Abstract
Bopyeo-tang (BPT), comprising six medicinal plants, has been used for the treatment of respiratory diseases such as pulmonary fibrosis and chronic obstructive pulmonary disease. In this study, we developed and validated a quantitative method for the quality assessment of BPT using ultra-performance liquid chromatography with tandem mass spectrometry (UPLC–MS/MS). Eighteen marker compounds were separated on an Acquity UPLC BEH C18 reversed-phase column (2.1 mm × 100 mm, 1.7 μm) via gradient elution with a 0.1% aqueous formic acid–acetonitrile mobile phase. The multiple-reaction monitoring mode was used to improve analysis speed and accuracy. The coefficients of determination, limits of detection, and limits of quantitation of the 18 marker compounds were 0.9991–0.9996, 0.36–24.45 μg/L, and 1.07–73.35 μg/L, respectively. The recovery was 85.19–110.25%, and the relative standard deviation of precision was ≤9.01%. When applied to a typical BPT sample, the method revealed a range of concentrations from below the quantitative limit (one compound only) to a maximum of 3.20 mg/freeze-dried g. This method will be used for quality control of BPT preparations.
1. Introduction
Bopyeo-tang (BPT), called Bufei-tang or simply Bufei in China, is a traditional herbal formula comprising parts or extracts of six medicinal plants: Morus alba L. (Mori Radicis Cortex), Rehmannia glutinosa (Gaertn.) DC. (Rehmanniae Radix Preparata), Panax ginseng C.A.Mey. (Ginseng Radix), Aster tataricus L.f. (Asteris Radix et Rhizoma), Astragalus propinquus Schischkin (Astragali Radix), and Schisandra chinensis (Turcz.) Baill. (Schisandrae Fructus). BPT has been widely used in the clinical treatment of respiratory diseases such as chronic obstructive pulmonary disease (COPD) [1,2,3].
COPD is a disease that causes airflow obstruction and breathing problems, with symptoms such as chronic coughing, phlegm production, wheezing, and dyspnea [2,4]. The disease kills approximately three million people worldwide a year. Smoking and exposure to harmful substances are the main causes; both are irreversible risk factors from exposure [2,4,5]. The worldwide prevalence of COPD is estimated at between 7.5% and 10% [6].
Among the many herbal formulas used as medicines, Yupingfeng san, the Bufei Yishen formula, the Jia-Wei-Bu-Shein-Yi-Qi formula, and San-Huang Gu-Ben Zhi-Ke have each been used to treat COPD [7,8,9,10]. All these formulas contain Astragali Radix. Astragali Radix extract, Astragali Radix polysaccharide fraction, and astragaloside, a major component of Astragali Radix, were reported to be effective when used as COPD treatments [11,12,13]. Mori Radicis Cortex, Rehmanniae Radix Preparata, Ginseng Radix, Asteris Radix et Rhizoma, and Schisandrae Fructus—which are other components of BPT—have also been reported to be effective as COPD treatments [14,15]. In addition to its efficacy against COPD, positive effects on lung cancer and pulmonary fibrosis have also been demonstrated [16,17,18,19].
We conducted a study to establish and verify an analytical method for the quality control of traditional herbal formulas using high-performance liquid chromatography or ultra-performance liquid chromatography with tandem mass spectrometry (UPLC–MS/MS) [20,21,22,23,24]. However, the only analytical study on BPT was conducted by He et al. [18]. This is the only study using a high-performance liquid chromatography–diode array detection electrospray ionization–hybrid ion trap–time of flight mass spectrometry method, detecting 89 peaks from BPT and identifying 47 of these [18]. In addition, they identified acetylene glycosides (from Cononopsis Radix), flavonoids and saponins (Astragali Radix), lignan derivatives (Schisandrae Fructus), organic acidic and iridoid compounds (Rehmanniae Radix Preparata), organic acidic and peptides compounds (Asteris Radix et Rhizoma), and styrenes (Mori Radicis Cortex). However, they used BPT containing Cononopsis Radix instead of Ginseng Radix, but more importantly, each analysis takes approximately 150 min [18]. Therefore, there is scope for a BPT analysis method capable of quantitatively analyzing BPT’s major components in a shorter time.
In the present study, a quantitative and simultaneous analyzing method for the efficient quality control of BPT is described and validated using a more sensitive and accurate technique—ultra-performance liquid chromatography with tandem mass spectrometry (UPLC–MS/MS). The marker analytes for simultaneous analysis were mulberroside A, hydroxymethylfurfural, chlorogenic acid, rutin, calycosin 7-O-glucoside, isoquercetin, 3,4-dicaffeoylquinic acid, 3,5-dicaffeoylquinic acid, ginsenoside Rg1, resveratrol, calycosin, quercetin, kaempferol, ginsenoside Rb1, astragaloside IV, schizandrin, gomisin A, and gomisin N.
2. Results and Discussion
2.1. Selection of Marker Compounds for Quality Assessment of BPT Using the UPLC–MS/MS Multiple-Reaction Monitoring (MRM) Method
Most traditional herbal formulas are complex preparations consisting of two or more different herbal medicines, contain numerous ingredients, and show various targets and various effects. Therefore, a scientific approach is needed to evaluate the quality of these traditional herbal formulas.
