Pharmacokinetics of Oral Cannabinoid Δ8-Tetrahydrocannabivarin and Its Main Metabolites in Healthy Participants
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Component | Internal Standard | LLOQ [ng/mL] | Analytical Measuring Range [ng/mL] |
|---|---|---|---|
| Δ8-THCV | Δ9-THCV-d5 | 0.5 | 0.5–400 |
| Δ9-THCV | Δ9-THCV-d5 | 0.5 | 0.5–400 |
| 11-OH-Δ8-THCV | 7-OH-CBD-d3 | 0.5 | 0.5–400 |
| Δ8-THCV-COOH | 7-CBD-COOH-d3 | 1.0 | 1.0–400 |
| Δ9-THCV-COOH | Δ9-THC-COOH-d3 | 1.0 | 1.0–400 |
| Δ8-THC | Δ8-THC-d9 | 0.5 | 0.5–400 |
| Δ9-THC | Δ9-THC-d9 | 0.5 | 0.5–400 |
| 9S-Δ10-THC | Δ8-THC-d9 | 0.5 | 0.5–400 |
| 9R-Δ10-THC | Δ8-THC-d9 | 0.5 | 0.5–400 |
| 11-OH-Δ8-THC | 11-OH-Δ9-THC-d3 | 1.0 | 1.0–400 |
| 11-OH-Δ9-THC | 11-OH-Δ9-THC-d3 | 1.0 | 1.0–400 |
| Δ8-THC-COOH | Δ9-THC-COOH-d3 | 1.0 | 1.0–400 |
| Δ9-THC-COOH | Δ9-THC-COOH-d3 | 1.0 | 1.0–400 |
| Intra-Day Summary | Inter-Day Summary | |||||||
|---|---|---|---|---|---|---|---|---|
| Component | Mean Accuracy | Accuracy Range | Mean Precision | Precision Range | Mean Accuracy | Accuracy Range | Mean Precision | Precision Range |
| Δ8-THCV | 103.1 | 95.0–106.4 | 4.66 | 3.19–18.2 | 103.1 | 94.2–106.7 | 4.91 | 4.33–14.7 |
| Δ9-THCV | 97.1 | 80.3–105.2 | 9.96 | 1.68–6.78 | 95.8 | 82.9–100.7 | 7.64 | 4.55–12.3 |
| 11-OH-Δ8-THCV | 100.8 | 96.4–104.1 | 2.90 | 3.91–9.69 | 99.0 | 96.3–102.7 | 2.58 | 5.09–12.4 |
| Δ8-THCV-COOH | 95.3 | 86.7–101.8 | 6.66 | 5.81–14.4 | 97.1 | 90.0–104.3 | 5.74 | 7.51–16.1 |
| Δ9-THCV-COOH | 97.1 | 93.2–100.0 | 3.22 | 5.90–18.3 | 96.0 | 91.6–99.7 | 4.16 | 7.80–17.5 |
| Δ8-THC | 105.1 | 102.7–109.9 | 2.66 | 4.09–18.5 | 105.6 | 101.6–108.3 | 2.34 | 5.65–15.5 |
| Δ9-THC | 101.6 | 98.2–106.6 | 3.05 | 4.38–14.5 | 99.5 | 97.9–101.6 | 1.35 | 5.78–11.3 |
| Δ10-9S-THC | 100.2 | 89.7–109.3 | 8.84 | 2.49–18.6 | 100.9 | 89.2–107.8 | 7.31 | 6.16–29.0 |
| Δ10-9R-THC | 102.5 | 88.9–110.2 | 8.81 | 3.76–43.0 | 103.5 | 91.6–108.8 | 7.08 | 4.78–27.1 |
| 11-OH-Δ8-THC | 95.9 | 83.6–101.0 | 7.34 | 6.70–17.7 | 98.6 | 87.5–103.1 | 6.62 | 7.20–16.5 |
| 11-OH-Δ9-THC | 106.7 | 100.5–119.2 | 6.88 | 4.32–23.3 | 101.8 | 95.7–105.2 | 3.82 | 4.71–25.3 |
| Δ8-THC-COOH | 104.5 | 99.8–114.1 | 5.42 | 3.58–15.6 | 100.4 | 96.9–103.5 | 2.66 | 6.24–15.7 |
| Δ9-THC-COOH | 99.8 | 96.0–102.0 | 2.47 | 3.37–15.5 | 96.1 | 88.8–99.4 | 4.72 | 5.97–12.4 |
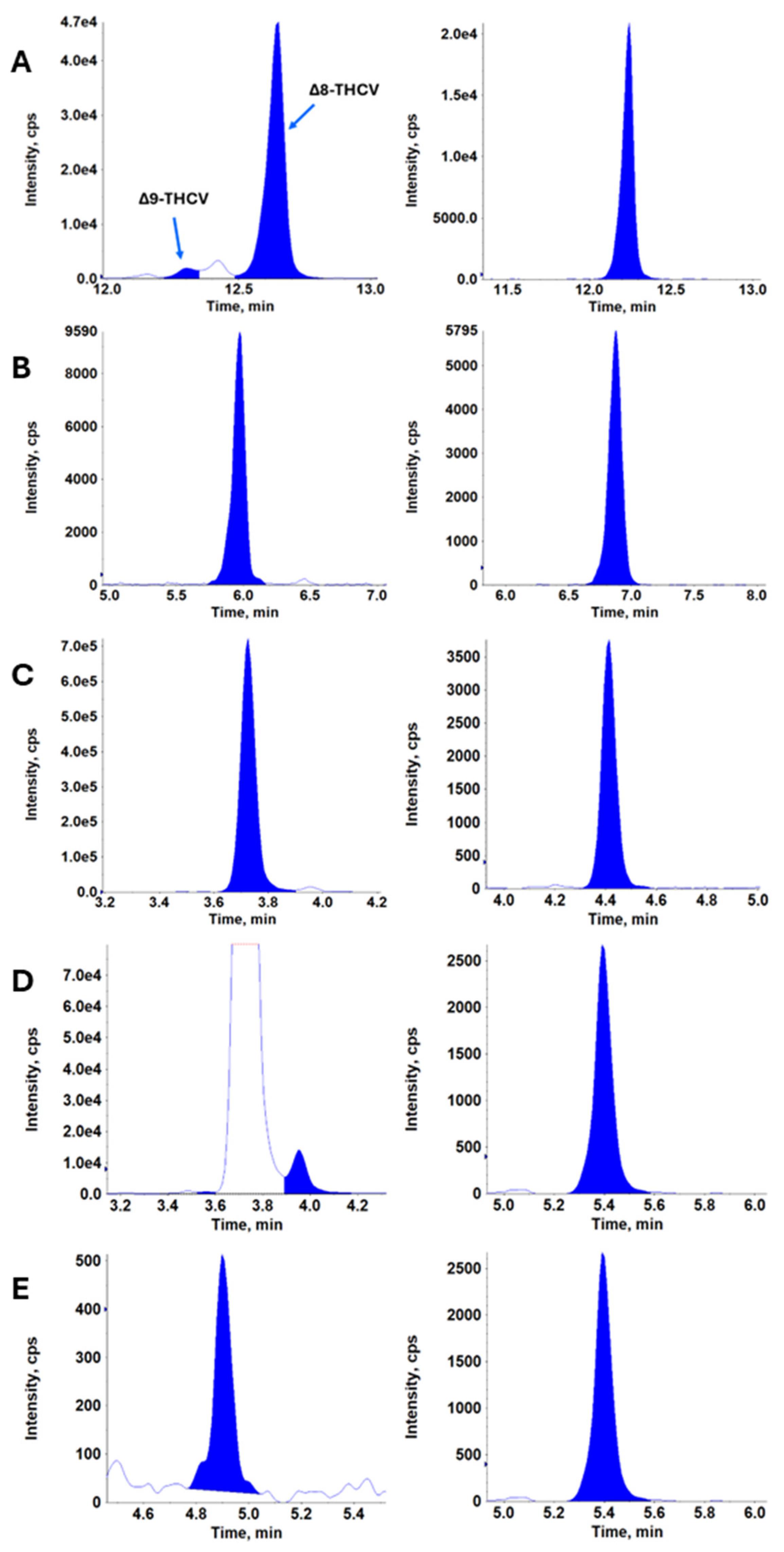
| Timepoint | 12.5 mg | 25 mg | 50 mg | 100 mg | 200 mg | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BLOQ (n = 15) | Mean (SD, %CV) | Median (IQ Range) | BLOQ (n = 14) | Mean (SD, %CV) | Median (IQ Range) | BLOQ (n = 14) | Mean (SD, %CV) | Median (IQ Range) | BLOQ (n = 14) | Mean (SD, %CV) | Median (IQ Range) | BLOQ (n = 15) | Mean (SD, %CV) | Median (IQ Range) | |
| Δ8-THCV | |||||||||||||||
| Predose | 15 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 15 | 0 (±0, --) | 0 (0–0) |
