Hypoxia Imaging in Lung Cancer: A PET-Based Narrative Review for Clinicians and Researchers
Abstract
:1. Introduction
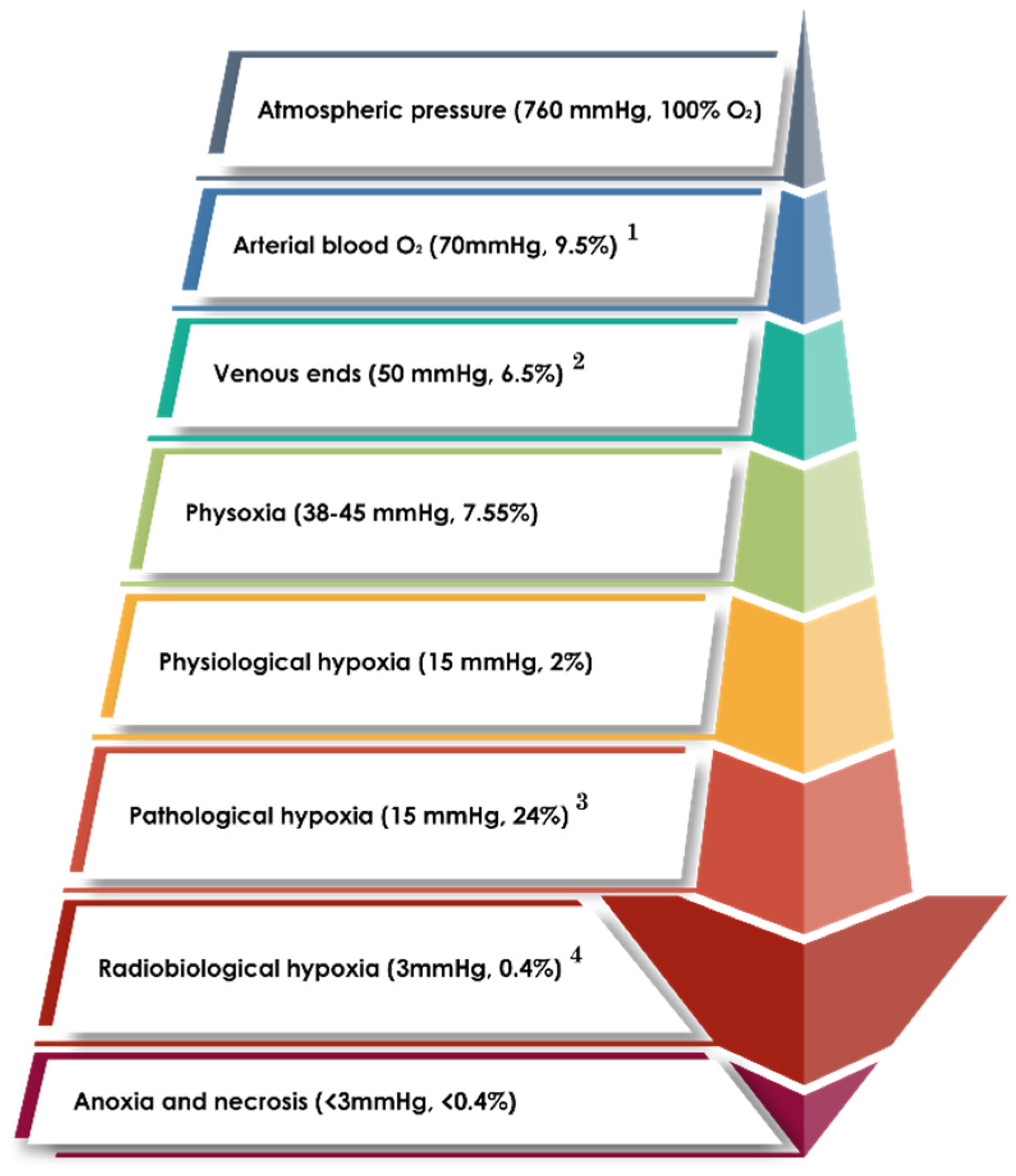
2. Pathophysiology of Hypoxia
3. Narrative Survey
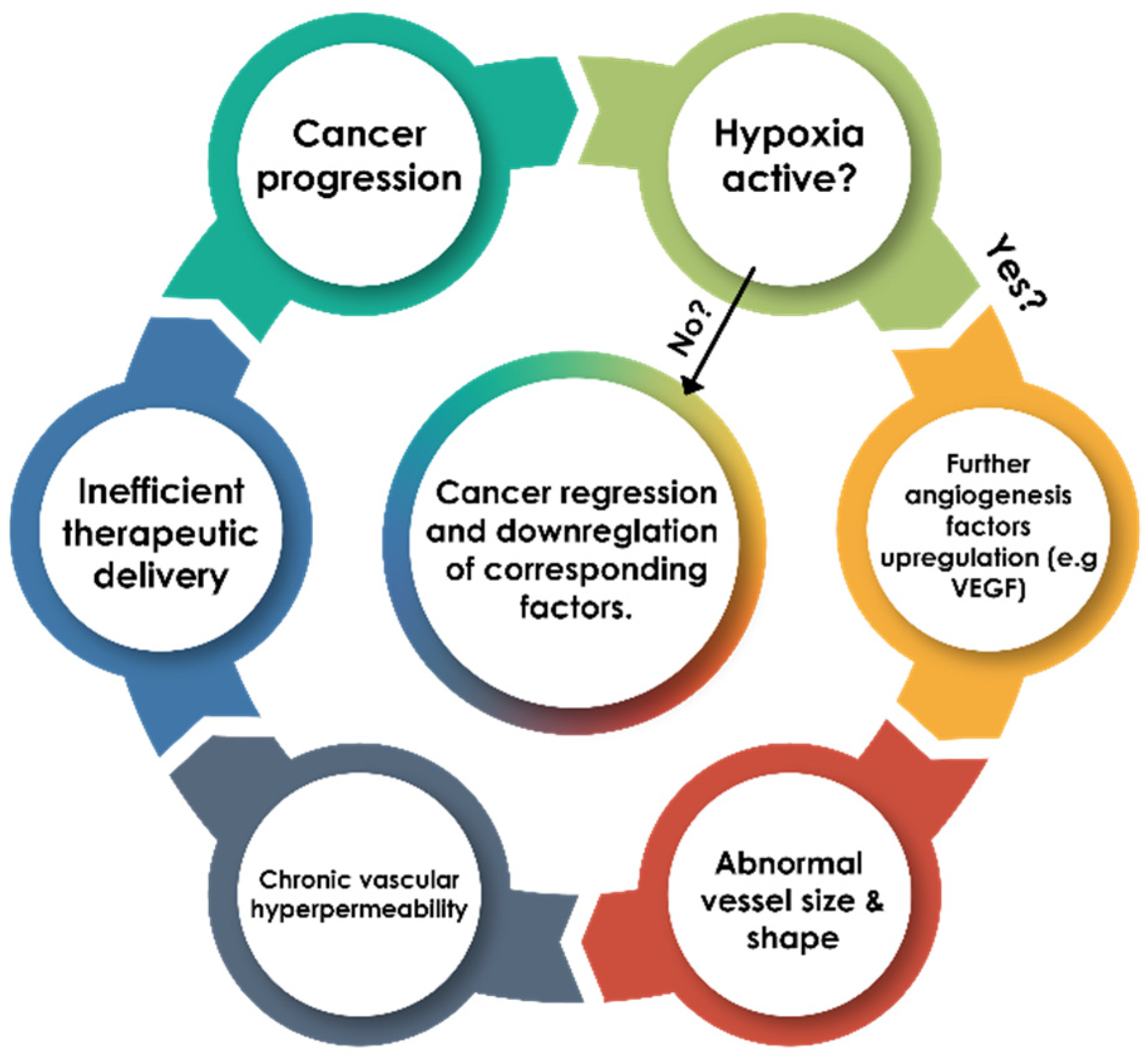
3.1. Hypoxia as a Limiting Factor for Cancer Therapeutics
3.2. Molecular Mechanisms of Cancer Resistance to Radiotherapy and Chemotherapy
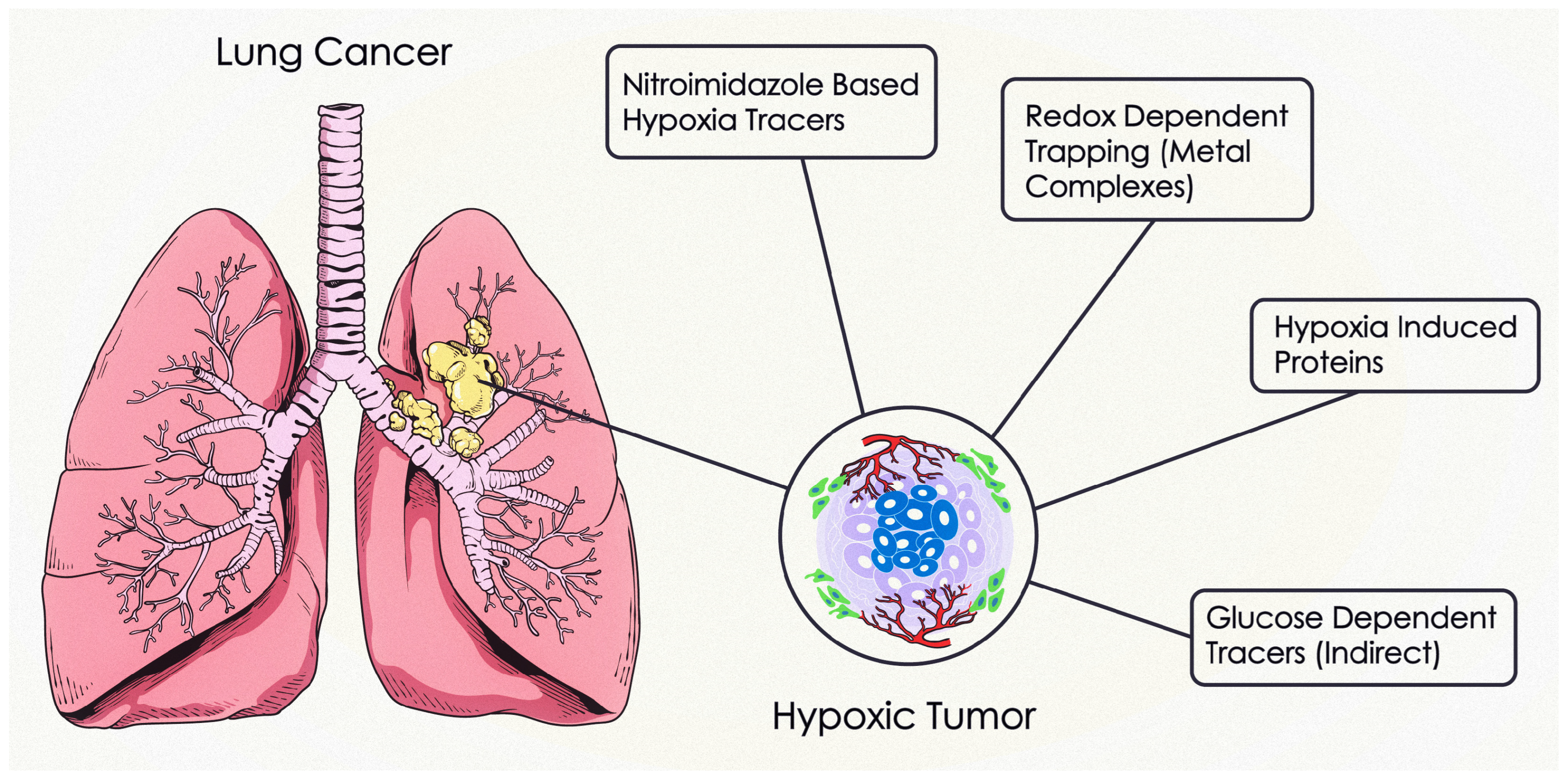
3.3. Hypoxia PET Imaging
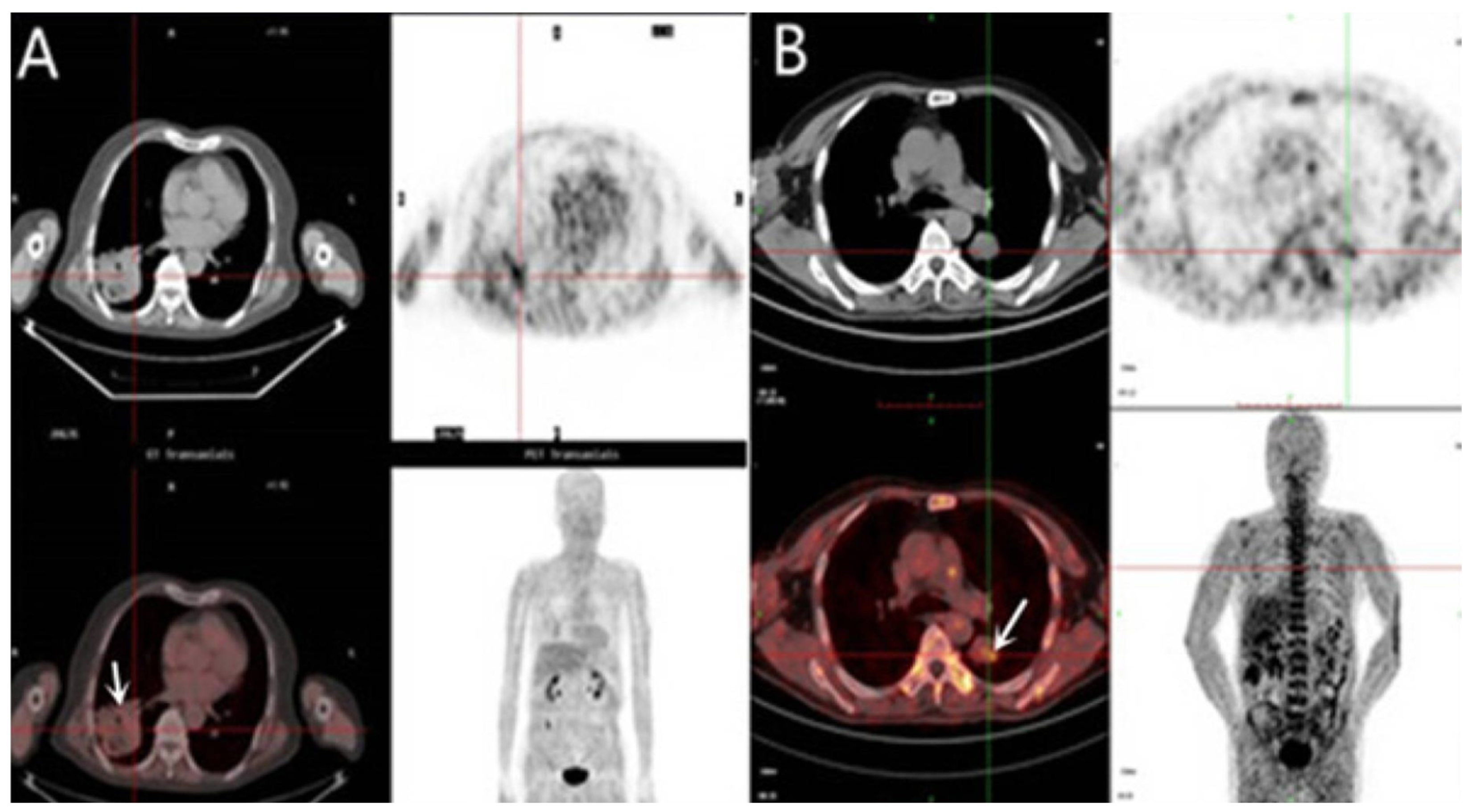
3.3.1. 18F-Fluromisonidazole (18F-FMISO)

3.3.2. 18F-FETNIM
3.3.3. 18F-FDG
3.3.4. 18F-FSPG
3.3.5. 18F-FLT
3.3.6. 18F-FAZA
3.3.7. 18F-EF3
3.3.8. 18F-EF5
3.3.9. 18F-HX4
3.3.10. Cu-ATSM

4. Discussion
4.1. Technical Consideration
4.2. Tumor Perfusion Versus Uptake
5. Limitations and Future Perspective
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LC | Lung cancer |
| NSCLC | Non-small cell lung cancer |
References
- National Cancer Institute. Lung and Bronchus Cancer; Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/lungb.html (accessed on 14 April 2024).
- Nicholson, A.G.; Tsao, M.S.; Beasley, M.B.; Borczuk, A.C.; Brambilla, E.; Cooper, W.A.; Dacic, S.; Jain, D.; Kerr, K.M.; Lantuejoul, S.; et al. The 2021 WHO classification of lung tumors: Impact of advances since 2015. J. Thorac. Oncol. 2022, 17, 362–387. [Google Scholar] [CrossRef] [PubMed]
- Ekinci, M.; Magne, T.M.; Alencar, L.M.R.; Fechine, P.B.A.; Santos-Oliveira, R.; Ilem-Özdemir, D. Molecular imaging for lung cancer: Exploring small molecules, peptides, and beyond in radiolabeled diagnostics. Pharmaceutics 2024, 16, 404. [Google Scholar] [CrossRef] [PubMed]
- Oprea-Lager, D.E.; MacLennan, S.; Bjartell, A.; Briganti, A.; Burger, I.A.; de Jong, I.; De Santis, M.; Eberlein, U.; Emmett, L.; Fizazi, K.; et al. European association of nuclear medicine focus 5: Consensus on molecular imaging and theranostics in prostate cancer. Eur. Urol. 2024, 85, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of cancer: New dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Voigt, W. Advanced PET imaging in oncology: Status and developments with current and future relevance to lung cancer care. Curr. Opin. Oncol. 2018, 30, 77–83. [Google Scholar] [CrossRef]
- Uusijärvi, H.; Bernhardt, P.; Ericsson, T.; Forssell-Aronsson, E. Dosimetric characterization of radionuclides for systemic tumor therapy: Influence of particle range, photon emission, and subcellular distribution. Med. Phys. 2006, 33, 3260–3269. [Google Scholar] [CrossRef]
- Kopecka, J.; Salaroglio, I.C.; Perez-Ruiz, E.; Sarmento-Ribeiro, A.B.; Saponara, S.; De Las Rivas, J.; Riganti, C. Hypoxia as a driver of resistance to immunotherapy. Drug Resist. Updat. 2021, 59, 100787. [Google Scholar] [CrossRef]
- Beckers, C.; Pruschy, M.; Vetrugno, I. Tumor hypoxia and radiotherapy: A major driver of resistance even for novel radiotherapy modalities. Semin. Cancer Biol. 2024, 98, 19–30. [Google Scholar] [CrossRef]
- Hopewell, J.W.; Trott, K.R. Volume effects in radiobiology as applied to radiotherapy. Radiother. Oncol. 2000, 56, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Grimes, D.R.; Warren, D.R.; Warren, S. Hypoxia imaging and radiotherapy: Bridging the resolution gap. Br. J. Radiol. 2017, 90, 20160939. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.; Dutreix, M. DNA repair inhibitors to enhance radiotherapy: Progresses and limitations. Cancer Radiother. 2019, 23, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Piert, M.; Machulla, H.-J.; Picchio, M.; Reischl, G.; Ziegler, S.; Kumar, P.; Wester, H.-J.; Beck, R.; McEwan, A.J.B.; I Wiebe, L.; et al. Hypoxia-Specific Tumor Imaging with 18F-Fluoroazomycin Arabinoside. J. Nucl. Med. 2005, 46, 106–113. [Google Scholar]
- Eschmann, S.M.; Paulsen, F.; Bedeshem, C.; Machulla, H.J.; Hehr, T.; Bamberg, M.; Bares, R. Hypoxia-imaging with 18F-misonidazole and PET: Changes of kinetics during radiotherapy of head-and-neck cancer. Radiother. Oncol. 2007, 83, 406–410. [Google Scholar] [CrossRef]
- Löck, S.; Linge, A.; Seidlitz, A.; Bandurska-Luque, A.; Nowak, A.; Gudziol, V.; Buchholz, F.; Aust, D.E.; Baretton, G.B.; Zöphel, K.; et al. Repeat FMISO-PET imaging weakly correlates with hypoxia-associated gene expressions for locally advanced HNSCC treated by primary radiochemotherapy. Radiother. Oncol. 2019, 135, 43–50. [Google Scholar] [CrossRef]
- Dierckx, R.A.; Van de Wiele, C. FDG uptake, a surrogate of tumour hypoxia? Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1544–1549. [Google Scholar] [CrossRef]
- Yip, C.; Blower, P.J.; Goh, V.; Landau, D.B.; Cook, G.J.R. Molecular imaging of hypoxia in non-small-cell lung cancer. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 956–976. [Google Scholar] [CrossRef]
- Kim, J.W.; Gao, P.; Dang, C.V. Effects of hypoxia on tumor metabolism. Cancer Metastasis Rev. 2007, 26, 291–298. [Google Scholar] [CrossRef]
- Takahashi, N.; Fujibayashi, Y.; Yonekura, Y.; Welch, M.J.; Waki, A.; Tsuchida, T.; Sadato, N.; Sugimoto, K.; Itoh, H. Evaluation of 62Cu labeled diacetyl-bis(N4-methylthiosemicarbazone) as a hypoxic tissue tracer in patients with lung cancer. Ann. Nucl. Med. 2000, 14, 323–328. [Google Scholar] [CrossRef]
- Kinoshita, T.; Fujii, H.; Hayashi, Y.; Kamiyama, I.; Ohtsuka, T.; Asamura, H. Prognostic significance of hypoxic PET using 18F-FAZA and 62Cu-ATSM in non-small-cell lung cancer. Lung Cancer 2016, 91, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Sun, J.; Zhu, W.; Yang, Z.; Wang, P.; Zeng, Y. Hypoxia studies in non-small cell lung cancer: Pathogenesis and clinical implications (Review). Oncol. Rep. 2025, 53, 29. [Google Scholar] [CrossRef] [PubMed]
- Challapalli, A.; Carroll, L.; Aboagye, E.O. Molecular mechanisms of hypoxia in cancer. Clin. Transl. Imaging 2017, 5, 225–253. [Google Scholar] [CrossRef] [PubMed]
- Cardenas-Navia, L.I.; Richardson, R.A.; Dewhirst, M.W. Targeting the molecular effects of a hypoxic tumor microenvironment. Front. Biosci. 2007, 12, 4061–4078. [Google Scholar] [CrossRef]
- Arneth, B. Tumor microenvironment. Medicina 2019, 56, 15. [Google Scholar] [CrossRef]
- Bernier, J.; Hall, E.J.; Giaccia, A. Radiation oncology: A century of achievements. Nat. Rev. Cancer 2004, 4, 737–747. [Google Scholar] [CrossRef]
- Read, J. The “oxygen effect” in radiation biology and radiotherapy. Australas. Radiol. 1959, 3, 115–119. [Google Scholar] [CrossRef]
- Bertout, J.A.; Patel, S.A.; Simon, M.C. The impact of O2 availability on human cancer. Nat. Rev. Cancer 2008, 8, 967–975. [Google Scholar] [CrossRef]
- Thomlinson, R.H.; Gray, L.H. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br. J. Cancer 1955, 9, 539–549. [Google Scholar] [CrossRef]
