Unraveling the Bioactive Potential of Camellia japonica Edible Flowers: Profiling Antioxidant Substances and In Vitro Bioactivity Assessment
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phytochemical Profile
2.2. Antioxidant Capacity
2.3. Antioxidant Activity
2.4. Antimicrobial Activity
2.5. Cytotoxic Activity
2.6. Neuroprotective Effects
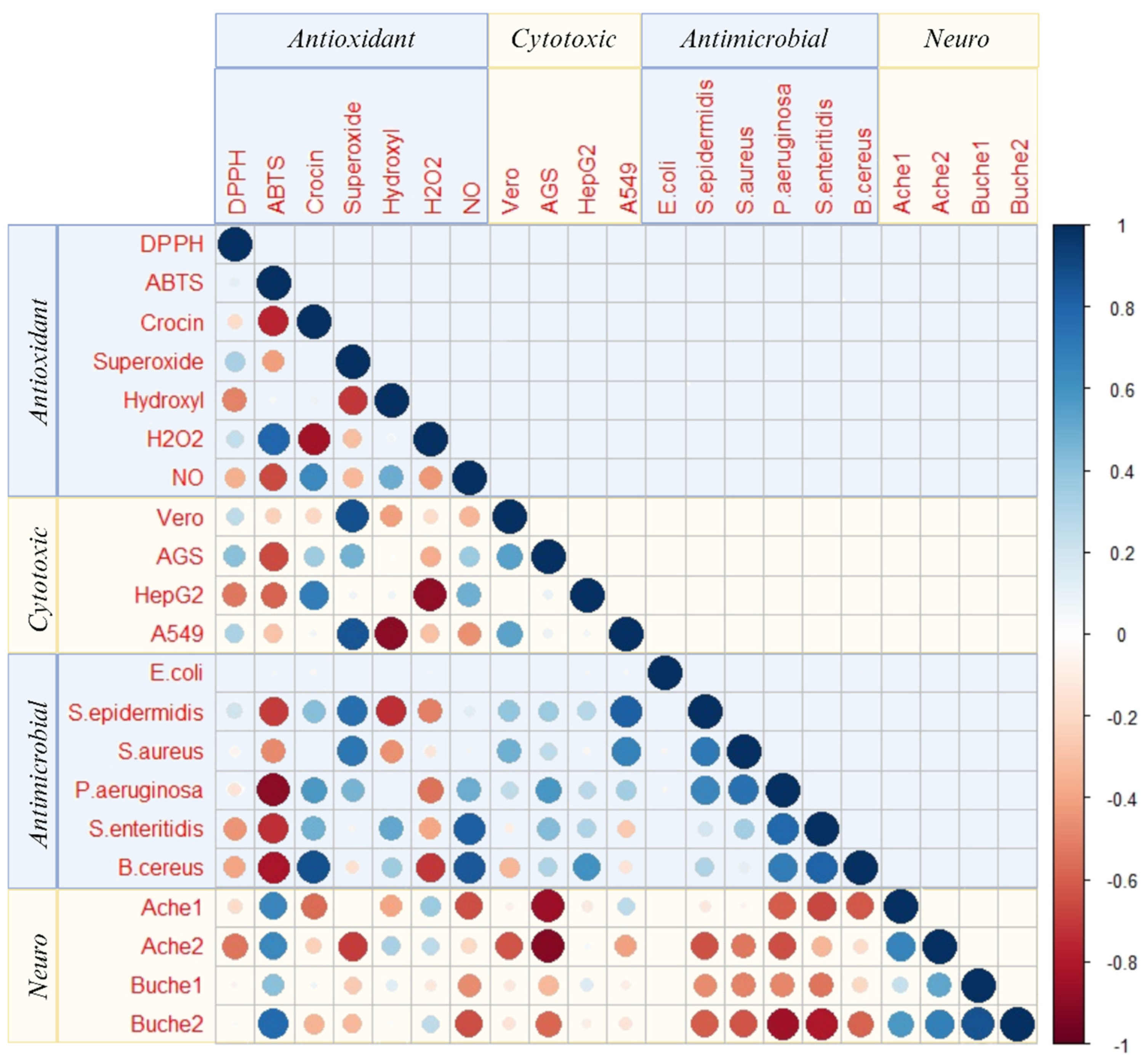
2.7. Pearson’s Correlation Analysis
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Camellia Sampling and Preparation
3.3. Extraction Procedure
3.4. Phytochemical Characterization
3.5. Antioxidant Capacity Determination
3.5.1. DPPH Radical Scavenging Activity
3.5.2. ABTS Radical Scavenging Activity
3.5.3. Crocin Bleaching Assay (CBA)
3.6. Antioxidant Activity Determination
3.6.1. Superoxide Radical Scavenging Activity (SRSA)
3.6.2. Hydroxyl Radical Scavenging Activity (OHSA)
3.6.3. Nitric Oxide Scavenging Assay (NOSA)
3.6.4. H2O2 Scavenging Assay
3.7. Antibacterial Tests
3.7.1. Microorganisms and Culture Conditions
3.7.2. Agar Diffusion Assay (ADA)
3.8. Cytotoxic Activity
3.9. Neuroprotective Activity
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Carocho, M.; Morales, P.; Ferreira, I.C.F.R. Natural food additives: Quo vadis? Trends Food Sci. Technol. 2015, 45, 284–295. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.B.P.P.; Barros, L.; Ferreira, I.C.F.R. Phenolic compounds: Current industrial applications, limitations and future challenges. Food Funct. 2021, 12, 14–29. [Google Scholar] [CrossRef]
- Wang, J.H.; Cai, Y.F.; Li, S.F.; Zhang, S.B. Differences in leaf physiological and morphological traits between Camellia japonica and Camellia reticulata. Plant Divers. 2020, 42, 181–188. [Google Scholar] [CrossRef]
- Park, J.; Kim, Y.; Xi, H.; Oh, Y.J.; Hahm, K.M.; Ko, J. The complete chloroplast genome of common camellia tree, Camellia japonica L. (Theaceae), adapted to cold environment in Korea. Mitochondrial DNA Part B Resour. 2019, 4, 1038–1040. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, L.; Ma, L.; Tian, X.; Li, R.; Zhou, C.; Cao, M. Virome of Camellia japonica: Discovery of and Molecular Characterization of New Viruses of Different Taxa in Camellias. Front. Microbiol. 2020, 11, 945. [Google Scholar] [CrossRef]
- Liu, H.; Wu, L.; Zheng, L.; Cao, M.; Li, R. Characterization of three new viruses of the family Betaflexiviridae associated with camellia ringspot disease. Virus Res. 2019, 272, 197668. [Google Scholar] [CrossRef]
- Meng, X.H.; Li, N.; Zhu, H.T.; Wang, D.; Yang, C.R.; Zhang, Y.J. Plant Resources, Chemical Constituents, and Bioactivities of Tea Plants from the Genus Camellia Section Thea. J. Agric. Food Chem. 2019, 67, 5318–5349. [Google Scholar] [CrossRef]
- Yoon, I.S.; Park, D.H.; Kim, J.E.; Yoo, J.C.; Bae, M.S.; Oh, D.S.; Shim, J.H.; Choi, C.Y.; An, K.W.; Kim, E.I.; et al. Identification of the biologically active constituents of Camellia japonica leaf and anti-hyperuricemic effect in vitro and in vivo. Int. J. Mol. Med. 2017, 39, 1613–1620. [Google Scholar] [CrossRef]
- Pereira, A.G.; Garcia-Perez, P.; Cassani, L.; Chamorro, F.; Cao, H.; Barba, F.J.; Simal-Gandara, J.; Prieto, M.A. Camellia japonica: A phytochemical perspective and current applications facing its industrial exploitation. Food Chem. X 2022, 13, 100258. [Google Scholar] [CrossRef]
- Pereira, A.G.; Silva, A.; Barral-Martinez, M.; Echave, J.; Chamorro, F.; Mansour, S.S.; Cassani, L.; Otero, P.; Xiao, J.; Barroso, F.; et al. Antimicrobial Activity Screening of Camellia japonica Flowers (var. Conde de la Torre). Med. Sci. Forum. 2022, 12, 15. [Google Scholar]
- WHO. The Fight against Antimicrobial Resistance Is Significant for Cancer Prevention and Treatment; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- WHO Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 30 June 2022).
