Antimicrobial Activity of Naphthyridine Derivatives
Abstract
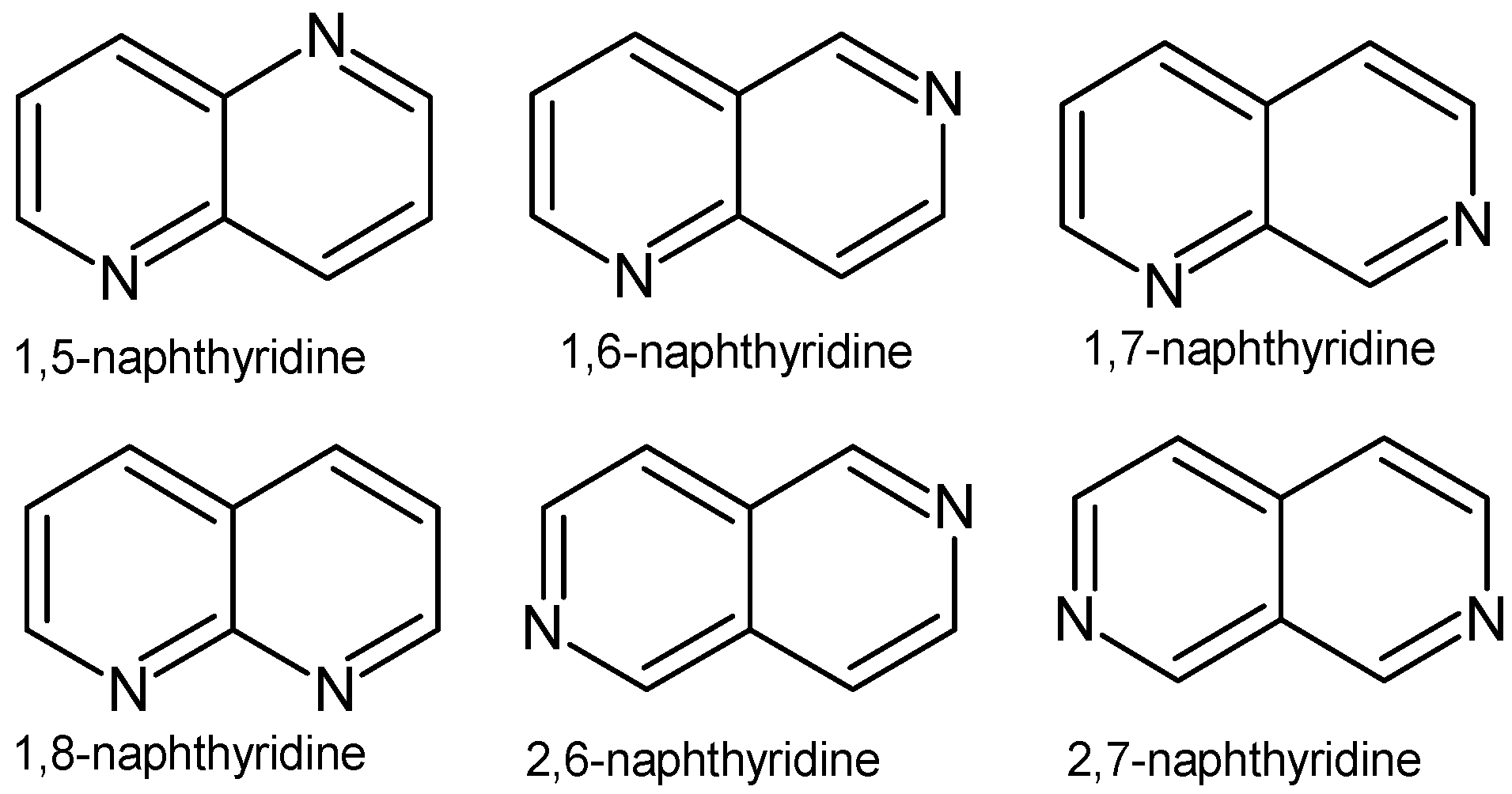
1. Introduction
2. 1,8-Naphthyridine Derivatives
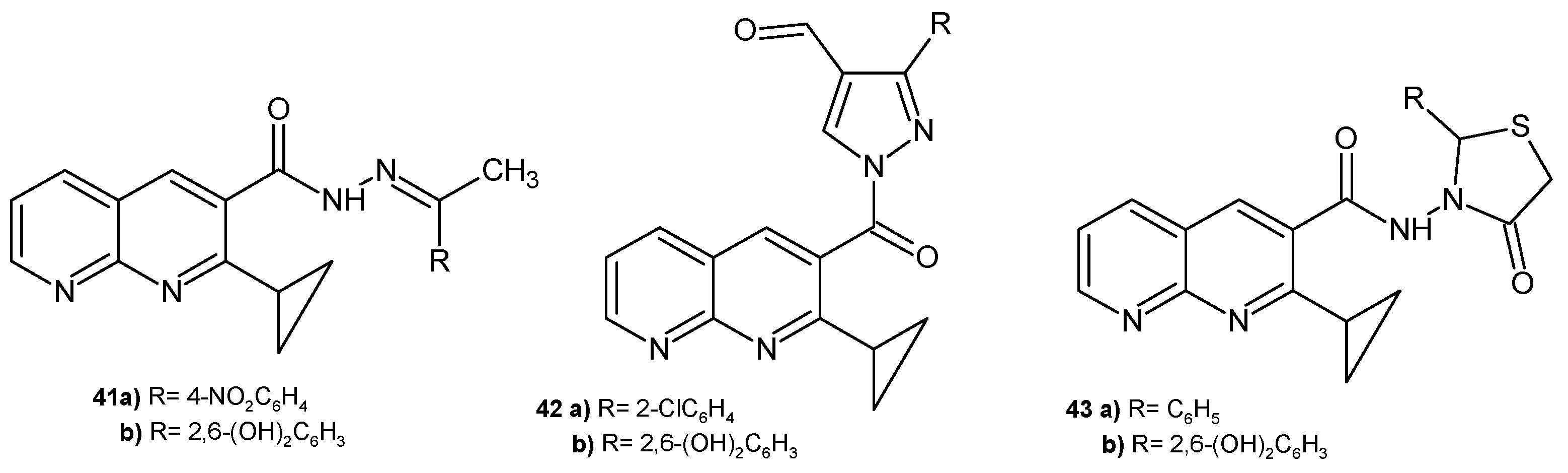
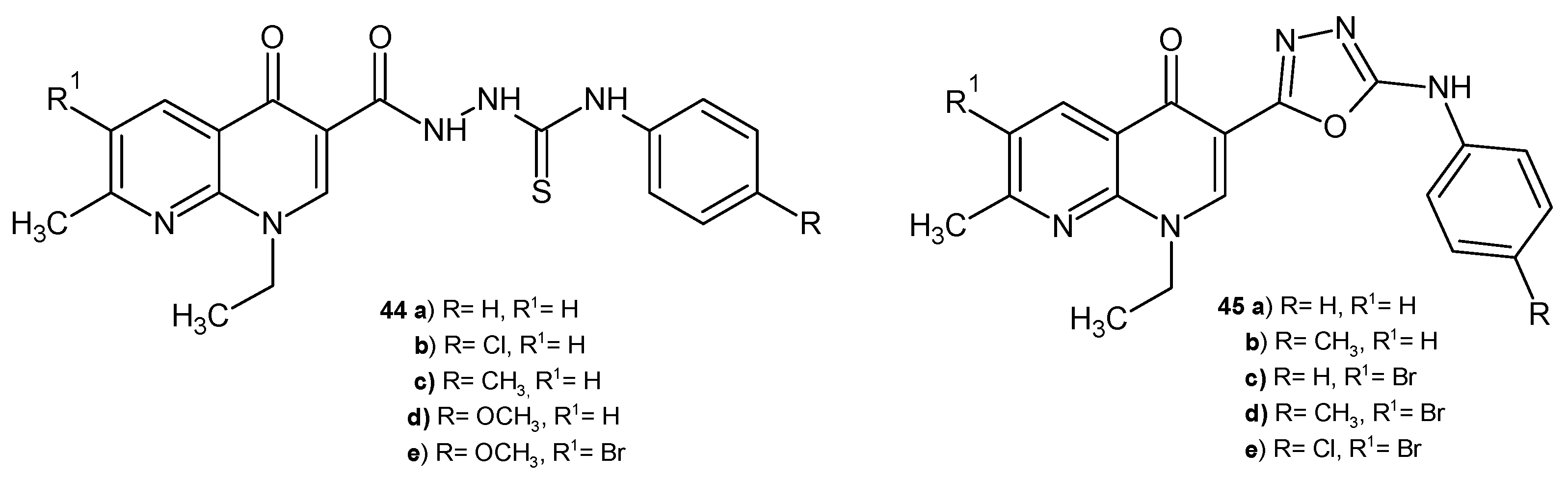
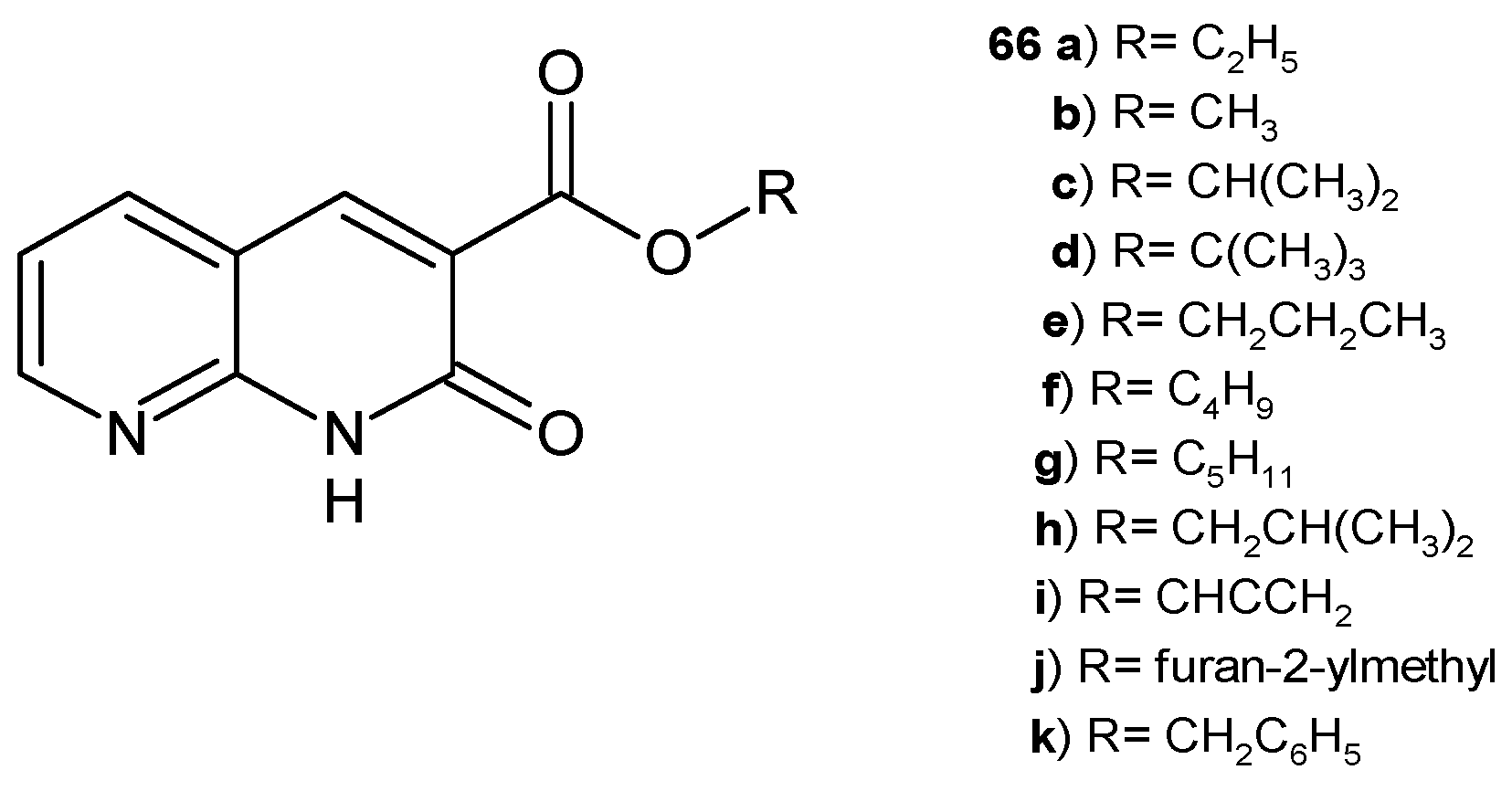
2.1. Antimicrobial Activity of Nalidixic Acid Derivatives
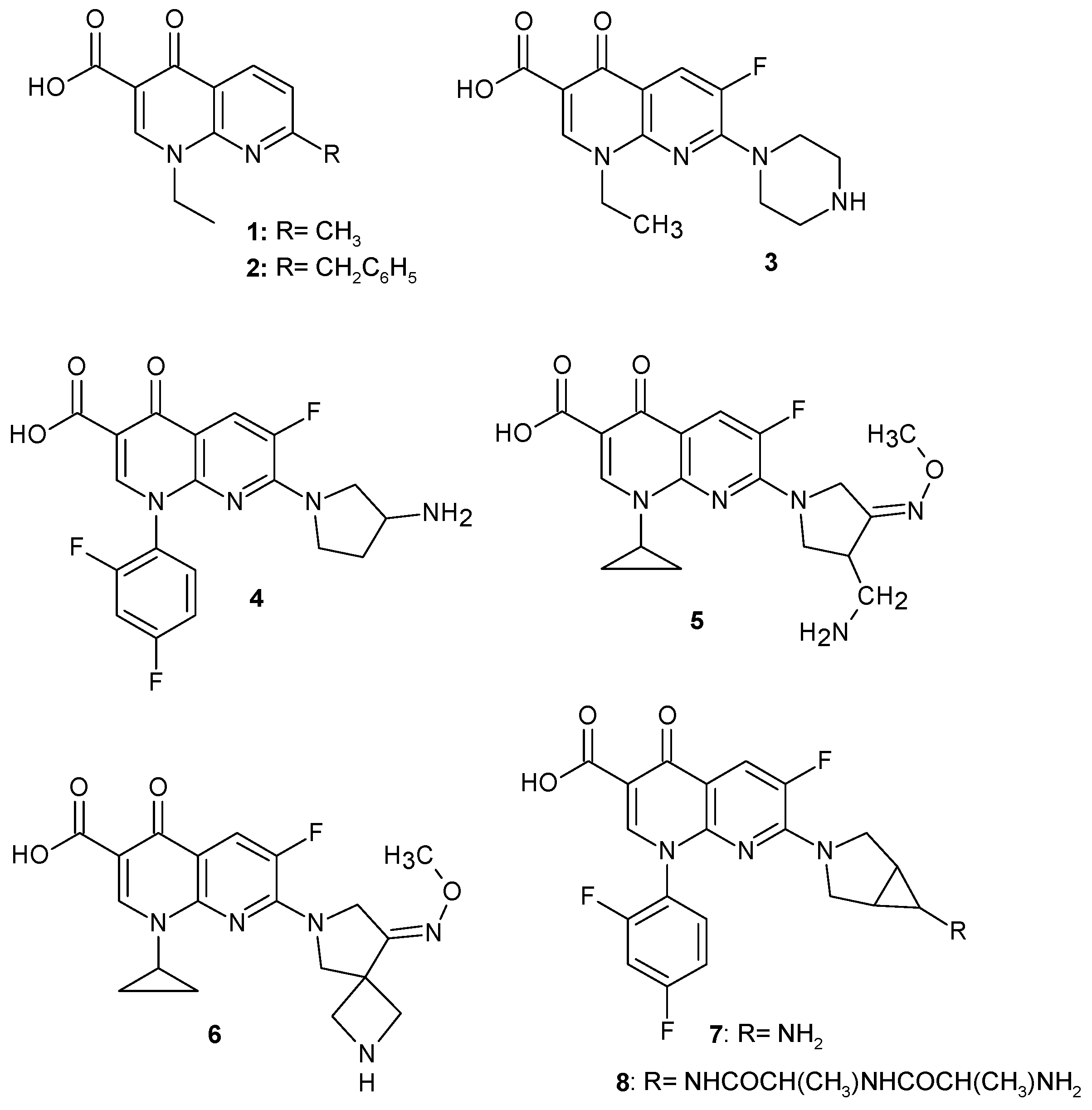
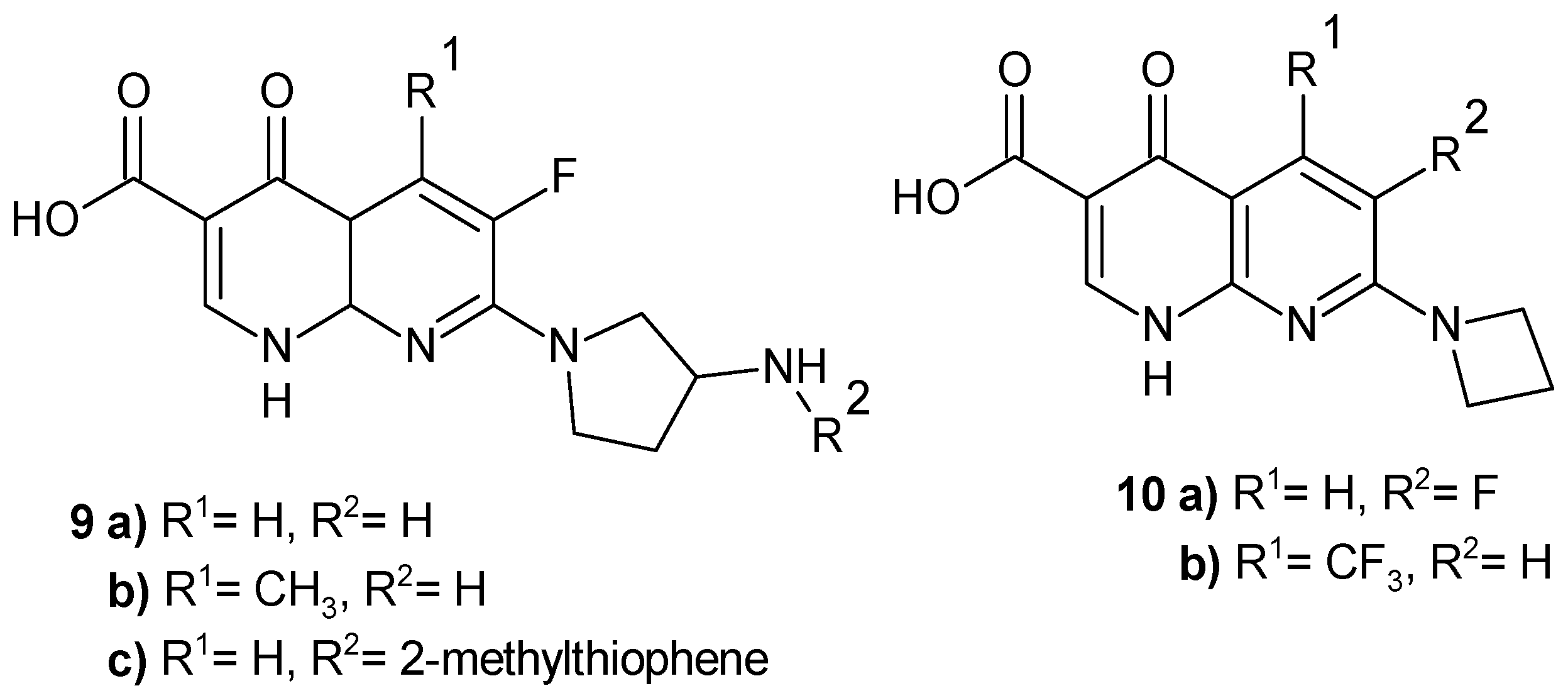
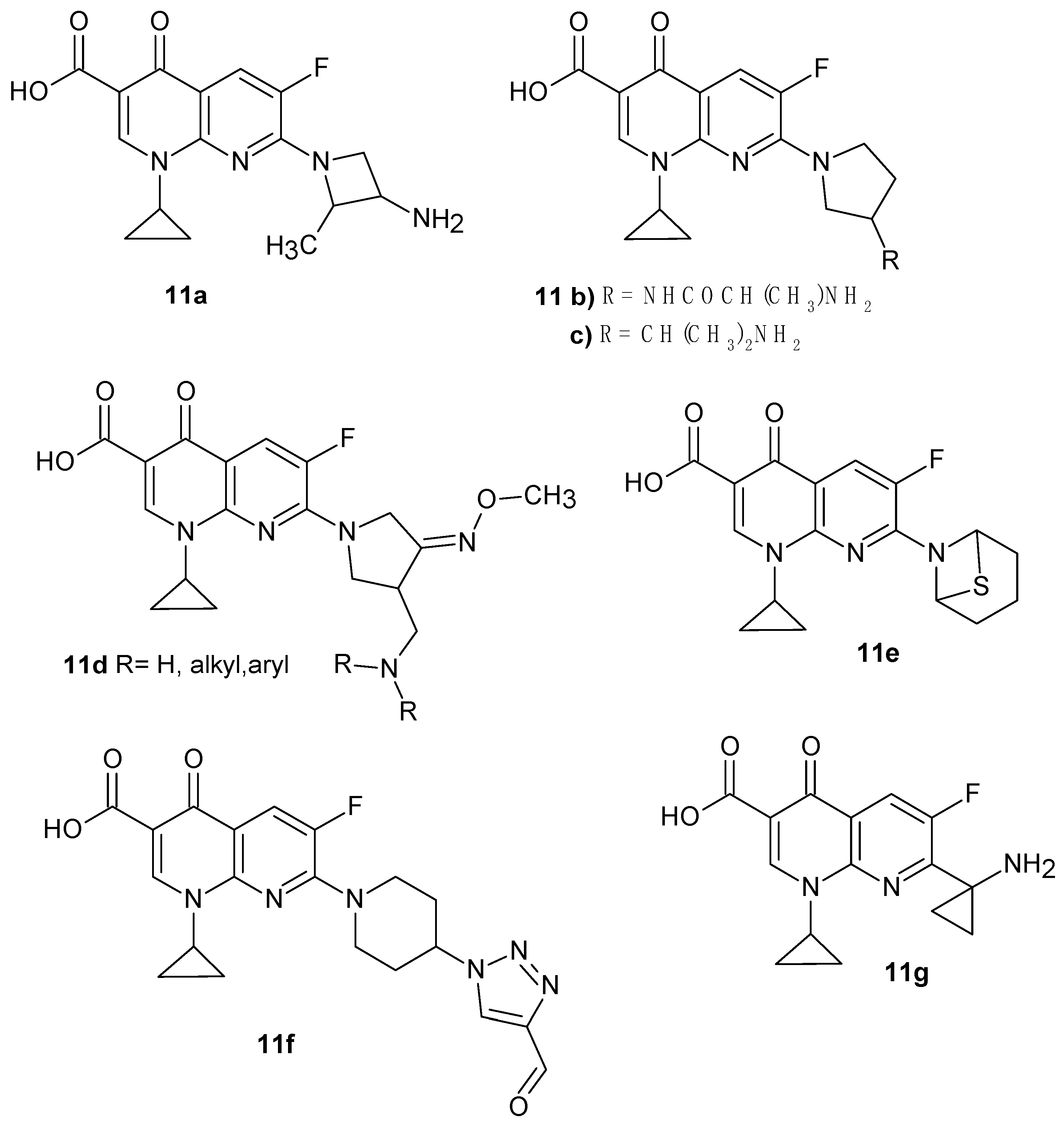
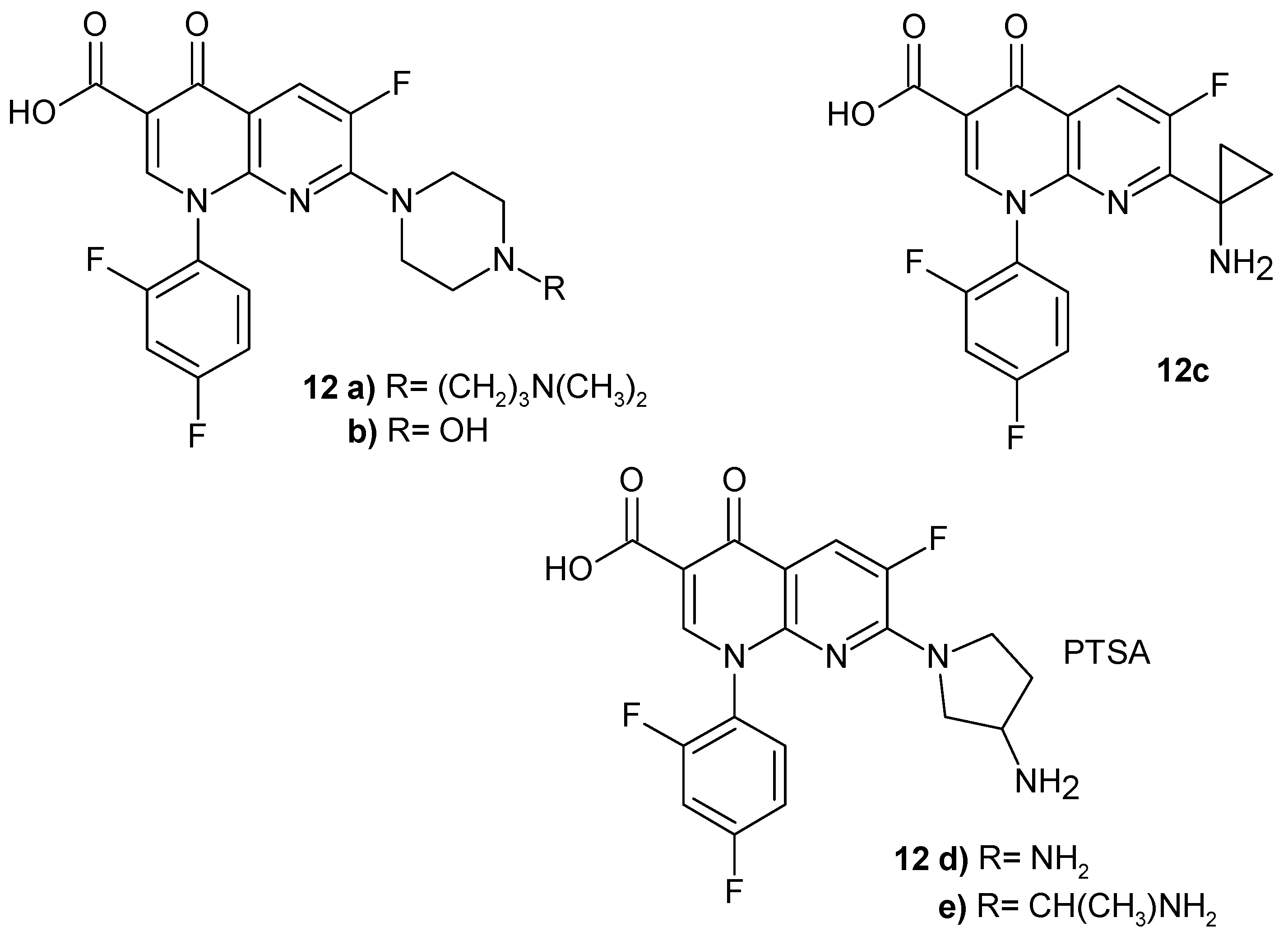
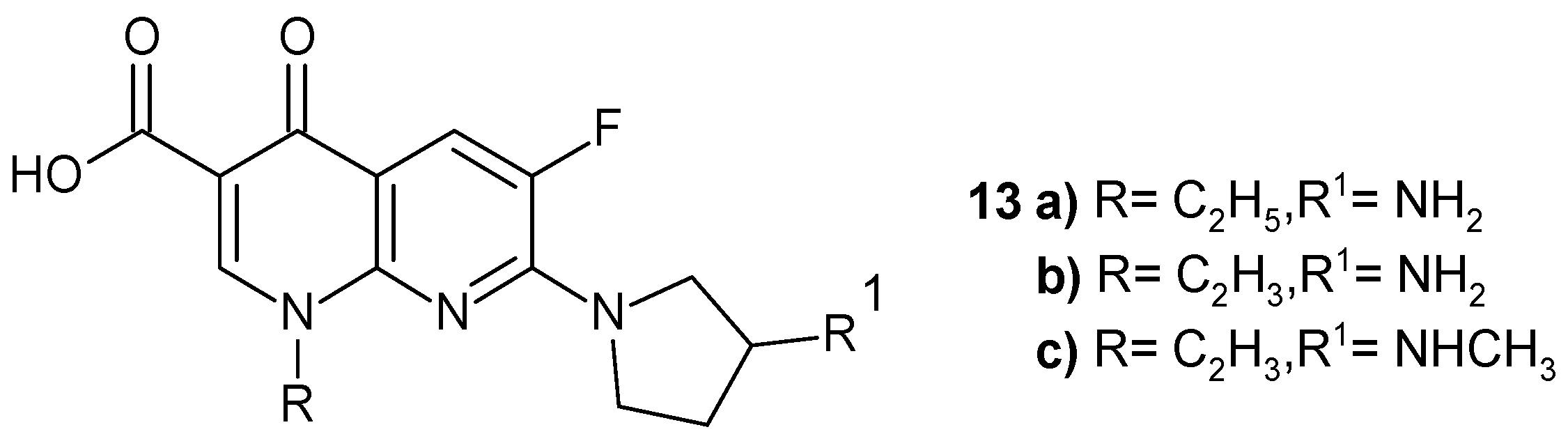
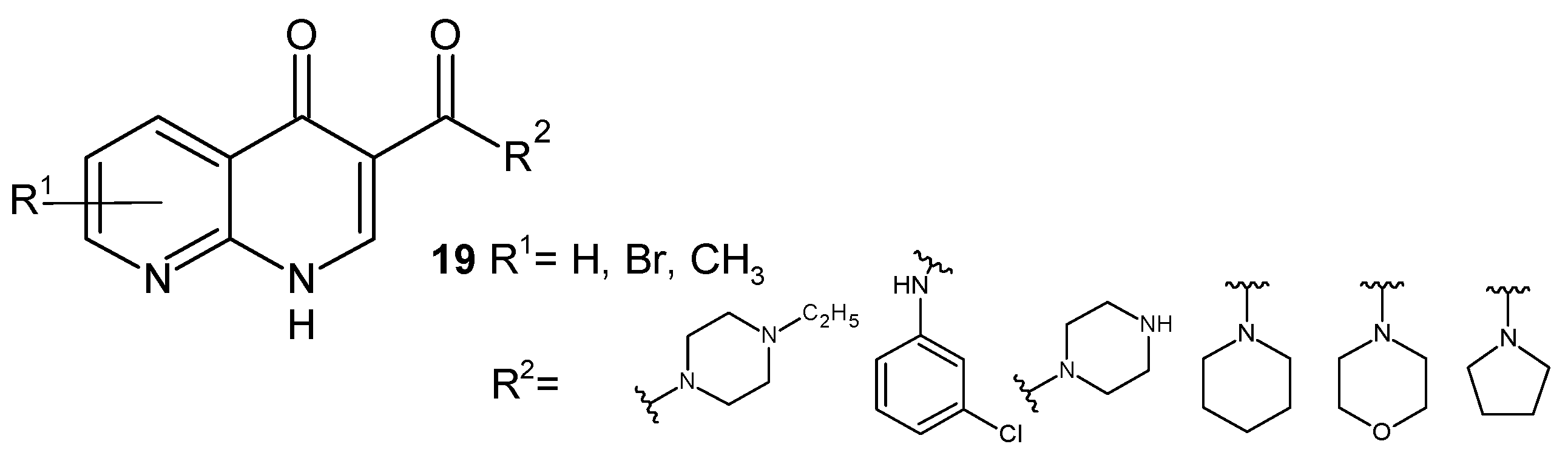
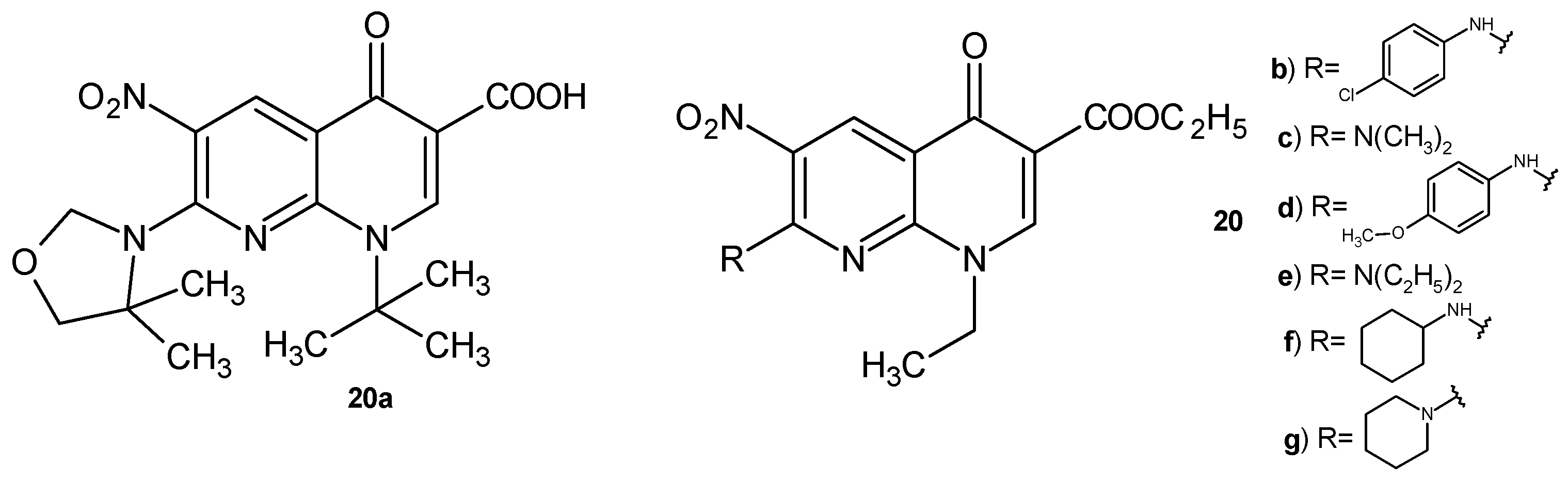
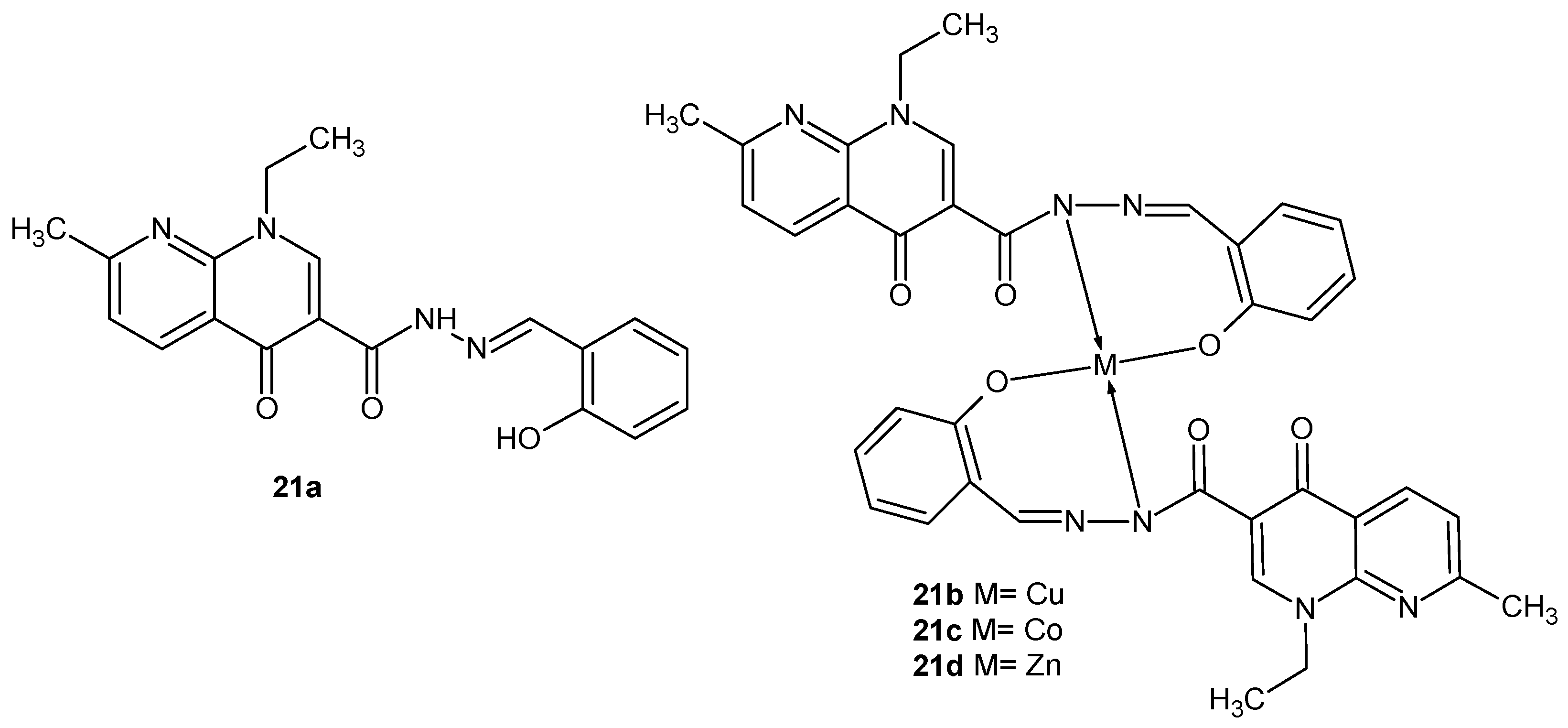
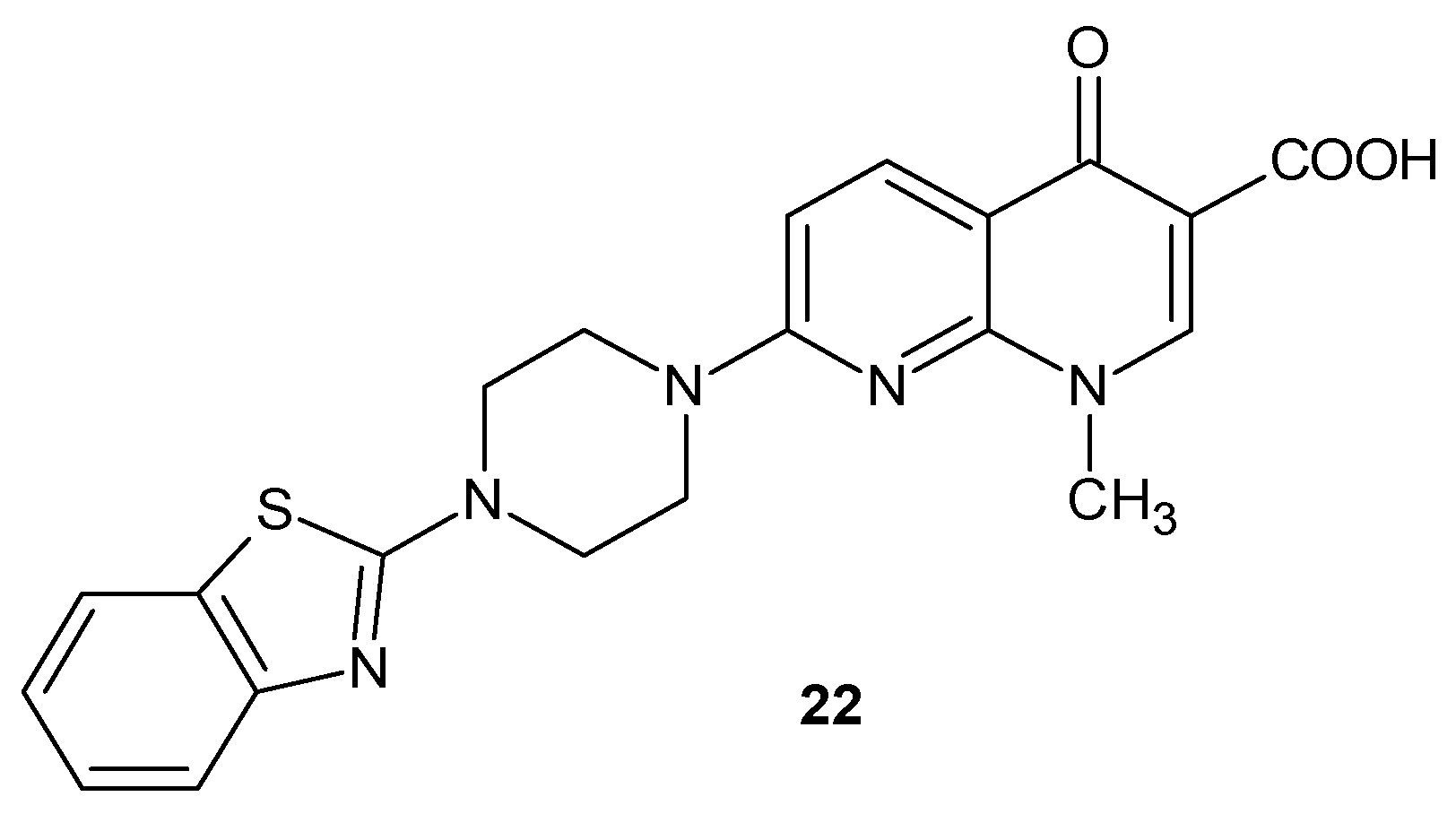
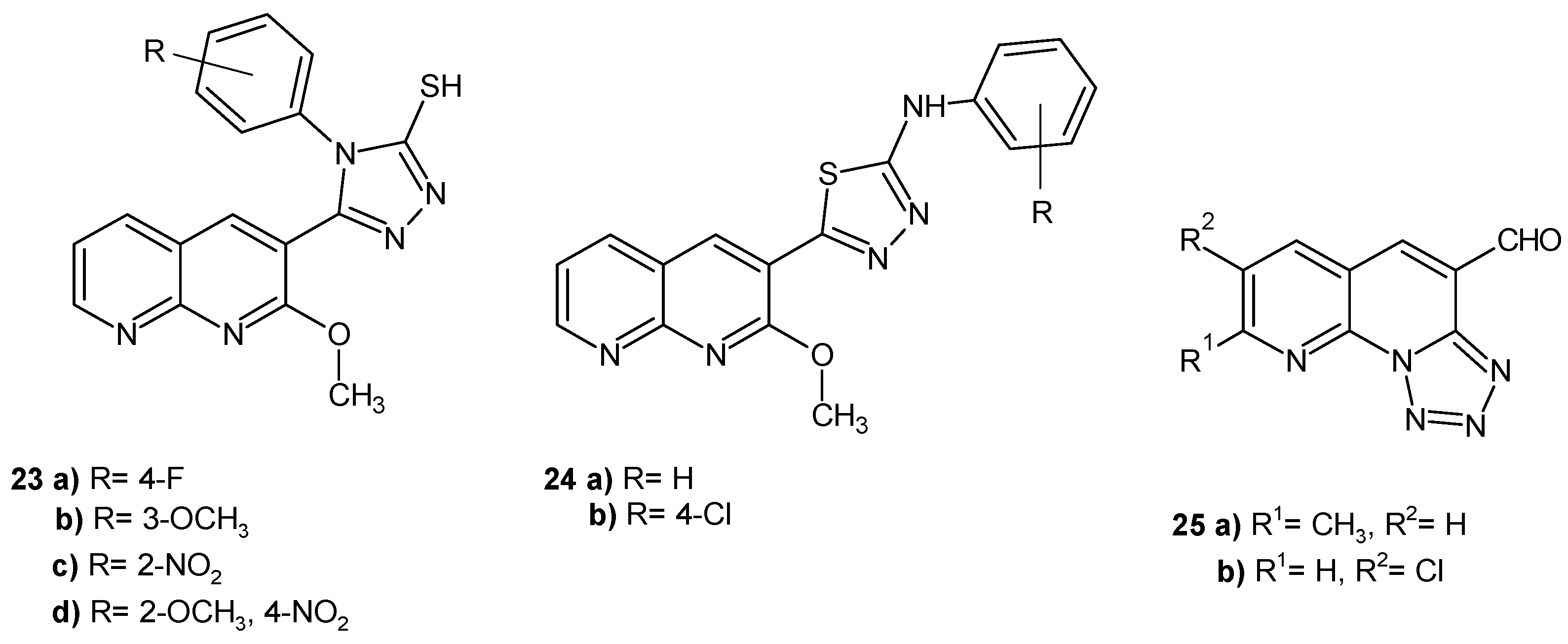
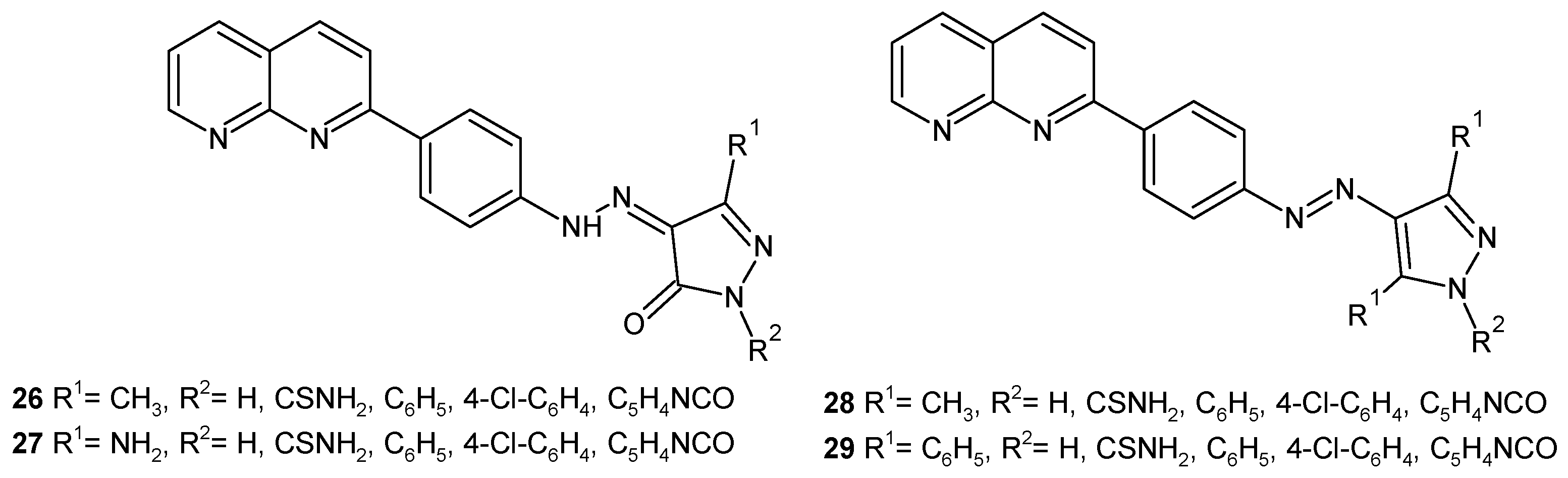
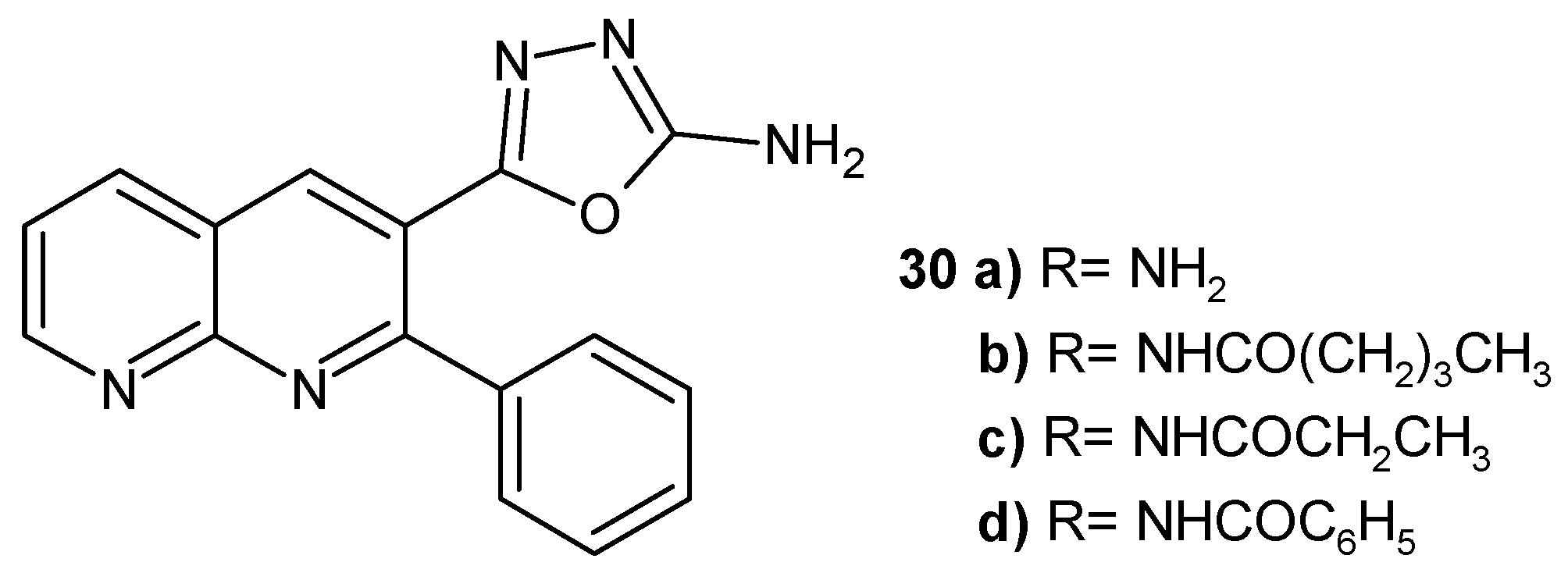
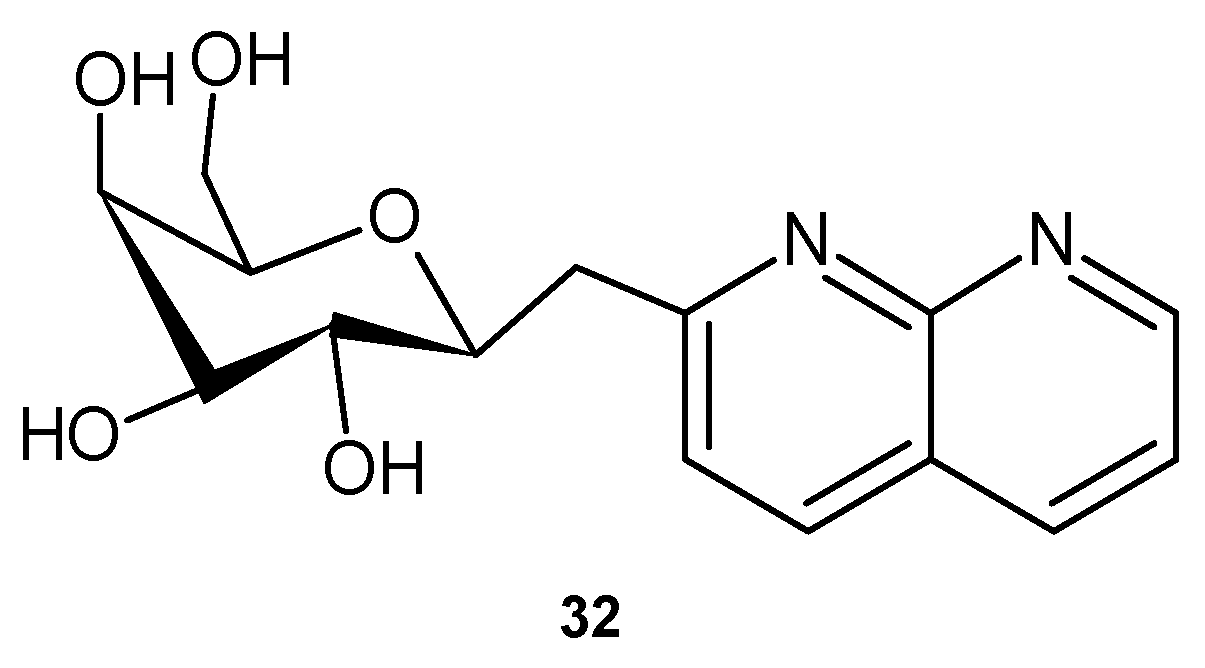
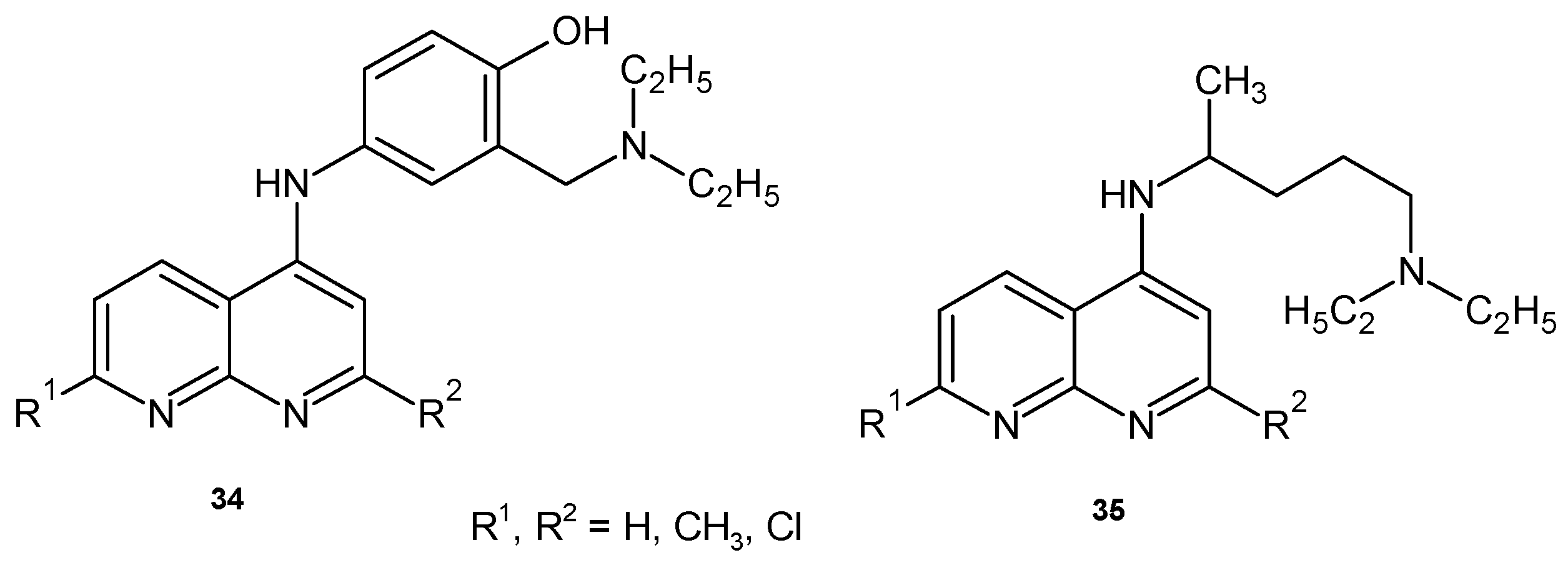
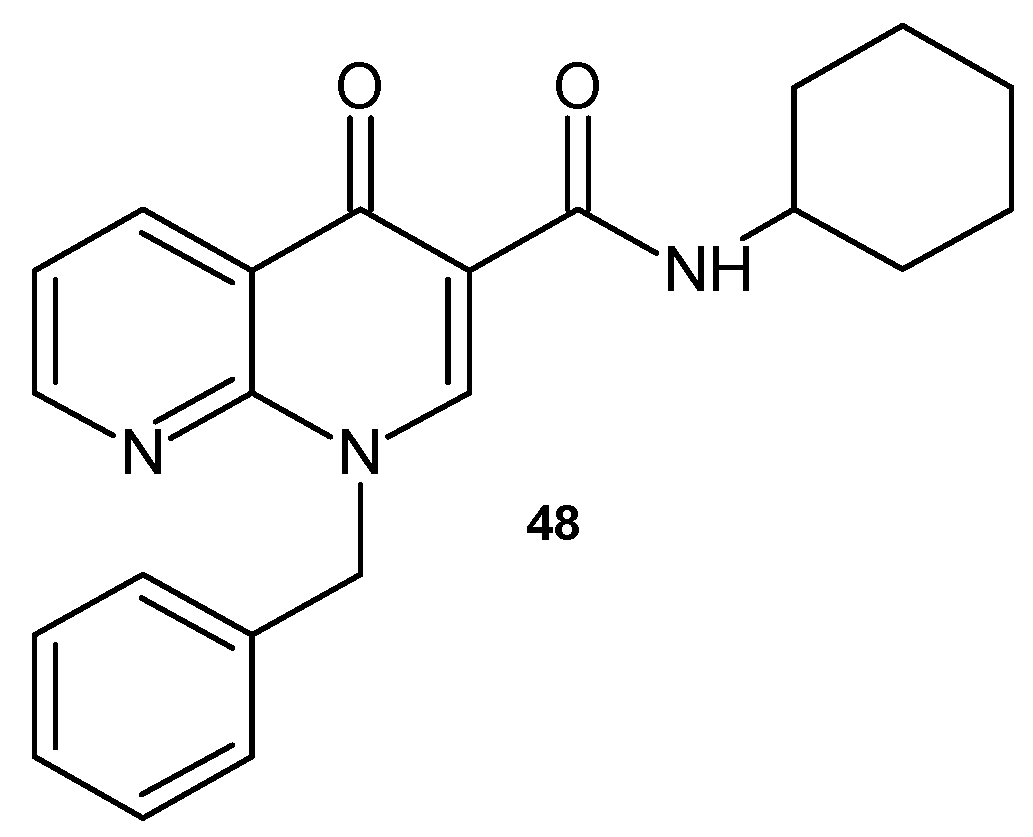
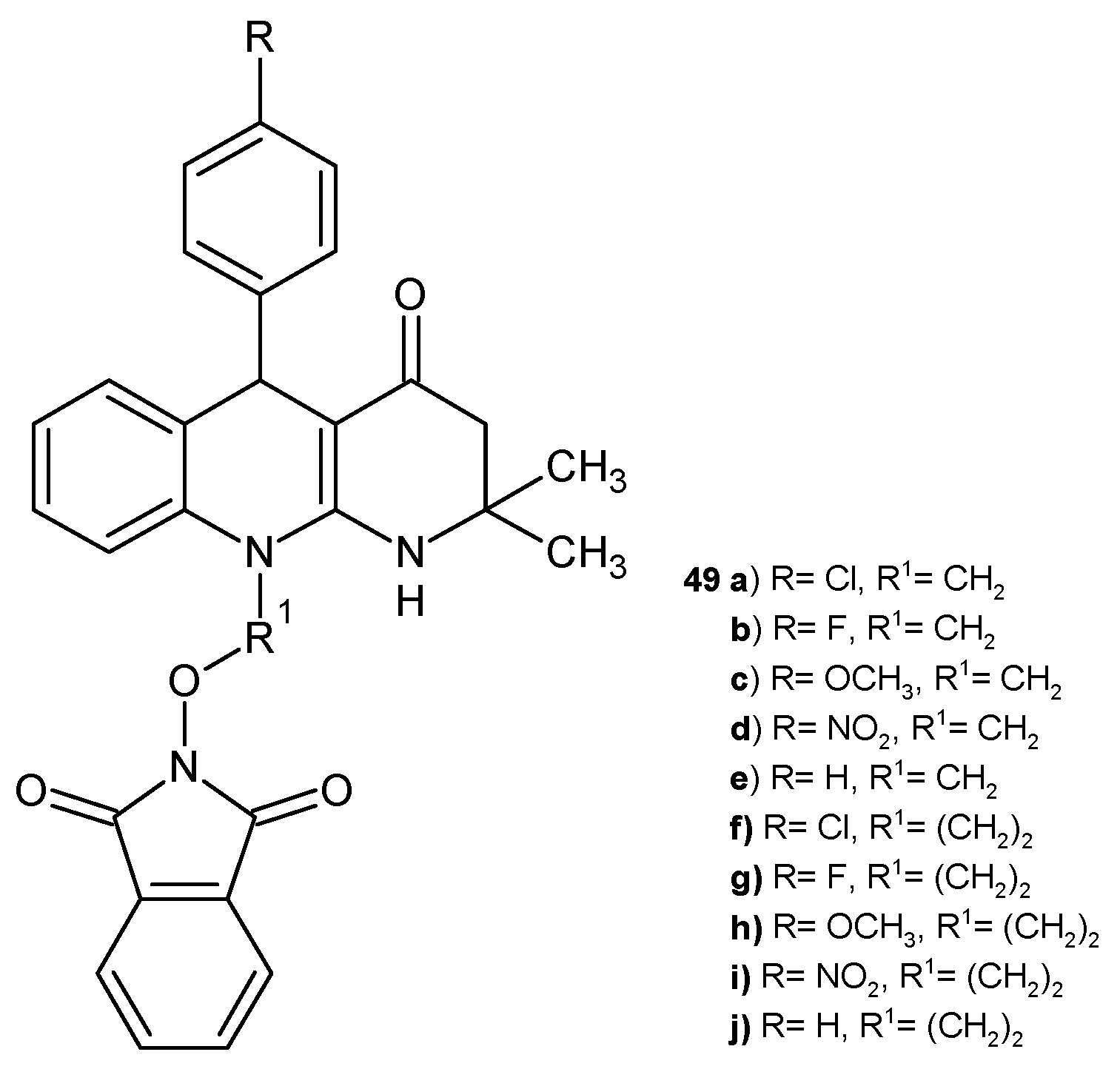
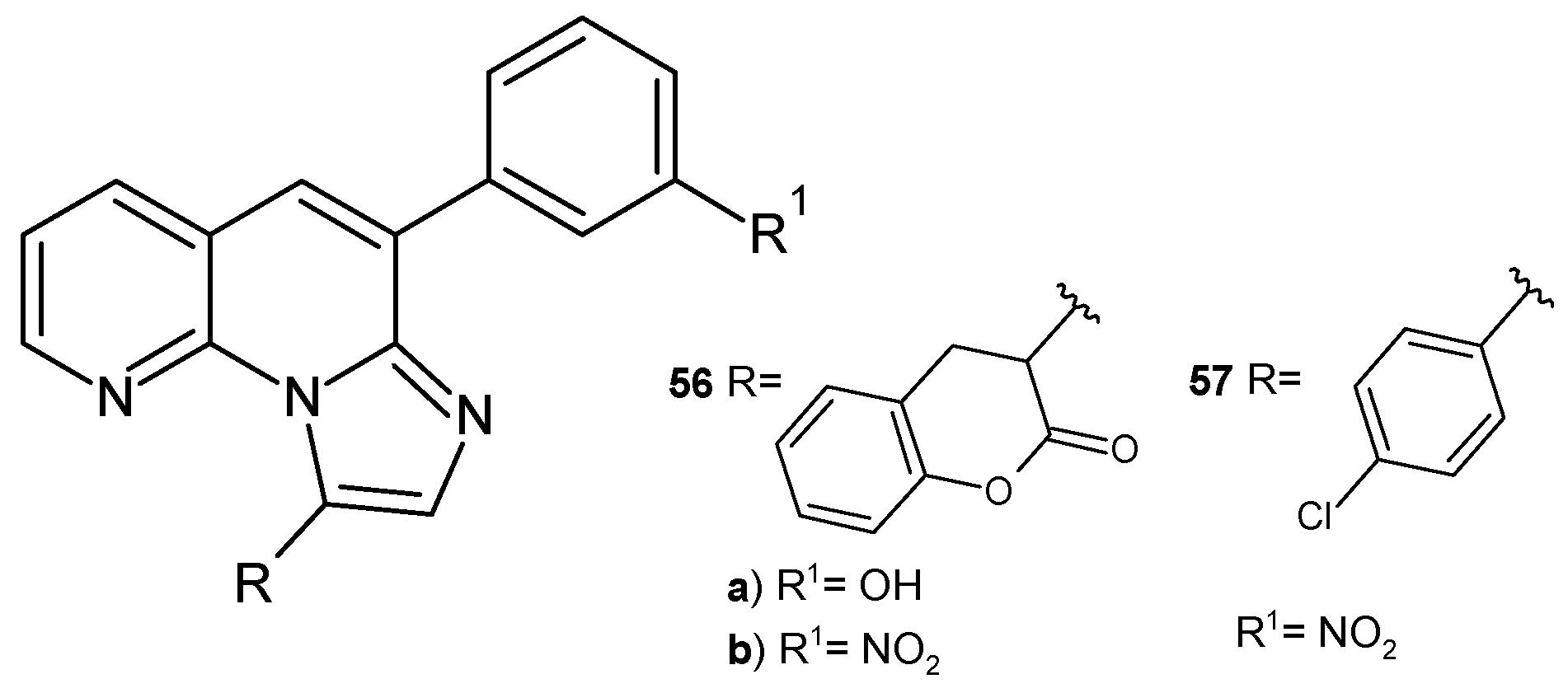
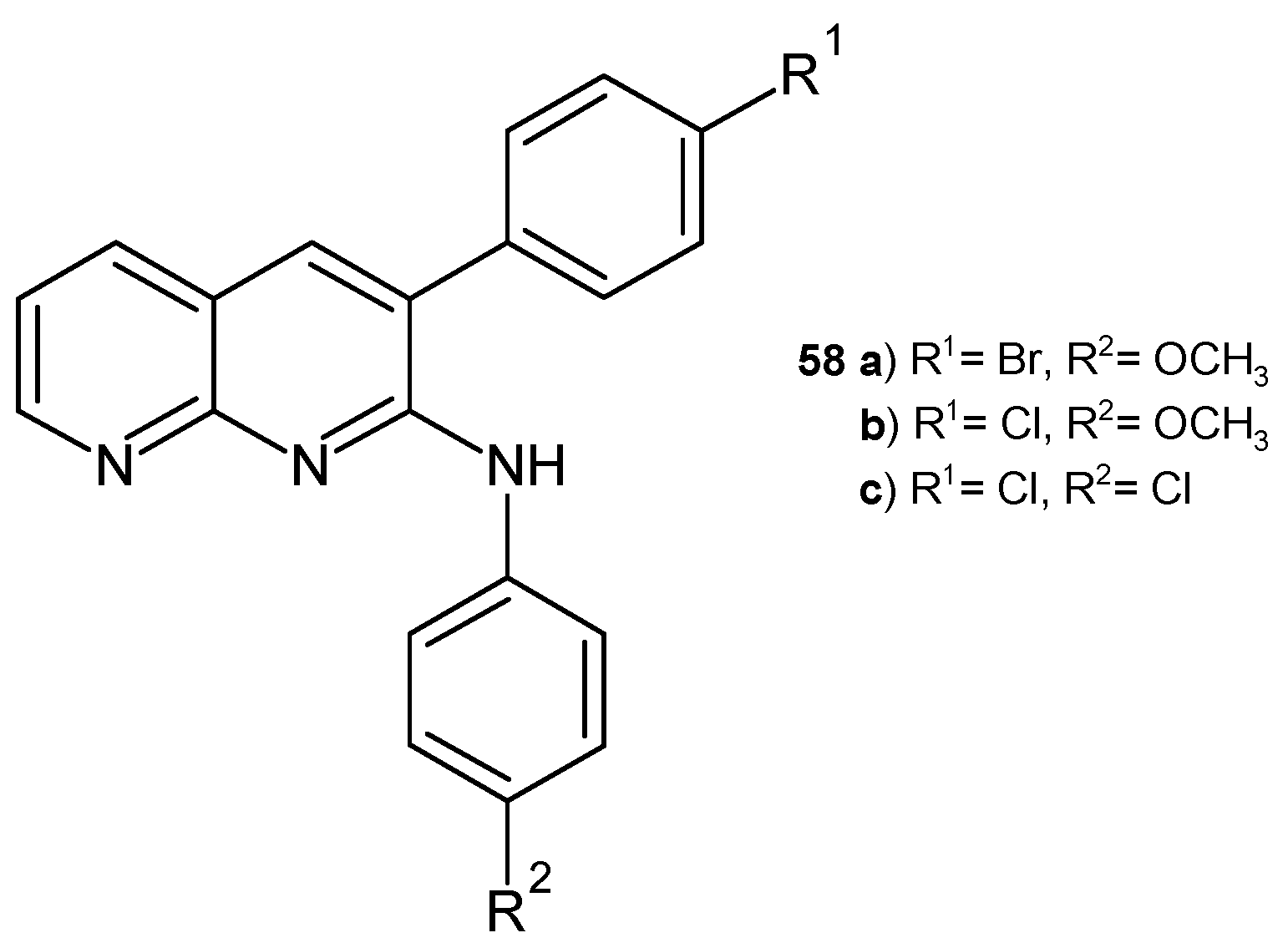
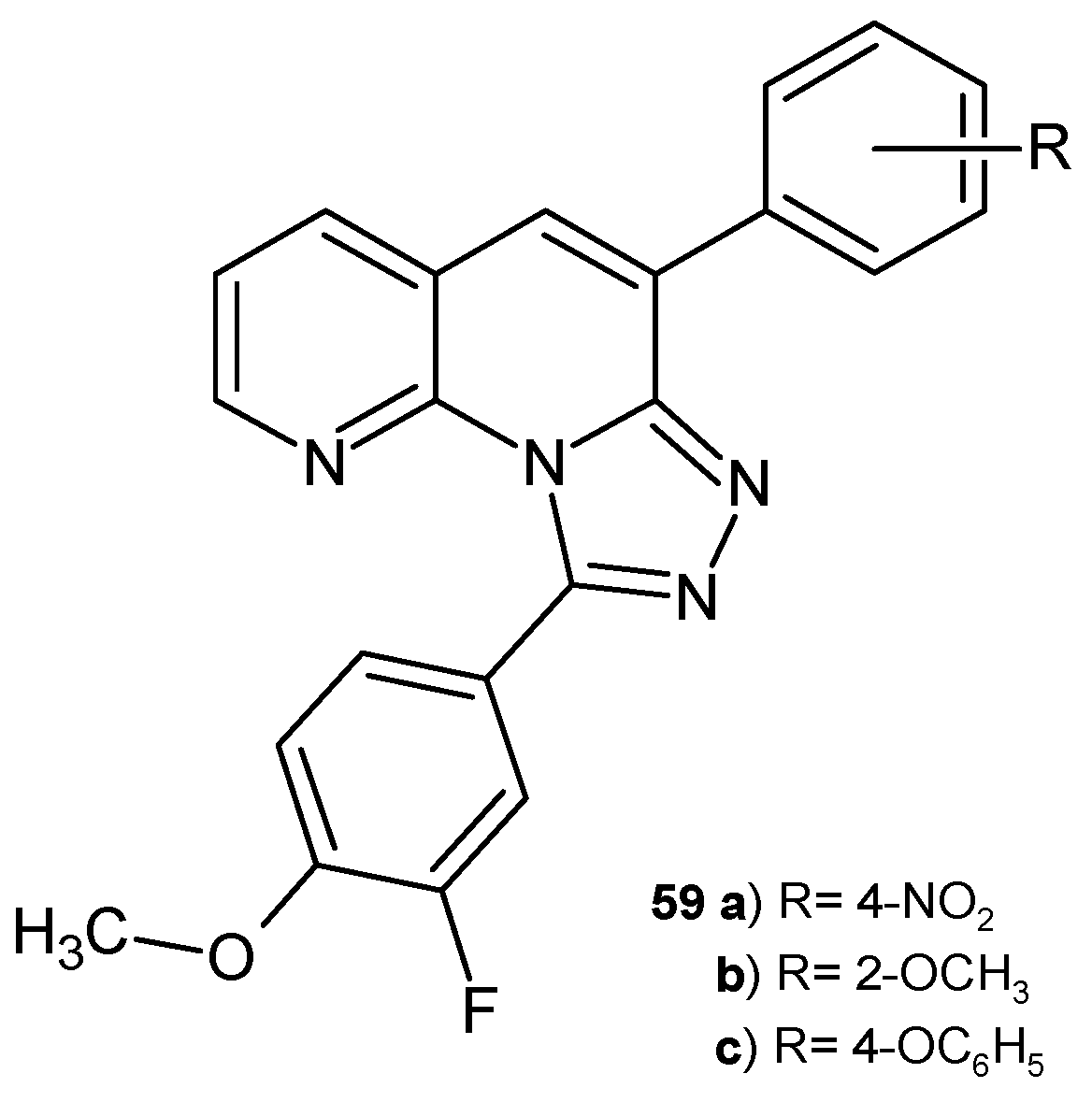
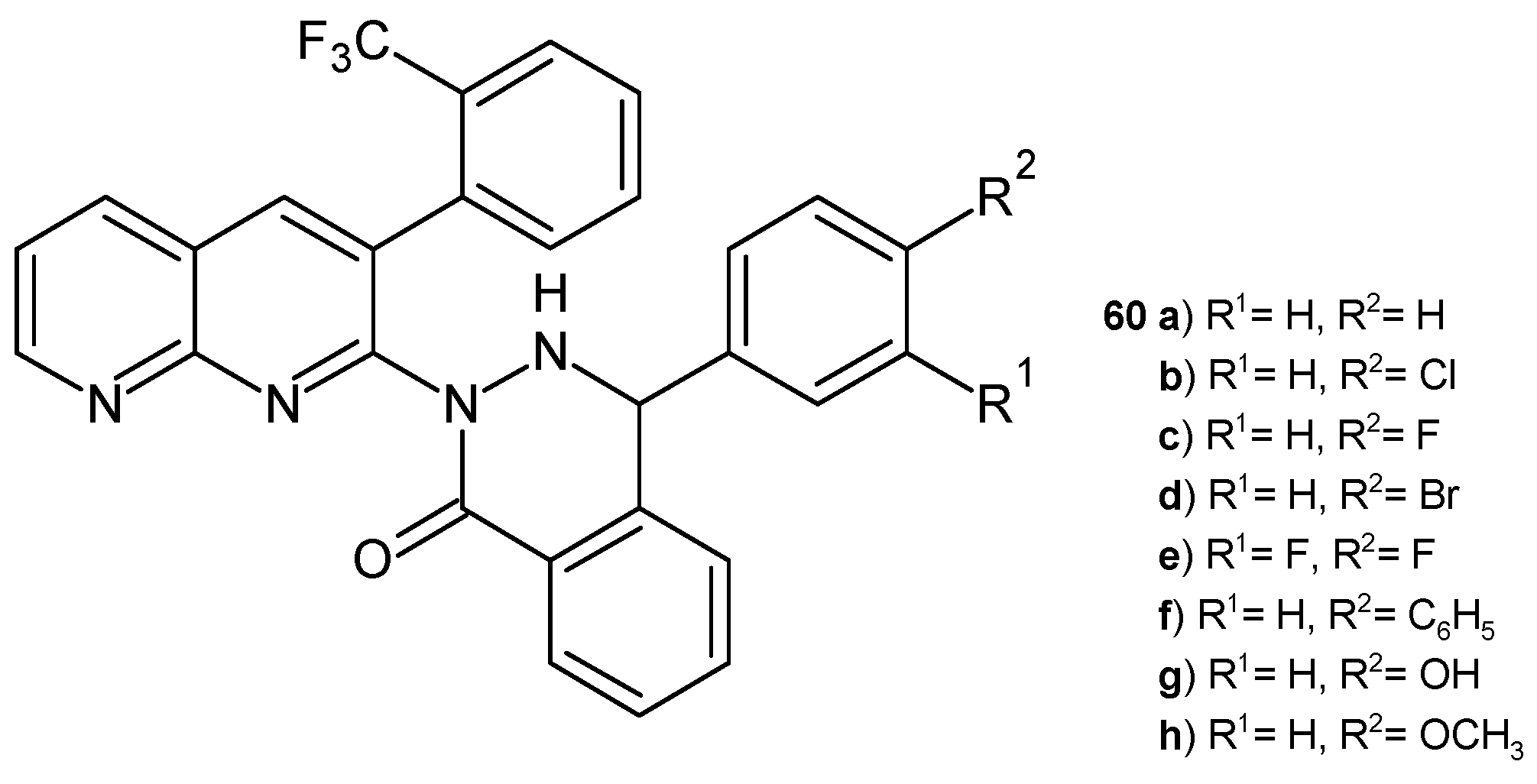
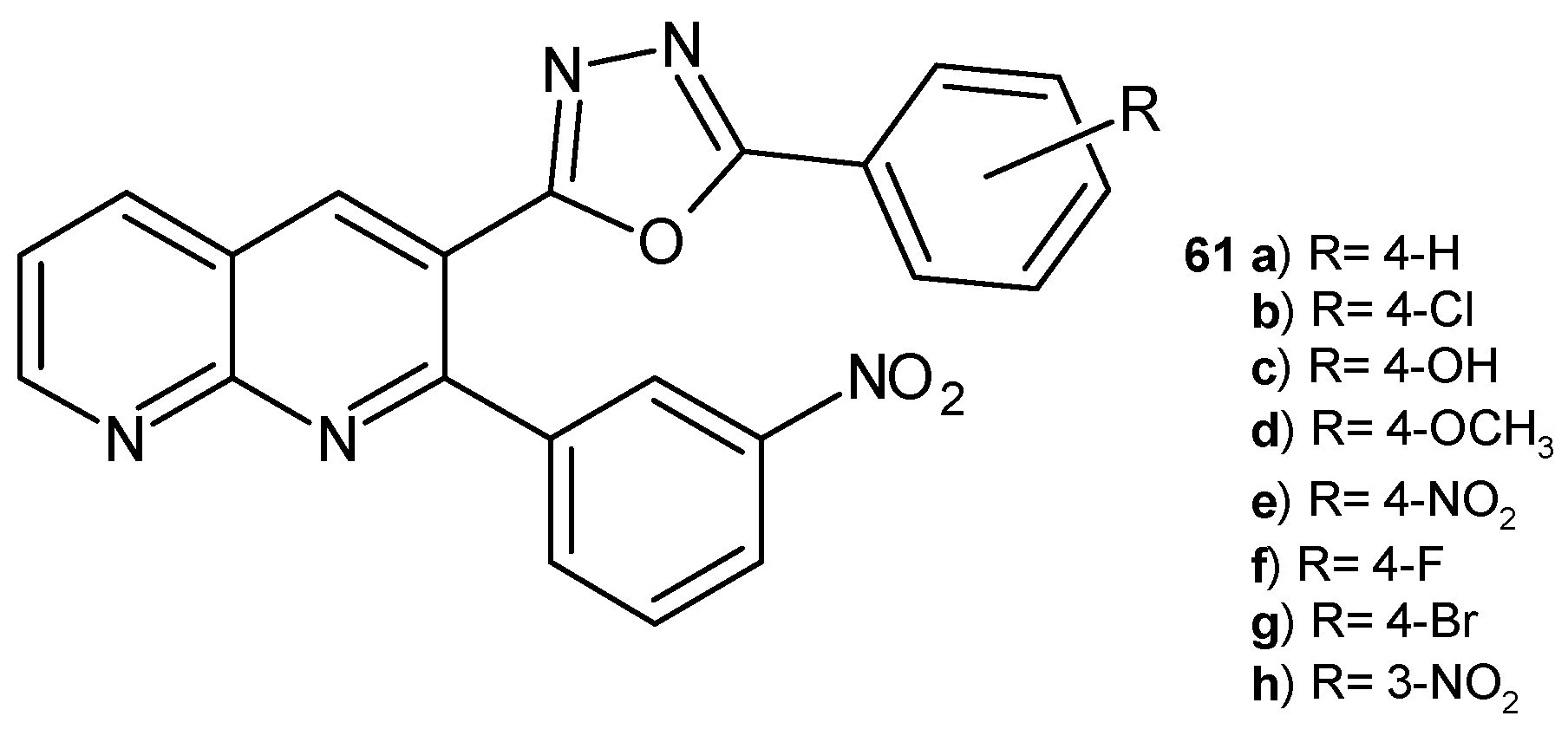
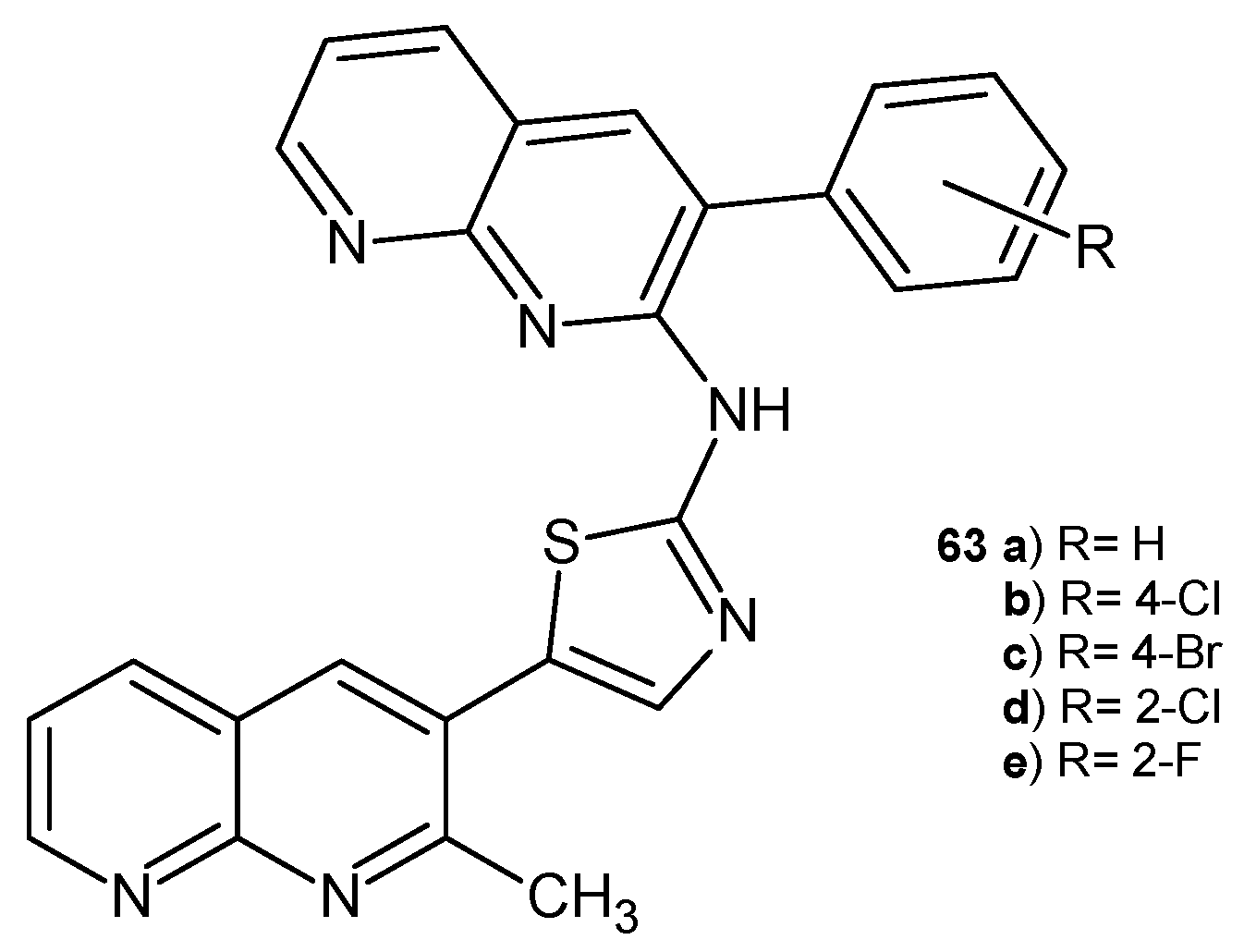
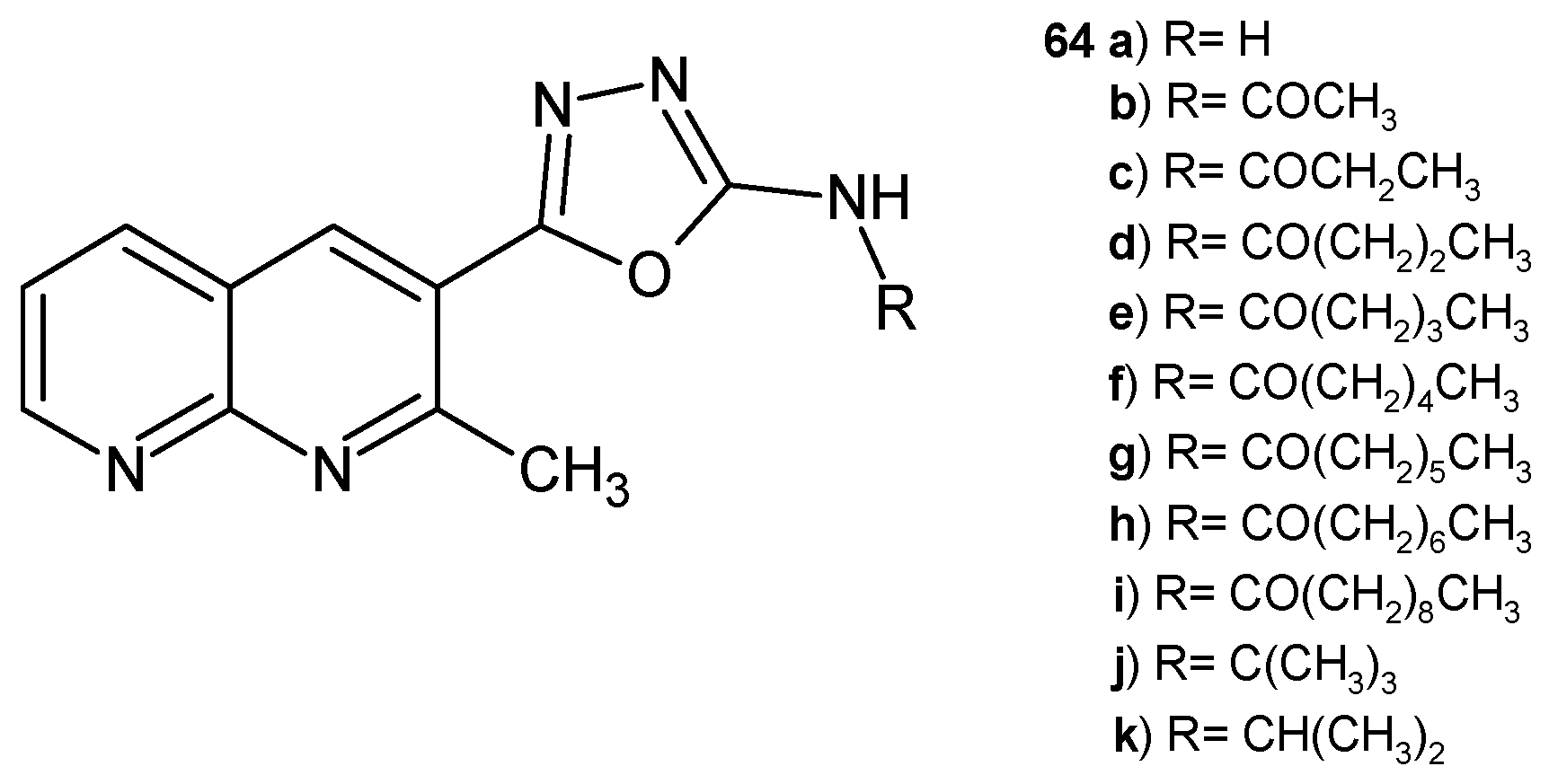
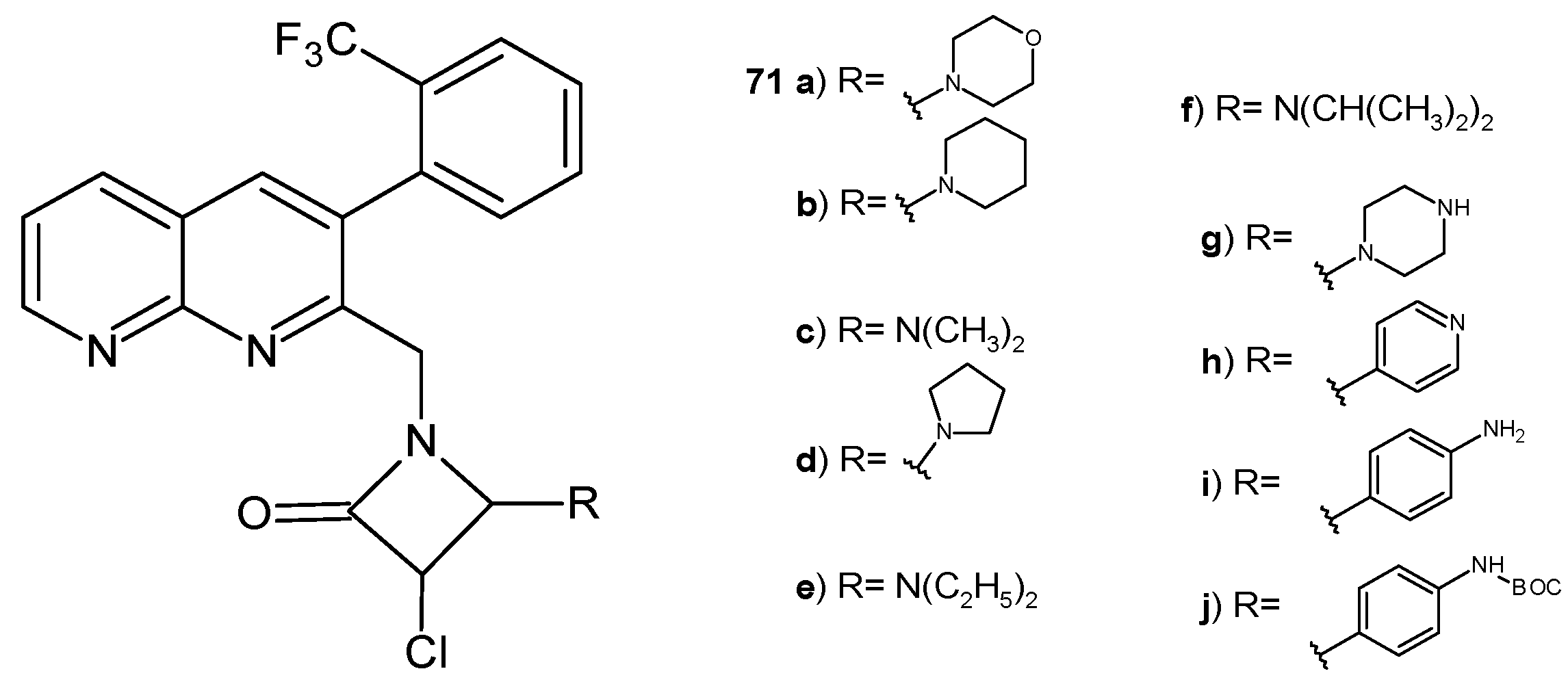
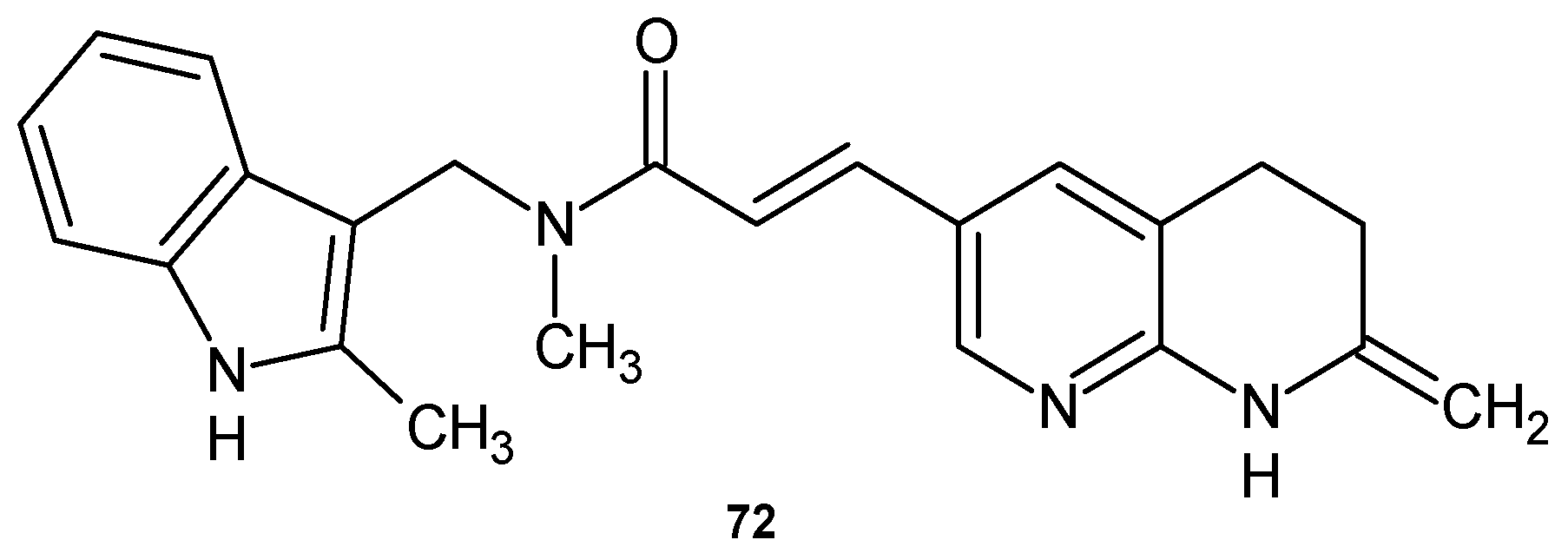
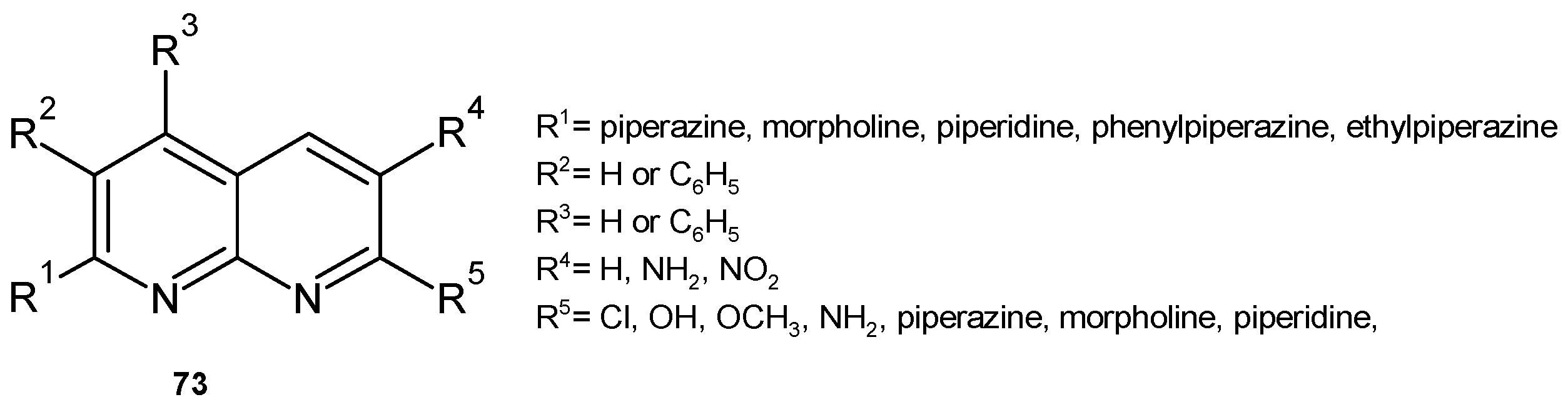
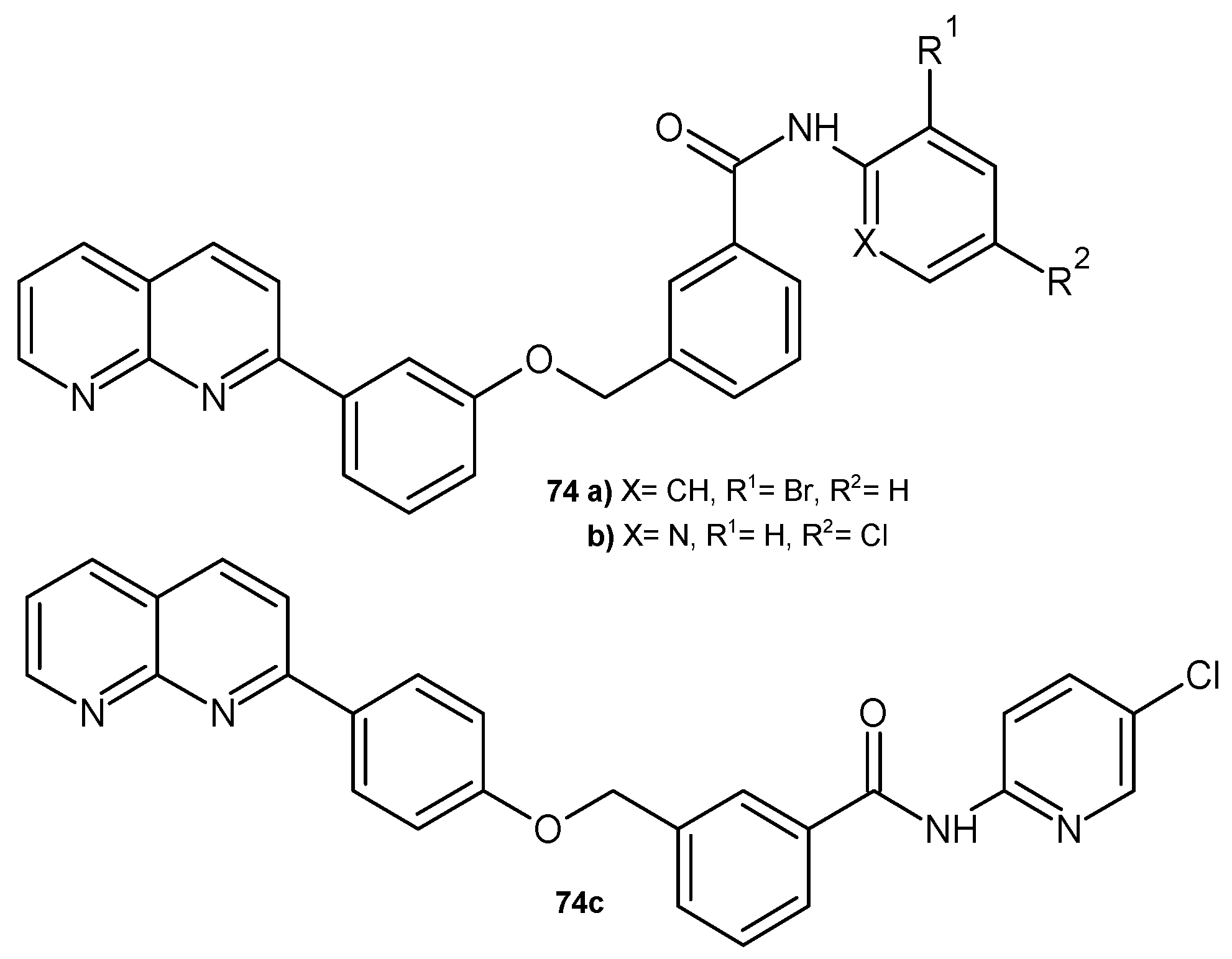
2.2. 1,8-Naphthyridine Derivatives with Antimicrobial Activity
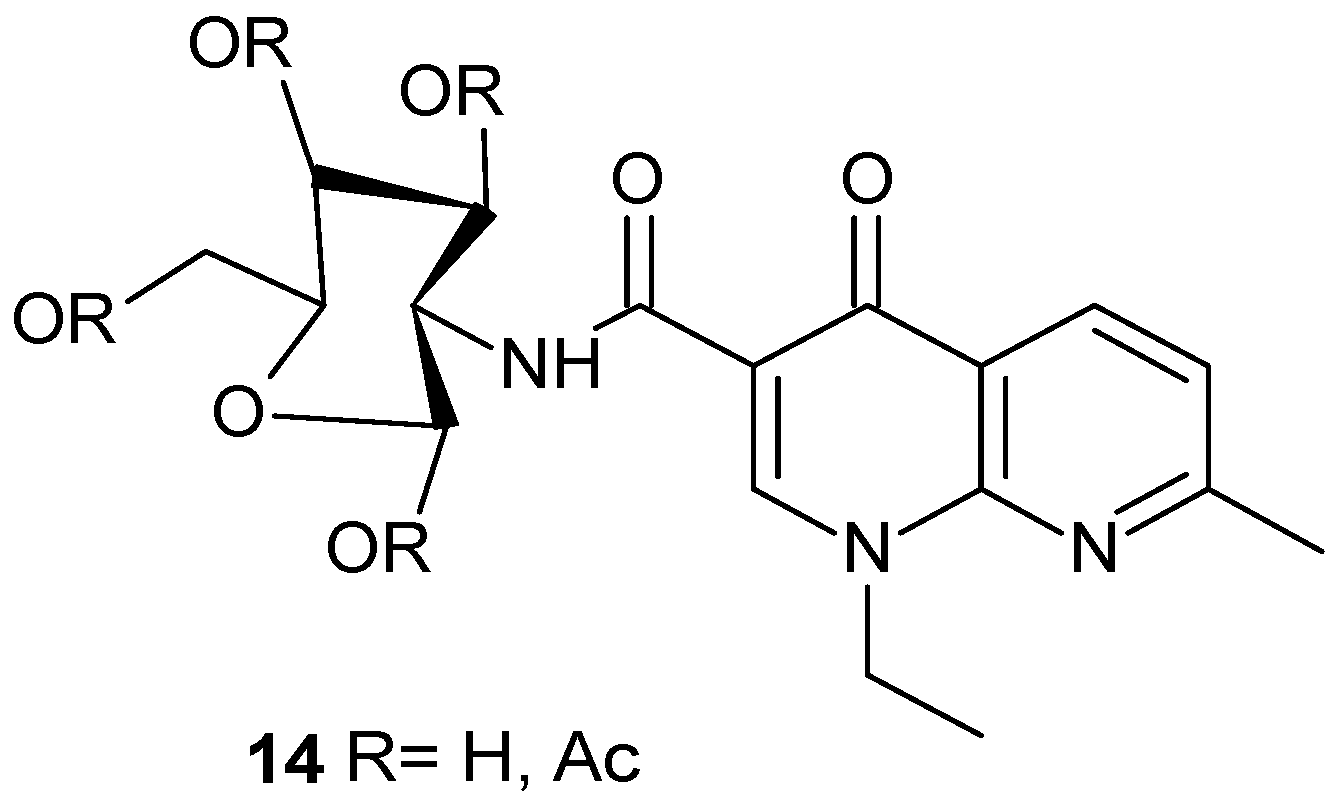
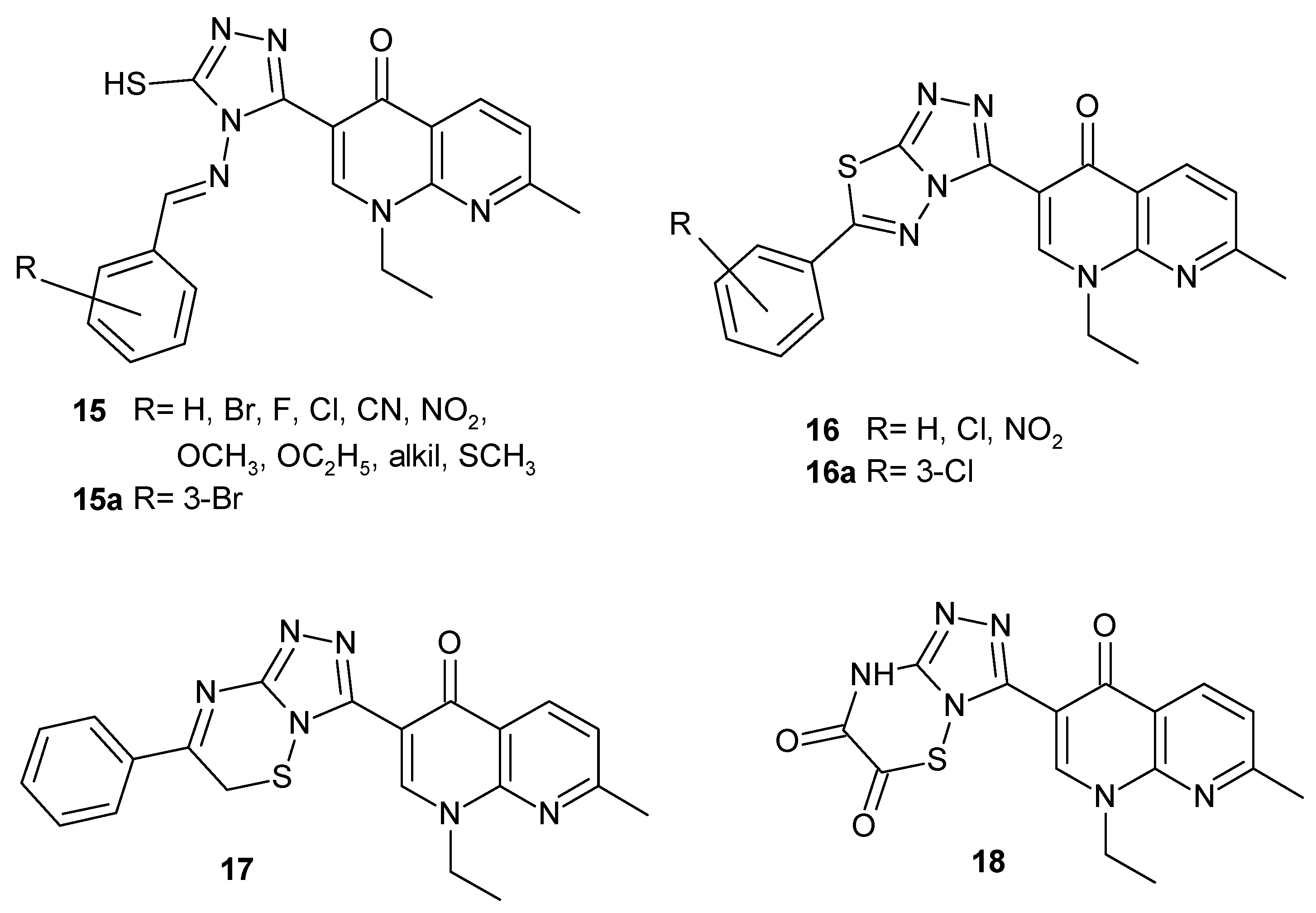
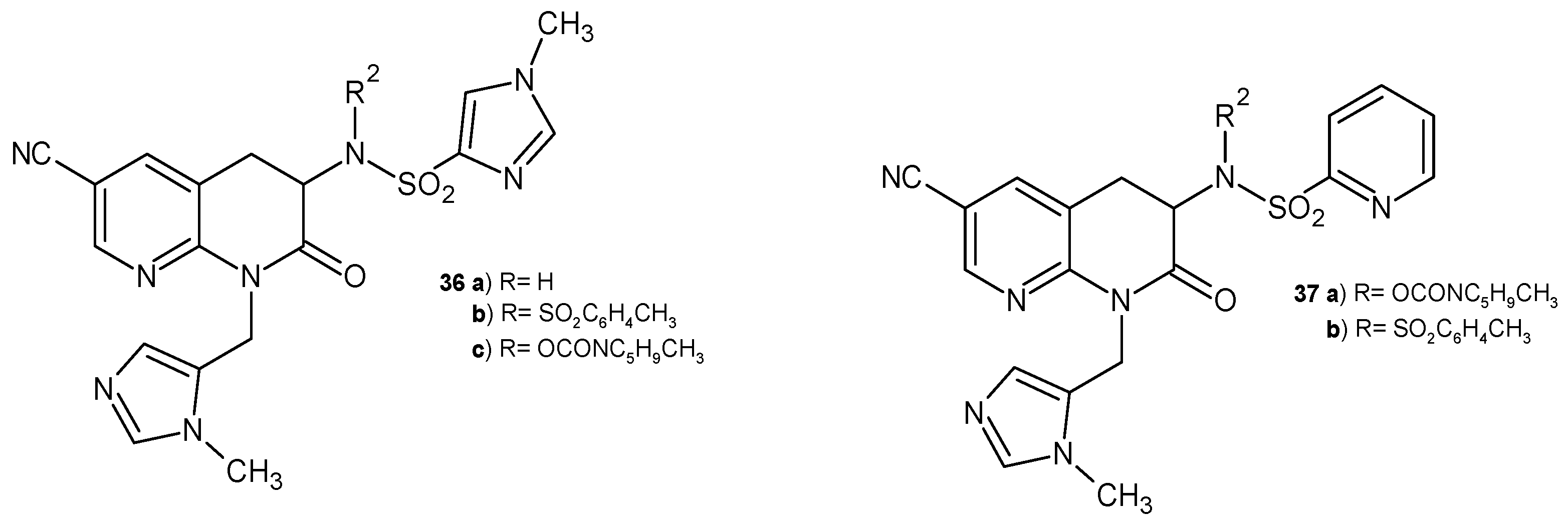
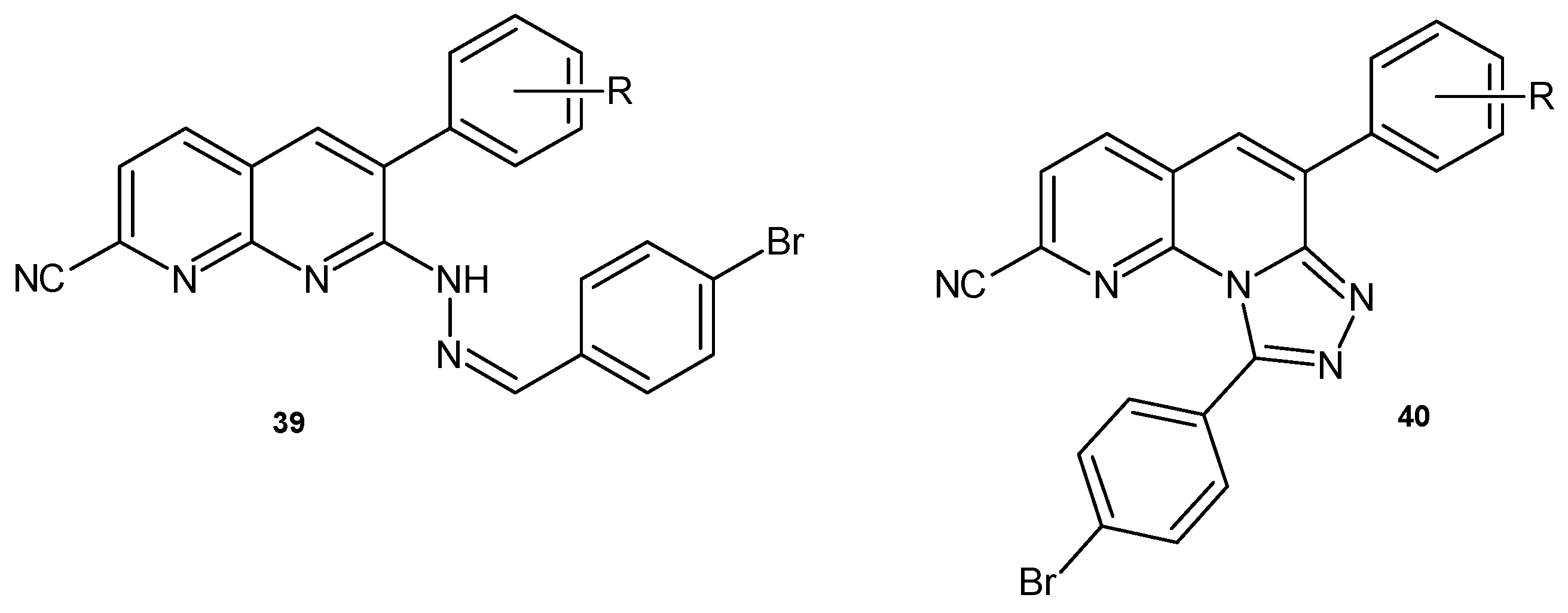
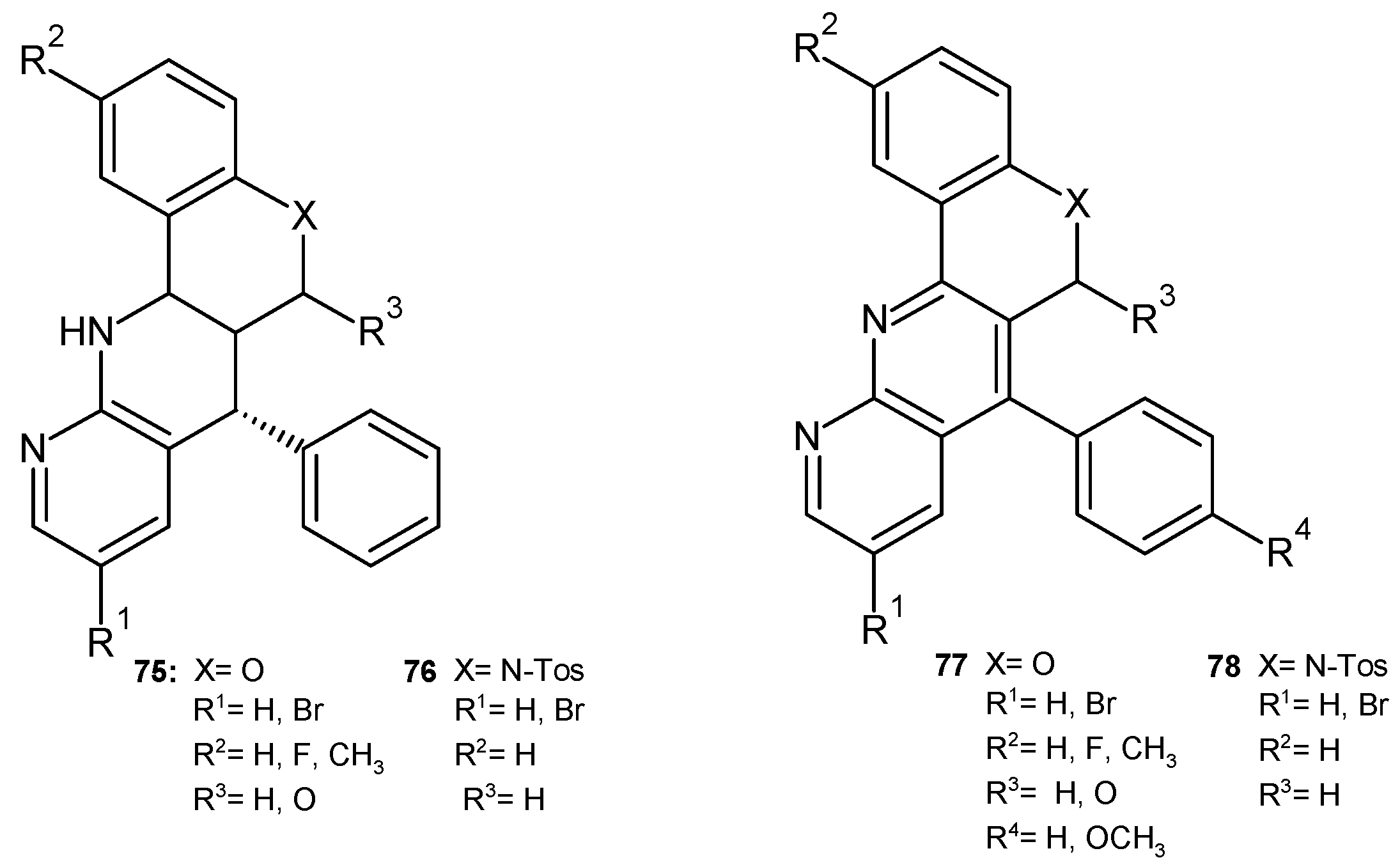
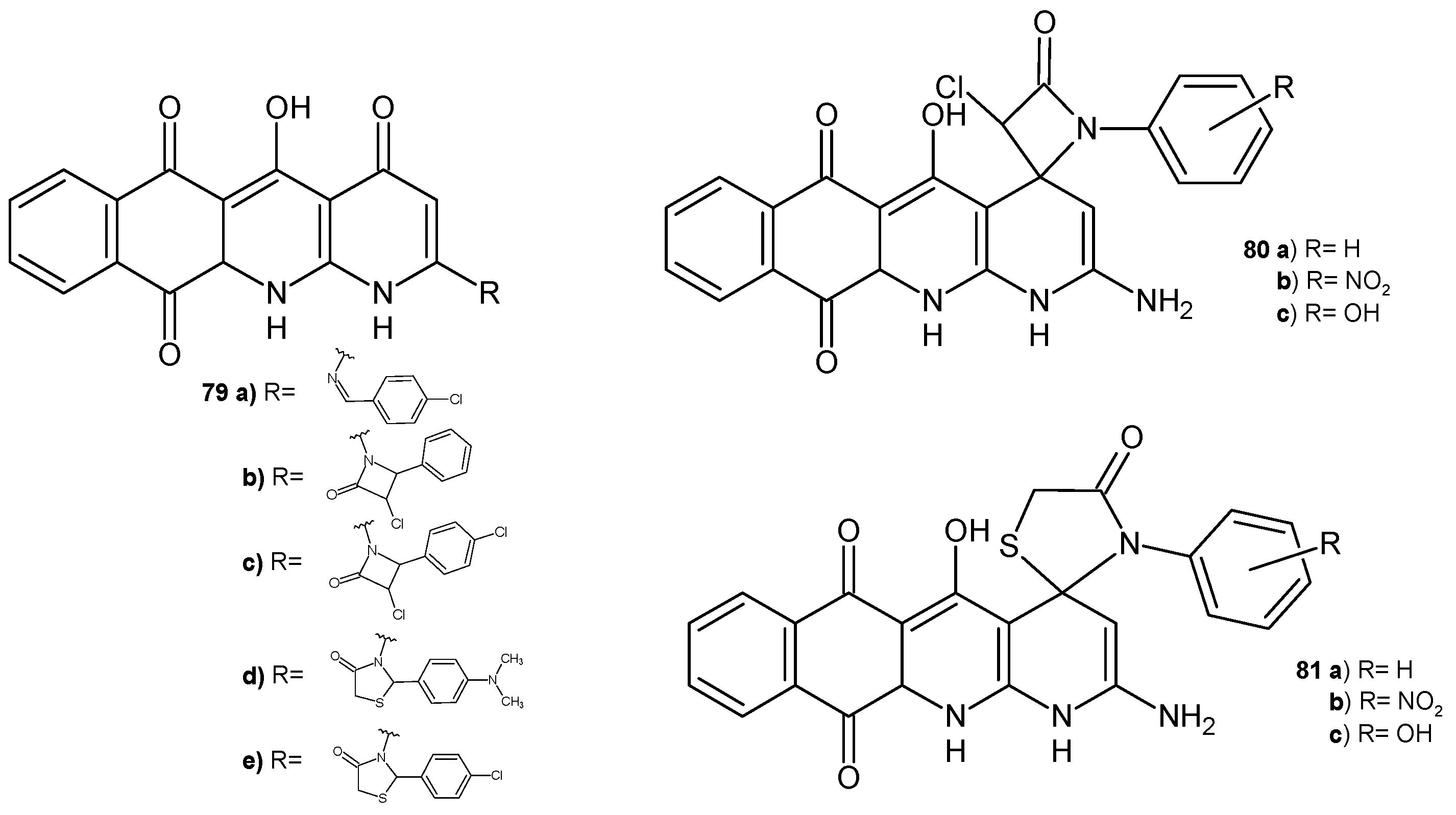
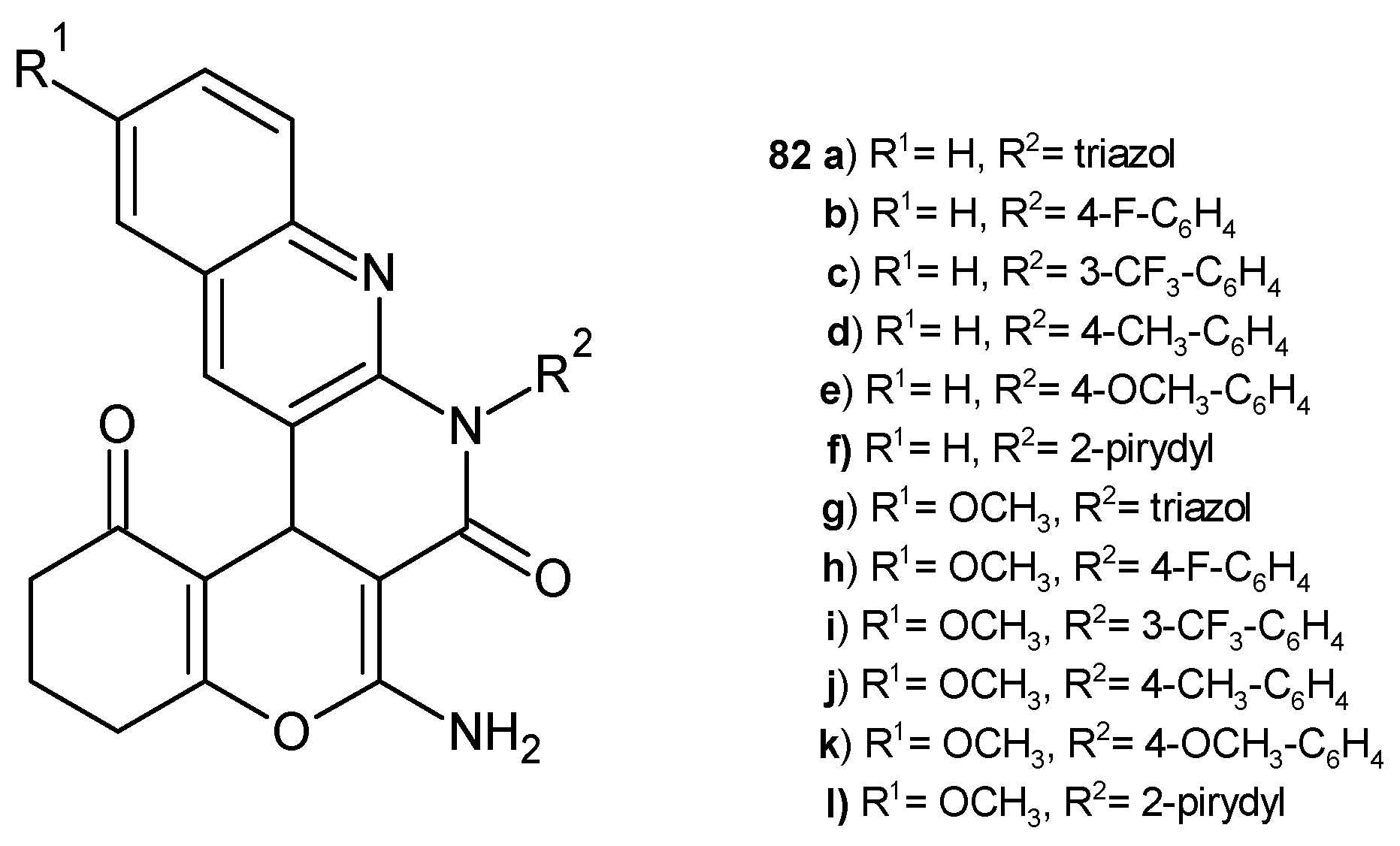
2.3. Polycyclic Derivatives of 1,8-Naphthyridine
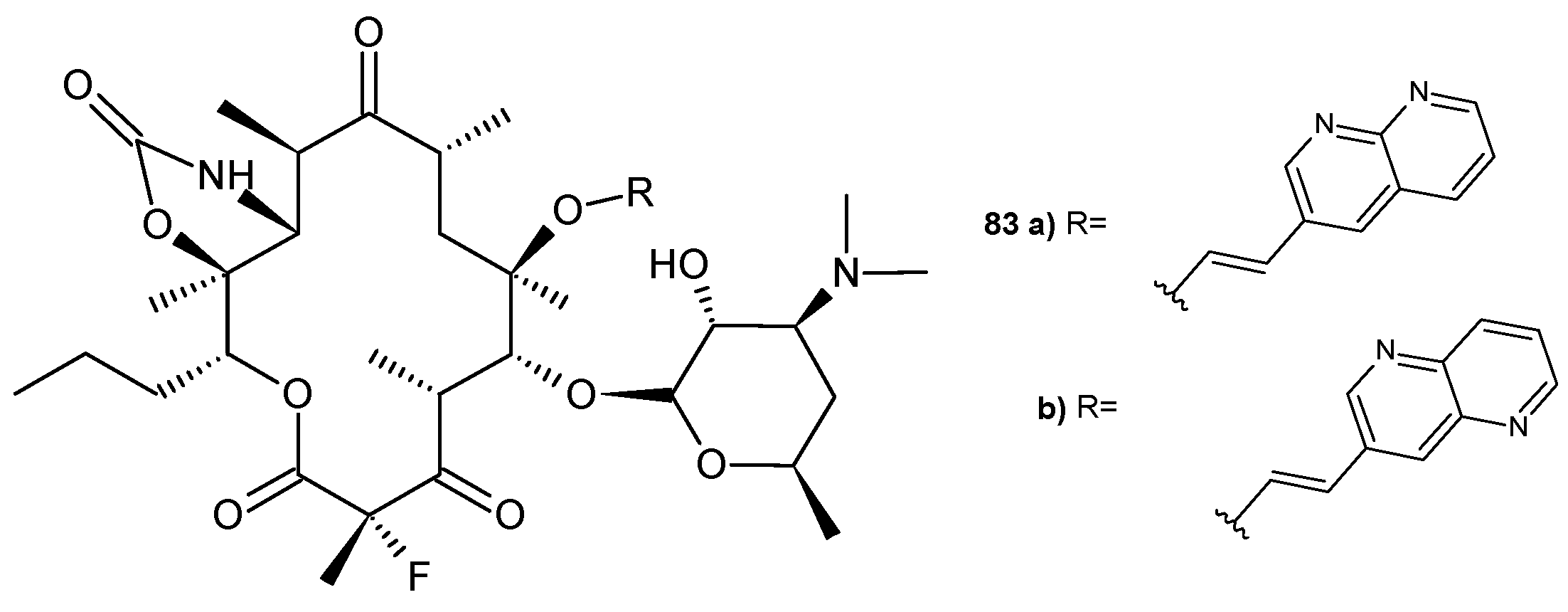
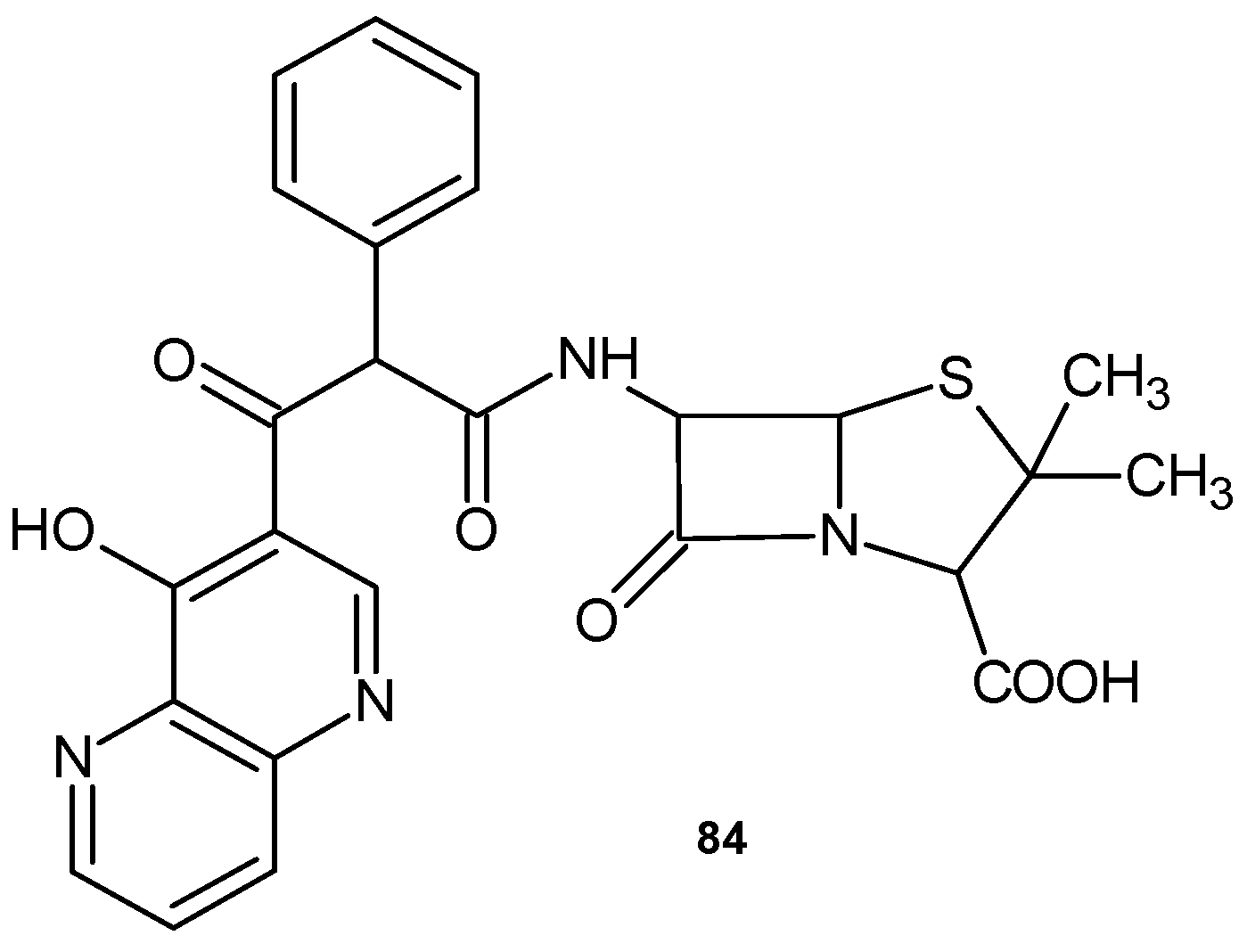
2.4. 1,8-Naphthyridine Conjugated with Ketolide
3. 1,5-Naphthyridine Derivatives
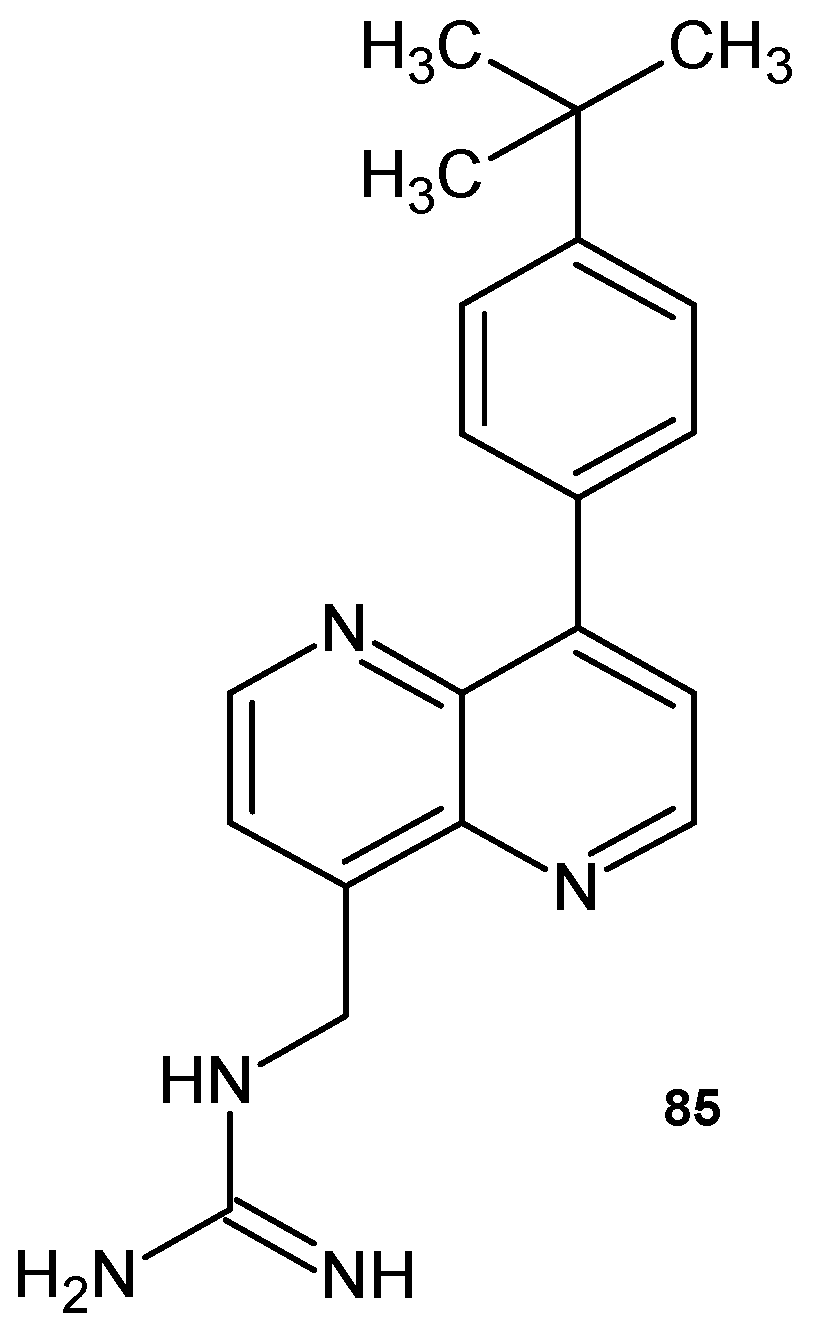
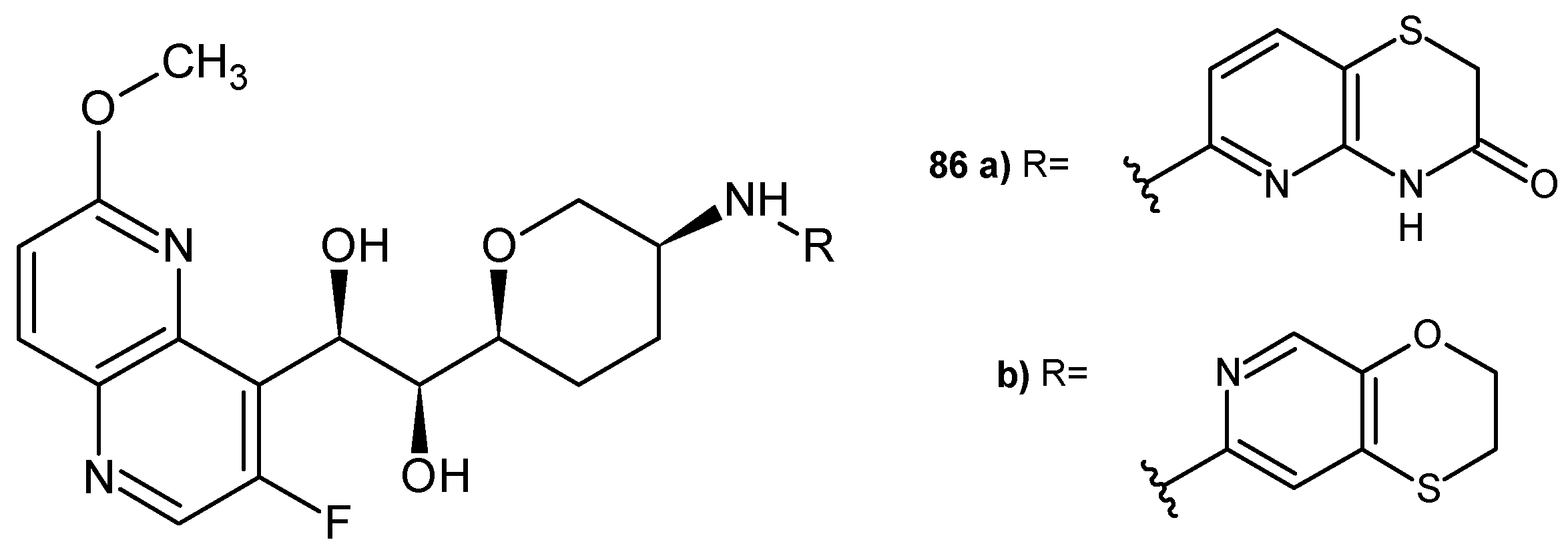
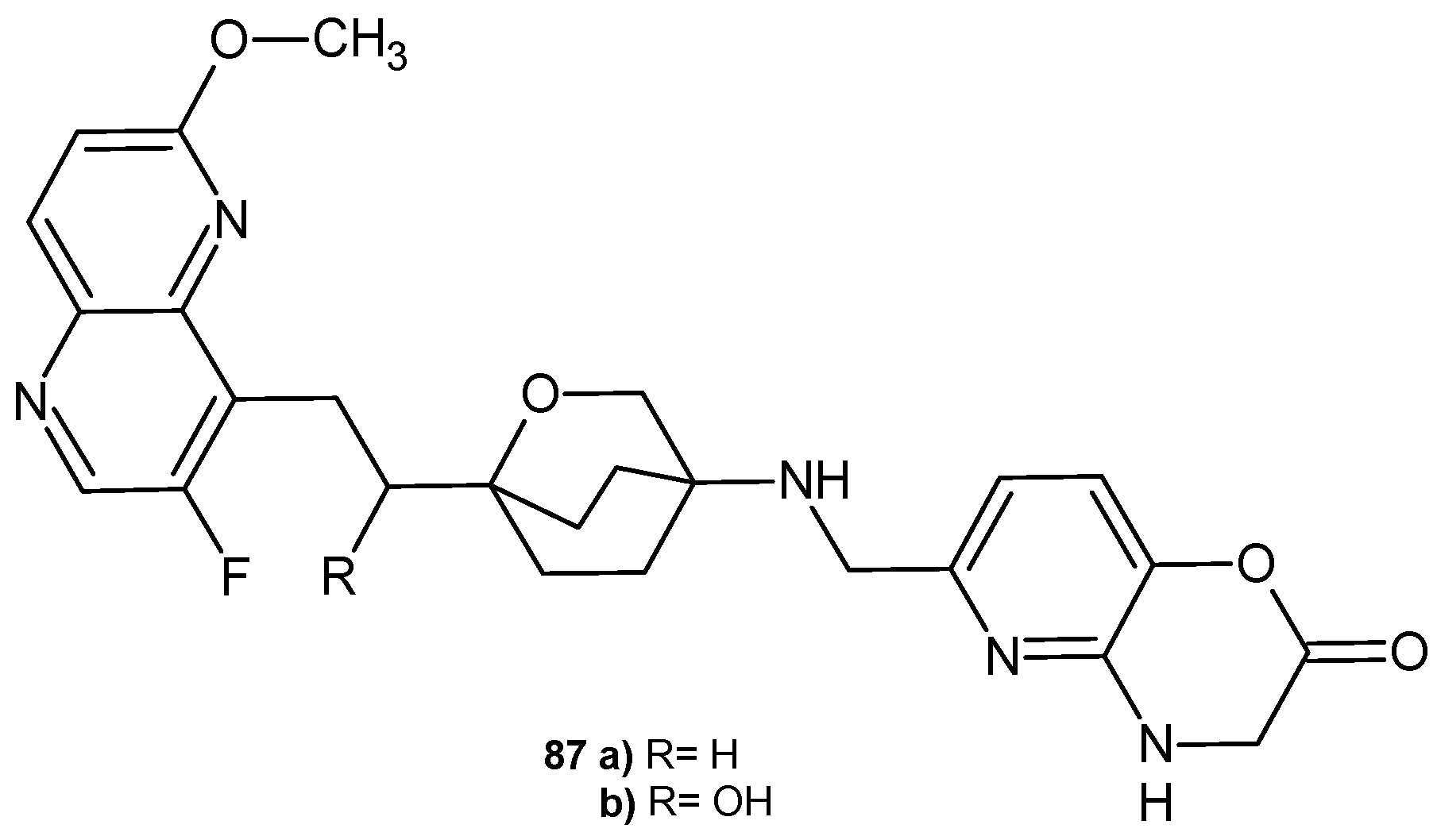
3.1. 1,5-Naphthyridines Conjugated with Antibiotics
3.2. Synthetic 1,5-Naphthyridine Derivatives
3.3. Alkaloids Containing a 1,5-Naphthyridine Scaffold
4. 1,6-Naphthyridine Derivatives
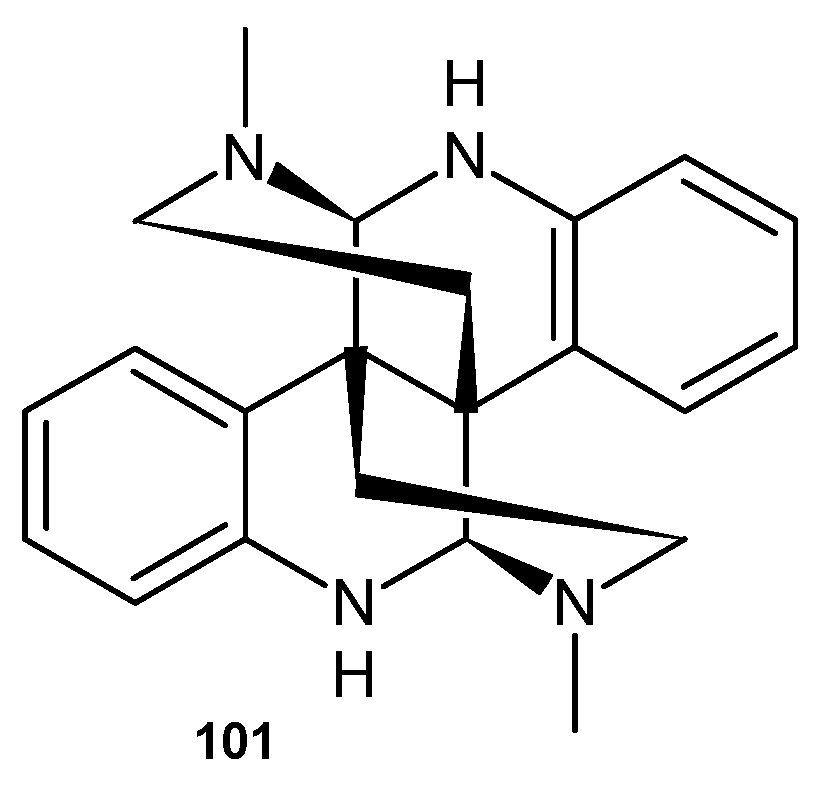
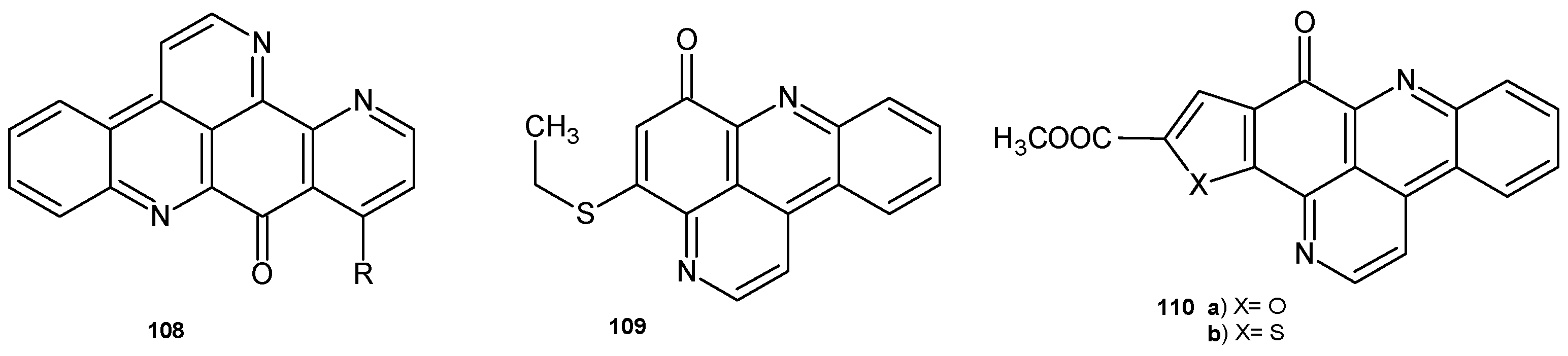
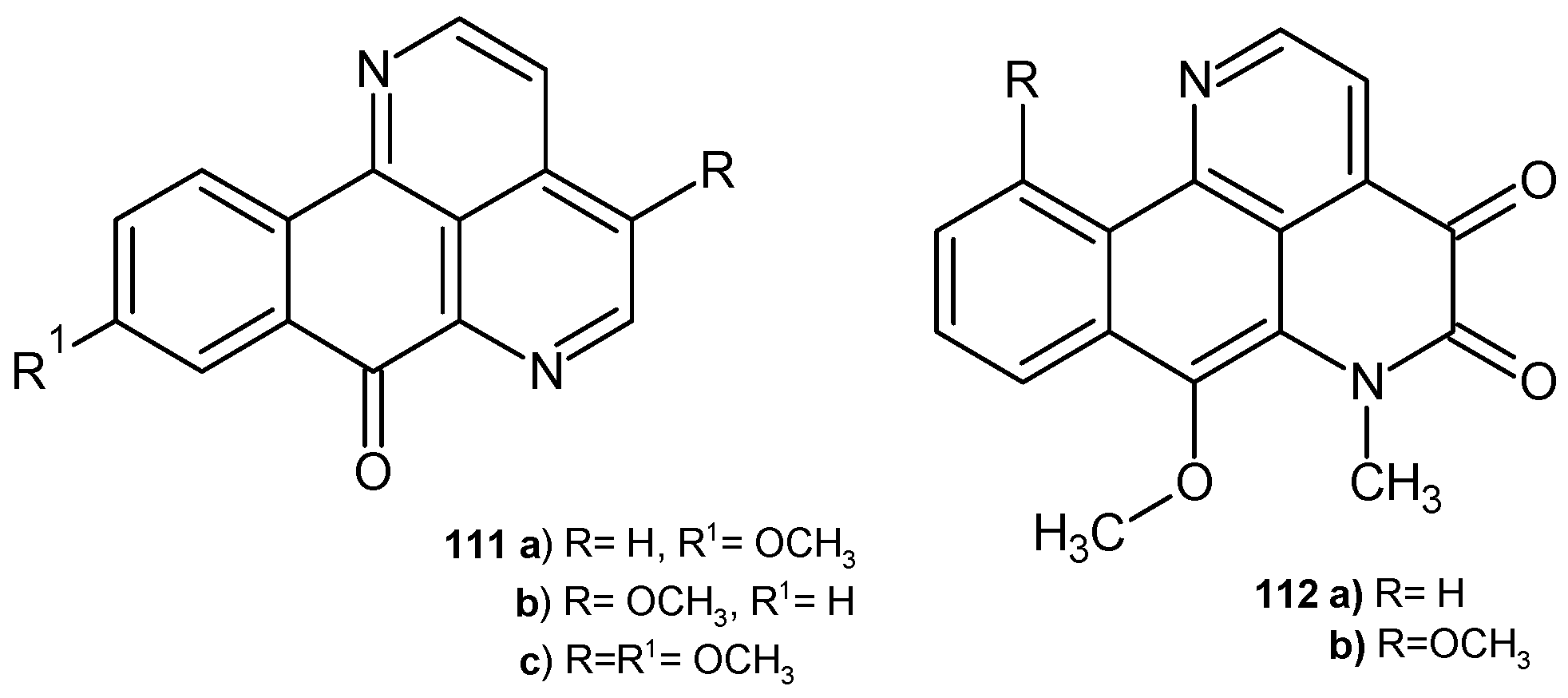
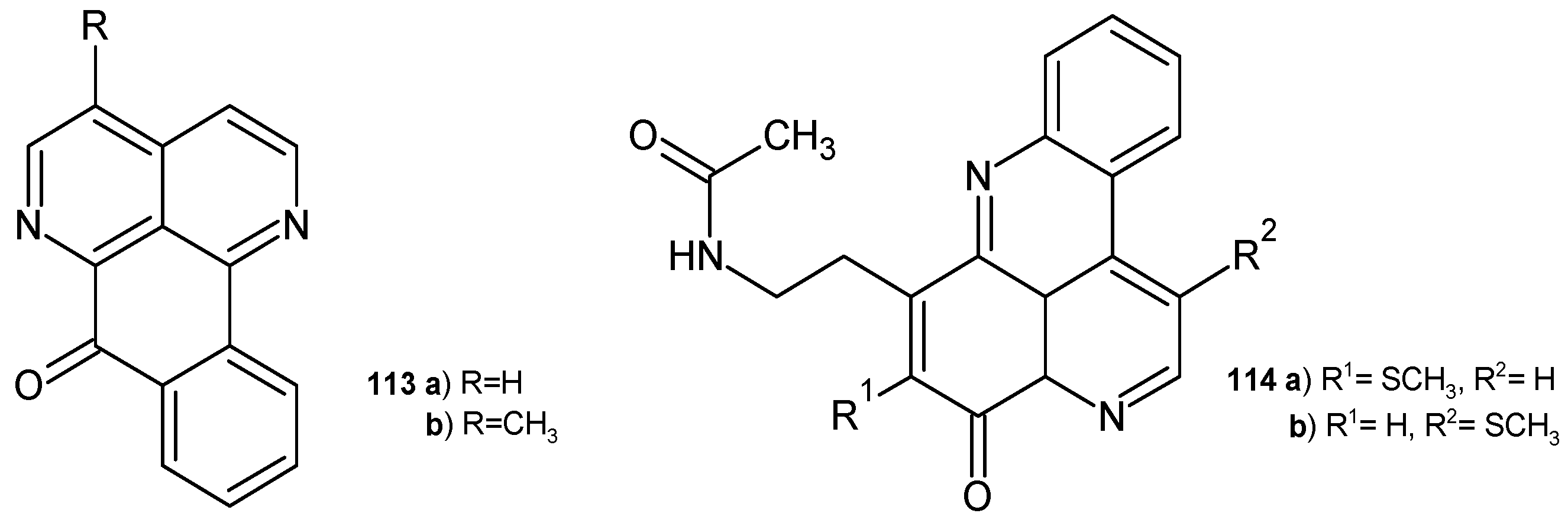
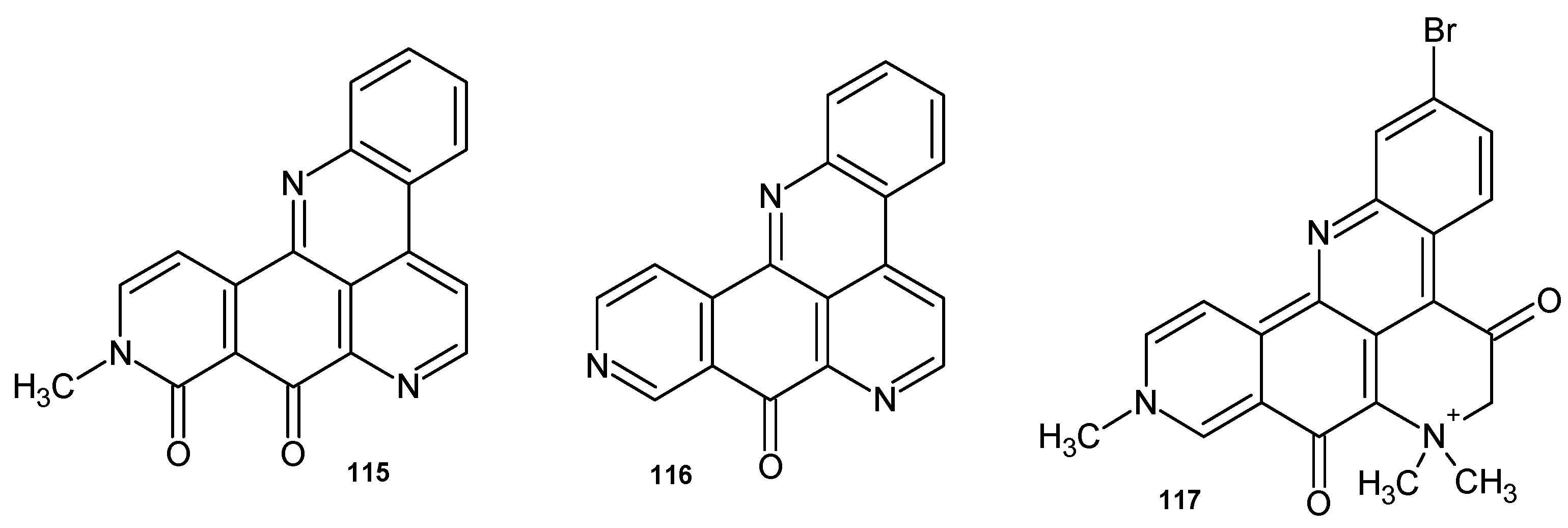
4.1. Alkaloids Containing a 1,6-Naphthyridine Scaffold
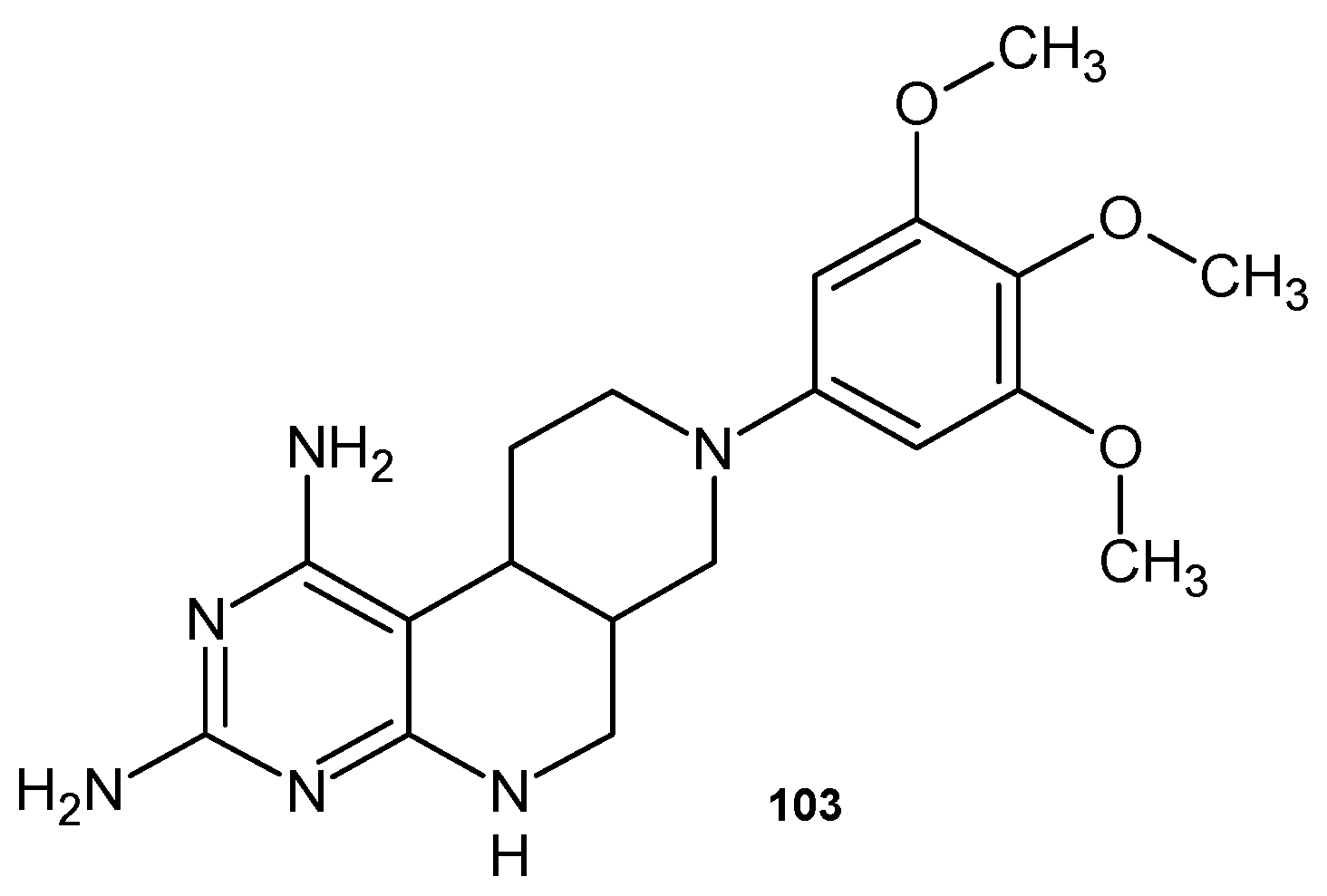
4.2. Synthetic 1,6-Naphthyridine Derivatives
5. 2,6-Naphthyridine Derivatives
5.1. Synthetic 2,6-Naphthyridine Derivatives
5.2. An Alkaloid Containing a 2,6-Naphthyridine Scaffold
6. 2,7-Naphthyridine Derivatives
6.1. Synthetic 2,7-Naphthyridine Derivatives
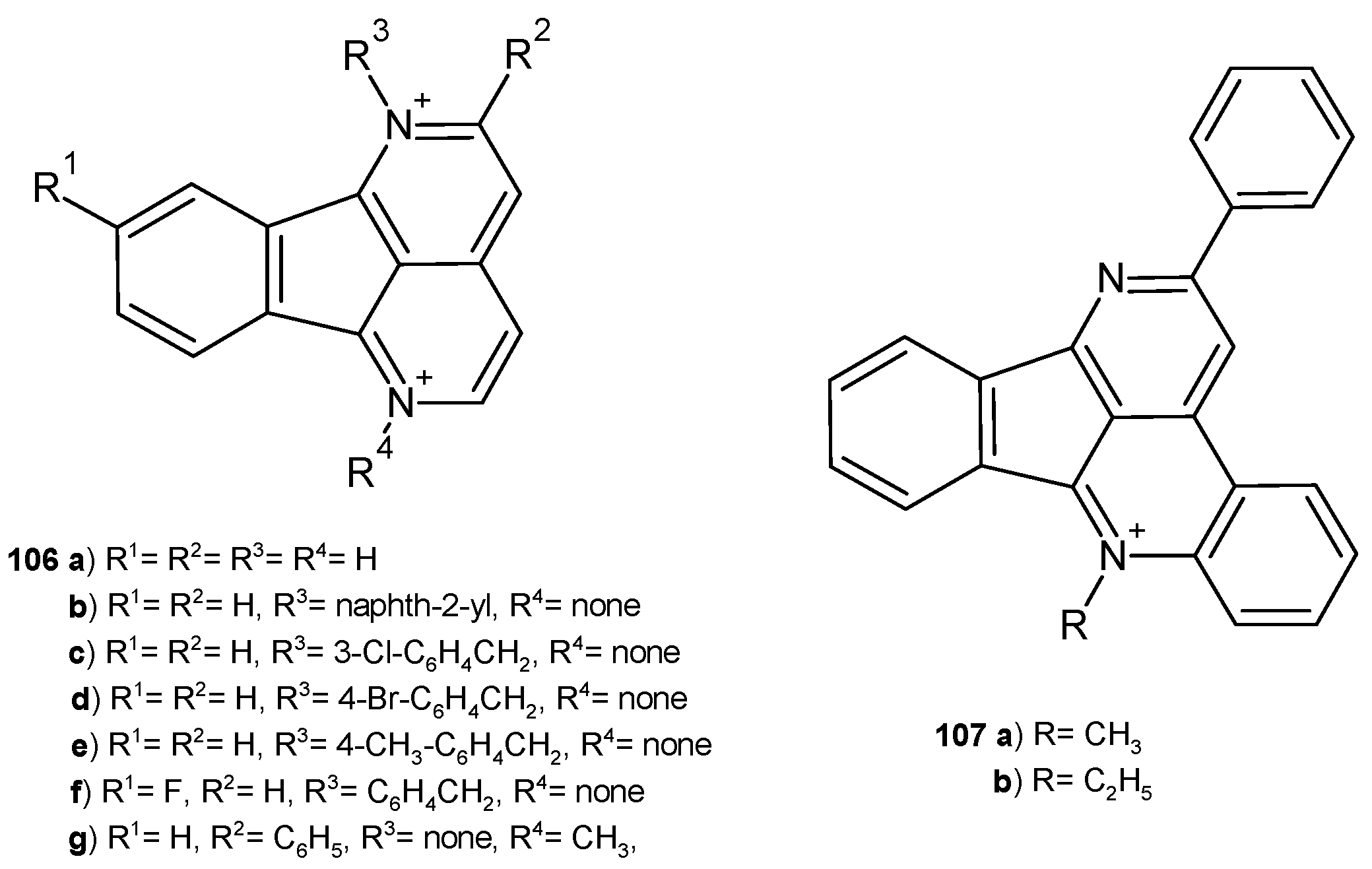
6.2. Alkaloids Containing a 2,7-Naphthyridine Scaffold
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hossain, T.J. Methods for screening and evaluation of antimicrobial activity: A review of protocols, advantages, and limitations. Eur. J. Microbiol. Immunol. 2024, 14, 97–115. [Google Scholar] [CrossRef] [PubMed]
- Madaan, A.; Verma, R.; Kumar, V.; Singh, A.T.; Jain, S.K.; Jaggi, M. 1,8-Naphthyridine Derivatives: A Review of Multiple Biological Activities. Arch. Pharm. 2015, 348, 837–860. [Google Scholar] [CrossRef]
- Lesher, G.Y.; Froelich, E.J.; Gruett, M.D.; Bailey, J.H.; Brundage, R.P. 1,8-naphthyridine derivatives. A new class of chemotherapeutic agents. J. Med. Chem. 1962, 5, 1063–1065. [Google Scholar] [CrossRef] [PubMed]
- Pedrini, A.M.; Geroldi, D.; Siccardi, A.; Falaschi, A. Studies on the Mode of Action of Nalidixic Acid. Eur. J. Biochem. 1972, 25, 359–365. [Google Scholar] [CrossRef]
- Wang, K.; Gong, C.; Xiao, W.; Abdukader, A.; Wang, D. Accessing 1,8-Naphthyridone-3-carboxylic Acid Derivatives and Application to the Synthesis of Amfonelic Acid. J. Org. Chem. 2024, 89, 5811–5824. [Google Scholar] [CrossRef] [PubMed]
- Wise, R.; Andrews, J.M.; Danks, G. In-vitro activity of enoxacin (CI-919), a new quinoline derivative, compared with that of other antimicrobial agents. J. Antimicrob. Chemother. 1984, 13, 237–244. [Google Scholar] [CrossRef]
- Jałbrzykowska, K.; Chrzanowska, A.; Roszkowski, P.; Struga, M. The New Face of a Well-Known Antibiotic: A Review of the Anticancer Activity of Enoxacin and Its Derivatives. Cancers 2022, 14, 3056. [Google Scholar] [CrossRef]
- Ethan Rubinstein. History of Quinolones and Their Side Effects. Chemotherapy 2001, 47 (Suppl. S3), 3–8. [Google Scholar] [CrossRef] [PubMed]
