Comparison of Glutathione Nanoparticles, CoEnzyme Q10, and Fish Oil for Prevention of Oxygen-Induced Retinopathy in Neonatal Rats
Abstract
:1. Introduction
2. Results
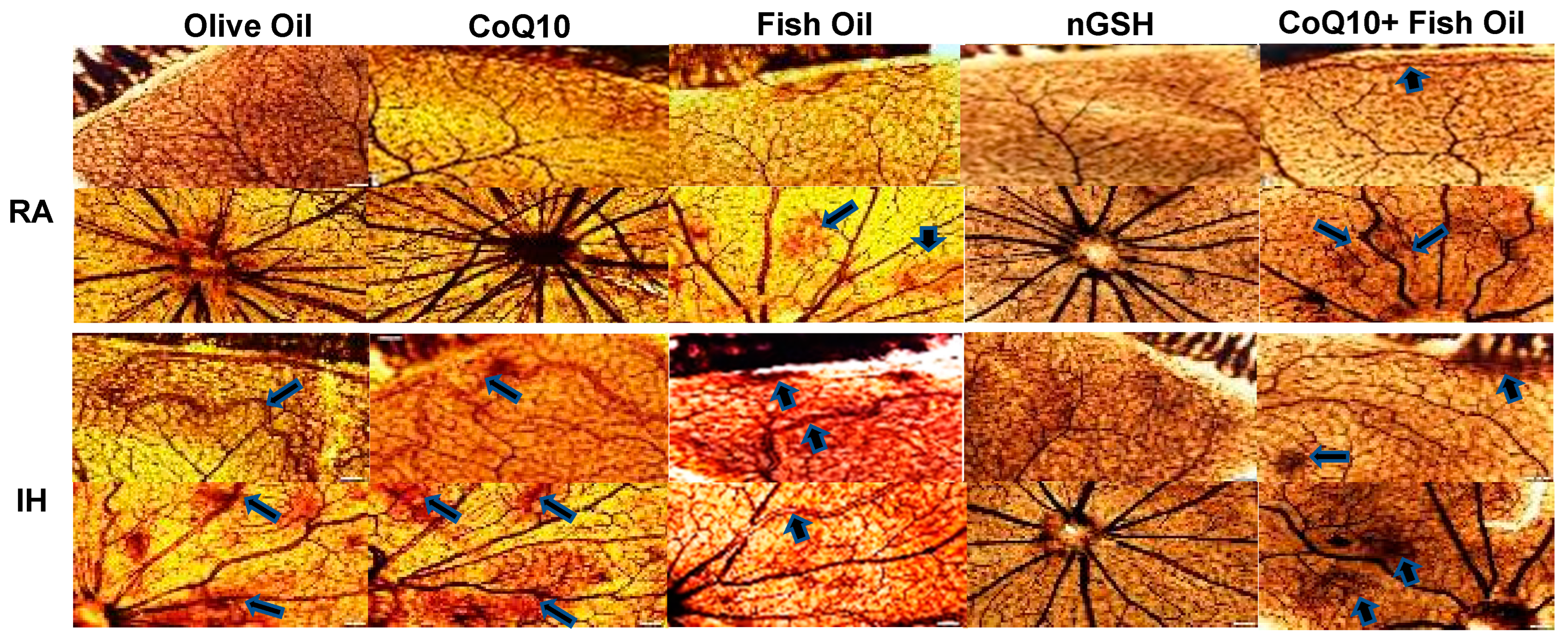
2.1. Retinal Vasculature
2.2. Retinal Astrocytes
2.3. Retinal Histopathology
2.4. Notch-1
2.5. Notch-4
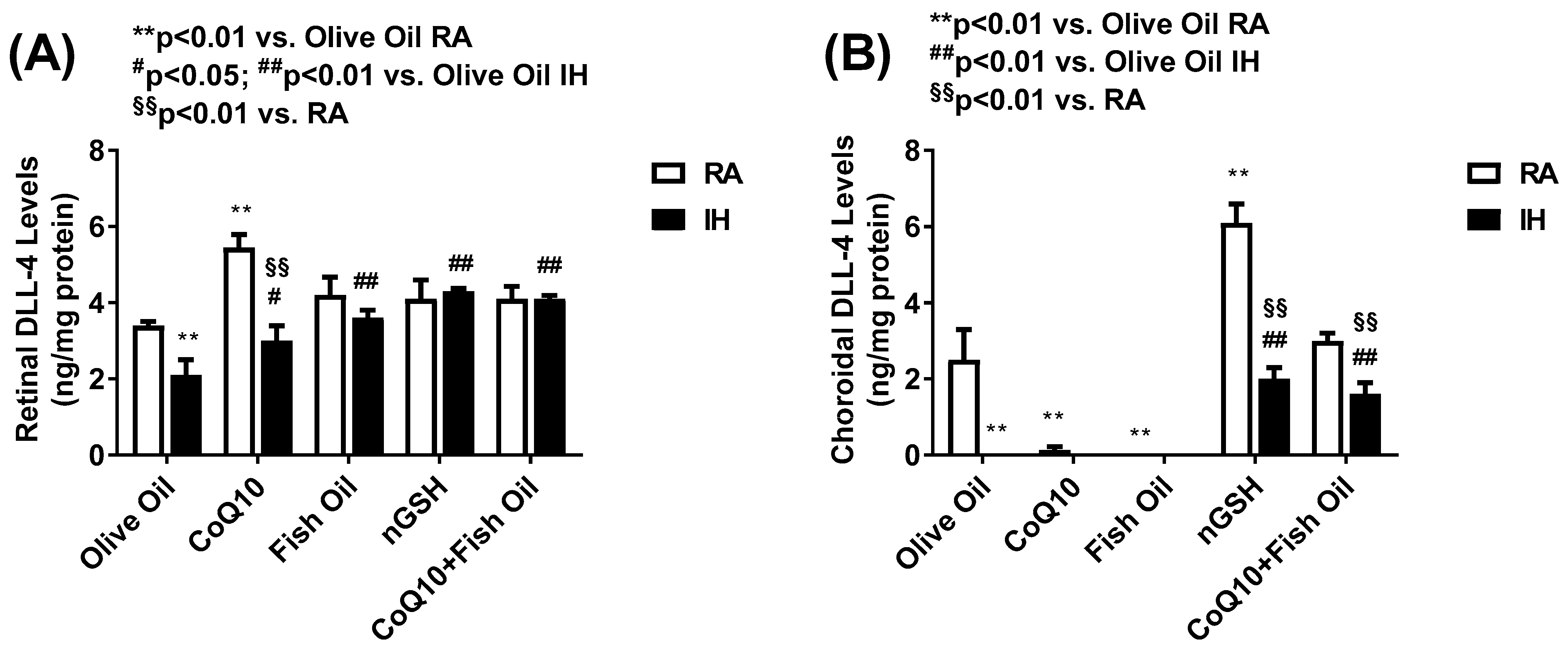
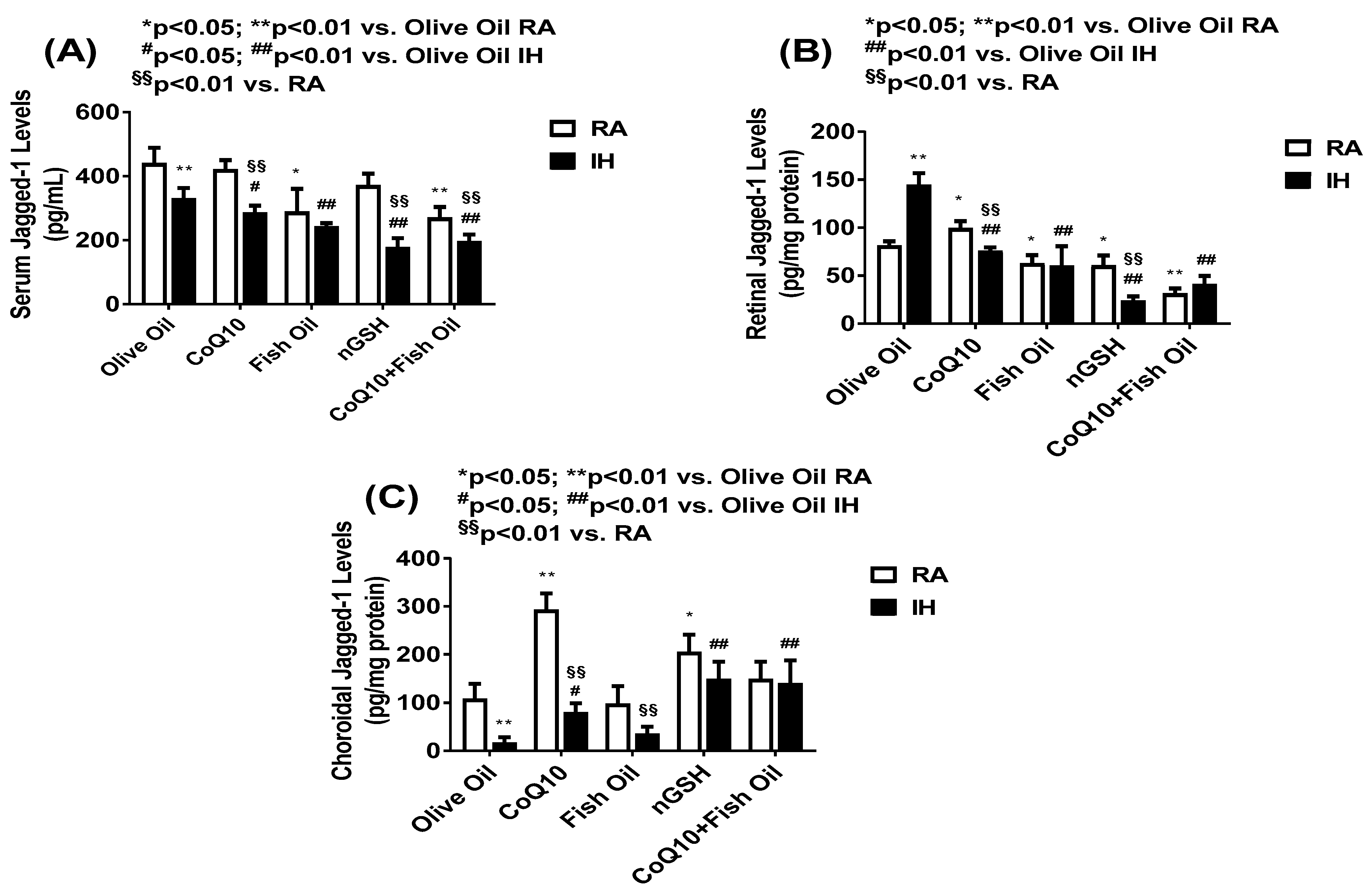
2.6. Notch Ligands
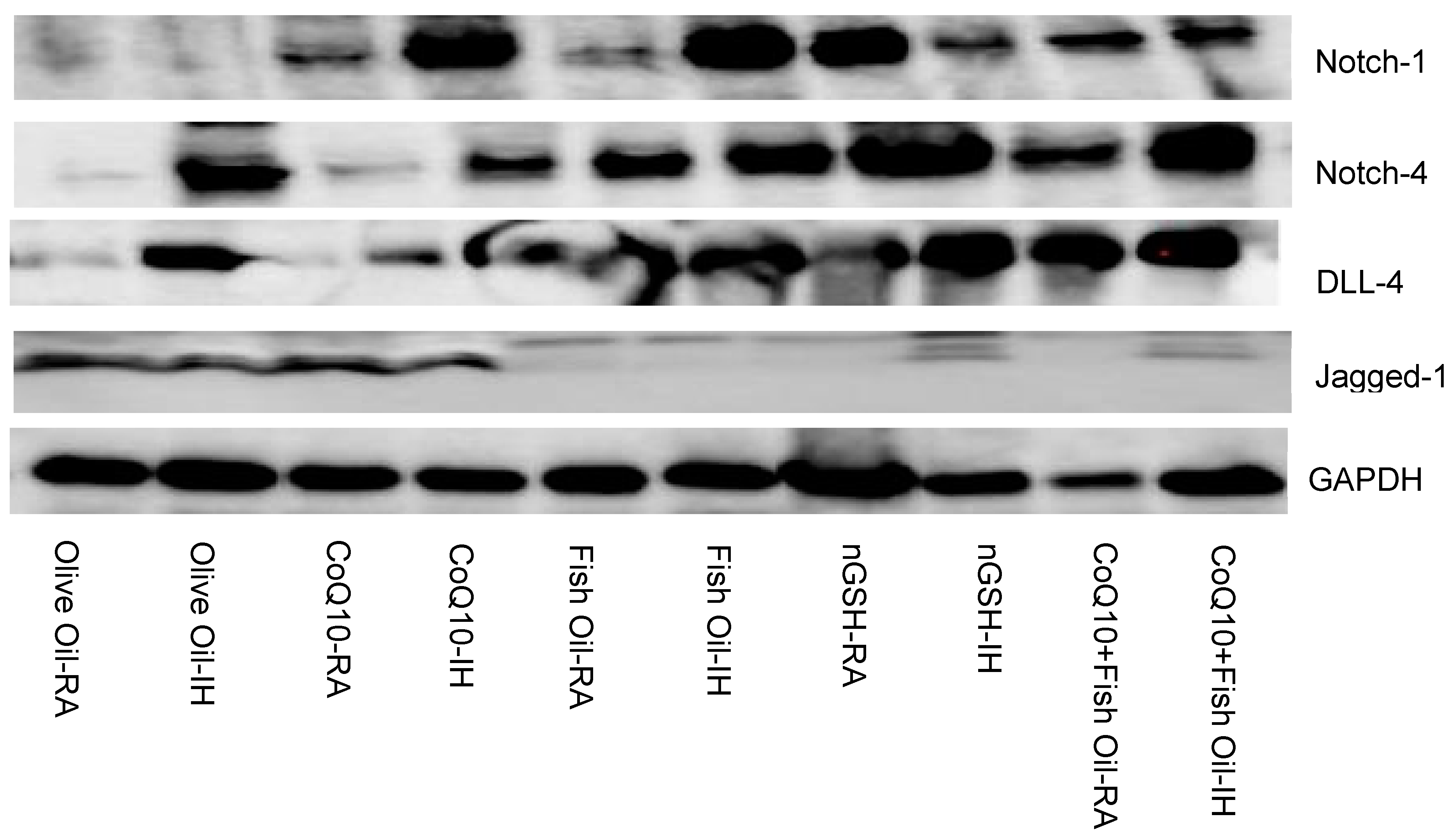
2.7. Western Blots
2.8. VEGF165
2.9. VEGFR-1
2.10. Lipid Peroxidation
2.11. Nestin
2.12. Apoptosis
2.13. HIF1α
2.14. Eye Opening
2.15. Somatic Growth
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Design
4.3. Neonatal Intermittent Hypoxia (IH) Profile
4.4. Sample Collection and Processing
4.5. Retinal Flatmounts
4.6. ADPase Staining of the Retinas
4.7. GFAP and Isolectin B4 Staining
4.8. Retinal Angiogenesis and Morphometric Analyses
4.9. Assay of MDA
4.10. Assay of Notch Ligands and Receptors
4.11. Total Cellular Protein Levels
4.12. IHC
4.13. Western Blots
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bashinsky, A.L. Retinopathy of Prematurity. N. C Med. J. 2017, 78, 124–12817. [Google Scholar] [CrossRef]
- Kim, S.J.; Port, A.D.; Swan, R.; Campbell, J.P.; Chan, R.V.P.; Chiang, M.F. Retinopathy of prematurity: A review of risk factors and their clinical significance. Surv. Ophthalmol. 2018, 63, 618–637. [Google Scholar] [CrossRef]
- Carroll, L.; Owen, L.A. Current evidence and outcomes for retinopathy of prematurity prevention: Insights into novel maternal and placental disease contributions. Explor. Med. 2020, 1, 4–26. [Google Scholar] [CrossRef]
- Hong, E.H.; Shin, Y.U.; Bae, G.H.; Choi, Y.J.; Ahn, S.J.; Kim, I.; Cho, H. Ophthalmic complications in retinopathy of prematurity in the first decade of life in Korea using the national health insurance database. Sci. Rep. 2022, 12, 911. [Google Scholar] [CrossRef] [PubMed]
- Gunay, M.; Sekeroglu, M.A.; Bardak, H.; Celik, G.; Esenulku, C.M.; Hekimoglu, E.; Bardak, Y. Evaluation of refractive errors and ocular biometric outcomes after intravitreal bevacizumab for retinopathy of prematurity. Strabismus 2016, 24, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Fielder, A.; Blencowe, H.; O’Connor, A.; Gilbert, C. Impact of retinopathy of prematurity on ocular structures and visual functions. Arch. Dis. Child. Fetal Neonatal Ed. 2015, 100, F179–F184. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, M.I.; Russ, R.; Brennan, K.A.; Williams, C.J.; Berrones, D.; Patel, B.; Martinez-Castellanos, M.A.; Fernandes, A.; Hubbard, G.B.; Chan, R.P.; et al. The Economic Model of Retinopathy of Prematurity (EcROP) Screening and Treatment: Mexico and the United States. Arch. Ophthalmol. 2016, 168, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Dave, H.B.; Gordillo, L.; Yang, Z.; Zhang, M.S.; Hubbard, G.B.; Olsen, T.W. The societal burden of blindness secondary to retinopathy of prematurity in Lima, Peru. Arch. Ophthalmol. 