Molecular Imaging of Fibrosis in Benign Diseases: An Overview of the State of the Art
Abstract
:1. Introduction
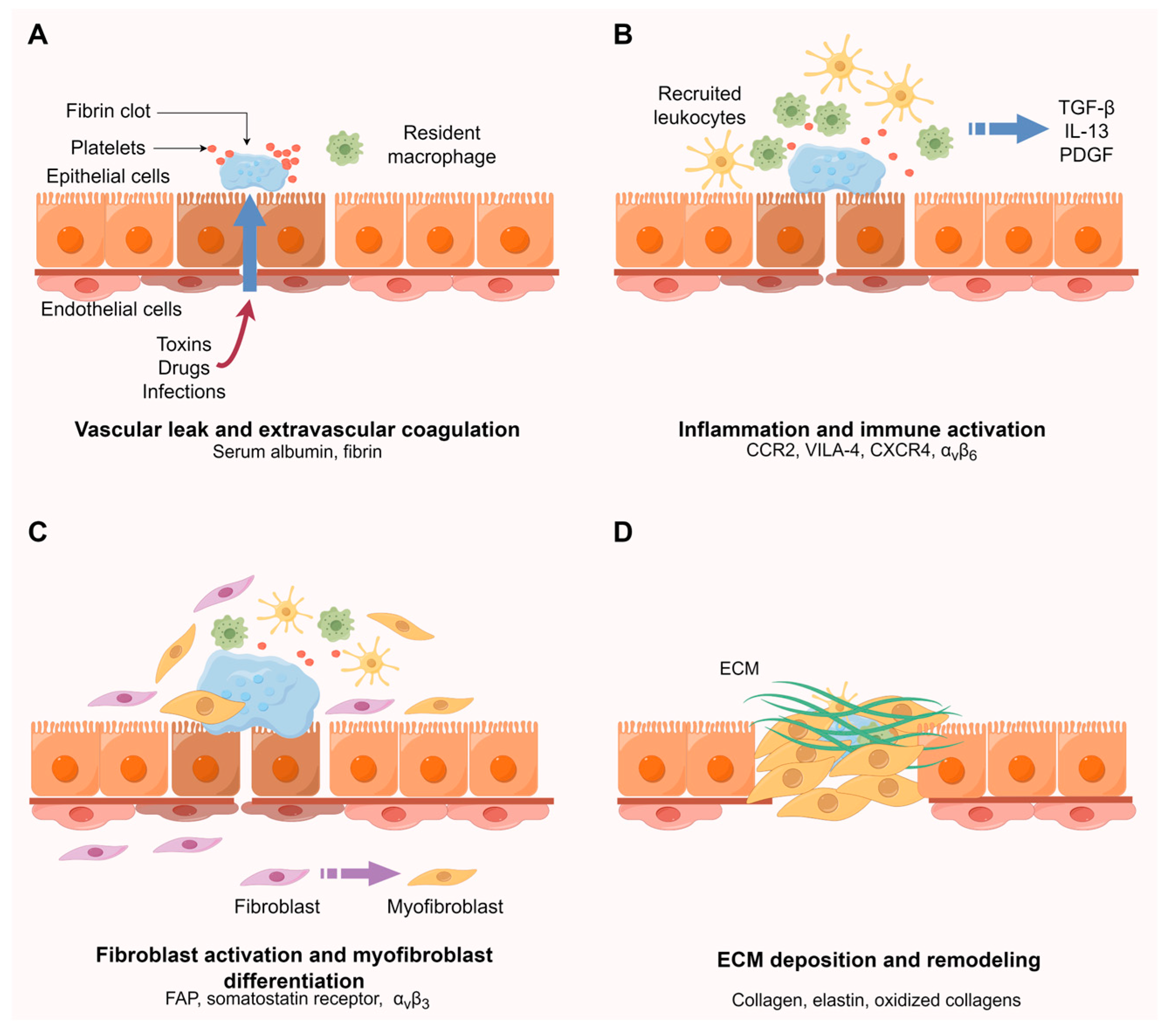
2. Molecular Mechanisms of Fibrosis
3. Molecular Probes for Imaging of Fibrosis and Fibrogenesis
| Probe | References | Molecular Process | Molecular/Cell Target | Stage of Development | Imaging Type | Disease | Potential Clinical Use |
|---|---|---|---|---|---|---|---|
| Gadofosveset | [31] | Vascular leak | Serum albumin | Human studies (FDA-approved) | MRI | Pulmonary fibrosis | Disease activity |
| EP-2104R | [32,33] | Extravascular coagulation | Fibrin | Animal studies | MRI | Pulmonary and liver fibrosis | Disease activity, treatment response |
| [64Cu]Cu-DOTA-ECL1i | [34] | Macrophage | CCR2 | Animal and human studies | PET | Pulmonary fibrosis | Diagnosis, disease activity, treatment response |
| BMV109/BMV101 | [35] | Macrophage | Cysteine cathepsin | Animal and human studies | PET | Pulmonary fibrosis | Disease activity |
| [64Cu]Cu-LLP2A | [36,37] | Recruitment of immune cells | VLA-4 | Animal studies | PET | Pulmonary fibrosis | Disease activity |
| [68Ga]Ga-pentixafor | [38,39] | Recruitment of immune cells | CXCR4 | Animal and human studies | PET | Pulmonary and myocardial fibrosis | Disease activity, treatment response, outcome prediction |
| A20FMDV2 | [40,41,42,43] | Activation of TGFβ | Integrin αvβ6 | Animal and human studies | PET and SPECT | Pulmonary fibrosis | Disease activity, treatment response |
| Knottin | [44] | Activation of TGFβ | Integrin αvβ6 | Animal and human studies | PET | Pulmonary fibrosis | Disease activity, treatment response |
| [68Ga]Ga-FAPI-04/46 | [45,46,47,48,49,50,51,52,53,54,55,56,57,58,59] | Activated fibroblasts | FAP | Animal and human studies | PET | Cardiac diseases, IgG4-RD, renal fibrosis, pulmonary fibrosis, and liver fibrosis | Diagnosis, disease activity, treatment response |
| [68Ga]Ga-MHLL1 | [60] | Activated fibroblasts | FAP | Animal studies | PET | Myocardial infarction | Diagnosis, disease activity |
| [111In]In-octreotide scintigraphy | [61,62] | Activated fibroblasts | Somatostatin receptor | FDA-approved | SPECT | Pulmonary fibrosis | Diagnosis |
| [68Ga]Ga-DOTANOC | [63] | Activated fibroblasts | Somatostatin receptor | Human studies | PET | Pulmonary fibrosis | Disease activity |
| [99mTc]Tc-cRGD | [64] | Activated HSCs | Integrin αvβ3 | Animal studies | SPECT | Liver fibrosis | Disease activity |
| [99mTc]Tc-3PRGD2 | [65] | Activated HSCs | Integrin αvβ3 | Animal studies | SPECT | Liver fibrosis | Disease activity, treatment response |
| [18F]-Alfatide | [66] | Activated HSCs | Integrin αvβ3 | Animal studies | PET | Liver fibrosis | Disease activity |
| [18F]FPP-RGD2 | [67,68] | Activated HSCs | Integrin αvβ3 | Animal studies | PET | Liver and pulmonary fibrosis | Disease activity |
| RGD-USPIO | [69] | Activated HSCs | Integrin αvβ3 | Animal studies | MRI | Liver fibrosis | Disease activity |
| Den-RGD | [70] | Activated HSCs | Integrin αvβ3 | Animal studies | MRI | Liver fibrosis | Disease activity |
| [99mTc]Tc-CRIP | [71,72] | Myofibroblasts | Integrin αvβ3 | Animal studies | SPECT | Myocardial fibrosis | Disease activity, treatment response |
| EP-3533 | [73,74,75,76,77,78,79,80] | Collagen deposition | Type I collagen | Animal studies | MRI | Myocardial, liver and pulmonary fibrosis | Diagnosis, disease activity, treatment response |
| CM-101 | [81,82] | Collagen deposition | Type I collagen | Animal studies | MRI | Liver and post-chemotherapy fibrosis | Disease activity |
| ProCA32.collagen1 | [83] | Collagen deposition | Type I collagen | Animal studies | MRI | Liver fibrosis | Disease activity |
| SNIO-CBP | [84] | Collagen deposition | Type I collagen | Animal studies | MRI | Liver fibrosis | Diagnosis and disease activity |
| [68Ga]Ga-CBP8 | [85] | Collagen deposition | Type I collagen | Animal studies | PET | Pulmonary fibrosis | Disease activity, treatment response |
| [64Cu]Cu-CBP7 | [86] | Collagen deposition | Type I collagen | Animal studies | PET | Pulmonary fibrosis | Disease activity |
| [99mTc]Tc-CBP1495 | [87] | Collagen deposition | Type I collagen | Animal studies | SPECT | Pulmonary and liver fibrosis | Disease activity |
| Collagelin | [88,89,90] | Collagen deposition | Type I and III collagen | Animal studies | SPECT and PET | Myocardial, pulmonary and liver fibrosis animal | Disease activity |
| PVD | [91] | ECM deposition | Type I collagen | Animal studies | NIRF | Pulmonary fibrosis | Disease activity |
| CNA35-AuNPs | [92,93] | ECM deposition | Type I collagen | Animal studies | CT | Myocardial fibrosis | Disease activity |
| CNA35-Cy7 | [94] | ECM deposition | Type I collagen | Animal studies | CT–fluorescence imaging | Renal fibrosis | Disease activity |
| CNA35-PFP NPs | [95]. | ECM deposition | Type I collagen | Animal studies | Ultrasound | Myocardial fibrosis | Disease activity |
| ESMA | [96,97,98,99] | ECM deposition | Elastin | Animal and human studies | MRI | Myocardial, renal and liver fibrosis animal | Disease activity, treatment response |
| [89Zr]Zr-pro-MMP-9 F(ab’)2 | [21] | ECM deposition | MMPs | Animal studies | PET | Intestinal fibrosis | Disease activity |
| [18F]MAGL-4-11 | [100] | ECM deposition | MAGL | Animal studies | PET | Liver fibrosis | Disease activity |
| Gd-Hyd | [76,101] | Crosslinking | Allysine aldehyde of oxidized collagens | Animal studies | MRI | Pulmonary and liver fibrosis | Diagnosis, disease activity, treatment response |
| Gd-CHyd | [12] | Crosslinking | Allysine aldehyde of oxidized collagens | Animal studies | MRI | Pulmonary fibrosis | Disease activity |
| Gd-1,4 | [102] | Crosslinking | Allysine aldehyde of oxidized collagens | Animal studies | MRI | Liver fibrosis | Disease activity |
| Gd-OA | [103] | Crosslinking | Allysine aldehyde of oxidized collagens | Animal studies | MRI | Pulmonary fibrosis | Disease activity |
| HTCDGd | [104] | Crosslinking | Allysine aldehyde of oxidized collagens | Animal studies | MRI and fluorescence imaging | Liver fibrosis | Disease activity, diagnosis |
3.1. Vascular Leak and Extravascular Coagulation
3.2. Inflammation and Immune Activation
3.3. Fibroblast Activation and Myofibroblast Differentiation
3.3.1. Targeting of Fibroblast Activation Protein
3.3.2. Targeting of Somatostatin Receptor
3.3.3. Targeting of Integrin αvβ3