| 0.5 h | 15 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 13 | 0.1 (±0.3, 374) | 0 (0–0) | 12 | 0.2 (±0.5, 254) | 0 (0–0) | 14 | 0.1 (±0.3, 387) | 0 (0–0) |
| 1 h | 12 | 0.1 (±0.3, 212) | 0 (0–0) | 11 | 0.3 (±0.6, 238) | 0 (0–0) | 10 | 0.5 (±1, 201) | 0 (0–0.5) | 6 | 4.2 (±6.1, 145) | 2.1 (0–4.9) | 6 | 3.6 (±4.5, 124) | 2 (0–5.2) |
| 2 h | 9 | 0.4 (±0.8, 184) | 0 (0–0.7) | 7 | 1.4 (±1.9, 140) | 0.3 (0–2.1) | 8 | 2.6 (±5.3, 204) | 0 (0–1.4) | 4 | 7.1 (±7.9, 113) | 5.5 (0.2–8) | 5 | 21.4 (±21.8, 102) | 14.3 (0–36.6) |
| 4 h | 7 | 0.7 (±1.1, 145) | 0.6 (0–0.9) | 1 | 1.8 (±1.4, 78.9) | 1.5 (1–2.3) | 3 | 4.3 (±4.6, 109) | 2.4 (1.3–6.8) | 1 | 11.4 (±12.4, 109) | 7.3 (4.6–11.6) | 1 | 29.4 (±21.2, 72.2) | 33.3 (12.6–38.6) |
| 6 h | 11 | 0.5 (±0.8, 178) | 0 (0–0.7) | 6 | 0.9 (±1.1, 120) | 0.7 (0–1.4) | 1 | 6.1 (±7.3, 120) | 2.8 (1.7–7.3) | 0 | 12.3 (±13.6, 110) | 7.7 (2.8–18.5) | 0 | 26.6 (±20.3, 76.2) | 21.8 (12.9–40.9) |
| 8 h | 11 | 0.2 (±0.3, 172) | 0 (0–0.3) | 7 | 0.4 (±0.5, 117) | 0.3 (0–0.7) | 1 | 2.2 (±1.6, 71.4) | 2.2 (0.8–3.2) | 0 | 4.1 (±3.1, 74.8) | 3.1 (1.8–5.9) | 0 | 21.2 (±23.2, 109) | 13.4 (5.3–28.3) |
| 11-OH-Δ8-THCV | |||||||||||||||
| Predose | 15 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 15 | 0 (±0, --) | 0 (0–0) |
| 0.5 h | 15 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 15 | 0 (±0, --) | 0 (0–0) |
| 1 h | 14 | 0 (±0.1, 387) | 0 (0–0) | 13 | 0.1 (±0.2, 374) | 0 (0–0) | 12 | 0.1 (±0.4, 274) | 0 (0–0) | 7 | 1.4 (±2.2, 161) | 0.5 (0–1.5) | 8 | 1.2 (±1.5, 126) | 0 (0–1.8) |
| 2 h | 13 | 0.1 (±0.3, 282) | 0 (0–0) | 10 | 0.3 (±0.4, 174) | 0 (0–0.4) | 12 | 0.4 (±1.1, 264) | 0 (0–0) | 4 | 1.8 (±2.3, 132) | 1 (0.1–1.5) | 5 | 4.5 (±5.7, 128) | 1.8 (0–5.9) |
| 4 h | 13 | 0.1 (±0.3, 264) | 0 (0–0) | 11 | 0.2 (±0.4, 203) | 0 (0–0) | 9 | 0.6 (±0.9, 154) | 0 (0–1.1) | 3 | 2.1 (±2.8, 129) | 1.3 (0.9–2.6) | 1 | 5.2 (±5.4, 104) | 3.4 (2.4–5.9) |
| 6 h | 14 | 0.1 (±0.2, 374) | 0 (0–0) | 11 | 0.2 (±0.3, 201) | 0 (0–0) | 5 | 1 (±1, 103) | 0.9 (0–1.5) | 2 | 2.4 (±2.3, 95.6) | 1.5 (1–2.9) | 0 | 4.6 (±2.9, 63.1) | 3.7 (2.2–6) |
| 8 h | 15 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 5 | 0.6 (±0.5, 87.9) | 0.6 (0–0.9) | 2 | 1.3 (±1, 80.3) | 1.1 (0.7–1.6) | 0 | 3.7 (±2.6, 71.6) | 3.1 (1.7–4.9) |
| Δ8-THCV-COOH | |||||||||||||||
| Predose | 13 | 0.2 (±0.4, 267) | 0 (0–0) | 13 | 0.3 (±1, 374) | 0 (0–0) | 10 | 0.8 (±1.6, 187) | 0 (0–0.9) | 10 | 0.5 (±0.8, 166) | 0 (0–1) | 14 | 0.1 (±0.3, 387) | 0 (0–0) |
| 0.5 h | 12 | 0.4 (±0.9, 237) | 0 (0–0) | 9 | 2.7 (±5.2, 194) | 0 (0–3.2) | 6 | 4.7 (±10.4, 223) | 1.3 (0–2.8) | 5 | 13 (±22.1, 170) | 1.7 (0–17.8) | 10 | 5.6 (±16.2, 289) | 0 (0–1.4) |
| 1 h | 5 | 15 (±22, 147) | 3 (0–19.9) | 2 | 28.5 (±35.7, 125) | 7.1 (2.3–61.6) | 4 | 27.3 (±42.3, 155) | 4.8 (0.6–36.7) | 2 | 202 (±229, 113) | 79.5 (4–426) | 3 | 158 (±231, 146) | 108.5 (4.7–224) |
