Comparison of Two Chelator Scaffolds as Basis for Cholecystokinin-2 Receptor Targeting Bimodal Imaging Probes
Abstract
:1. Introduction
2. Results
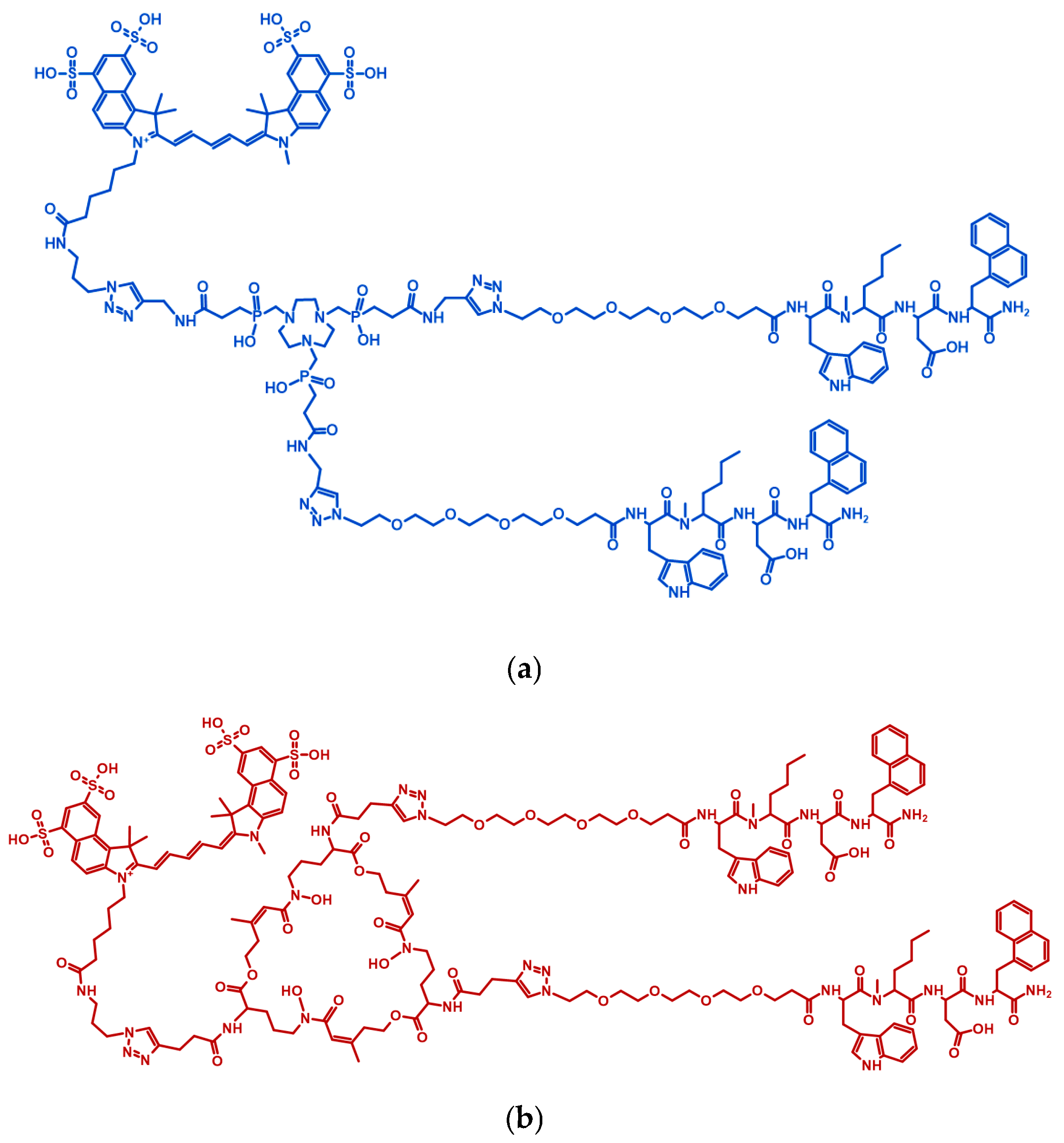
2.1. Precursor Preparation
2.2. Radiolabelling with Gallium-68
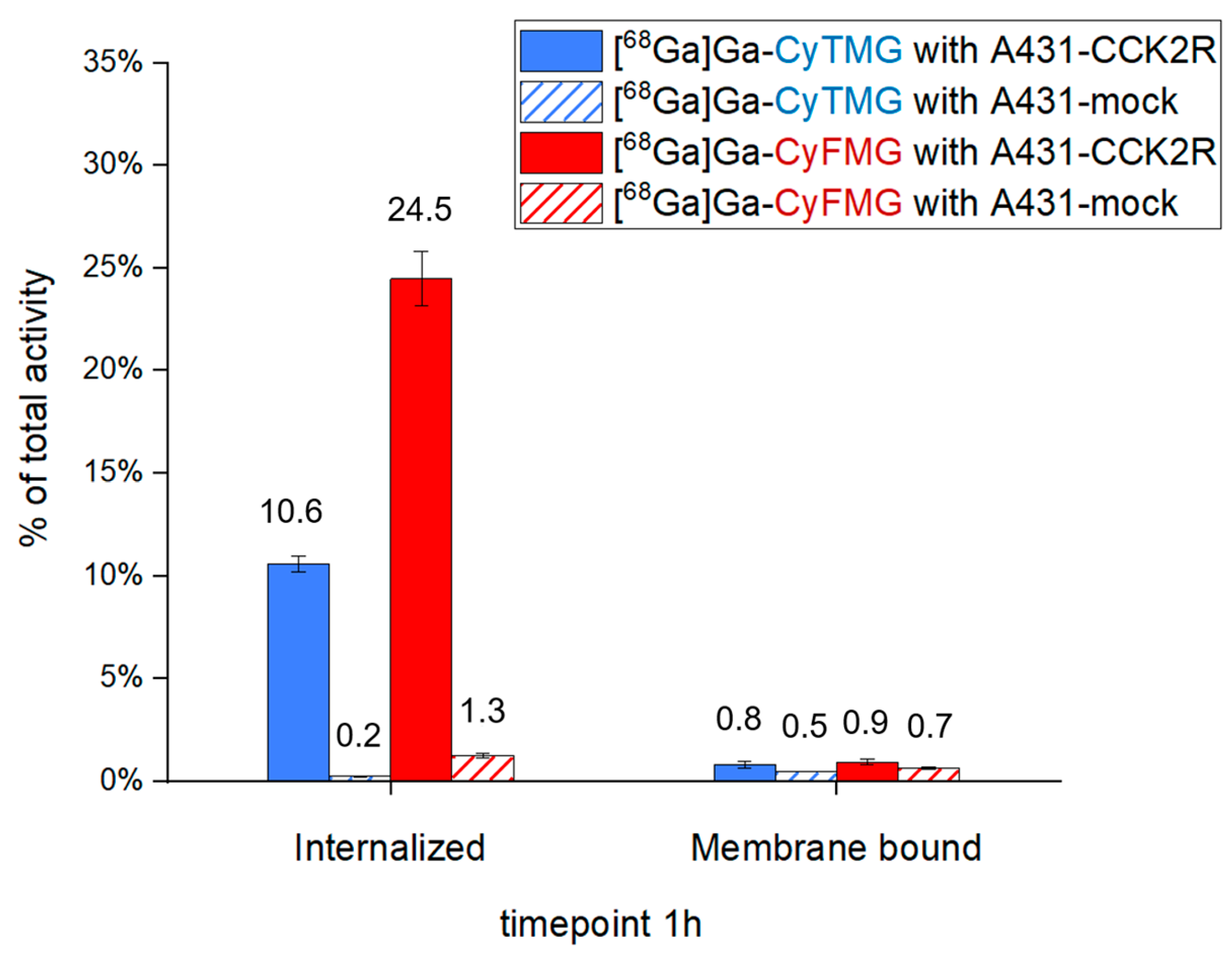
2.3. In Vitro Characterization
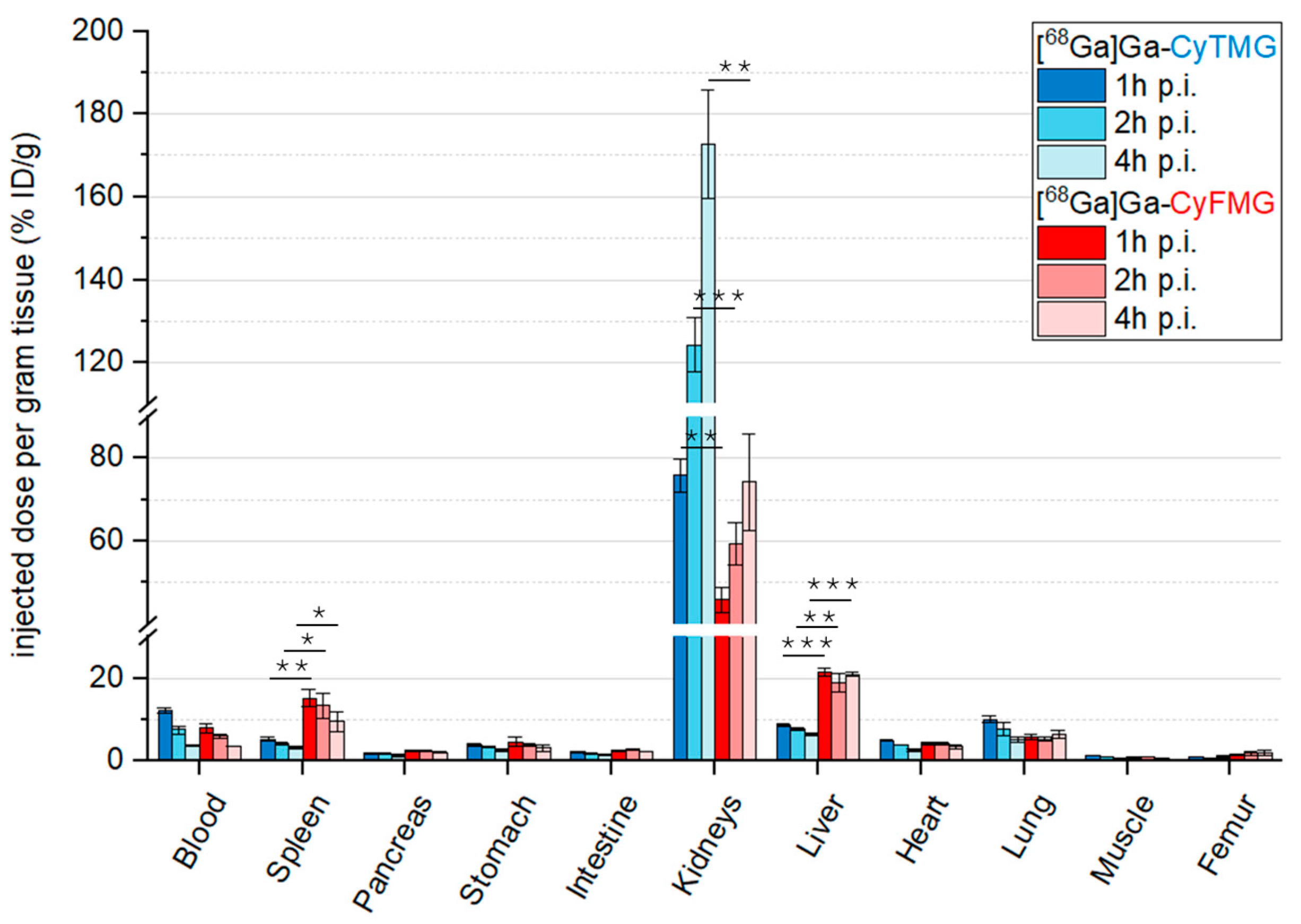
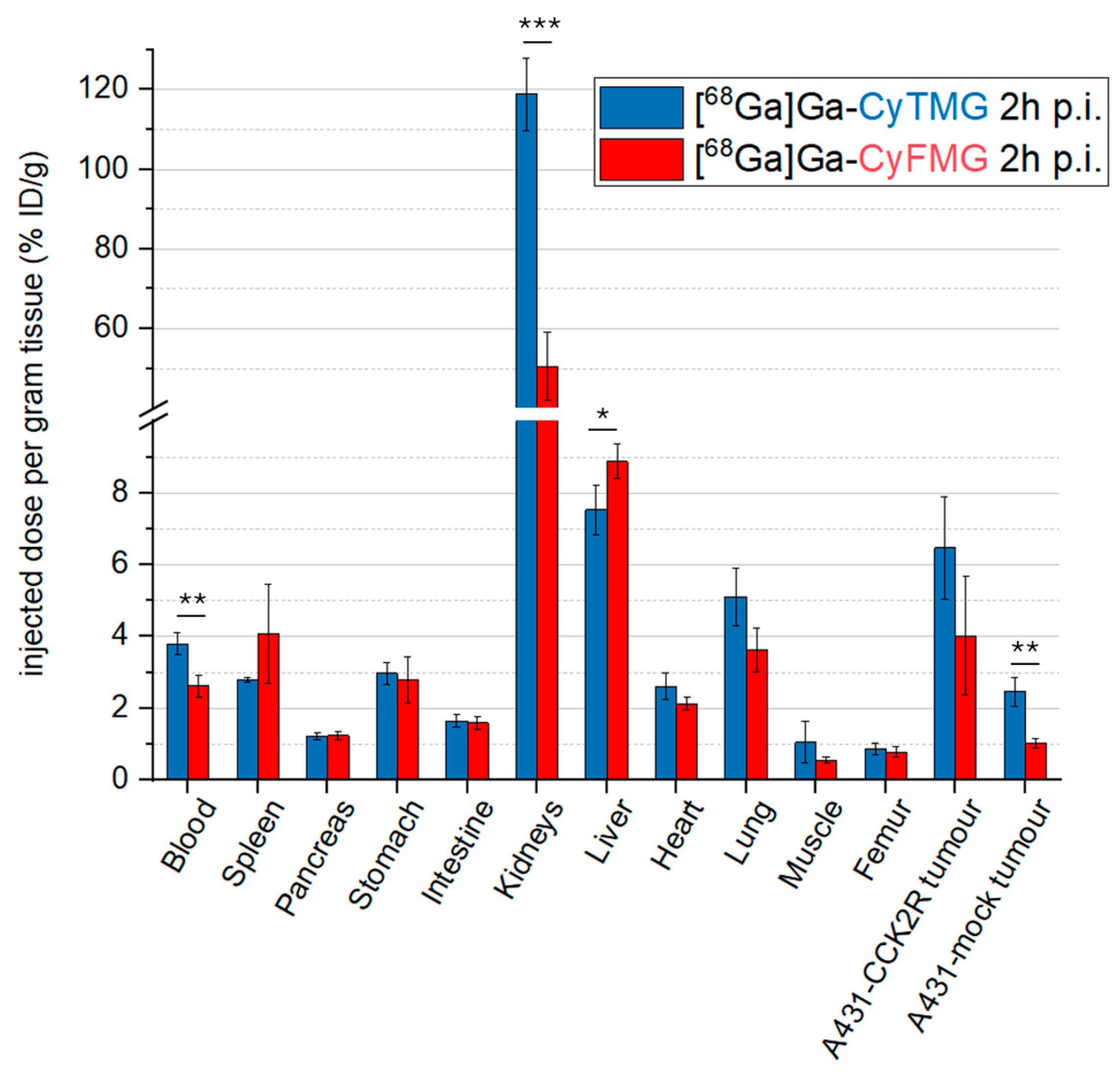
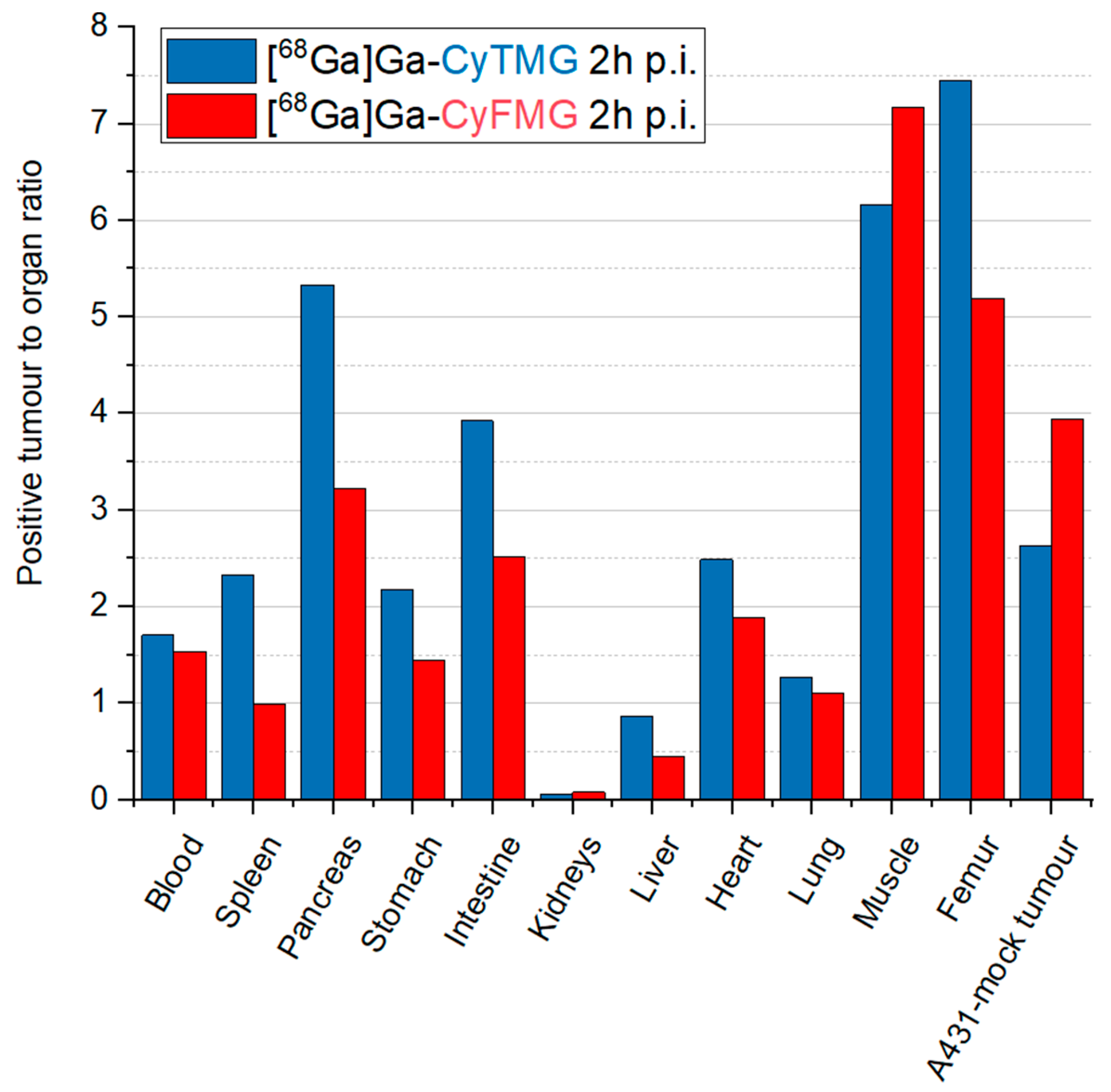
2.4. In Vivo Characterization
3. Discussion
4. Materials and Methods
4.1. Instrumentation
4.1.1. Analytical [Radio]-RP-HPLC
4.1.2. MALDI-TOF MS
4.1.3. 68Ge/68Ga Generator
4.1.4. γ- Counter
4.1.5. Radio-iTLC
4.1.6. Confocal Imaging
4.1.7. Cryo-Fluorescence Tomography (CFT)
4.2. In Vitro Methods
4.2.1. Radiolabelling with Gallium-68/Radiochemistry
4.2.2. Distribution Coefficient (LogD), Stability in Human Serum and PBS, and Protein Binding
4.2.3. Tumour Cell Lines and Cell Culture
4.2.4. Cell Uptake Studies
4.2.5. Fluorescence Microscopy Studies
4.2.6. Receptor-Binding Affinity Studies
4.3. Animal Experiments
4.3.1. Ex Vivo Biodistribution Experiments, Small Animal Imaging Studies
4.3.2. Cryo-Fluorescence Tomography (CFT) Data Acquisition
4.4. Statistical and Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACN | Acetonitrile |
| BSA | Bovine serum albumin |
| CCK2R | Cholecystokinin-2 receptor |
| CFT | Cryo-fluorescence tomography |
| CuAAC | Cu(I)-catalyzed azide–alkyne cycloaddition |
| DAFC | Diacetylated Fusarinine C |
| DMEM | Dulbecco’s Modified Eagle’s medium |
| DOTA | Dodecane tetraacetic acid |
| EDTA | Ethylenediaminetetraacetic acid |
| FDA | Food and Drug Administration |
| FI | Fluorescence imaging |