In this study, to develop a simultaneous determination analysis method using UPLC–MS/MS, the 18 dominant compounds comprising BPT were chosen as markers. These were mulberroside A, chlorogenic acid, rutin, isoquercetin, and resveratrol from M. alba [25,26,27], hydroxymethylfurfural from R. glutinosa [28], ginsenoside Rb1 and ginsenoside Rg1 from P. ginseng [29], chlorogenic acid, isochlorogenic acid A, isochlorogenic acid B, quercetin, and kaempferol from A. tataricus [30,31], astragaloside IV, calycosin, and calycosin 7-O-glucoside from A. propinquus [32,33], and schizandrin, gomisin A, and gomisin N from S. chinensis [34].
2.2. Identification of the 18 Marker Compounds via the UPLC–MS/MS MRM Method
In the UPLC–MS/MS electrospray ionization mode, 9 of the 18 markers—mulberroside A, hydroxymethylfurfural, calycosin 7-O-glucoside, quercetin, kaempferol, astragaloside IV, schizandrin, gomisin A, and gomisin N—were detected in positive-ion mode as [M+H]+. The other nine compounds—chlorogenic acid, rutin, iosquercetin, isochlorogenic acid B, isochlorogenic acid A, ginsenoside Rg1, resveratrol, calycosin, and ginsenoside Rb1—were detected in negative-ion mode as the [M–H]− form (Table 1, Figure 1, Figures S1 and S2).