2012, 154, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Eckert, K.A.; Carter, M.J.; Lansingh, V.C.; Wilson, D.A.; Furtado, J.M.; Frick, K.D.; Resnikoff, S. A simple method for estimating the economic cost of productivity loss due to blindness and moderate to severe visual impairment. Ophthalmic Epidemiol. 2015, 22, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Hellström, A.; Smith, L.E.; Dammann, O. Retinopathy of prematurity. Lancet 2012, 382, 1445–1457. [Google Scholar] [CrossRef]
- Owen, L.A.; Morrison, M.A.; Hoffman, R.O.; Yoder, B.A.; DeAngelis, M.M. Retinopathy of prematurity: A comprehensive risk analysis for prevention and prediction of disease. PLoS ONE 2017, 12, e0171467. [Google Scholar] [CrossRef]
- Slidsborg, C.; Jensen, A.; Forman, J.L.; Rasmussen, S.; Bangsgaard, R.; Fledelius, H.C.; Greisen, G.; la Cour, M. Neonatal risk factors for treatment-demanding retinopathy of prematurity: A Danish national study. Ophthalmology 2016, 123, 796–803. [Google Scholar] [CrossRef]
- Gunn, T.R.; Easdown, J.; Outerbridge, E.W.; Aranda, J.V. Risk factors in retrolental fibroplasia. Pediatrics 1980, 65, 1096–1100. [Google Scholar] [CrossRef]
- DiFiore, J.M.; Martin, R.J.; Gauda, E.B. Apnea of prematurity-perfect storm. Respir Physiol. Neurobiol. 2013, 189, 213–222. [Google Scholar] [CrossRef]
- Di Fiore, J.M.; Bloom, J.N.; Orge, F.; Schutt, A.; Schluchter, M.; Cheruvu, V.K.; Walsh, M.; Finer, N.; Martin, R.J. A higher incidence of intermittent hypoxemic episodes is associated with severe retinopathy of prematurity. J. Pediatr. 2010, 157, 69–73. [Google Scholar] [CrossRef]
- Di Fiore, J.M.; Kaffashi, F.; Loparo, K.; Sattar, A.; Schluchter, M.; Foglyano, R.; Martin, R.J.; Wilson, C.G. The relationship between patterns of intermittent hypoxia and retinopathy of prematurity in preterm infants. Pediatr. Res. 2012, 72, 606–612. [Google Scholar] [CrossRef]
- Beharry, K.D.; Cai, C.L.; Ahmad, T.; Guzel, S.; Valencia, G.B.; Aranda, J.V. Impact of chronic neonatal intermittent hypoxia on severity of retinal damage in a rat model of oxygen-induced retinopathy. J. Nat. Sci. 2018, 4, e488. [Google Scholar]
- Beharry, K.D.; Cai, C.L.; Skelton, J.; Siddiqui, F.; D’agrosa, C.; Calo, J.; Valencia, G.B.; Aranda, J.V. Oxygen-induced retinopathy from recurrent intermittent hypoxia is not dependent on resolution with room air or oxygen, in neonatal rats. Int. J. Mol. Sci. 2018, 19, 1337. [Google Scholar] [CrossRef] [PubMed]
- Melincovici, C.S.; Boşca, A.B.; Şuşman, S.; Mărginean, M.; Mihu, C.; Istrate, M.; Moldovan, I.M.; Roman, A.L.; Mihu, C.M. Vascular endothelial growth factor (VEGF)—Key factor in normal and pathological angiogenesis. Rom. J. Morphol. Embryol. 2018, 59, 455–467. [Google Scholar] [PubMed]
