Comparison of Drug Delivery Systems with Different Types of Nanoparticles in Terms of Cellular Uptake and Responses in Human Endothelial Cells, Pericytes, and Astrocytes
Abstract
:1. Introduction
2. Results
2.1. Synthesis and Characterization of Nanoparticles for In Vitro Imaging Studies
2.1.1. Particle Size and Polydispersity of Nanoparticles
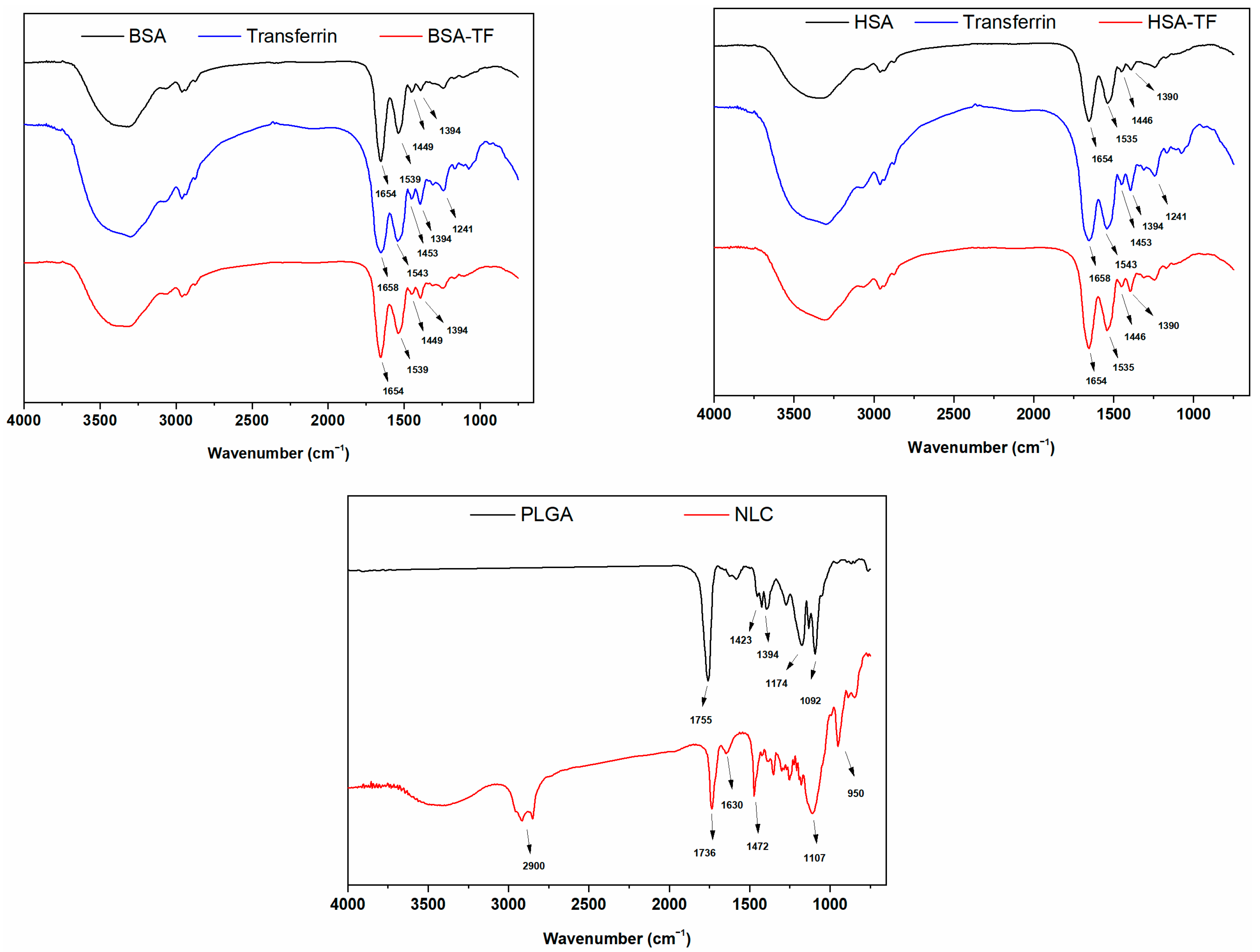
2.1.2. Characterization of the Nanoparticles by FTIR
2.2. In Vitro Studies
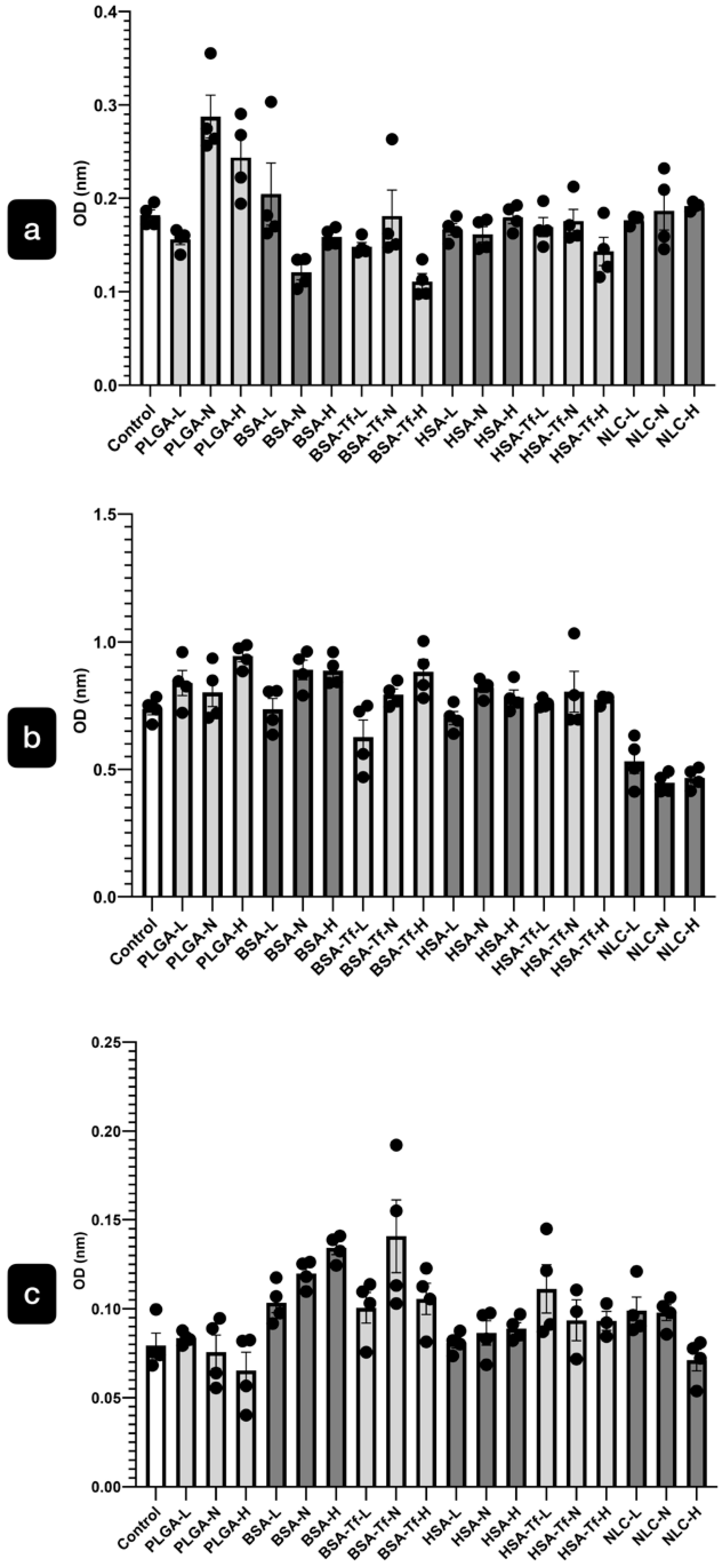
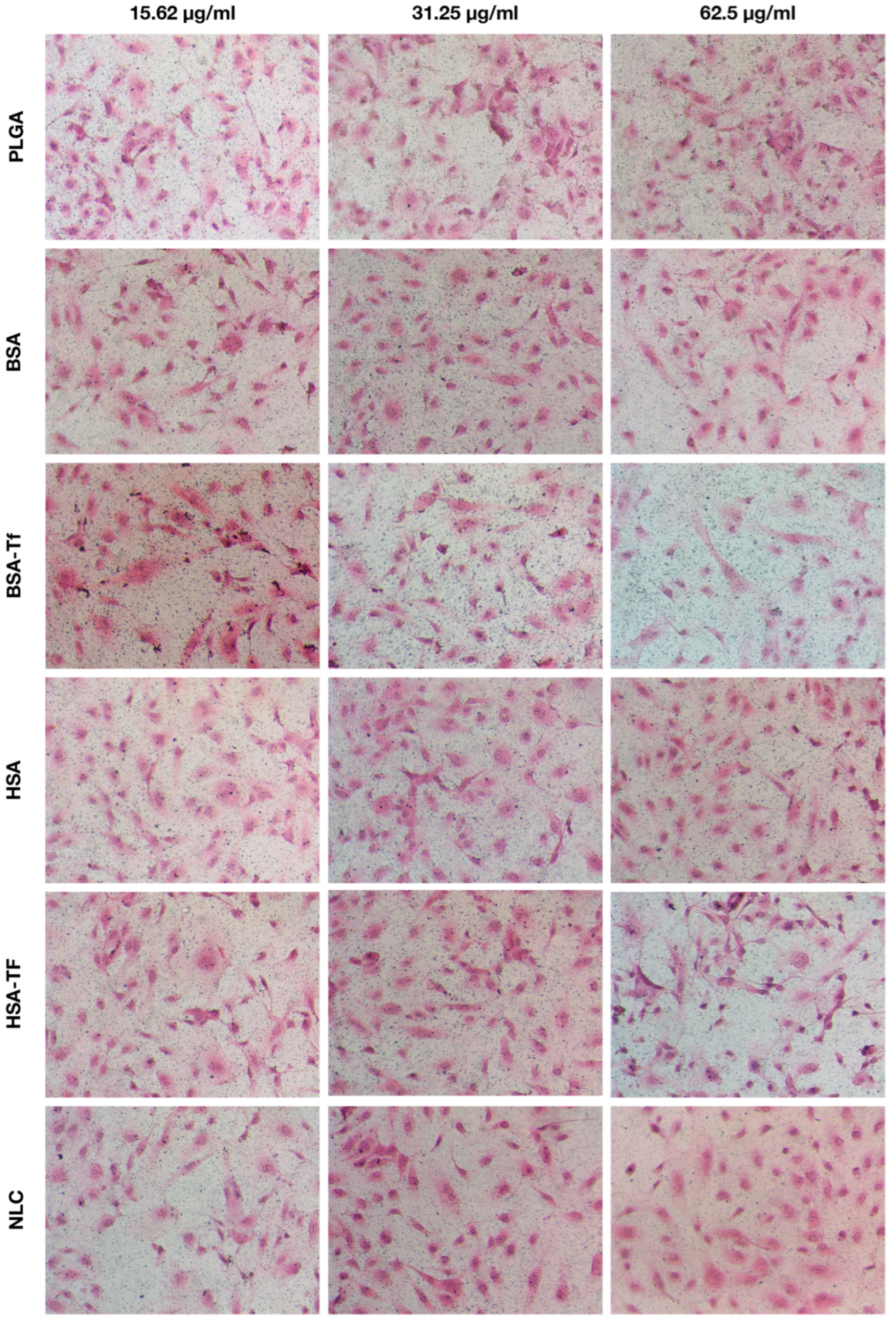
2.2.1. Cytotoxicity of the NPs
2.2.2. NP Uptake
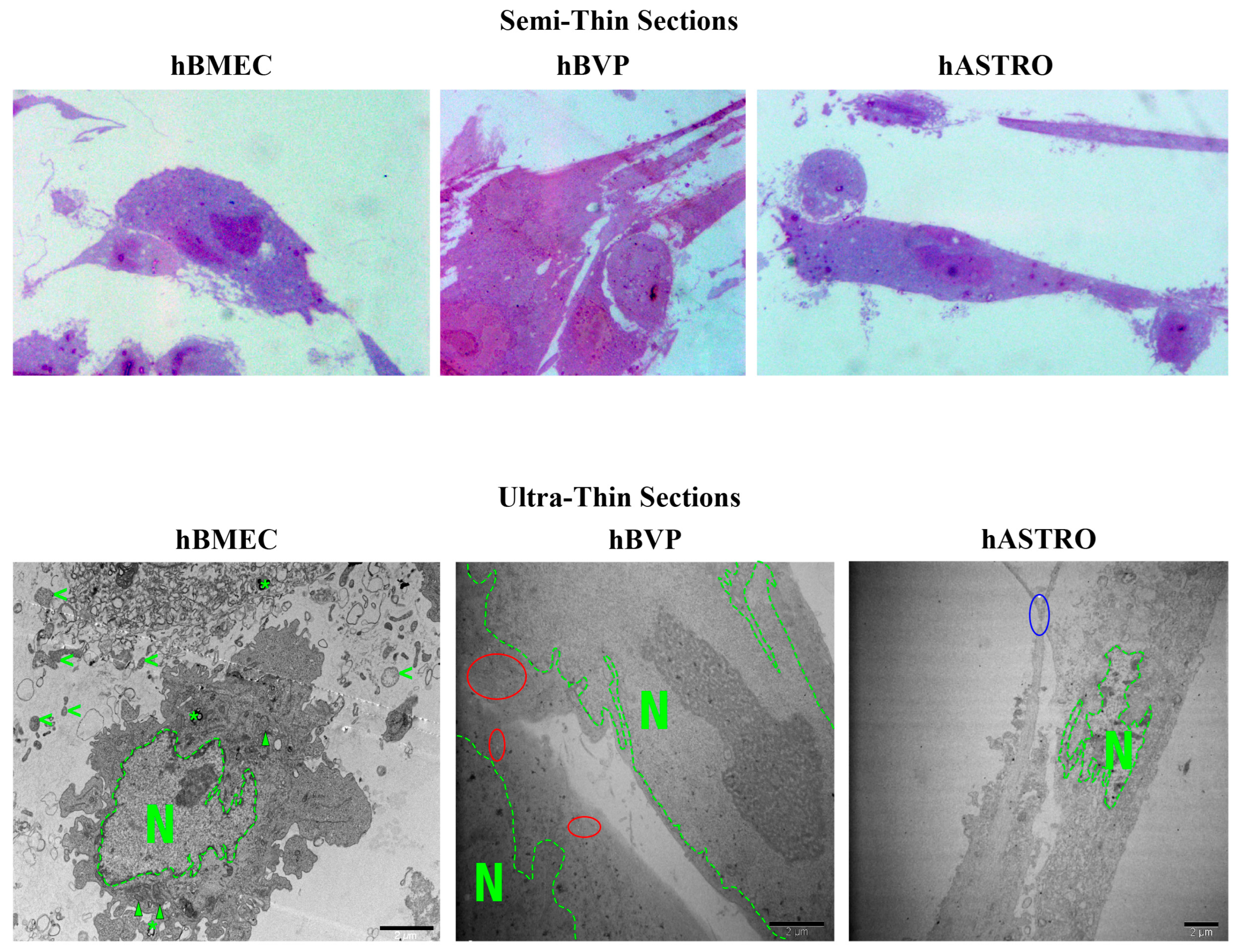
2.2.3. Ultrastructural Features of hBMECs, hBVPs, and hASTROs
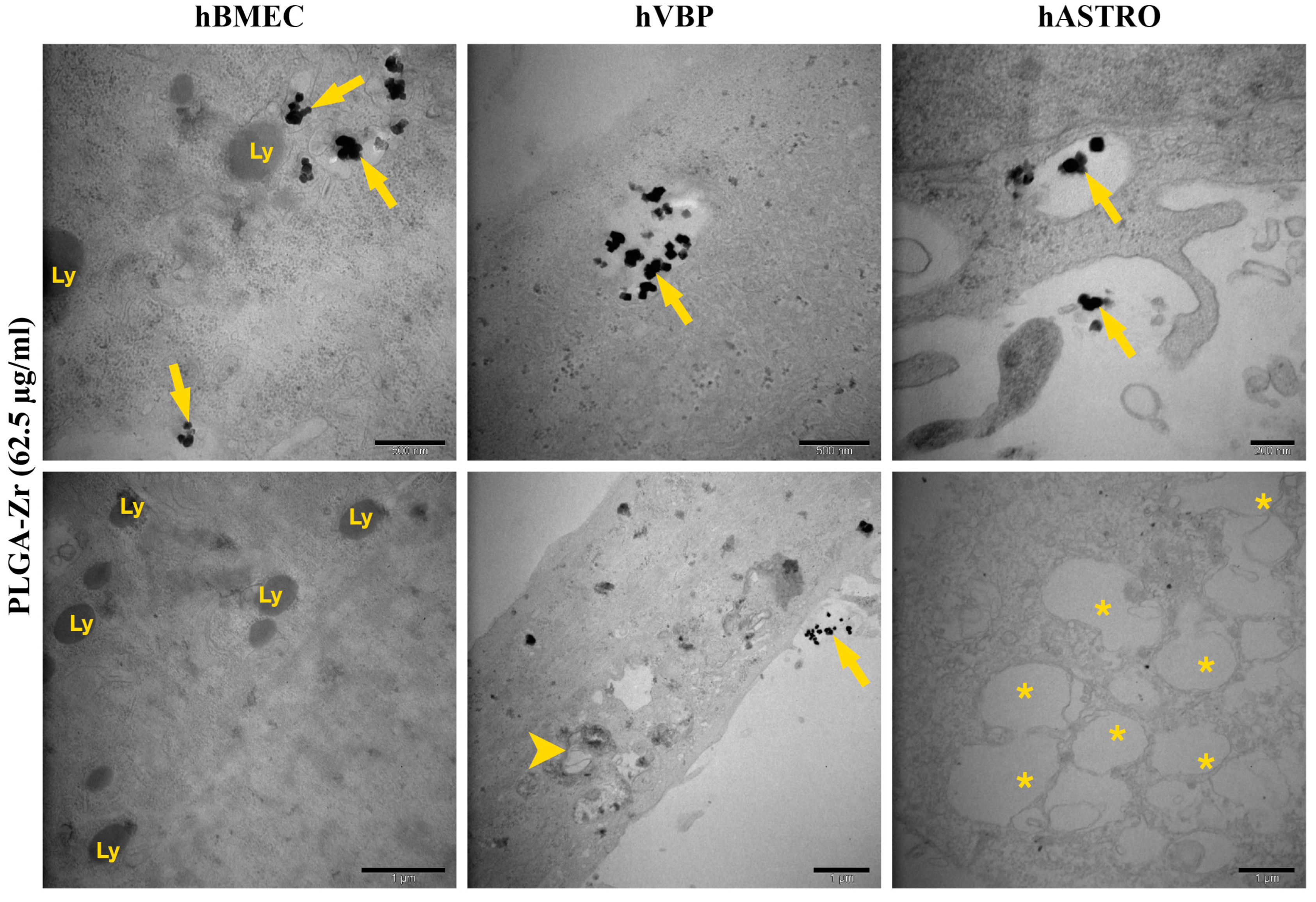
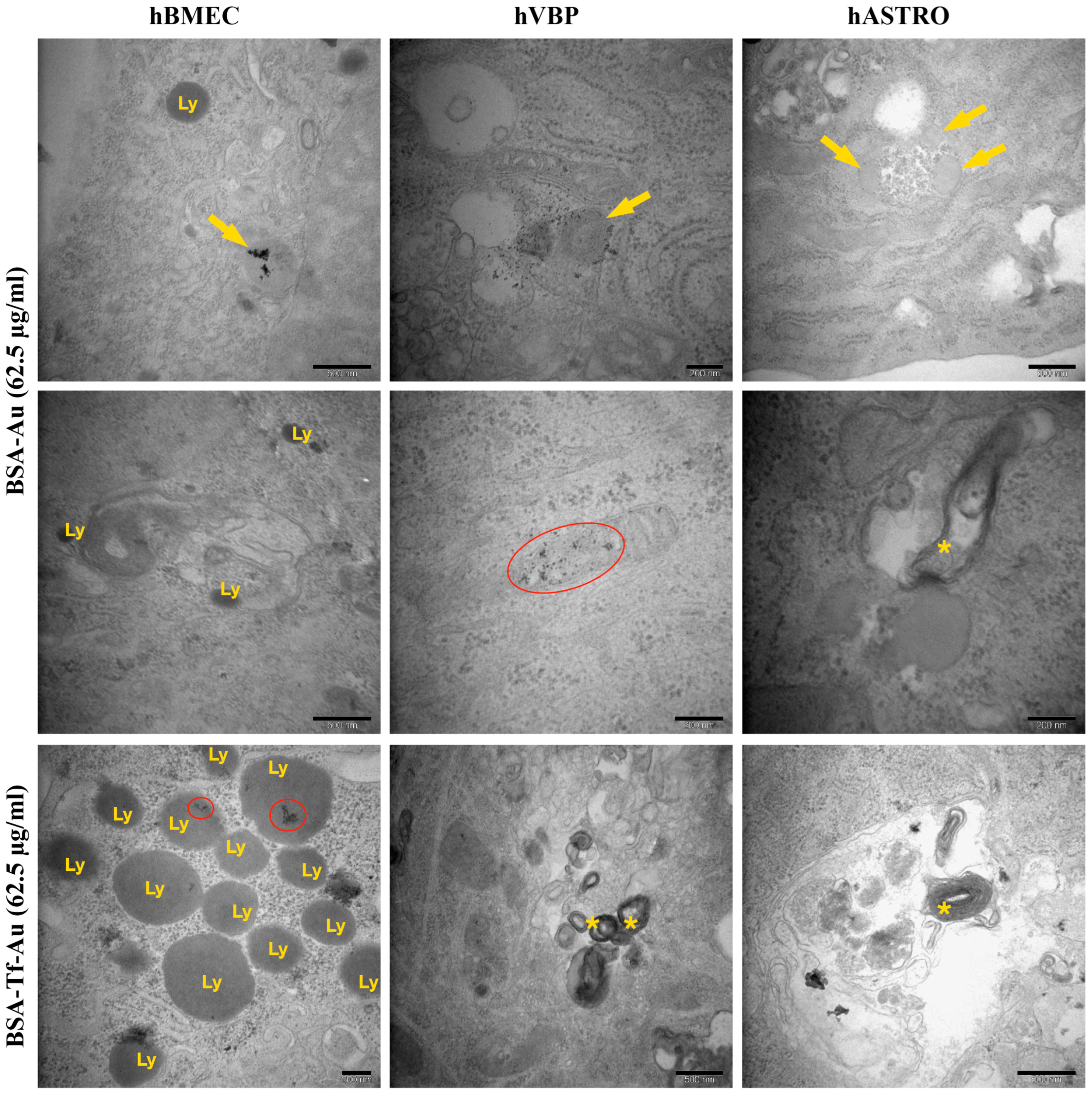
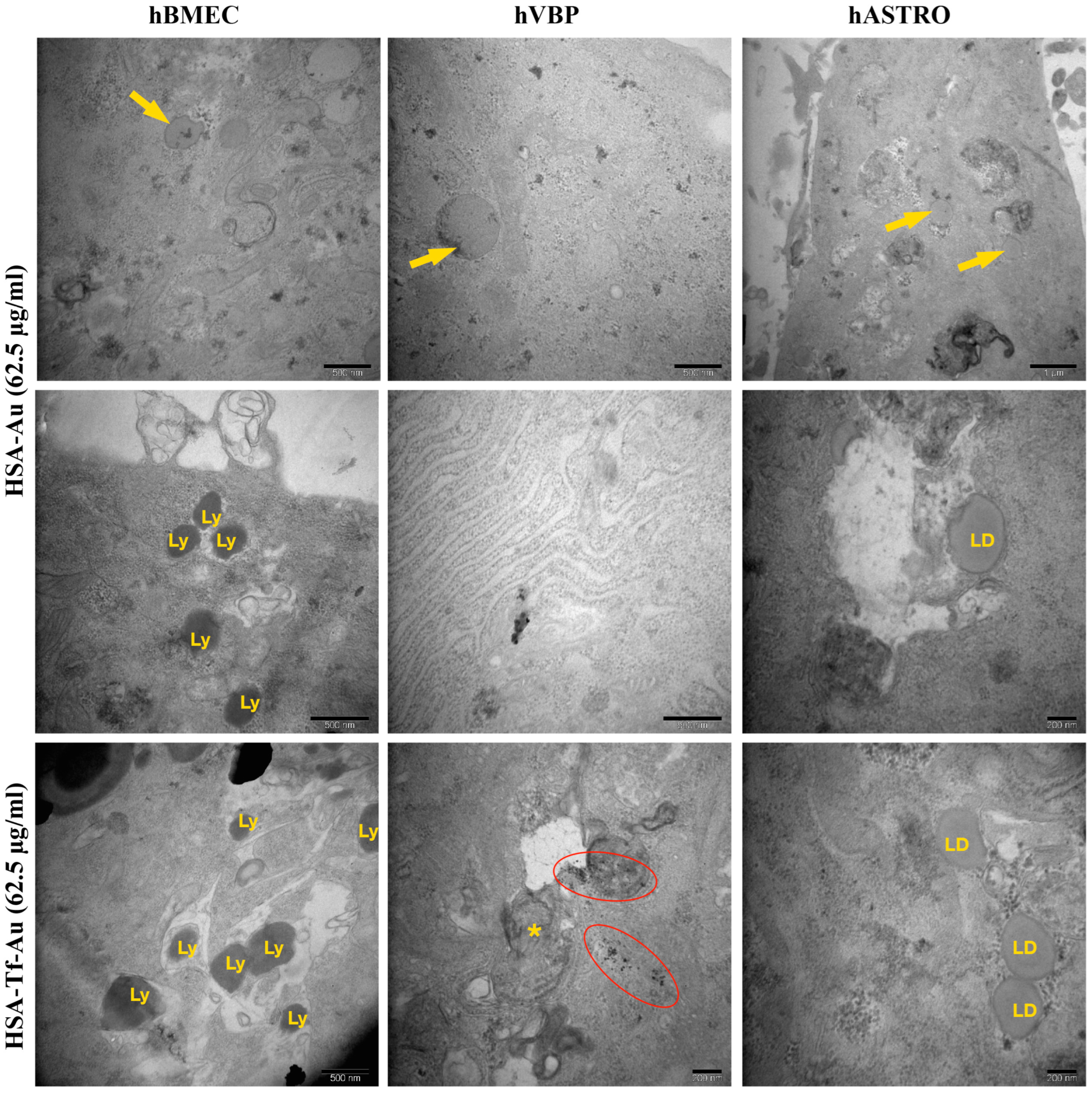
2.2.4. Ultrastructural Analysis of Cellular Uptake for NP Formulations
3. Discussion
4. Materials and Methods
4.1. Synthesis of Markers (Gold Nanoparticles and Zr-Based Metal–Organic Frameworks) for In Vitro Imaging Studies
4.2. Synthesis of BSA and HSA Nanoparticles
4.3. Transferrin Conjugation of HSA and BSA Nanoparticles
4.4. Synthesis of NLCs
4.5. Synthesis of PLGA Nanoparticles
4.6. Cell Culture
4.7. In Vitro Application of Nanoparticles
4.8. Cytotoxic Assay
4.9. Histological Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dotiwala, A.K.; McCausland, C.; Samra, N.S. Anatomy, Head and Neck: Blood Brain Barrier. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and function of the blood–brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef] [PubMed]
- Hervé, F.; Ghinea, N.; Scherrmann, J.-M. CNS Delivery Via Adsorptive Transcytosis. AAPS J. 2008, 10, 455–472. [Google Scholar] [CrossRef] [PubMed]
- Park, K. The Controlled Drug Delivery Systems: Past Forward and Future Back. J. Control Release 2014, 190, 3. [Google Scholar] [CrossRef]
- De Jong, W.H.; Borm, P.J.A. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The blood–brain barrier: Structure, regulation, and drug delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef]
- Joudeh, N.; Linke, D. Nanoparticle classification, physicochemical properties, characterization, and applications: A comprehensive review for biologists. J. Nanobiotechnol. 2022, 20, 262. [Google Scholar] [CrossRef]
- Sun, R.; Chen, Y.; Pei, Y.; Wang, W.; Zhu, Z.; Zheng, Z.; Yang, L.; Sun, L. The drug release of PLGA-based nanoparticles and their application in treatment of gastrointestinal cancers. Heliyon 2024, 10, e38165. [Google Scholar] [CrossRef]