- Calvo, A.; Giménez, M.J.; Alou, L.; Gómez-Lus, M.L.; Aguilar, L.; Prieto, J. Ex vivo serum activity (killing rates) after gemifloxacin 320 mg versus trovafloxacin 200 mg single doses against ciprofloxacin-susceptible and -resistant Streptococcus pneumoniae. Int. J. Antimicrob. Agents 2002, 20, 144–146. [Google Scholar] [CrossRef]
- Goldstein, E.J.C.; Citron, D.M.; Warren, Y.; Tyrrell, K.; Merriam, C.V. In Vitro Activity of Gemifloxacin (SB 265805) against Anaerobes. Antimicrob. Agents Chemother. 1999, 43, 2231–2235. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yague, G.; Morris, J.E.; Pan, X.; Gould, K.A.; Fisher, L.M. Cleavable-Complex Formation by Wild-Type and Quinolone-Resistant Streptococcus pneumoniae Type II Topoisomerases Mediated by Gemifloxacin and Other Fluoroquinolones. Antimicrob. Agents Chemother. 2002, 46, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Kim, H.; Seol, M.; Choi, D.; Choi, E.; Kwak, J. In Vitro and In Vivo Antibacterial Activities of DW-224a, a New Fluoronaphthyridone. Antimicrob. Agents Chemother. 2006, 50, 2261–2264. [Google Scholar] [CrossRef]
- Kwon, A.-R.; Min, Y.-H.; Ryu, J.-M.; Choi, D.-R.; Shim, M.-J.; Choi, E.-C. In vitro and in vivo activities of DW-224a, a novel fluoroquinolone antibiotic agent. J. Antimicrob. Chemother. 2006, 58, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.N.; Biedenbach, D.J.; Ambrose, P.G.; Wikler, M.A. Zabofloxacin (DW-224a) activity against Neisseria gonorrhoeae including quinolone-resistant strains. Diagn. Microbiol. Infect. Dis. 2008, 62, 110–112. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-S.; Oh, S.-H.; Kim, H.-S.; Choi, D.-R.; Kwak, J.-H. Antimicrobial Activity of Zabofloxacin against Clinically Isolated Streptococcus pneumoniae. Molecules 2016, 21, 1562. [Google Scholar] [CrossRef]
- Babich, J.W.; Rubin, R.H.; Graham, W.A.; Wilkinson, R.A.; Vincent, J.; Fischman, A.J. 18F-Labeling and Biodistribution of the Novel Fluoro-Quinolone Antimicrobial Agent, Trovafloxacin (CP 99,219). Nucl. Med. Biol. 1996, 23, 995–998. [Google Scholar] [CrossRef]
- Jones, R.N.; Barrett, M.S.; Deguchi, T. Antimicrobial Activity of Trovafloxacin Tested against Ciprofloxacin-Susceptible and-Resistant Neisseria gonomhoeae Interpretive Criteria and Comparisons with Etest Results. Diagn. Microbiol. Infect. Dis. 1997, 28, 193–200. [Google Scholar] [CrossRef]
- Qureshi, Z.P.; Seoane-Vazquez, E.; Rodriguez-Monguio, R.; Stevenson, K.B.; Szeinbach, S.L. Market withdrawal of new molecular entities approved in the United States from 1980 to 2009. Pharmacoepidemiol. Drug Saf. 2011, 20, 772–777. [Google Scholar] [CrossRef]
- Clark, R.F.; Wang, S.; Ma, Z.; Weitzberg, M.; Motter, C.; Tufano, M.; Wagner, R.; Gu, Y.G.; Dandliker, P.J.; Lerner, C.G.; et al. Novel inhibitors of bacterial protein synthesis: Structure-activity relationships for 1,8-naphthyridine derivatives incorporating position 3 and 4 variants. Bioorg. Med. Chem. Lett. 2004, 14, 3299–3302. [Google Scholar] [CrossRef]
- Shen, L.L.; Black-Schaefer, C.; Cai, Y.; Dandliker, P.J.; Beutel, B.A. Mechanism of action of a novel series of naphthyridine-type ribosome inhibitors: Enhancement of tRNA footprinting at the decoding site of 16S rRNA. Antimicrob. Agents Chemother. 2005, 49, 1890–1897. [Google Scholar] [CrossRef]
- Dandliker, P.J.; Pratt, S.D.; Nilius, A.M.; Black-Schaefer, C.; Ruan, X.; Towne, D.L.; Clark, R.F.; Englund, E.E.; Wagner, R.; Weitzberg, M.; et al. Novel Antibacterial Class. Antimicrob. Agents Chemother. 2003, 47, 3831–3839. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.N. In vitro evaluation of E-4695, a new fluoro-naphthyridine. Eur. J. Clin. Microbiol. Infect. Dis. 1992, 11, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Guinea, J.; Gargallo-Viola, D.; Robert, M.; Tudela, E.; Xicota, M.A.; Garcia, J.; Esteve, M.; Coll, R.; Pares, M.; Roser, R. E-4695, a new C-7 azetidinyl fluoronaphthyridine with enhanced activity against gram-positive and anaerobic pathogens. Antimicrob. Agents Chemother. 1995, 39, 413–421. [Google Scholar] [CrossRef]
- Cohen, M.A.; Huband, M.D.; Mailloux, G.B.; Yoder, S.L.; Roland, G.E.; Domagala, J.M.; Heifetz, C.L. In Vitro Antibacterial Activities of PD 131628, a New 1,8-Naphthyridine Anti-Infective Agent. Antimicrob. Agents Chemother. 1991, 35, 141–146. [Google Scholar] [CrossRef]
- Hussain Qadri, S.M.; Ueno, Y.; Saldin, H.; Burdette, J.M.; Lee, G.C. CI-990 (PD 131112): A New Quinolone Prodrug. Ann. Saudi Med. 1993, 13, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.A.; Yoder, S.L.; Huband, M.D.; Roland, G.E.; Courtney, C.L. In vitro and in vivo activities of clinafloxacin, CI-990 (PD 131112), and PD 138312 versus enterococci. Antimicrob. Agents Chemother. 1995, 39, 2123–2127. [Google Scholar] [CrossRef]
- Lewin, C.S. Antibacterial activity of a 18-Naphthyridine quinolone. J. Med. Microbiol. 1992, 36, 353–357. [Google Scholar] [CrossRef]