- Gerhardt, H.; Golding, M.; Fruttiger, M.; Ruhrberg, C.; Lundkvist, A.; Abramsson, A.; Jeltsch, M.; Mitchell, C.; Alitalo, K.; Shima, D.; et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 2003, 161, 1163–1177. [Google Scholar] [CrossRef] [PubMed]
- Betsholtz, C. Cell–cell signaling in blood vessel development and function. EMBO Mol. Med. 2018, 10, e8610. [Google Scholar] [CrossRef]
- Dou, G.-R.; Wang, L.; Wang, Y.-S.; Han, H. Notch signaling in ocular vasculature development and diseases. Mol. Med. 2011, 18, 47–55. [Google Scholar] [CrossRef]
- Ahmad, I.; Balasubramanian, S.; Del Debbio, C.B.; Parameswaran, S.; Katz, A.R.; Toris, C.; Fariss, R.N. Regulation of ocular angiogenesis by notch signaling: Implications in neovascular age-related macular degeneration. Investig. Opthalmology Vis. Sci. 2011, 52, 2868–2878. [Google Scholar] [CrossRef]
- Ehling, M.; Adams, S.; Benedito, R.; Adams, R.H. Notch controls retinal blood vessel maturation and quiescence. Development 2013, 140, 3051–3061. [Google Scholar] [CrossRef] [PubMed]
- Benedito, R.; Rocha, S.F.; Woeste, M.; Zamykal, M.; Radtke, F.; Casanovas, O.; Duarte, A.; Pytowski, B.; Adams, R.H. Notch-dependent VEGFR3 upregulation allows angiogenesis without VEGF–VEGFR2 signalling. Nature 2012, 484, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Hellström, M.; Phng, L.K.; Hofmann, J.J.; Wallgard, E.; Coultas, L.; Lindblom, P.; Alva, J.; Nilsson, A.K.; Karlsson, L.; Gaiano, N.; et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 2007, 445, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Benedito, R.; Roca, C.; Sörensen, I.; Adams, S.; Gossler, A.; Fruttiger, M.; Adams, R.H. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell 2009, 137, 1124–1135. [Google Scholar] [CrossRef] [PubMed]
- Raghuram, K.; Isaac, M.; Yang, J.; AlAli, A.; Mireskandari, K.; Ly, L.G.; Kelly, E.; Banihani, R.; Shah, P.S.; Tehrani, N. Neurodevelopmental outcomes in infants treated with intravitreal bevacizumab versus laser. J. Perinatol. 2019, 39, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, G.; Shankaran, S.; Nolen, T.L.; Sridhar, A.; Kennedy, K.A.; Hintz, S.R.; Phelps, D.L.; DeMauro, S.B.; Carlo, W.A.; Gantz, M.G.; et al. Neurodevelopmental outcomes of preterm infants with retinopathy of prematurity by treatment. Pediatrics 2019, 144, e20183537. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-S.; Chen, Y.-T.; Lai, T.-T.; Chou, H.-C.; Chen, C.-Y.; Hsieh, W.-S.; Yang, C.-M.; Yeh, P.-T.; Tsao, P.-N. Involution of retinopathy of prematurity and neurodevelopmental outcomes after intravitreal bevacizumab treatment. PLoS ONE 2019, 14, e0223972. [Google Scholar] [CrossRef]
- Barry, G.P.; Tauber, K.A.; Fisher, M.; Greenberg, S.; Zobal-Ratner, J.; Binenbaum, G. Short-term retinal detachment risk after treatment of type 1 retinopathy of prematurity with laser photocoagulation versus intravitreal bevacizumab. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2019, 23, 260. [Google Scholar] [CrossRef]