| FSC | Fusarinine C |
| HEPES | 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid |
| IC50 | Half-maximal inhibitory concentration |
| ID | Injected dose |
| iTLC | Instant thin-layer chromatography |
| MALDI-TOF MS | Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry |
| MG | Minigastrin |
| MIP | Maximum-intensity projection |
| MRI | Magnetic resonance imaging |
| MTC | Medullary thyroid carcinoma |
| PBS | Phosphate-buffered saline |
| PET | Positron emission tomography |
| PSG | Penicillin–streptomycin–glutamine |
| Rf | Retention factor |
| ROI | Region of interest |
| RP-HPLC | Reversed phase high-performance liquid chromatography |
| RT | Room temperature |
| SCLC | Small-cell lung cancer |
| SPECT | Single-photon emission computed tomography |
| SulfoCy5.5 | Sulfo-Cyanine5.5 |
| TRAP | Triazacyclononane-phosphinic acid |
| TRIS | Tris(hydroxymethyl)aminomethan |
| USI | Ultrasound imaging |
| WGA-Alexa | Wheat germ agglutinin–Alexa |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Soerjomataram, I.; Bray, F. Planning for Tomorrow: Global Cancer Incidence and the Role of Prevention 2020–2070. Nat. Rev. Clin. Oncol. 2021, 18, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Rowe, S.P.; Pomper, M.G. Molecular Imaging in Oncology: Current Impact and Future Directions. CA Cancer J. Clin. 2022, 72, 333–352. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.T.; Tsien, R.Y. Fluorescence-Guided Surgery with Live Molecular Navigation—A New Cutting Edge. Nat. Rev. Cancer 2013, 13, 653–662. [Google Scholar] [CrossRef]