| 2 h | 2 | 30.1 (±34.2, 114) | 15.5 (2.1–44.4) | 0 | 54.6 (±48.9, 89.6) | 55.6 (5.2–94.4) | 1 | 84.1 (±118, 140) | 26.5 (8.7–101) | 0 | 306 (±371, 121) | 275 (27.5–372) | 1 | 653 (±732, 112) | 318.8 (8.5–1009) |
| 4 h | 0 | 84.6 (±47.9, 56.7) | 73.1 (47.9–116) | 0 | 136 (±83.3, 61.1) | 108 (94.1–143) | 0 | 214 (±157, 73.2) | 177 (93.5–307) | 0 | 556 (±333, 59.9) | 546.6 (439–755) | 0 | 1302 (±838, 64.4) | 1111 (530–1902) |
| 6 h | 1 | 62.2 (±39.8, 63.9) | 53.3 (37.5–66.4) | 0 | 96.3 (±50.9, 52.9) | 82.6 (64.7–132) | 0 | 233 (±173, 74) | 200 (131–276) | 0 | 512 (±259, 50.7) | 496.8 (401–602) | 0 | 1167 (±508, 43.6) | 1023 (841–1310) |
| 8 h | 0 | 46.8 (±23, 49.1) | 38.2 (34.6–51.8) | 0 | 71.6 (±33.8, 47.2) | 78.3 (43.9–86.7) | 0 | 166 (±60.3, 36.4) | 148 (125–208) | 0 | 319 (±118, 37.1) | 303.5 (241–359) | 0 | 917 (±582, 63.4) | 855 (467–1040) |
| Δ9-THCV | |||||||||||||||
| Predose | 15 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 15 | 0 (±0, --) | 0 (0–0) |
| 0.5 h | 15 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 15 | 0 (±0, --) | 0 (0–0) |
| 1 h | 15 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 13 | 0.1 (±0.3, 374) | 0 (0–0) | 14 | 0 (±0.2, 387) | 0 (0–0) |
| 2 h | 15 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 12 | 0.1 (±0.3, 258) | 0 (0–0) | 9 | 0.6 (±0.8, 134) | 0 (0–1.5) |
| 4 h | 15 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 10 | 0.3 (±0.6, 183) | 0 (0–0) | 6 | 0.9 (±1, 112) | 0.7 (0–1.5) |
| 6 h | 15 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 13 | 0.1 (±0.3, 374) | 0 (0–0) | 10 | 0.3 (±0.6, 194) | 0 (0–0) | 7 | 0.7 (±0.8, 112) | 0.6 (0–1.5) |
| 8 h | 15 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 9 | 0.6 (±1.1, 186) | 0 (0–0.7) |
| Δ9-THCV-COOH | |||||||||||||||
| Predose | 15 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 15 | 0 (±0, --) | 0 (0–0) |
| 0.5 h | 15 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 12 | 0.3 (±0.7, 257) | 0 (0–0) | 14 | 0.1 (±0.6, 387) | 0 (0–0) |
| 1 h | 14 | 0.1 (±0.3, 387) | 0 (0–0) | 9 | 0.7 (±1.1, 144) | 0 (0–1.7) | 11 | 0.6 (±1.3, 204) | 0 (0–0) | 6 | 5.8 (±6.9, 118) | 1.9 (0–13.7) | 7 | 4.9 (±7, 143) | 2.7 (0–7.6) |
| 2 h | 12 | 0.5 (±1.1, 217) | 0 (0–0) | 6 | 1.6 (±1.5, 96.6) | 1.7 (0–2.9) | 8 | 2.3 (±3.9, 170) | 0 (0–2.1) | 4 | 9.2 (±10.2, 111) | 8.7 (0.6–11.6) | 5 | 19.1 (±21.6, 113) | 9.4 (0–30.7) |
| 4 h | 3 | 1.9 (±1.3, 70.7) | 1.8 (1.1–3.1) | 0 | 3.7 (±1.8, 48.1) | 3.6 (2.5–4.9) | 2 | 5.4 (±4.2, 78.3) | 5 (2.4–7.9) | 0 | 16.1 (±11.1, 68.7) | 16 (11.7–19) | 1 | 37.1 (±22.4, 60.4) | 40 (17.6–57.4) |
| 6 h | 6 | 1.5 (±1.6, 107) | 1.3 (0–1.9) | 1 | 3 (±1.6, 53.2) | 2.4 (2.1–4.5) | 0 | 6.7 (±3.9, 58.4) | 5.9 (4.1–8) | 0 | 15.6 (±6.9, 44.2) | 17.5 (11.1–20.1) | 0 | 32.3 (±13.1, 40.4) | 28.5 (24.2–35.6) |
| 8 h | 6 | 1.2 (±1.1, 92.5) | 1.4 (0–2.1) | 2 | 2.3 (±1.4, 61.7) | 2.3 (1.4–3.2) | 0 | 5.4 (±1.9, 36) | 5.2 (4.2–6.5) | 0 | 10.8 (±3.7, 34.7) | 10.5 (7.7–14.1) | 0 | 29.2 (±16.1, 55.3) | 26.3 (17.4–35.5) |
