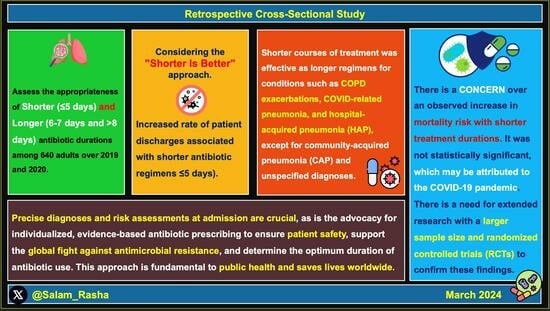
Shorter and Longer Antibiotic Durations for Respiratory Infections: To Fight Antimicrobial Resistance—A Retrospective Cross-Sectional Study in a Secondary Care Setting in the UK
Abstract
:1. Introduction
2. Results
2.1. Categorising Antibiotic Treatment Durations: Shorter and Longer
2.2. Clinical and Demographic Characteristics
2.3. Most Frequently Prescribed Antibiotics for Respiratory Infections
2.4. Shorter versus Longer Antibiotic Courses in Respiratory Infections
3. Discussion
Strengths and Limitations
4. Materials and Methods
4.1. Study Design and Setting
4.2. Participants
4.3. Data Sources and Variables
4.4. Statistical Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coque, T.M.; Cantón, R.; Pérez-Cobas, A.E.; Fernández-de-Bobadilla, M.D.; Baquero, F. Antimicrobial Resistance in the Global Health Network: Known Unknowns and Challenges for Efficient Responses in the 21st Century. Microorganisms 2023, 11, 1050. [Google Scholar] [CrossRef]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef]
- WHO. Antimicrobial Resistance. World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 20 February 2024).
- European Commission. EU Action on Antimicrobial Resistance. Available online: https://health.ec.europa.eu/antimicrobial-resistance/eu-action-antimicrobial-resistance_en (accessed on 10 February 2024).
- Wellcome. The Global Response to AMR|Reports. Available online: https://wellcome.org/reports/global-response-amr-momentum-success-and-critical-gaps (accessed on 5 February 2024).
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial Resistance: A Global Multifaceted Phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Bond, S.E.; Bakhit, M.; Hasan, S.S.; Sadeq, A.A.; Conway, B.R.; Aldeyab, M.A. COVID-19 Mixed Impact on Hospital Antimicrobial Stewardship Activities: A Qualitative Study in UK-Based Hospitals. Antibiotics 2022, 11, 1600. [Google Scholar] [CrossRef]
- Abdelsalam Elshenawy, R.; Umaru, N.; Aslanpour, Z. Impact of COVID-19 on “Start Smart, Then Focus” Antimicrobial Stewardship at One NHS Foundation Trust in England Prior to and during the Pandemic. COVID 2024, 4, 102–116. [Google Scholar] [CrossRef]
- Majumder, M.A.A.; Rahman, S.; Cohall, D.; Bharatha, A.; Singh, K.; Haque, M.; Gittens-St Hilaire, M. Antimicrobial Stewardship: Fighting Antimicrobial Resistance and Protecting Global Public Health. Infect. Drug Resist. 2020, 13, 4713–4738. [Google Scholar] [CrossRef]
- Llor, C.; Bjerrum, L. Antimicrobial Resistance: Risk Associated with Antibiotic Overuse and Initiatives to Reduce the Problem. Ther. Adv. Drug Saf. 2014, 5, 229–241. [Google Scholar] [CrossRef]
- Dadgostar, P. Antimicrobial Resistance: Implications and Costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef]
- Elshenawy, R.A.; Umaru, N.; Alharbi, A.B.; Aslanpour, Z. Antimicrobial Stewardship Implementation before and during the COVID-19 Pandemic in the Acute Care Settings: A Systematic Review. BMC Public Health 2023, 23, 309. [Google Scholar] [CrossRef] [PubMed]
- UKHSA. Start Smart Then Focus: Antimicrobial Stewardship Toolkit for Inpatient Care Settings. Available online: https://www.gov.uk/government/publications/antimicrobial-stewardship-start-smart-then-focus/start-smart-then-focus-antimicrobial-stewardship-toolkit-for-inpatient-care-settings#:~:text=Evidence%20consistently%20shows%20that%20short (accessed on 13 February 2024).