3.4. ECM Deposition and Remodeling
3.4.1. Targeting of Collagen
3.4.2. Other Targets Associated with ECM Deposition
3.4.3. Targeting of Oxidized Collagens
4. Current Challenges and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ΔR2* | T2* relaxation rate change |
| [18F]FDG | 2-Deoxy-2-[fluorine-18]-fluoro-d-glucose |
| α-SMA | α-smooth muscle actin |
| AI | Artificial intelligence |
| AuNP | Gold nanoparticle |
| BDL | Bile duct ligation |
| CAF | Cancer-associated fibroblast |
| CCL2 | C-C motif chemokine ligand 2 |
| CCl4 | Carbon tetrachloride |
| CCR2 | C-C motif chemokine receptor 2 |
| CDAHFD | Choline-deficient, L-amino acid-defined, high-fat diet |
| CKD | Chronic kidney disease |
| CMR | Cardiac magnetic resonance |
| CT | Computed tomography |
| CT-FMT | Hybrid computed tomography–fluorescence molecular tomography |
| COPD | Chronic obstructive pulmonary disease |
| CXCR4 | CXC-motif receptor 4 |
| DPP | Dipeptidyl peptidase |
| ECL1 | Extracellular loop one |
| ECM | Extracellular matrix |
| EMT | Epithelial–mesenchymal transition |
| EndMT | Endothelial–mesenchymal transition |
| FAP | Fibroblast activation protein |
| FAPI | Fibroblast activation protein inhibition |
| FDA | Food and Drug Administration |
| fILD | Fibrotic interstitial lung diseases |
| FITC | Fluorescein isothiocyanate |
| Gd | Gadolinium |
| GF | Growth factor |
| GPVI | Glycoprotein VI |
| HRCT | High-resolution computed tomography |
| HSC | Hepatic stellate cell |
| LC | Lung cancer |
| LDBVL | Low-dose bleomycin vascular leak |
| LGE | Late gadolinium enhancement |
| LIFU | Low-intensity focused ultrasound |
| IL-13 | Interleukin 13 |
| LOX | Lysyl oxidase |
| LOXL | Lysyl oxidase-like protein |
| IPF | Idiopathic pulmonary fibrosis |
| MAGL | Monoacylglycerol lipase |
| MCT | Monocrotaline |
| MI | Myocardial infarction |
| MMP | Matrix metalloproteinase |
| MRI | Magnetic resonance imaging |
| NASH | Non-alcoholic steatohepatitis |
| NIRF | Near-infrared fluorescence |
| PDGF | Platelet-derived growth factor |
| PET | Positron emission tomography |
| PFD | Antifibrotic pirfenidone |
| RGD | Arginine-glycine-aspartic |
| SLE | Systemic lupus erythematosus |
| SPECT | Single-photon emission computed tomography |
| SSc | Systemic sclerosis |
| SSTR | Somatostatin receptor |
| SUV | Standardized uptake values |
| TAA | Thioacetamide |
| TBR | Target-to-background ratio |
| TGF-β | Transforming growth factor beta |
| USPIO | Ultrasmall superparamagnetic iron oxide nanoparticle |
| UTE | Ultrashort echo time |
| VT | Volume of distribution |
| VCAM-1 | Vascular cell adhesion molecule-1 |
| VLA-4 | Very late antigen-4 |
References
- Borthwick, L.A.; Wynn, T.A.; Fisher, A.J. Cytokine mediated tissue fibrosis. Biochim. Et Biophys. Acta-Mol. Basis Dis. 2013, 1832, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Urban, M.L.; Manenti, L.; Vaglio, A. Fibrosis—A Common Pathway to Organ Injury and Failure. N. Engl. J. Med. 2015, 373, 95–96. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, M. Fibrosis and diseases of the eye. J. Clin. Investig. 2007, 117, 576–586. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat. Rev. Immunol. 2004, 4, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Chang, X.; Shen, L.J.; Zhang, C.L.; Fan, Y.T.; Cho, C.S.; Zhang, Z.Q.; Jiang, H.L. Progress in drug delivery system for fibrosis therapy. Asian J. Pharm. Sci. 2021, 16, 47–61. [Google Scholar] [CrossRef]
- Montesi, S.B.; Désogère, P.; Fuchs, B.C.; Caravan, P. Molecular imaging of fibrosis: Recent advances and future directions. J. Clin. Investig. 2019, 129, 24–33. [Google Scholar] [CrossRef]
- Hood, L.; Friend, S.H. Predictive, personalized, preventive, participatory (P4) cancer medicine. Nat. Rev. Clin. Oncol. 2011, 8, 184–187. [Google Scholar] [CrossRef]
- Smith, M.; Dalurzo, M.; Panse, P.; Parish, J.; Leslie, K. Usual interstitial pneumonia-pattern fibrosis in surgical lung biopsies. Clinical, radiological and histopathological clues to aetiology. J. Clin. Pathol. 2013, 66, 896–903. [Google Scholar] [CrossRef]
- Lynch, D.A.; Sverzellati, N.; Travis, W.D.; Brown, K.K.; Colby, T.V.; Galvin, J.R.; Goldin, J.G.; Hansell, D.M.; Inoue, Y.; Johkoh, T.; et al. Diagnostic criteria for idiopathic pulmonary fibrosis: A Fleischner Society White Paper. Lancet Respir. Med. 2018, 6, 138–153. [Google Scholar] [CrossRef]
- Ordovas, K.G.; Higgins, C.B. Delayed contrast enhancement on MR images of myocardium: Past, present, future. Radiology 2011, 261, 358–374. [Google Scholar] [CrossRef]
- Akam, E.A.; Abston, E.; Rotile, N.J.; Slattery, H.R.; Zhou, I.Y.; Lanuti, M.; Caravan, P. Improving the reactivity of hydrazine-bearing MRI probes for in vivo imaging of lung fibrogenesis. Chem. Sci. 2020, 11, 224–231. [Google Scholar] [CrossRef]
- Thakur, M.L.; Lentle, B.C. Joint SNM/RSNA Molecular Imaging Summit Statement. J. Nucl. Med. 2005, 46, 11n–13n, 42n. [Google Scholar]
- Rowe, S.P.; Pomper, M.G. Molecular imaging in oncology: Current impact and future directions. CA Cancer J. Clin. 2022, 72, 333–352. [Google Scholar] [CrossRef]
- Klinkhammer, B.M.; Lammers, T.; Mottaghy, F.M.; Kiessling, F.; Floege, J.; Boor, P. Non-invasive molecular imaging of kidney diseases. Nat. Rev. Nephrol. 2021, 17, 688–703. [Google Scholar] [CrossRef]
- Wynn, T.A. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J. Clin. Investig. 2007, 117, 524–529. [Google Scholar] [CrossRef]
- Talbott, H.E.; Mascharak, S.; Griffin, M.; Wan, D.C.; Longaker, M.T. Wound healing, fibroblast heterogeneity, and fibrosis. Cell Stem Cell 2022, 29, 1161–1180. [Google Scholar] [CrossRef]
- Wynn, T.A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-wound Healing Phenotypes. Front. Physiol. 2018, 9, 419. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, N.; Shea, B.S.; Tager, A.M. New therapeutic targets in idiopathic pulmonary fibrosis. Aiming to rein in runaway wound-healing responses. Am. J. Respir. Crit. Care Med. 2014, 190, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Dmochowska, N.; Tieu, W.; Keller, M.D.; Hollis, C.A.; Campaniello, M.A.; Mavrangelos, C.; Takhar, P.; Hughes, P.A. Zr-89-pro-MMP-9 F(ab‘)(2) detects colitis induced intestinal and kidney fibrosis. Sci. Rep. 2020, 10, 20372. [Google Scholar] [CrossRef] [PubMed]