| Δ8-THC-COOH | |||||||||||||||
| Predose | 15 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 15 | 0 (±0, --) | 0 (0–0) |
| 0.5 h | 15 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 15 | 0 (±0, --) | 0 (0–0) |
| 1 h | 15 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 15 | 0 (±0, --) | 0 (0–0) |
| 2 h | 15 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 11 | 0.4 (±0.7, 175) | 0 (0–0.5) |
| 4 h | 15 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 10 | 0.4 (±0.7, 167) | 0 (0–0.8) | 6 | 1.3 (±1.2, 90.5) | 1.7 (0–2.2) |
| 6 h | 15 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 8 | 0.6 (±0.7, 124) | 0 (0–1.2) | 2 | 1.8 (±1, 55.2) | 2 (1.3–2.4) |
| 8 h | 15 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 13 | 0.1 (±0.3, 374) | 0 (0–0) | 3 | 1.6 (±1, 62.6) | 1.6 (1.1–2.2) |
References
- Hanus, L.O. Pharmacological and therapeutic secrets of plant and brain (endo)cannabinoids. Med. Res. Rev. 2009, 29, 213–271. [Google Scholar] [CrossRef] [PubMed]
- Potter, D.J. A review of the cultivation and processing of cannabis (Cannabis sativa L.) for production of prescription medicines in the UK. Drug Test. Anal. 2014, 6, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Hill, K.P. Medical Use of Cannabis in 2019. JAMA 2019, 322, 974–975. [Google Scholar] [CrossRef] [PubMed]
- Walsh, K.B.; McKinney, A.E.; Holmes, A.E. Minor cannabinoids: Biosynthesis, molecular pharmacology and potential therapeutic uses. Front. Pharmacol. 2021, 12, 777804. [Google Scholar] [CrossRef]
- Zagzoog, A.; Mohamed, K.A.; Kim, H.J.J.; Kim, E.D.; Frank, C.S.; Black, T.; Jadhav, P.D.; Holbrook, L.A.; Laprairie, R.B. In vitro and in vivo pharmacological activity of minor cannabinoids isolated from Cannabis sativa. Sci. Rep. 2020, 10, 20405. [Google Scholar] [CrossRef]
- Peters, E.N.; MacNair, L.; Harrison, A.; Feldner, M.T.; Eglit, G.M.L.; Babalonis, S.; Turcotte, C.; Bonn-Miller, M.O. A two-phase, dose-ranging, placebo-controlled study of the safety and preliminary test of acute effects of oral Δ8-tetrahydrocannabivarin in healthy participants. Cannabis Cannabinoid Res. 2023, 8, S71–S82. [Google Scholar] [CrossRef]
- Mehmedic, Z.; Chandra, S.; Slade, D.; Denham, H.; Foster, S.; Patel, A.S.; Ross, S.A.; Khan, I.A.; ElSohly, M.A. Potency trends of Δ9-THC and other cannabinoids in confiscated cannabis preparations from 1993 to 2008. J. Forensic. Sci. 2010, 55, 1209–1217. [Google Scholar] [CrossRef]
- Haghdoost, M.; Peters, E.N.; Roberts, M.; Bonn-Miller, M.O. Tetrahydrocannabivarin is not tetrahydrocannabinol. Cannabis Cannabinoid Res. 2024. online ahead of print. [Google Scholar] [CrossRef]
- Haghdoost, M.; Lopez de Los Santos, Y.; Brunstetter, M.; Ferretti, M.L.; Roberts, M.; Bonn-Miller, M.O. Using in silico molecular docking to explain differences in receptor binding behavior of HHC and THCV isomers: Revealing new binding modes. Pharmaceuticals 2024, 17, 637. [Google Scholar] [CrossRef]