- GOV.UK. Government Response to Results of Antimicrobial Resistance (AMR) Call for Evidence. Available online: https://www.gov.uk/government/calls-for-evidence/antimicrobial-resistance-national-action-plan-call-for-evidence/outcome/government-response-to-results-of-antimicrobial-resistance-amr-call-for-evidence (accessed on 13 February 2024).
- Annunziato, G. Strategies to Overcome Antimicrobial Resistance (AMR) Making Use of Non-Essential Target Inhibitors: A Review. Int. J. Mol. Sci. 2019, 20, 5844. [Google Scholar] [CrossRef]
- Spellberg, B.; Rice, L.B. Duration of Antibiotic Therapy: Shorter Is Better. Ann. Intern. Med. 2019, 171, 210. [Google Scholar] [CrossRef]
- Palin, V.; Welfare, W.; Ashcroft, D.M.; van Staa, T.P. Shorter and Longer Courses of Antibiotics for Common Infections and the Association with Reductions of Infection-Related Complications Including Hospital Admissions. Clin. Infect. Dis. 2021, 73, 1805–1812. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.A.; Stripling, J.T.; Spellberg, B.; Centor, R.M. Short-Course Antibiotics for Common Infections: What Do We Know and Where Do We Go from Here? Clin. Microbiol. Infect. 2022, 29, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Public Health England. English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR) Report. 2014. Available online: https://www.gov.uk/government/publications/english-surveillance-programme-antimicrobial-utilisation-and-resistance-espaur-report (accessed on 13 February 2024).
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.R.M.; Mitra, S.; Emran, T.B.; Dhama, K.; Ripon, M.K.H.; Gajdács, M.; Sahibzada, M.U.K.; et al. Antibiotic Resistance in Microbes: History, Mechanisms, Therapeutic Strategies and Future Prospects. J. Infect. Public Health 2021, 14, 1750–1766. [Google Scholar] [CrossRef]
- Mullish, B.H.; Williams, H.R. Clostridium Difficile Infection and Antibiotic-Associated Diarrhoea. Clin. Med. 2018, 18, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Cella, E.; Giovanetti, M.; Benedetti, F.; Scarpa, F.; Johnston, C.; Borsetti, A.; Ceccarelli, G.; Azarian, T.; Zella, D.; Ciccozzi, M. Joining Forces against Antibiotic Resistance: The One Health Solution. Pathogens 2023, 12, 1074. [Google Scholar] [CrossRef] [PubMed]
- Skouras, V.; Chatzivasiloglou, F.; Iliopoulou, M.; Rimpa, T. Medical Treatment of Pleural Infection: Antibiotic Duration and Corticosteroid Usefulness. Breathe 2023, 19, 230134. [Google Scholar] [CrossRef]
- WHO. Global Action Plan on Antimicrobial Resistance. 2016. Available online: https://www.who.int/publications/i/item/9789241509763 (accessed on 20 February 2024).