- Micallef, L.; Vedrenne, N.; Billet, F.; Coulomb, B.; Darby, I.A.; Desmoulière, A. The myofibroblast, multiple origins for major roles in normal and pathological tissue repair. Fibrogenesis Tissue Repair 2012, 5, S5. [Google Scholar] [CrossRef] [PubMed]
- Marconi, G.D.; Fonticoli, L.; Rajan, T.S.; Pierdomenico, S.D.; Trubiani, O.; Pizzicannella, J.; Diomede, F. Epithelial-Mesenchymal Transition (EMT): The Type-2 EMT in Wound Healing, Tissue Regeneration and Organ Fibrosis. Cells 2021, 10, 1587. [Google Scholar] [CrossRef] [PubMed]
- Jonckheere, S.; Adams, J.; De Groote, D.; Campbell, K.; Berx, G.; Goossens, S. Epithelial-Mesenchymal Transition (EMT) as a Therapeutic Target. Cells Tissues Organs 2022, 211, 157–182. [Google Scholar] [CrossRef]
- Désogère, P.; Montesi, S.B.; Caravan, P. Molecular Probes for Imaging Fibrosis and Fibrogenesis. Chemistry 2019, 25, 1128–1141. [Google Scholar] [CrossRef]
- Nastase, M.V.; Zeng-Brouwers, J.; Wygrecka, M.; Schaefer, L. Targeting renal fibrosis: Mechanisms and drug delivery systems. Adv. Drug Deliv. Rev. 2018, 129, 295–307. [Google Scholar] [CrossRef]
- Kircher, M.F.; Willmann, J.K. Molecular body imaging: MR imaging, CT, and US. part I. principles. Radiology 2012, 263, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Sawall, S.; Amato, C.; Klein, L.; Wehrse, E.; Maier, J.; Kachelrieß, M. Toward molecular imaging using spectral photon-counting computed tomography? Curr. Opin. Chem. Biol. 2021, 63, 163–170. [Google Scholar] [CrossRef]
- He, H.; Zhang, X.; Du, L.; Ye, M.; Lu, Y.; Xue, J.; Wu, J.; Shuai, X. Molecular imaging nanoprobes for theranostic applications. Adv. Drug Deliv. Rev. 2022, 186, 114320. [Google Scholar] [CrossRef]
- James, M.L.; Gambhir, S.S. A molecular imaging primer: Modalities, imaging agents, and applications. Physiol. Rev. 2012, 92, 897–965. [Google Scholar] [CrossRef]
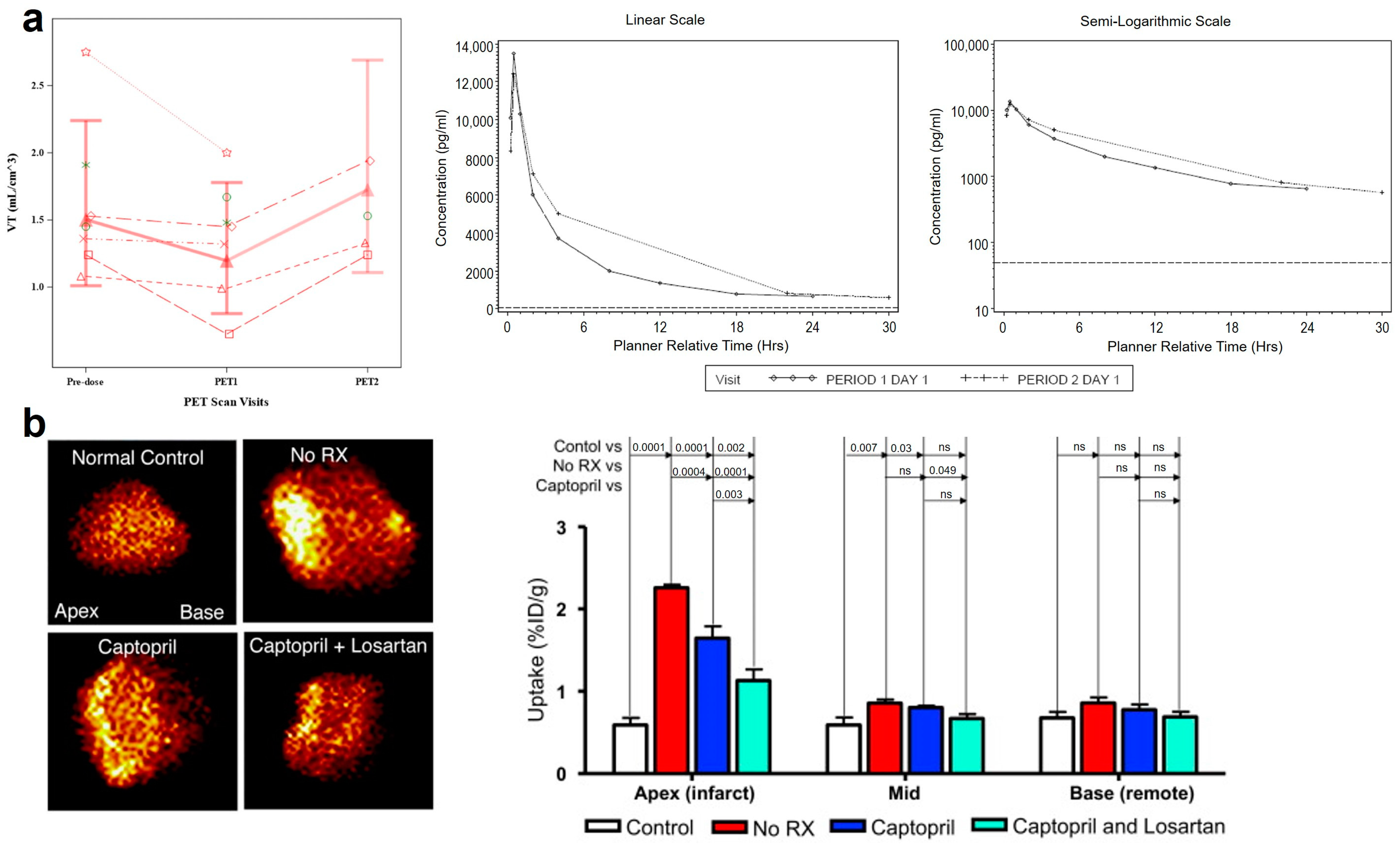
- Montesi, S.B.; Rao, R.; Liang, L.L.; Goulart, H.E.; Sharma, A.; Digumarthy, S.R.; Shea, B.S.; Seethamraju, R.T.; Caravan, P.; Tager, A.M. Gadofosveset-enhanced lung magnetic resonance imaging to detect ongoing vascular leak in pulmonary fibrosis. Eur. Respir. J. 2018, 51, 1800171. [Google Scholar] [CrossRef]
- Shea, B.S.; Probst, C.K.; Brazee, P.L.; Rotile, N.J.; Blasi, F.; Weinreb, P.H.; Black, K.E.; Sosnovik, D.E.; Van Cott, E.M.; Violette, S.M.; et al. Uncoupling of the profibrotic and hemostatic effects of thrombin in lung fibrosis. JCI Insight 2017, 2, e86608. [Google Scholar] [CrossRef] [PubMed]
- Atanasova, I.; Sojoodi, M.; Leitão, H.S.; Shuvaev, S.; Geraldes, C.; Masia, R.; Guimaraes, A.S.; Tanabe, K.K.; Fuchs, B.C.; Caravan, P. Molecular Magnetic Resonance Imaging of Fibrin Deposition in the Liver as an Indicator of Tissue Injury and Inflammation. Investig. Radiol. 2020, 55, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Brody, S.L.; Gunsten, S.P.; Luehmann, H.P.; Sultan, D.H.; Hoelscher, M.; Heo, G.S.; Pan, J.H.; Koenitzer, J.R.; Lee, E.C.; Huang, T.; et al. Chemokine Receptor 2-targeted Molecular Imaging in Pulmonary Fibrosis A Clinical Trial. Am. J. Respir. Crit. Care Med. 2021, 203, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Withana, N.P.; Ma, X.; McGuire, H.M.; Verdoes, M.; van der Linden, W.A.; Ofori, L.O.; Zhang, R.; Li, H.; Sanman, L.E.; Wei, K.; et al. Non-invasive Imaging of Idiopathic Pulmonary Fibrosis Using Cathepsin Protease Probes. Sci. Rep. 2016, 6, 19755. [Google Scholar] [CrossRef]
- Zhu, Q.; Barnes, C.E.; Mannes, P.Z.; Latoche, J.D.; Day, K.E.; Nedrow, J.R.; Novelli, E.M.; Anderson, C.J.; Tavakoli, S. Targeted imaging of very late antigen-4 for noninvasive assessment of lung inflammation-fibrosis axis. EJNMMI Res. 2023, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- Haddad, J.; Latoche, J.D.; Nigam, S.; Bellavia, M.C.; Day, K.E.; Zhu, Q.; Edwards, W.B.; Anderson, C.J.; Tavakoli, S. Molecular Imaging of Very Late Antigen-4 in Acute Lung Injury. J. Nucl. Med. 2021, 62, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Glasenapp, A.; Derlin, K.; Gutberlet, M.; Hess, A.; Ross, T.L.; Wester, H.J.; Bengel, F.M.; Thackeray, J.T. Molecular Imaging of Inflammation and Fibrosis in Pressure Overload Heart Failure. Circ. Res. 2021, 129, 369–382. [Google Scholar] [CrossRef]
- Derlin, T.; Jaeger, B.; Jonigk, D.; Apel, R.M.; Freise, J.; Shin, H.O.; Weiberg, D.; Warnecke, G.; Ross, T.L.; Wester, H.J.; et al. Clinical Molecular Imaging of Pulmonary CXCR4 Expression to Predict Outcome of Pirfenidone Treatment in Idiopathic Pulmonary Fibrosis. Chest 2021, 159, 1094–1106. [Google Scholar] [CrossRef]
- John, A.; Luckett, J.; Awas, R.; Habgood, A.; Ludbrook, S.; Blanchard, A.; Perkins, A.; Jenkins, R.; Marshall, J. S66 Targeted in Vivo Imaging of the αvβ6 Integrin in Mice with Bleomycin-Induced Lung Fibrosis. Thorax 2012, 67, A33. [Google Scholar] [CrossRef]
- John, A.E.; Luckett, J.C.; Tatler, A.L.; Awais, R.O.; Desai, A.; Habgood, A.; Ludbrook, S.; Blanchard, A.D.; Perkins, A.C.; Jenkins, R.G.; et al. Preclinical SPECT/CT imaging of αvβ6 integrins for molecular stratification of idiopathic pulmonary fibrosis. J. Nucl. Med. 2013, 54, 2146–2152. [Google Scholar] [CrossRef] [PubMed]
- Lukey, P.T.; Coello, C.; Gunn, R.; Parker, C.; Wilson, F.J.; Saleem, A.; Garman, N.; Costa, M.; Kendrick, S.; Onega, M.; et al. Clinical quantification of the integrin αvβ6 by [(18)F]FB-A20FMDV2 positron emission tomography in healthy and fibrotic human lung (PETAL Study). Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 967–979. [Google Scholar] [CrossRef]
- Maher, T.M.; Simpson, J.K.; Porter, J.C.; Wilson, F.J.; Chan, R.; Eames, R.; Cui, Y.; Siederer, S.; Parry, S.; Kenny, J.; et al. A positron emission tomography imaging study to confirm target engagement in the lungs of patients with idiopathic pulmonary fibrosis following a single dose of a novel inhaled αvβ6 integrin inhibitor. Respir. Res. 2020, 21, 75. [Google Scholar] [CrossRef] [PubMed]
- Kimura, R.H.; Wang, L.; Shen, B.; Huo, L.; Tummers, W.; Filipp, F.V.; Guo, H.W.H.; Haywood, T.; Abou-Elkacem, L.; Baratto, L.; et al. Evaluation of integrin alpha v beta(6) cystine knot PET tracers to detect cancer and idiopathic pulmonary fibrosis. Nat. Commun. 2019, 10, 4673. [Google Scholar] [CrossRef] [PubMed]
- Siebermair, J.; Köhler, M.I.; Kupusovic, J.; Nekolla, S.G.; Kessler, L.; Ferdinandus, J.; Guberina, N.; Stuschke, M.; Grafe, H.; Siveke, J.T.; et al. Cardiac fibroblast activation detected by Ga-68 FAPI PET imaging as a potential novel biomarker of cardiac injury/remodeling. J. Nucl. Cardiol. 2021, 28, 812–821. [Google Scholar] [CrossRef] [PubMed]