- Jiao, J.; Zhang, J.; Yang, F.; Song, W.; Han, D.; Wen, W.; Qin, W. Quicker, Deeper and Stronger Imaging: A Review of Tumor-Targeted, near-Infrared Fluorescent Dyes for Fluorescence Guided Surgery in the Preclinical and Clinical Stages. Eur. J. Pharm. Biopharm. 2020, 152, 123–143. [Google Scholar] [CrossRef]
- Dindere, M.E.; Tanca, A.; Rusu, M.; Liehn, E.A.; Bucur, O. Intraoperative Tumor Detection Using Pafolacianine. Int. J. Mol. Sci. 2022, 23, 12842. [Google Scholar] [CrossRef]
- Ariztia, J.; Solmont, K.; Moïse, N.P.; Specklin, S.; Heck, M.P.; Lamande-Langle, S.; Kuhnast, B. PET/Fluorescence Imaging: An Overview of the Chemical Strategies to Build Dual Imaging Tools. Bioconjug. Chem. 2022, 33, 24–52. [Google Scholar] [CrossRef]
- Kubeil, M.; Martínez, I.I.S.; Bachmann, M.; Kopka, K.; Tuck, K.L.; Stephan, H. Dual-Labelling Strategies for Nuclear and Fluorescence Molecular Imaging: Current Status and Future Perspectives. Pharmaceuticals 2022, 15, 432. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, J.; Ma, S.; Liu, Q.; Huang, L.; Chen, X.; Lou, K.; Wang, W. Recent Developments in Multimodality Fluorescence Imaging Probes. Acta Pharm. Sin. B 2018, 8, 320–338. [Google Scholar] [CrossRef]
- Von Guggenberg, E.; Kolenc, P.; Rottenburger, C.; Mikołajczak, R.; Hubalewska-Dydejczyk, A. Update on Preclinical Development and Clinical Translation of Cholecystokinin-2 Receptor Targeting Radiopharmaceuticals. Cancers 2021, 13, 5776. [Google Scholar] [CrossRef] [PubMed]
- Sosabowski, J.K.; Matzow, T.; Foster, J.M.; Finucane, C.; Ellison, D.; Watson, S.A.; Mather, S.J. Targeting of CCK-2 Receptor–Expressing Tumors Using a Radiolabeled Divalent Gastrin Peptide. J. Nucl. Med. 2009, 50, 2082–2089. [Google Scholar] [CrossRef] [PubMed]
