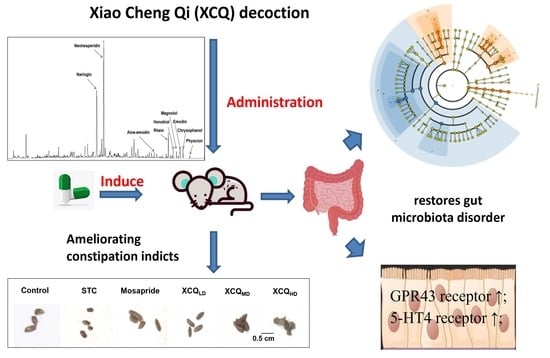
Xiao Cheng Qi Decoction, an Ancient Chinese Herbal Mixture, Relieves Loperamide-Induced Slow-Transit Constipation in Mice: An Action Mediated by Gut Microbiota
Abstract
:1. Introduction
2. Results
2.1. Effects of XCQ on Constipation Indices in STC Mice
2.2. XCQ Restores Gut Microbiota Disorder of STC Mice
2.3. XCQ Restores the Levels of 5-HT, 5-HT4, and GPR43 Receptors in STC Mice
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Preparation of XCQ
3.3. Qualitative Analysis of XCQ Extract via HPLC
3.4. Experimental Environment
3.5. Animal Experimental Design
3.6. Measurement of Constipation-Related Indicators and Pathological Analysis
3.7. Quantification of Short-Chain Fatty Acids in Feces
3.8. Bacterial Community Analysis Based on 16S rRNA Gene
3.9. Western Blotting Assay
3.10. Immunohistochemistry Assay
3.11. Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Camilleri, M.; Ford, A.C.; Mawe, G.M.; Dinning, P.G.; Rao, S.S.; Chey, W.D.; Simrén, M.; Lembo, A.; Young-Fadok, T.M.; Chang, L. Chronic constipation. Nat. Rev. Dis. Primer 2017, 3, 17095. [Google Scholar] [CrossRef] [PubMed]
- Wang, H. Understanding the pathogenesis of slow-transit constipation: One step forward. Dig. Dis. Sci. 2015, 60, 2216–2218. [Google Scholar] [CrossRef] [PubMed]
- Johanson, J.F. Review of the treatment options for chronic constipation. Med. Gen. Med. 2007, 9, 25. [Google Scholar]
- Yarullina, D.R.; Shafigullin, M.U.; Sakulin, K.A.; Arzamastseva, A.A.; Shaidullov, I.F.; Markelova, M.I.; Grigoryeva, T.V.; Karpukhin, O.Y.; Sitdikova, G.F. Characterization of gut contractility and microbiota in patients with severe chronic constipation. PLoS ONE 2020, 15, 0235985. [Google Scholar] [CrossRef] [PubMed]
- Dimidi, E.; Christodoulides, S.; Scott, S.M.; Whelan, K. Mechanisms of action of probiotics and the gastrointestinal microbiota on gut motility and constipation. Adv. Nutr. 2017, 8, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, M.; Shang, W.; Ma, Q.; Strappe, P.; Zhou, Z. Abundance of probiotics and butyrate-production microbiome manages constipation via short-chain fatty acids production and hormones secretion. Mol. Nutr. Food. Res. 2019, 63, 1801187. [Google Scholar] [CrossRef] [PubMed]
- Segers, A.; Desmet, L.; Thijs, T.; Verbeke, K.; Tack, J.; Depoortere, I. The circadian clock regulates the diurnal levels of microbial short-chain fatty acids and their rhythmic effects on colon contractility in mice. Acta Physiol. 2019, 225, 13193. [Google Scholar] [CrossRef]
- Gershon, M.D.; Tack, J. The serotonin signaling system: From basic understanding to drug development for functional GI disorders. Gastroenterology 2007, 132, 397–414. [Google Scholar] [CrossRef]
- Li, W.; Meng, Y.; Chen, H.; Chen, H.; Huang, X.; Yang, M.; Yang, W. Research overview of Xiaochengqi decoction. J. Exp. Formulae 2020, 26, 10. [Google Scholar]
- Yin, D.; Cai, M.; Hu, X.; Wang, X.; Liu, M.; Zhu, R.; Fu, T.; Dong, X.; Ni, J.; Yin, X. Research progress on pharmacodynamic components and clinical application of Xiaochengqi Decoction. J. Liaoning Univ. Tradit. Chin. Med. 2023, 25, 8. [Google Scholar]
- Gao, C.C.; Li, G.W.; Wang, T.T.; Gao, L.; Wang, F.F.; Shang, H.W.; Yang, Z.J.; Guo, Y.X.; Wang, B.Y.; Xu, J.D. Rhubarb extract relieves constipation by stimulating mucus production in the colon and altering the intestinal flora. Biomed. Pharmacother. 2021, 138, 111479. [Google Scholar] [CrossRef] [PubMed]