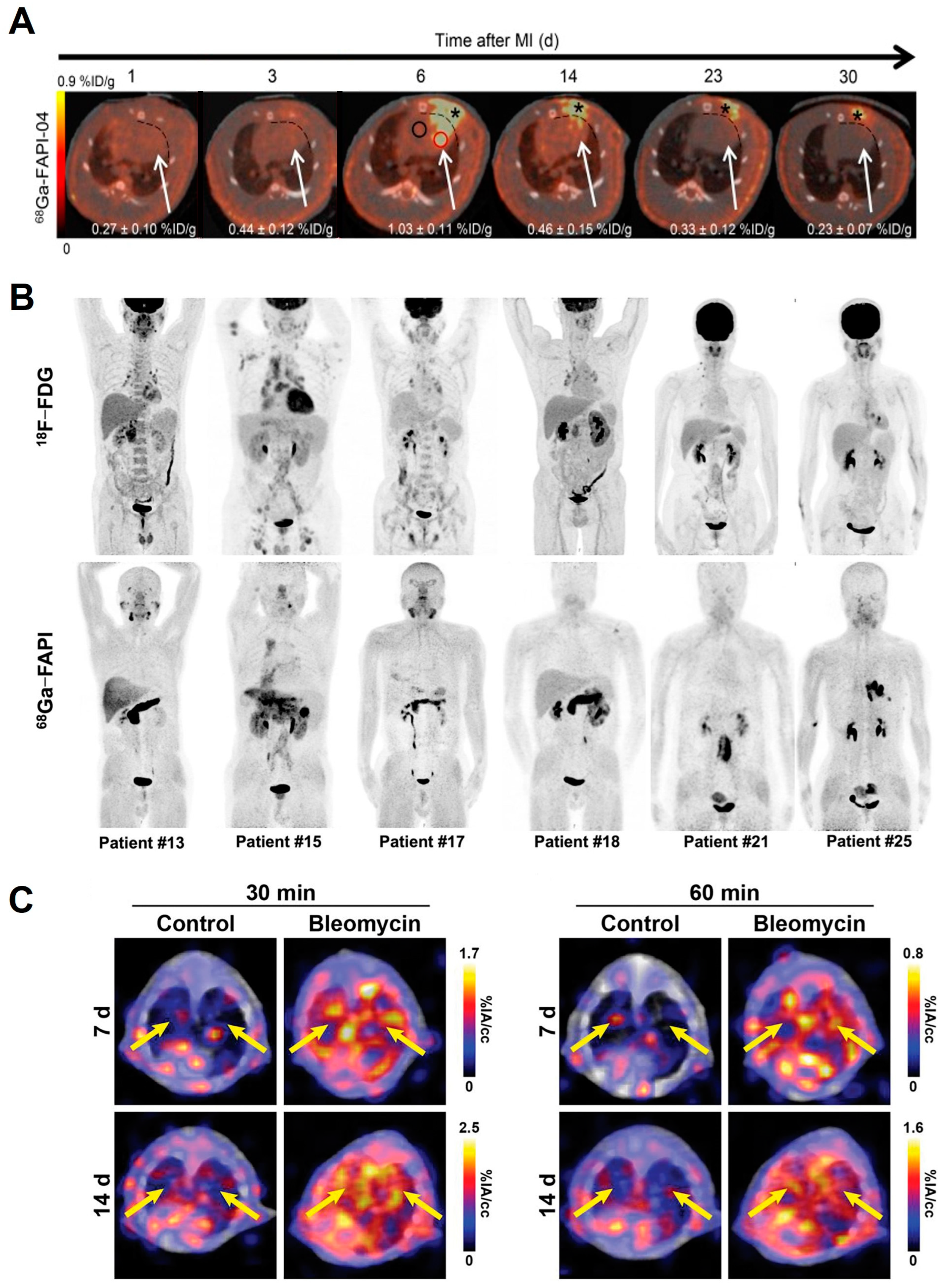
- Varasteh, Z.; Mohanta, S.; Robu, S.; Braeuer, M.; Li, Y.; Omidvari, N.; Topping, G.; Sun, T.; Nekolla, S.G.; Richter, A.; et al. Molecular Imaging of Fibroblast Activity After Myocardial Infarction Using a (68)Ga-Labeled Fibroblast Activation Protein Inhibitor, FAPI-04. J. Nucl. Med. 2019, 60, 1743–1749. [Google Scholar] [CrossRef]
- Heckmann, M.B.; Reinhardt, F.; Finke, D.; Katus, H.A.; Haberkorn, U.; Leuschner, F.; Lehmann, L.H. Relationship Between Cardiac Fibroblast Activation Protein Activity by Positron Emission Tomography and Cardiovascular Disease. Circ. Cardiovasc. Imaging 2020, 13, e010628. [Google Scholar] [CrossRef]
- Diekmann, J.; Koenig, T.; Thackeray, J.T.; Derlin, T.; Czerner, C.; Neuser, J.; Ross, T.L.; Schäfer, A.; Tillmanns, J.; Bauersachs, J.; et al. Cardiac Fibroblast Activation in Patients Early After Acute Myocardial Infarction: Integration with MR Tissue Characterization and Subsequent Functional Outcome. J. Nucl. Med. 2022, 63, 1415–1423. [Google Scholar] [CrossRef]
- Gu, Y.; Han, K.; Zhang, Z.; Zhao, Z.; Yan, C.; Wang, L.; Fang, W. 68Ga-FAPI PET/CT for molecular assessment of fibroblast activation in right heart in pulmonary arterial hypertension: A single-center, pilot study. J. Nucl. Cardiol. 2023, 30, 495–503. [Google Scholar] [CrossRef]
- Luo, Y.; Pan, Q.; Yang, H.; Peng, L.; Zhang, W.; Li, F. Fibroblast Activation Protein-Targeted PET/CT with (68)Ga-FAPI for Imaging IgG4-Related Disease: Comparison to (18)F-FDG PET/CT. J. Nucl. Med. 2021, 62, 266–271. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, X.; Liu, H.P.; Luo, W.B.; Liu, H.X.; Lv, T.Y.; Wang, J.Z.; Qin, J.H.; Ou, S.T.; Chen, Y. Value of Ga-68 Ga-FAPI-04 imaging in the diagnosis of renal fibrosis. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3493–3501. [Google Scholar] [CrossRef]
- Conen, P.; Pennetta, F.; Dendl, K.; Hertel, F.; Vogg, A.; Haberkorn, U.; Giesel, F.L.; Mottaghy, F.M. (68) Ga Ga-FAPI uptake correlates with the state of chronic kidney disease. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3365–3372. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.X.; Chen, L.M.; Wu, W.H.; Zhang, L.L.; Li, X.; Chen, Y.; Huang, Z.W.; Ou, S.T. Noninvasive Assessment of Renal Fibrosis of Chronic Kidney Disease in Rats by 68Ga Ga-FAPI-04 Small Animal PET/CT and Biomarkers. Mol. Pharm. 2023, 20, 2714–2725. [Google Scholar] [CrossRef] [PubMed]
- Rohrich, M.; Leitz, D.; Glatting, F.M.; Wefers, A.K.; Weinheimer, O.; Flechsig, P.; Kahn, N.; Mall, M.A.; Giesel, F.L.; Kratochwil, C.; et al. Fibroblast Activation Protein-Specific PET/CT Imaging in Fibrotic Interstitial Lung Diseases and Lung Cancer: A Translational Exploratory Study. J. Nucl. Med. 2022, 63, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Rosenkrans, Z.T.; Massey, C.F.; Bernau, K.; Ferreira, C.A.; Jeffery, J.J.; Schulte, J.J.; Moore, M.; Valla, F.; Batterton, J.M.; Drake, C.R.; et al. Ga-68 Ga-FAPI-46 PET for non-invasive detection of pulmonary fibrosis disease activity. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3705–3716. [Google Scholar] [CrossRef] [PubMed]
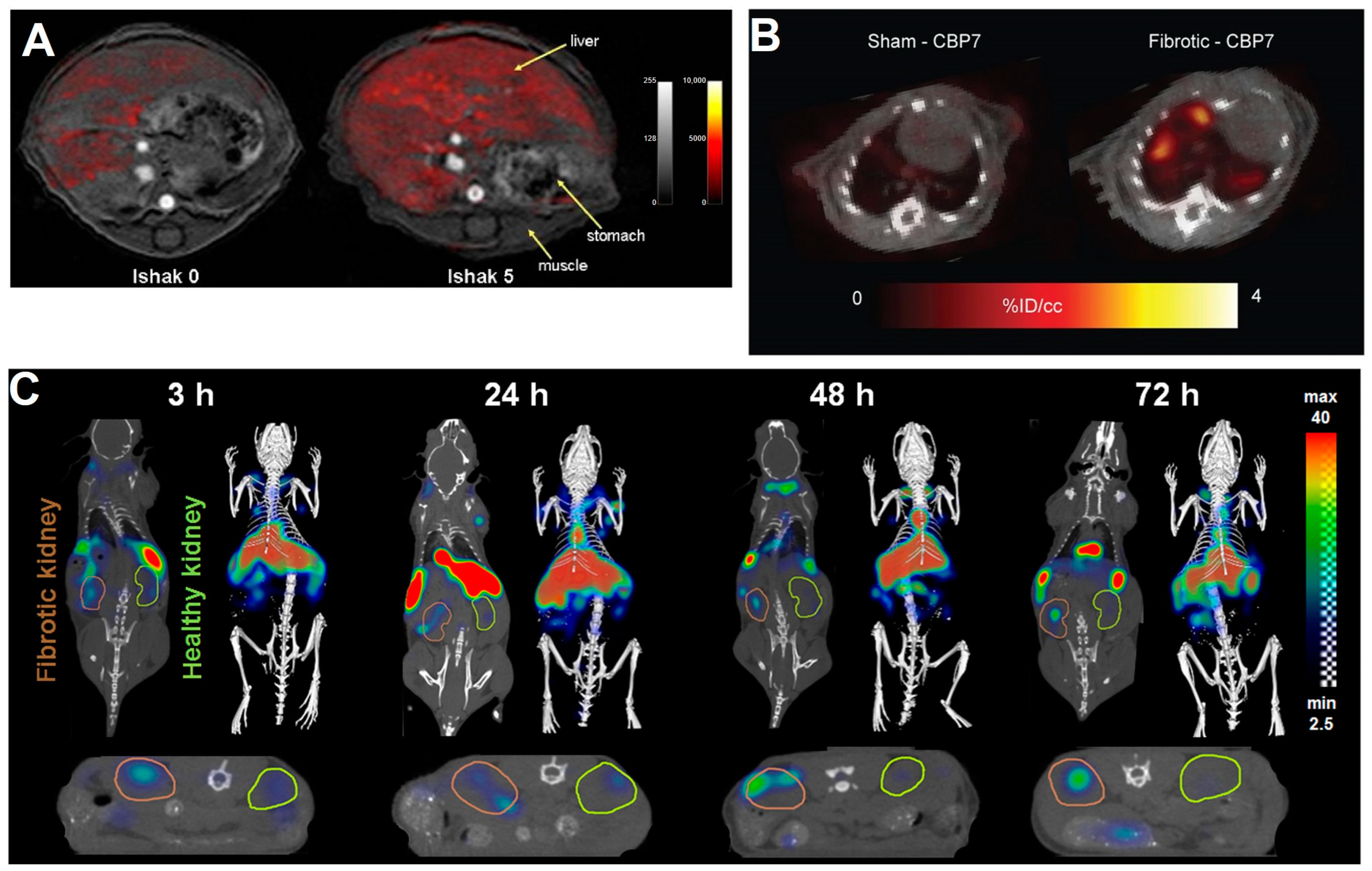
- Pirasteh, A.; Periyasamy, S.; Meudt, J.J.; Liu, Y.J.; Lee, L.M.; Schachtschneider, K.M.; Schook, L.B.; Gaba, R.C.; Mao, L.; Said, A.; et al. Staging Liver Fibrosis by Fibroblast Activation Protein Inhibitor PET in a Human-Sized Swine Model. J. Nucl. Med. 2022, 63, 1956–1961. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.M.; Zhong, X.L.; Li, L.M.; Li, X.; Liu, Y.; Guo, C.M.; Chen, Y.; Huang, Z.W. Ga-68 Ga-FAPI-04 PET/CT on assessing Crohn’s disease intestinal lesions. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 1360–1370. [Google Scholar] [CrossRef]
- Treutlein, C.; Distler, J.H.W.; Tascilar, K.; Fakhouri, S.C.; Gyorfi, A.H.; Atzinger, A.; Matei, A.E.; Dees, C.; Buttner-Herold, M.; Kuwert, T.; et al. Assessment of myocardial fibrosis in patients with systemic sclerosis using Ga-68 Ga-FAPI-04-PET-CT. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 1629–1635. [Google Scholar] [CrossRef]
- Bergmann, C.; Distler, J.H.; Treutlein, C.; Tascilar, K.; Müller, A.-T.; Atzinger, A.; Matei, A.-E.; Knitza, J.; Györfi, A.-H.; Lück, A. 68Ga-FAPI-04 PET-CT for molecular assessment of fibroblast activation and risk evaluation in systemic sclerosis-associated interstitial lung disease: A single-centre, pilot study. Lancet Rheumatol. 2021, 3, e185–e194. [Google Scholar] [CrossRef]
- Langer, L.B.N.; Hess, A.; Korkmaz, Z.; Tillmanns, J.; Reffert, L.M.; Bankstahl, J.P.; Bengel, F.M.; Thackeray, J.T.; Ross, T.L. Molecular imaging of fibroblast activation protein after myocardial infarction using the novel radiotracer Ga-68 MHLL1. Theranostics 2021, 11, 7755–7766. [Google Scholar] [CrossRef]
- Carbone, R.; Filiberti, R.; Grosso, M.; Paredi, P.; Peano, L.; Cantalupi, D.; Villa, G.; Monselise, A.; Bottino, G.; Shah, P. Octreoscan perspectives in sarcoidosis and idiopathic interstitial pneumonia. Eur. Rev. Med. Pharmacol. Sci. 2003, 7, 97–105. [Google Scholar]
- Lebtahi, R.; Moreau, S.; Marchand-Adam, S.; Debray, M.P.; Brauner, M.; Soler, P.; Marchal, J.; Raguin, O.; Gruaz-Guyon, A.; Reubi, J.C.; et al. Increased uptake of 111In-octreotide in idiopathic pulmonary fibrosis. J. Nucl. Med. 2006, 47, 1281–1287. [Google Scholar]