- Viola, D.; Elisei, R. Management of Medullary Thyroid Cancer. Endocrinol. Metab. Clin. N. Am. 2019, 48, 285–301. [Google Scholar] [CrossRef] [PubMed]
- Machens, A.; Dralle, H. Biomarker-Based Risk Stratification for Previously Untreated Medullary Thyroid Cancer. J. Clin. Endocrinol. Metab. 2010, 95, 2655–2663. [Google Scholar] [CrossRef]
- Maia, A.L.; Wajner, S.M.; Vargas, C.V.F. Advances and Controversies in the Management of Medullary Thyroid Carcinoma. Curr. Opin. Oncol. 2017, 29, 25–32. [Google Scholar] [CrossRef]
- Ganeshan, D.; Paulson, E.; Duran, C.; Cabanillas, M.E.; Busaidy, N.L.; Charnsangavej, C. Current Update on Medullary Thyroid Carcinoma. Am. J. Roentgenol. 2013, 201, W867–W876. [Google Scholar] [CrossRef]
- Treglia, G.; Castaldi, P.; Villani, M.F.; Perotti, G.; De Waure, C.; Filice, A.; Ambrosini, V.; Cremonini, N.; Santimaria, M.; Versari, A.; et al. Comparison of 18F-DOPA, 18F-FDG and 68Ga-Somatostatin Analogue PET/CT in Patients with Recurrent Medullary Thyroid Carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 569–580. [Google Scholar] [CrossRef]
- Kossatz, S.; Béhé, M.; Mansi, R.; Saur, D.; Czerney, P.; Kaiser, W.A.; Hilger, I. Multifactorial Diagnostic NIR Imaging of CCK2R Expressing Tumors. Biomaterials 2013, 34, 5172–5180. [Google Scholar] [CrossRef]
- Kossatz, S.; Mansi, R.; Béhé, M.; Czerney, P.; Hilger, I. Influence of D-Glutamine and d-Glutamic Acid Sequences in Optical Peptide Probes Targeted against the Cholecystokinin-2/Gastrin-Receptor on Binding Affinity, Specificity and Pharmacokinetic Properties. EJNMMI Res. 2013, 3, 75. [Google Scholar] [CrossRef]