- Zhi, K.; Raji, B.; Nookala, A.R.; Khan, M.M.; Nguyen, X.H.; Sakshi, S.; Pourmotabbed, T.; Yallapu, M.M.; Kochat, H.; Tadrous, E.; et al. PLGA Nanoparticle-Based Formulations to Cross the Blood–Brain Barrier for Drug Delivery: From R&D to cGMP. Pharmaceutics 2021, 13, 500. [Google Scholar] [CrossRef]
- Hoyos-Ceballos, G.P.; Ruozi, B.; Ottonelli, I.; Da Ros, F.; Vandelli, M.A.; Forni, F.; Daini, E.; Vilella, A.; Zoli, M.; Tosi, G.; et al. PLGA-PEG-ANG-2 Nanoparticles for Blood–Brain Barrier Crossing: Proof-of-Concept Study. Pharmaceutics 2020, 12, 72. [Google Scholar] [CrossRef]
- Pinto, M.; Silva, V.; Barreiro, S.; Silva, R.; Remião, F.; Borges, F.; Fernandes, C. Brain drug delivery and neurodegenerative diseases: Polymeric PLGA-based nanoparticles as a forefront platform. Ageing Res. Rev. 2022, 79, 101658. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, R.; Somoza, Á. Albumin Nanostructures for Nucleic Acid Delivery in Cancer: Current Trend, Emerging Issues, and Possible Solutions. Cancers 2021, 13, 3454. [Google Scholar] [CrossRef]
- Duan, L.; Hao, Z.; Ji, R.; Li, X.; Wang, H.; Su, Y.; Guan, F.; Ma, S. Glucose-modified BSA/procyanidin C1 NPs penetrate the blood-brain barrier and alleviate neuroinflammation in Alzheimer’s disease models. Int. J. Biol. Macromol. 2024, 268, 131739. [Google Scholar] [CrossRef]
- Sahin, H.; Yucel, O.; Emik, S.; Senturk, G.E. Protective Effects of Intranasally Administrated Oxytocin-Loaded Nanoparticles on Pentylenetetrazole-Kindling Epilepsy in Terms of Seizure Severity, Memory, Neurogenesis, and Neuronal Damage. ACS Chem. Neurosci. 2022, 13, 1923–1937. [Google Scholar] [CrossRef]
- Thi, T.T.H.; Suys, E.J.A.; Lee, J.S.; Nguyen, D.H.; Park, K.D.; Truong, N.P. Lipid-Based Nanoparticles in the Clinic and Clinical Trials: From Cancer Nanomedicine to COVID-19 Vaccines. Vaccines 2021, 9, 359. [Google Scholar] [CrossRef]
- Khare, P.; Edgecomb, S.X.; Hamadani, C.M.; Tanner, E.E.L.; Manickam, D.S. Lipid nanoparticle-mediated drug delivery to the brain. Adv. Drug Deliv. Rev. 2023, 197, 114861. [Google Scholar] [CrossRef]
- Li, J.; Kataoka, K. Chemo-physical Strategies to Advance the in Vivo Functionality of Targeted Nanomedicine: The Next Generation. J. Am. Chem. Soc. 2021, 143, 538–559. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.; Ke, W.; Dirisala, A.; Toh, K.; Tanaka, M.; Li, J. Stealth and pseudo-stealth nanocarriers. Adv. Drug Deliv. Rev. 2023, 198, 114895. [Google Scholar] [CrossRef]