- Roohipoor, R.; Karkhaneh, R.; Riazi-Esfahani, M.; Farahani, A.D.; Khodabandeh, A.; Adib, N.E.; Imani, M.; Jafari, H.K.; Riazi-Esfahani, H.; Hosseinpour, J.; et al. Comparison of intravitreal bevacizumab and laser photocoagulation in the treatment of retinopathy of prematurity. Ophthalmol. Retin. 2018, 2, 942–948. [Google Scholar] [CrossRef]
- Mansukhani, S.A.; Hutchinson, A.K.; Neustein, R.; Schertzer, J.; Allen, J.C.; Hubbard, G.B. Fluorescein angiography in retinopathy of prematurity: Comparison of infants treated with bevacizumab to those with spontaneous regression. Ophthalmol. Retin. 2019, 3, 436–443. [Google Scholar] [CrossRef]
- Vayalthrikkovil, S.; Bashir, R.A.; Rabi, Y.; Amin, H.; Spence, J.M.; Robertson, H.L.; Lodha, A. Parenteral Fish-Oil Lipid Emulsions in the Prevention of Severe Retinopathy of Prematurity: A Systematic Review and Meta-Analysis. Am. J. Perinatol. 2017, 34, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, D.; Lauterbach, R.; Turyk, E. Fish-oil fat emulsion supplementation may reduce the risk of severe retinopathy in VLBW infants. Pediatrics 2011, 127, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, D.; Lauterbach, R.; Walczak, M.; Hurkala, J.; Sherman, M.P. Fish-oil fat emulsion supplementation reduces the risk of retinopathy in very low birth weight infants: A prospective, randomized study. JPEN J Parenter. Enteral Nutr 2014, 38, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Beken, S.; Dilli, D.; Fettah, N.D.; Kabataş, E.U.; Zenciroğlu, A.; Okumuş, N. The influence of fish-oil lipid emulsions on retinopathy of prematurity in very low birth weight infants: A randomized controlled trial. Early Hum. Dev. 2014, 90, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Ding, H.; Shan, J.; Li, X.; Zhang, J.; Liu, G.; Zheng, H.; Su, Y.; Yao, H.; Qi, K. Association of fish oil containing lipid emulsions with retinopathy of prematurity: A retrospective observational study. BMC Pediatr. 2022, 22, 113. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.-F.; Lee, C.-H.; Chen, H.-N.; Tsao, L.-Y.; Chen, J.-Y.; Hsiao, C.-C. Effects of fish oil-containing lipid emulsions on retinopathy of prematurity in very low birth weight infants. Pediatr. Neonatol. 2020, 61, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Beharry, K.D.; Cai, C.L.; Siddiqui, F.; Chowdhury, S.; D’agrosa, C.; Valencia, G.B.; Aranda, J.V. Comparative effects of coenzyme Q10 or n-3 polyunsaturated fatty acid supplementation on retinal angiogenesis in a rat model of oxygen-induced retinopathy. Antioxidants 2018, 7, 160. [Google Scholar] [CrossRef]