- Ambrosini, V.; Zompatori, M.; De Luca, F.; Antonia, D.; Allegri, V.; Nanni, C.; Malvi, D.; Tonveronachi, E.; Fasano, L.; Fabbri, M.; et al. 68Ga-DOTANOC PET/CT allows somatostatin receptor imaging in idiopathic pulmonary fibrosis: Preliminary results. J. Nucl. Med. 2010, 51, 1950–1955. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Song, Z.; Li, Q.; Wu, J.; Wang, J.; Xie, C.; Tu, C.; Wang, J.; Huang, X.; Lu, W. Molecular imaging of hepatic stellate cell activity by visualization of hepatic integrin αvβ3 expression with SPECT in rat. Hepatology 2011, 54, 1020–1030. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wu, Y.; Liu, H.; Gao, L.; Sun, X.; Zhang, C.; Shi, J.; Zhao, H.; Jia, B.; Liu, Z.; et al. Small-Animal SPECT/CT of the Progression and Recovery of Rat Liver Fibrosis by Using an Integrin αvβ3-targeting Radiotracer. Radiology 2016, 279, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Shao, T.; Chen, Z.; Belov, V.; Wang, X.H.; Rwema, S.H.; Kumar, V.; Fu, H.L.; Deng, X.Y.; Rong, J.; Yu, Q.Z.; et al. F-18 -Alfatide PET imaging of integrin alpha v beta 3 for the non-invasive quantification of liver fibrosis. J. Hepatol. 2020, 73, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Rokugawa, T.; Konishi, H.; Ito, M.; Iimori, H.; Nagai, R.; Shimosegawa, E.; Hatazawa, J.; Abe, K. Evaluation of hepatic integrin αvβ3 expression in non-alcoholic steatohepatitis (NASH) model mouse by (18)F-FPP-RGD(2) PET. EJNMMI Res. 2018, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Hiroyama, S.; Matsunaga, K.; Ito, M.; Iimori, H.; Tajiri, M.; Nakano, Y.; Shimosegawa, E.; Abe, K. Usefulness of F-18-FPP-RGD(2) PET in pathophysiological evaluation of lung fibrosis using a bleomycin-induced rat model. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 4358–4368. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, H.; Cui, Y.; Li, X.; Zhang, Z.; Zhang, Y.; Wang, D. Molecular magnetic resonance imaging of activated hepatic stellate cells with ultrasmall superparamagnetic iron oxide targeting integrin αvβ₃ for staging liver fibrosis in rat model. Int. J. Nanomed. 2016, 11, 1097–1108. [Google Scholar] [CrossRef]
- Li, F.; Yan, H.; Wang, J.; Li, C.; Wu, J.; Wu, S.; Rao, S.; Gao, X.; Jin, Q. Non-invasively differentiating extent of liver fibrosis by visualizing hepatic integrin αvβ3 expression with an MRI modality in mice. Biomaterials 2016, 102, 162–174. [Google Scholar] [CrossRef]
- van den Borne, S.W.; Isobe, S.; Verjans, J.W.; Petrov, A.; Lovhaug, D.; Li, P.; Zandbergen, H.R.; Ni, Y.; Frederik, P.; Zhou, J.; et al. Molecular imaging of interstitial alterations in remodeling myocardium after myocardial infarction. J. Am. Coll. Cardiol. 2008, 52, 2017–2028. [Google Scholar] [CrossRef]
- van den Borne, S.W.; Isobe, S.; Zandbergen, H.R.; Li, P.; Petrov, A.; Wong, N.D.; Fujimoto, S.; Fujimoto, A.; Lovhaug, D.; Smits, J.F.; et al. Molecular imaging for efficacy of pharmacologic intervention in myocardial remodeling. JACC Cardiovasc. Imaging 2009, 2, 187–198. [Google Scholar] [CrossRef]
- Caravan, P.; Das, B.; Dumas, S.; Epstein, F.H.; Helm, P.A.; Jacques, V.; Koerner, S.; Kolodziej, A.; Shen, L.; Sun, W.C.; et al. Collagen-targeted MRI contrast agent for molecular imaging of fibrosis. Angew. Chem. Int. Ed. Engl. 2007, 46, 8171–8173. [Google Scholar] [CrossRef]
- Helm, P.A.; Caravan, P.; French, B.A.; Jacques, V.; Shen, L.; Xu, Y.; Beyers, R.J.; Roy, R.J.; Kramer, C.M.; Epstein, F.H. Postinfarction myocardial scarring in mice: Molecular MR imaging with use of a collagen-targeting contrast agent. Radiology 2008, 247, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Polasek, M.; Fuchs, B.C.; Uppal, R.; Schühle, D.T.; Alford, J.K.; Loving, G.S.; Yamada, S.; Wei, L.; Lauwers, G.Y.; Guimaraes, A.R.; et al. Molecular MR imaging of liver fibrosis: A feasibility study using rat and mouse models. J. Hepatol. 2012, 57, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Zhou, I.Y.; Clavijo Jordan, V.; Rotile, N.J.; Akam, E.; Krishnan, S.; Arora, G.; Krishnan, H.; Slattery, H.; Warner, N.; Mercaldo, N.; et al. Advanced MRI of Liver Fibrosis and Treatment Response in a Rat Model of Nonalcoholic Steatohepatitis. Radiology 2020, 296, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Wei, L.; Rotile, N.; Day, H.; Rietz, T.; Farrar, C.T.; Lauwers, G.Y.; Tanabe, K.K.; Rosen, B.; Fuchs, B.C.; et al. Combined magnetic resonance elastography and collagen molecular magnetic resonance imaging accurately stage liver fibrosis in a rat model. Hepatology 2017, 65, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, B.C.; Wang, H.; Yang, Y.; Wei, L.; Polasek, M.; Schühle, D.T.; Lauwers, G.Y.; Parkar, A.; Sinskey, A.J.; Tanabe, K.K.; et al. Molecular MRI of collagen to diagnose and stage liver fibrosis. J. Hepatol. 2013, 59, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Farrar, C.T.; DePeralta, D.K.; Day, H.; Rietz, T.A.; Wei, L.; Lauwers, G.Y.; Keil, B.; Subramaniam, A.; Sinskey, A.J.; Tanabe, K.K.; et al. 3D molecular MR imaging of liver fibrosis and response to rapamycin therapy in a bile duct ligation rat model. J. Hepatol. 2015, 63, 689–696. [Google Scholar] [CrossRef]
- Erstad, D.J.; Farrar, C.T.; Ghoshal, S.; Masia, R.; Ferreira, D.S.; Chen, Y.I.; Choi, J.K.; Wei, L.; Waghorn, P.A.; Rotile, N.J.; et al. Molecular magnetic resonance imaging accurately measures the antifibrotic effect of EDP-305, a novel farnesoid X receptor agonist. Hepatol. Commun. 2018, 2, 821–835. [Google Scholar] [CrossRef]
- Marckmann, P.; Skov, L.; Rossen, K.; Dupont, A.; Damholt, M.B.; Heaf, J.G.; Thomsen, H.S. Nephrogenic systemic fibrosis: Suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J. Am. Soc. Nephrol. 2006, 17, 2359–2362. [Google Scholar] [CrossRef]
- Erstad, D.J.; Sojoodi, M.; Taylor, M.S.; Jordan, V.C.; Farrar, C.T.; Axtell, A.L.; Rotile, N.J.; Jones, C.; Graham-O’Regan, K.A.; Ferreira, D.S.; et al. Fibrotic Response to Neoadjuvant Therapy Predicts Survival in Pancreatic Cancer and Is Measurable with Collagen-Targeted Molecular MRI. Clin. Cancer Res. 2020, 26, 5007–5018. [Google Scholar] [CrossRef] [PubMed]
- Salarian, M.; Turaga, R.C.; Xue, S.H.; Nezafati, M.; Hekmatyar, K.; Qiao, J.J.; Zhang, Y.W.; Tan, S.S.; Ibhagui, O.Y.; Hai, Y.; et al. Early detection and staging of chronic liver diseases with a protein MRI contrast agent. Nat. Commun. 2019, 10, 4777. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ning, Y.; Zhu, H.; Rotile, N.J.; Wei, H.; Diyabalanage, H.; Hansen, E.C.; Zhou, I.Y.; Barrett, S.C.; Sojoodi, M.; et al. Fast detection of liver fibrosis with collagen-binding single-nanometer iron oxide nanoparticles via T(1)-weighted MRI. Proc. Natl. Acad. Sci. USA 2023, 120, e2220036120. [Google Scholar] [CrossRef]
- Désogère, P.; Tapias, L.F.; Hariri, L.P.; Rotile, N.J.; Rietz, T.A.; Probst, C.K.; Blasi, F.; Day, H.; Mino-Kenudson, M.; Weinreb, P.; et al. Type I collagen-targeted PET probe for pulmonary fibrosis detection and staging in preclinical models. Sci. Transl. Med. 2017, 9, eaaf4696. [Google Scholar] [CrossRef] [PubMed]