- Garcia-Oliveira, P.; Otero, P.; Pereira, A.G.; Chamorro, F.; Carpena, M.; Echave, J.; Fraga-Corral, M.; Simal-Gandara, J.; Prieto, M.A. Status and Challenges of Plant-Anticancer Compounds in Cancer Treatment. Pharmaceuticals 2021, 14, 157. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Lopez, C.; Pereira, A.G.; Lourenço-Lopes, C.; Garcia-Oliveira, P.; Cassani, L.; Fraga-Corral, M.; Prieto, M.A.; Simal-Gandara, J. Main bioactive phenolic compounds in marine algae and their mechanisms of action supporting potential health benefits. Food Chem. 2021, 341, 128262. [Google Scholar] [CrossRef] [PubMed]
- Stuart, G.A. Chinese Materia Medica: Vegetable Kingdom; Presbyterian Mission Press: Shanghai, China, 1976; ISBN 0879684690. [Google Scholar]
- Trinh, L.T.P.; Choi, Y.S.; Bae, H.J. Production of phenolic compounds and biosugars from flower resources via several extraction processes. Ind. Crop. Prod. 2018, 125, 261–268. [Google Scholar] [CrossRef]
- Lee, H.H.; Cho, J.Y.; Moon, J.H.; Park, K.H. Isolation and identification of antioxidative phenolic acids and flavonoid glycosides from Camellia japonica flowers. Hortic. Environ. Biotechnol. 2011, 52, 270–277. [Google Scholar] [CrossRef]
- Pereira, A.G.; Cassani, L.; Oludemi, T.; Chamorro, F.; Calhelha, R.C.; Prieto, M.A.; Barros, L.; Simal-Gandara, J.; Lucini, L.; Garcia-Perez, P. Untargeted metabolomics and in vitro functional analysis unravel the intraspecific bioactive potential of flowers from underexplored Camellia japonica cultivars facing their industrial application. Ind. Crop. Prod. 2023, 204, 117389. [Google Scholar] [CrossRef]
- Majumder, S.; Ghosh, A.; Chakraborty, S.; Bhattacharya, M. Brewing and biochemical characterization of Camellia japonica petal wine with comprehensive discussion on metabolomics. Food Prod. Process. Nutr. 2022, 4, 29. [Google Scholar] [CrossRef]
- Haiyan, Z.; Bedgood, D.R.; Bishop, A.G.; Prenzler, P.D.; Robards, K. Effect of added caffeic acid and tyrosol on the fatty acid and volatile profiles of camellia oil following heating. J. Agric. Food Chem. 2006, 54, 9551–9558. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Wang, J.; Yin, H.F.; Fan, Z.Q.; Li, J.Y. Variation of flower colors and their relationships with anthocyanins in cultivars of Camellia japonica. J. Ecol. Rural Environ. 2019, 35, 1307–1313. [Google Scholar]
- Tateishi, N.; Ozaki, Y.; Okubo, H. Molecular cloning of the genes involved in anthocyanin biosynthesis in Camellia japonica. J. Fac. Agric. Kyushu Univ. 2010, 55, 21–28. [Google Scholar] [CrossRef]
- Fu, M.; Yang, X.; Zheng, J.; Wang, L.; Yang, X.; Tu, Y.; Ye, J.; Zhang, W.; Liao, Y.; Cheng, S.; et al. Unraveling the Regulatory Mechanism of Color Diversity in Camellia japonica Petals by Integrative Transcriptome and Metabolome Analysis. Front. Plant Sci. 2021, 12, 1119. [Google Scholar] [CrossRef]
- Sakata, Y.; Arisumi, K.; Miyajima, I. Cyanidin 3-Galactoside, a New Anthocyanin from Camellia japonica subsp. rusticana (Honda) Kitamura and Its Occurrence in the Garden Forms of Camellia of Japanese Origin. J. Jpn. Soc. Hortic. Sci. 1986, 55, 82–88. [Google Scholar] [CrossRef]
- Tanikawa, N.; Inoue, H.; Nakayama, M. Aluminum ions are involved in purple flower coloration in Camellia japonica ‘sennen-fujimurasaki’. Hortic. J. 2016, 85, 331–339. [Google Scholar] [CrossRef]
- Forsyth, A.C.; Hayward, J.B.; Roberts, G.C.; June, T. Separation of Anthocyanin and Leucoanthocyanin in Flowers of Camellia japonica. Nature 1958, 182, 801. [Google Scholar]
- Zhou, C.; Mei, X.; Rothenberg, N.; Yang, Z.; Zhang, W.; Wan, S.; Yang, H.; Zhang, L. Metabolome and Transcriptome Analysis Reveals Accumulation and Coloration in White and Pink Tea. Molecules 2020, 25, 190. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Woo, J.; Roh, K.B.; Ryu, D.; Jang, Y.; Cho, E.; Park, D.; Jung, E. Assessment of the anti-hair loss potential of Camellia japonica fruit shell extract in vitro. Int. J. Cosmet. Sci. 2022, 45, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.G.; Cruz, L.; Cassani, L.; Chamorro, F.; Lourenço-Lopes, C.; Freitas, V.; Otero, P.; Fraga-Corral, M.; Prieto, M.A.; Simal-Gandara, J.; et al. Comparative Study of Microwave-Assisted Extraction and Ultrasound-Assisted Extraction Techniques (MAE vs. UAE) for the Optimized Production of Enriched Extracts in Phenolic Compounds of Camellia japonica var Eugenia de Montijo. Eng. Proc. 2023, 37, 124. [Google Scholar] [CrossRef]