- Hong, C.Y.; Kim, Y.K.; Chang, J.H.; Kim, S.H.; Choi, H.; Nam, D.H.; Kim, Y.Z.; Kwak, J.K. Novel Fluoroquinolone Antibacterial Agents Containing Oxime-Substituted (Aminomethyl)pyrrolidines: Synthesis and Antibacterial Activity of 7-(4-(Aminomethyl)-3-(methoxyimino)pyrrolidin-1-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydro[1,8]naphthyridine-3-carboxylic Acid (LB20304). J. Med. Chem. 1997, 40, 3584–3593. [Google Scholar]
- Huang, X.; Chen, D.; Wu, N.; Zhang, A.; Jia, Z.; Li, X. The synthesis and biological evaluation of a novel series of C7 non-basic substituted fluoroquinolones as antibacterial agents. Bioorg. Med. Chem. Lett. 2009, 19, 4130–4133. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhang, A.; Chen, D.; Jia, Z.; Li, X. 4-Substituted 4-(1H-1,2,3-triazol-1-yl)piperidine: Novel C7 moieties of fluoroquinolones as antibacterial agents. Bioorg. Med. Chem. Lett. 2010, 20, 2859–2863. [Google Scholar] [CrossRef]
- Todo, Y.; Takagi, H.; Iino, F.; Fukuoka, Y.; Takahata, M.; Okamoto, S.; Saikawa, I.; Narita, H. Pyridonecarboxylic acids as antibacterial agents. IX. Synthesis and structure-activity relationship of 3-substituted 10-(1-aminocyclopropyl)-9-fluoro-7-oxo-2,3-dihydro-7H-pyrido[1,2,3-de]- 1,4-benzoxazine-6-carboxylic acids and their 1-thio and 1-aza analogues. Chem. Pharm. Bull. 1994, 42, 2569–2574. [Google Scholar] [CrossRef][Green Version]
- Frigola, J.; Torrens, A.; Castrillo, J.A.; Mas, J.; Vañó, D.; Berrocal, J.M.; Calvet, C.; Salgado, L.; Redondo, J.; Garcia-Granda, S.; et al. 7-Azetidinylquinolones as Antibacterial Agents. 2.1 Synthesis and Biological Activity of 7-(2,3-Disubstituted-l-azetidinyl)-4-oxoquinoline-and-1,8-naphthyridine-3-carboxylic Acids. Properties and Structure-Activity Relationships of Quinolones with an Azetidine Moiety2 Chart 1. J. Med. Chem 1994, 37, 4195–4210. [Google Scholar]
- Gençer, H.K.; Levent, S.; Acar Çevik, U.; Özkay, Y.; Ilgın, S. New 1,4-dihydro[1,8]naphthyridine derivatives as DNA gyrase inhibitors. Bioorg. Med. Chem. Lett. 2017, 27, 1162–1168. [Google Scholar] [CrossRef]
- Domagala, J.M.; Hagen, S.E.; Joannides, T.; Kiely, J.S.; Laborde, E.; Schroeder, M.C.; Sesnie, J.A.; Shapiro, M.A.; Suto, M.J.; Vanderroest, S. Quinolone Antibacterials Containing the New 7-[3-(l-Aminoethyl)-l-pyrrolidinyl] Side Chain: The Effects of the 1-Aminoethyl Moiety and Its Stereochemical Configurations on Potency and in Vivo Efficacy H R = Et. J. Med. Chem. 1993, 36, 871–882. [Google Scholar] [CrossRef]
- Takahata, M.; Otsuki, M.; Nishino, T. In-vitro and in-vivo activities of T-3262, a new pyridone carboxylic acid. J. Antimicrob. Chemother. 1988, 22, 143–154. [Google Scholar] [CrossRef]
- Espinoza, A.M.; Chin, N.-X.; Novelli, A.; Neu, H.C. Comparative In Vitro Activity of a New Fluorinated 4-Quinolone, T-3262 (A-60969). Antimicrob. Agents Chemother. 1988, 32, 663–670. [Google Scholar] [CrossRef]
- Cooper, C.S.; Klock, P.L.; Chu, D.T.W.; Hardy, D.J.; Swanson, R.N.; Plattner, J.J. Preparation and in Vitro and in Vivo Evaluation of Quinolones with Selective Activity against Gram-Positive Organisms. J. Med. Chem. 1992, 35, 1392–1398. [Google Scholar] [CrossRef]
- Egawa, H.; Miyamoto, T.; Minamida, A.; Nishimura, Y.; Okada, H.; Uno, H.; Matsumoto, J. Pyridonecarboxylic Acids as Antibacterial Agents. 4.1 Synthesis and Antibacterial Activity of 7-(3-Amino-l-pyrrolidinyl)-l-ethyl-6-fluoro-l,4-dihydro-4-oxol,8-naphthyridine-3-carboxylic Acid and Its Analogues. J. Med. Chem. 1984, 27, 1543–1548. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.A.M.; Suaifan, G.A.R.Y.; Shehadeh, M.B.; Okechukwu, P.N. Design, synthesis, and biological evaluation of 1,8-naphthyridine glucosamine conjugates as antimicrobial agents. Drug. Dev. Res. 2019, 80, 179–186. [Google Scholar] [CrossRef]
- Aggarwal, N.; Kumar, R.; Dureja, P.; Khurana, J.M. Synthesis, antimicrobial evaluation and QSAR analysis of novel nalidixic acid based 1,2,4-triazole derivatives. Eur. J. Med. Chem. 2011, 46, 4089–4099. [Google Scholar] [CrossRef]
- Gurjar, V.K.; Shukla, S.; Gondaliya, N.; Puwar, N. Design, Synthesis, in Silico Study and Biological Evaluation of 1,8-Naphthyridine Derivatives as Potential Antibacterial Agents. Orient. J. Chem. 2023, 39, 320–334. [Google Scholar] [CrossRef]
- Sriram, D.; Senthilkumar, P.; Dinakaran, M.; Yogeeswari, P.; China, A.; Nagaraja, V. Antimycobacterial activities of novel 1-(cyclopropyl/tert-butyl/4- fluorophenyl)-1,4-dihydro-6-nitro-4-oxo-7-(substituted secondary amino)-1,8-naphthyridine-3-carboxylic acid. J. Med. Chem. 2007, 50, 6232–6239. [Google Scholar] [CrossRef]
- Suzuki, N.; Kato, M.; Dohmori, R. Synthesis of 1,8-naphthyridine derivatives and their activities against Trichomonas vaginalis. Yakugaku Zasshi 1979, 99, 155–164. [Google Scholar] [CrossRef]