- Désogère, P.; Tapias, L.F.; Rietz, T.A.; Rotile, N.; Blasi, F.; Day, H.; Elliott, J.; Fuchs, B.C.; Lanuti, M.; Caravan, P. Optimization of a Collagen-Targeted PET Probe for Molecular Imaging of Pulmonary Fibrosis. J. Nucl. Med. 2017, 58, 1991–1996. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Ding, X.; Liu, K.; Feng, S.; Tang, B.; Li, Q.; Huang, D.; Yang, S. Molecular imaging of fibrosis using a novel collagen-binding peptide labelled with (99m)Tc on SPECT/CT. Amino Acids 2017, 49, 89–101. [Google Scholar] [CrossRef]
- Muzard, J.; Sarda-Mantel, L.; Loyau, S.; Meulemans, A.; Louedec, L.; Bantsimba-Malanda, C.; Hervatin, F.; Marchal-Somme, J.; Michel, J.B.; Le Guludec, D.; et al. Non-invasive molecular imaging of fibrosis using a collagen-targeted peptidomimetic of the platelet collagen receptor glycoprotein VI. PLoS ONE 2009, 4, e5585. [Google Scholar] [CrossRef]
- Velikyan, I.; Rosenström, U.; Estrada, S.; Ljungvall, I.; Häggström, J.; Eriksson, O.; Antoni, G. Synthesis and preclinical evaluation of 68Ga-labeled collagelin analogs for imaging and quantification of fibrosis. Nucl. Med. Biol. 2014, 41, 728–736. [Google Scholar] [CrossRef]
- Velikyan, I.; Rosenström, U.; Rosestedt, M.; Eriksson, O.; Antoni, G. Improved Radiolytic Stability of a (68)Ga-labelled Collagelin Analogue for the Imaging of Fibrosis. Pharmaceuticals 2021, 14, 990. [Google Scholar] [CrossRef]
- Li, G.L.; He, H.Q.; Zheng, G.D.; Jiang, W.J.; Du, S.W.; Tao, H.; Xiao, T.; Zhou, D.Z.; Ding, S.W.; Yu, X.Y.; et al. Direct Detection of Pulmonary Fibrosis by Near-Infrared-Responsive Biomimetic Platelets. Int. J. Nanomed. 2022, 17, 151–162. [Google Scholar] [CrossRef]
- Kee, P.H.; Danila, D. CT imaging of myocardial scar burden with CNA35-conjugated gold nanoparticles. Nanomedicine 2018, 14, 1941–1947. [Google Scholar] [CrossRef] [PubMed]
- Danila, D.; Johnson, E.; Kee, P. CT imaging of myocardial scars with collagen-targeting gold nanoparticles. Nanomedicine 2013, 9, 1067–1076. [Google Scholar] [CrossRef]
- Baues, M.; Klinkhammer, B.M.; Ehling, J.; Gremse, F.; van Zandvoort, M.; Reutelingsperger, C.P.M.; Daniel, C.; Amann, K.; Babickova, J.; Kiessling, F.; et al. A collagen-binding protein enables molecular imaging of kidney fibrosis in vivo. Kidney Int. 2020, 97, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zeng, Y.L.; Xiong, Q.S.; Zhong, S.G.; Li, P.; Ran, H.T.; Yin, Y.H.; Reutelingsperger, C.; Prinze, F.W.; Ling, Z.Y. Construction of CNA35 Collagen-Targeted Phase-Changeable Nanoagents for Low-Intensity Focused Ultrasound-Triggered Ultrasound Molecular Imaging of Myocardial Fibrosis in Rabbits. Acs Appl. Mater. Interfaces 2019, 11, 23006–23017. [Google Scholar] [CrossRef]
- Wildgruber, M.; Bielicki, I.; Aichler, M.; Kosanke, K.; Feuchtinger, A.; Settles, M.; Onthank, D.C.; Cesati, R.R.; Robinson, S.P.; Huber, A.M.; et al. Assessment of myocardial infarction and postinfarction scar remodeling with an elastin-specific magnetic resonance agent. Circ. Cardiovasc. Imaging 2014, 7, 321–329. [Google Scholar] [CrossRef]
- Ramos, I.T.; Henningsson, M.; Nezafat, M.; Lavin, B.; Lorrio, S.; Gebhardt, P.; Protti, A.; Eykyn, T.R.; Andia, M.E.; Flögel, U.; et al. Simultaneous Assessment of Cardiac Inflammation and Extracellular Matrix Remodeling after Myocardial Infarction. Circ. Cardiovasc. Imaging 2018, 11, e007453. [Google Scholar] [CrossRef]
- Ehling, J.; Bartneck, M.; Fech, V.; Butzbach, B.; Cesati, R.; Botnar, R.; Lammers, T.; Tacke, F. Elastin-based molecular MRI of liver fibrosis. Hepatology 2013, 58, 1517–1518. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.X.; Baues, M.; Klinkhammer, B.M.; Ehling, J.; Djudjaj, S.; Drude, N.I.; Daniel, C.; Amann, K.; Kramann, R.; Kim, H.; et al. Elastin imaging enables noninvasive staging and treatment monitoring of kidney fibrosis. Sci. Transl. Med. 2019, 11, eaat4865. [Google Scholar] [CrossRef]
- Shao, T.; Chen, Z.; Rong, J.; Belov, V.; Chen, J.H.; Jeyarajan, A.; Deng, X.Y.; Fu, H.L.; Yu, Q.Z.; Rwema, S.H.; et al. F-18 MAGL-4-11 positron emission tomography molecular imaging of monoacylglycerol lipase changes in preclinical liver fibrosis models. Acta Pharm. Sin. B 2022, 12, 308–315. [Google Scholar] [CrossRef]
- Chen, H.H.; Waghorn, P.A.; Wei, L.; Tapias, L.F.; Schühle, D.T.; Rotile, N.J.; Jones, C.M.; Looby, R.J.; Zhao, G.; Elliott, J.M.; et al. Molecular imaging of oxidized collagen quantifies pulmonary and hepatic fibrogenesis. JCI Insight 2017, 2, e91506. [Google Scholar] [CrossRef]
- Ning, Y.Y.; Zhou, I.Y.; Roberts, J.D.; Rotile, N.J.; Akam, E.; Barrett, S.C.; Sojoodi, M.; Barr, M.N.; Punshon, T.; Pantazopoulos, P.; et al. Molecular MRI quantification of extracellular aldehyde pairs for early detection of liver fibrogenesis and response to treatment. Sci. Transl. Med. 2022, 14, eabq6297. [Google Scholar] [CrossRef]
- Waghorn, P.A.; Jones, C.M.; Rotile, N.J.; Koerner, S.K.; Ferreira, D.S.; Chen, H.H.; Probst, C.K.; Tager, A.M.; Caravan, P. Molecular Magnetic Resonance Imaging of Lung Fibrogenesis with an Oxyamine-Based Probe. Angew. Chem. Int. Ed. Engl. 2017, 56, 9825–9828. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.M.; Zhang, X.Q.; Li, Z.Q.; Xiao, X.Y.; Guo, S.W.; Pan, D.Y.; Zhang, H.; Tian, X.H.; Gong, Q.Y.; Gu, Z.W.; et al. A hyaluronic acid-derived imaging probe for enhanced imaging and accurate staging of liver fibrosis. Carbohydr. Polym. 2022, 295, 119870. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.J.; Clark, R.A. Cutaneous wound healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Shea, B.S.; Brooks, S.F.; Fontaine, B.A.; Chun, J.; Luster, A.D.; Tager, A.M. Prolonged exposure to sphingosine 1-phosphate receptor-1 agonists exacerbates vascular leak, fibrosis, and mortality after lung injury. Am. J. Respir. Cell Mol. Biol. 2010, 43, 662–673. [Google Scholar] [CrossRef]
- Phinikaridou, A.; Andia, M.E.; Protti, A.; Indermuehle, A.; Shah, A.; Smith, A.; Warley, A.; Botnar, R.M. Noninvasive magnetic resonance imaging evaluation of endothelial permeability in murine atherosclerosis using an albumin-binding contrast agent. Circulation 2012, 126, 707–719. [Google Scholar] [CrossRef]
- Chambers, R.C.; Laurent, G.J. Coagulation cascade proteases and tissue fibrosis. Biochem. Soc. Trans. 2002, 30, 194–200. [Google Scholar] [CrossRef]
- McDougall, S.; Dallon, J.; Sherratt, J.; Maini, P. Fibroblast migration and collagen deposition during dermal wound healing: Mathematical modelling and clinical implications. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2006, 364, 1385–1405. [Google Scholar] [CrossRef]