- Summer, D.; Grossrubatscher, L.; Petrik, M.; Michalcikova, T.; Novy, Z.; Rangger, C.; Klingler, M.; Haas, H.; Kaeopookum, P.; von Guggenberg, E.; et al. Developing Targeted Hybrid Imaging Probes by Chelator Scaffolding. Bioconjug. Chem. 2017, 28, 1722–1733. [Google Scholar] [CrossRef]
- Klingler, M.; Summer, D.; Rangger, C.; Haubner, R.; Foster, J.; Sosabowski, J.; Decristoforo, C.; Virgolini, I.; von Guggenberg, E. DOTA-MGS5, a New Cholecystokinin-2 Receptor-Targeting Peptide Analog with an Optimized Targeting Profile for Theranostic Use. J. Nucl. Med. 2019, 60, 1010–1016. [Google Scholar] [CrossRef] [PubMed]
- Summer, D.; Kroess, A.; Woerndle, R.; Rangger, C.; Klingler, M.; Haas, H.; Kremser, L.; Lindner, H.H.; von Guggenberg, E.; Decristoforo, C. Multimerization Results in Formation of Re-Bindable Metabolites: A Proof of Concept Study with FSC-Based Minigastrin Imaging Probes Targeting CCK2R Expression. PLoS ONE 2018, 13, e0201224. [Google Scholar] [CrossRef] [PubMed]
- Carlucci, G.; Ananias, H.J.K.; Yu, Z.; Van de Wiele, C.; Dierckx, R.A.; de Jong, I.J.; Elsinga, H.P. Multimerization Improves Targeting of Peptide Radio-Pharmaceuticals. Curr. Pharm. Des. 2012, 18, 2501–2516. [Google Scholar] [CrossRef]
- Lindner, S.; Michler, C.; Wangler, B.; Bartenstein, P.; Fischer, G.; Schirrmacher, R.; Wangler, C. PESIN Multimerization Improves Receptor Avidities and in Vivo Tumor Targeting Properties to GRPR-Overexpressing Tumors. Bioconjug. Chem. 2014, 25, 489–500. [Google Scholar] [CrossRef]
- Summer, D.; Rangger, C.; Klingler, M.; Laverman, P.; Franssen, G.M.; Lechner, B.E.; Orasch, T.; Haas, H.; von Guggenberg, E.; Decristoforo, C. Exploiting the Concept of Multivalency with 68 Ga- and 89 Zr-Labelled Fusarinine C-Minigastrin Bioconjugates for Targeting CCK2R Expression. Contrast Media Mol. Imaging 2018, 2018, 3171794. [Google Scholar] [CrossRef]