- Jing, X.; Yang, F.; Shao, C.; Wei, K.; Xie, M.; Shen, H.; Shu, Y. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol. Cancer 2019, 18, 157. [Google Scholar] [CrossRef]
- Begg, K.; Tavassoli, M. Inside the hypoxic tumour: Reprogramming of the DDR and radioresistance. Cell Death Discov. 2020, 6, 77. [Google Scholar] [CrossRef] [PubMed]
- Bristow, R.G.; Hill, R.P. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat. Rev. Cancer 2008, 8, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Siemann, D.W.; Horsman, M.R. Modulation of the tumor vasculature and oxygenation to improve therapy. Pharmacol. Ther. 2015, 153, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Höckel, M.; Vaupel, P. Tumor hypoxia: Definitions and current clinical, biologic, and molecular aspects. J. Natl. Cancer Inst. 2001, 93, 266–276. [Google Scholar] [CrossRef]
- Lasalle, C.; Onwuka, O.; Saddemi, J.; Aydogmus, F.S.A.; Ramsey, D.J. A comparison of symptoms at diagnosis in sequential neovascular age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2022, 63, 3759–F0180. [Google Scholar]
- Carlin, S.; Zhang, H.; Reese, M.; Ramos, N.N.; Chen, Q.; Ricketts, S.A. A comparison of the imaging characteristics and microregional distribution of 4 hypoxia PET tracers. J. Nucl. Med. 2014, 55, 515–521. [Google Scholar] [CrossRef]
- Lee, S.Y.; Ju, M.K.; Jeon, H.M.; Lee, Y.J.; Kim, C.H.; Park, H.G.; Han, S.I.; Kang, H.S. Oncogenic metabolism acts as a prerequisite step for induction of cancer metastasis and cancer stem cell phenotype. Oxid. Med. Cell. Longev. 2018, 2018, 1027453. [Google Scholar] [CrossRef]
- Monge-Cadet, J.; Moyal, E.; Supiot, S.; Guimas, V. DNA repair inhibitors and radiotherapy. Cancer Radiother. 2022, 26, 947–954. [Google Scholar] [CrossRef]
- Evans, C.E. Hypoxia-inducible factor signaling in inflammatory lung injury and repair. Cells 2022, 11, 183. [Google Scholar] [CrossRef]
- Wu, Q.; You, L.; Nepovimova, E.; Heger, Z.; Wu, W.; Kuca, K.; Adam, V. Hypoxia-inducible factors: Master regulators of hypoxic tumor immune escape. J. Hematol. Oncol. 2022, 15, 77. [Google Scholar] [CrossRef]
- Chapman, J.D. Measurement of tumor hypoxia by invasive and non-invasive procedures: A review of recent clinical studies. Radiother. Oncol. 1991, 20 (Suppl. S1), 13–19. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, B.S.; Horsman, M.R. Tumor hypoxia: Impact on radiation therapy and molecular pathways. Front. Oncol. 2020, 10, 562. [Google Scholar] [CrossRef]
- Sun, X.; Niu, G.; Chan, N.; Shen, B.; Chen, X. Tumor hypoxia imaging. Mol. Imaging Biol. 2011, 13, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chu, T. Recent advances on radionuclide labeled hypoxia-imaging agents. Curr. Pharm. Des. 2012, 18, 1084–1097. [Google Scholar] [CrossRef]
- Ueda, M.; Kudo, T.; Mutou, Y.; Umeda, I.O.; Miyano, A.; Ogawa, K.; Ono, M.; Fujii, H.; Kizaka-Kondoh, S.; Hiraoka, M.; et al. Evaluation of [125I]IPOS as a molecular imaging probe for hypoxia-inducible factor-1-active regions in a tumor: Comparison among single-photon emission computed tomography/X-ray computed tomography imaging, autoradiography, and immunohistochemistry. Cancer Sci. 2011, 102, 2090–2096. [Google Scholar] [CrossRef]
- Lin, X.; Tan, X.; Wu, F.; Yu, Q. A novel tumor hypoxia imaging agent: [99mTc]Tc(CO)3-CPA-2-NIM. Curr. Radiopharm. 2023, 16, 300–307. [Google Scholar] [CrossRef]
- Chitneni, S.K.; Palmer, G.M.; Zalutsky, M.R.; Dewhirst, M.W. Molecular imaging of hypoxia. J. Nucl. Med. 2011, 52, 165–168. [Google Scholar] [CrossRef]
- Dubois, L.; Landuyt, W.; Cloetens, L.; Bol, A.; Bormans, G.; Haustermans, K.; Labar, D.; Nuyts, J.; Grégoire, V.; Mortelmans, L. [18F]EF3 is not superior to [18F]FMISO for PET-based hypoxia evaluation as measured in a rat rhabdomyosarcoma tumour model. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 209–218. [Google Scholar] [CrossRef]
- Ollinger, J.M.; Fessler, J.A. Positron-emission tomography. IEEE Signal Process Mag. 1997, 14, 43–55. [Google Scholar] [CrossRef]
- Mokoala, K.M.G.; Sathekge, M.M. Non-FDG hypoxia tracers. Semin. Nucl. Med. 2024, 54, 827–844. [Google Scholar] [CrossRef]
- Bekaert, L.; Valable, S.; Lechapt-Zalcman, E.; Ponte, K.; Collet, S.; Constans, J.M.; Levallet, G.; Bordji, K.; Petit, E.; Branger, P.; et al. [18F]-FMISO PET study of hypoxia in gliomas before surgery: Correlation with molecular markers of hypoxia and angiogenesis. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Bruehlmeier, M.; Roelcke, U.; Schubiger, P.A.; Ametamey, S.M. Assessment of hypoxia and perfusion in human brain tumors using PET with 18F-Fluoromisonidazole and 15O-H2O. J. Nucl. Med. 2004, 45, 1851–1859. [Google Scholar] [PubMed]
- Hirata, K.; Yamaguchi, S.; Shiga, T.; Kuge, Y.; Tamaki, N. The roles of hypoxia imaging using 18F-fluoromisonidazole positron emission tomography in glioma treatment. J. Clin. Med. 2019, 8, 1088. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, S.N.; Wuest, M.; Jans, H.-S.; Woodfield, J.; Nario, A.P.; Krys, D.; Dufour, J.; Glubrecht, D.; Bergman, C.; Bernardes, E.S.; et al. Comparison of three 18F-labeled 2-nitroimidazoles for imaging hypoxia in breast cancer xenografts: [18F]FBNA, [18F]FAZA and [18F]FMISO. Nucl. Med. Biol. 2023, 124–125, 108383. [Google Scholar] [CrossRef]
- Cheng, J.; Lei, L.; Xu, J.; Sun, Y.; Zhang, Y.; Wang, X.; Pan, L.; Shao, Z.; Zhang, Y.; Liu, G. 18F-Fluoromisonidazole PET/CT: A Potential Tool for Predicting Primary Endocrine Therapy Resistance in Breast Cancer. J. Nucl. Med. 2013, 333, 54. [Google Scholar]
- Thorwarth, D.; Welz, S.; Mönnich, D.; Pfannenberg, C.; Nikolaou, K.; Reimold, M.; La Fougère, C.; Reischl, G.; Mauz, P.S.; Paulsen, F.; et al. Prospective evaluation of a tumor control probability model based on dynamic 18F-FMISO PET for head and neck cancer radiotherapy. J. Nucl. Med. 2019, 60, 1698–1704. [Google Scholar] [CrossRef]
- Watanabe, S.; Inoue, T.; Okamoto, S.; Magota, K.; Takayanagi, A.; Sakakibara-Konishi, J.; Katoh, N.; Hirata, K.; Manabe, O.; Toyonaga, T.; et al. Combination of FDG-PET and FMISO-PET as a treatment strategy for patients undergoing early-stage NSCLC stereotactic radiotherapy. EJNMMI Res. 2019, 9, 104. [Google Scholar] [CrossRef]
- Troost, E.G.C.; Laverman, P.; Philippens, M.E.P.; Lok, J.; van der Kogel, A.J.; Oyen, W.J.G.; Boerman, O.C.; Kaanders, J.H.A.M.; Bussink, J. Correlation of [18F]FMISO autoradiography and pimonodazole immunohistochemistry in human head and neck carcinoma xenografts. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1803–1811. [Google Scholar] [CrossRef]
