discov. 2024, 21, 829–835. [Google Scholar] [CrossRef]
- Ortiz, M.A.; Piedrafita, F.J.; Nefzi, A. 1,5-Disubstituted Acylated 2-Amino-4,5-dihydroimidazoles as a New Class of Retinoic Acid Receptor-Related Orphan Receptor (ROR) Inhibitors. Int. J. Mol. Sci. 2022, 23, 4433. [Google Scholar] [CrossRef]
- Blondelle, S.E.; Crooks, E.; Ostresh, J.M.; Houghten, R.A. Mixture-based heterocyclic combinatorial positional scanning libraries: Discovery of bicyclic guanidines having potent antifungal activities against Candida albicans and Cryptococcus neoformans. Antimicrob. Agents Chemother. 1999, 43, 106–114. [Google Scholar] [CrossRef]
- Kowalska, D.; Liu, J.; Appel, J.R.; Ozawa, A.; Nefzi, A.; Mackin, R.B.; Houghten, R.A.; Lindberg, I. Synthetic small-molecule prohormone convertase 2 inhibitors. Mol. Pharmacol. 2009, 75, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Nefzi, A.; Santos, R.T. Efficient approaches toward the solid-phase synthesis of new heterocyclic azoniaspiro ring systems: Synthesis of tri- and tetrasubstituted 10-oxo-3,9-diaza-6-azoniaspiro[5.5]undecanes. J. Org. Chem. 2005, 70, 9622–9625. [Google Scholar] [CrossRef] [PubMed]
- Hoesl, C.E.; Nefzi, A.; Houghten, R.A. Halogenoalkyl isocyanates as bifunctional reagents in an Aza-Wittig/heterocyclization reaction on the solid phase: Efficient entry into new tetracyclic benzimidazole systems. J. Comb. Chem. 2004, 6, 220–223. [Google Scholar] [CrossRef]
- Hoesl, C.E.; Nefzi, A.; Houghten, R.A. Parallel solid-phase synthesis of 2-imino-4-oxo-1,3,5-triazino[1,2-a]benzimidazoles via tandem aza-Wittig/heterocumulene-mediated annulation reaction. J. Comb. Chem. 2003, 5, 155–160. [Google Scholar] [CrossRef]
- Hoesl, C.E.; Ostresh, J.M.; Houghten, R.A.; Nefzi, A. Solid phase synthesis of 3,4,7-trisubstituted 4,5,8,9-tetrahydro-3H-imidazo [1,2-a][1,3,5]triazepin-2(7H)-thiones and N-alkyl-4,5,7,8-tetrahydro-3H-imidazo[1,2-a][1,3,5]triazepin-2-amines. J. Comb. Chem. 2006, 8, 127–131. [Google Scholar] [CrossRef]
- Eans, S.O.; Ganno, M.L.; Mizrachi, E.; Houghten, R.A.; Dooley, C.T.; McLaughlin, J.P.; Nefzi, A. Parallel Synthesis of Hexahydrodiimidazodiazepines Heterocyclic Peptidomimetics and Their in Vitro and in Vivo Activities at μ (MOR), δ (DOR), and κ (KOR) Opioid Receptors. J. Med. Chem. 2015, 58, 4905–4917. [Google Scholar] [CrossRef]
- Hartrick, C.T.; Poulin, D.; Molenaar, R.; Hartrick, A. Dual-Acting Peripherally Restricted Delta/Kappa Opioid (CAV1001) Produces Antinociception in Animal Models of Sub-Acute and Chronic Pain. J. Pain Res. 2020, 13, 2461–2474. [Google Scholar] [CrossRef]
- Hartrick, C.; Van Valkenburg, T.; Kartrick, A.; Pomonis, J. (257) The Efficacy of a Dual-Acting, Peripherally-Restricted Kappa/Delta Opioid Agonist (CA1001) in Neuropathic Pain in the Rat. J. Pain 2019, 20, S38–S39. [Google Scholar] [CrossRef]
- Hartrick, C.; Molenaar, R.; Hartrick, A.; Pomonis, J. (258) Reversal of Mechanical Hyperalgesia by a Dual-Acting, Peripherally-Restricted Kappa/Delta Opioid Agonist (CA1001) in a Rat Model of Inflammatory Arthritis. J. Pain 2019, 20, S39. [Google Scholar] [CrossRef]