- Batkai, S.; Mukhopadhyay, P.; Horvath, B.; Rajesh, M.; Gao, R.Y.; Mahadevan, A.; Amere, M.; Battista, N.; Lichtman, A.H.; Gauson, L.A.; et al. Δ8-Tetrahydrocannabivarin prevents hepatic ischaemia/reperfusion injury by decreasing oxidative stress and inflammatory responses through cannabinoid CB2 receptors. Br. J. Pharmacol. 2012, 165, 2450–2461. [Google Scholar] [CrossRef]
- Abioye, A.; Ayodele, O.; Marinkovic, A.; Patidar, R.; Akinwekomi, A.; Sanyaolu, A. Δ9-Tetrahydrocannabivarin (THCV): A commentary on potential therapeutic benefit for the management of obesity and diabetes. J. Cannabis Res. 2020, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Sempio, C.; Campos-Palomino, J.; Klawitter, J.; Harrison, A.; Peters, E.N.; MacNair, L.; Haghdoost, M.; Bonn-Miller, M.; Babalonis, S.; Huestis, M.A.; et al. LC-MS-MS quantification of Delta8-THC, Delta9-THC, THCV isomers and their main metabolites in human plasma. J. Anal. Toxicol. 2024, 48, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Assanangkornchai, S.; Kalayasiri, R.; Ratta-Apha, W.; Tanaree, A. Effects of cannabis legalization on the use of cannabis and other substances. Curr. Opin. Psychiatry 2023, 36, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, J.; Rosado, T.; Soares, S.; Simao, A.Y.; Caramelo, D.; Luis, A.; Fernandez, N.; Barroso, M.; Gallardo, E.; Duarte, A.P. Cannabis and its secondary metabolites: Their use as therapeutic drugs, toxicological aspects, and analytical determination. Medicines 2019, 6, 31. [Google Scholar] [CrossRef]
- Englund, A.; Atakan, Z.; Kralj, A.; Tunstall, N.; Murray, R.; Morrison, P. The effect of five day dosing with THCV on THC-induced cognitive, psychological and physiological effects in healthy male human volunteers: A placebo-controlled, double-blind, crossover pilot trial. J. Psychopharmacol. 2016, 30, 140–151. [Google Scholar] [CrossRef]
- Jadoon, K.A.; Ratcliffe, S.H.; Barrett, D.A.; Thomas, E.L.; Stott, C.; Bell, J.D.; O’Sullivan, S.E.; Tan, G.D. Efficacy and safety of cannabidiol and tetrahydrocannabivarin on glycemic and lipid parameters in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled, parallel group pilot study. Diabetes Care 2016, 39, 1777–1786. [Google Scholar] [CrossRef]
- Newmeyer, M.N.; Swortwood, M.J.; Barnes, A.J.; Abulseoud, O.A.; Scheidweiler, K.B.; Huestis, M.A. Free and glucuronide whole blood cannabinoids’ pharmacokinetics after controlled smoked, vaporized, and oral cannabis administration in frequent and occasional cannabis users: Identification of recent cannabis intake. Clin. Chem. 2016, 62, 1579–1592. [Google Scholar] [CrossRef]