- Martin, G.V.; Caldwell, J.H.; Graham, M.M.; Grierson, J.R.; Kroll, K.; Cowan, M.J.; Lewellen, T.K.; Rasey, J.S.; Casciari, J.J.; Krohn, K.A. Noninvasive detection of hypoxic myocardium using fluorine-18-fluoromisonidazole and positron emission tomography. J. Nucl. Med. 1992, 33, 2202–2208. [Google Scholar]
- Bittner, M.I.; Wiedenmann, N.; Bucher, S.; Hentschel, M.; Mix, M.; Rücker, G.; Weber, W.A.; Meyer, P.T.; Werner, M.; Grosu, A.L.; et al. Analysis of relation between hypoxia PET imaging and tissue-based biomarkers during head and neck radiochemotherapy. Acta Oncol. 2016, 55, 1299–1304. [Google Scholar] [CrossRef]
- Norikane, T.; Yamamoto, Y.; Maeda, Y.; Kudomi, N.; Matsunaga, T.; Haba, R.; Iwasaki, A.; Hoshikawa, H.; Nishiyama, Y. Correlation of 18F-fluoromisonidazole PET findings with HIF-1α and p53 expressions in head and neck cancer: Comparison with. Nucl. Med. Commun. 2014, 18, 30–35. [Google Scholar] [CrossRef]
- Tachibana, I.; Nishimura, Y.; Hanaoka, K.; Inada, M.; Fukuda, K.; Tatebe, H.; Ishikawa, K.; Nakamatsu, K.; Kanamori, S.; Hosono, M. Tumor hypoxia detected by 18F-fluoromisonidazole Positron Emission Tomography (FMISO PET) as a prognostic indicator of radiotherapy (RT). Anticancer Res. 2018, 38, 1775–1781. [Google Scholar] [CrossRef] [PubMed]
- Zschaeck, S.; Haase, R.; Abolmaali, N.; Perrin, R.; Stützer, K.; Appold, S.; Steinbach, J.; Kotzerke, J.; Zips, D.; Richter, C.; et al. Spatial distribution of FMISO in head and neck squamous cell carcinomas during radio-chemotherapy and its correlation to pattern of failure. Acta Oncol. 2015, 54, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Ma, N.; Ren, C.; Song, S.; Wu, K.; Sun, Y.; Mao, J.; Cheng, J. Hypoxia predicts favorable response to carbon ion radiotherapy in non-small cell lung cancer (NSCLC) defined by 18F-FMISO positron emission tomography/computed tomography (PET/CT) imaging. Quant. Imaging Med. Surg. 2024, 14, 3489–3500. [Google Scholar] [CrossRef]
- Gagel, B.; Reinartz, P.; Demirel, C.; Kaiser, H.J.; Zimny, M.; Piroth, M.; Pinkawa, M.; Stanzel, S.; Asadpour, B.; Hamacher, K.; et al. [18F]fluoromisonidazole and [18F]fluorodeoxyglucose positron emission tomography in response evaluation after chemo-/radiotherapy of non-small-cell lung cancer: A feasibility study. BMC Cancer 2006, 6, 51. [Google Scholar] [CrossRef]
- Couturier, O.; Luxen, A.; Chatal, J.-F.; Vuillez, J.-P.; Rigo, P.; Hustinx, R. Fluorinated tracers for imaging cancer with positron emission tomography. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 1182–1206. [Google Scholar] [CrossRef]
- Yuan, H.; Palmer, G.M.; Dewhirst, M.W. Imaging hypoxia. In Molecular Imaging: Principles and Practice; Elsevier: Amsterdam, The Netherlands, 2021; pp. 869–895. [Google Scholar] [CrossRef]
- Thureau, S.; Piton, N.; Gouel, P.; Modzelewski, R.; Dujon, A.; Baste, J.M.; Melki, J.; Rinieri, P.; Peillon, C.; Rastelli, O.; et al. First comparison between [18F]-FMISO and [18F]-Faza for preoperative pet imaging of hypoxia in lung cancer. Cancers 2021, 13, 4101. [Google Scholar] [CrossRef]
- Yang, D.J.; Wallace, S.; Cherif, A.; Li, C.; Gretzer, M.B.; Kim, E.E.; Podoloff, D.A. Development of F-18-labeled fluoroerythronitroimidazole as a PET agent for imaging tumor hypoxia. Radiology 1995, 194, 795–800. [Google Scholar] [CrossRef]
- Grönroos, T.; Bentzen, L.; Marjamäki, P.; Murata, R.; Horsman, M.R.; Keiding, S.; Eskola, O.; Haaparanta, M.; Minn, H.; Solin, O. Comparison of the biodistribution of two hypoxia markers [18F]FETNIM and [18F]FMISO in an experimental mammary carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 513–520. [Google Scholar] [CrossRef]
- Hu, M.; Kong, L.; Zhao, S.Q.; Yang, G.R.; Yang, W.F.; Han, A.Q.; Fu, Z.; Ma, L.; Zheng, J.S.; Yu, J.M. Value of 18F-FETNIM PET-CT for detection of tumor hypoxia in non-small-cell lung cancer. Zhonghua Zhong Liu Za Zhi 2010, 32, 463–466. [Google Scholar]
- Wei, Y.; Zhao, W.; Huang, Y.; Yu, Q.; Zhu, S.; Wang, S.; Zhao, S.; Hu, X.; Yu, J.; Yuan, S. A comparative study of noninvasive hypoxia imaging with 18F-Fluoroerythronitroimidazole and 18F-Fluoromisonidazole PET/CT in patients with lung cancer. PLoS ONE 2016, 11, e0157606. [Google Scholar] [CrossRef]
- Wei, S.; Lin, H.; Shi, C.; Xiong, W.; Chen, C.C.; Huang, S.; Press, R.H.; Hasan, S.; Chhabra, A.M.; Choi, J.I.; et al. Use of single-energy proton pencil beam scanning Bragg peak for intensity-modulated proton therapy FLASH treatment planning in liver-hypofractionated radiation therapy. Med. Phys. 2022, 49, 6560–6574. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, V.; McCook, B.M.; Torok, F.S. An introduction to PET-CT imaging. RadioGraphics 2004, 24, 523–543. [Google Scholar] [CrossRef] [PubMed]
- Telo, S.; Calderoni, L.; Vichi, S.; Zagni, F.; Castellucci, P.; Fanti, S. Alternative and new radiopharmaceutical agents for lung cancer. Curr. Radiopharm. 2020, 13, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Verhagen, A.F.T.; Bootsma, G.P.; Tjan-Heijnen, V.C.G.; van der Wilt, G.J.; Cox, A.L.; Brouwer, M.H.; Corstens, F.H.; Oyen, W.J. FDG-PET in staging lung cancer: How does it change the algorithm? Lung Cancer 2004, 44, 175–181. [Google Scholar] [CrossRef]
- Cuaron, J.; Dunphy, M.; Rimner, A. Role of FDG-PET scans in staging, response assessment, and follow-up care for non-small cell lung cancer. Front. Oncol. 2012, 2, 208. [Google Scholar] [CrossRef]
- Park, J.; Kang, S.K.; Hwang, D.; Choi, H.; Ha, S.; Seo, J.M.; Eo, J.S.; Lee, J.S. Automatic lung cancer segmentation in [18F]FDG PET/CT using a two-stage deep learning approach. Nucl. Med. Mol. Imaging 2023, 57, 86–93. [Google Scholar] [CrossRef]
- Clavo, A.C.; Brown, R.S.; Wahl, R.L. Fluorodeoxyglucose uptake in human cancer cell lines is increased by hypoxia. J. Nucl. Med. 1995, 36, 1625–1632. [Google Scholar]
- Zhao, S.; Kuge, Y.; Mochizuki, T.; Takahashi, T.; Nakada, K.; Sato, M.; Takei, T.; Tamaki, N. Biologic correlates of intratumoral heterogeneity in 18F-FDG distribution with regional expression of glucose transporters and hexokinase-II in experimental tumor. J. Nucl. Med. 2005, 46, 675–682. [Google Scholar]
- Van Laere, K.; Koole, M.; Lemahieu, I.; Dierckx, R. Image filtering in single-photon emission computed tomography: Principles and applications. Comput. Med. Imaging Graph. 2001, 25, 127–133. [Google Scholar] [CrossRef]
- Lopci, E.; Grassi, I.; Chiti, A.; Nanni, C.; Cicoria, G.; Toschi, L.; Fonti, C.; Lodi, F.; Mattioli, S.; Fanti, S. Review Article PET Radiopharmaceuticals for Imaging of Tumor Hypoxia: A Review of the Evidence. Am. J. Nucl. Med. Mol. Imaging. 2014, 4, 365–384. [Google Scholar] [PubMed] [PubMed Central]