- Behrendt, R.; White, P.; Offer, J. Advances in Fmoc solid-phase peptide synthesis. J. Pept. Sci. 2016, 22, 4–27. [Google Scholar] [CrossRef]
- Acharya, A.N.; Ostresh, J.M.; Houghten, R.A. Solid-phase synthesis of substituted imidazoline-tethered 2,3-diketopiperazines, cyclic ureas, and cyclic thioureas. J. Comb. Chem. 2001, 3, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Nefzi, A.; Marconi, G.D.; Ortiz, M.A.; Davis, J.C.; Piedrafita, F.J. Synthesis of dihydroimidazole tethered imidazolinethiones and their activity as novel antagonists of the nuclear retinoic acid receptor-related orphan receptors (RORs). Bioorg. Med. Chem. Lett. 2017, 27, 1608–1610. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Al-Ali, H.; Debevec, G.; Santos, R.G.; Houghten, R.A.; Davis, J.C.; Nefzi, A.; Lemmon, V.P.; Bixby, J.L.; Giulianotti, M.A. Scaffold Ranking and Positional Scanning Identify Novel Neurite Outgrowth Promoters with Nanomolar Potency. ACS Med. Chem. Lett. 2018, 9, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Nash, I.A.; Bycroft, B.W.; Chan, W.C. Dde—A selective primary amine protecting group: A facile solid phase synthetic approach to polyamine conjugates. Tetrahedron Lett. 1996, 37, 2625–2628. [Google Scholar] [CrossRef]
- Hensler, M.E.; Bernstein, G.; Nizet, V.; Nefzi, A. Pyrrolidine bis-cyclic guanidines with antimicrobial activity against drug-resistant Gram-positive pathogens identified from a mixture-based combinatorial library. Bioorg. Med. Chem. Lett. 2006, 16, 5073–5079. [Google Scholar] [CrossRef]
- Fleeman, R.; LaVoi, T.M.; Santos, R.G.; Morales, A.; Nefzi, A.; Welmaker, G.S.; Medina-Franco, J.L.; Giulianotti, M.A.; Houghten, R.A.; Shaw, L.N. Combinatorial Libraries As a Tool for the Discovery of Novel, Broad-Spectrum Antibacterial Agents Targeting the ESKAPE Pathogens. J. Med. Chem. 2015, 58, 3340–3355. [Google Scholar] [CrossRef]
- Perry, D.L., Jr.; Roberts, B.F.; Debevec, G.; Michaels, H.A.; Chakrabarti, D.; Nefzi, A. Identification of Bis-Cyclic Guanidines as Antiplasmodial Compounds from Positional Scanning Mixture-Based Libraries. Molecules 2019, 24, 1100. [Google Scholar] [CrossRef]
- McLaughlin, J.P.; Rayala, R.; Bunnell, A.J.; Tantak, M.P.; Eans, S.O.; Nefzi, K.; Ganno, M.L.; Dooley, C.T.; Nefzi, A. Bis-Cyclic Guanidine Heterocyclic Peptidomimetics as Opioid Ligands with Mixed μ-, κ- and δ-Opioid Receptor Interactions: A Potential Approach to Novel Analgesics. Int. J. Mol. Sci. 2022, 23, 9623. [Google Scholar] [CrossRef]
- Reilley, K.J.; Giulianotti, M.; Dooley, C.T.; Nefzi, A.; McLaughlin, J.P.; Houghten, R.A. Identification of two novel, potent, low-liability antinociceptive compounds from the direct in vivo screening of a large mixture-based combinatorial library. AAPS J. 2010, 12, 318–329. [Google Scholar] [CrossRef]