- Chennam, K.P.; Ravi, M.; Ushaiah, B.; Srinu, V.; Eslavath, R.K.; Devi, C.S. Synthesis, characterization, DNA interactions, DNA cleavage, radical scavenging activity, antibacterial, anti-proliferative and docking studies of new transition metal complexes. J. Fluoresc. 2016, 26, 189–205. [Google Scholar] [CrossRef]
- Massari, S.; Daelemans, D.; Barreca, M.L.; Knezevich, A.; Sabatini, S.; Cecchetti, V.; Marcello, A.; Pannecouque, C.; Tabarrini, O. A 1,8-naphthyridone derivative targets the HIV-1 Tat-mediated transcription and potently inhibits the HIV-1 replication. J. Med. Chem. 2010, 53, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Laxminarayana, E.; Karunakar, T.; Shankar, S.S.; Chary, M.T. A study on antibacterial activity of substituted 1, 8-naphthyridines containing carbaldehydes, methylene hydrazines, thiadiazol amines and triazole thiols. J. Adv. Drug Res. 2012, 2, 6–11. [Google Scholar]
- Raja, S.; Jayaveera, K.N.; Subramanyam, S.; Sunil, A.; Reddy, K.; Prakash, C.R. Synthesis and biological evaluation of novel 1,8-naphthyridines containing pyrazolinone, pyrazole, isoxazolinone, isoxazole and pyrimidine-2-ones. Int. J. Pharm. Sci. Res. 2016, 7, 2573–2585. [Google Scholar] [CrossRef]
- Md, K.; Domala, R. Synthesis, Antimicrobial Activities and Molecular Docking Studies of New N-Acylated Derivatives of 5-(2-Phenyl-1,8-naphthyridin-3-yl)-1,3,4-oxadiazol-2-amine. Asian J. Chem. 2024, 36, 628–634. [Google Scholar] [CrossRef]
- Mohamed, N.G.; Sheha, M.M.; Hassan, H.Y.; Abdel-Hafez, L.J.M.; Omar, F.A. Synthesis, antimicrobial activity and molecular modeling study of 3-(5-amino-(2H)-1,2,4-triazol-3-yl]-naphthyridinones as potential DNA-gyrase inhibitors. Bioorg. Chem. 2018, 81, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, S.; Arjun, P.; Raaman, N.; Das, T.M. Regioselective facile one-pot Friedländer synthesis of sugar-based heterocyclic biomolecules. Carbohydr. Res. 2010, 345, 1988–1997. [Google Scholar] [CrossRef]
- Hussein, M.A.; Abdel-Rahman, M.A.; Geies, A.A. New heteroaromatic polyazomethines containing naphthyridine moieties: Synthesis, characterization, and biological screening. J. Appl. Polym. Sci. 2012, 126, 2–12. [Google Scholar] [CrossRef]
- Bavlin, G.B.; Tan, W.-L. Potential Antimalarials. I 1,s-Naphthyridines. Aust. J. Chem 1984, 37, 1065–1073. [Google Scholar]
- Olepu, S.; Suryadevara, P.K.; Rivas, K.; Yokoyama, K.; Verlinde, C.L.M.J.; Chakrabarti, D.; van Voorhis, W.C.; Gelb, M.H. 2-Oxo-tetrahydro-1,8-naphthyridines as selective inhibitors of malarial protein farnesyltransferase and as anti-malarials. Bioorg. Med. Chem. Lett. 2008, 18, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Quintela, J.M.; Peinador, C.; González, L.; Iglesias, R.; Paramá, A.; Álvarez, F.; Sanmartín, M.L.; Riguera, R. Piperazine N-substituted naphthyridines, pyridothienopyrimidines and pyridothienotriazines: New antiprotozoals active against Philasterides dicentrarchi. Eur. J. Med. Chem. 2003, 38, 265–275. [Google Scholar] [CrossRef]
- Priya, K.K.; Jella, K.S. Synthesis and Biological Evaluation of 1,2,4-Triazolo[4,3-a][1,8] Naphthyridines under Microwave Condition. Chem. Biol. Lett. 2024, 11, 664–667. [Google Scholar] [CrossRef]
- Narender, A.; Kumar, M.R.; Laxminarayana, E.; Chary, M.T. Synthesis and antimicrobial activity of pyrazole derivatives of 2-cyclopropyl-1,8-naphthyridines. Asian J. Chem. 2009, 21, 6885–6903. [Google Scholar]
- Omar, F.A.; Abelrasoul, M.; Sheha, M.M.; Hassan, H.Y.; Ibrahiem, Y.M. Synthesis, Antibacterial Activity and Molecular Docking of Substituted Naphthyridines as Potential DNA Gyrase Inhibitors. Chem. Select 2018, 3, 2604–2612. [Google Scholar] [CrossRef]
- Turan-Zitouni, G.; Dilek Altintop, M.; Özdemir, A.; Demirci, F.; Abu Mohsen, U.; Asım Kaplancıklı, Z. Cukurova Medical Journal Novel Naphthyridine-Hydrazone Derivatives and their Antimicrobial Activity Yeni Naftiridin Hidrazon Türevleri ve Anti-Mikrobiyal Aktiviteleri. Cukurova Med. J. 2014, 39, 234–239. [Google Scholar] [CrossRef]
- Ramesh, D.; Chary, M.T.; Laxminarayana, E.; Sreenivasulu, B. Synthesis and antimicrobial activity of 1-alkyl and aryl-3-(2-methyl-1,8-naphthyridin-3-yl)ureas. Indian J. Chem. 2010, 49B, 1271–1273. [Google Scholar] [CrossRef]
- Gurjar, V.K.; Pal, D.; Mazumder, A.; Mazumder, A.R. Research Paper Synthesis, Biological Evaluation and Molecular Docking Studies of Novel 1,8-Naphthyridine-3-carboxylic Acid Derivatives as Potential Antimicrobial Agents (Part-1). Indian J. Pharm. Sci. 2021, 82, 41–53. [Google Scholar] [CrossRef]
- Bhambi, D.; Salvi, V.K.; Bapna, A.; Pemawat, G.; Talesara, G.L. Synthesis and antimicrobial evaluation of some alkoxyphthalimide derivatives of naphthyridine. Indian J. Chem. 2009, 48, 607–704. [Google Scholar]