Table 1.
Parameters for UPLC–MS/MS MRM of the 18 marker compounds.
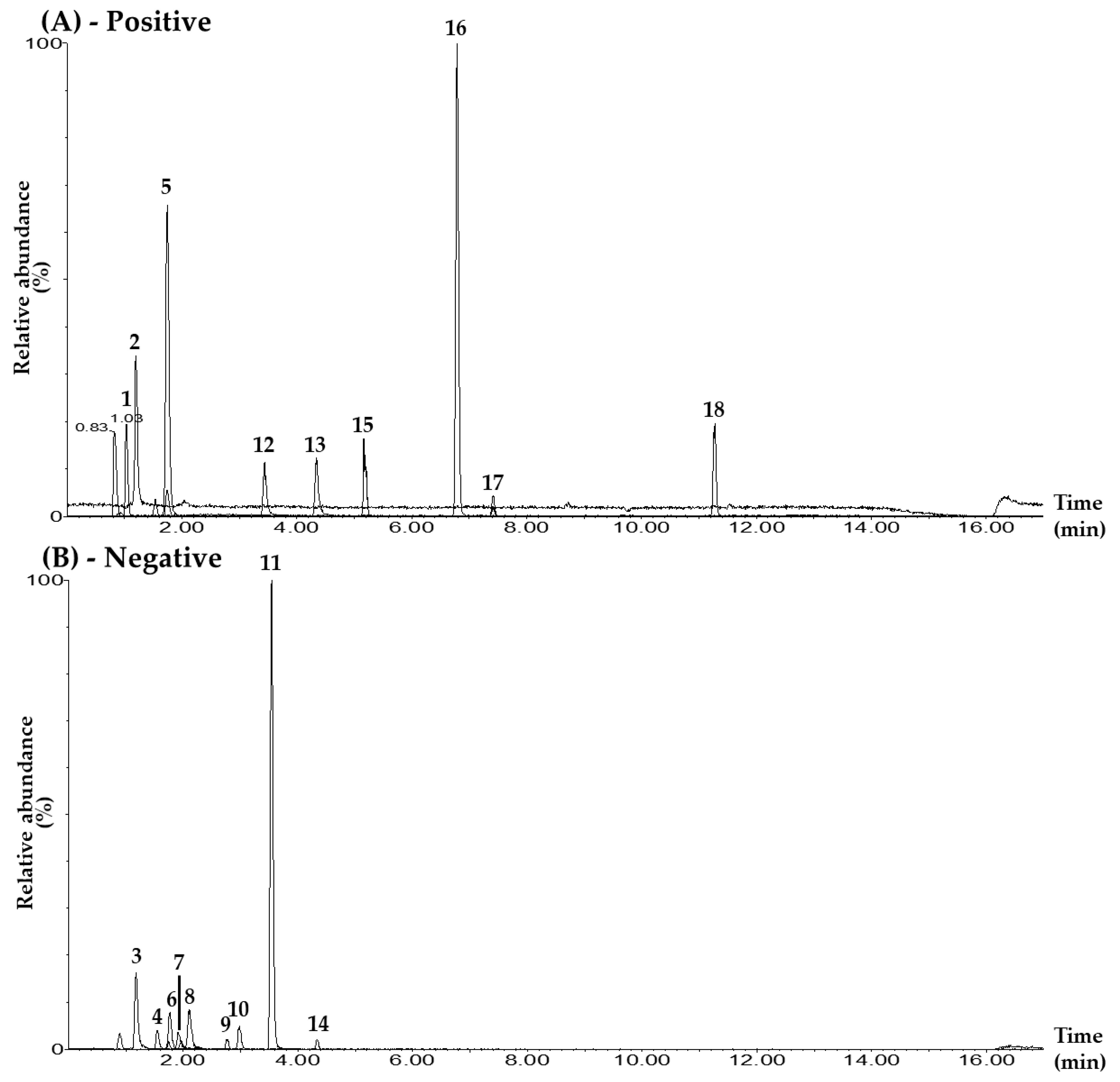
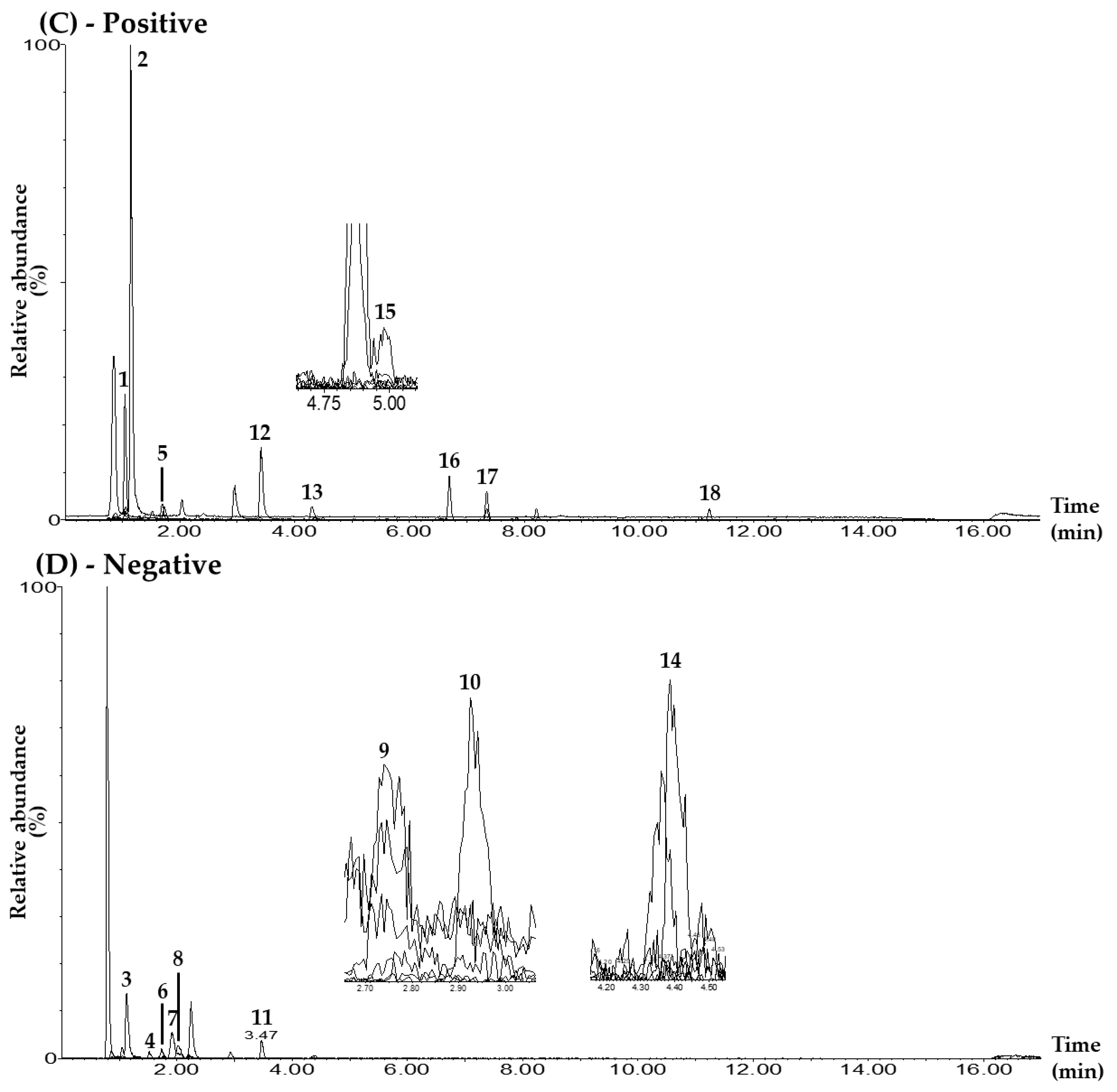
Figure 1.
Representative total ion chromatograms of the mixed standard solution (A,B) and the BPT sample (C,D) under the UPLC–MS/MS MRM method in positive-ion (A,C) and negative-ion (B,D) modes. Mulberroside A (1), hydroxymethylfurfural (2), chlorogenic acid (3), rutin (4), calycosin 7-O-glucoside (5), isoquercetin (6), isochlorogenic acid B (7), isochlorogenic acid A (8), ginsenoside Rg1 (9), resveratrol (10), calycosin (11), quercetin (12), kaempferol (13), ginsenoside Rb1 (14), astragaloside IV (15), schizandrin (16), gomisin A (17), and gomisin N (18). The concentrations of each compound in the mixed standard solution were as follows: 10.00 μg/L (hydroxymethylfurfural, chlorogenic acid, rutin, calycosin 7-O-glucoside, isoquercetin, isochlorogenic acid A, resveratrol, calycosin, kaempferol, schizandrin, gomisin A, and gomisin N); 50.00 μg/L (quercetin); 100.00 μg/L (isochlorogenic acid B); 500.00 μg/L (ginsenoside Rb1); 2500.00 μg/L (mulberroside A); and 5000.00 μg/L (ginsenoside Rg1 and astragaloside IV).