- Mo, Y.; Booraphun, S.; Li, A.Y.; Domthong, P.; Kayastha, G.; Lau, Y.H.; Chetchotisakd, P.; Limmathurotsakul, D.; Tambyah, P.A.; Cooper, B.S.; et al. Individualised, Short-Course Antibiotic Treatment versus Usual Long-Course Treatment for Ventilator-Associated Pneumonia (REGARD-VAP): A Multicentre, Individually Randomised, Open-Label, Non-Inferiority Trial. Lancet Respir. Med. 2024. [Google Scholar] [CrossRef] [PubMed]
- Gjini, E.; Paupério, F.F.S.; Ganusov, V.V. Treatment Timing Shifts the Benefits of Short and Long Antibiotic Treatment over Infection. Evol. Med. Public Health 2020, 2020, 249–263. [Google Scholar] [CrossRef]
- Spellberg, B. The New Antibiotic Mantra—“Shorter Is Better”. JAMA Intern. Med. 2016, 176, 1254. [Google Scholar] [CrossRef]
- Public Health Ontario. Antimicrobial Stewardship Strategies|Public Health Ontario. Public Health Ontario. Available online: https://www.publichealthontario.ca/en/health-topics/antimicrobial-stewardship/asp-strategies (accessed on 13 February 2024).
- Public Health Ontario. Shorter Is Smarter. 2018. Available online: https://www.publichealthontario.ca/-/media/Documents/I/2018/infographic-duration-antibiotics-ltc.pdf?rev=ef04bab2b8a8463f90b731079cd50157&sc_lang=en (accessed on 13 February 2024).
- Wald-Dickler, N.; Spellberg, B. Short-Course Antibiotic Therapy—Replacing Constantine Units with “Shorter Is Better”. Clin. Infect. Dis. 2019, 69, 1476–1479. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, S. 3 Days’ Antibiotic Is Effective in Childhood Pneumonia. NIHR Evidence. Available online: https://evidence.nihr.ac.uk/alert/short-course-antibiotics-effective-in-childhood-pneumonia/ (accessed on 13 February 2024).
- Dunbar, L.M.; Khashab, M.M.; Kahn, J.B.; Zadeikis, N.; Xiang, J.X.; Tennenberg, A.M. Efficacy of 750-Mg, 5-Day Levofloxacin in the Treatment of Community-Acquired Pneumonia Caused by Atypical Pathogens. Curr. Med. Res. Opin. 2004, 20, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wu, J.; Xiu, Q.; Wang, C.; Zhang, D.; Huang, J.; Xie, C.; Sun, S.; Lv, X.; Si, B.; et al. A Randomized Controlled Clinical Trial of Levofloxacin 750 Mg versus 500 Mg Intravenous Infusion in the Treatment of Community-Acquired Pneumonia. Diagn. Microbiol. Infect. Dis. 2014, 80, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Pakistan Multicentre Amoxicillin Short Course Therapy (MASCOT) Pneumonia Study Group. Clinical Efficacy of 3 Days versus 5 Days of Oral Amoxicillin for Treatment of Childhood Pneumonia: A Multicentre Double-Blind Trial. Lancet 2002, 360, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Moussaoui, R.; de Borgie, C.A.J.M.; van den Broek, P.; Hustinx, W.N.; Bresser, P.; van den Berk, G.E.L.; Poley, J.-W.; van den Berg, B.; Krouwels, F.H.; Bonten, M.J.M.; et al. Effectiveness of Discontinuing Antibiotic Treatment after Three Days versus Eight Days in Mild to Moderate-Severe Community Acquired Pneumonia: Randomised, Double Blind Study. BMJ 2006, 332, 1355. [Google Scholar] [CrossRef] [PubMed]
- Uranga, A.; España, P.P.; Bilbao, A.; Quintana, J.M.; Arriaga, I.; Intxausti, M.; Lobo, J.L.; Tomás, L.; Camino, J.; Nuñez, J.; et al. Duration of Antibiotic Treatment in Community-Acquired Pneumonia. JAMA Intern. Med. 2016, 176, 1257. [Google Scholar] [CrossRef]
- Israelsen, S.B.; Tingsgård, S.; Thorlacius-Ussing, L.; Knudsen, A.; Lindegaard, B.; Johansen, I.S.; Mygind, L.H.; Ravn, P.; Benfield, T. Short-Course Antibiotic Therapy of 5 Days in Community-Acquired Pneumonia (CAP5): Study Protocol for a Randomised Controlled Trial. BMJ Open 2023, 13, e069013. [Google Scholar] [CrossRef]
- Patangia, D.V.; Anthony Ryan, C.; Dempsey, E.; Paul Ross, R.; Stanton, C. Impact of Antibiotics on the Human Microbiome and Consequences for Host Health. MicrobiologyOpen 2022, 11, e1260. [Google Scholar] [CrossRef]
- Hassan, M.; Gad-Allah, M.; El-Shaarawy, B.; El-Shazly, A.M.; Daneshvar, C.; Sadaka, A.S. The Short versus Long Antibiotic Course for Pleural Infection Management (SLIM) Randomised Controlled Open Label Trial. ERJ Open Res. 2023, 9, 00635–2022. [Google Scholar] [CrossRef]
- World Health Organization. The True Death Toll of COVID-19: Estimating Global Excess Mortality. World Health Organization. Available online: https://www.who.int/data/stories/the-true-death-toll-of-covid-19-estimating-global-excess-mortality (accessed on 16 February 2024).