- Zimny, M.; Gagel, B.; DiMartino, E.; Hamacher, K.; Coenen, H.H.; Westhofen, M.; Eble, M.; Buell, U.; Reinartz, P. FDG—A marker of tumour hypoxia? A comparison with [18F]fluoromisonidazole and pO2-polarography in metastatic head and neck cancer. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 1426–1431. [Google Scholar] [CrossRef]
- Bury, T.; Corhay, J.L.; Duysinx, B.; Daenen, F.; Ghaye, B.; Barthelemy, N.; Rigo, P.; Bartsch, P. Value of FDG-PET in detecting residual or recurrent nonsmall cell lung cancer. Eur. Respir. J. 1999, 14, 1376–1380. [Google Scholar] [CrossRef]
- Machtay, M.; Duan, F.; Siegel, B.A.; Snyder, B.S.; Gorelick, J.J.; Reddin, J.S.; Munden, R.; Johnson, D.W.; Wilf, L.H.; DeNittis, A.; et al. Prediction of survival by [18F]fluorodeoxyglucose positron emission tomography in patients with locally advanced non-small-cell lung cancer undergoing definitive chemoradiation therapy: Results of the ACRIN 6668/RTOG 0235 trial. J. Clin. Oncol. 2013, 31, 3823–3830. [Google Scholar] [CrossRef]
- Vera, P.; Mezzani-Saillard, S.; Edet-Sanson, A.; Ménard, J.F.; Modzelewski, R.; Thureau, S.; Meyer, M.E.; Jalali, K.; Bardet, S.; Lerouge, D.; et al. FDG PET during radiochemotherapy is predictive of outcome at 1 year in non-small-cell lung cancer patients: A prospective multicentre study (RTEP2). Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1057–1065. [Google Scholar] [CrossRef]
- Derlin, T.; Grünwald, V.; Steinbach, J.; Wester, H.J.; Ross, T.L. Molecular imaging in oncology using positron emission tomography. Dtsch. Ärztebl. Int. 2018, 115, 175–181. [Google Scholar] [CrossRef]
- Rivera, M.P.; Mehta, A.C.; Wahidi, M.M. Establishing the diagnosis of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143, e142S–e165S. [Google Scholar] [CrossRef]
- Cegla, P.; Bos-Liedke, A.; Burchardt, E.; Konstanty, E.; Piotrowski, A.; Kozak, M.; Cholewinski, W. Diagnosis and treatment of lung cancer using nuclear medicine techniques—Current state of the art. Nucl. Med. Rev. Cent. East. Eur. 2023, 26, 77–84. [Google Scholar] [CrossRef]
- Paez, R.; Shah, C.; Cords, A.J.; Muterspaugh, A.; Helton, J.E.; Antic, S.; Eisenberg, R.; Chen, H.; Grogan, E.L.; Manning, H.C.; et al. 18F-FSPG PET imaging for the evaluation of indeterminate pulmonary nodules. PLoS ONE 2022, 17, e0265427. [Google Scholar] [CrossRef]
- Lo, M.; Wang, Y.-Z.; Gout, P.W. The x cystine/glutamate antiporter: A potential target for therapy of cancer and other diseases. J. Cell Physiol. 2008, 215, 593–602. [Google Scholar] [CrossRef]
- Park, S.Y.; Mosci, C.; Kumar, M.; Wardak, M.; Koglin, N.; Bullich, S.; Mueller, A.; Berndt, M.; Stephens, A.W.; Chin, F.T.; et al. Initial evaluation of (4S)-4-(3-[18F]fluoropropyl)-l-glutamate (FSPG) PET/CT imaging in patients with head and neck cancer, colorectal cancer, or non-Hodgkin lymphoma. EJNMMI Res. 2020, 10, 100. [Google Scholar] [CrossRef]
- Baek, S.; Choi, C.-M.; Ahn, S.H.; Lee, J.W.; Gong, G.; Ryu, J.-S.; Oh, S.J.; Bacher-Stier, C.; Fels, L.; Koglin, N.; et al. Exploratory clinical trial of (4S)-4-(3-[18F]fluoropropyl)-l-glutamate for imaging xC− transporter using positron emission tomography in patients with non–small cell lung or breast cancer. Clin. Cancer Res. 2012, 18, 5427–5437. [Google Scholar] [CrossRef]
- Park, S.Y.; Na, S.J.; Kumar, M.; Mosci, C.; Wardak, M.; Koglin, N.; Bullich, S.; Mueller, A.; Berndt, M.; Stephens, A.W.; et al. Clinical evaluation of (4S)-4-(3-[18F]fluoropropyl)-L-glutamate (18F-FSPG) for PET/CT imaging in patients with newly diagnosed and recurrent prostate cancer. Clin. Cancer Res. 2020, 26, 5380–5387. [Google Scholar] [CrossRef]
- Mittra, E.S.; Koglin, N.; Mosci, C.; Kumar, M.; Hoehne, A.; Keu, K.V.; Iagaru, A.H.; Mueller, A.; Berndt, M.; Bullich, S.; et al. Pilot preclinical and clinical evaluation of (4S)-4-(3-[18F]fluoropropyl)-L-glutamate (18F-FSPG) for PET/CT imaging of intracranial malignancies. PLoS ONE 2016, 11, e0148628. [Google Scholar] [CrossRef]
- Kavanaugh, G.; Williams, J.; Morris, A.S.; Nickels, M.L.; Walker, R.; Koglin, N.; Stephens, A.W.; Washington, M.K.; Geevarghese, S.K.; Liu, Q.; et al. Utility of [18F]FSPG PET to image hepatocellular carcinoma: First clinical evaluation in a US population. Mol. Imaging Biol. 2016, 18, 924–934. [Google Scholar] [CrossRef]
- Sharkey, A.R.; Koglin, N.; Mittra, E.S.; Han, S.; Cook, G.J.R.; Witney, T.H. Clinical [18F]FSPG positron emission tomography Imaging Reveals Heterogeneity in Tumor-Associated System xc− activity. Cancers 2024, 16, 1437. [Google Scholar] [CrossRef]
- Chae, S.Y.; Choi, C.-M.; Shim, T.S.; Park, Y.; Park, C.-S.; Lee, H.S.; Lee, S.J.; Oh, S.J.; Kim, S.-Y.; Baek, S.; et al. Exploratory Clinical Investigation of (4S)-4-(3-18F-Fluoropropyl)-L-Glutamate PET of Inflammatory and Infectious Lesions. J. Nucl. Med. 2016, 57, 67–69. [Google Scholar] [CrossRef]
- Krarup, M.M.K.; Fischer, B.M.; Christensen, T.N. New PET tracers: Current knowledge and perspectives in lung cancer. Semin. Nucl. Med. 2022, 52, 781–796. [Google Scholar] [CrossRef]
- Kairemo, K.; Santos, E.B.; Macapinlac, H.A.; Subbiah, V. Early Response Assessment to Targeted Therapy Using 3′-deoxy-3′[18F]-Fluorothymidine (18F-FLT) PET/CT in Lung Cancer. Diagnostics 2020, 10, 26. [Google Scholar] [CrossRef]
- Rasey, J.S.; Grierson, J.R.; Wiens, L.W.; Kolb, P.D.; Schwartz, J.L. Validation of FLT uptake as a measure of thymidine Kinase-1 activity in A549 carcinoma cells. J. Nucl. Med. 2002, 43, 1210–1217. [Google Scholar]
- Vera, P.; Bohn, P.; Edet-Sanson, A.; Salles, A.; Hapdey, S.; Gardin, I.; Ménard, J.F.; Modzelewski, R.; Thiberville, L.; Dubray, B. Siultaneous positron emission tomography (PET) assessment of metabolism with 18F-fluoro-2-deoxy-d-glucose (FDG), proliferation with 18F-fluoro-thymidine (FLT), and hypoxia with ¹⁸fluoro-misonidazole (F-miso) before and during radiotherapy in patients with non-small-cell lung cancer (NSCLC): A pilot study. Radiother. Oncol. 2011, 98, 109–116. [Google Scholar] [CrossRef]
- Alwadani, B.; Dall’Angelo, S.; Fleming, I.N. Clinical value of 3′-deoxy-3′-[18F]fluorothymidine-positron emission tomography for diagnosis, staging and assessing therapy response in lung cancer. Insights Imaging 2021, 12, 90. [Google Scholar] [CrossRef]
- Buck, A.K.; Schirrmeister, H.; Hetzel, M.; von der Heide, M.; Halter, G.; Glatting, G.; Mattfeldt, T.; Liewald, F.; Reske, S.N.; Neumaier, B. 3-Deoxy-3-[18F]Fluorothymidine-Positron Emission Tomography for Noninvasive Assessment of Proliferation in Pulmonary Nodules. Cancer Res. 2002, 62, 3331–3334. Available online: https://aacrjournals.org/cancerres/article-pdf/62/12/3331/2495004/ch1202003331.pdf (accessed on 19 January 2025).