For quantitative analysis, the conditions of the MRM transitions (precursor ion (Q1) and product ion (Q3)) of each marker compound were set as shown in Table 1 and Figure S3. The MRM analysis method has the advantage of being able to quantify multiple compounds simultaneously by setting the cone voltage and collision energy for each compound separately in UPLC–MS/MS analysis. The Q3 peaks of mulberroside A and isoquercetin were set to m/z 245.0 and 300.0 in the form of [M+H–2Glu]+ and [M–H–Glu]–, respectively, where two glucose groups and one glucose group were lost from each Q1 peak [35,36]. The Q3 peaks of hydroxymethylfurfural, schizandrin, and gomisin A were set to the [M+H–H2O]+ form generated at m/z 109.0, 415.0, and 399.0 via the loss of one water molecule from each Q1 peak, respectively [37,38]. The Q3 peak for chlorogenic acid was set at m/z 191.0 for the [M–H–C9H7O3]– form, with the caffeoyl group removed from the Q1 peak [39]. The Q3 peaks for isochlorogenic acid B and astragaloside IV were set at m/z 173.0 and 142.9 for the [M–H–2caffeoyl]– and [M+H–C33H53O12]+ forms, where two caffeoyl groups and the glucose-xylose-C22H33O2 functional group were removed from the Q1 peak at m/z 515.5 and 785.4, respectively [39,40]. For the three compounds, rutin, isochlorogenic acid A, and gomisin N, Q3 peaks were set to [M–H–Rhm–Glc]–, [Caffeoyl group]–, and [M+H–(OCH3–H)]+ forms at m/z 300.5, 352.7, and 371.0, respectively [36,39,41]. The Q3 peaks of calycosin 7-O-glucoside and ginsenoside Rg1 were set to m/z 285.1 and 636.8, respectively, in the form of [M+H–Glu]+ and [M–H–Glu]–, in which a glucose molecule was lost from each Q1 peak [42,43]. The Q3 peaks for resveratrol and calycosin were set at m/z 185.0 and 267.9 for the [M–H–C2H2O]– and [M–H–CH3]− forms, where the C2H2O moiety and methyl group were removed from the Q1 peak at m/z 227.2 and 283.4, respectively [44,45]. For MRM transitions of the flavonoids quercetin and kaempferol, the Q3 peaks were detected at m/z 153.0 and 153.2, and were both generated via the cleavage of the C-ring through the retro Diels–Alder reaction [46]. For the MRM transition of ginsenoside Rb1, the peak at m/z 179.0—where glucose was detected in the Q1 peak—was selected as the Q3 peak [47].
2.3. Validation of the UPLC–MS/MS MRM Method
In the developed analytical method, validation is a very important factor in the standardization of traditional herbal formulas. This is necessary to ensure the ultimate validity, practicality, and reproducibility of the scientific methods used in the developed assay.
The regression equations and values of the coefficient of determination (r2), limit of detection (LOD), and limit of quantitation (LOQ) tested for different concentrations of each marker are summarized in Table 2. The r2 values spanned from 0.9991 to 0.9996, showing high linearity, and the LOD and LOQ concentrations were 0.36–24.45 μg/L and 1.07–73.35 μg/L, respectively. Table 3 shows the recovery, precision, and repeatability results for the markers. The recoveries were 85.19–110.25% (relative standard deviation (RSD) ≤ 6.60%), which were within the acceptable range of 80–120%. The RSD value of precision was ≤9.01%, which was within the tolerance level of ±20%. In the stability test, these 18 marker compounds were considered stable for at least 3 days as the change in peak area content was less than 3.0% within 3 days (Table 3).

Table 2.
Retention times, linear ranges, regression equations, coefficients of determination (r2), limits of detection (LOD), and limits of quantitation (LOQ) for the simultaneous determination of the 18 marker compounds using the UPLC–MS/MS MRM method.

Table 3.
Recovery, precision, and stability data of the 18 marker compounds for the UPLC–MS/MS MRM method.
2.4. Simultaneous Determination of the 18 Marker Compounds in a BPT Sample via the UPLC–MS/MS MRM Method
The new method was applied for the simultaneous determination of the 18 compounds in a BPT sample (Table 4). Among these compounds, gomisin A was detected below the LOQ. The other compounds were detected at 0.001–3.20 mg/freeze-dried g of BPT. Hydroxymethylfurfural—a major component of R. glutinosa—was by far the most abundant compound, at 3.20 mg/g, in this BPT sample.

Table 4.
Amounts of the 18 marker compounds in a BPT sample, determined using the UPLC–MS/MS MRM method.
3. Materials and Methods
3.1. Plant Materials
The six raw medicinal herbs that BPT is composed of—Mori Radicis Cortex (Moraceae, China), Rehmanniae Radix Preparata (Plantaginaceae, Gunwi, Republic of Korea), Ginseng Radix (Araliaceae, Geumsan, Republic of Korea), Asteris Radix et Rhizoma (Compositae, China), Astragali Radix (Lequminosae, Jecheon, Republic of Korea), and Schisandrae Fructus (Schisandraceae, Samcheok, Republic of Korea)—were purchased from Kwangmyungdang Pharmaceutical (Ulsan, Republic of Korea) in 2018. They were used for this research after morphological identity confirmation by Dr. Goya Choi, a herbalist at the Korea Institute of Oriental Medicine (KIOM, Daejeon, Republic of Korea). Six crude material specimens (CA05–1 to CA05–6) were kept at the KM Science Research Division, KIOM.