- Deiana, S.; Watanabe, A.; Yamasaki, Y.; Amada, N.; Arthur, M.; Fleming, S.; Woodcock, H.; Dorward, P.; Pigliacampo, B.; Close, S.; et al. Plasma and brain pharmacokinetic profile of cannabidiol (CBD), cannabidivarine (CBDV), Δ9-tetrahydrocannabivarin (THCV) and cannabigerol (CBG) in rats and mice following oral and intraperitoneal administration and CBD action on obsessive-compulsive behaviour. Psychopharmacology 2012, 219, 859–873. [Google Scholar] [CrossRef]
- Moore, C.F.; Weerts, E.M.; Kulpa, J.; Schwotzer, D.; Dye, W.; Jantzi, J.; McDonald, J.D.; Lefever, T.W.; Bonn-Miller, M.O. Pharmacokinetics of oral minor cannabinoids in blood and brain. Cannabis Cannabinoid Res. 2023, 8, S51–S61. [Google Scholar] [CrossRef]
- ElSohly, M.A.; Feng, S.; Murphy, T.P.; Warrington, A.W.; Ross, S.; Nimrod, A.; Mehmedic, Z.; Fortner, N. Identification and quantitation of 11-nor-delta9-tetrahydrocannabivarin-9-carboxylic acid, a major metabolite of delta9-tetrahydrocannabivarin. J. Anal. Toxicol. 2001, 25, 476–480. [Google Scholar] [CrossRef]
- Rao, Q.; Zhang, T.; Pu, Q.L.; Li, B.; Zhao, Q.; Yan, D.M.; Wu, Z.E.; Li, F. Comparative metabolism of THCA and THCV using UHPLC-Q-Exactive Orbitrap-MS. Xenobiotica 2023, 53, 46–59. [Google Scholar] [CrossRef]
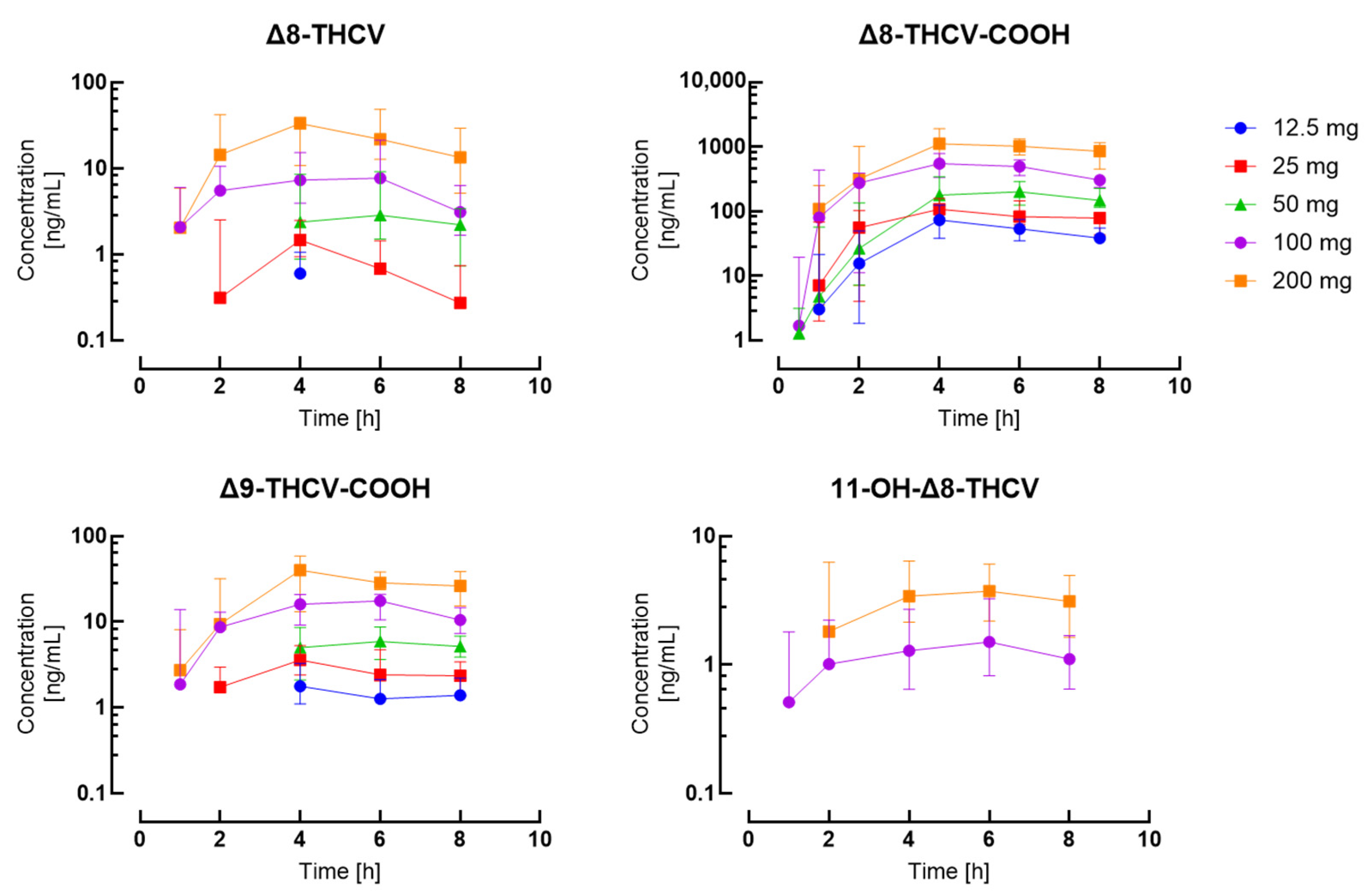
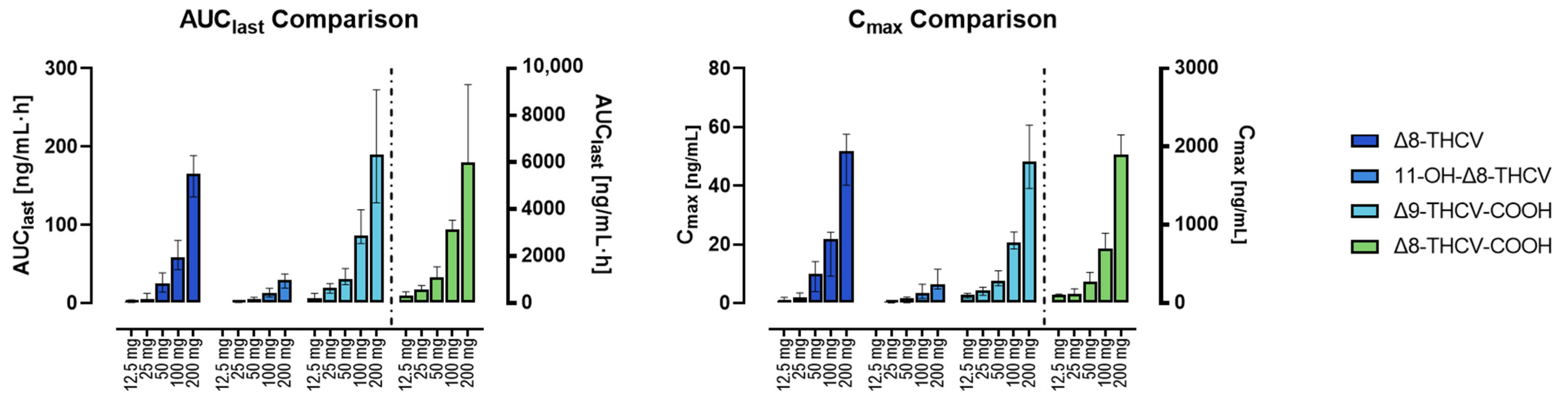
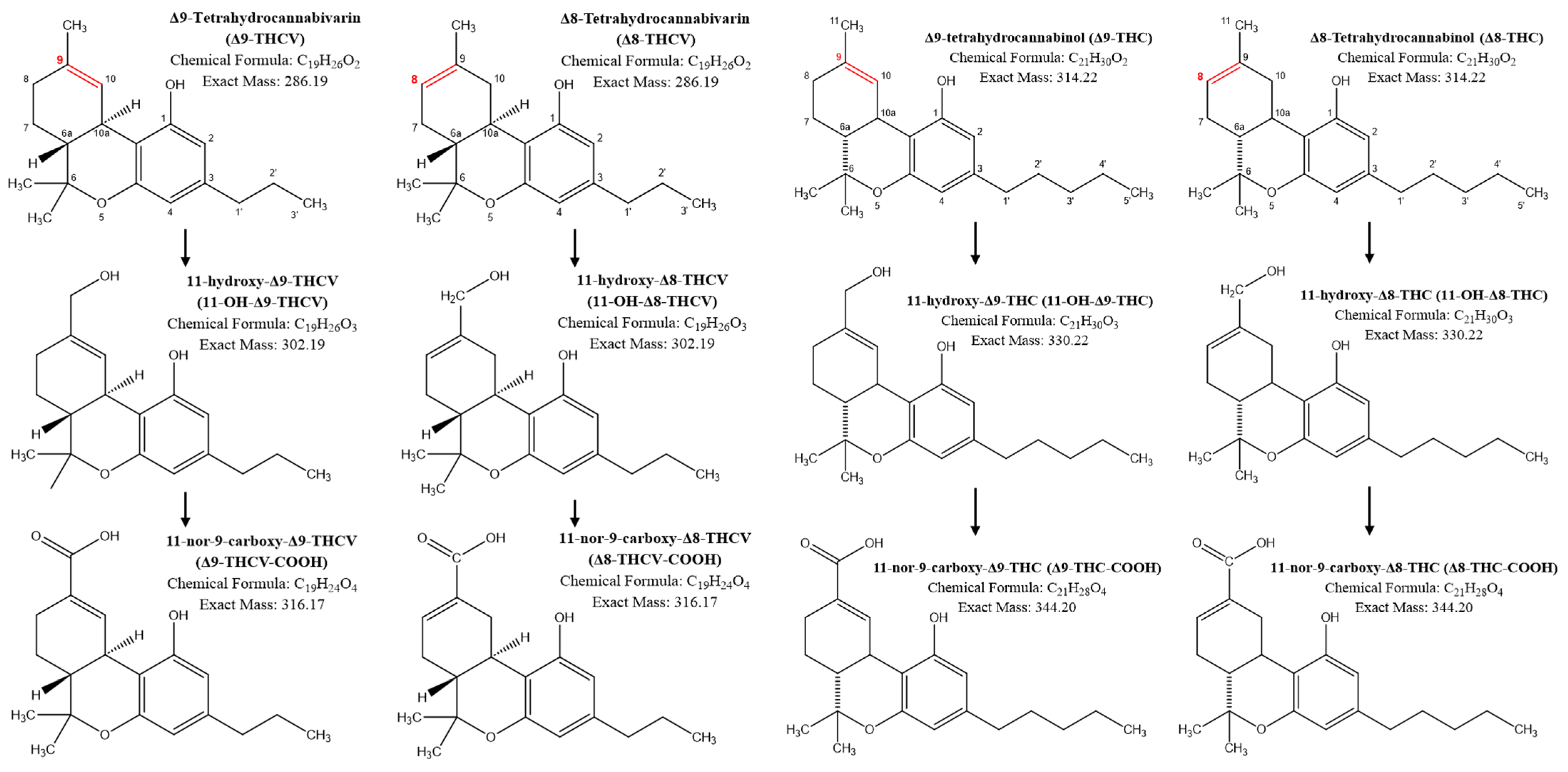
| Timepoint | 12.5 mg | 25 mg | 50 mg | 100 mg | 200 mg | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (±SD) | Median (Range) | n | Mean (±SD) | Median (Range) | n | Mean (±SD) | Median (Range) | n | Mean (±SD) | Median (Range) | n | Mean (±SD) | Median (Range) | |
| Δ8-THCV | |||||||||||||||
| tmax | 12 | 3.9 (±2) | 4 (1–8) | 14 | 3.8 (±1.8) | 4 (1–6) | 14 | 5 (±2) | 6 (2–8) | 14 | 4.2 (±2) | 4 (1–8) | 15 | 4.7 (±2.2) | 4 (2–8) |
| Cmax | 12 | 1.6 (±1.1) | 1.1 (0.6–3.3) | 14 | 2.6 (±1.7) | 2.1 (0.9–5.7) | 14 | 10 (±6.8) | 10 (2.4–24.7) | 14 | 21.5 (±13.3) | 22 (5.7–50.4) | 15 | 52.9 (±16.3) | 51.7 (33.3–87.6) |
| tlast | 12 | 5.3 (±2.3) | 5 (2–8) | 14 | 6.3 (±1.9) | 7 (4–8) | 14 | 7.9 (±0.5) | 8 (6–8) | 14 | 8 (±0) | 8 (8–8) | 15 | 8 (±0) | 8 (8–8) |
| Clast | 12 | 0.9 (±0.3) | 0.7 (0.6–1.5) | 14 | 1.1 (±0.4) | 1 (0.5–1.8) | 14 | 2.3 (±1.5) | 2.2 (0.6–5.5) | 14 | 4.1 (±3.1) | 3.1 (0.8–9.9) | 15 | 21.2 (±23.2) | 13.4 (1.5–87.6) |
| AUClast | 12 | 3.5 (±3.1) | 2.1 (0.3–9.3) | 14 | 7.4 (±5.5) | 5.2 (1.4–16.5) | 14 | 27.3 (±18.5) | 24.9 (2.6–68.6) | 14 | 65.5 (±34.3) | 58.4 (15.7–137) | 15 | 168 (±54.3) | 165 (93.9–299) |
| MRT | 12 | 4 (±1.9) | 4 (1.4–8) | 14 | 4.1 (±1.1) | 4.2 (2.4–5.8) | 14 | 5.2 (±1.4) | 5.4 (2.5–8) | 14 | 4.5 (±1.3) | 4.1 (2.4–7.1) | 15 | 4.7 (±1.3) | 4.7 (2.7–6.5) |
| 11-OH-Δ8-THCV | |||||||||||||||
| tmax | 5 | 3.4 (±1.9) | 4 (1–6) | 7 | 3.7 (±1.8) | 4 (2–6) | 10 | 5 (±1.9) | 6 (2–8) | 14 | 4.2 (±2.4) | 5 (1–8) | 15 | 4.7 (±2) | 4 (2–8) |
| Cmax | 5 | 0.8 (±0.2) | 0.8 (0.6–1.1) | 7 | 0.9 (±0.2) | 0.8 (0.6–1.3) | 10 | 2 (±1) | 1.9 (0.6–3.7) | 14 | 4.3 (±3) | 3.4 (1.3–10.6) | 15 | 8.7 (±5.4) | 6.4 (2.6–22.2) |