- Mohamed, E.A.; Abdelmajeid, A.; Behalo, M.S.; Aly, A.A.; Hebash, K.A. Green Synthesis, Cytotoxicity and Antimicrobial Activities of Some New Pyrazolines, Pyrimidines and Naphthyridines Based on 1,3-Di(thien-2-yl)prop-2-en-1-one Using Choline Chloride-Urea Mixture As A Deep Eutectic Solvent. Egypt. J. Chem. 2022, 65, 651–663. [Google Scholar] [CrossRef]
- De Morais Oliveira-Tintino, C.D.; Muniz, D.F.; Dos Santos Barbosa, C.R.; Pereira, R.L.S.; Begnini, I.M.; Rebelo, R.A.; da Silva, L.E.; Mireski, S.L.; Nasato, M.C.; Krautler, M.I.L.; et al. The 1,8-naphthyridines sulfonamides are NorA efflux pump inhibitors. J. Glob. Antimicrob. Resist. 2021, 24, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Fayyadh, K.M.; Mayeed, H.S. Synthesis and Spectroscopic Studies of Some Heterocyclic Compounds (Oxazepane -4, 7-Dione, Azetidin-2-one) Derived from 2-Chloro -1, 8-Naphthyridine-3-Carbaldyhyd and Studying their Bacterial Activity. J. Glob. Pharma Technol. 2018, 10, 353–359. [Google Scholar]
- Seefeld, M.A.; Miller, W.H.; Newlander, K.A.; Burgess, W.J.; DeWolf, W.E., Jr.; Elkins, P.A.; Head, M.S.; Jakas, D.R.; Janson, C.A.; Keller, P.M.; et al. Indole naphthyridinones as inhibitors of bacterial enoyl-ACP reductases FabI and FabK. J. Med. Chem. 2003, 46, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Al-Omran, F.; Mohareb, R.M.; El-Khair, A.A. Synthesis and biological effects of new derivatives of benzotriazole as antimicrobial and antifungal agents. J. Heterocycl. Chem. 2002, 39, 877–883. [Google Scholar] [CrossRef]
- El-Remaily, M.A.A.; Elhady, O.M.; Abdel-Raheem, E.M.M. Synthesis and Antimicrobial Screening of Fused Heterocyclic Pyridines. J. Heterocycl. Chem. 2017, 54, 871–878. [Google Scholar] [CrossRef]
- Banoth, S.; Perugu, S.; Boda, S. Green Synthesis of Fused Imidazo[1,2-a][1,8]naphthyridine Derivatives Catalyzed by DABCO under Solvent-Free Solid-State Conditions and Their Biological Evaluation. J. Heterocycl. Chem. 2018, 55, 709–715. [Google Scholar] [CrossRef]
- Ravi, D.; Rambabu, S.; Ashok, K.; Madhu, P.; Sakram, B. An Exceedingly Mild, Green Synthesis of Substituted N-3-diaryl-1,8-naphthyridin-2-amine Derivatives and Their Antimicrobial Activity. J. Heterocycl. Chem. 2018, 55, 957–963. [Google Scholar] [CrossRef]
- Sakram, B.; Ashok, K.; Rambabu, S.; Ravi, D.; Kurumanna, A.; Abbas, N.K. Molecular Modeling, Ionic Liquid Cu(II)-Catalyzed Synthesis of 9-(3-Fluoro-4-methoxyphenyl)-6-aryl-[1,2,4]triazolo[4,3-a][1,8] Naphthyridines under Microwave Irradiation and Their Antimicrobial Activity. J. Heterocycl. Chem. 2018, 55, 2392–2399. [Google Scholar] [CrossRef]
- Sakram, B.; Ravi, D.; Raghupathi, M.; Kumar, B.S.; Anantha Lakshmi, P. A facile greener synthesis, antimicrobial evaluation and molecular modelling of new 4-aryl-2-(3-(2-(trifluoromethyl)phenyl)-1,8-naphthyridin-2-yl)phthalazin-1(2H)-one derivatives. Res. Chem. Intermed. 2019, 45, 2007–2022. [Google Scholar] [CrossRef]
- Sakram, B.; Sonyanaik, B.; Ashok, K.; Rambabu, S.; Ravi, D.; Kurumanna, A.; Shyam, P. Eco-friendly synthesis of 1,8-naphthyridine 5-aryl-1,3,4-oxadiazole derivatives under solvent-free solid-state conditions and their antimicrobial activity. Res. Chem. Intermed. 2017, 43, 1881–1892. [Google Scholar] [CrossRef]
- Sonyanaik, B.; Ravi, D.; Kishore, K.; Shyam, P.; Sakram, B. Green pathway for the construction of aryl-1,8-naphthyridine-thiazole scaffolds and in vitro-antimicrobial evaluation, DNA binding interactions, viscosity measurements, molecular modeling studies and ADME properties. Res. Chem. Intermed. 2024, 50, 265–279. [Google Scholar] [CrossRef]
- Ashok, A.; Sonyanaik, B.; Sakram, B. Green synthesis of substituted 1,8-naphthyridin-thiazole scaffolds, molecular docking studies and biological evaluation. Res. Chem. Intermed. 2023, 49, 1029–1041. [Google Scholar] [CrossRef]
- Kumar Parangi, R.; Domala, R. Synthesis, characterization, biological evaluation and docking studies of novel 5-(2-methyl-1,8-naphthyridin-3-yl)-1,3,4-oxadiazol-2-amine and some of its derivatives using symmetrical anhydrides. Results Chem. 2023, 5, 100795. [Google Scholar] [CrossRef]
- Ravi, D.; Sonyanaik, B.; Sakram, B. Green design, synthesis of novel 2-methoxy-3-aryl-1,8-naphthyridines and their biological and molecular docking study. Results Chem. 2022, 4, 100611. [Google Scholar] [CrossRef]
- Sakram, B.; Madhu, P.; Sonyanaik, B.; Rambabu, S.; Ravi, D.; Kurumanna, A. Ferric Chloride-catalyzed Synthesis of 2-Oxo-1,2-dihydro-1,8-naphthyridine-3-carboxylate Derivatives and Their Biological Evaluation. Russ. J. Gen. Chem. 2018, 88, 1224–1227. [Google Scholar] [CrossRef]
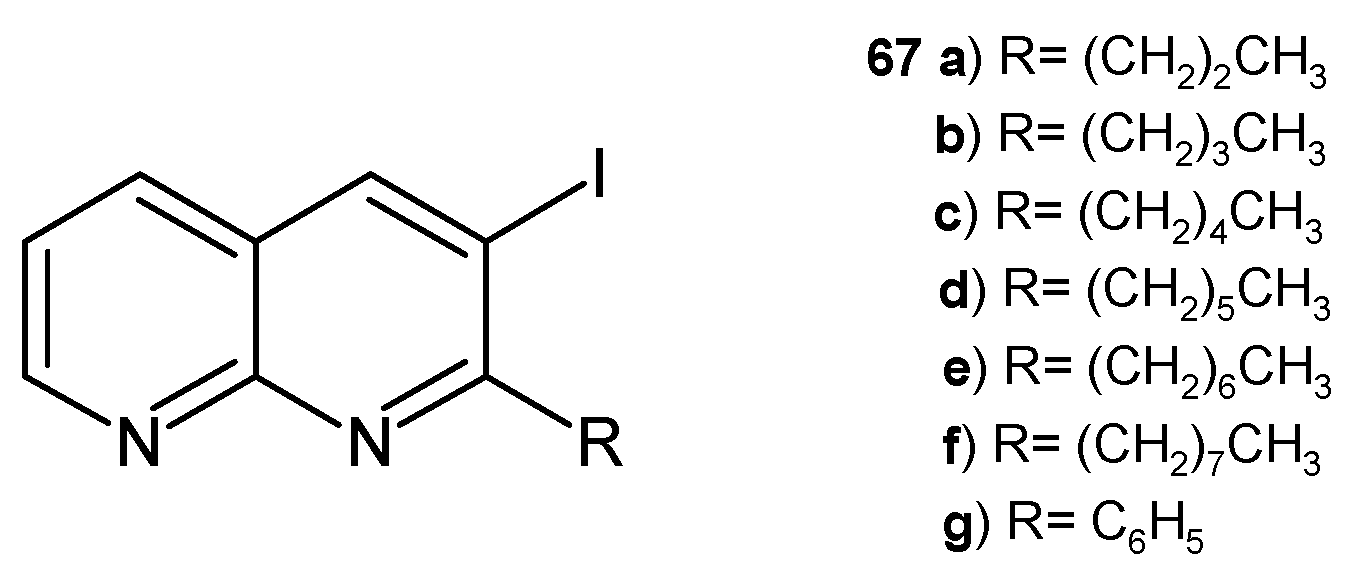
- Sakram, B.; Ashok, K.; Rambabu, S.; Sonyanaik, B.; Ravi, D. A novel and efficient synthesis of 3-iodo substituted 1,8-naphthyridines by electrophilic cyclization of 2-amino nicotinaldehyde and their antimicrobial activity. Russ. J. Gen. Chem. 2017, 87, 1794–1799. [Google Scholar] [CrossRef]