- Azuma, C.M.; dos Santos, F.C.S.; Lago, J.H.G. Flavonoids and fatty acids of Camellia japonica leaves extract. Rev. Bras. Farmacogn. 2011, 21, 1159–1162. [Google Scholar] [CrossRef]
- Peterson, J.; Dwyer, J.; Bhagwat, S.; Haytowitz, D.; Holden, J.; Eldridge, A.L.; Beecher, G.; Aladesanmi, J. Major flavonoids in dry tea. J. Food Compos. Anal. 2005, 18, 487–501. [Google Scholar] [CrossRef]
- Wang, H.; Provan, G.J.; Helliwell, K. Tea flavonoids: Their functions, utilisation and analysis. Trends Food Sci. Technol. 2000, 11, 152–160. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, J.; Wei, S.; Jiang, W.; Li, Y.; Guo, W.; Dai, W.; Liao, B. Metabolomic Study of Flavonoids in Camellia drupifera under Aluminum Stress by UPLC-MS/MS. Plants 2023, 12, 1432. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xie, G.L.; Ma, J.L.; Huang, X.Q.; Gu, Y.; Huang, L.; Chen, H.Y.; Ouyang, X.L. Phytochemical constituents of Camellia osmantha fruit cores with antithrombotic activity. Food Sci. Nutr. 2022, 10, 1510–1519. [Google Scholar] [CrossRef]
- Gosch, C.; Halbwirth, H.; Stich, K. Phloridzin: Biosynthesis, distribution and physiological relevance in plants. Phytochemistry 2010, 71, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Cao, J.; Zhao, T.; Liu, Y.; Khan, A.; Cheng, G. The bioavailability, extraction, biosynthesis and distribution of natural dihydrochalcone: Phloridzin. Int. J. Mol. Sci. 2021, 22, 962. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Luo, L.; Zeng, L. Characterization of key sweet taste compounds in Camellia nanchuanica black tea. LWT 2023, 182, 114858. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, S.R.; Sun, K.; Wang, X.H.; Jiang, J.-L.; Luo, L.-Y.; Zeng, L. Characterization of bitter taste theacrine in Pu-erh tea. J. Food Compos. Anal. 2022, 106, 104331. [Google Scholar] [CrossRef]
- Li, J.-y.; Zhou, X.-w.; Fan, Z.; Yin, H.-f.; Sun, Y.-k.; Li, X.-l.; Chen, Y. A flavonol synthase gene related to yellow flowers in Camellia nitidissima. Int. Camellia Soc. 2022, 1, 10–20. [Google Scholar]
- Fan, M.; Zhang, Y.; Yang, M.; Wu, S.; Yin, H.; Li, J.; Li, X. Transcriptomic and Chemical Analyses Reveal the Hub Regulators of Flower Color Variation from Camellia japonica Bud Sport. Horticulturae 2022, 8, 129. [Google Scholar] [CrossRef]
- Orlova, S.V.; Tatarinov, V.V.; Nikitina, E.A.; Sheremeta, A.V.; Ivlev, V.A.; Vasil’ev, V.G.; Paliy, K.V.; Goryainov, S.V. Bioavailability and Safety of Dihydroquercetin (Review). Pharm. Chem. J. 2022, 55, 1133–1137. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Torres, J.L. Analysis of nonextractable phenolic compounds in foods: The current state of the art. J. Agric. Food Chem. 2011, 59, 12713–12724. [Google Scholar] [CrossRef]
- Wang, W.; Guo, J.; Zhang, J.; Peng, J.; Liu, T.; Xin, Z. Isolation, identification and antioxidant activity of bound phenolic compounds present in rice bran. Food Chem. 2015, 171, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Liu, C.; Luo, S.; Chen, J.; Gong, E. The profile and bioaccessibility of phenolic compounds in cereals influenced by improved extrusion cooking treatment. PLoS ONE 2016, 11, e0161086. [Google Scholar] [CrossRef]
- Borgonovi, S.M.; Chiarello, E.; Pasini, F.; Picone, G.; Marzocchi, S.; Capozzi, F.; Bordoni, A.; Barbiroli, A.; Marti, A.; Iametti, S.; et al. Effect of Sprouting on Biomolecular and Antioxidant Features of Common Buckwheat (Fagopyrum esculentum). Foods 2023, 12, 2047. [Google Scholar] [CrossRef]
- Kim, M.; Son, D.; Shin, S.; Park, D.; Byun, S.; Jung, E. Protective effects of Camellia japonica flower extract against urban air pollutants. BMC Complement. Altern. Med. 2019, 19, 30. [Google Scholar] [CrossRef]
- Kanth, B.K.; Lee, K.Y.; Lee, G.J. Antioxidant and radical-scavenging activities of petal extracts of Camellia japonica ecotypes. Hortic. Environ. Biotechnol. 2014, 55, 335–341. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Yin, C.P.; Kong, L.C.; Jiang, D.H. Extraction optimisation, purification and major antioxidant component of red pigments extracted from Camellia japonica. Food Chem. 2011, 129, 660–664. [Google Scholar] [CrossRef]
- Yang, F.; Yang, Z.; Xiao, J. Radical scavenging activity of crude polysaccharides from Camellia sinensis. Arch. Biol. Sci. 2011, 63, 717–721. [Google Scholar] [CrossRef]
- Bhebhe, M.; Füller, T.N.; Chipurura, B.; Muchuweti, M. Effect of Solvent Type on Total Phenolic Content and Free Radical Scavenging Activity of Black Tea and Herbal Infusions. Food Anal. Methods 2016, 9, 1060–1067. [Google Scholar] [CrossRef]