| tlast | 5 | 3.6 (±1.7) | 4 (2–6) | 7 | 4.3 (±1.8) | 4 (2–6) | 10 | 7.8 (±0.6) | 8 (6–8) | 14 | 7.6 (±1.2) | 8 (4–8) | 15 | 8 (±0) | 8 (8–8) |
| Clast | 5 | 0.8 (±0.2) | 0.8 (0.6–1.1) | 7 | 0.8 (±0.1) | 0.8 (0.6–1) | 10 | 0.9 (±0.3) | 0.8 (0.6–1.5) | 14 | 1.4 (±0.9) | 1.1 (0.5–3.8) | 15 | 3.7 (±2.6) | 3.1 (1.2–11.3) |
| AUClast | 5 | 0.7 (±0.1) | 0.8 (0.6–0.8) | 7 | 1.5 (±1.9) | 0.8 (0.4–5.8) | 10 | 6.2 (±4) | 5.3 (0.6–14.7) | 14 | 13.9 (±9.9) | 12.4 (1.5–40.3) | 15 | 30.8 (±18.6) | 29.5 (10.6–87.8) |
| MRT | 5 | 1.5 (±1.7) | 1.4 (0–4) | 7 | 3.6 (±1.4) | 3.6 (2–6) | 10 | 5.7 (±1.5) | 6.1 (3–8) | 14 | 4.6 (±1.6) | 4.3 (2.3–8) | 15 | 4.8 (±1.2) | 4.7 (3.2–6.6) |
| Δ8-THCV-COOH | |||||||||||||||
| tmax | 15 | 4.9 (±1.7) | 4 (2–8) | 14 | 4.6 (±0.9) | 4 (4–6) | 14 | 5.3 (±1.3) | 6 (4–8) | 14 | 4.7 (±1.9) | 4 (2–8) | 15 | 5.3 (±1.8) | 6 (2–8) |
| Cmax | 15 | 99 (±37) | 101 (39.2–178) | 14 | 151 (±76.8) | 116 (91.7–357) | 14 | 324 (±165) | 275 (114–765) | 14 | 756 (±302) | 699 (307–1392) | 15 | 1738 (±579) | 1899 (868–2594) |
| tlast | 15 | 8 (±0) | 8 (8–8) | 14 | 8 (±0) | 8 (8–8) | 14 | 8 (±0) | 8 (8–8) | 14 | 8 (±0) | 8 (8–8) | 15 | 8 (±0) | 8 (8–8) |
| Clast | 15 | 46.8 (±23) | 38.2 (20.1–102) | 14 | 71.6 (±33.8) | 78.3 (25.5–146) | 14 | 166 (±60.3) | 148 (78.9–264) | 14 | 319 (±118) | 304 (153–590) | 15 | 917 (±582) | 855 (364–2402) |
| AUClast | 15 | 398 (±151) | 331 (216–787) | 14 | 642 (±298) | 574 (264–1311) | 14 | 1209 (±582) | 1093 (364–2194) | 14 | 3071 (±1101) | 3132 (577–4951) | 15 | 6955 (±2671) | 5999 (3345–10759) |
| MRT | 15 | 4.9 (±0.8) | 4.9 (3.7–6.6) | 14 | 4.8 (±0.6) | 4.6 (3.8–5.9) | 14 | 5.3 (±0.9) | 5.3 (4–7.4) | 14 | 4.8 (±1) | 4.6 (3.6–6.7) | 15 | 5.1 (±1) | 5.1 (3.6–6.6) |
| Δ9-THCV | |||||||||||||||
| tmax | -- | -- | -- | -- | -- | -- | -- | -- | -- | 8 | 4.6 (±1.8) | 5 (1–6) | 13 | 4.9 (±2.3) | 4 (2–8) |
| Cmax | -- | -- | -- | -- | -- | -- | -- | -- | -- | 8 | 1.1 (±0.5) | 0.9 (0.5–1.9) | 13 | 2 (±0.9) | 1.7 (0.8–4.1) |
| tlast | -- | -- | -- | -- | -- | -- | -- | -- | -- | 8 | 4.8 (±1.5) | 5 (2–6) | 13 | 6.2 (±2.1) | 6 (2–8) |
| Clast | -- | -- | -- | -- | -- | -- | -- | -- | -- | 8 | 1.1 (±0.5) | 0.8 (0.5–1.9) | 13 | 1.5 (±1) | 1.4 (0.5–4.1) |
| AUClast | -- | -- | -- | -- | -- | -- | -- | -- | -- | 8 | 1.4 (±0.8) | 1.2 (0.5–3.1) | 13 | 4.8 (±2.9) | 4.5 (1–10.2) |
| MRT | -- | -- | -- | -- | -- | -- | -- | -- | -- | 8 | 4.4 (±1.7) | 4.4 (1.3–6) | 13 | 4.5 (±1.6) | 4.6 (2–7.2) |
| Δ9-THCV-COOH | |||||||||||||||
| tmax | 15 | 5.1 (±2) | 4 (2–8) | 14 | 4.6 (±1.5) | 4 (2–8) | 14 | 5.1 (±1.9) | 6 (2–8) | 14 | 4.5 (±2) | 4 (1–8) | 15 | 5.3 (±2.1) | 6 (2–8) |
| Cmax | 15 | 2.7 (±1.2) | 2.8 (1.1–5) | 14 | 4.2 (±1.6) | 4.4 (1.9–7.5) | 14 | 8.8 (±3.7) | 7.7 (3.7–16.1) | 14 | 22.5 (±8.3) | 20.6 (11.3–41.6) | 15 | 50.3 (±13.8) | 48.2 (31.4–71.1) |