- Trigonis, I.; Koh, P.K.; Taylor, B.; Tamal, M.; Ryder, D.; Earl, M.; Anton-Rodriguez, J.; Haslett, K.; Young, H.; Faivre-Finn, C.; et al. Early reduction in tumour [18F]fluorothymidine (FLT) uptake in patients with non-small cell lung cancer (NSCLC) treated with radiotherapy alone. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 682–693. [Google Scholar] [CrossRef]
- Allen, M.D.; Luong, P.; Hudson, C.; Leyton, J.; Delage, B.; Ghazaly, E.; Cutts, R.; Yuan, M.; Syed, N.; Lo Nigro, C.; et al. Prognostic and therapeutic impact of argininosuccinate synthetase 1 control in bladder cancer as monitored longitudinally by PET imaging. Cancer Res. 2014, 74, 896–907. [Google Scholar] [CrossRef]
- Huang, Y.; Fan, J.; Li, Y.; Fu, S.; Chen, Y.; Wu, J. Imaging of tumor hypoxia with radionuclide-labeled tracers for PET. Front. Oncol. 2021, 11, 731503. [Google Scholar] [CrossRef]
- Sorger, D.; Patt, M.; Kumar, P.; Wiebe, L.I.; Barthel, H.; Seese, A.; Dannenberg, C.; Tannapfel, A.; Kluge, R.; Sabri, O. [18F]Fluoroazomycinarabinofuranoside (18FAZA) and [18F]Fluoromisonidazole (18FMISO): A comparative study of their selective uptake in hypoxic cells and PET imaging in experimental rat tumors. Nucl. Med. Biol. 2003, 30, 317–326. [Google Scholar] [CrossRef]
- Postema, E.J.; McEwan, A.J.B.; Riauka, T.A.; Kumar, P.; Richmond, D.A.; Abrams, D.N.; Wiebe, L.I. Initial results of hypoxia imaging using 1-α-d-(5-deoxy-5-[18F]-fluoroarabinofuranosyl)-2-nitroimidazole (18F-FAZA). Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1565–1573. [Google Scholar] [CrossRef]
- Trinkaus, M.E.; Blum, R.; Rischin, D.; Callahan, J.; Bressel, M.; Segard, T.; Roselt, P.; Eu, P.; Binns, D.; MacManus, M.P.; et al. Imaging of hypoxia with 18F-FAZA PET in patients with locally advanced non-small cell lung cancer treated with definitive chemoradiotherapy. J. Med. Imaging Radiat. Oncol. 2013, 57, 475–481. [Google Scholar] [CrossRef]
- Graves, E.E.; Hicks, R.J.; Binns, D.; Bressel, M.; Le, Q.T.; Peters, L.; Young, R.J.; Rischin, D. Quantitative and qualitative analysis of [18F]FDG and [18F]FAZA positron emission tomography of head and neck cancers and associations with HPV status and treatment outcome. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 617–625. [Google Scholar] [CrossRef]
- Mortensen, L.S.; Johansen, J.; Kallehauge, J.; Primdahl, H.; Busk, M.; Lassen, P.; Alsner, J.; Sørensen, B.S.; Toustrup, K.; Jakobsen, S.; et al. FAZA PET/CT hypoxia imaging in patients with squamous cell carcinoma of the head and neck treated with radiotherapy: Results from the DAHANCA 24 trial. Radiother. Oncol. 2012, 105, 14–20. [Google Scholar] [CrossRef]
- Saga, T.; Inubushi, M.; Koizumi, M.; Yoshikawa, K.; Zhang, M.R.; Tanimoto, K.; Horiike, A.; Yanagitani, N.; Ohyanagi, F.; Nishio, M. Prognostic value of 18F-fluoroazomycin arabinoside PET/CT in patients with advanced non-small-cell lung cancer. Cancer Sci. 2015, 106, 1554–1560. [Google Scholar] [CrossRef]
- Saksø, M.; Mortensen, L.S.; Primdahl, H.; Johansen, J.; Kallehauge, J.; Hansen, C.R.; Overgaard, J. Influence of FAZA PET hypoxia and HPV-status for the outcome of head and neck squamous cell carcinoma (HNSCC) treated with radiotherapy: Long-term results from the DAHANCA 24 trial (NCT01017224). Radiother. Oncol. 2020, 151, 126–133. [Google Scholar] [CrossRef]
- Busk, M.; Mortensen, L.S.; Nordsmark, M.; Overgaard, J.; Jakobsen, S.; Hansen, K.V.; Theil, J.; Kallehauge, J.F.; D’Andrea, F.P.; Steiniche, T.; et al. PET hypoxia imaging with FAZA: Reproducibility at baseline and during fractionated radiotherapy in tumour-bearing mice. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 186–197. [Google Scholar] [CrossRef]
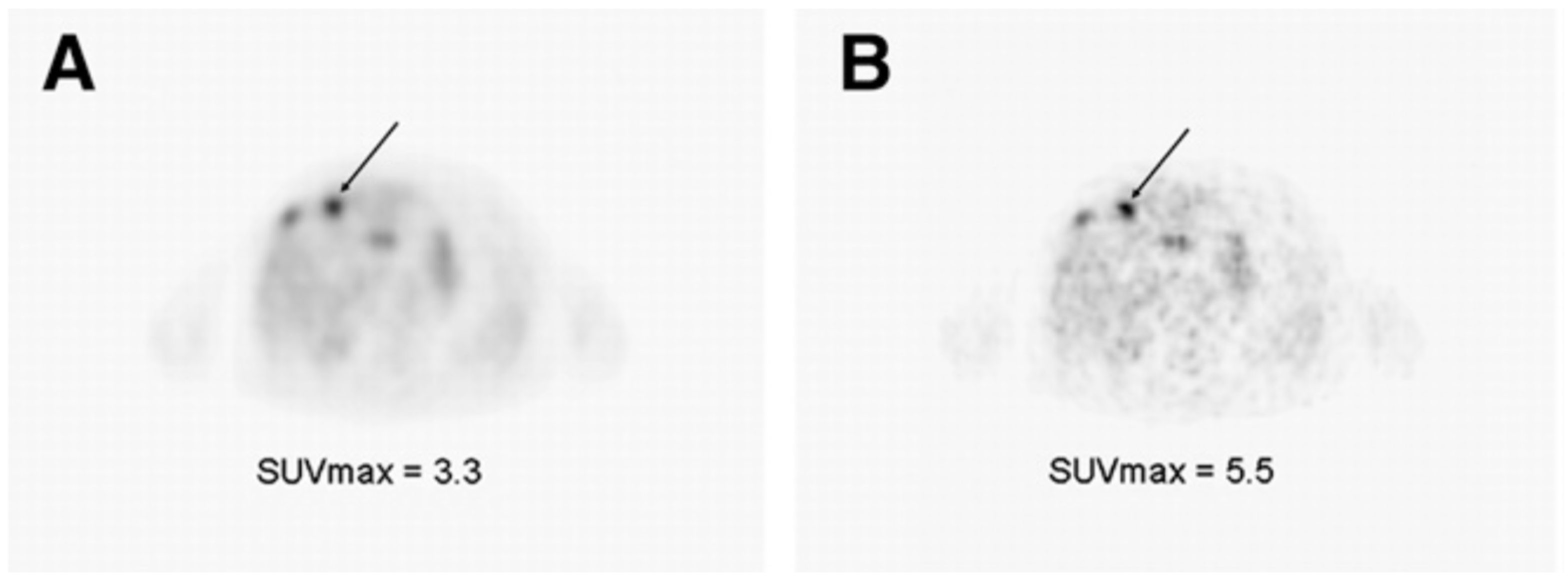
- Bollineni, V.R.; Kerner, G.S.M.A.; Pruim, J.; Steenbakkers, R.J.H.M.; Wiegman, E.M.; Koole, M.J.B.; de Groot, E.H.; Willemsen, A.T.M.; Luurtsema, G.; Widder, J.; et al. PET imaging of tumor hypoxia using 18F-fluoroazomycin arabinoside in stage III-IV non-small cell lung cancer patients. J. Nucl. Med. 2013, 54, 1175–1180. [Google Scholar] [CrossRef]
- Beck, R.; Röper, B.; Carlsen, J.M.; Huisman, M.C.; Lebschi, J.A.; Andratschke, N.; Picchio, M.; Souvatzoglou, M.; Machulla, H.J.; Piert, M. Pretreatment 18F-FAZA PET predicts success of hypoxia-directed radiochemotherapy using tirapazamine. J. Nucl. Med. 2007, 48, 973–980. [Google Scholar] [CrossRef]
- Varia, M.A.; Lee, D.Y.W.; Raleigh, J.A. Oral pimonidazole HCl as tumor hypoxia marker: Report of first clinical use—Pharmacokinetics and tolerance. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, S539–S540. [Google Scholar] [CrossRef]
- Rios-Colon, L.; Kim, S.; Su, Y.; Kumar, D.; Deep, G. Immunofluorescence-based method to assess cancer biomarker in the hypoxic region of the tumor. In Methods in Molecular Biology; Humana Press Incorporated: New York, NY, USA, 2022; Volume 2413, pp. 37–43. [Google Scholar] [CrossRef]
- Labiano, S.; Serrano-Mendioroz, I.; Rodriguez-Ruiz, M.E. Assessment of hypoxia by pimonidazole staining following radiotherapy. Methods Cell Biol. 2022, 172, 179–189. [Google Scholar] [CrossRef]
- Bussink, J.; Kaanders, J.H.A.M.; Strik, A.M.; Vojnovic, B.; van Der Kogel, A.J. Optical sensor-based oxygen tension measurements correspond with hypoxia marker binding in three human tumor xenograft lines. Radiat. Res. 2000, 154, 547–555. [Google Scholar] [CrossRef]
- Verwer, E.E.; Boellaard, R.; Van Der Veldt, A.A.M. Positron emission tomography to assess hypoxia and perfusion in lung cancer. World J. Clin. Oncol. 2014, 5, 824–844. [Google Scholar] [CrossRef]
- Halmos, G.B.; Bruine de Bruin, L.; Langendijk, J.A.; van der Laan, B.F.A.M.; Pruim, J.; Steenbakkers, R.J.H.M. Head and neck tumor hypoxia imaging by 18F-Fluoroazomycin-arabinoside (18F-FAZA)-PET: A review. Clin. Nucl. Med. 2014, 39, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Mahy, P.; Geets, X.; Lonneux, M.; Levêque, P.; Christian, N.; De Bast, M.; Gillart, J.; Labar, D.; Lee, J.; Grégoire, V. Determination of tumour hypoxia with [18F]EF3 in patients with head and neck tumours: A phase I study to assess the tracer pharmacokinetics, biodistribution and metabolism. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1282–1289. [Google Scholar] [CrossRef] [PubMed]
- Komar, G.; Seppänen, M.; Eskola, O.; Lindholm, P.; Grönroos, T.J.; Forsback, S.; Sipilä, H.; Evans, S.M.; Solin, O.; Minn, H. 18F-EF5: A new PET tracer for imaging hypoxia in head and neck cancer. J. Nucl. Med. 1944, 49, 1944–1951. [Google Scholar]
- Koch, C.J.; Scheuermann, J.S.; Divgi, C.; Judy, K.D.; Kachur, A.V.; Freifelder, R.; Reddin, J.S.; Karp, J.; Stubbs, J.B.; Hahn, S.M.; et al. Biodistribution and dosimetry of 18F-EF5 in cancer patients with preliminary comparison of 18F-EF5 uptake versus EF5 binding in human glioblastoma. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 2048–2059. [Google Scholar] [CrossRef]
- Qian, Y.; Von Eyben, R.; Liu, Y.; Chin, F.T.; Miao, Z.; Apte, S.; Carter, J.N.; Binkley, M.S.; Pollom, E.L.; Harris, J.P.; et al. 18F-EF5 PET-based imageable hypoxia predicts local recurrence in tumors treated with highly conformal radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 1183–1192. [Google Scholar] [CrossRef]