- WHO. COVID-19 Deaths|WHO COVID-19 Dashboard. Datadot. Available online: https://data.who.int/dashboards/covid19/deaths?n=c (accessed on 16 February 2024).
- Furukawa, Y.; Luo, Y.; Funada, S.; Onishi, A.; Ostinelli, E.; Hamza, T.; Furukawa, T.A.; Kataoka, Y. Optimal Duration of Antibiotic Treatment for Community-Acquired Pneumonia in Adults: A Systematic Review and Duration-Effect Meta-Analysis. BMJ Open 2023, 13, e061023. [Google Scholar] [CrossRef]
- Abdelsalam Elshenawy, R.; Umaru, N.; Aslanpour, Z. WHO AWaRe Classification for Antibiotic Stewardship: Tackling Antimicrobial Resistance—A Descriptive Study from an English NHS Foundation Trust Prior to and during the COVID-19 Pandemic. Front. Microbiol. 2023, 14, 1298858. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. 2021 AWaRe Classification. Available online: https://www.who.int/publications/i/item/2021-aware-classification (accessed on 13 February 2024).
- Wehrenberg, K.; Mitchell, M.; Zembles, T.; Yan, K.; Zhang, L.; Thompson, N. Antibiotic Treatment Duration for Culture-Negative Sepsis in the Pediatric Intensive Care Unit. Antimicrob. Steward. Healthc. Epidemiol. 2023, 3, e249. [Google Scholar] [CrossRef]
- Elshenawy, R.A.; Umaru, N.; Aslanpour, Z. An Evaluation of the Five Rights Antibiotic Safety before and during COVID-19 at an NHS Foundation Trust in the United Kingdom. J. Glob. Antimicrob. Resist. 2024, 36, 188–189. [Google Scholar] [CrossRef]
- Dinh, A.; Davido, B.; Bouchand, F.; Duran, C.; Ropers, J.; Crémieux, A.-C. Honey, I Shrunk the Antibiotic Therapy. Clin. Infect. Dis. 2018, 66, 1981–1982. [Google Scholar] [CrossRef]
- Dinh, A.; Ropers, J.; Duran, C.; Davido, B.; Deconinck, L.; Matt, M.; Senard, O.; Lagrange, A.; Makhloufi, S.; Mellon, G.; et al. Discontinuing β-Lactam Treatment after 3 Days for Patients with Community-Acquired Pneumonia in Non-Critical Care Wards (PTC): A Double-Blind, Randomised, Placebo-Controlled, Non-Inferiority Trial. Lancet 2021, 397, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and Elaboration. PLoS Med. 2007, 4, e297. [Google Scholar] [CrossRef] [PubMed]
- ISRCTN. Antibiotic Prescribing in an English Secondary Care Setting before and during the COVID-19 Pandemic. Available online: https://www.isrctn.com/ISRCTN14825813 (accessed on 1 February 2024).