- Beharry, K.D.; Cai, C.L.; Siddiqui, F.; D’agrosa, C.; Zangaladze, A.; Mustafa, G.; Qadri, A.; Duggan, T.J.; Aranda, J.V. Combination Antioxidant/NSAID Therapies and Oral/Topical Ocular Delivery Modes for Prevention of Oxygen-Induced Retinopathy in a Rat Model. Nutrients 2020, 12, 1980. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, F.; Cai, C.; Aranda, J.V.; Beharry, K.D. Coenzyme Q10 and Fish Oil Supplementation for Reducing Retinal Oxidative Stress in a Rat Model. Vision 2023, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Lobov, I.; Mikhailova, N. The role of Dll4/Notch signaling in normal and pathological ocular angiogenesis: Dll4 controls blood vessel sprouting and vessel remodeling in normal and pathological conditions. J. Ophthalmol. 2018, 2018, 3565292. [Google Scholar] [CrossRef]
- Miloudi, K.; Oubaha, M.; Ménard, C.; Dejda, A.; Guber, V.; Cagnone, G.; Wilson, A.M.; Tétreault, N.; Mawambo, G.; Binet, F.; et al. NOTCH1 signaling induces pathological vascular permeability in diabetic retinopathy. Proc. Natl. Acad. Sci. USA 2019, 116, 4538–4547. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, R.S.; O’connell, R.; Ascher, S.B.; Sarin, N.; Freedman, S.F.; Wallace, D.K.; Chiu, S.J.; Farsiu, S.; Cotten, M.; Toth, C.A. Spectral-domain optical coherence tomographic assessment of severity of cystoid macular edema in retinopathy of prematurity. Arch. Ophthalmol. 2012, 130, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Lavoie, P.M.; Lacaze-Masmonteil, T.; Rhainds, M.; Marc, I. Omega-3 long-chain polyunsaturated fatty acids for extremely preterm infants: A systematic review. Pediatrics 2014, 134, 120–134. [Google Scholar] [CrossRef]
- Lapillonne, A.; Moltu, S.J. Long-Chain Polyunsaturated fatty acids and clinical outcomes of preterm infants. Ann. Nutr. Metab. 2016, 69, 35–44. [Google Scholar] [CrossRef]
- Sarmiento, A.; Diaz-Castro, J.; Pulido-Moran, M.; Kajarabille, N.; Guisado, R.; Ochoa, J.J. Coenzyme Q10 supplementation and exercise in healthy humans: A systematic review. Curr. Drug Metab. 2016, 17, 345–358. [Google Scholar] [CrossRef]
- Garrido-Maraver, J.; Cordero, M.D.; Oropesa-Avila, M.; Vega, A.F.; de la Mata, M.; Pavon, A.D.; Alcocer-Gomez, E.; Calero, C.P.; Paz, M.V.; Alanis, M.; et al. Clinical applications of coenzyme Q10. Front. Biosci. 2014, 19, 619–633. [Google Scholar] [CrossRef]
- Lee, D.; Kim, K.-Y.; Shim, M.S.; Kim, S.Y.; Ellisman, M.H.; Weinreb, R.N.; Ju, W.-K. Coenzyme Q10 ameliorates oxidative stress and prevents mitochondrial alteration in ischemic retinal injury. Apoptosis 2013, 19, 603–614. [Google Scholar] [CrossRef]
- Bergamini, C.; Moruzzi, N.; Sblendido, A.; Lenaz, G.; Fato, R. A Water soluble CoQ10 formulation improves intracellular distribution and promotes mitochondrial respiration in cultured cells. PLoS ONE 2012, 7, e33712. [Google Scholar] [CrossRef]