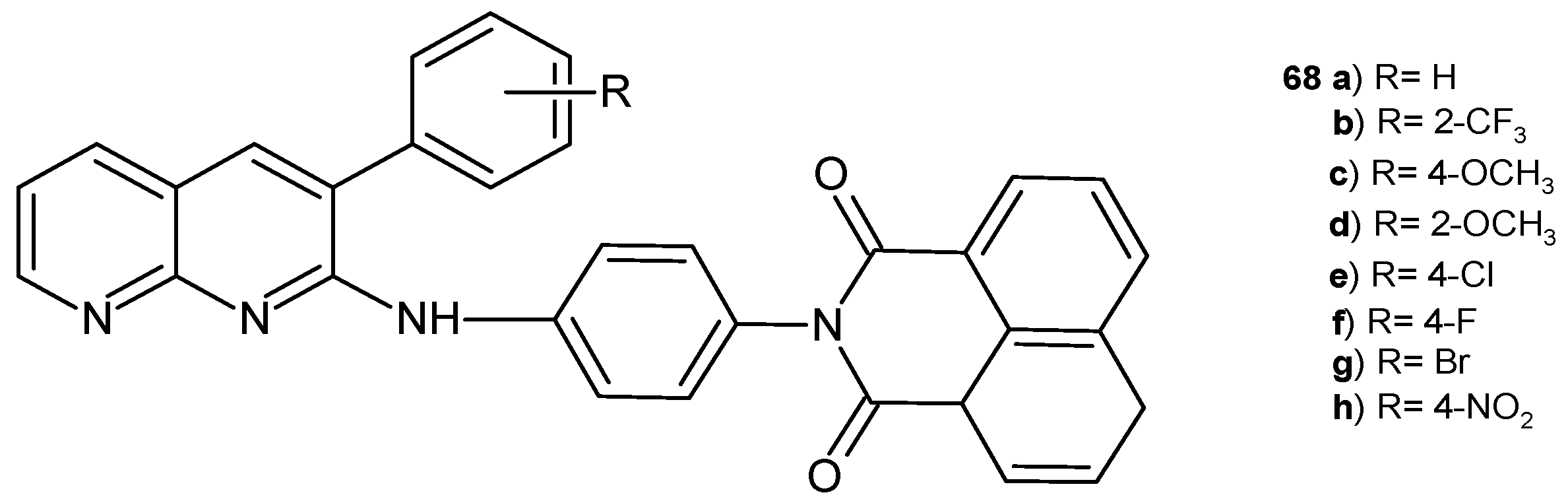
- Sakram, B.; Ravi, D.; Ashok, K.; Rambabu, S.; Sonyanaik, B.; Kurumanna, A. An Efficient Microwave-Assisted Synthesis of Novel 2-{4-[(3-Aryl-1,8-naphthyridin-2-yl)amino]phenyl}-1H-benzo[de]isoquinoline-1,3(2H)-diones and Their Antimicrobial Activity. Russ. J. Gen. Chem. 2018, 88, 780–788. [Google Scholar] [CrossRef]
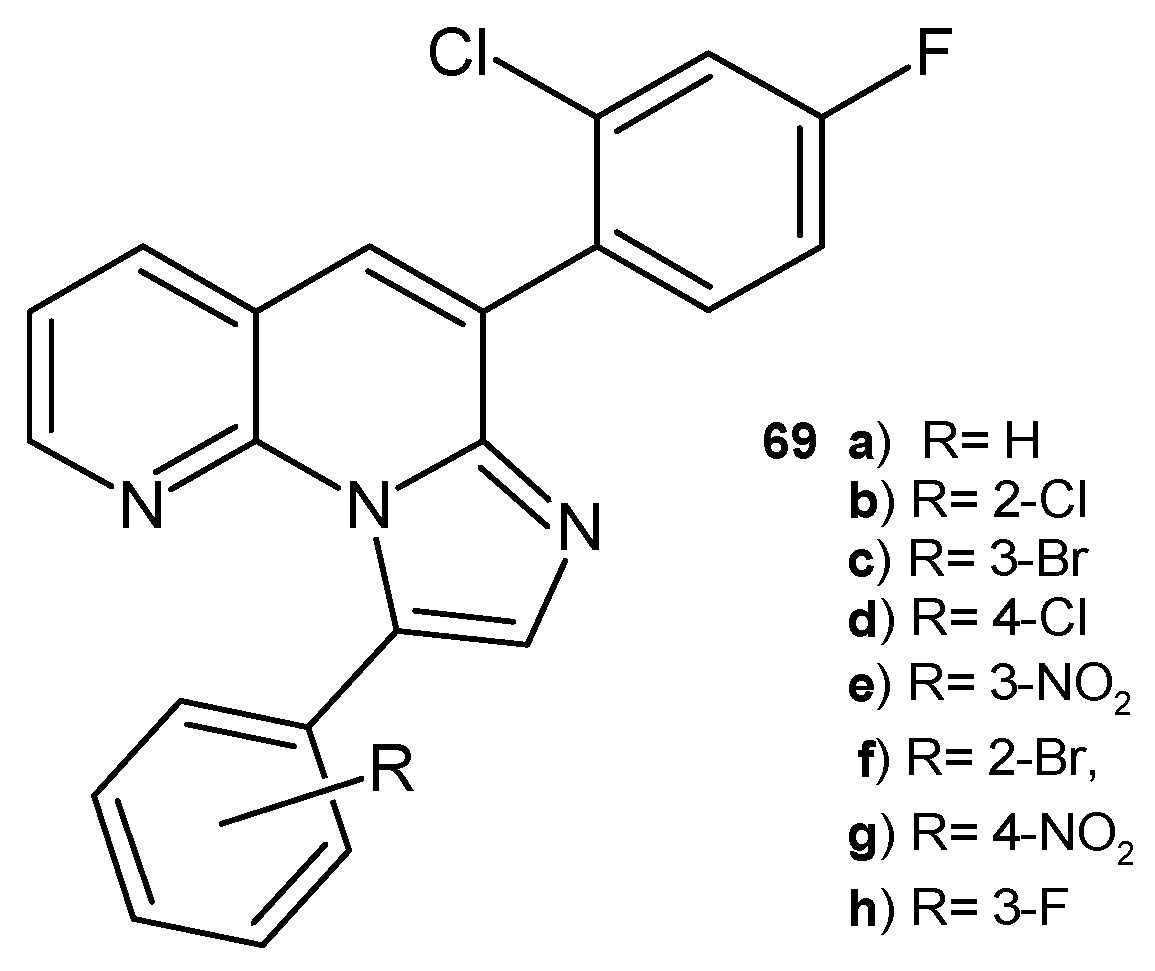
- Sonyanaik, B.; Sakram, B.; Shyam, P.; Madhu, P.; Govan, M. Green Synthesis and Biological Evaluation of Novel Fused 6-(2-Chloro-4-fluorophenyl)-9-arylimidazo[1,2-a][1,8]naphthyridine Derivatives Catalyzed by DABCO. Russ. J. Gen. Chem. 2018, 88, 1495–1501. [Google Scholar] [CrossRef]
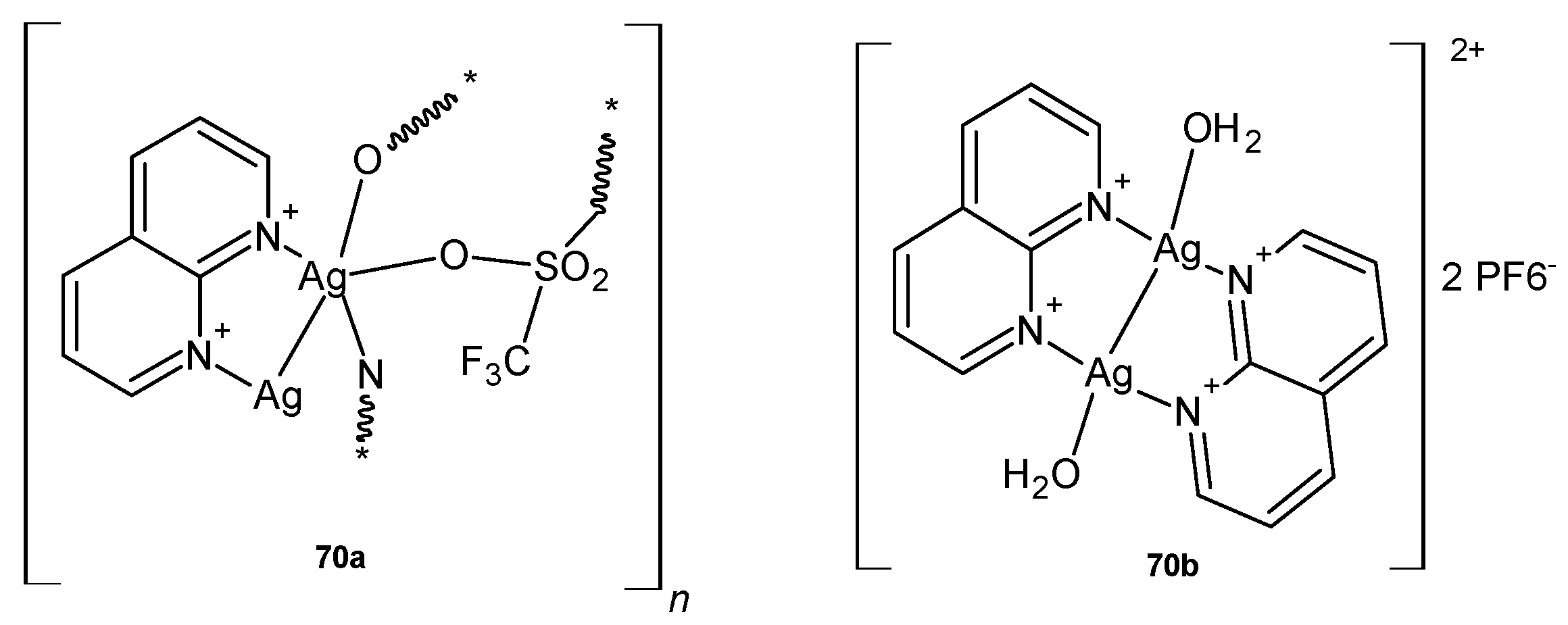
- Ašanin, D.P.; Bogojevic, S.S.; Perdih, F.; Andrejević, T.P.; Milivojevic, D.; Aleksic, I.; Nikodinovic-Runic, J.; Glišić, B.; Turel, I.; Djuran, M.I. Structural characterization, antimicrobial activity and BSA/DNA binding affinity of new silver(I) complexes with thianthrene and 1,8-naphthyridine. Molecules 2021, 26, 1871. [Google Scholar] [CrossRef] [PubMed]
- Pandya, K.M.; Battula, S.; Kumar, K.A.A.; Patel, R.J.; Patel, N.B. Design & synthesis of hybrid pharmacophore of β-lactam, 1,8-naphthyridine, and secondary amines; Discover their in vitro antimicrobial, anticancer properties & DFT and ADMET prediction studies. Med. Chem. Res. 2023, 32, 1098–1108. [Google Scholar] [CrossRef]
- Payne, D.J.; Miller, W.H.; Berry, V.; Brosky, J.; Burgess, W.J.; Chen, E.; DeWolf, W.E.; Fosberry, A.P.; Greenwood, R.; Head, M.S.; et al. Discovery of a novel and potent class of fabI-directed antibacterial agents. Antimicrob. Agents Chemother. 2002, 46, 3118–3124. [Google Scholar] [CrossRef]
- Sivakumar, P.M.; Iyer, G.; Doble, M. QSAR studies on substituted 3- or 4-phenyl-1,8-naphthyridine derivatives as antimicrobial agents. Med. Chem. Res. 2012, 21, 788–795. [Google Scholar] [CrossRef]
- Bhasker, G.V.; Satyanarayana, G.V.; Laxminarayana, E.; Latha, A.; Chary, M.T. Synthesis, Antibacterial Activity, and Docking Studies of Some New 2-Substituted-1,8-naphthyridine Derivatives. Indian J. Chem. 2018, 28, 227–242. [Google Scholar]
- Melcón-Fernandez, E.; Martín-Encinas, E.; Palacios, F.; Galli, G.; Reguera, R.M.; Martínez-Valladares, M.; Balaña-Fouce, R.; Alonso, C.; Pérez-Pertejo, Y. Antileishmanial Effect of 1,5- and 1,8-Substituted Fused Naphthyridines. Molecules 2024, 29, 74. [Google Scholar] [CrossRef] [PubMed]
- Elkanzi, N.A.A.; Hrichi, H.; Muqbil Alsirhani, A.; Bakr, R.B. Antioxidant and Antimicrobial Potential of 1,8-Naphthyridine Based Scaffolds: Design, Synthesis and in Silico Simulation Studies within Topoisomerase II. Chem. Biodivers. 2024, 21, e202301746. [Google Scholar] [CrossRef]
- Gohil, J.D.; Patel, H.B.; Patel, M.P. Synthesis and evaluation of new chromene based [1,8]naphthyridines derivatives as potential antimicrobial agents. RSC Adv. 2016, 6, 74726–74733. [Google Scholar] [CrossRef]
- Abbanat, D.; Webb, G.; Foleno, B.; Li, Y.; Macielag, M.; Montenegro, D.; Wira, E.; Bush, K. In vitro activities of novel 2-fluoro-naphthyridine-containing ketolides. Antimicrob. Agents Chemother. 2005, 49, 309–315. [Google Scholar] [CrossRef]
- Bodey, G.P.; Weaver, S.; Pan, T. PC-904, a New Semisynthetic Penicillin. Antimicrob. Agents Chemother. 1978, 13, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Parhi, A.K.; Zhang, Y.; Saionz, K.W.; Pradhan, P.; Kaul, M.; Trivedi, K.; Pilch, D.S.; Lavoie, E.J. Antibacterial activity of quinoxalines, quinazolines, and 1,5-naphthyridines. Bioorg. Med. Chem. Lett. 2013, 23, 4968–4974. [Google Scholar] [CrossRef] [PubMed]
- Surivet, J.P.; Zumbrunn, C.; Rueedi, G.; Hubschwerlen, C.; Bur, D.; Bruyère, T.; Locher, H.; Ritz, D.; Keck, W.; Seiler, P.; et al. Design, synthesis, and characterization of novel tetrahydropyran-based bacterial topoisomerase inhibitors with potent anti-gram-positive activity. J. Med. Chem. 2013, 56, 7396–7415. [Google Scholar] [CrossRef]
- Singh, S.B.; Kaelin, D.E.; Wu, J.; Miesel, L.; Tan, C.M.; Meinke, P.T.; Olsen, D.; Lagrutta, A.; Bradley, P.; Lu, J.; et al. Oxabicyclooctane-linked novel bacterial topoisomerase inhibitors as broad spectrum antibacterial agents. ACS Med. Chem. Lett. 2014, 5, 609–614. [Google Scholar] [CrossRef]
- Singh, S.B.; Kaelin, D.E.; Wu, J.; Miesel, L.; Tan, C.M.; Meinke, P.T.; Olsen, D.; Lagrutta, A.; Wei, C.; Liao, Y.; et al. C1-C2-linker substituted 1,5-naphthyridine analogues of oxabicyclooctane-linked NBTIs as broad-spectrum antibacterial agents (part 7). MedChemComm 2015, 6, 1773–1780. [Google Scholar] [CrossRef]