- Elshenawy, R. How Did the COVID-19 Pandemic Impact Antibiotic Prescribing and Antimicrobial Stewardship in Acute Care Settings?—Octopus|Built for Researchers. 2022. Available online: https://www.octopus.ac/publications/372b-6747/versions/1 (accessed on 10 February 2024).
- Public Health England. Research Reveals Levels of Inappropriate Prescriptions in England. Available online: https://www.gov.uk/government/news/research-reveals-levels-of-inappropriate-prescriptions-in-england (accessed on 11 February 2024).
- IBM. SPSS Statistics 22.0. Available online: https://www.ibm.com/support/pages/spss-statistics-220-available-download (accessed on 3 January 2024).
- RStudio. Previous Releases of R for Windows. Available online: https://cran.r-project.org/bin/windows/base/old/ (accessed on 20 January 2024).
| Duration of Antibiotic Use | p-Value | |||
|---|---|---|---|---|
| ≤5 Days | 6–7 Days | >8 Days | ||
| n (%) | n (%) | n (%) | ||
| Age (Median, IQR) | 79 (21) | 80 (17) | 79.5 (19) | 0.8 |
| WBCs (Median, IQR) | 34.1 (123) | 46 (131) | 16.5 (117) | 0.3 |
| CRP (Median, IQR) | 76 (206) | 91 (291) | 103.5 (240) | 0.7 |
| LOS (Median, IQR) | 8 (10) | 9 (10) | 15 (17) | <0.01 |
| Patient Characteristics | Duration of Antibiotic Use | p-Value | ||||
|---|---|---|---|---|---|---|
| ≤5 Days n = 463 | 6–7 Days n = 109 | >8 Days n = 68 | ||||
| n (%) | n (%) | n (%) | ||||
| Demographic characteristics | Year | 2019 | 237 (51.2) | 53 (48.6) | 32 (47.1) | 0.3 |
| 2020 | 226 (48.8) | 56 (51.4) | 36 (52.9) | |||
| Gender | Male | 227 (49) | 60 (55) | 34 (50) | 0.6 | |
| Female | 236 (51) | 49 (45) | 34 (50) | |||
| Patient Outcomes | Discharged | 384 (82.9) | 97 (89) | 61 (89.7) | 0.9 | |
| Died | 79 (17.1) | 12 (11) | 7 (10.3) | |||
| Allergy | 36 (7.8) | 11 (10.1) | 6 (8.82) | 0.6 | ||