- Lin, L.L.; Silvoniemi, A.; Stubbs, J.B.; Rengan, R.; Suilamo, S.; Solin, O.; Divgi, C.; Eskola, O.; Sorger, J.M.; Stabin, M.G.; et al. Radiation dosimetry and biodistribution of the hypoxia tracer 18F-EF5 in oncologic patients. Cancer Biother. Radiopharm. 2012, 27, 412–419. [Google Scholar] [CrossRef]
- Novruzov, E.; Mori, Y.; Antke, C.; Dabir, M.; Schmitt, D.; Kratochwil, C.; Koerber, S.A.; Haberkorn, U.; Giesel, F.L. A role of non-FDG tracers in lung cancer? Semin. Nucl. Med. 2022, 52, 720–733. [Google Scholar] [CrossRef]
- Dubois, L.J.; Lieuwes, N.G.; Janssen, M.H.M.; Peeters, W.J.M.; Windhorst, A.D.; Walsh, J.C.; Kolb, H.C.; Ollers, M.C.; Bussink, J.; van Dongen, G.A.M.S.; et al. Preclinical evaluation and validation of [18F]HX4,a promising hypoxia marker for imaging. Proc. Natl. Acad. Sci. USA 2011, 108, 14620–14625. [Google Scholar] [CrossRef]
- van Loon, J.; Janssen, M.H.M.; Öllers, M.; Aerts, H.J.W.L.; Dubois, L.; Hochstenbag, M.; Dingemans, A.M.C.; Lalisang, R.; Brans, B.; Windhorst, B.; et al. PET imaging of hypoxia using [18F]HX4: A phase I trial. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1663–1668. [Google Scholar] [CrossRef]
- Sanduleanu, S.; Wiel, A.M.A.V.; Lieverse, R.I.Y.; Marcus, D.; Ibrahim, A.; Primakov, S.; Wu, G.; Theys, J.; Yaromina, A.; Dubois, L.J.; et al. Hypoxia PET Imaging with [18F]-HX4-A Promising Next-Generation Tracer. Cancers 2020, 12, 1322. [Google Scholar] [CrossRef]
- Ureba, A.; Lindblom, E.; Dasu, A.; Uhrdin, J.; Even, A.J.G.; van Elmpt, W.; Lambin, P.; Wersäll, P.; Toma-Dasu, I. Non-linear conversion of HX4 uptake for automatic segmentation of hypoxic volumes and dose prescription. Acta Oncol. 2018, 57, 485–490. [Google Scholar] [CrossRef] [PubMed]
- van Elmpt, W.; Zegers, C.M.L.; Reymen, B.; Even, A.J.G.; Dingemans, A.C.; Oellers, M.; Wildberger, J.E.; Mottaghy, F.M.; Das, M.; Troost, E.G.C.; et al. Multiparametric imaging of patient and tumour heterogeneity in non-small-cell lung cancer: Quantification of tumour hypoxia, metabolism and perfusion. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Zegers, C.M.L.; van Elmpt, W.; Wierts, R.; Reymen, B.; Sharifi, H.; Öllers, M.C.; Hoebers, F.; Troost, E.G.C.; Wanders, R.; van Baardwijk, A.; et al. Hypoxia imaging with [18F]HX4 PET in NSCLC patients: Defining optimal imaging parameters. Radiother. Oncol. 2013, 109, 58–64. [Google Scholar] [CrossRef]
- Zegers, C.M.L.; van Elmpt, W.; Reymen, B.; Even, A.J.G.; Troost, E.G.C.; Ollers, M.C.; Hoebers, F.J.P.; Houben, R.M.A.; Eriksson, J.; Windhorst, A.D.; et al. In vivo quantification of hypoxic and metabolic status of NSCLC tumors using [18F]HX4 and [18F]FDG-PET/CT imaging. Clin. Cancer Res. 2014, 20, 6389–6397. [Google Scholar] [CrossRef] [PubMed]
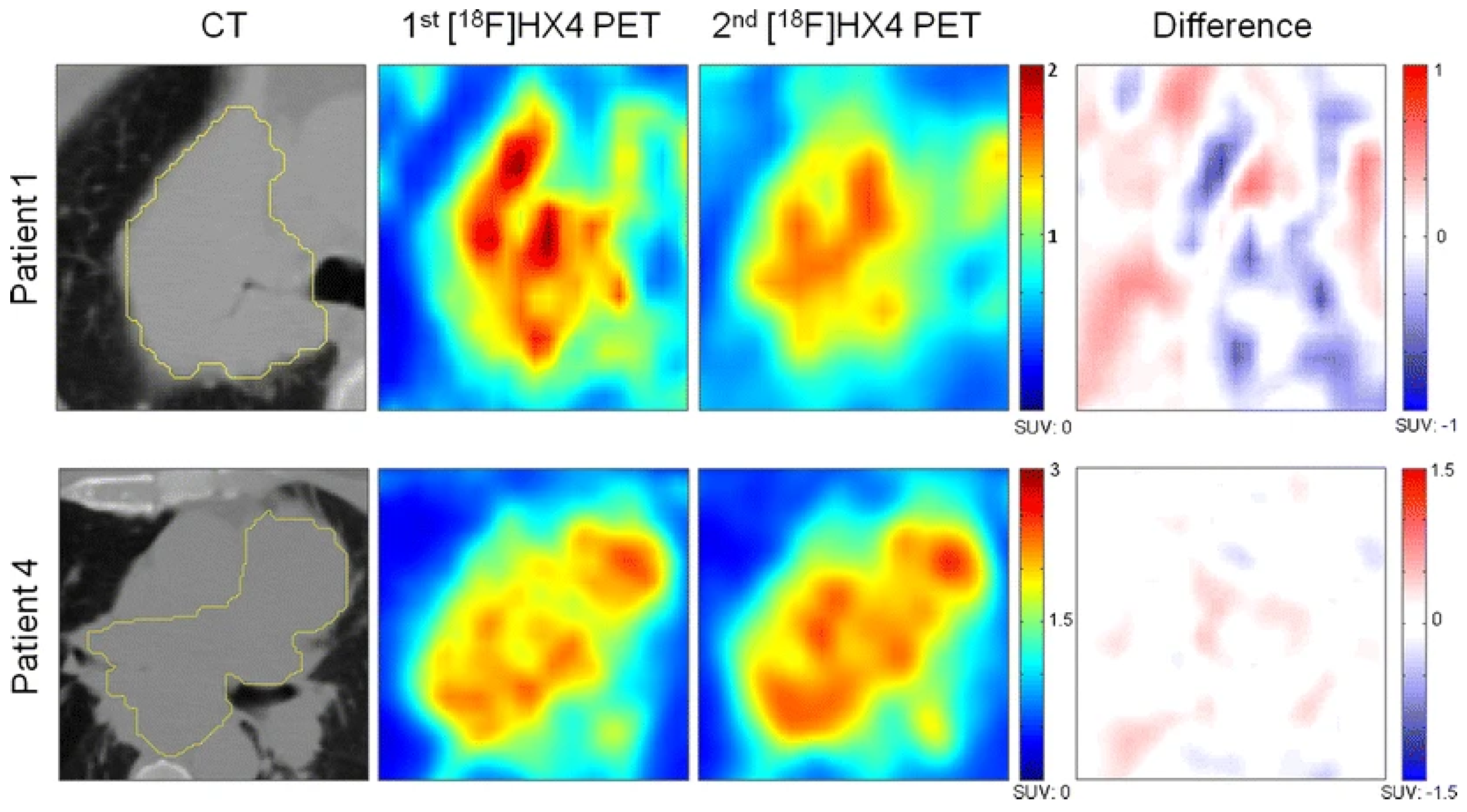
- Zegers, C.M.L.; van Elmpt, W.; Szardenings, K.; Kolb, H.; Waxman, A.; Subramaniam, R.M.; Moon, D.H.; Brunetti, J.C.; Srinivas, S.M.; Lambin, P.; et al. Repeatability of hypoxia PET imaging using [18F]HX4 in lung and head and neck cancer patients: A prospective multicenter trial. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1840–1849. [Google Scholar] [CrossRef]
- Perez, R.C.; Kim, D.; Maxwell, A.W.P.; Camacho, J.C. Functional imaging of hypoxia: PET and MRI. Cancers 2023, 15, 3336. [Google Scholar] [CrossRef]
- Dearling, J.L.J.; Packard, A.B. Some thoughts on the mechanism of cellular trapping of Cu(II)-ATSM. Nucl. Med. Biol. 2010, 37, 237–243. [Google Scholar] [CrossRef]
- Lowe, V.J.; DeLong, D.M.; Hoffman, J.M.; Coleman, R.E. Optimum scanning protocol for FDG-PET evaluation of pulmonary malignancy. J. Nucl. Med. 1995, 36, 883–887. [Google Scholar]
- Folkman, J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1995, 1, 27–31. [Google Scholar] [CrossRef]
- Pan, Y.; Yu, Y.; Wang, X.; Zhang, T. Tumor-associated macrophages in tumor immunity. Front. Immunol. 2020, 11, 583084. [Google Scholar] [CrossRef]
- Holland, J.P.; Lewis, J.S.; Dehdashti, F. Assessing Tumor Hypoxia by Positron Emission Tomography with Cu-ATSM. Q. J. Nucl. Med. Mol. Imaging 2009, 53, 193–200. [Google Scholar]
- Yoshii, Y.; Matsumoto, H.; Yoshimoto, M.; Furukawa, T.; Morokoshi, Y.; Sogawa, C.; Zhang, M.R.; Wakizaka, H.; Yoshii, H.; Fujibayashi, Y.; et al. Controlled administration of penicillamine reduces radiation exposure in critical organs during64Cu-ATSM internal radiotherapy: A novel strategy for liver protection. PLoS ONE 2014, 9, e86996. [Google Scholar] [CrossRef]
- Wong, T.Z.; Lacy, J.L.; Petry, N.A.; Hawk, T.C.; Sporn, T.A.; Dewhirst, M.W.; Vlahovic, G. PET of hypoxia and perfusion with 62Cu-ATSM and 62Cu-PTSM using a 62Zn/62Cu generator. AJR Am. J. Roentgenol. 2008, 190, 427–432. [Google Scholar] [CrossRef]
- Firth, G.; Blower, J.E.; Bartnicka, J.J.; Mishra, A.; Michaels, A.M.; Rigby, A.; Darwesh, A.; Al-Salemee, F.; Blower, P.J. Non-invasive radionuclide imaging of trace metal trafficking in health and disease: “PET metallomics”. RSC Chem. Biol. 2022, 3, 495–518. [Google Scholar] [CrossRef]
- Bourgeois, M.; Rajerison, H.; Guerard, F.; Mougin-Degraef, M.; Barbet, J.; Michel, N.; Cherel, M.; Faivre-Chauvet, A. Contribution of [164Cu]-ATSM PET in molecular imaging of tumour hypoxia compared to classical [18F]MISO—A selected review. Nucl. Med. Rev. Cent. East. Eur. 2011, 14, 90–95. [Google Scholar] [CrossRef]
- Okazawa, H.; Fujibayashi, Y.; Yonekura, Y.; Tamaki, N.; Nishizawa, S.; Magata, Y.; Ishizu, K.; Tsuchida, T.; Sadato, N.; Konishi, J. Clinical application of 62Zn/62Cu positron generator: Perfusion and plasma pool images in normal subjects. Ann. Nucl. Med. 1995, 9, 81–87. [Google Scholar] [CrossRef]
- Zhang, T.; Das, S.K.; Fels, D.R.; Hansen, K.S.; Wong, T.Z.; Dewhirst, M.W.; Vlahovic, G. PET with 62Cu-ATSM and 62Cu-PTSM is a useful imaging tool for hypoxia and perfusion in pulmonary lesions. AJR Am. J. Roentgenol. 2013, 201, W698–W706. [Google Scholar] [CrossRef]
- Dehdashti, F.; Mintun, M.A.; Lewis, J.S.; Bradley, J.; Govindan, R.; Laforest, R.; Welch, M.J.; Siegel, B.A. In vivo assessment of tumor hypoxia in lung cancer with 60Cu-ATSM. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 844–850. [Google Scholar] [CrossRef]
- Lohith, T.G.; Kudo, T.; Demura, Y.; Umeda, Y.; Kiyono, Y.; Fujibayashi, Y.; Okazawa, H. Pathophysiologic correlation between 62Cu-ATSM and 18F-FDG in lung cancer. J. Nucl. Med. 2009, 50, 1948–1953. [Google Scholar] [CrossRef]
- Sun, W.; Falzon, C.; Naimi, E.; Akbari, A.; Wiebe, L.I.; Tandon, M.; Kumar, P. Synthesis of [18F]FAZA using nosyl and iodo precursors for nucleophilic radiofluorination. Curr. Radiopharm. 2019, 12, 49–57. [Google Scholar] [CrossRef]
- Sharma, R. Nitroimidazole radiopharmaceuticals in hypoxia: Part II cytotoxicity and radiosensitization applications. Curr. Radiopharm. 2011, 4, 379–393. [Google Scholar] [CrossRef]
- Sharma, R. Nitroimidazole radiopharmaceuticals in bioimaging: Part I: Synthesis and imaging applications. Curr. Radiopharm. 2011, 4, 361–378. [Google Scholar] [CrossRef]
- Russell, J.; Carlin, S.; Burke, S.A.; Wen, B.; Yang, K.M.; Ling, C.C. Immunohistochemical detection of changes in tumor hypoxia. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 1177–1186. [Google Scholar] [CrossRef]
- Godet, I.; Doctorman, S.; Wu, F.; Gilkes, D.M. Detection of hypoxia in cancer models: Significance, challenges, and advances. Cells 2022, 11, 686. [Google Scholar] [CrossRef]
- Close, D.A.; Johnston, P.A. Detection and impact of hypoxic regions in multicellular tumor spheroid cultures formed by head and neck squamous cell carcinoma cells lines. SLAS Discov. 2022, 27, 39–54. [Google Scholar] [CrossRef]