- Buckle, T.; Van Willigen, D.M.; Spa, S.J.; Hensbergen, A.W.; van der Wal, S.; de Korne, C.M.; Welling, M.M.; van der Poel, H.G.; Hardwick, J.C.; van Leeuwen, F.W. Tracers for Fluorescence-Guided Surgery: How Elongation of the Polymethine Chain in Cyanine Dyes Alters the Pharmacokinetics of a Dual-Modality c[RGDyK] Tracer. J. Nucl. Med. 2018, 59, 986–992. [Google Scholar] [CrossRef]
- Sauter, A.W.; Mansi, R.; Hassiepen, U.; Muller, L.; Panigada, T.; Wiehr, S.; Wild, A.M.; Geistlich, S.; Béhé, M.; Rottenburger, C.; et al. Targeting of the Cholecystokinin-2 Receptor with the Minigastrin Analog 177 Lu-DOTA-PP-F11N: Does the Use of Protease Inhibitors Further Improve In Vivo Distribution? J. Nucl. Med. 2019, 60, 393–399. [Google Scholar] [CrossRef]
- Notni, J.; Šimeček, J.; Hermann, P.; Wester, H.J. TRAP, a Powerful and Versatile Framework for Gallium-68 Radiopharmaceuticals. Chem. Eur. J. 2011, 17, 14718–14722. [Google Scholar] [CrossRef]
- Notni, J.; Hermann, P.; Havlíčková, J.; Kotek, J.; Kubíček, V.; Plutnar, J.; Loktionova, N.; Riss, P.J.; Rösch, F.; Lukeš, I. A Triazacyclononane-Based Bifunctional Phosphinate Ligand for the Preparation of Multimeric 68 Ga Tracers for Positron Emission Tomography. Chem. Eur. J. 2010, 16, 7174–7185. [Google Scholar] [CrossRef]
- Quigley, N.G.; Steiger, K.; Hoberück, S.; Czech, N.; Zierke, M.A.; Kossatz, S.; Pretze, M.; Richter, F.; Weichert, W.; Pox, C.; et al. PET/CT Imaging of Head-and-Neck and Pancreatic Cancer in Humans by Targeting the “Cancer Integrin” Avβ6 with Ga-68-Trivehexin. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1136–1147. [Google Scholar] [CrossRef]
