Transcriptome-Wide 5-Methylcytosine Profiling of lncRNAs in the Mouse Cerebral Ischemia Model
Abstract
:1. Introduction
2. Results
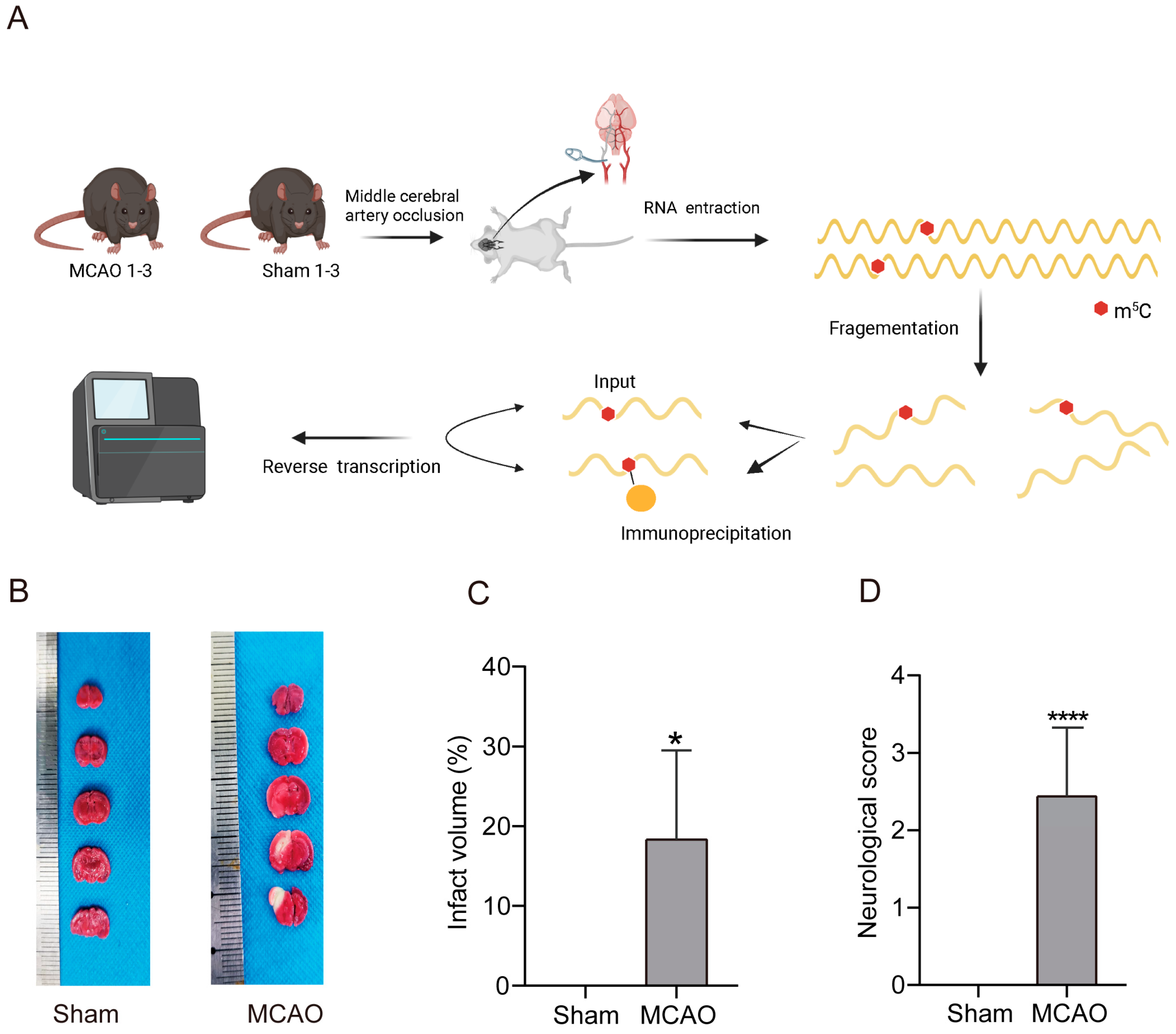
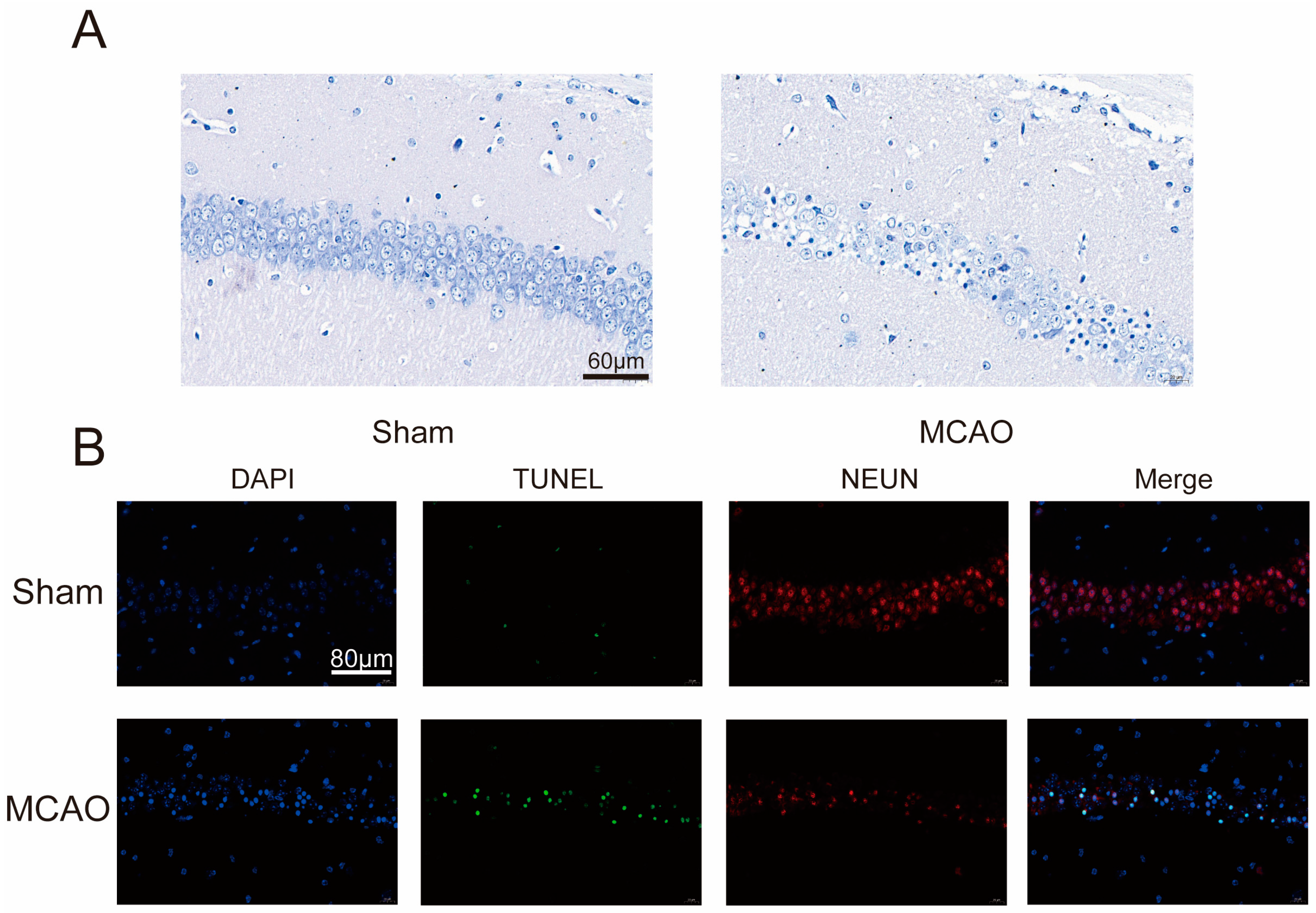
2.1. Increase inCerebral Infarction Area and Aggravation of Neurological Impairment in MCAO Group