- Ye, D.; Nic Raghnaill, M.; Bramini, M.; Mahon, E.; Åberg, C.; Salvati, A.; Dawson, K.A. Nanoparticle accumulation and transcytosis in brain endothelial cell layers. Nanoscale 2013, 5, 11153. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huo, Y.; Yao, L.; Xu, Y.; Meng, F.; Li, H.; Sun, K.; Zhou, G.; Kohane, D.S.; Tao, K. Transcytosis of Nanomedicine for Tumor Penetration. Nano Lett. 2019, 19, 8010–8020. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, Y.; Shen, Y.; Zhou, Z. Stimuli-Responsive Nanocarriers for Transcytosis-Based Cancer Drug Delivery. Adv. NanoBiomed Res. 2024, 4, 2300125. [Google Scholar] [CrossRef]
- Nag, O.K.; Delehanty, J.B. Active Cellular and Subcellular Targeting of Nanoparticles for Drug Delivery. Pharmaceutics 2019, 11, 543. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101. [Google Scholar] [CrossRef]
- Zomuansangi, R.; Singh, B.P.; Singh, G.; Zothanpuia; Singh, P.K.; Song, J.J.; Kharat, A.S.; Deka, P.; Yadav, M.K. Role of nanoparticles in the treatment of human disease: A comprehensive review. In Nanotechnology and Human Health; Elsevier: Amsterdam, The Netherlands, 2023; pp. 381–404. [Google Scholar] [CrossRef]
- Khan, S.; Sharma, A.; Jain, V. An Overview of Nanostructured Lipid Carriers and its Application in Drug Delivery through Different Routes. Adv. Pharm. Bull. 2023, 13, 446–460. [Google Scholar] [CrossRef] [PubMed]
- Hadden, J.M.; Bloemendal, M.; Haris, P.I.; Srai, S.K.S.; Chapman, D. Fourier transform infrared spectroscopy and differential scanning calorimetry of transferrins: Human serum transferrin, rabbit serum transferrin and human lactoferrin. Biochim. Biophys. Acta (BBA) Protein Struct. Mol. Enzymol. 1994, 1205, 59–67. [Google Scholar] [CrossRef]
- Andrade, S.; Loureiro, J.A.; Pereira, M.C. Transferrin-Functionalized Liposomes for the Delivery of Gallic Acid: A Therapeutic Approach for Alzheimer’s Disease. Pharmaceutics 2022, 14, 2163. [Google Scholar] [CrossRef]
- de Figueiredo, D.B.; Kaneko, K.; Rodrigues, T.d.C.; MacLoughlin, R.; Miyaji, E.N.; Saleem, I.; Gonçalves, V.M. Pneumococcal Surface Protein A-Hybrid Nanoparticles Protect Mice from Lethal Challenge after Mucosal Immunization Targeting the Lungs. Pharmaceutics 2022, 14, 1238. [Google Scholar] [CrossRef]