- Quigley, N.G.; Czech, N.; Sendt, W.; Notni, J. PET/CT Imaging of Pancreatic Carcinoma Targeting the “Cancer Integrin” Avβ6. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4107–4108. [Google Scholar] [CrossRef] [PubMed]
- Pfister, J.; Summer, D.; Petrik, M.; Khoylou, M.; Lichius, A.; Kaeopookum, P.; Kochinke, L.; Orasch, T.; Haas, H.; Decristoforo, C. Hybrid Imaging of Aspergillus Fumigatus Pulmonary Infection with Fluorescent, 68Ga-Labelled Siderophores. Biomolecules 2020, 10, 168. [Google Scholar] [CrossRef] [PubMed]
- Zavvar, T.S.; Hörmann, A.A.; Klingler, M.; Summer, D.; Rangger, C.; Desrues, L.; Castel, H.; Gandolfo, P.; von Guggenberg, E. Effects of Side Chain and Peptide Bond Modifications on the Targeting Properties of Stabilized Minigastrin Analogs. Pharmaceuticals 2023, 16, 278. [Google Scholar] [CrossRef] [PubMed]
- Aloj, L.; Caracò, C.; Panico, M.; Zannetti, A.; Del Vecchio, S.; Tesauro, D.; De Luca, S.; Arra, C.; Pedone, C.; Morelli, G.; et al. In Vitro and in Vivo Evaluation of 111In-DTPAGlu-G-CCK8 for Cholecystokinin-B Receptor Imaging. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2004, 45, 485–494. [Google Scholar]
- Leach, B.I.; Lister, D.; Adams, S.R.; Bykowski, J.; Schwartz, A.B.; McConville, P.; Dimant, H.; Ahrens, E.T. Cryo-Fluorescence Tomography as a Tool for Visualizing Whole-Body Inflammation Using Perfluorocarbon Nanoemulsion Tracers. Mol. Imaging Biol. 2024, 26, 888–898. [Google Scholar] [CrossRef]




| [68Ga]Ga-CyTMG | [68Ga]Ga-CyFMG | ||
|---|---|---|---|
| Lipophilicity | LogDpH7.4 ± SD | −1.78 ± 0.16 | −1.53 ± 0.10 |
| 1 h | 56.68 ± 6.90 | 61.39 ± 1.34 | |
| Protein binding (%) | 2 h | 62.26 ± 2.74 | 65.64 ± 6.01 |