- Singh, S.B.; Kaelin, D.E.; Wu, J.; Miesel, L.; Tan, C.M.; Meinke, P.T.; Olsen, D.; Lagrutta, A.; Wei, C.; Peng, X.; et al. Structure activity relationship of substituted 1,5-naphthyridine analogs of oxabicyclooctane-linked novel bacterial topoisomerase inhibitors as broad-spectrum antibacterial agents (Part-4). Bioorg. Med. Chem. Lett. 2015, 25, 2409–2415. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Okumu, A.A.; Nolan, S.; English, A.; Vibhute, S.; Lu, Y.; Hervert-Thomas, K.; Seffernick, J.T.; Azap, L.; Cole, S.L.; et al. 1,3-Dioxane-Linked Bacterial Topoisomerase Inhibitors with Enhanced Antibacterial Activity and Reduced hERG Inhibition. ACS Infect. Dis. 2019, 5, 1115–1128. [Google Scholar] [CrossRef]
- Auparakkitanon, S.; Chapoomram, S.; Kuaha, K.; Chirachariyavej, T.; Wilairat, P. Targeting of hematin by the antimalarial pyronaridine. Antimicrob. Agents Chemother. 2006, 50, 2197–2200. [Google Scholar] [CrossRef]
- Krishna, S.; Augustin, Y.; Wang, J.; Xu, C.; Staines, H.M.; Platteeuw, H.; Kamarulzaman, A.; Sall, A.; Kremsner, P. Repurposing Antimalarials to Tackle the COVID-19 Pandemic. Trends Parasitol. 2021, 37, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Đurić, S.; Vojnovic, S.; Pavic, A.; Mojicevic, M.; Wadepohl, H.; Savić, N.D.; Popsavin, M.; Nikodinovic-Runic, J.; Djuran, M.I.; Glišić, B. New polynuclear 1,5-naphthyridine-silver(I) complexes as potential antimicrobial agents: The key role of the nature of donor coordinated to the metal center. J. Inorg. Biochem. 2020, 203, 110872. [Google Scholar] [CrossRef] [PubMed]
- Kaigongi, M.M.; Lukhoba, C.W.; Yaouba, S.; Makunga, N.P.; Githiomi, J.; Yenesew, A. In Vitro Antimicrobial and Antiproliferative Activities of the Root Bark Extract and Isolated Chemical Constituents of Zanthoxylum Paracanthum Kokwaro (Rutaceae). Plants 2020, 9, 920. [Google Scholar] [CrossRef]
- Zhao, F.; Dai, J.-K.; Liu, D.; Wang, S.-J.; Wang, J.-R. Synthesis and Evaluation of Ester Derivatives of 10-Hydroxycanthin-6-One as Potential Antimicrobial Agents. Molecules 2016, 21, 390. [Google Scholar] [CrossRef]
- O’Donnell, G.; Gibbons, S. Antibacterial Activity of Two Canthin-6-One Alkaloids FromAllium Neapolitanum. Phyther. Res. 2007, 21, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.E.; Nakayama, H.; de Arias, A.R.; Schinini, A.; de Bilbao, N.V.; Serna, E.; Lagoutte, D.; Soriano-Agatón, F.; Poupon, E.; Hocquemiller, R.; et al. Effects of Canthin-6-One Alkaloids from Zanthoxylum Chiloperone on Trypanosoma Cruzi-Infected Mice. J. Ethnopharmacol. 2007, 109, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Bowling, J.J.; Pennaka, H.K.; Ivey, K.; Wahyuono, S.; Kelly, M.; Schinazi, R.F.; Valeriote, F.A.; Graves, D.E.; Hamann, M.T. Antiviral and Anticancer Optimization Studies of the DNA-Binding Marine Natural Product Aaptamine. Chem. Biol. Drug Des. 2008, 71, 205–215. [Google Scholar] [CrossRef]
- Amin, N.M.; Hamdin, M.S.; Amir, M.; Azarnudin, T.; Asari, A.; Nur, F.; Rashid, A.A.; Mariam, S.; Nor, M. Cytotoxicity effect of Aaptamine and its derivatives on Acanthamoeba castellanii (IMR isolate). Sci. Int. 2018, 30, 309–313. [Google Scholar]
- Ašanin, D.P.; Andrejević, T.P.; Nenadovic, M.; Rodić, M.; Vojnovic, S.; Djuran, M.I.; Glišić, B. Comparative study of antimicrobial potential and DNA/BSA binding affinity of silver(I) and gold(III) coordination compounds with 1,6-naphthyridine. Polyhedron 2023, 244, 116585. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; El-Gohary, N.M. Chemical behavior of 4,9-dimethoxy-5-oxo-5H-furo[3,2-g]chromene-6-carboxaldehyde towards carbon nucleophilic reagents. J. Heterocycl. Chem. 2020, 57, 2815–2830. [Google Scholar] [CrossRef]
- Wall, R.J.; Moniz, S.; Thomas, M.G.; Norval, S.; Ko, E.-J.; Marco, M.; Miles, T.J.; Gilbert, I.H.; Horn, D.; Fairlamb, A.H.; et al. Antitrypanosomal 8-Hydroxy-Naphthyridines Are Chelators of Divalent Transition Metals. Antimicrob. Agents Chemother. 2018, 62, e00235-18. [Google Scholar] [CrossRef] [PubMed]
- Bishnoi, A.; Tiwari, A.K.; Singh, S.; Sethi, A.; Tripathi, C.M.; Banerjee, B. Synthesis, characterization, and biological evaluation of novel thiazole and pyrazole derivatives of quinoline-4-carboxylic acid as potential antimicrobial agents. Med. Chem. Res. 2013, 22, 3527–3535. [Google Scholar] [CrossRef]
- Zhang, J.W.; Gao, J.M.; Xua, T.; Zhang, X.C.; Ma, Y.T.; Jarussophon, S.; Konishi, Y. Antifungal Activity of Alkaloids from the Seeds of Chimonanthus Praecox. Chem. Biodivers. 2009, 6, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Hossaini, Z.; Tabarsaei, N.; Khandan, S.; Valipour, P.; Ghorchibeigi, M. ZnO/Ag/Fe3O4 nanoparticles supported on carbon nanotubes employing Petasites hybridus rhizome water extract: A novel organometallic nanocatalyst for the synthesis of new naphthyridines. Appl. Organomet. Chem. 2021, 35, e6114. [Google Scholar] [CrossRef]
- Gangjee, A.; Shi, J.; Queener, S.F. Synthesis and Biological Activities of Conformationally Restricted, Tricyclic Nonclassical Antifolates as Inhibitors of Dihydrofolate Reductases 1. J. Med. Chem. 1997, 40, 1930–1936. [Google Scholar] [CrossRef] [PubMed]