3.2. Chemicals and Reagents
The 18 reference marker compounds (Figure S4) used for simultaneous determination in BPT via the UPLC–MS/MS system were purchased from high-purity natural product manufacturers such as EnsolBioSciences (Daejeon, Republic of Korea), Merck KGaA (Darmstadt, Germany), Shanghai Sunny Biotech (Shanghai, China), Biopurify Phytochemicals (Chengdu, China), and Wuhan ChemFaces Biochemical (Wuhan, China). Detailed information on these marker compounds is summarized in Table S1. LC–MS-grade organic solvents (methanol and acetonitrile) and reagents (formic acid) were purchased from Thermo Fisher Scientific (Cleveland, OH, USA). Ultrapure deionized water was obtained using the Elix Technology Inside system (Milli-Q Integral 15, Merck, Millipore, France).
3.3. Preparation of a BPT Water Extract
A BPT water extract was prepared by KIOM based on previously reported extraction protocols [20,21,22,23,24]. Briefly, Mori Radicis Cortex (1500 g, Moraceae, China), Rehmanniae Radix Preparata (1500 g, Scrophulariaceae, Gunwi, Korea), Ginseng Radix (500 g, Ariliaceae, Geumsan, Republic of Korea), Asteris Radix et Rhizoma (500 g, Compositae, China), Astragali Radix (500 g, Leguminosae, Jecheon, Republic of Korea), and Schisandrae Fructus (500 g, Schisandraceae, Samcheok, Republic of Korea) were well mixed and extracted with 50 L of (deionized) water in a COSMOS-660 extractor (Kyungseo E&P, Incheon, Republic of Korea) at 100 °C for 2 h. The cooled extract was lyophilized using an LP100R freeze-dryer (IlShinBioBase, Dongducheon, Republic of Korea) to obtain a powder sample (1600 g, a 32% yield) that was held at −20 °C before use.
3.4. Analytical Conditions for the Simultaneous Quantification of Markers in a BPT Sample via the UPLC–MS/MS MRM Method
The simultaneous quantification of the 18 marker compounds was performed by modifying analytical protocols previously reported to the UPLC–MS/MS system comprising a Waters Acquity UPLC I-Class Plus system and a Xevo TQ-XS triple quadrupole mass spectrometry system (Waters, Milford, MA, USA) [23,24]. The chromatographic separation of all markers was carried out using a 0.1% (v/v) aqueous formic acid–acetonitrile mobile phase system and an Acquity UPLC BEH reverse-phase column (Waters) maintained at 45 °C. Detailed operating conditions for the UPLC and mass spectrometry systems are described in Table 5. In addition, the operating parameters for the MRM of each compound are listed in Table 1.

Table 5.
Operating conditions for the UPLC–MS/MS multiple-reaction monitoring (MRM) simultaneous analysis of BPT.
Standard stock solutions of each reference standard compound were prepared at a concentration of 1.0 mg/mL using methanol, stored in a refrigerator (approximately 4 °C), and used for analysis.
The sample solution for UPLC–MS/MS analysis was prepared at a concentration of 0.05 mg/mL with 70% methanol. That is, we took exactly 0.5 mg of BPT sample, placed it in a 10 mL volumetric flask, and added 70% methanol. The mixed sample solution was continuously subjected to ultrasonic extraction for 5 min and vortexing for 1 min. The solution was filtered through a 0.22 μm polytetrafluoroethylene hydrophobic membrane filter (catalog No. SSKPTFE13022B, SsolKorea, Daejeon, Republic of Korea) before use in UPLC–MS/MS analysis.
3.5. Validation of the UPLC–MS/MS MRM Method
For validation linearity, sensitivities (LOD and LOQ), accuracy, and precision parameters were evaluated using the International Conference on Harmonisation guidelines, specifically the methods for there Q2B validation of analytical procedures [48]. Briefly, linearity was evaluated using the r2 value of the calibration regression equation for each marker compound. The sensitivities, LOD and LOQ, were calculated from signal-to-noise ratios of 3:1 and 10:1, respectively.
Recovery was determined using a standard addition method. In general, it is difficult to source reference samples to be used as blanks in herbal medicine analysis that do not contain marker components. Therefore, in this study, recoveries were calculated by adding the authentic compounds the BPT extract at three concentrations (low, medium, and high) and subtracting the endogenous quantity in the sample. The calculation equation was as follows:
Intraday and interday precisions were determined in the same way recovery was, within one day and over three consecutive days, and then evaluated as RSD (%), which was calculated as follows:
Finally, the stability of 18 marker compounds was tested for 3 days at room temperature (23 ± 1 °C) using the sample solution and was evaluated using the RSD values.
4. Conclusions
We developed and validated a fast, sensitive, and accurate UPLC–MS/MS MRM method to quantify the quality of BPT, which is widely prescribed in oriental medicine for respiratory diseases. This method was validated for linearity, sensitivities (LOD and LOQ), accuracy, and precision. In a practical trial with a BPT sample, hydroxymethylfurfural, a major component of R. glutinosa, was detected in the highest abundance. We believe that the knowledge developed in this study will be useful for efficacy studies.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ph17030352/s1: Figure S1: Total ion chromatograms of a blank UPLC–MS/MS MRM trial in positive- and negative-ion modes; Figure S2: Extracted ion chromatograms of each standard compound (A) and of a BPT sample (B) obtained via the UPLC–MS/MS MRM method in positive- or negative-ion modes; Figure S3: Precursor ion (Q1) and product ion (Q3) peaks for each marker compound. Mulberroside A (A), hydroxymethylfurfural (B), chlorogenic acid (C), rutin (D), calycosin 7-O-glucoside (E), isoquercetin (F), 3,4-dicaffeoylquinic acid (G), 3,5-dicaffeoylquinic acid (H), ginsenoside Rg1 (I), resveratrol (J), calycosin (K), quercetin (L), kaempferol (M), ginsenoside Rb1 (N), astragaloside IV (O), schizandrin (P), gomisin A (Q) and gomisin N (R); Figure S4: Chemical structures of the 18 marker compounds selected for simultaneous determination in BPT; Table S1: Information about the 18 reference standard compounds.