- Lemli, J. Metabolism of sennosides–an overview. Pharmacology 1988, 36 (Suppl. S1), 126–128. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Li, Y.; Gu, Y. Hesperidin improves colonic motility in loeramide-induced constipation rat model via 5-hydroxytryptamine 4R/cAMP signaling pathway. Digestion 2020, 101, 692–705. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Wu, C.; Wei, J.; Xian, M.; Wang, T.; Yang, B.; Chen, M. An orally administered magnoloside A ameliorates functional dyspepsia by modulating brain-gut peptides and gut microbiota. Life Sci. 2019, 233, 116749. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.P.; Yuan, X.B.; Cheng, G.; Jiao, S.M.; Feng, C.; Zhao, X.M.; Yin, H.; Du, Y.; Liu, H. Chitosan oligosaccharides improve the disturbance in glucose metabolism and reverse the dysbiosis of gut microbiota in diabetic mice. Carbohydr. Polym. 2018, 190, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2017, 23, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Routy, B.; le Chatelier, E.; DeRosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Reynaud, Y.; Fakhry, J.; Fothergill, L.; Callaghan, B.; Ringuet, M.; Hunne, B.; Bravo, D.M.; Furness, J.B. The chemical coding of 5-hydroxytryptamine containing enteroendocrine cells in the mouse gastrointestinal tract. Cell Tissue Res. 2016, 364, 489–497. [Google Scholar] [CrossRef]
- Ren, X.; Liu, L.; Gamallat, Y.; Zhang, B.; Xin, Y. Enteromorpha and polysaccharides from enteromorpha ameliorate loperamide-induced constipation in mice. Biomed. Pharmacother. 2017, 96, 1075–1081. [Google Scholar] [CrossRef]
- Wang, L.; Hu, L.; Xu, Q.; Yin, B.; Fang, D.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Bifidobacterium adolescentis exerts strain-specific effects on constipation induced by loperamide in BALB/c mice. Int. J. Mol. Sci. 2017, 18, 318. [Google Scholar] [CrossRef]
- Zhang, R.; Zhu, X.; Bai, H.; Ning, K. Network pharmacology databases for traditional Chinese medicine: Review and assessment. Front. Pharmacol. 2019, 10, 123. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Shi, Y.; Ye, L.; Yang, Q.; Li, C.; Chen, X.; Jing, Y. Near-infrared spectroscopy for rapid and simultaneous determination of five main active components in rhubarb of different geographical origins and processing. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 205, 419–427. [Google Scholar] [CrossRef]
- Xiao, P.; He, L.; Wang, L. Ethnopharmacologic study of Chinese rhubarb. J Ethnopharmacol 1984, 10, 275–293. [Google Scholar] [CrossRef] [PubMed]
- Suntar, I.; Khan, H.; Patel, S.; Celano, R.; Rastrelli, L. An overview on Citrus aurantium L.: Its functions as food ingredient and therapeutic agent. Oxid. Med. Cell. Longev. 2018, 2018, 7864269. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Liu, J.; Shen, P.; Cai, J.; Han, Y.; Zhu, K.; Fu, Y.; Zhang, N.; Zhang, Z.; Cao, Y. Protective effect of naringin on DSS-induced ulcerative colitis in mice. J. Agric. Food Chem. 2018, 66, 13133–13140. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Yi, L.T.; Pan, Y.; Wang, X.; Li, Y.C.; Li, J.M.; Wang, C.P.; Kong, L.D. Antidepressant-like effects of the mixture of honokiol and magnolol from the barks of Magnolia officinalis in stressed rodents. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, A.; Ohno, K.; Maeda, S.; Nakashima, K.; Fukushima, K.; Fujino, Y.; Tsujimoto, H. Prokinetic effect of the 5-HT4R agonist mosapride on canine gastric motility. J. Vet. Med. Sci. 2011, 73, 1635–1637. [Google Scholar] [CrossRef] [PubMed]
- Wouters, M.M.; Farrugia, G.; Schemann, M. 5-HT receptors on interstitial cells of Cajal, smooth muscle and enteric nerves. Neurogastroenterol. Motil. 2007, 19 (Suppl. S2), 5–12. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef]