| tlast | 15 | 6.8 (±1.7) | 8 (4–8) | 14 | 7.6 (±1.2) | 8 (4–8) | 14 | 8 (±0) | 8 (8–8) | 14 | 8 (±0) | 8 (8–8) | 15 | 8 (±0) | 8 (8–8) |
| Clast | 15 | 1.9 (±0.8) | 1.7 (1.1–3.3) | 14 | 2.6 (±1) | 2.4 (1.3–4.4) | 14 | 5.4 (±1.9) | 5.2 (2.4–8.5) | 14 | 10.8 (±3.7) | 10.5 (6.2–16.1) | 15 | 29.2 (±16.1) | 26.3 (10.9–63.8) |
| AUClast | 15 | 7.9 (±5.7) | 5.6 (1.1–20.1) | 14 | 18.3 (±8.7) | 19.6 (2.5–30.6) | 14 | 33.4 (±15.8) | 30.8 (8.7–62.7) | 14 | 92.5 (±35.3) | 86.5 (17.6–149.2) | 15 | 201 (±77.9) | 190 (89.7–336) |
| MRT | 15 | 5.1 (±1.3) | 5.3 (3.2–8) | 14 | 4.8 (±0.7) | 4.6 (3.9–6.1) | 14 | 5.5 (±1) | 5.5 (4–7.4) | 14 | 4.9 (±1) | 4.6 (3.7–7) | 15 | 5.1 (±1) | 5.2 (3.7–6.7) |
| Δ8-THC-COOH | |||||||||||||||
| tmax | -- | -- | -- | -- | -- | -- | -- | -- | -- | 8 | 5 (±1.1) | 5 (4–6) | 15 | 6.1 (±1.6) | 6 (4–8) |
| Cmax | -- | -- | -- | -- | -- | -- | -- | -- | -- | 8 | 1.4 (±0.3) | 1.4 (1–1.7) | 15 | 2.4 (±0.7) | 2.5 (1.4–3.8) |
| tlast | -- | -- | -- | -- | -- | -- | -- | -- | -- | 8 | 5.8 (±1.3) | 6 (4–8) | 15 | 7.5 (±1.2) | 8 (4–8) |
| Clast | -- | -- | -- | -- | -- | -- | -- | -- | -- | 8 | 1.3 (±0.2) | 1.3 (1–1.7) | 15 | 1.9 (±0.6) | 1.8 (1.1–2.8) |
| AUClast | -- | -- | -- | -- | -- | -- | -- | -- | -- | 8 | 2.4 (±1.5) | 1.6 (1–4.7) | 15 | 8.1 (±4.5) | 9.6 (1.4–15) |
| MRT | -- | -- | -- | -- | -- | -- | -- | -- | -- | 8 | 5.2 (±1) | 5.3 (4–6.5) | 15 | 5.7 (±1.3) | 5.8 (3.1–8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sempio, C.; Campos-Palomino, J.; Klawitter, J.; Peters, E.N.; MacNair, L.; Haghdoost, M.; Bonn-Miller, M.O.; Harrison, A.; Babalonis, S.; Christians, U.; et al. Pharmacokinetics of Oral Cannabinoid Δ8-Tetrahydrocannabivarin and Its Main Metabolites in Healthy Participants. Pharmaceuticals 2024, 17, 1603. https://doi.org/10.3390/ph17121603
Sempio C, Campos-Palomino J, Klawitter J, Peters EN, MacNair L, Haghdoost M, Bonn-Miller MO, Harrison A, Babalonis S, Christians U, et al. Pharmacokinetics of Oral Cannabinoid Δ8-Tetrahydrocannabivarin and Its Main Metabolites in Healthy Participants. Pharmaceuticals. 2024; 17(12):1603. https://doi.org/10.3390/ph17121603
Chicago/Turabian StyleSempio, Cristina, Jorge Campos-Palomino, Jelena Klawitter, Erica N. Peters, Laura MacNair, Mehdi Haghdoost, Marcel O. Bonn-Miller, Amy Harrison, Shanna Babalonis, Uwe Christians, and et al. 2024. "Pharmacokinetics of Oral Cannabinoid Δ8-Tetrahydrocannabivarin and Its Main Metabolites in Healthy Participants" Pharmaceuticals 17, no. 12: 1603. https://doi.org/10.3390/ph17121603
APA StyleSempio, C., Campos-Palomino, J., Klawitter, J., Peters, E. N., MacNair, L., Haghdoost, M., Bonn-Miller, M. O., Harrison, A., Babalonis, S., Christians, U., & Klawitter, J. (2024). Pharmacokinetics of Oral Cannabinoid Δ8-Tetrahydrocannabivarin and Its Main Metabolites in Healthy Participants. Pharmaceuticals, 17(12), 1603. https://doi.org/10.3390/ph17121603