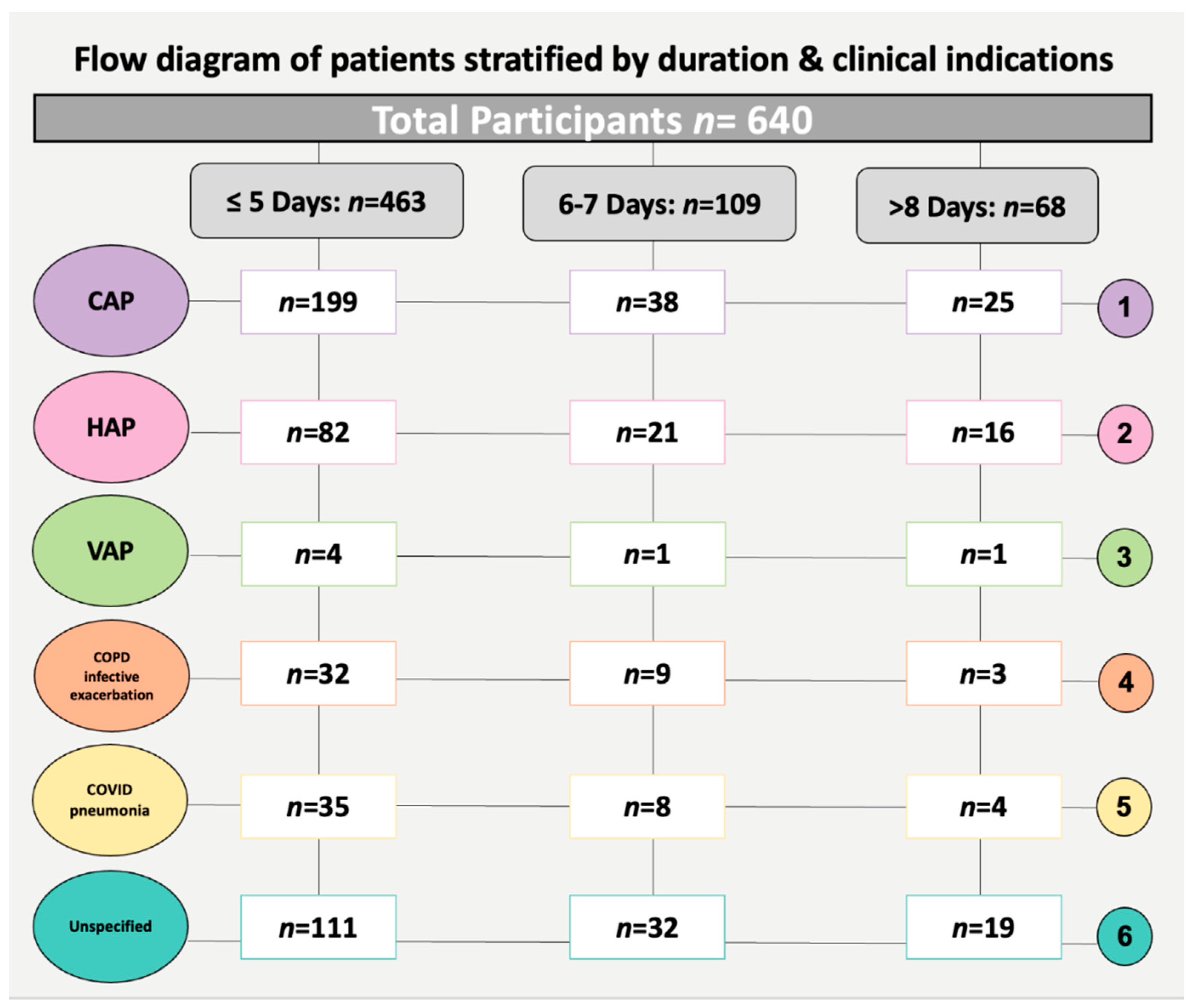
| Clinical characteristics | Indication | CAP | 199 (43) | 38 (34.9) | 25 (36.8) | 0.9 |
| HAP | 82 (17.7) | 21 (19.3) | 16 (23.5) | 0.7 | ||
| VAP | 4 (0.9) | 1 (0.9) | 1 (1.5) | - | ||
| COPD infective exacerbation | 32 (6.9) | 9 (8.3) | 3 (4.4) | 0.6 | ||
| COVID-19 pneumonia | 35 (7.6) | 8 (7.3) | 4 (5.9) | 0.6 | ||
| Unspecified | 111 (24) | 32 (29.4) | 19 (27.9) | 0.4 | ||
| Comorbidities | Hypertension (HPN) | 212 (45.8) | 47 (43.1) | 32 (47) | 0.5 | |
| Heart failure (HF) | 63 (13.6) | 24 (22) | 8 (11.8) | 0.3 | ||
| Hypercholesterolemia | 69 (14.9) | 17 (15.6) | 12 (17.6) | 0.3 | ||
| Diabetes mellitus (DM) | 79 (17.1) | 22 (20.1) | 18 (26.5) | 0.6 | ||
| Asthma | 41 (8.9) | 11 (10.1) | 4 (5.9) | 0.1 | ||
| Chest X-rays | Pneumonia | 66 (14.3) | 16 (14.5) | 11 (16.2) | 0.3 | |
| No pneumonia | 107 (23.1) | 22 (20.1) | 18 (26.5) | |||
| Antibiotic | Duration Category | p-Value | ||
|---|---|---|---|---|
| ≤5 Days n = 463 | 6–7 Days n = 109 | >8 Days n = 68 | ||
| n (%) | n (%) | n (%) | ||
| Amoxicillin | 16 (3.5) | 4 (3.7) | 1 (1.5) | - |
| Amoxicillin/Clavulanic Acid | 283 (61.1) | 61 (56) | 36 (52.9) | - |
| Azithromycin | 5 (1.1) | 0 (0) | 2 (2.9) | - |
| Benzylpenicillin | 3 (0.6) | 1 (0.9) | 1 (1.5) | - |
| Ceftazidime | 6 (1.3) | 1 (0.9) | 2 (2.9) | - |
| Ciprofloxacin | 11 (2.4) | 2 (1.8) | 4 (5.9) | - |
| Clarithromycin | 23 (5) | 7 (6.4) | 2 (2.9) | - |
| Levofloxacin | 50 (10.8) | 6 (5.5) | 3 (4.4) | - |
| Metronidazole | 4 (0.9) | 1 (0.9) | 4 (5.9) | 0.01 |
| Piperacillin/Tazobactam | 53 (11.4) | 22 (20.2) | 12 (17.6) | 0.007 |
| Indication (n, %) | Duration of Antibiotic Use | p-Value | |||
|---|---|---|---|---|---|
| ≤5 Days n = 463 | 6–7 Days n = 109 | >8 Days n = 68 | |||
| n (%) | n (%) | n (%) | |||