- Wan, C.-P.; Yu, Y.; Zhou, S.-R.; Cao, S.-W. Antioxidant and Free Radical Scavenging Activity of Camellia nitidissima Chi. Asian J. Chem. 2011, 23, 2893–2897. [Google Scholar]
- Olugbami, J.; Gbadegesin, M.; Odunola, O. In vitro free radical scavenging and antioxidant properties of ethanol extract of Terminalia glaucescens. Pharmacognosy Res. 2015, 7, 49–56. [Google Scholar]
- Benslama, A.; Harrar, A. Free radicals scavenging activity and reducing power of two Algerian Sahara medicinal plants extracts. Int. J. Herb. Med. 2016, 4, 158–161. [Google Scholar] [CrossRef]
- Dhalwal, K.; Deshpande, Y.S.; Purohit, A.P. Evaluation of in vitro antioxidant activity of Sida rhombifolia (L.) Ssp. retusa (L.). J. Med. Food 2007, 10, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Yang, H.; Kim, K.K. Camellia japonica Root Extract Increases Antioxidant Genes by Induction of NRF2 in HeLa Cells. Plants 2022, 11, 2914. [Google Scholar] [CrossRef]
- Choi, M.-H.; Min, M.-J.; Oh, D.-S.; Shin, H.-J. Antimicrobial and Antioxidant Activity of Camellia japonica Extracts for Cosmetic Applications. KSBB J. 2013, 28, 99–105. [Google Scholar] [CrossRef]
- Zheng, J.; Yu, X.; Maninder, M.; Xu, B. Total phenolics and antioxidants profiles of commonly consumed edible flowers in China. Int. J. Food Prop. 2018, 21, 1524–1540. [Google Scholar] [CrossRef]
- Luo, F.; Fei, X. Distribution and Antioxidant Activities of Free, Conjugated, and Insoluble-Bound Phenolics from Seven Species of the Genus Camellia. J. Am. Oil Chem. Soc. 2019, 96, 159–170. [Google Scholar] [CrossRef]
- Moon, S.H.; Kim, M.Y. Phytochemical profile, antioxidant, antimicrobial and antipancreatic lipase activities of fermented Camellia japonica L. leaf extracts. Trop. J. Pharm. Res. 2018, 17, 905–912. [Google Scholar] [CrossRef]
- Feng, S.; Cheng, H.; Fu, L.; Ding, C.; Zhang, L.; Yang, R.; Zhou, Y. Ultrasonic-assisted extraction and antioxidant activities of polysaccharides from Camellia oleifera leaves. Int. J. Biol. Macromol. 2014, 68, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Xu, Y.; Jie, G.; He, P.; Tu, Y. Study on the antioxidant activity of tea flowers (Camellia sinensis). Asia Pac. J. Clin. Nutr. 2007, 16, 148–152. [Google Scholar]
- Kim, K.Y.; Davidson, P.M.; Chung, H.J. Antibacterial activity in extracts of Camellia japonica L. petals and its application to a model food system. J. Food Prot. 2001, 64, 1255–1260. [Google Scholar]
- Pradhan, S.; Dubey, R.C. Deciphering antimicrobial, phytochemical, GC–MS and pharmacokinetic properties of Camellia sinensis from high-altitude region. Vegetos 2022, 35, 895–902. [Google Scholar] [CrossRef]
- Das, A.; Parashar, D.P.; Raychaiudhuri, U.; Chakraborty, R. Studying the effect of different drying methods on phenolic content, antioxidant activity, color and antimicrobial activity in Assam tea (Camellia assamica). J. Plant Biochem. Biotechnol. 2022, 31, 615–624. [Google Scholar] [CrossRef]
- Khan, A.; Shabir, D.; Ahmad, P.; Khandaker, M.U.; Faruque, M.R.I.; Din, I.U. Biosynthesis and antibacterial activity of MgO-NPs produced from Camellia sinensis leaves extract. Mater. Res. Express 2020, 8, 015402. [Google Scholar] [CrossRef]
- Boudou, F.; Belakredar, A. In vitro and In Silico Screening of Antibacterial Compounds from Camellia sinensis Against Bacillus cereus and Escherichia coli. Res. Sq. 2022. [CrossRef]
- Chan, E.W.C.; Soh, E.Y.; Tie, P.P.; Law, Y.P. Antioxidant and antibacterial properties of green, black, and herbal teas of Camellia sinensis. Pharmacognosy Res. 2011, 3, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-Y.; Hwang, E.-J.; Kim, G.-H.; Choi, Y.-B.; Lim, C.-Y.; Kim, S.-M. Antifungal and Antioxidant Activities of Extracts from Leaves and Flowers of Camellia japonica L. Korean J. Med. Crop Sci. 2005, 13, 93–100. [Google Scholar]
- Sun, H.; Yin, M.; Hao, D.; Shen, Y. Anti-cancer activity of catechin against A549 lung carcinoma cells by induction of cyclin kinase inhibitor p21 and suppression of cyclin E1 and P-AKT. Appl. Sci. 2020, 10, 2065. [Google Scholar] [CrossRef]
- Di, T.M.; Yang, S.L.; Du, F.Y.; Zhao, L.; Xia, T.; Zhang, X.F. Cytotoxic and hypoglycemic activity of triterpenoid saponins from Camellia oleifera abel. Seed pomace. Molecules 2017, 22, 1562. [Google Scholar] [CrossRef]
- Zhang, L.; Santos, J.S.; Cruz, T.M.; Marques, M.B.; do Carmo, M.A.V.; Azevedo, L.; Wang, Y.; Granato, D. Multivariate effects of Chinese keemun black tea grades (Camellia sinensis var. sinensis) on the phenolic composition, antioxidant, antihemolytic and cytotoxic/cytoprotection activities. Food Res. Int. 2019, 125, 108516. [Google Scholar] [CrossRef]