- Coleman, R.J.; Beharry, K.D.A.; Brock, R.S.; Abad-Santos, P.; Abad-Santos, M.; Modanlou, H.D. Effects of brief, clustered versus dispersed hypoxic episodes on systemic and ocular growth factors in a rat model of oxygen-induced retinopathy. Pediatr. Res. 2008, 64, 50–55. [Google Scholar] [CrossRef]
- Zhu, G.; Lin, Y.; Ge, T.; Singh, S.; Liu, H.; Fan, L.; Wang, S.; Rhen, J.; Jiang, D.; Lyu, Y.; et al. A novel peptide inhibitor of Dll4-Notch1 signalling and its pro-angiogenic functions. Br. J. Pharmacol. 2022, 179, 1716–1731. [Google Scholar] [CrossRef]
- Kofler, N.M.; Shawber, C.J.; Kangsamaksin, T.; Reed, H.O.; Galatioto, J.; Kitajewski, J. Notch Signaling in Developmental and Tumor Angiogenesis. Genes. Cancer 2011, 2, 1106–1116. [Google Scholar] [CrossRef]
- Fong, G.-H.; Rossant, J.; Gertsenstein, M.; Breitman, M.L. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature 1995, 376, 66–70. [Google Scholar] [CrossRef]
- Funahashi, Y.; Shawber, C.J.; Vorontchikhina, M.; Sharma, A.; Outtz, H.H.; Kitajewski, J. Notch regulates the angiogenic response via induction of VEGFR-1. J. Angiogenes Res. 2010, 2, 3. [Google Scholar] [CrossRef]
- Li, D.; Masiero, M.; Banham, A.H.; Harris, A.L. The Notch ligand Jagged1 as a target for anti-tumor therapy. Front. Oncol. 2014, 4, 254. [Google Scholar] [CrossRef]
- Tualeka, A.R.; Martiana, T.; Ahsan, A.; Russeng, S.S.; Meidikayanti, W. Association between malondialdehyde and glutathione (L-gamma-glutamyl-cysteinyl-glycine/GSH) levels on workers exposed to benzene in Indonesia. Open Access Maced. J. Med. Sci. 2019, 7, 1198–1202. [Google Scholar] [CrossRef]
- Tekin, S.; Seven, E. Assessment of serum catalase, reduced glutathione, and superoxide dismutase activities and malondialdehyde levels in keratoconus patients. Eye 2022, 36, 2062–2066. [Google Scholar] [CrossRef]
- Bruggeman, B.K.; Storo, K.E.; Fair, H.M.; Wommack, A.J.; Carriker, C.R.; Smoliga, J.M. The absorptive effects of orobuccal non-liposomal nano-sized glutathione on blood glutathione parameters in healthy individuals: A pilot study. PLoS ONE 2019, 14, e0215815. [Google Scholar] [CrossRef]
- Nakano, A.; Nakahara, T.; Mori, A.; Ushikubo, H.; Sakamoto, K.; Ishii, K. Short-term treatment with VEGF receptor inhibitors induces retinopathy of prematurity-like abnormal vascular growth in neonatal rats. Exp. Eye Res. 2015, 143, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Fiúza, U.-M.; Arias, A.M. Cell and molecular biology of Notch. J. Endocrinol. 2007, 194, 459–474. [Google Scholar] [CrossRef] [PubMed]
- Bray, S.J. Notch signalling: A simple pathway becomes complex. Nat. Rev. Mol. Cell Biol. 2006, 7, 678–689. [Google Scholar] [CrossRef]
- Suchting, S.; Freitas, C.; le Noble, F.; Benedito, R.; Bréant, C.; Duarte, A.; Eichmann, A. The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc. Natl. Acad. Sci. USA 2007, 104, 3225–3230. [Google Scholar] [CrossRef]






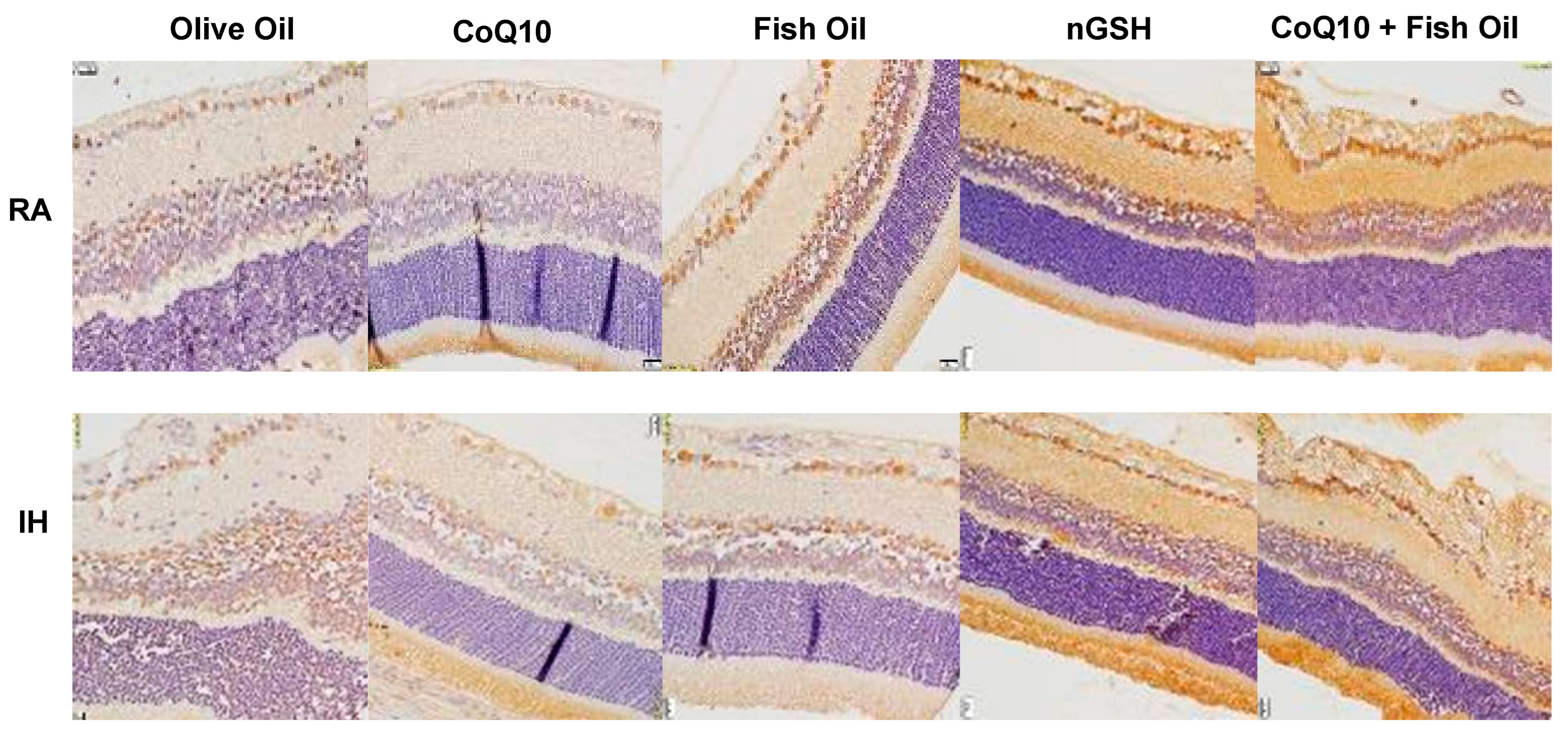

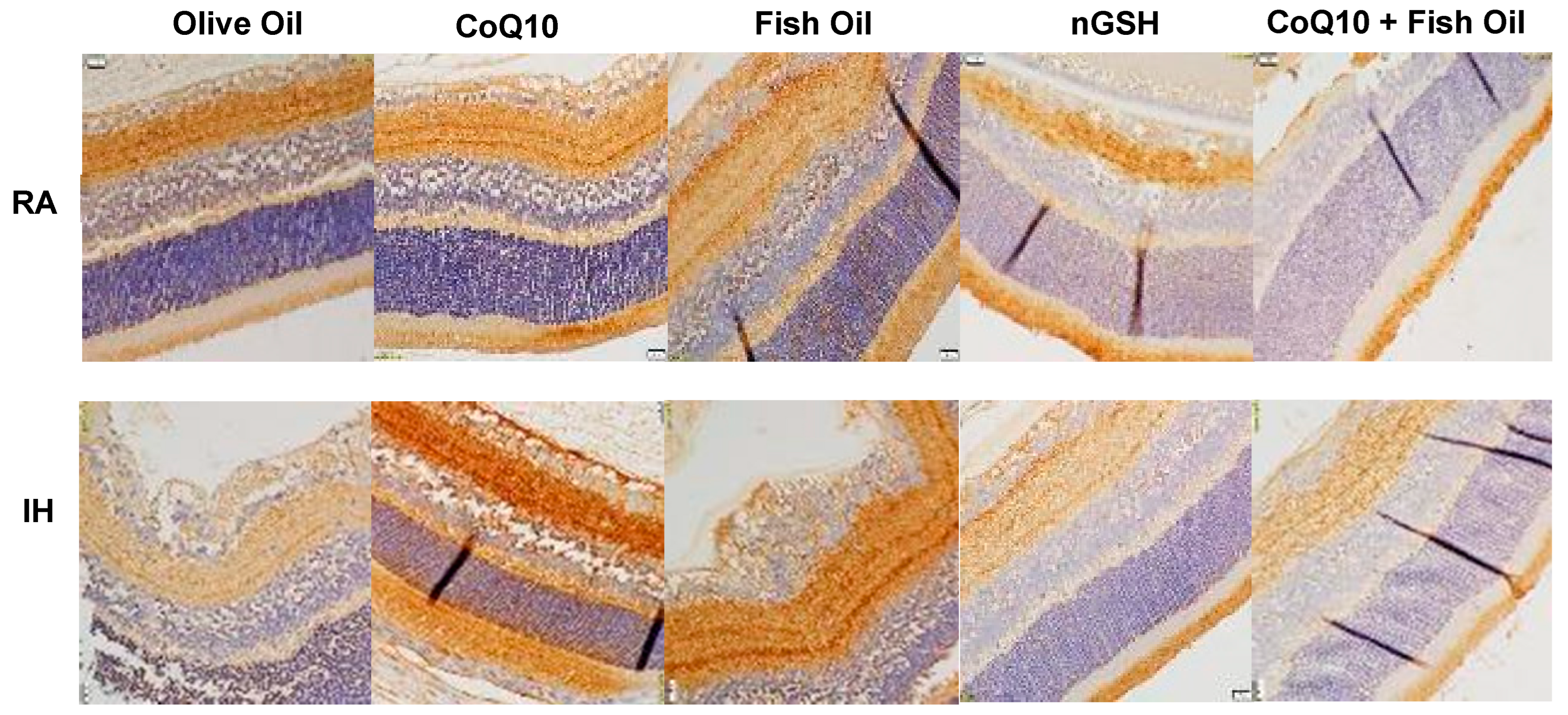