- Shah, B.; Khunt, D.; Bhatt, H.; Misra, M.; Padh, H. Intranasal delivery of venlafaxine loaded nanostructured lipid carrier: Risk assessment and QbD based optimization. J. Drug Deliv. Sci. Technol. 2016, 33, 37–50. [Google Scholar] [CrossRef]
- Mirakabad, F.S.T.; Nejati-Koshki, K.; Akbarzadeh, A.; Yamchi, M.R.; Milani, M.; Zarghami, N.; Zeighamian, V.; Rahimzadeh, A.; Alimohammadi, S.; Hanifehpour, Y.; et al. PLGA-based nanoparticles as cancer drug delivery systems. Asian Pac. J. Cancer Prev. 2014, 15, 517–535. [Google Scholar] [CrossRef]
- Goldberg, M.; Langer, R.; Jia, X. Nanostructured materials for applications in drug delivery and tissue engineering. J. Biomater. Sci. Polym. Ed. 2007, 18, 241–268. [Google Scholar] [CrossRef]
- Guo, X.; Zuo, X.; Zhou, Z.; Gu, Y.; Zheng, H.; Wang, X.; Wang, G.; Xu, C.; Wang, F. PLGA-Based Micro/Nanoparticles: An Overview of Their Applications in Respiratory Diseases. Int. J. Mol. Sci. 2023, 24, 4333. [Google Scholar] [CrossRef] [PubMed]
- Dhuri, K.; Vyas, R.N.; Blumenfeld, L.; Verma, R.; Bahal, R. Nanoparticle Delivered Anti-miR-141-3p for Stroke Therapy. Cells 2021, 10, 1011. [Google Scholar] [CrossRef]
- Katila, N.; Duwa, R.; Bhurtel, S.; Khanal, S.; Maharjan, S.; Jeong, J.-H.; Lee, S.; Choi, D.-Y.; Yook, S. Enhancement of blood–brain barrier penetration and the neuroprotective effect of resveratrol. J. Control. Release 2022, 346, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.P.; Cotter, J.D.; Prakapenka, A.V.; Cook, R.L.; DiPerna, D.M.; Sirianni, R.W. Targeting Small Molecule Delivery to the Brain and Spinal Cord via Intranasal Administration of Rabies Virus Glycoprotein (RVG29)-Modified PLGA Nanoparticles. Pharmaceutics 2020, 12, 93. [Google Scholar] [CrossRef]
- Solanki, R.; Rostamabadi, H.; Patel, S.; Jafari, S.M. Anticancer nano-delivery systems based on bovine serum albumin nanoparticles: A critical review. Int. J. Biol. Macromol. 2021, 193, 528–540. [Google Scholar] [CrossRef]
- Kulig, K.; Morak-Młodawska, B.; Jeleń, M.; Ziąbka, M.; Owczarzy, A.; Rogóż, W.; Maciążek-Jurczyk, M. Bovine Serum Albumin Nanoparticles as a Proposed Drug Formulation for the Delivery of 10H-2,7-diazaphenothiazine. J. Clust. Sci. 2024, 35, 2353–2362. [Google Scholar] [CrossRef]
- Paramasivam, S.; Kundal, K.; Sarkar, N. Human Serum Albumin Aggregation and its Modulation Using Nanoparticles: A Review. Protein Pept. Lett. 2022, 29, 11–21. [Google Scholar] [CrossRef]