- Overoye-Chan, K.; Koerner, S.; Looby, R.J.; Kolodziej, A.F.; Zech, S.G.; Deng, Q.; Chasse, J.M.; McMurry, T.J.; Caravan, P. EP-2104R: A fibrin-specific gadolinium-Based MRI contrast agent for detection of thrombus. J. Am. Chem. Soc. 2008, 130, 6025–6039. [Google Scholar] [CrossRef]
- Stracke, C.P.; Katoh, M.; Wiethoff, A.J.; Parsons, E.C.; Spangenberg, P.; Spüntrup, E. Molecular MRI of cerebral venous sinus thrombosis using a new fibrin-specific MR contrast agent. Stroke 2007, 38, 1476–1481. [Google Scholar] [CrossRef]
- Spuentrup, E.; Botnar, R.M.; Wiethoff, A.J.; Ibrahim, T.; Kelle, S.; Katoh, M.; Ozgun, M.; Nagel, E.; Vymazal, J.; Graham, P.B.; et al. MR imaging of thrombi using EP-2104R, a fibrin-specific contrast agent: Initial results in patients. Eur. Radiol. 2008, 18, 1995–2005. [Google Scholar] [CrossRef]
- Vymazal, J.; Spuentrup, E.; Cardenas-Molina, G.; Wiethoff, A.J.; Hartmann, M.G.; Caravan, P.; Parsons, E.C., Jr. Thrombus imaging with fibrin-specific gadolinium-based MR contrast agent EP-2104R: Results of a phase II clinical study of feasibility. Investig. Radiol. 2009, 44, 697–704. [Google Scholar] [CrossRef]
- Gibbons, M.A.; MacKinnon, A.C.; Ramachandran, P.; Dhaliwal, K.; Duffin, R.; Phythian-Adams, A.T.; van Rooijen, N.; Haslett, C.; Howie, S.E.; Simpson, A.J.; et al. Ly6Chi monocytes direct alternatively activated profibrotic macrophage regulation of lung fibrosis. Am. J. Respir. Crit. Care Med. 2011, 184, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Misharin, A.V.; Morales-Nebreda, L.; Reyfman, P.A.; Cuda, C.M.; Walter, J.M.; McQuattie-Pimentel, A.C.; Chen, C.I.; Anekalla, K.R.; Joshi, N.; Williams, K.J.N.; et al. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J. Exp. Med. 2017, 214, 2387–2404. [Google Scholar] [CrossRef] [PubMed]
- Moore, B.B.; Paine, R., 3rd; Christensen, P.J.; Moore, T.A.; Sitterding, S.; Ngan, R.; Wilke, C.A.; Kuziel, W.A.; Toews, G.B. Protection from pulmonary fibrosis in the absence of CCR2 signaling. J. Immunol. 2001, 167, 4368–4377. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gunsten, S.P.; Sultan, D.H.; Luehmann, H.P.; Zhao, Y.; Blackwell, T.S.; Bollermann-Nowlis, Z.; Pan, J.H.; Byers, D.E.; Atkinson, J.J.; et al. PET-based Imaging of Chemokine Receptor 2 in Experimental and Disease-related Lung Inflammation. Radiology 2017, 283, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Kadioglu, A.; De Filippo, K.; Bangert, M.; Fernandes, V.E.; Richards, L.; Jones, K.; Andrew, P.W.; Hogg, N. The integrins Mac-1 and alpha4beta1 perform crucial roles in neutrophil and T cell recruitment to lungs during Streptococcus pneumoniae infection. J. Immunol. 2011, 186, 5907–5915. [Google Scholar] [CrossRef] [PubMed]
- Bitterman, P.; Rennard, S.; Adelberg, S.; Crystal, R.G. Role of fibronectin in fibrotic lung disease. A growth factor for human lung fibroblasts. Chest 1983, 83, 96s. [Google Scholar] [CrossRef] [PubMed]
- Agassandian, M.; Tedrow, J.R.; Sembrat, J.; Kass, D.J.; Zhang, Y.; Goncharova, E.A.; Kaminski, N.; Mallampalli, R.K.; Vuga, L.J. VCAM-1 is a TGF-β1 inducible gene upregulated in idiopathic pulmonary fibrosis. Cell. Signal. 2015, 27, 2467–2473. [Google Scholar] [CrossRef]
- Soodgupta, D.; Hurchla, M.A.; Jiang, M.; Zheleznyak, A.; Weilbaecher, K.N.; Anderson, C.J.; Tomasson, M.H.; Shokeen, M. Very late antigen-4 (α(4)β(1) Integrin) targeted PET imaging of multiple myeloma. PLoS ONE 2013, 8, e55841. [Google Scholar] [CrossRef]
- Beaino, W.; Anderson, C.J. PET imaging of very late antigen-4 in melanoma: Comparison of 68Ga- and 64Cu-labeled NODAGA and CB-TE1A1P-LLP2A conjugates. J. Nucl. Med. 2014, 55, 1856–1863. [Google Scholar] [CrossRef] [PubMed]
- Laforest, R.; Ghai, A.; Fraum, T.J.; Oyama, R.; Frye, J.; Kaemmerer, H.; Gaehle, G.; Voller, T.; Mpoy, C.; Rogers, B.E.; et al. First-in-Humans Evaluation of Safety and Dosimetry of (64)Cu-LLP2A for PET Imaging. J. Nucl. Med. 2023, 64, 320–328. [Google Scholar] [CrossRef]
- Burger, J.A.; Kipps, T.J. CXCR4: A key receptor in the crosstalk between tumor cells and their microenvironment. Blood 2006, 107, 1761–1767. [Google Scholar] [CrossRef]
- Weiskirchen, R.; Weiskirchen, S.; Tacke, F. Organ and tissue fibrosis: Molecular signals, cellular mechanisms and translational implications. Mol. Aspects Med. 2019, 65, 2–15. [Google Scholar] [CrossRef]
- Williamson, J.D.; Sadofsky, L.R.; Hart, S.P. The pathogenesis of bleomycin-induced lung injury in animals and its applicability to human idiopathic pulmonary fibrosis. Exp. Lung Res. 2015, 41, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Hausner, S.H.; DiCara, D.; Marik, J.; Marshall, J.F.; Sutcliffe, J.L. Use of a peptide derived from foot-and-mouth disease virus for the noninvasive imaging of human cancer: Generation and evaluation of 4-[18F]fluorobenzoyl A20FMDV2 for in vivo imaging of integrin alphavbeta6 expression with positron emission tomography. Cancer Res. 2007, 67, 7833–7840. [Google Scholar] [CrossRef]
- Onega, M.; Parker, C.A.; Coello, C.; Rizzo, G.; Keat, N.; Ramada-Magalhaes, J.; Moz, S.; Tang, S.P.; Plisson, C.; Wells, L.; et al. Preclinical evaluation of [(18)F]FB-A20FMDV2 as a selective marker for measuring α(V)β(6) integrin occupancy using positron emission tomography in rodent lung. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 958–966. [Google Scholar] [CrossRef]
- Kendall, R.T.; Feghali-Bostwick, C.A. Fibroblasts in fibrosis: Novel roles and mediators. Front. Pharmacol. 2014, 5, 123. [Google Scholar] [CrossRef]
- Park, J.E.; Lenter, M.C.; Zimmermann, R.N.; Garin-Chesa, P.; Old, L.J.; Rettig, W.J. Fibroblast activation protein, a dual specificity serine protease expressed in reactive human tumor stromal fibroblasts. J. Biol. Chem. 1999, 274, 36505–36512. [Google Scholar] [CrossRef]
- Loktev, A.; Lindner, T.; Burger, E.M.; Altmann, A.; Giesel, F.; Kratochwil, C.; Debus, J.; Marmé, F.; Jäger, D.; Mier, W.; et al. Development of Fibroblast Activation Protein-Targeted Radiotracers with Improved Tumor Retention. J. Nucl. Med. 2019, 60, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Lindner, T.; Loktev, A.; Altmann, A.; Giesel, F.; Kratochwil, C.; Debus, J.; Jaeger, D.; Mier, W.; Haberkorn, U. Development of Quinoline-Based Theranostic Ligands for the Targeting of Fibroblast Activation Protein. J. Nucl. Med. 2018, 59, 1415–1422. [Google Scholar] [CrossRef]