| Olive Oil | CoQ10 | Fish Oil | nGSH | CoQ10 + Fish Oil | |
|---|---|---|---|---|---|
| Room Air (RA) | |||||
| Tortuosity index | 0.96 ± 0.05 | 0.97 ± 0.05 | 0.98 ± 0.03 | 1.0 ± 0.02 | 1.0 ± 0.03 |
| Artery diameter (µm) | 55.3 ± 9.3 | 50.6 ± 11.8 | 46.1 ± 7.3 | 27.6 ± 0.69 * | 26.6 ± 0.63 * |
| Vein diameter (µm) | 41.9 ± 10.3 | 38.4 ± 9.8 | 32.2 ± 5.9 | 40.6 ± 1.5 | 43.8 ± 1.2 |
| No. ECs in NFL/GCL layer | 181.1 ± 16.6 | 173.0 ± 16.6 | 176.1 ± 12.8 | 211.0 ± 28 | 166.8 ± 14.9 |
| Total retinal thickness (µm) | 269.3 ± 6.8 | 248.2 ± 5.0 | 343.3 ± 11.6 ** | 261.6 ± 9.2 | 303.0 ± 9.0 * |
| NFL/GCL thickness (µm) | 34.1 ± 8.1 | 29.5 ± 1.8 | 50.9 ± 4.1 * | 26.8 ± 3.1 | 41.8 ± 3.0 |
| IPL thickness (µm) | 53.3 ± 1.6 | 47.9 ± 0.84 | 67.2 ± 4.0 ** | 51.6 ± 2.3 | 48.1 ± 3.7 |
| INL thickness (µm) | 57.7 ± 0.98 | 48.3 ± 1.2 | 59.8 ± 2.3 | 43.4 ± 3.4 ** | 52.9 ± 4.2 |
| OPL thickness (µm) | 14.7 ± 0.7 | 14.1 ± 0.56 | 17.6 ± 0.7 ** | 12.4 ± 0.74 | 14.5 ± 0.63 |
| ONL thickness (µm) | 81.6 ± 1.1 | 71.4 ± 1.4 * | 82.8 ± 2.4 | 73.7 ± 2.9 | 87.3 ± 4.8 |
| R/C thickness (µm) | 37.1 ± 1.1 | 32.5 ± 1.3 | 45.5 ± 2.3 ** | 32.0 ± 2.6 | 40.1 ± 1.8 |
| Intermittent Hypoxia (IH) | |||||
| Tortuosity index | 1.52 ± 0.24 §§ | 1.1 ± 0.1 ## | 1.08 ± 0.04 ## | 1.03 ± 0.03 ## | 1.08 ± 0.08 ## |
| Artery diameter (µm) | 60.9 ± 3.4 | 45.1 ± 12.2 | 42.7 ± 10.3 | 29.5 ± 0.57 ##,§ | 29.2 ± 0.65 ##,§§ |
| Vein diameter (µm) | 48.3 ± 8.3 | 37.6 ± 5.9 | 34.9 ± 7.3 | 41.6 ± 0.95 | 44.2 ± 1.03 |
| No. ECs in NFL/GCL layer | 639.5 ± 148.4 §§ | 274.2 ± 32.7 #,§§ | 610.3 ± 81.5 §§ | 300.0 ± 41.9 # | 770.3 ± 73.1 §§ |
| Total retinal thickness (µm) | 379.4 ± 10.6 §§ | 330.8 ± 11.7 ##,§§ | 308.6 ± 13.1 ##,§ | 307.0 ± 16.7 ##,§ | 286.7 ± 11.5 ## |
| NFL/GCL thickness (µm) | 72.4 ± 7.9 §§ | 49.2 ± 4.0 ##,§§ | 41.2 ± 2.7 ##,§ | 42.0 ± 3.4 ##,§§ | 54.1 ± 4.6#,§ |
| IPL thickness (µm) | 63.0 ± 2.2 §§ | 61.0 ± 2.4 §§ | 52.3 ± 2.5 §§ | 61.0 ± 5.1 | 46.0 ± 2.8 ## |
| INL thickness (µm) | 57.5 ± 1.5 §§ | 73.4 ± 2.4 §§ | 59.1 ± 4.2 | 59.5 ± 3.5 §§ | 49.9 ± 2.2 ## |
| OPL thickness (µm) | 18.1 ± 0.65 §§ | 17.7 ± 3.6 §§ | 15.6 ± 0.6 §§ | 16.6 ± 1.6 §§ | 16.5 ± 0.82 |
| ONL thickness (µm) | 94.1 ± 2.3 §§ | 90.8 ± 5.1 §§ | 109.5 ± 7.0 §§ | 86.6 ± 2.9 §§ | 95.4 ± 7.2 |
| R/C thickness (µm) | 58.2 ± 2.7 §§ | 44.9 ± 2.0 ##,§§ | 48.0 ± 4.0 # | 40.7 ± 1.9 ##,§§ | 40.2 ± 1.9 ## |
| Olive Oil | CoQ10 | Fish Oil | nGSH | CoQ10 + Fish Oil | |
|---|---|---|---|---|---|
| Room Air (RA) | |||||
| Notch-1 | 12,221.9 ± 404.6 | 14,501.7 ± 378.6 * | 14,784.6 ± 379.2 ** | 23,151.2 ± 808.7 ** | 18,245.5 ± 750.7 ** |
| DLL-4 | 6915.3 ± 306.9 | 3325.1 ± 159.9 ** | 2317.1 ± 86.9 ** | 16,473.9 ± 696.9 ** | 16,907.0 ± 681.2 ** |
| Jagged-1 | 12,481.7 ± 744.8 | 12,722.4 ± 347.3 | 19,277.8 ± 600.1 ** | 12,166.4 ± 684.0 | 12,517.7 ± 619.6 |
| GFAP | 26,370.4 ± 555.9 | 20,405.6 ± 433.6 ** | 21,796.3 ± 604.1 ** | 20,876.6 ± 1027.7 ** | 28,311.1 ± 1290.8 |