Funding
This research was funded by the Korea Institute of Oriental Medicine (KSN2022220 and KSN1823221).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data of this study can be found in this paper.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Heo, J. Donguibogan; Namsandang: Seoul, Republic of Korea, 2007; p. 470. [Google Scholar]
- Shen, J.; Zhu, X.; Chen, Y.; Li, W.; Liu, H.; Chu, C.; Zhang, Y.; Xu, C.; Tong, P.; Yu, X.; et al. Bufei decoction improves lung-qi deficiency syndrome of chronic obstructive pulmonary disease in rats by regulating the balance of Th17/Treg cells. Evid. Based Complement. Alternat. Med. 2022, 2022, 1459232. [Google Scholar] [CrossRef]
- Zhang, L.; Tian, Y.; Zhao, P.; Feng, S.; Han, X.; Li, J. Network pharmacology analysis uncovers the effect on apoptotic pathway by Bu-Fei formula for COPD treatment. J. Ethnopharmacol. 2022, 289, 115022. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Liu, X.; Jiang, Y.; Guan, Q.; Tian, Y.; Li, J.; Zhao, P. Maintenance of airway epithelial barrier integrity via the inhibition of AHR/EGFR activation ameliorates chronic obstructive pulmonary disease using effective-component combination. Phytomedicine 2023, 118, 154980. [Google Scholar] [CrossRef] [PubMed]
- Stone, P.W.; Osen, M.; Ellis, A.; Coaker, R.; Quint, J.K. Prevalence of chronic obstructive pulmonary disease in England from 2000 to 2019. Int. J. Chron. Obstruct. Pulmon. Dis. 2023, 18, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.I.; Park, Y.B.; Yoo, K.H. Recent trends in the prevalence of chronic obstructive pulmonary disease in Korea. Tuberc. Respir. Dis. 2017, 80, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Wei, W.; Yao, C.; Wu, S.; Wang, W.; Guo, D. Advances in the chemical constituents, pharmacological properties and clinical applications of TCM formula Yupingfeng San. Fitoterapia 2023, 164, 105385. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, S.; Cui, L.; Chen, Y.; Xu, X.; Wu, L. Bufei Tishen formula inhibits the cell senescence in COPD by up-regulating the znf263 and Klotho expression. Int. J. Chron. Obstruct. Pulmon. Dis. 2023, 18, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Deng, L.; Zhou, Y.; Yuy, H.; Huang, X.; Chen, M.; Lei, Y.; Dong, J. Network pharmacology and experiments in vivo and in vitro reveal that the Jia-Wei-Bu-Shen-Yi-Qi formula (JWBSYQF) and its active ingredient baicalein ameliorate BLM-induced lung fibrosis in mice via PI3K/Akt signaling pathway. J. Ethnopharmacol. 2023, 315, 116691. [Google Scholar] [CrossRef] [PubMed]
- Lyu, T.; Li, D.; Lei, X.; Zhang, Y.; Cheng, S.; Shu, X.; Zhang, H. Effects of the Chinese herbal formula San-Huang Gu-Ben Zhi-Ke treatment on stable chronic obstructive pulmonary disease: A randomized, double-blind, placebo-controlled trial. Front. Pharmacol. 2023, 14, 1164818. [Google Scholar] [CrossRef]
- Wu, L.; Chen, Y.; Xu, Y.; Guo, X.; Li, X.; Zhang, A.L.; May, B.H.; Xue, C.C.; Wen, Z.; Lin, L. Oral Guangqi formulae for stable chronic obstructive pulmonary disease: A systematic review and meta-analysis. Evid. Based Complement. Alternat. Med. 2013, 2013, 705315. [Google Scholar]
- Chu, X.; Liu, J.M.; Zeng, X.L.; Bao, H.R.; Shu, J. Effects of Astragalus and Codonopis pilosula polysaccharides on alveolar macrophage phagocytosis and inflammation in chronic obstructive pulmonary disease mice exposed to PM2.5. Environ. Toxicol. Pharmacol. 2016, 48, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tan, Y.; Gao, K.; Lei, J.; Chen, C.; Shi, Y. Effect of astragaloside on diaphragm cell apoptosis in chronic obstructive pulmonary disease. Food Sci. Nutr. 2020, 8, 6357–6366. [Google Scholar] [CrossRef] [PubMed]
- Timalsina, D.; Pokhrel, K.P.; Bhusal, D. Pharmacologic activities of plant-derived natural products on respiratory diseases and inflammations. Biomed Res. Int. 2021, 2021, 1636816. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, H.; Li, Y.; Liu, J.; Jia, Z.; Xu, W.; Xiao, H.; Wang, W. Aster tataricus attenuates asthma efficiently by simultaneously inhibiting tracheal ring contraction and inflammation. Biomed. Pharmacother. 2020, 130, 110616. [Google Scholar] [CrossRef]
- Jiang, S.T.; Han, S.Y.; Pang, L.N.; Jiao, Y.N.; He, X.R.; Li, P.P. Bu-Fei decoction and modified Bu-Fei decoction inhibit the growth of non-small cell lung cancer, possibly via inhibition of apurinic/apyrimidinic endonuclease 1. Int. J. Mol. Med. 2018, 41, 2128–2138. [Google Scholar] [CrossRef]