- El-Dean, A.M.K.; Geies, A.A.; Hassanien, R.; Abdel-Wadood, F.K.; El-Naeem, E.E.A. Novel Synthesis, Reactions, and Biological Study of New Morpholino-Thieno[2,3-c][2,7]Naphthyridines as Anti-Cancer and Anti-Microbial Agents. Russ. J. Bioorg. Chem. 2022, 48, 821–834. [Google Scholar] [CrossRef]
- Hufford, C.D.; Liu, S.; Clark, A.M.; Oguntimein, B.O. Anticandidal activity of eupolauridine and onychine, alkaloids from Cleistopholis patens. J. Nat. Prod. 1987, 50, 961–964. [Google Scholar] [CrossRef]
- Khan, S.I.; Nimrod, A.C.; Mehrpooya, M.; Nitiss, J.L.; Walker, L.A.; Clark, A.M. Antifungal activity of eupolauridine and its action on DNA topoisomerases. Antimicrob. Agents Chemother. 2002, 46, 1785–1792. [Google Scholar] [CrossRef] [PubMed]
- Taghavi-Moghadam, S.; Kwong, C.D.; Secrist, J.A.; Khan, S.I.; Clark, A.M. The synthesis and biological evaluation of alkyl and benzyl naphthyridinium analogs of eupolauridine as potential antimicrobial and cytotoxic agents. Bioorg. Med. Chem. 2016, 24, 6119–6130. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, B.S.; Barrows, L.R.; Copp, B.R. Structural Requirements for Biological Activity of the Marine Alkaloid Ascididemin. Bioorg. Med. Chem. Lett. 1995, 5, 739–742. [Google Scholar] [CrossRef]
- Appleton, D.R.; Pearce, A.N.; Copp, B.R. Anti-Tuberculosis Natural Products: Synthesis and Biological Evaluation of Pyridoacridine Alkaloids Related to Ascididemin. Tetrahedron 2010, 66, 4977–4986. [Google Scholar] [CrossRef]
- Kitahara, Y.; Onikura, H.; Shibano, Y.; Watanabe, S.; Mikami, Y.; Kuboa, A. Synthesis of Eupomatidines 1,2 and 3 and Related Compounds Including Iminoquinolinequinone Structure. Yoshiyasu 1997, 53, 6001–6010. [Google Scholar]
- Muhammad, I.; Dunbar, D.C.; Takamatsu, S.; Walker, L.A.; Clark, A.M. Antimalarial, Cytotoxic, and Antifungal Alkaloids from Duguetia Hadrantha. J. Nat. Prod. 2001, 64, 559–562. [Google Scholar] [CrossRef]
- Peterson, J.R.; Zjawiony, J.K.; Liu, S.; Hufford, C.D.; Clark, A.M.; Rogers, R.D. Copyrine Alkaloids: Synthesis, Spectroscopic Characterization, and Antimycotic/Antimycobacterial Activity of A- and B-Ring-Functionalized Sampangines. J. Med. Chem. 1992, 35, 4069–4077. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Oguntimein, B.; Hufford, C.D.; Clark, A.M. 3-Methoxysampangine, a Novel Antifungal Copyrine Alkaloid from Cleistopholis Patens. Antimicrob. Agents Chemother. 1990, 34, 529–533. [Google Scholar] [CrossRef]
- Charyulu, G.A.; McKee, T.C.; Ireland, C.M. Diplamine, a Cytotoxic Polyaromatic Alkaloid from the Tunicate Diplosoma sp. Tetrahedron Lett. 1989, 30, 4201–4202. [Google Scholar] [CrossRef]
- Appleton, D.R.; Pearce, A.N.; Lambert, G.; Babcock, R.C.; Copp, B.R. Isodiplamine, Cystodytin K and Lissoclinidine: Novel Bioactive Alkaloids from the New Zealand Ascidian Lissoclinum Notti. Tetrahedron 2002, 58, 9779–9783. [Google Scholar] [CrossRef]
- Bry, D.; Banaigs, B.; Long, C.; Bontemps, N. New Pyridoacridine Alkaloids from the Purple Morph of the Ascidian Cystodytes Dellechiajei. Tetrahedron Lett. 2011, 52, 3041–3044. [Google Scholar] [CrossRef]
- Carroll, A.R.; Ngo, A.; Quinn, R.J.; Redburn, J.; Hooper, J.N.A. Petrosamine B, an Inhibitor of the Helicobacter Pylori Enzyme Aspartyl Semialdehyde Dehydrogenase from the Australian Sponge Oceanapia sp. J. Nat. Prod. 2005, 68, 804–806. [Google Scholar] [CrossRef]
- Feng, Y.; Davis, R.A.; Sykes, M.L.; Avery, V.M.; Carroll, A.R.; Camp, D.; Quinn, R.J. Antitrypanosomal Pyridoacridine Alkaloids from the Australian Ascidian Polysyncraton Echinatum. Tetrahedron Lett. 2010, 51, 2477–2479. [Google Scholar] [CrossRef]
- Chabowska, G.; Barg, E.; Wójcicka, A. Biological activity of naturally derived naphthyridines. Molecules 2021, 26, 4324. [Google Scholar] [CrossRef]
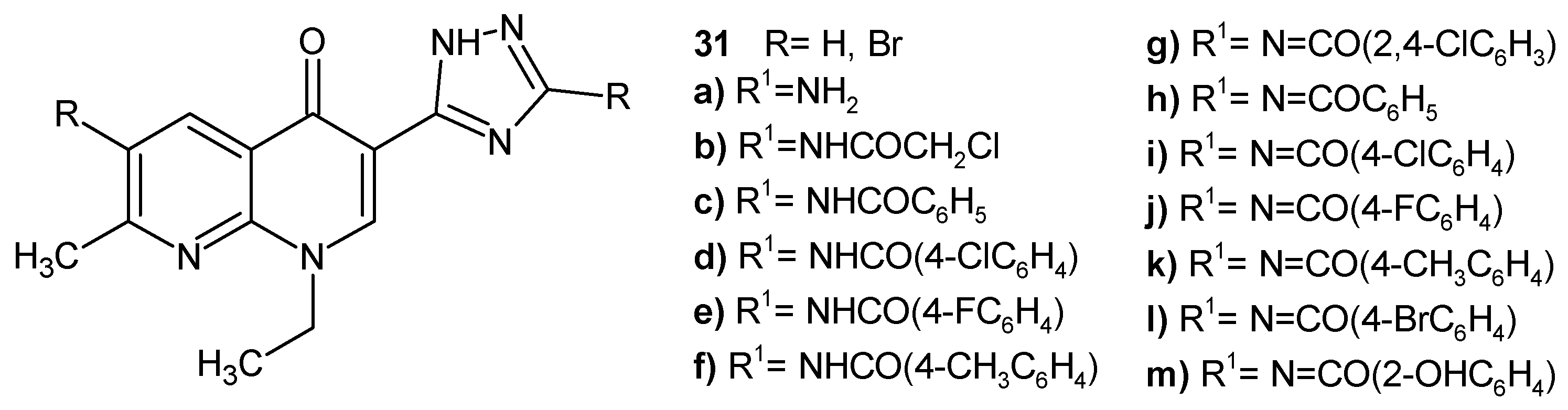








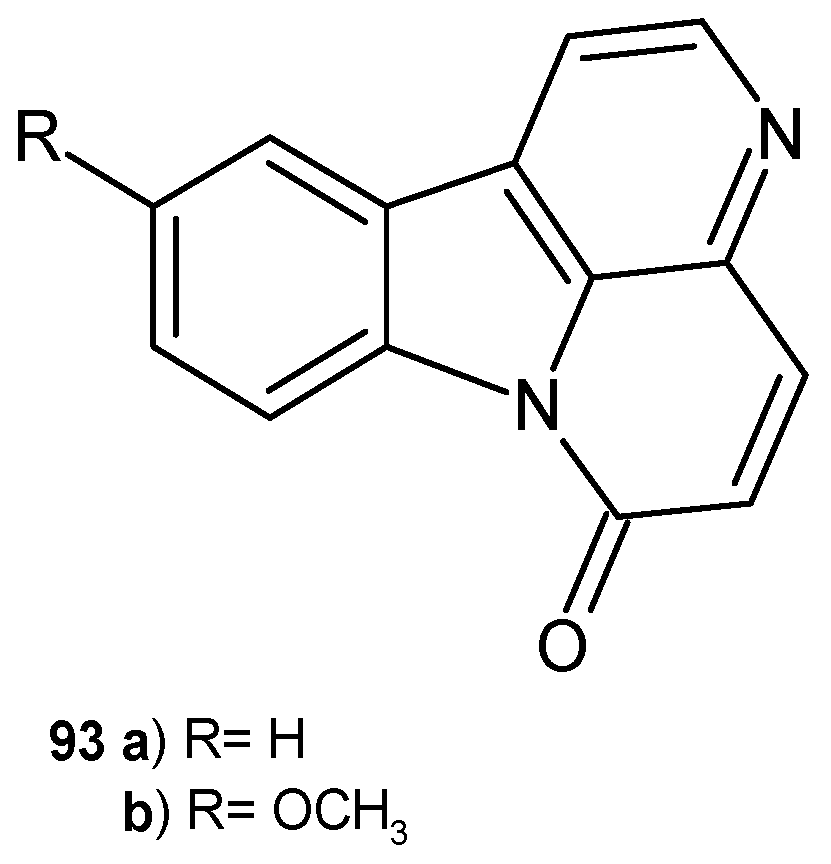
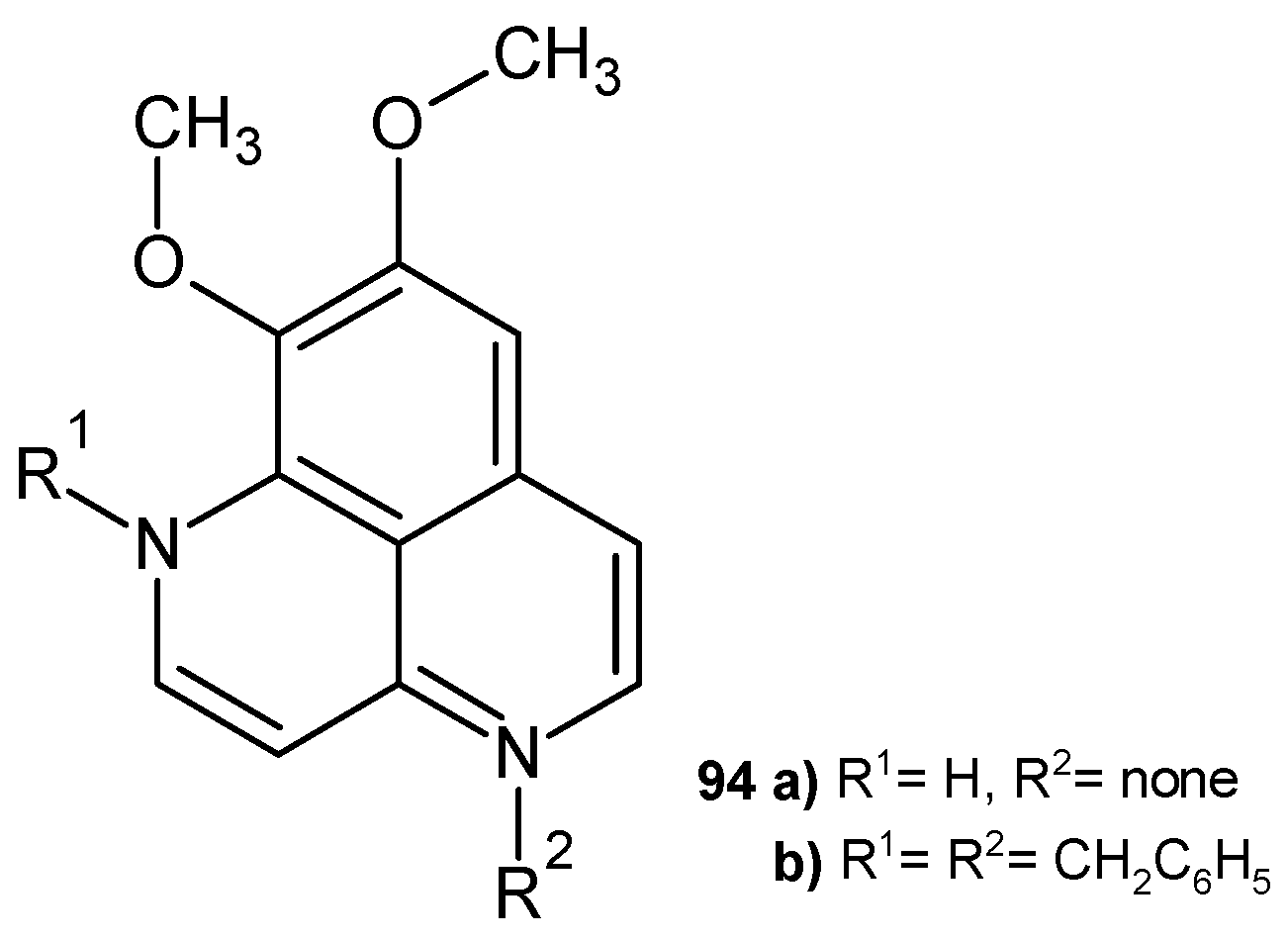


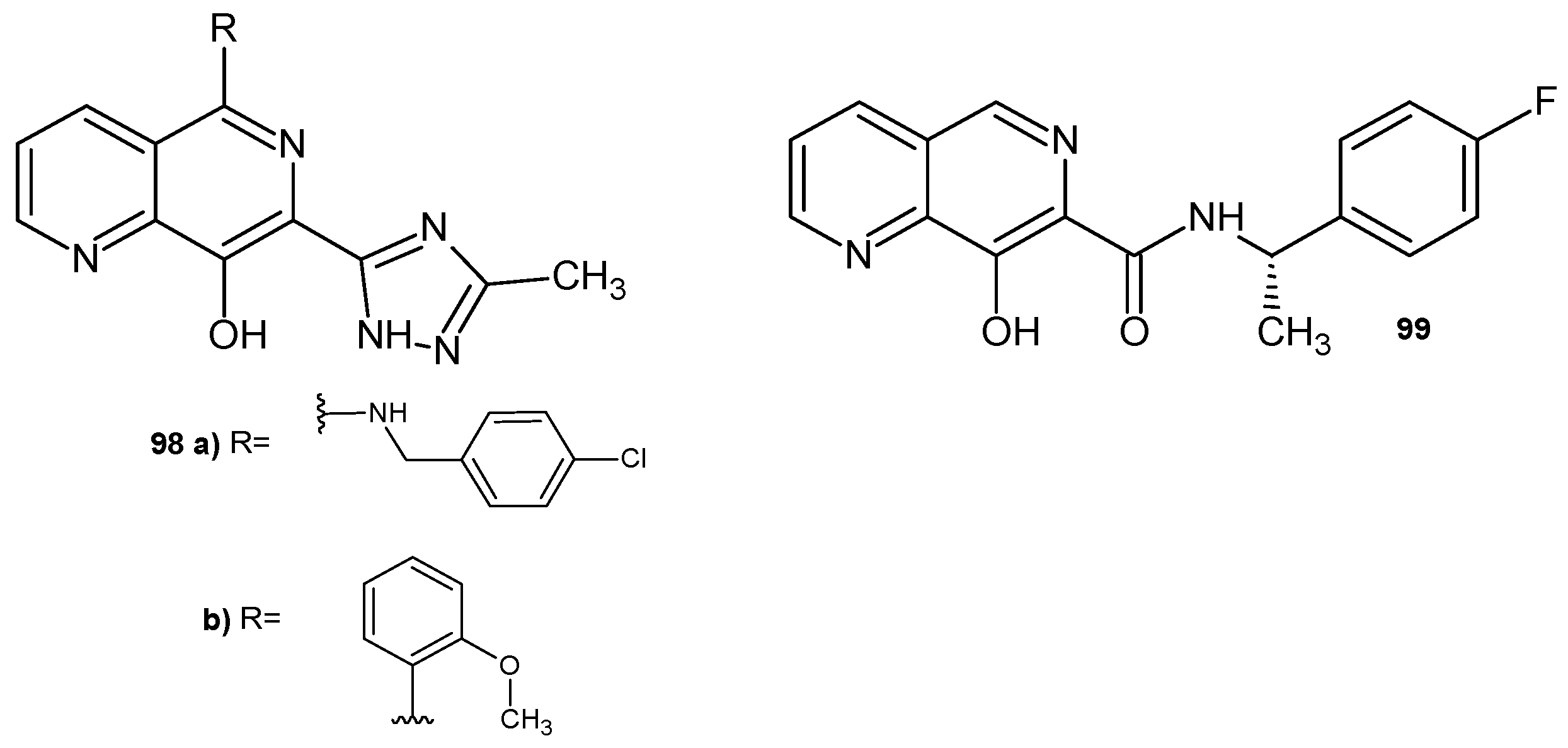
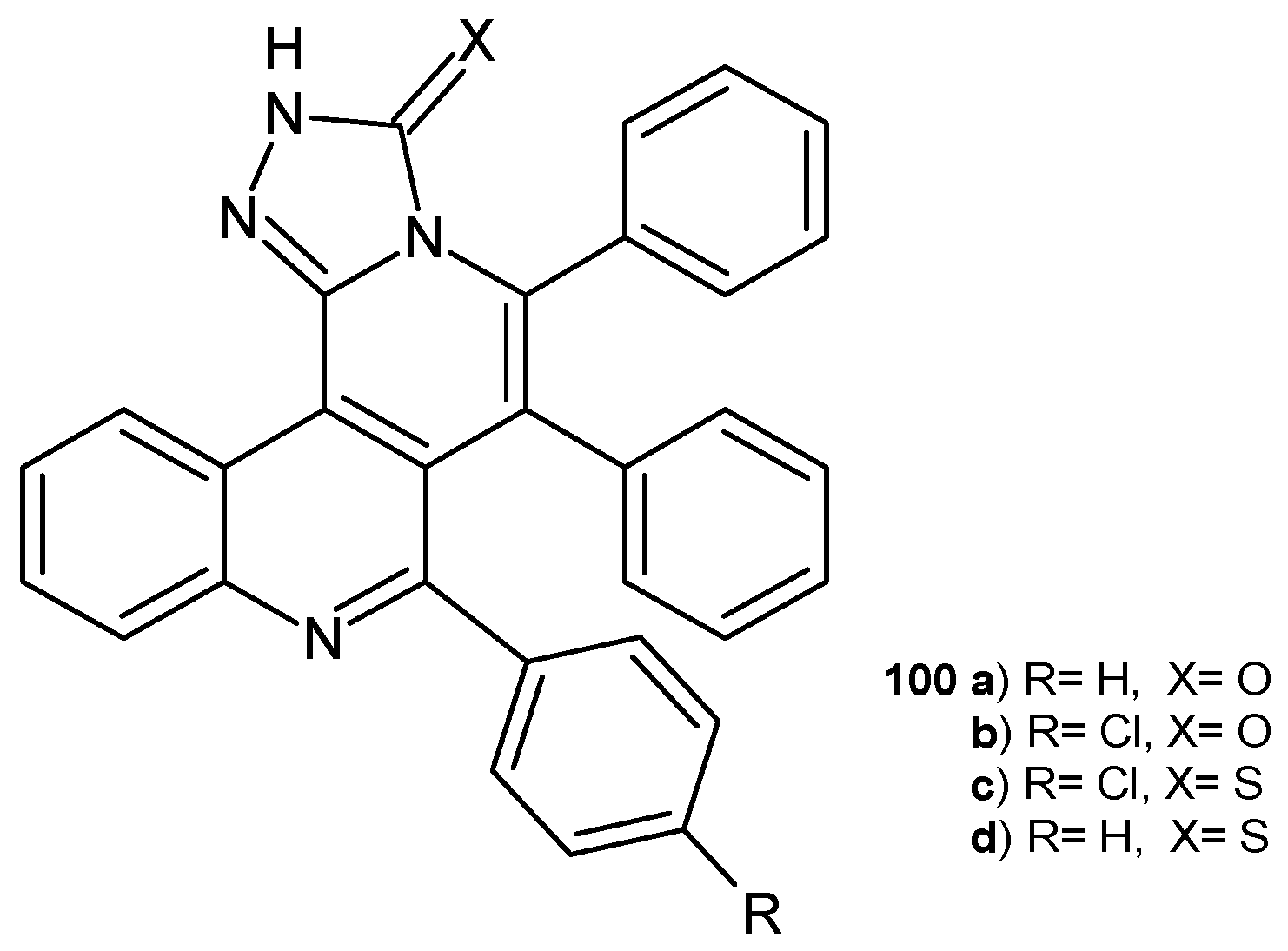
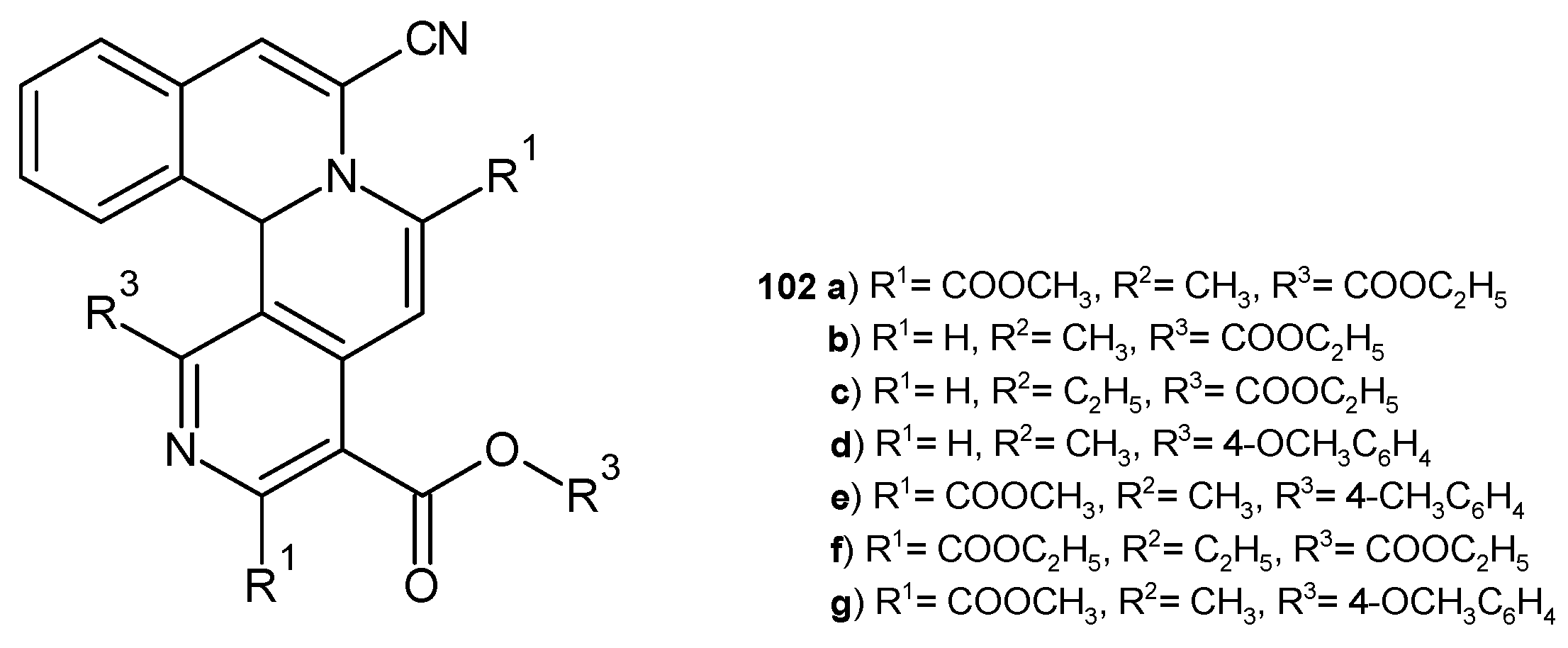

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wójcicka, A.; Mączyński, M. Antimicrobial Activity of Naphthyridine Derivatives. Pharmaceuticals 2024, 17, 1705. https://doi.org/10.3390/ph17121705
Wójcicka A, Mączyński M. Antimicrobial Activity of Naphthyridine Derivatives. Pharmaceuticals. 2024; 17(12):1705. https://doi.org/10.3390/ph17121705
Chicago/Turabian StyleWójcicka, Anna, and Marcin Mączyński. 2024. "Antimicrobial Activity of Naphthyridine Derivatives" Pharmaceuticals 17, no. 12: 1705. https://doi.org/10.3390/ph17121705
APA StyleWójcicka, A., & Mączyński, M. (2024). Antimicrobial Activity of Naphthyridine Derivatives. Pharmaceuticals, 17(12), 1705. https://doi.org/10.3390/ph17121705