- Shen, X.; Liu, X.; Li, T.; Chen, Y.; Chen, Y.; Wang, P.; Zheng, L.; Yang, H.; Wu, C.; Deng, S.; et al. Recent Advancements in Serum Albumin-Based Nanovehicles Toward Potential Cancer Diagnosis and Therapy. Front. Chem. 2021, 9, 746646. [Google Scholar] [CrossRef]
- Mogues, T.; Li, J.; Coburn, J.; Kuter, D.J. IgG antibodies against bovine serum albumin in humans—Their prevalence and response to exposure to bovine serum albumin. J. Immunol. Methods 2005, 300, 1–11. [Google Scholar] [CrossRef]
- Ulbrich, K.; Hekmatara, T.; Herbert, E.; Kreuter, J. Transferrin- and transferrin-receptor-antibody-modified nanoparticles enable drug delivery across the blood–brain barrier (BBB). Eur. J. Pharm. Biopharm. 2009, 71, 251–256. [Google Scholar] [CrossRef]
- Zhang, S.; Asghar, S.; Yang, L.; Hu, Z.; Chen, Z.; Shao, F.; Xiao, Y. Borneol and poly (ethylene glycol) dual modified BSA nanoparticles as an itraconazole vehicle for brain targeting. Int. J. Pharm. 2020, 575, 119002. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.-P.; Ahmadvand, D.; Su, J.; Hall, A.; Tan, X.; Farhangrazi, Z.S.; Moghimi, S.M. Crossing the blood-brain-barrier with nanoligand drug carriers self-assembled from a phage display peptide. Nat. Commun. 2019, 10, 4635. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, R.; Granja, A.; Loureiro, J.; Pereira, M.; Pinheiro, M.; Neves, A.; Reis, S. Quercetin lipid nanoparticles functionalized with transferrin for Alzheimer’s disease. Eur. J. Pharm. Sci. 2020, 148, 105314. [Google Scholar] [CrossRef]
- Choi, C.H.J.; Alabi, C.A.; Webster, P.; Davis, M.E. Mechanism of active targeting in solid tumors with transferrin-containing gold nanoparticles. Proc. Natl. Acad. Sci. USA 2010, 107, 1235–1240. [Google Scholar] [CrossRef]
- Nogueira-Librelotto, D.R.; Codevilla, C.F.; Farooqi, A.; Rolim, C.M.B. Transferrin-Conjugated Nanocarriers as Active-Targeted Drug Delivery Platforms for Cancer Therapy. Curr. Pharm. Des. 2017, 23, 454–466. [Google Scholar] [CrossRef]
- Sahin, A.; Esendagli, G.; Yerlikaya, F.; Caban-Toktas, S.; Yoyen-Ermis, D.; Horzum, U.; Aktas, Y.; Khan, M.; Couvreur, P.; Capan, Y. A small variation in average particle size of PLGA nanoparticles prepared by nanoprecipitation leads to considerable change in nanoparticles’ characteristics and efficacy of intracellular delivery. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1657–1664. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Y.; Cao, W.; Xie, S.; Wen, L.; Chen, G. Understanding the translocation mechanism of PLGA nanoparticles across round window membrane into the inner ear: A guideline for inner ear drug delivery based on nanomedicine. Int. J. Nanomed. 2018, 13, 479–492. [Google Scholar] [CrossRef]