- Tantak, M.; Rayala, R.; Deng, Z.; Bunnell, A.; Wang, T.; Chaudhari, P.; Leng, F.; Nefzi, A. Polyheterocyclic peptidomimetics: Parallel solid phase synthesis of oligo cyclic guanidines and their inhibition activity against Mycobacterium tuberculosis DNA gyrase. Bioorg. Med. Chem. Lett. 2023, 93, 129439. [Google Scholar] [CrossRef]
- Doering, S.R.; Freeman, K.; Debevec, G.; Geer, P.; Santos, R.G.; Lavoi, T.M.; Giulianotti, M.A.; Pinilla, C.; Appel, J.R.; Houghten, R.A.; et al. Discovery of Nanomolar Melanocortin-3 Receptor (MC3R)-Selective Small Molecule Pyrrolidine Bis-Cyclic Guanidine Agonist Compounds Via a High-Throughput “Unbiased” Screening Campaign. J. Med. Chem. 2021, 64, 5577–5592. [Google Scholar] [CrossRef] [PubMed]
- Hastings, R.H.; Burton, D.W.; Nefzi, A.; Montgrain, P.R.; Quintana, R.; Deftos, L.J. Combinatorial library discovery of small molecule inhibitors of lung cancer proliferation and parathyroid hormone-related protein expression. Cancer Biol. Ther. 2010, 10, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Onwuha-Ekpete, L.; Tack, L.; Knapinska, A.; Smith, L.; Kaushik, G.; Lavoi, T.; Giulianotti, M.; Houghten, R.A.; Fields, G.B.; Minond, D. Novel pyrrolidine diketopiperazines selectively inhibit melanoma cells via induction of late-onset apoptosis. J. Med. Chem. 2014, 57, 1599–1608. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, S.W.; Armishaw, C.J.; Strømgaard, K. Synthesis of peptides using tert-butyloxycarbonyl (Boc) as the α-amino protection group. Methods Mol. Biol. 2013, 1047, 65–80. [Google Scholar] [CrossRef]
- Nefzi, A.; Santos, R.T. A versatile access to new macrocyclic oligoheterocycles (MOH). Bioorg. Med. Chem. Lett. 2006, 16, 3358–3361. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tantak, M.P.; Rayala, R.; Chaudhari, P.; Danta, C.C.; Nefzi, A. Synthesis of Diazacyclic and Triazacyclic Small-Molecule Libraries Using Vicinal Chiral Diamines Generated from Modified Short Peptides and Their Application for Drug Discovery. Pharmaceuticals 2024, 17, 1566. https://doi.org/10.3390/ph17121566
Tantak MP, Rayala R, Chaudhari P, Danta CC, Nefzi A. Synthesis of Diazacyclic and Triazacyclic Small-Molecule Libraries Using Vicinal Chiral Diamines Generated from Modified Short Peptides and Their Application for Drug Discovery. Pharmaceuticals. 2024; 17(12):1566. https://doi.org/10.3390/ph17121566
Chicago/Turabian StyleTantak, Mukund P., Ramanjaneyulu Rayala, Prakash Chaudhari, Chhanda C. Danta, and Adel Nefzi. 2024. "Synthesis of Diazacyclic and Triazacyclic Small-Molecule Libraries Using Vicinal Chiral Diamines Generated from Modified Short Peptides and Their Application for Drug Discovery" Pharmaceuticals 17, no. 12: 1566. https://doi.org/10.3390/ph17121566
APA StyleTantak, M. P., Rayala, R., Chaudhari, P., Danta, C. C., & Nefzi, A. (2024). Synthesis of Diazacyclic and Triazacyclic Small-Molecule Libraries Using Vicinal Chiral Diamines Generated from Modified Short Peptides and Their Application for Drug Discovery. Pharmaceuticals, 17(12), 1566. https://doi.org/10.3390/ph17121566