- Yi, R.; Peng, P.; Zhang, J.; Du, M.; Lan, L.; Qian, Y.; Zhou, J.; Zhao, X. Lactobacillus plantarum CQPCo2-fermented soybean milk improves loperamide-induced constipation in mice. J. Med. Food 2019, 22, 1208–1221. [Google Scholar] [CrossRef]
- Chai, M.; Wang, L.; Li, X.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. Different Bifidobacterium bifidum strains change the intestinal flora composition of mice via different mechanisms to alleviate loperamide-induced constipation. Food Funct. 2021, 12, 6058–6069. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, S.; Tatewaki, M.; Yamada, T.; Fujimiya, M.; Mantyh, C.; Voss, M.; Eubanks, S.; Harris, M.; Pappas, T.N.; Takahashi, T. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Akiba, Y.; Kaunitz, J.D. Duodenal chemosensing of short-chain fatty acids: Implications for GI diseases. Curr. Gastroenterol. Rep. 2019, 21, 35. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, C.; Monaghan, P.J.; Morris, J.; Issa, B.; Whorwell, P.J.; Keevil, B.; Houghton, L.A. Rome III functional constipation and irritable bowel syndrome with constipation are similar disorders within a spectrum of sensitization, regulated by serotonin. Gastroenterology 2013, 145, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Costedio, M.M.; Coates, M.D.; Brooks, E.M.; Glass, L.M.; Ganguly, E.K.; Blaszyk, H.; Ciolino, A.L.; Wood, M.J.; Strader, D.; Hyman, N.H.; et al. Mucosal serotonin signaling is altered in chronic constipation but not in opiate-induced constipation. Am. J. Gastroenterol. 2010, 105, 1173–1180. [Google Scholar] [CrossRef]
- Wei, L.; Singh, R.; Ha, S.E.; Martin, A.M.; Jones, L.A.; Jin, B.; Jorgensen, B.G.; Zogg, H.; Chervo, T.; Gottfried-Blackmore, A.; et al. Serotonin deficiency is associated with delayed gastric emptying. Gastroenterology 2021, 160, 2451–2466. [Google Scholar] [CrossRef]
- Qiu, B.; Zhu, L.; Zhang, S.; Han, S.; Fei, Y.; Ba, F.; Berglund, B.; Li, L.; Yao, M. Prevention of loperamide-induced constipation in mice and alteration of 5-hydroxytryotamine signaling by Ligilactobacillus salivarius Li01. Nutrients 2022, 14, 4083. [Google Scholar] [CrossRef]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuohongerbieke, A.; Wang, H.; Wu, J.; Wang, Z.; Dong, T.; Huang, Y.; Zhu, D.; Sun, D.; Tsim, K.W.K. Xiao Cheng Qi Decoction, an Ancient Chinese Herbal Mixture, Relieves Loperamide-Induced Slow-Transit Constipation in Mice: An Action Mediated by Gut Microbiota. Pharmaceuticals 2024, 17, 153. https://doi.org/10.3390/ph17020153
Tuohongerbieke A, Wang H, Wu J, Wang Z, Dong T, Huang Y, Zhu D, Sun D, Tsim KWK. Xiao Cheng Qi Decoction, an Ancient Chinese Herbal Mixture, Relieves Loperamide-Induced Slow-Transit Constipation in Mice: An Action Mediated by Gut Microbiota. Pharmaceuticals. 2024; 17(2):153. https://doi.org/10.3390/ph17020153
Chicago/Turabian StyleTuohongerbieke, Amanguli, Huaiyou Wang, Jiahui Wu, Zhengqi Wang, Tingxia Dong, Yamiao Huang, Dequan Zhu, Dongmei Sun, and Karl Wah Keung Tsim. 2024. "Xiao Cheng Qi Decoction, an Ancient Chinese Herbal Mixture, Relieves Loperamide-Induced Slow-Transit Constipation in Mice: An Action Mediated by Gut Microbiota" Pharmaceuticals 17, no. 2: 153. https://doi.org/10.3390/ph17020153
APA StyleTuohongerbieke, A., Wang, H., Wu, J., Wang, Z., Dong, T., Huang, Y., Zhu, D., Sun, D., & Tsim, K. W. K. (2024). Xiao Cheng Qi Decoction, an Ancient Chinese Herbal Mixture, Relieves Loperamide-Induced Slow-Transit Constipation in Mice: An Action Mediated by Gut Microbiota. Pharmaceuticals, 17(2), 153. https://doi.org/10.3390/ph17020153