- Sousa de Almeida, M.; Susnik, E.; Drasler, B.; Taladriz-Blanco, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Understanding nanoparticle endocytosis to improve targeting strategies in nanomedicine. Chem. Soc. Rev. 2021, 50, 5397–5434. [Google Scholar] [CrossRef]
- Trivedi, P.C.; Bartlett, J.J.; Pulinilkunnil, T. Lysosomal Biology and Function: Modern View of Cellular Debris Bin. Cells 2020, 9, 1131. [Google Scholar] [CrossRef]
- Florance, I.; Cordani, M.; Pashootan, P.; Moosavi, M.A.; Zarrabi, A.; Chandrasekaran, N. The impact of nanomaterials on autophagy across health and disease conditions. Cell. Mol. Life Sci. 2024, 81, 184. [Google Scholar] [CrossRef]
- Karimi, M.; Bahrami, S.; Ravari, S.B.; Zangabad, P.S.; Mirshekari, H.; Bozorgomid, M.; Shahreza, S.; Sori, M.; Hamblin, M.R. Albumin nanostructures as advanced drug delivery systems. Expert Opin. Drug Deliv. 2016, 13, 1609–1623. [Google Scholar] [CrossRef] [PubMed]
- Patrón-Romero, L.; Luque-Morales, P.A.; Loera-Castañeda, V.; Lares-Asseff, I.; Leal-Ávila, M.; Alvelais-Palacios, J.A.; Plasencia-López, I.; Almanza-Reyes, H. Mitochondrial Dysfunction Induced by Zinc Oxide Nanoparticles. Crystals 2022, 12, 1089. [Google Scholar] [CrossRef]
- Nalika, N.; Parvez, S. Mitochondrial dysfunction in titanium dioxide nanoparticle-induced neurotoxicity. Toxicol. Mech. Methods 2015, 25, 355–363. [Google Scholar] [CrossRef]
- Cameron, S.J.; Sheng, J.; Hosseinian, F.; Willmore, W.G. Nanoparticle Effects on Stress Response Pathways and Nanoparticle-Protein Interactions. Int. J. Mol. Sci. 2022, 23, 7962. [Google Scholar] [CrossRef]
- Gómez-Virgilio, L.; Silva-Lucero, M.-D.; Flores-Morelos, D.-S.; Gallardo-Nieto, J.; Lopez-Toledo, G.; Abarca-Fernandez, A.-M.; Zacapala-Gómez, A.-E.; Luna-Muñoz, J.; Montiel-Sosa, F.; Soto-Rojas, L.O.; et al. Autophagy: A Key Regulator of Homeostasis and Disease: An Overview of Molecular Mechanisms and Modulators. Cells 2022, 11, 2262. [Google Scholar] [CrossRef] [PubMed]
- Fransen, M.; Nordgren, M.; Wang, B.; Apanasets, O. Role of peroxisomes in ROS/RNS-metabolism: Implications for human disease. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2012, 1822, 1363–1373. [Google Scholar] [CrossRef]