- Li, X.; Zhao, J.; Peng, C.; Chen, Z.; Liu, Y.; Xu, Q.; Khan, I.A.; Yang, S. Cytotoxic triterpenoid glycosides from the roots of Camellia oleifera. Planta Med. 2014, 80, 590–598. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, C.Z.; Ye, J.Z.; Chen, H.X. New triterpene saponins from the seed cake of Camellia oleifera and their cytotoxic activity. Phytochem. Lett. 2014, 8, 46–51. [Google Scholar] [CrossRef]
- Di, T.M.; Yang, S.L.; Du, F.Y.; Zhao, L.; Li, X.H.; Xia, T.; Zhang, X.F. Oleiferasaponin A2, a Novel Saponin from Camellia oleifera Abel. Seeds, inhibits lipid accumulation of HepG2 cells through regulating fatty acid metabolism. Molecules 2018, 23, 3296. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, Y.; La, K.K.; Peng, L.; Song, X.; Shi, X.; Zhu, X.; Leung, P.; Ko, C.; Ye, C. Inhibitory effects of cocoa tea (Camellia ptilophylla) in human hepatocellular carcinoma HepG2 in vitro and in vivo through apoptosis. J. Nutr. Biochem. 2012, 23, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalapathy, D.; Shivamallu, C.; Prasad, S.K.; Saradha, G.T.; Rudrapathy, P.; Amachawadi, R.G.; Patil, S.S.; Syed, A.; Elgorban, A.M.; Bahkali, A.H.; et al. Assessment of chemopreventive potential of the plant extracts against liver cancer using hepg2 cell line. Molecules 2021, 26, 4593. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.R.; Scott, E.; Brown, V.A.; Gescher, A.J.; Steward, W.P.; Brown, K. Clinical trials of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.M.; Ha, T.K.Q.; Doan, T.P.; Dhodary, B.; An, J.P.; Lee, B.W.; Yang, J.L.; Oh, W.K. Neuroprotective Effects of Triterpenoids from Camellia japonica against Amyloid β-Induced Neuronal Damage. J. Nat. Prod. 2020, 83, 2076–2086. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Zhang, W.; Ma, G.; Wang, K.; Ji, Y.; Ren, H.; Wang, Y. Neuroprotective effects of Camellia nitidissima Chi leaf extract in hydrogen peroxide-treated human neuroblastoma cells and its molecule mechanisms. Food Sci. Nutr. 2020, 8, 4782–4793. [Google Scholar] [CrossRef] [PubMed]
- Schimidt, H.L.; Garcia, A.; Martins, A.; Mello-Carpes, P.B.; Carpes, F.P. Green tea supplementation produces better neuroprotective effects than red and black tea in Alzheimer-like rat model. Food Res. Int. 2017, 100, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Fazeli-Nasab, B.; Rahnama, M.; Mazarei, A. Correlation between antioxidant activity and antibacterial activity of nine medicinal plant extracts. J. Maz. Univ. Med. Sci. 2017, 27, 63–78. [Google Scholar]
- Marzoug, H.N.B.; Romdhane, M.; Lebrihi, A.; Lebrihi, F.; Couderc, F.; Abderraba, M.; Abderraba, M.L.; Bouajila, J. Eucalyptus oleosa essential oils: Chemical composition and antimicrobial and antioxidant activities of the oils from different plant parts (stems, leaves, flowers and fruits). Molecules 2011, 16, 1695–1709. [Google Scholar] [CrossRef]
- Corral, M.d.C.S.; Fernández, P.V.; Vázquez, J.P.M.; Gesto, M.J.L. La Camelia en la Colección de la Diputación de Pontevedra; Editorial Deputación de Pontevedra: Pontevedra, Spain, 2004; Volume 53, ISBN 9788578110796. [Google Scholar]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Cassani, L.; Lourenço-Lopes, C.; Barral-Martinez, M.; Chamorro, F.; Garcia-Perez, P.; Simal-Gandara, J.; Prieto, M.A. Thermochemical Characterization of Eight Seaweed Species and Evaluation of Their Potential Use as an Alternative for Biofuel Production and Source of Bioactive Compounds. Int. J. Mol. Sci. 2022, 23, 2355. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Arnao, M.B. ABTS/TEAC (2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)/Trolox®-equivalent antioxidant capacity) radical scavenging mixed-mode assay. In Measurement of Antioxidant Activity and Capacity: Recent Trends and Applications; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 117–139. ISBN 9781119135388. [Google Scholar]
- Prieto, M.A.; Vázquez, J.A.; Murado, M.A. Crocin bleaching antioxidant assay revisited: Application to microplate to analyse antioxidant and pro-oxidant activities. Food Chem. 2015, 1, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Hazra, B.; Biswas, S.; Mandal, N. Antioxidant and free radical scavenging activity of Spondias pinnata. BMC Complement. Altern. Med. 2008, 8, 63. [Google Scholar] [CrossRef]
- Soares, C.; Paíga, P.; Marques, M.; Neto, T.; Carvalho, A.P.; Paiva, A.; Simões, P.; Costa, L.; Bernardo, A.; Fernández, N.; et al. Multi-Step Subcritical Water Extracts of Fucus vesiculosus L. and Codium tomentosum Stackhouse: Composition, Health-Benefits and Safety. Processes 2021, 9, 893. [Google Scholar] [CrossRef]