| HIF1α | 3114.1 ± 351.1 | 3028.8 ± 190.6 | 2061.0 ± 304.8 * | 681.8 ± 49.6 ** | 2794.4 ± 446.7 |
| VEGF165 | 9395.5 ± 284.4 | 20,770.9 ± 1146.3 ** | 15,096.4 ± 799.1 ** | 14,043.8 ± 900.5 ** | 5879.1 ± 984.0 ** |
| VEGFR-1 | 2549.6 ± 112.0 | 1939.7 ± 77.6 | 3644.7 ± 199.4 | 9991.0 ± 631.3 ** | 7485.3 ± 357.4 ** |
| Nestin | 17,562.0 ± 514.2 | 20,229.1 ± 578.9 | 13,373.8 ± 854.5 ** | 13,690.9 ± 900.9 ** | 9490.6 ± 1007.6 ** |
| Apoptosis | 2629.1 ± 319.5 | 2855.0 ± 188.8 | 745.8 ± 124.0 ** | 524.9 ± 72.4 ** | 1321.6 ± 151.6 ** |
| Intermittent Hypoxia (IH) | |||||
| Notch-1 | 14,804.0 ± 379.5 §§ | 13,479.1 ± 554.3 | 14,482.5 ± 748.9 | 15,798.2 ± 367.7 §§ | 17,638.2 ± 912.7 ##,§§ |
| DLL-4 | 5749.8 ± 336.1§ | 3028.5 ± 133.5 ## | 2829.8 ± 214.1 ##,§ | 13,985.6 ± 527.2 ##,§§ | 1480.9 ± 678.4 ##,§§ |
| Jagged-1 | 19,913.3 ± 217.1 §§ | 14,091.6 ± 401.8 ##§ | 15,960.2 ± 821.8 ##,§§ | 12,341.0 ± 542.4 ##,§§ | 15,610.7 ± 362.4 ##,§§ |
| GFAP | 22,055.9 ± 336.0 §§ | 25,644.4 ± 604.3 #,§§ | 22,582.2 ± 766.8 | 19,288.8 ± 1562.6 | 18,498.6 ± 951.3 # |
| HIF1α | 7694.6 ± 444.6 §§ | 1353.7 ± 321.0 ##,§§ | 4740.5 ± 449.3 ##,§§ | 1851.3 ± 113.7 ##,§§ | 4395.5 ± 566.7 ##,§ |
| VEGF165 | 14,909.4 ± 793.0 §§ | 18,180.6 ± 817.8 ## | 18,207.6 ± 548.5 ##,§§ | 5175.2 ± 521.3 ##,§§ | 5031.7 ± 331.6 ## |
| VEGFR-1 | 1932.7 ± 81.5 § | 2051.9 ± 219.1 §§ | 1186.9 ± 57.5 §§ | 9246.0 ± 490.9 ## | 7213.0 ± 445.6 ## |
| Nestin | 15,755.4 ± 560.5 §§ | 25,224.1 ± 682.9 | 14,154.7 ± 1081.7 | 14,856.1 ± 464.3 | 10,295.0 ± 656.2 ## |
| Apoptosis | 3696.1 ± 204.9 §§ | 2920.5 ± 181.7 | 2882.2 ± 241.3 §§ | 1914.9 ± 216.8 ##,§§ | 3785.8 ± 332.2 §§ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bashir, S.; Cai, C.L.; Marcelino, M.; Aranda, J.V.; Beharry, K.D. Comparison of Glutathione Nanoparticles, CoEnzyme Q10, and Fish Oil for Prevention of Oxygen-Induced Retinopathy in Neonatal Rats. Pharmaceuticals 2024, 17, 381. https://doi.org/10.3390/ph17030381
Bashir S, Cai CL, Marcelino M, Aranda JV, Beharry KD. Comparison of Glutathione Nanoparticles, CoEnzyme Q10, and Fish Oil for Prevention of Oxygen-Induced Retinopathy in Neonatal Rats. Pharmaceuticals. 2024; 17(3):381. https://doi.org/10.3390/ph17030381
Chicago/Turabian StyleBashir, Sidra, Charles L. Cai, Matthew Marcelino, Jacob V. Aranda, and Kay D. Beharry. 2024. "Comparison of Glutathione Nanoparticles, CoEnzyme Q10, and Fish Oil for Prevention of Oxygen-Induced Retinopathy in Neonatal Rats" Pharmaceuticals 17, no. 3: 381. https://doi.org/10.3390/ph17030381
APA StyleBashir, S., Cai, C. L., Marcelino, M., Aranda, J. V., & Beharry, K. D. (2024). Comparison of Glutathione Nanoparticles, CoEnzyme Q10, and Fish Oil for Prevention of Oxygen-Induced Retinopathy in Neonatal Rats. Pharmaceuticals, 17(3), 381. https://doi.org/10.3390/ph17030381