- Hamson, E.J.; Keane, F.M.; Tholen, S.; Schilling, O.; Gorrell, M.D. Understanding fibroblast activation protein (FAP): Substrates, activities, expression and targeting for cancer therapy. Proteom. Clin. Appl. 2014, 8, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Watabe, T.; Liu, Y.W.; Kaneda-Nakashima, K.; Shirakami, Y.; Lindner, T.; Ooe, K.; Toyoshima, A.; Nagata, K.; Shimosegawa, E.; Haberkorn, U.; et al. Theranostics Targeting Fibroblast Activation Protein in the Tumor Stroma: Cu-64- and Ac-225-Labeled FAPI-04 in Pancreatic Cancer Xenograft Mouse Models. J. Nucl. Med. 2020, 61, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Kosmala, A.; Serfling, S.E.; Schlotelburg, W.; Lindner, T.; Michalski, K.; Schirbel, A.; Higuchi, T.; Hartrampf, P.E.; Buck, A.K.; Weich, A.; et al. Impact of Ga-68-FAPI-04 PET/CT on Staging and Therapeutic Management in Patients With Digestive System Tumors. Clin. Nucl. Med. 2023, 48, 35–42. [Google Scholar] [CrossRef]
- Kratochwil, C.; Flechsig, P.; Lindner, T.; Abderrahim, L.; Altmann, A.; Mier, W.; Adeberg, S.; Rathke, H.; Rohrich, M.; Winter, H.; et al. Ga-68-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J. Nucl. Med. 2019, 60, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.J.; Pang, Y.Z.; Wu, J.X.; Zhao, L.; Hao, B.; Wu, J.; Wei, J.H.; Wu, S.M.; Zhao, L.; Luo, Z.M.; et al. Comparison of Ga-68 Ga-DOTA-FAPI-04 and F-18 FDG PET/CT for the diagnosis of primary and metastatic lesions in patients with various types of cancer. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1820–1832. [Google Scholar] [CrossRef]
- Chen, H.J.; Zhao, L.; Ruan, D.; Pang, Y.Z.; Hao, B.; Dai, Y.Q.; Wu, X.R.; Guo, W.; Fan, C.L.; Wu, J.X.; et al. Usefulness of Ga-68 Ga-DOTA-FAPI-04 PET/CT in patients presenting with inconclusive F-18 FDG PET/CT findings. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 73–86. [Google Scholar] [CrossRef]
- Borie, R.; Fabre, A.; Prost, F.; Marchal-Somme, J.; Lebtahi, R.; Marchand-Adam, S.; Aubier, M.; Soler, P.; Crestani, B. Activation of somatostatin receptors attenuates pulmonary fibrosis. Thorax 2008, 63, 251–258. [Google Scholar] [CrossRef]
- Buscail, L.; Estève, J.P.; Saint-Laurent, N.; Bertrand, V.; Reisine, T.; O’Carroll, A.M.; Bell, G.I.; Schally, A.V.; Vaysse, N.; Susini, C. Inhibition of cell proliferation by the somatostatin analogue RC-160 is mediated by somatostatin receptor subtypes SSTR2 and SSTR5 through different mechanisms. Proc. Natl. Acad. Sci. USA 1995, 92, 1580–1584. [Google Scholar] [CrossRef]
- Pettinato, C.; Sarnelli, A.; Di Donna, M.; Civollani, S.; Nanni, C.; Montini, G.; Di Pierro, D.; Ferrari, M.; Marengo, M.; Bergamini, C. 68Ga-DOTANOC: Biodistribution and dosimetry in patients affected by neuroendocrine tumors. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 72–79. [Google Scholar] [CrossRef]
- Kaushik, P.; Patel, C.; Gulati, G.S.; Seth, S.; Parakh, N.; Randeep, G.; Kumar, R.; Gupta, P.; Bal, C. Comparison of (68)Ga-DOTANOC PET/CT with cardiac MRI in patients with clinical suspicion of cardiac sarcoidosis. Ann. Nucl. Med. 2021, 35, 1058–1065. [Google Scholar] [CrossRef]
- Friedman, S.L. Liver fibrosis from bench to bedside. J. Hepatol. 2003, 38 (Suppl. S1), S38–S53. [Google Scholar] [CrossRef]
- Zhou, X.; Murphy, F.R.; Gehdu, N.; Zhang, J.; Iredale, J.P.; Benyon, R.C. Engagement of alphavbeta3 integrin regulates proliferation and apoptosis of hepatic stellate cells. J. Biol. Chem. 2004, 279, 23996–24006. [Google Scholar] [CrossRef]
- Belkahla, H.; Antunes, J.C.; Lalatonne, Y.; Sainte Catherine, O.; Illoul, C.; Journe, C.; Jandrot-Perrus, M.; Coradin, T.; Gigoux, V.; Guenin, E.; et al. USPIO-PEG nanoparticles functionalized with a highly specific collagen-binding peptide: A step towards MRI diagnosis of fibrosis. J. Mater. Chem. B 2020, 8, 5515–5528. [Google Scholar] [CrossRef]
- van der Meijden, P.E.J.; Heemskerk, J.W.M. Platelet biology and functions: New concepts and clinical perspectives. Nat. Rev. Cardiol. 2019, 16, 166–179. [Google Scholar] [CrossRef]
- Patti, J.M.; Bremell, T.; Krajewska-Pietrasik, D.; Abdelnour, A.; Tarkowski, A.; Rydén, C.; Höök, M. The Staphylococcus aureus collagen adhesin is a virulence determinant in experimental septic arthritis. Infect. Immun. 1994, 62, 152–161. [Google Scholar] [CrossRef]
- Pellicoro, A.; Aucott, R.L.; Ramachandran, P.; Robson, A.J.; Fallowfield, J.A.; Snowdon, V.K.; Hartland, S.N.; Vernon, M.; Duffield, J.S.; Benyon, R.C.; et al. Elastin accumulation is regulated at the level of degradation by macrophage metalloelastase (MMP-12) during experimental liver fibrosis. Hepatology 2012, 55, 1965–1975. [Google Scholar] [CrossRef]
- Jun, J.I.; Lau, L.F. Resolution of organ fibrosis. J. Clin. Investig. 2018, 128, 97–107. [Google Scholar] [CrossRef]
- Habib, A.; Chokr, D.; Wan, J.; Hegde, P.; Mabire, M.; Siebert, M.; Ribeiro-Parenti, L.; Le Gall, M.; Lettéron, P.; Pilard, N.; et al. Inhibition of monoacylglycerol lipase, an anti-inflammatory and antifibrogenic strategy in the liver. Gut 2019, 68, 522–532. [Google Scholar] [CrossRef]
- Vallet, S.D.; Ricard-Blum, S. Lysyl oxidases: From enzyme activity to extracellular matrix cross-links. Essays Biochem. 2019, 63, 349–364. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Bhatt, R.; Brown, C.; Brown, E.A.; Buhr, D.L.; Chantranuvatana, K.; Danaher, P.; Dunaway, D.; Garrison, R.G.; Geiss, G.; et al. High-plex imaging of RNA and proteins at subcellular resolution in fixed tissue by spatial molecular imaging. Nat. Biotechnol. 2022, 40, 1794–1806. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Huang, W.; Jiao, H.; Song, L.; Kang, L. Molecular Imaging of Fibrosis in Benign Diseases: An Overview of the State of the Art. Pharmaceuticals 2024, 17, 296. https://doi.org/10.3390/ph17030296
Zhang Y, Huang W, Jiao H, Song L, Kang L. Molecular Imaging of Fibrosis in Benign Diseases: An Overview of the State of the Art. Pharmaceuticals. 2024; 17(3):296. https://doi.org/10.3390/ph17030296
Chicago/Turabian StyleZhang, Yongbai, Wenpeng Huang, Hao Jiao, Lele Song, and Lei Kang. 2024. "Molecular Imaging of Fibrosis in Benign Diseases: An Overview of the State of the Art" Pharmaceuticals 17, no. 3: 296. https://doi.org/10.3390/ph17030296
APA StyleZhang, Y., Huang, W., Jiao, H., Song, L., & Kang, L. (2024). Molecular Imaging of Fibrosis in Benign Diseases: An Overview of the State of the Art. Pharmaceuticals, 17(3), 296. https://doi.org/10.3390/ph17030296