- Corroyer-Dulmont, A.; Valable, S.; Fantin, J.; Chatre, L.; Toutain, J.; Teulier, S.; Bazille, C.; Letissier, E.; Levallet, J.; Divoux, D.; et al. Multimodal evaluation of hypoxia in brain metastases of lung cancer and interest of hypoxia image-guided radiotherapy. Sci. Rep. 2021, 11, 11239. [Google Scholar] [CrossRef]
- Rudà, R.; Franchino, F.; Soffietti, R. Treatment of brain metastasis: Current status and future directions. Curr. Opin. Oncol. 2016, 28, 502–510. [Google Scholar] [CrossRef]
- Rankin, E.B.; Giaccia, A.J. Hypoxic control of metastasis. Science 2016, 352, 175–180. [Google Scholar] [CrossRef]
- Saxena, P.; Gambhir, S.; Dixit, M. Insight into tumor hypoxia: Radionuclide-based biomarker as diagnostic tools. Curr. Top. Med. Chem. 2023, 23, 1136–1154. [Google Scholar] [CrossRef]
- Cabral, P.; Cerecetto, H. Radiopharmaceuticals in tumor hypoxia imaging: A review focused on medicinal chemistry aspects. Anti Cancer Agents Med. Chem. 2017, 17, 318–332. [Google Scholar] [CrossRef]
- Bonnitcha, P.; Grieve, S.; Figtree, G. Clinical imaging of hypoxia: Current status and future directions. Free Radic. Biol. Med. 2018, 126, 296–312. [Google Scholar] [CrossRef]
- Padhani, A.R.; Liu, G.; Koh, D.M.; Chenevert, T.L.; Thoeny, H.C.; Takahara, T.; Dzik-Jurasz, A.; Ross, B.D.; Van Cauteren, M.; Collins, D.; et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: Consensus and recommendations. Neoplasia 2009, 11, 102–125. [Google Scholar] [CrossRef]
- Juweid, M.E.; Hoekstra, O.S. Positron emission tomography. J. Nucl. Med. 2012, 53, 1002–1003. [Google Scholar] [CrossRef]
- Lasne, M.-C.; Perrio, C.; Rouden, J.; Barré, L.; Roeda, D.; Dolle, F.; Crouzel, C. Chemistry of β +-Emitting Compounds Based on Fluorine-18; Springer: Berlin/Heidelberg, Germany, 2002; pp. 201–258. [Google Scholar]
- Jødal, L.; Le Loirec, C.; Champion, C. Positron range in PET imaging: Non-conventional isotopes. Phys. Med. Biol. 2014, 59, 7419–7434. [Google Scholar] [CrossRef]
- Carter, L.M.; Kesner, A.L.; Pratt, E.C.; Sanders, V.A.; Massicano, A.V.F.; Cutler, C.S.; Lapi, S.E.; Lewis, J.S. The impact of positron range on PET resolution, evaluated with phantoms and PHITS Monte Carlo simulations for conventional and non-conventional radionuclides. Mol. Imaging Biol. 2020, 22, 73–84. [Google Scholar] [CrossRef]
- De Palma, M.; Biziato, D.; Petrova, T.V. Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer 2017, 17, 457–474. [Google Scholar] [CrossRef]
- Lin, Q.; Choyke, P.L.; Sato, N. Visualizing vasculature and its response to therapy in the tumor microenvironment. Theranostics 2023, 13, 5223–5246. [Google Scholar] [CrossRef]
- Ruoslahti, E. Specialization of tumour vasculature. Nat. Rev. Cancer 2002, 2, 83–90. [Google Scholar] [CrossRef]
- Goel, S.; Wong, A.H.K.; Jain, R.K. Vascular normalization as a therapeutic strategy for malignant and nonmalignant disease. Cold Spring Harb. Perspect. Med. 2012, 2, a006486. [Google Scholar] [CrossRef]
- Rieger, H.; Welter, M. Integrative models of vascular remodeling during tumor growth. Wiley Interdiscip. Rev. Syst. Biol. Med. 2015, 7, 113–129. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef]
- Fishman, J.E.; Joseph, P.M.; Floyd, T.F.; Mukherji, B.; Sloviter, H.A. Oxygen-sensitive 19F NMR imaging of the vascular system in vivo. Magn. Reson. Imaging 1987, 5, 279–285. [Google Scholar] [CrossRef]
- Jirak, D.; Galisova, A.; Kolouchova, K.; Babuka, D.; Hruby, M. Fluorine polymer probes for magnetic resonance imaging: Quo vadis? Magma 2019, 32, 173–185. [Google Scholar] [CrossRef]
- Carmona-Bozo, J.C.; Manavaki, R.; Miller, J.L.; Brodie, C.; Caracò, C.; Woitek, R.; Baxter, G.C.; Graves, M.J.; Fryer, T.D.; Provenzano, E.; et al. PET/MRI of hypoxia and vascular function in ER-positive breast cancer: Correlations with immunohistochemistry. Eur. Radiol. 2023, 33, 6168–6178. [Google Scholar] [CrossRef]
- Ohno, Y.; Chen, Q.; Hatabu, H. Oxygen-enhanced magnetic resonance ventilation imaging of lung. Eur. J. Radiol. 2001, 37, 164–171. [Google Scholar] [CrossRef]
- Michalski, M.H.; Chen, X. Molecular imaging in cancer treatment. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 358–377. [Google Scholar] [CrossRef]
- Büsing, K.A.; Schönberg, S.O.; Brade, J.; Wasser, K. Impact of blood glucose, diabetes, insulin, and obesity on standardized uptake values in tumors and healthy organs on 18F-FDG PET/CT. Nucl. Med. Biol. 2013, 40, 206–213. [Google Scholar] [CrossRef]
- Smith, T.A. FDG uptake, tumour characteristics and response to therapy: A review. Nucl. Med. Commun. 1998, 19, 97–105. [Google Scholar] [CrossRef]
- Miwa, K.; Wagatsuma, K.; Iimori, T.; Sawada, K.; Kamiya, T.; Sakurai, M.; Miyaji, N.; Murata, T.; Sato, E. Multicenter study of quantitative PET system harmonization using NIST-traceable 68Ge/68Ga cross-calibration kit. Phys. Med. 2018, 52, 98–103. [Google Scholar] [CrossRef]
- Aide, N.; Lasnon, C.; Veit-Haibach, P.; Sera, T.; Sattler, B.; Boellaard, R. EANM/EARL harmonization strategies in PET quantification: From daily practice to multicentre oncological studies. Eur. J. Nucl. Med. Mol. Imaging 2017, 44 (Suppl. S1), 17–31. [Google Scholar] [CrossRef]
- Scheuermann, J.S.; Saffer, J.R.; Karp, J.S.; Levering, A.M.; Siegel, B.A. Qualification of PET scanners for use in multicenter cancer clinical trials: The American College of Radiology Imaging Network experience. J. Nucl. Med. 2009, 50, 1187–1193. [Google Scholar] [CrossRef]
- Adams, M.C.; Turkington, T.G.; Wilson, J.M.; Wong, T.Z. A systematic review of the factors affecting accuracy of SUV measurements. AJR Am. J. Roentgenol. 2010, 195, 310–320. [Google Scholar] [CrossRef]
- Juweid, M.E.; Wiseman, G.A.; Vose, J.M.; Ritchie, J.M.; Menda, Y.; Wooldridge, J.E.; Mottaghy, F.M.; Rohren, E.M.; Blumstein, N.M.; Stolpen, A.; et al. Response assessment of aggressive non-Hodgkin’s lymphoma by integrated international workshop criteria and fluorine-18–fluorodeoxyglucose positron emission tomography. J. Clin. Oncol. 2005, 23, 4652–4661. [Google Scholar] [CrossRef]
- Han, H.S.; Escalón, M.P.; Hsiao, B.; Serafini, A.; Lossos, I.S. High incidence of false-positive PET scans in patients with aggressive non-Hodgkin’s lymphoma treated with rituximab-containing regimens. Ann. Oncol. 2009, 20, 309–318. [Google Scholar] [CrossRef]
- Kinahan, P.E.; Fletcher, J.W. Positron emission tomography-computed tomography standardized uptake values in clinical practice and assessing response to therapy. Semin. Ultrasound CT MR 2010, 31, 496–505. [Google Scholar] [CrossRef]
- Weber, W.A.; Grosu, A.L.; Czernin, J. Technology Insight: Advances in molecular imaging and an appraisal of PET/CT scanning. Nat. Clin. Pract. Oncol. 2008, 5, 160–170. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Ueda, O.; Hara, H.; Sakai, H.; Kida, T.; Suzuki, K.; Adachi, S.; Ishii, K. Usefulness of a breath-holding acquisition method in PET/CT for pulmonary lesions. Ann. Nucl. Med. 2009, 23, 65–71. [Google Scholar] [CrossRef]
- Cheng, N.M.; Yu, C.T.; Ho, K.C.; Wu, Y.C.; Liu, Y.C.; Wang, C.W.; Yen, T.C. Respiration-averaged CT for attenuation correction in non-small-cell lung cancer. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 607–615. [Google Scholar] [CrossRef]