- Pang, L.; Han, S.; Jiao, Y.; Jiang, S.; He, X.; Li, P. Bu Fei Decoction attenuates the tumor associated macrophage stimulated proliferation, migration, invasion and immunosuppression of non-small cell lung cancer, partially via IL-10 and PD-L1 regulation. Int. J. Oncol. 2017, 51, 25–38. [Google Scholar] [CrossRef]
- He, X.R.; Han, S.Y.; Li, X.H.; Zheng, W.X.; Pang, L.N.; Jiang, S.T.; Li, P.P. Chinese medicine Bu-Fei decoction attenuates epithelial-mesenchymal transition of non-small cell lung cancer via inhibition of transforming growth factor β1 signaling pathway in vitro and in vivo. J. Ethnopharmacol. 2017, 204, 45–57. [Google Scholar] [CrossRef]
- Yang, S.; Cui, W.; Wang, M.; Xing, L.; Wang, Y.; Zhu, P.; Qu, Q.; Tang, Q. Bufei decoction alleviated bleomycin-induced idiopathic pulmonary fibrosis in mice by anti-inflammation. Evid. Based Complement. Alternat. Med. 2020, 2020, 7483278. [Google Scholar] [CrossRef]
- Seo, C.S.; Lee, M.Y. Simultaneous quantification of eight marker components in traditional herbal formula, Haepyoyijin-tang using HPLC–PDA. Appl. Sci. 2020, 10, 3888. [Google Scholar] [CrossRef]
- Seo, C.S.; Lee, M.Y. Quality assessment of Insamyangpye decoction by liquid chromatography tandem mass spectrometry multiple reaction monitoring. Processes 2021, 9, 831. [Google Scholar] [CrossRef]
- Seo, C.S.; Lee, M.Y. Method development and validation for simultaneous analysis of eleven components for quality control of Geumgwesingihwan using HPLC–DAD and UPLC–MS/MS. Separations 2022, 9, 213. [Google Scholar] [CrossRef]
- Seo, C.S.; Shin, H.K. Validation of the ultra-performance liquid chromatography with tandem mass spectrometry method for simultaneous analysis of eighteen compounds in the traditional herbal prescription, Sanjoin-tang. Separations 2023, 10, 411. [Google Scholar] [CrossRef]
- Seo, C.S.; Shin, H.K. Ultra-performance liquid chromatography with tandem mass spectrometry for simultaneous analysis of 22 analytes of Oncheong-eum, a traditional Korean herbal formula. Processes 2023, 11, 2906. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, K.; Kweon, H.Y.; Kim, H.; Lee, J.H. Inhibitory effect of mulberry root bark extract and its derived compounds on cholesterol regulation, inflammation, and platelet aggregation. J. Korean Soc. Food Sci. Nutr. 2022, 51, 633–639. [Google Scholar] [CrossRef]
- Kim, J.H.; Doh, E.J.; Lee, G. Quantitative comparison of the marker compounds in different medicinal parts of Morus alba L. using high-performance liquid chromatography-diode array detector with chemometric analysis. Molecules 2020, 25, 5592. [Google Scholar] [CrossRef] [PubMed]
- Eom, J.H.; Vu, T.P.D.; Cai, L.; Zhao, Y.; Li, H.X.; Yang, S.Y.; Kim, Y.H.; Kim, S.J.; Cho, H.S.; Bao, H.; et al. Development of HPLC method for differentiation of three parts of mulberry tree. Anal. Sci. Technol. 2017, 30, 130–137. [Google Scholar]
- Hwang, S.Y.; Hwang, B.Y.; Choi, W.H.; Jung, H.J.; Huh, J.D.; Lee, K.S.; Ro, J.S. Quantitative determination of 5-hydroxymethyl-2-furaldehyde in the Rehmanniae Radix Preparata samples at various processing stages. Kor. J. Pharmacogn. 2001, 32, 116–120. [Google Scholar]
- Wang, H.P.; Zhang, Y.B.; Yang, X.W.; Zhao, D.Q.; Wang, Y.P. Rapid characterization of ginsenosides in the roots and rhizomes of Panax ginseng by UPLC–DAD–QTOF–MS/MS and simultaneous determination of 19 ginsenosides by HPLC-ESI-MS. J. Ginseng Res. 2016, 40, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Shi, H.; Zhang, Q.; Liu, Y.; Wan, C.; Zhang, L. Simultaneous determination of five components in Aster tataricus by ultra performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. Sci. 2016, 54, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.X.; Hu, B.Q.; Zhang, M.; Zhang, C.F.; Xu, X.H. Simultaneous separation and determination of phenolic acids, pentapeptides, and triterpenoid saponins in the root of Aster tataricus by high-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight mass spectrometry. J. Sep. Sci. 2015, 38, 571–575. [Google Scholar] [CrossRef]
- Song, J.Z.; Yiu, H.H.W.; Qiao, C.F.; Han, Q.B.; Xu, H.X. Chemical comparison and classification of Radix Astragali by determination of isoflavonoids and astragalosides. J. Pharm. Biomed. Anal. 2008, 47, 399–406. [Google Scholar] [CrossRef]
- Lin, L.Z.; He, X.G.; Lindenmaier, M.; Nolan, G.; Yang, J.; Cleary, M.; Qiu, S.X.; Cordell, G.A. Liquid chromatography–electrospray ionization mass spectrometry study of the flavonoids of the roots of Astragalus mongholicus and A. membranaceus. J. Chromatogr. A 2000, 876, 87–95. [Google Scholar] [CrossRef]