- Yücel, O.; Aksüt, Y.; Şengelen, A.; Yıldırım, E.; Emik, S.; Arda, N.; Gürdağ, G. Folate receptor-targeted indomethacin-loaded gold nanoparticles enhance drug chemotherapeutic efficacy in glioblastoma cells and spheroids. J. Drug Deliv. Sci. Technol. 2024, 100, 106025. [Google Scholar] [CrossRef]
- Duskey, J.T.; Rinaldi, A.; Ottonelli, I.; Caraffi, R.; De Benedictis, C.A.; Sauer, A.K.; Tosi, G.; Vandelli, M.A.; Ruozi, B.; Grabrucker, A.M. Glioblastoma Multiforme Selective Nanomedicines for Improved Anti-Cancer Treatments. Pharmaceutics 2022, 14, 1450. [Google Scholar] [CrossRef]
- Gartziandia, O.; Herran, E.; Pedraz, J.L.; Carro, E.; Igartua, M.; Hernandez, R.M. Chitosan coated nanostructured lipid carriers for brain delivery of proteins by intranasal administration. Colloids Surf. B Biointerfaces 2015, 134, 304–313. [Google Scholar] [CrossRef]
- Danz, K.; Fleddermann, J.; Koch, M.; Fecioru, E.; Maahs, L.; Kinsinger, N.; Krämer, J.; Kraegeloh, A.; Wagner, S. Evaluation of the Transport and Binding of Dopamine-Loaded PLGA Nanoparticles for the Treatment of Parkinson’s Disease Using In Vitro Model Systems. Pharmaceutics 2024, 16, 571. [Google Scholar] [CrossRef]
- Seko, I.; Tonbul, H.; Tavukçuoğlu, E.; Şahin, A.; Akbas, S.; Yanık, H.; Öztürk, S.C.; Esendagli, G.; Khan, M.; Capan, Y. Development of curcumin and docetaxel co-loaded actively targeted PLGA nanoparticles to overcome blood brain barrier. J. Drug Deliv. Sci. Technol. 2021, 66, 102867. [Google Scholar] [CrossRef]
- Wagner, S.; Zensi, A.; Wien, S.L.; Tschickardt, S.E.; Maier, W.; Vogel, T.; Worek, F.; Pietrzik, C.U.; Kreuter, J.; von Briesen, H. Uptake Mechanism of ApoE-Modified Nanoparticles on Brain Capillary Endothelial Cells as a Blood-Brain Barrier Model. PLoS ONE 2012, 7, e32568. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zuo, H.; Fu, Y.; Cao, Y.; Li, Q.; Zhang, Q.; Zheng, Y.; Wang, Y.; Wu, D.; Chen, W.; et al. Intranasal delivery of phenytoin loaded layered double hydroxide nanoparticles improves therapeutic effect on epileptic seizures. J. Nanobiotechnol. 2024, 22, 144. [Google Scholar] [CrossRef] [PubMed]
- Zaman, R.U.; Mulla, N.S.; Braz Gomes, K.; D’Souza, C.; Murnane, K.S.; D’Souza, M.J. Nanoparticle formulations that allow for sustained delivery and brain targeting of the neuropeptide oxytocin. Int. J. Pharm. 2018, 548, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Stierhof, Y.-D.; Humbel, B.; Hermann, R.; Otten, M.; Schwarz, H. Direct Visualization and Silver Enhancement of Ultra-Small Antibody-Bound Gold Particles on Immunolabeled Ultrathin Resin Sections. Scanning Microsc. 1992, 6, 12. [Google Scholar]