- Yang, T.; Hu, Y.; Yan, Y.; Zhou, W.; Chen, G.; Zeng, X.; Cao, Y. Characterization and Evaluation of Antioxidant and Anti-Inflammatory Activities of Flavonoids from the Fruits of Lycium barbarum. Foods 2022, 11, 306. [Google Scholar] [CrossRef] [PubMed]
- Smirnoff, N.; Cumbes, Q.J. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 1989, 28, 1057–1060. [Google Scholar] [CrossRef]
- Geng, M. Free radical scavenging activities of pigment extract from Hibiscus syriacus L. petals in vitro. Afr. J. Biotechnol. 2012, 11, 429–435. [Google Scholar]
- Gülçin, İ.; Mshvildadze, V.; Gepdiremen, A.; Elias, R. Screening of antiradical and antioxidant activity of monodesmosides and crude extract from Leontice smirnowii tuber. Phytomedicine 2006, 13, 343–351. [Google Scholar] [CrossRef]
- Mancini, S.; Nardo, L.; Gregori, M.; Ribeiro, I.; Mantegazza, F.; Delerue-Matos, C.; Masserini, M.; Grosso, C. Functionalized liposomes and phytosomes loading Annona muricata L. aqueous extract: Potential nanoshuttles for brain-delivery of phenolic compounds. Phytomedicine 2018, 42, 233–244. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk Susceptibility Tests, Approved Standard, 11th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012; Volume 32, ISBN 1562387812. [Google Scholar]
- Otto, M. Staphylococcus epidermidis—The “accidental” pathogen. Nat. Rev. Microbiol. 2009, 7, 555–567. [Google Scholar] [CrossRef]
- Silva, A.; Silva, S.A.; Lourenço-Lopes, C.; Jimenez-Lopez, C.; Carpena, M.; Gullón, P.; Fraga-Corral, M.; Domingues, V.F.; Barroso, M.F.; Simal-Gandara, J.; et al. Antibacterial Use of Macroalgae Compounds against Foodborne Pathogens. Antibiotics 2020, 9, 712. [Google Scholar] [CrossRef]
- Silva, A.; Rodrigues, C.; Garcia-Oliveira, P.; Lourenço-Lopes, C.; Silva, S.A.; Garcia-Perez, P.; Carvalho, A.P.; Domingues, V.F.; Barroso, M.F.; Delerue-Matos, C.; et al. Screening of bioactive properties in brown algae from the northwest iberian peninsula. Foods 2021, 10, 1915. [Google Scholar] [CrossRef]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicityscreening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Ingkaninan, K.; Temkitthawon, P.; Chuenchom, K.; Yuyaem, T.; Thongnoi, W. Screening for acetylcholinesterase inhibitory activity in plants used in Thai traditional rejuvenating and neurotonic remedies. J. Ethnopharmacol. 2003, 89, 261–264. [Google Scholar] [CrossRef]


| ID | Class | Compound | Formula | Precursor | Products | Col. | RF | Rt | Flowers of C. japonica Cultivars | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EV | DT | EM | CT | DD | HA | GS | TU | |||||||||
| (m/z) | (m/z) | (V) | (V) | (min) | (μg/100 g Extract) | |||||||||||
| P1 | Anthocyanins | Cyanidin 3-O-arabinoside | C20H19ClO10 | 449.233 | 287.083 | 20 | 107 | 4.6 | 0.098 | 0.171 | 0.192 | 0.144 | 0.007 | 0.268 | 0.225 | 0.145 |
| P2 | Pelargonidin-3-O-rutinoside (isomer) | C27H31O14+ | 579.383 | 289.167/291.167/409.167/427.25 | 15–20 | 188 | 6.19 | 0.073 | 0.058 | 0.098 | 0.093 | 0.052 | 0.088 | 0.065 | 0.053 | |
| P3 | Pelargonidin-3-O-rutinoside (isomer) | C27H31O14+ | 579.383 | 289.167/291.167/409.167/427.25 | 15–20 | 188 | 6.41 | 0.043 | 0.039 | 0.043 | 0.032 | 0.054 | 0.040 | 0.035 | 0.001 | |
| P4 | Pelargonidin 3-O-arabinoside | C20H19O9+ | 402.833 | 258.167/313.25 | 10 | 110 | 9.71 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | |
| P5 | Pelargonidin-3-O-sambubioside | C26H29O14+ | 579.333 | 222.667/408.167 | 15 | 163 | 12.63 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | |
| P6 | Peonidin | C16H13O6+ | 304.43 | 178.083/219/260.25 | 5–15 | 93 | 15.25 | 3.291 | 3.132 | 12.256 | 15.965 | 2.118 | 4.051 | 1.348 | 1.359 | |
| P7 | Peonidin derivate | C27H31O15+ | 579.383 | 287.083 | 28 | 171 | 22.12 | 1.548 | 1.496 | 7.017 | 1.508 | 1.489 | 1.531 | 1.472 | 1.525 | |
| P8 | Malvidin-3-O-glucoside | C23H25O12+ | 449.2 | 258.167/291/373.167 | 12–17 | 107 | 16.61 | 0.655 | 0.613 | 6.364 | 1.069 | 0.521 | 0.575 | 0.588 | 0.638 | |
| P9 | Malvidin 3-O-(6″-caffeoyl-glucoside) | C32H31O15 | 671.317 | 365.167/475.25/565.333 | 11–14 | 128 | 18.68 | 1.458 | 1.424 | 5.128 | 1.421 | 1.644 | 1.474 | 1.374 | 1.472 | |
| P10 | Cyanidin 3-O-glucosyl-rutinoside | C33H41O20+ | 717.617 | 303.25/577.25 | 15–24 | 166 | 19.75 | 0.960 | 0.928 | 4.475 | 0.915 | 0.916 | 0.956 | 0.963 | 1.007 | |