- Tsai, Y.J.; Liu, C. Pitfalls on PET/CT due to artifacts and instrumentation. Semin. Nucl. Med. 2021, 51, 646–656. [Google Scholar] [CrossRef]
- Rahmim, A.; Zaidi, H. Pet versus spect: Strengths, limitations and challenges. Nucl. Med. Commun. 2008, 29, 193–207. [Google Scholar] [CrossRef]
- Zeng, Y.; Ma, J.; Zhang, S.; Wu, D. Imaging agents in targeting tumor hypoxia. Curr. Med. Chem. 2016, 23, 1775–1800. [Google Scholar] [CrossRef]
- Shi, H.; Wang, Z.; Huang, C.; Gu, X.; Jia, T.; Zhang, A.; Wu, Z.; Zhu, L.; Luo, X.; Zhao, X.; et al. A functional CT contrast agent for in vivo imaging of tumor hypoxia. Small 2016, 12, 3995–4006. [Google Scholar] [CrossRef]
- Peng, C.; Zheng, L.; Chen, Q.; Shen, M.; Guo, R.; Wang, H.; Cao, X.; Zhang, G.; Shi, X. Pegylated dendrimer-entrapped gold nanoparticles for in vivo blood pool and tumor imaging by computed tomography. Biomaterials 2012, 33, 1107–1119. [Google Scholar] [CrossRef]
- Boellaard, R. Standards for PET image acquisition and quantitative data analysis. J. Nucl. Med. 2009, 50 (Suppl. S1), 11S–20S. [Google Scholar] [CrossRef]
- Mawlawi, O.; Kappadath, S.C.; Pan, T.; Rohren, E.; Macapinlac, H. Factors affecting quantification in PET/CT imaging. Curr. Med. Imaging Rev. 2008, 4, 34–45. [Google Scholar] [CrossRef]
- Ebbesen, P.; Pettersen, E.O.; Gorr, T.A.; Jobst, G.; Williams, K.; Kieninger, J.; Wenger, R.H.; Pastorekova, S.; Dubois, L.; Lambin, P.; et al. Taking advantage of tumor cell adaptations to hypoxia for developing new tumor markers and treatment strategies. J. Enzyme Inhib. Med. Chem. 2009, 24 (Suppl. S1), 1–39. [Google Scholar] [CrossRef]
- Østergaard, L.; Tietze, A.; Nielsen, T.; Drasbek, K.R.; Mouridsen, K.; Jespersen, S.N.; Horsman, M.R. The Relationship between tumor blood flow, angiogenesis, tumor hypoxia, and aerobic glycolysis. Cancer Res. 2013, 73, 5618–5624. [Google Scholar] [CrossRef]
- Angleys, H.; Jespersen, S.N.; Østergaard, L. The effects of capillary transit time heterogeneity (CTH) on the cerebral uptake of glucose and glucose analogs: Application to FDG and comparison to oxygen uptake. Front. Comput. Neurosci. 2016, 10, 103. [Google Scholar] [CrossRef]
- Sutherland, R.M.; Ausserer, W.A.; Murphy, B.J.; Laderoute, K.R. Tumor hypoxia and heterogeneity: Challenges and opportunities for the future. Semin. Radiat. Oncol. 1996, 6, 59–70. [Google Scholar] [CrossRef]
- Johnson, G.B.; Harms, H.J.; Johnson, D.R.; Jacobson, M.S. PET imaging of tumor perfusion: A potential cancer biomarker? Semin. Nucl. Med. 2020, 50, 549–561. [Google Scholar] [CrossRef]
- Bao, M.H.R.; Wong, C.C.L. Hypoxia, metabolic reprogramming, and drug resistance in liver cancer. Cells 2021, 10, 1715. [Google Scholar] [CrossRef] [PubMed]
- Milotti, E.; Fredrich, T.; Chignola, R.; Rieger, H. Oxygen in the tumor microenvironment: Mathematical and numerical modeling. Adv. Exp. Med. Biol. 2020, 1259, 53–76. [Google Scholar] [CrossRef] [PubMed]
- Malossini, A.; Blanzieri, E.; Ng, R.T. Detecting potential labeling errors in microarrays by data perturbation. Bioinformatics 2006, 22, 2114–2121. [Google Scholar] [CrossRef]



| Radiopharmaceutical | Mechanism of Uptake | Biochemical Characteristics | 3D Conformer | Pharmacokinetics and Pharmacodynamics |
|---|---|---|---|---|
| 18F-Fluoromisonidazole (18F-FMISO) | Under hypoxic conditions, FMISO undergoes bioreduction, forming reactive intermediates which bind to intracellular macromolecules, trapping the tracer in hypoxic cells. | Moderately lipophilic | 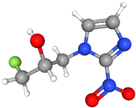 | Slow clearance from normoxic tissues, leading to modest tumor-to-background contrast. Primarily excreted via the kidneys and liver. |
| 18F-Fluoroazomycin Arabinoside (18F-FAZA) | Reduced in hypoxic cells, leading to covalent binding and retention in hypoxic tissues. | More hydrophilic than FMISO |  | Faster clearance from non-hypoxic tissues than FMISO, improving imaging contrast. Excreted primarily through the renal pathway. |
| 18F-EF3 | Hypoxia-specific reduction and trapping, resulting in accumulation in hypoxic tumor cells. | Moderately lipophilic | 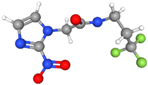 | Retained in hypoxic tissues with effective clearance from normoxic areas. Excreted primarily via the renal system. |
| 18F-EF5 | Bioreduced in hypoxic cells, forming stable adducts retained within hypoxic tumor regions. | Moderately lipophilic | 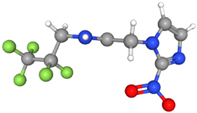 | High retention in hypoxic tissues but limited by slow plasma clearance as a result of its lipophilicity. Excreted via the renal system. |
| 18F-FSPG (4-(3-[18F]Fluoropropyl)-l-glutamate) | Targets the xC− cystine/glutamate antiporter. Upregulated in hypoxic and metabolically active tumor cells. | Hydrophilic |  | Rapid blood clearance and renal excretion. Provides specific imaging of metabolic and hypoxic regions. |
| 64Cu-ATSM | Reduced and trapped within hypoxic cells, selectively accumulating in hypoxic tumor areas. | Lipophilic |  | Rapid clearance from blood and high tumor uptake in hypoxic regions. Primarily metabolized in the liver and excreted via the biliary route. |
| 18F-FETNIM (Fluoroerythronitroimidazole) | Undergoes bioreduction in hypoxic cells, forming stable adducts retained in hypoxic tissues. | Hydrophilic | 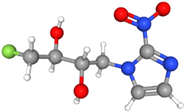 | Rapid clearance from normoxic tissues and prolonged retention in hypoxic regions. Primarily excreted via the renal system. |
| [18F]HX4 | Similar to FMISO, HX4 is bioreduced in hypoxic cells, resulting in covalent binding and retention in hypoxic regions. | More hydrophilic than FMISO | 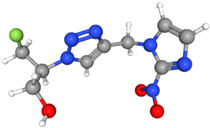 | Rapid clearance from normoxic tissues. High retention in hypoxic areas, providing excellent tumor-to-background contrast. Excreted via kidneys. |
| Category | Key Points |
|---|---|
| Recommendations | Use the same scanner and protocols for baseline and follow-up hypoxia PET/CT studies. Ensure precise calibration of dose calibrators for low-activity tracers. Adopt standardized postinjection uptake times specific to the hypoxia tracer used. Correct for respiratory motion in thoracic imaging to improve SUV accuracy. Document tumor oxygenation context and perfusion characteristics to aid interpretation. Avoid confounding factors such as recent oxygen supplementation or hyperbaric oxygen therapy. Provide explicit reporting of region-of-interest (ROI) definitions and imaging protocols for reproducibility. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alenezi, A.; Alhamad, H.; Alenezi, A.; Khan, M.U. Hypoxia Imaging in Lung Cancer: A PET-Based Narrative Review for Clinicians and Researchers. Pharmaceuticals 2025, 18, 459. https://doi.org/10.3390/ph18040459
Alenezi A, Alhamad H, Alenezi A, Khan MU. Hypoxia Imaging in Lung Cancer: A PET-Based Narrative Review for Clinicians and Researchers. Pharmaceuticals. 2025; 18(4):459. https://doi.org/10.3390/ph18040459
Chicago/Turabian StyleAlenezi, Ahmad, Hamad Alhamad, Aishah Alenezi, and Muhammad Umar Khan. 2025. "Hypoxia Imaging in Lung Cancer: A PET-Based Narrative Review for Clinicians and Researchers" Pharmaceuticals 18, no. 4: 459. https://doi.org/10.3390/ph18040459
APA StyleAlenezi, A., Alhamad, H., Alenezi, A., & Khan, M. U. (2025). Hypoxia Imaging in Lung Cancer: A PET-Based Narrative Review for Clinicians and Researchers. Pharmaceuticals, 18(4), 459. https://doi.org/10.3390/ph18040459