- Koo, D.C.; Suh, W.S.; Baek, S.Y.; Shim, S.H. Quantitative determination of lignans from Schizandra chinensis by HPLC. Kor. J. Pharmacogn. 2011, 42, 233–239. [Google Scholar]
- Wang, S.; Liu, X.M.; Zhang, J.; Zhang, Y.Q. An efficient preparation of mulberroside A from the branch bark of mulberry and its effect on the inhibition of tyrosinase activity. PLoS ONE 2014, 9, e109396. [Google Scholar] [CrossRef]
- Li, J.; Jiang, K.; Wang, L.J.; Yin, G.; Wang, J.; Wang, Y.; Jin, B.J.; Li, Q.; Wang, T.J. HPLC–MS/MS determination of flavonoids in Gleditsiae Spina for its quality assessment. J. Sep. Sci. 2018, 41, 1752–1763. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, X.; Dai, W.; Yan, S.; Huang, H.; Liang, X.; Li, Y.; Zhang, W. Chemical fingerprinting of Liuwei Dihuang Pill and simultaneous determination of its major bioactive constituents by HPLC coupled with multiple detections of DAD, ELSD and ESI–MS. J. Pharm. Biomed. Anal. 2009, 49, 638–645. [Google Scholar] [CrossRef]
- Qi, Y.; Cheng, X.; Jing, H.; Yan, T.; Xiao, F.; Wu, B.; Bi, K.; Jia, Y. Comparative pharmacokinetic study of the components in Alpinia oxyphylla Miq. -Schisandra chinensis (Turcz.) Baill. herb pair and its single herb between normal and Alzheimer’s disease rats by UPLC–MS/MS. J. Pharm. Biomed. Anal. 2020, 177, 112874. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Geng, C.A.; Ma, Y.B.; Huang, X.Y.; Chen, H.; Cao, T.W.; He, K.; Wang, H.; Zhang, X.M.; Chen, J.J. UFLC/MS–IT–TOF guided isolation of anti-HBV active chlorogenic acid analogues from Artemisia capillaris as a traditional Chinese herb for the treatment of hepatitis. J. Ethnopharmacol. 2014, 156, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Kafle, B.; Baak, J.; Brede, C. Quantification by LC–MS/MS of astragaloside IV and isoflavones in Astragali Radix can be more accurate by using standard addition. Phytochem. Anal. 2021, 32, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.C.; Lai, Y.C.; Chang, C.L. High throughput screening and antioxidant assay of dibenzo[a,c]cyclooctadiene lignans in modified-ultrasonic and supercritical fluid extracts of Schisandra chinensis Baill by liquid chromatography–mass spectrometry and a free radical-scavenging method. J. Sep. Sci. 2008, 31, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xiao, H.B.; Xue, X.Y.; Sun, Y.G.; Liang, X.M. Simultaneous characterization of isoflavonoids and astragalosides in two Astragalus species by high–performance liquid chromatography coupled with atmospheric pressure chemical ionization tandem mass spectrometry. J. Sep. Sci. 2007, 30, 2059–2069. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.M.; Lu, J.; Gao, O.P.; Li, S.P. Rapid method for simultaneous determination of flavonoid, saponins and polyacetylenes in Folium Ginseng and Radix Ginseng by pressurized liquid diode array detection and mass spectrometry. J. Chromatogr. A 2009, 1216, 3825–3830. [Google Scholar] [CrossRef] [PubMed]
- Avula, B.; Bae, J.Y.; Zhao, J.; Wang, Y.H.; Wang, M.; Zhang, Z.; Ali, Z.; Chittiboyina, A.G.; Khan, I.A. Quantitative determination and characterization of polyphenols from Cissus quadrangularis L. and dietary supplements using UHPLC–PDA–MS, LCQ–ToF and HPTLC. J. Pharm. Biomed. Anal. 2021, 199, 114036. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.Q.; Xiong, Y.J.; Xue, Y.; Wang, Y.; Yan, C. Using UPLC–MS/MS for characterization of active components in extracts of Yupingfeng and application to a comparative pharmacokinetic study in rat plasma after oral administration. Molecules 2017, 22, 810. [Google Scholar] [CrossRef]
- Tsimogiannis, D.; Samiotaki, M.; Panayotou, G.; Oreopoulou, V. Characterization of flavonoid subgroups and hydroxy substitution by HPLC–MS/MS. Molecules 2007, 12, 593–606. [Google Scholar] [CrossRef]
- Zhao, J.; Su, C.; Yang, C.; Liu, M.; Tang, L.; Su, W.; Liu, Z. Determination of ginsenosides Rb1, Rb2, and Rb3 in rat plasma by a rapid and sensitive liquid chromatography tandem mass spectrometry method: Application in a pharmacokinetic study. J. Pharm. Biomed. Anal. 2012, 64–65, 94–97. [Google Scholar] [CrossRef]
- International Conference on Harmonisation (ICH), Guidance for Industry, Q2B, Validation of Analytical Procedures: Methodology; Food and Drug Administration: Rockville, MD, USA, 1996.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).