| P11 | Curcuminoids | Curcumin | C21H20O6 | 381.15 | 204.917/277.083/349.167 | 9 -21 | 104 | 12.47 | 0.001 | 0.001 | 0.021 | 0.001 | 0.013 | 0.002 | 0.005 | 0.001 |
| P12 | Dihydrochalcones | 3-hydroxyphloretin-2-O-glucoside | C21H24O11 | 584.5 | 405/423.083/564.5 | 8–15 | 120 | 13.51 | 0.002 | 0.003 | 0.013 | 0.001 | 0.004 | 0.002 | 0.003 | 0.002 |
| P13 | 3-hydroxyphloretin 2-O-xylosyl-glucoside | C26H32O15 | 579.3 | 405.167/564.583 | 5–15 | 124 | 13.7 | 0.005 | 0.004 | 0.030 | 0.005 | 0.004 | 0.005 | 0.006 | 0.006 | |
| P14 | Phloridzin | C21H24O10 | 449.317 | 200/255/279.083/309.083 | 14–18 | 91 | 18.25 | 0.410 | 0.367 | 1.163 | 0.414 | 0.335 | 0.363 | 0.411 | 0.352 | |
| P15 | Dihydroflavonols | Dihydroquercetin (isomer 1) | C15H12O7 | 304.433 | 58.083/91.083/212.333 | 21–30 | 145 | 14.82 | 8.895 | 8.571 | 1.404 | 22.652 | 0.055 | 17.573 | 4.622 | 5.024 |
| P16 | Dihydroquercetin (isomer 2) | C15H12O7 | 304.433 | 58.083/91.083/212.333 | 21–30 | 145 | 14.39 | 0.119 | 0.045 | 0.352 | 34.284 | 0.043 | 33.313 | 0.109 | 0.095 | |
| P17 | Dihydroquercetin (isomer 3) | C15H12O7 | 304.433 | 58.083/91.083/212.333 | 21–30 | 145 | 14.6 | 13.351 | 0.151 | 0.376 | 28.479 | 0.059 | 27.941 | 0.165 | 0.100 | |
| P18 | Dihydroquercetin (isomer 4) | C15H12O7 | 304.433 | 58.083/91.083/212.333 | 21–30 | 145 | 17.19 | 0.674 | 0.658 | 2.993 | 0.562 | 0.418 | 0.459 | 0.630 | 0.666 | |
| P19 | Flavonols | Quercetin-3-O-arabinose | C20H18O11 | 449.15 | 254.667/302.833/309.167 | 12–19 | 101 | 15.46 | 1.386 | 1.352 | 7.854 | 9.322 | 1.138 | 1.265 | 0.634 | 0.592 |
| P20 | Kaempferol 3-O-acetyl-glucoside | C23H22O12 | 449.267 | 148.167/200.083/255/399.667 | 10–19 | 99 | 18.04 | 2.075 | 1.860 | 9.890 | 1.843 | 1.532 | 1.846 | 1.862 | 1.886 | |
| P21 | Flavone | Nobiletin | C21H22O8 | 449.233 | 245.167/369 | 10–21 | 114 | 15.99 | 0.478 | 0.100 | 0.642 | 1.583 | 0.185 | 1.337 | 0.085 | 0.077 |
| P22 | Apigenin 7-O-glucoside | C21H20O10 | 453.583 | 171.25/249/379.083 | 6–26 | 101 | 17.31 | 0.138 | 0.129 | 0.557 | 0.104 | 0.082 | 0.089 | 0.129 | 0.135 | |
| P23 | Apigenin 7-O-glucuronide | C21H18O11 | 449.2 | 326.75/365.333/406 | 6–18 | 91 | 21.98 | 0.230 | 0.219 | 1.044 | 0.214 | 0.214 | 0.224 | 0.215 | 0.222 | |
| P24 | Hydroxybenzoic acids | Ellagic acid acetyl-arabinose | C21H16O13 | 449.233 | 134 | 15 | 112 | 17.05 | 2.360 | 2.177 | 14.208 | 2.553 | 1.624 | 1.753 | 2.105 | 2.216 |
| P25 | Gallic acid 4-O-glucoside | C13H16O10 | 332.3 | 91.083/240.333 | 22–31 | 152 | 25.77 | 20.838 | 21.114 | 52.515 | 20.207 | 20.826 | 21.787 | 21.159 | 22.521 | |
| P26 | Hydroxycinnamic acids | p-coumaroylquinic acid | C16H18O8 | 381.267 | 173/219.083/249/298 | 5 -27 | 85 | 16.36 | 0.420 | 0.439 | 1.311 | 1.363 | 0.360 | 0.474 | 0.320 | 0.380 |
| P27 | Rosmarinic acid | C18H16O8 | 332.517 | 105–319 | 5–32 | 87 | 19.12 | 0.448 | 0.446 | 1.867 | 0.439 | 0.465 | 0.450 | 0.431 | 0.444 | |
| P28 | 3,4-dicaffeoylquinic acid | C25H24O12 | 503.35 | 217.083/337.167 | 13–31 | 91 | 20.4 | 0.552 | 0.509 | 2.104 | 0.488 | 0.495 | 0.539 | 0.546 | 0.575 | |
| P29 | Isoflavonoids | 6-O-malonylglycitin | C25H24O13 | 532.1 | 337.083/351.083 | 14 | 113 | 13.9 | 0.007 | 0.005 | 0.042 | 0.006 | 0.007 | 0.005 | 0.006 | 0.007 |
| P30 | 6-O-acetyldaidzin | C23H22O10 | 449.233 | 115/367.333/381.083 | 12–16 | 157 | 15.03 | 1.009 | 0.918 | 2.032 | 3.486 | 1.904 | 1.773 | 0.445 | 0.616 | |
| P31 | Daidzin | C21H20O9 | 449.233 | 295.083/325 | 11–18 | 128 | 15.78 | 0.137 | 0.135 | 0.939 | 2.477 | 0.126 | 2.325 | 0.090 | 0.092 | |
| P32 | Glycitin | C22H22O10 | 449.2 | 257.167/267 | 20–24 | 96 | 16.6 | 0.115 | 0.111 | 1.433 | 0.270 | 0.103 | 0.100 | 0.092 | 0.100 | |
| P33 | 6-O-malonyldaidzin | C24H22O12 | 503.283 | 308.833/323/471.167 | 16–28 | 114 | 20.61 | 0.189 | 0.177 | 0.923 | 0.179 | 0.177 | 0.180 | 0.174 | 0.176 | |
| P34 | Stilbenes | Pallidol | C28H22O6 | 449.1333 | 112.917 | 15 | 115 | 16.17 | 0.159 | 0.118 | 0.949 | 0.144 | 0.148 | 0.105 | 0.127 | 0.167 |
| P35 | Tyrosols | Oleuropein | C25H32O13 | 579.3 | 361.167/379.25/407.25 | 9–16 | 118 | 12.84 | 22.900 | 20.833 | 46.113 | 79.092 | 43.211 | 40.230 | 10.109 | 13.968 |
| P36 | Demethyloleuropein | C24H30O13 | 503.497 | 347.083/365.083 | 15–29 | 105 | 18.47 | 3.103 | 3.073 | 21.304 | 56.205 | 2.857 | 52.751 | 2.045 | 2.087 | |
| Control | EV | DT | EM | CT | DD | HA | GS | TU | p-Value | |
|---|---|---|---|---|---|---|---|---|---|---|
| (A) ANTIOXIDANT CAPACITY | ||||||||||
| DPPH (μg TE/mL) | - | 35 abc ± 2 | 33 bc ± 2 | 30 c ± 2 | 39 a ± 1 | 37 ab ± 3 | 30 c ± 4 | 30 c ± 1 | 35 abc ± 4 | 2.7 × 10−4 |