- Gunduz, N.; Ceylan, H.; Guler, M.O.; Tekinay, A.B. Intracellular Accumulation of Gold Nanoparticles Leads to Inhibition of Macropinocytosis to Reduce the Endoplasmic Reticulum Stress. Sci. Rep. 2017, 7, 40493. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
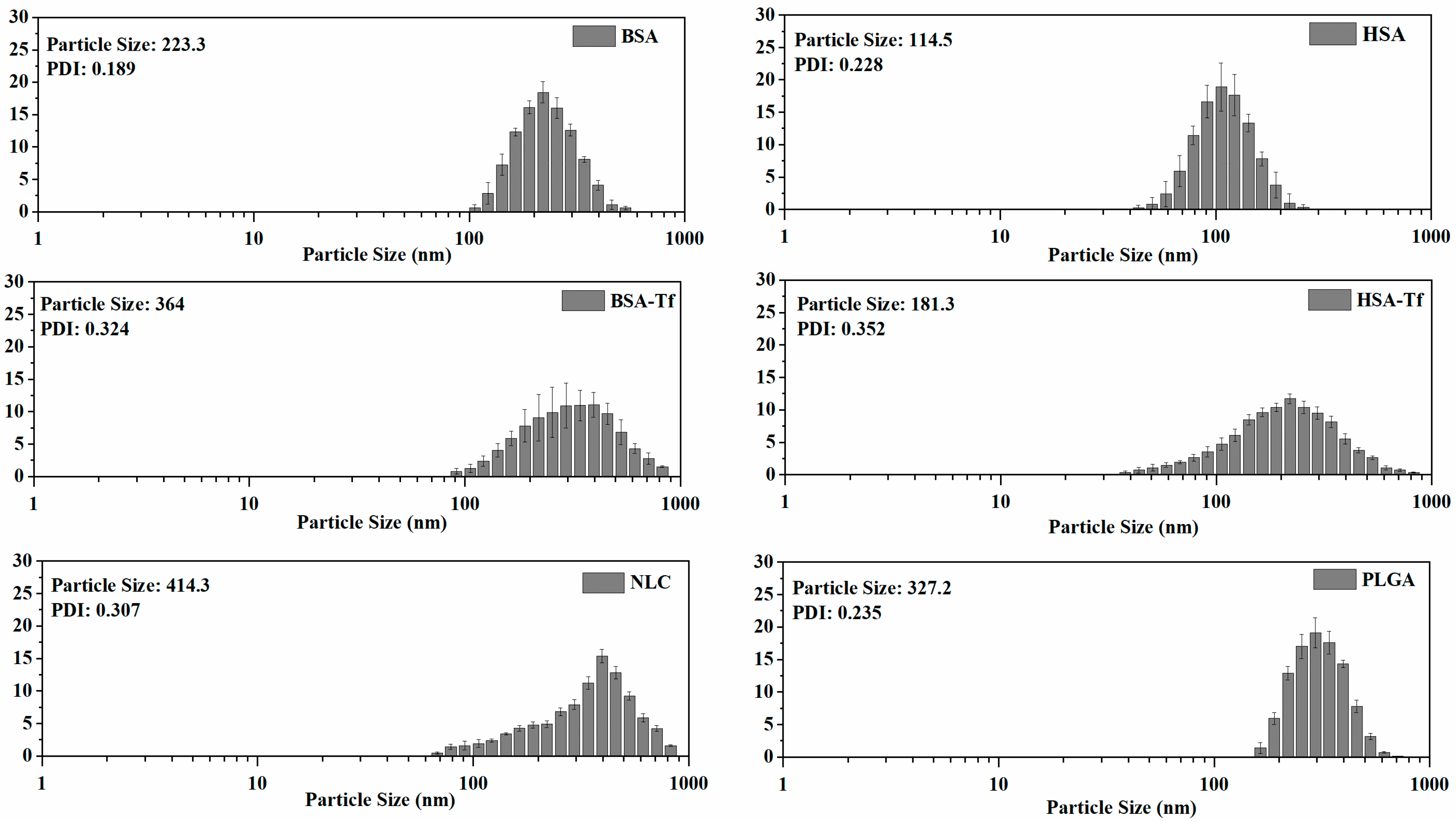




| NP | Particle Size (nm) | PDI |
|---|---|---|
| PLGA | 327.2 ± 35.3 | 0.235 ± 0.016 |
| BSA | 223.3 ± 15.8 | 0.189 ± 0.020 |
| BSA-Tf | 364.0 ± 22.1 | 0.324 ± 0.045 |
| HSA | 114.5 ± 7.5 | 0.228 ± 0.018 |
| HSA-Tf | 181.3 ± 14.2 | 0.352 ± 0.033 |
| NLC | 414.3 ± 28.6 | 0.307 ± 0.061 |
| Cell | hBMEC | hBVP | hASTRO | |
|---|---|---|---|---|
| NP | ||||
| PLGA |
|
|
| |
| BSA |
|
|
| |
| BSA-Tf |
|
|
| |
| HSA |
|
|
| |
| HSA-Tf |
|
|
| |
| NLC |
|
|
| |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahin, H.; Yucel, O.; Holloway, P.; Yildirim, E.; Emik, S.; Gurdag, G.; Tanriverdi, G.; Erkanli Senturk, G. Comparison of Drug Delivery Systems with Different Types of Nanoparticles in Terms of Cellular Uptake and Responses in Human Endothelial Cells, Pericytes, and Astrocytes. Pharmaceuticals 2024, 17, 1567. https://doi.org/10.3390/ph17121567
Sahin H, Yucel O, Holloway P, Yildirim E, Emik S, Gurdag G, Tanriverdi G, Erkanli Senturk G. Comparison of Drug Delivery Systems with Different Types of Nanoparticles in Terms of Cellular Uptake and Responses in Human Endothelial Cells, Pericytes, and Astrocytes. Pharmaceuticals. 2024; 17(12):1567. https://doi.org/10.3390/ph17121567
Chicago/Turabian StyleSahin, Hakan, Oguz Yucel, Paul Holloway, Eren Yildirim, Serkan Emik, Gulten Gurdag, Gamze Tanriverdi, and Gozde Erkanli Senturk. 2024. "Comparison of Drug Delivery Systems with Different Types of Nanoparticles in Terms of Cellular Uptake and Responses in Human Endothelial Cells, Pericytes, and Astrocytes" Pharmaceuticals 17, no. 12: 1567. https://doi.org/10.3390/ph17121567
APA StyleSahin, H., Yucel, O., Holloway, P., Yildirim, E., Emik, S., Gurdag, G., Tanriverdi, G., & Erkanli Senturk, G. (2024). Comparison of Drug Delivery Systems with Different Types of Nanoparticles in Terms of Cellular Uptake and Responses in Human Endothelial Cells, Pericytes, and Astrocytes. Pharmaceuticals, 17(12), 1567. https://doi.org/10.3390/ph17121567