| Appropriateness of antibiotics | CAP (262, 40.9%) | 84 (18.1) | 25 (22.9) | 14 (20.6) | 0.02 |
| HAP (119, 18.6%) | 45 (9.7) | 11 (10.1) | 7 (10.3) | 0.7 | |
| VAP (6, 0.9%) | 3 (0.6) | 1 (0.9) | 1 (1.5) | 0.6 | |
| COPD infective exacerbation (44, 6.9%) | 17 (3.7) | 5 (4.6) | 1 (1.5) | 0.6 | |
| COVID-19 pneumonia (47, 7.3%) | 8 (1.7) | 1 (0.9) | 1 (1.5) | 0.4 | |
| Unspecified (162, 25.3%) | 7 (1.5) | 2 (1.8) | 0 (0) | 0.07 | |
| Overall (640, 100%) | 164 (35.4) | 45 (41.3) | 24 (35.3) | 0.5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelsalam Elshenawy, R.; Umaru, N.; Aslanpour, Z. Shorter and Longer Antibiotic Durations for Respiratory Infections: To Fight Antimicrobial Resistance—A Retrospective Cross-Sectional Study in a Secondary Care Setting in the UK. Pharmaceuticals 2024, 17, 339. https://doi.org/10.3390/ph17030339
Abdelsalam Elshenawy R, Umaru N, Aslanpour Z. Shorter and Longer Antibiotic Durations for Respiratory Infections: To Fight Antimicrobial Resistance—A Retrospective Cross-Sectional Study in a Secondary Care Setting in the UK. Pharmaceuticals. 2024; 17(3):339. https://doi.org/10.3390/ph17030339
Chicago/Turabian StyleAbdelsalam Elshenawy, Rasha, Nkiruka Umaru, and Zoe Aslanpour. 2024. "Shorter and Longer Antibiotic Durations for Respiratory Infections: To Fight Antimicrobial Resistance—A Retrospective Cross-Sectional Study in a Secondary Care Setting in the UK" Pharmaceuticals 17, no. 3: 339. https://doi.org/10.3390/ph17030339
APA StyleAbdelsalam Elshenawy, R., Umaru, N., & Aslanpour, Z. (2024). Shorter and Longer Antibiotic Durations for Respiratory Infections: To Fight Antimicrobial Resistance—A Retrospective Cross-Sectional Study in a Secondary Care Setting in the UK. Pharmaceuticals, 17(3), 339. https://doi.org/10.3390/ph17030339