| ABTS (μg TE/mL) | - | 121 a ± 9 | 88 ab ± 21 | 81 b ± 19 | 77 b ± 7 | 80 b ± 6 | 86 b ± 11 | 96 ab ± 11 | 121 a ± 18 | 5.1 × 10−4 |
| Crocin (min/g dw) | - | 0.2 b ± 0.2 | 0.27 ab ± 0.06 | 0.30 ab ± 0.08 | 0.31 ab ± 0.02 | 0.23 b ± 0.05 | 0.43 a ± 0.07 | 0.01 c ± 0.01 | 0.02 c ± 0.01 | 1.5 × 10−7 |
| (B) ANTIOXIDANT ACTIVITY | ||||||||||
| Superoxide (mg/mL) | - | 0.022 a ± 0.001 | 0.0670 b ± 0.0033 | 0.040 c ± 0.002 | 0.1120 d ± 0.0056 | 0.0180 a ± 0.0009 | 0.029 a ± 0.001 | 0.096 e ± 0.005 | 0.028 a ± 0.028 | 1 × 10−4 |
| Hydroxyl (mg/mL) | - | 0.862 a ± 0.043 | 0.662 b ± 0.031 | 0.942 a ± 0.047 | 0.587 b ± 0.029 | 1.240 c ± 0.062 | 1.344 c ± 0.067 | 1.138 cd ± 0.057 | 1.017 cd ± 0.051 | 1 × 10−4 |
| H2O2 (mg/mL) | - | 0.0660 a ± 0.0033 | 0.0250 b ± 0.0012 | 0.0560 ac ± 0.0028 | 0.039 d ± 0.0019 | 0.0330 bd ± 0.0016 | 0.031 bd ± 0.0015 | 0.049 cd ± 0.002 | 0.161 e ± 0.0081 | 1 × 10−4 |
| NO (mg/mL) | 1.08 | 0.760 a ± 0.038 | 0.417 b ± 0.021 | 2.23 c ± 0.11 | 0.785 a ± 0.039 | 1.578 c ± 0.078 | 1.221 c ± 0.061 | 0.546 b ± 0.027 | 0.597 ab ± 0.029 | 1 × 10−4 |
| (C) ANTIMICROBIAL ACTIVITY (mm) | ||||||||||
| E. coli | 17.7 | nd | nd | nd | nd | nd | nd | nd | nd | - |
| S. epidermidis | 24.4 | nd | 11 a ± 2 | 11 a ± 3 | 14 a ± 1 | nd | nd | nd | nd | 0.02 |
| S. aureus | 19.0 | nd | 11.0 a ± 0.8 | 9.7 a ± 0.6 | 11 a ± 1 | nd | 9.7 a ± 0.1 | 9.4 a ± 0.6 | 10.3 a ± 0.5 | 1.9 × 10−13 |
| P. aeruginosa | 19.2 | 7 b ± 3 | 10.0 ab ± 0.8 | 9.8 ab ± 0.6 | 10.4 a ± 0.7 | 9.2 ab ± 0.4 | 10.8 a ± 0.8 | 9.3 ab ± 0.7 | 9.2 ab ± 0.5 | 0.04 |
| S. enteritidis | 18.7 | nd | 6 a ± 2 | 10 a ± 1 | 8 a ± 2 | 10.5 a ± 0.3 | 11 a ± 1 | 8.4 a ± 0.7 | 7 a ± 1 | 1.2 × 10−4 |
| B. cereus | 17.4 | nd | 6.5 ab ± 0.4 | 7 a ± 1 | 3 b ± 3 | 9 a ± 1 | 9.4 a ± 0.7 | nd | nd | 1.9 × 10−8 |
| (D) CYTOTOXIC ACTIVITY (μg/mL) | ||||||||||
| Vero | 9.0 | 18 b ± 1 | 9 b ± 8 | 18 b ± 1 | 52 a ± 25 | 6.23 b ± 0.03 | 17 b ± 11 | 69 a ± 12 | 9 b ± 2 | 1.7 × 10−5 |
| AGS | 3.7 | 22 ab ± 5 | 9 b ± 8 | 27 ab ± 5 | 52 a ± 25 | 36 ab ± 7 | 34 ab ± 8 | 32 ab ± 16 | 23 ab ± 1 | 0.02 |
| HepG2 | 3.9 | 40 a ± 7 | 38 a ± 20 | 45 a ± 13 | 33 a ± 17 | 35 a ± 5 | 39 a ± 3 | 41 a ± 10 | 13 a ± 4 | 0.11 |
| A549 | 1.9 | 15.0 ab ± 0.3 | 1.65 a ± 0.08 | 12.4 ab ± 0.9 | 22 ab ± 2 | 13.40 b ± 0.05 | 10.56 ab ± 0.4 | 19 ab ± 3 | 13.3 ab ± 0.6 | 0.02 |
| (E) NEUROPROTECTIVE ACTIVITY (g/mL) | ||||||||||
| AchE (%) 1 mg/mL | 0.92 | 20 ab ± 3 | 25 a ± 2 | 16 ab ± 2 | 8 b ± 1 | 10 b ± 3 | 7 b ± 1 | 20 ab ± 6 | 16 ab ± 8 | 2.1 × 10−3 |
| 2 mg/mL | 25 ab ± 2 | 26 a ± 7 | 20 ab ± 5 | 9 b ± 3 | 19 ab ± 5 | 22 ab ± 6 | 20 ab ± 8 | 21 ab ± 3 | 0.09 | |
| BuChE (%) 1 mg/mL | 4.92 | 33 ab ± 5 | 25 abc ± 7 | 4 c ± 2 | 20 abc ± 6 | 16 bc ± 8 | 37 a ± 7 | 20 abc ± 4 | 14 c ± 6 | 5.8 × 10−4 |
| 2 mg/mL | 47 a ± 2 | 29 ab ± 16 | 11 b ± 3 | 20 b ± 8 | 21 b ± 2 | 31 ab ± 4 | 28 ab ± 5 | 23 b ± 2 | 3.6 × 10−3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, A.G.; Fraga-Corral, M.; Silva, A.; Barroso, M.F.; Grosso, C.; Carpena, M.; Garcia-Perez, P.; Perez-Gregorio, R.; Cassani, L.; Simal-Gandara, J.; et al. Unraveling the Bioactive Potential of Camellia japonica Edible Flowers: Profiling Antioxidant Substances and In Vitro Bioactivity Assessment. Pharmaceuticals 2024, 17, 946. https://doi.org/10.3390/ph17070946
Pereira AG, Fraga-Corral M, Silva A, Barroso MF, Grosso C, Carpena M, Garcia-Perez P, Perez-Gregorio R, Cassani L, Simal-Gandara J, et al. Unraveling the Bioactive Potential of Camellia japonica Edible Flowers: Profiling Antioxidant Substances and In Vitro Bioactivity Assessment. Pharmaceuticals. 2024; 17(7):946. https://doi.org/10.3390/ph17070946
Chicago/Turabian StylePereira, Antia G., Maria Fraga-Corral, Aurora Silva, Maria Fatima Barroso, Clara Grosso, Maria Carpena, Pascual Garcia-Perez, Rosa Perez-Gregorio, Lucia Cassani, Jesus Simal-Gandara, and et al. 2024. "Unraveling the Bioactive Potential of Camellia japonica Edible Flowers: Profiling Antioxidant Substances and In Vitro Bioactivity Assessment" Pharmaceuticals 17, no. 7: 946. https://doi.org/10.3390/ph17070946
APA StylePereira, A. G., Fraga-Corral, M., Silva, A., Barroso, M. F., Grosso, C., Carpena, M., Garcia-Perez, P., Perez-Gregorio, R., Cassani, L., Simal-Gandara, J., & Prieto, M. A. (2024). Unraveling the Bioactive Potential of Camellia japonica Edible Flowers: Profiling Antioxidant Substances and In Vitro Bioactivity Assessment. Pharmaceuticals, 17(7), 946. https://doi.org/10.3390/ph17070946