| 4 h | 64.09 ± 7.12 | 66.07 ± 1.26 | |
| 1 h | 97.98 ± 0.20 | 99.06 ± 1.07 | |
| Stability in PBS (% ± SD) | 2 h | 97.56 ± 0.09 | 98.57 ± 0.47 |
| 4 h | 96.70 ± 1.15 | 99.3 ± 0.71 | |
| 1 h | 98.97 ± 0.81 | 98.21 ± 1.09 | |
| Stability in human serum (% ± SD) | 2 h | 98.04 ± 0.23 | 96.80 ± 0.53 |
| 4 h | 97.51 ± 0.06 | 96.94 ± 0.94 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gariglio, G.; Bendova, K.; Hermann, M.; Olafsdottir, A.; Sosabowski, J.K.; Petrik, M.; von Guggenberg, E.; Decristoforo, C. Comparison of Two Chelator Scaffolds as Basis for Cholecystokinin-2 Receptor Targeting Bimodal Imaging Probes. Pharmaceuticals 2024, 17, 1569. https://doi.org/10.3390/ph17121569
Gariglio G, Bendova K, Hermann M, Olafsdottir A, Sosabowski JK, Petrik M, von Guggenberg E, Decristoforo C. Comparison of Two Chelator Scaffolds as Basis for Cholecystokinin-2 Receptor Targeting Bimodal Imaging Probes. Pharmaceuticals. 2024; 17(12):1569. https://doi.org/10.3390/ph17121569
Chicago/Turabian StyleGariglio, Giacomo, Katerina Bendova, Martin Hermann, Asta Olafsdottir, Jane K. Sosabowski, Milos Petrik, Elisabeth von Guggenberg, and Clemens Decristoforo. 2024. "Comparison of Two Chelator Scaffolds as Basis for Cholecystokinin-2 Receptor Targeting Bimodal Imaging Probes" Pharmaceuticals 17, no. 12: 1569. https://doi.org/10.3390/ph17121569
APA StyleGariglio, G., Bendova, K., Hermann, M., Olafsdottir, A., Sosabowski, J. K., Petrik, M., von Guggenberg, E., & Decristoforo, C. (2024). Comparison of Two Chelator Scaffolds as Basis for Cholecystokinin-2 Receptor Targeting Bimodal Imaging Probes. Pharmaceuticals, 17(12), 1569. https://doi.org/10.3390/ph17121569