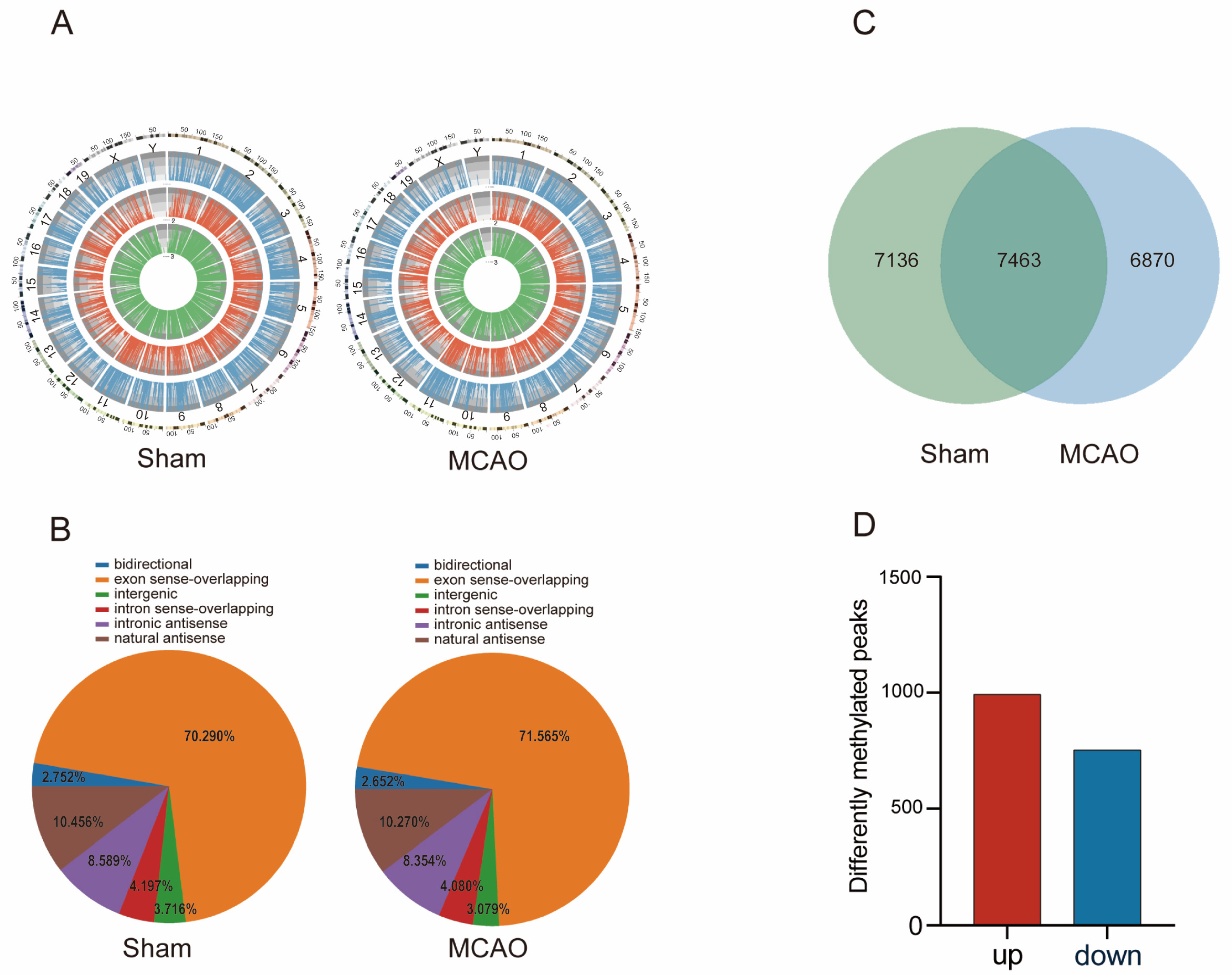
2.2. General Characteristics of lncRNAs m5C Methylation in MCAO Model and Normal Mice
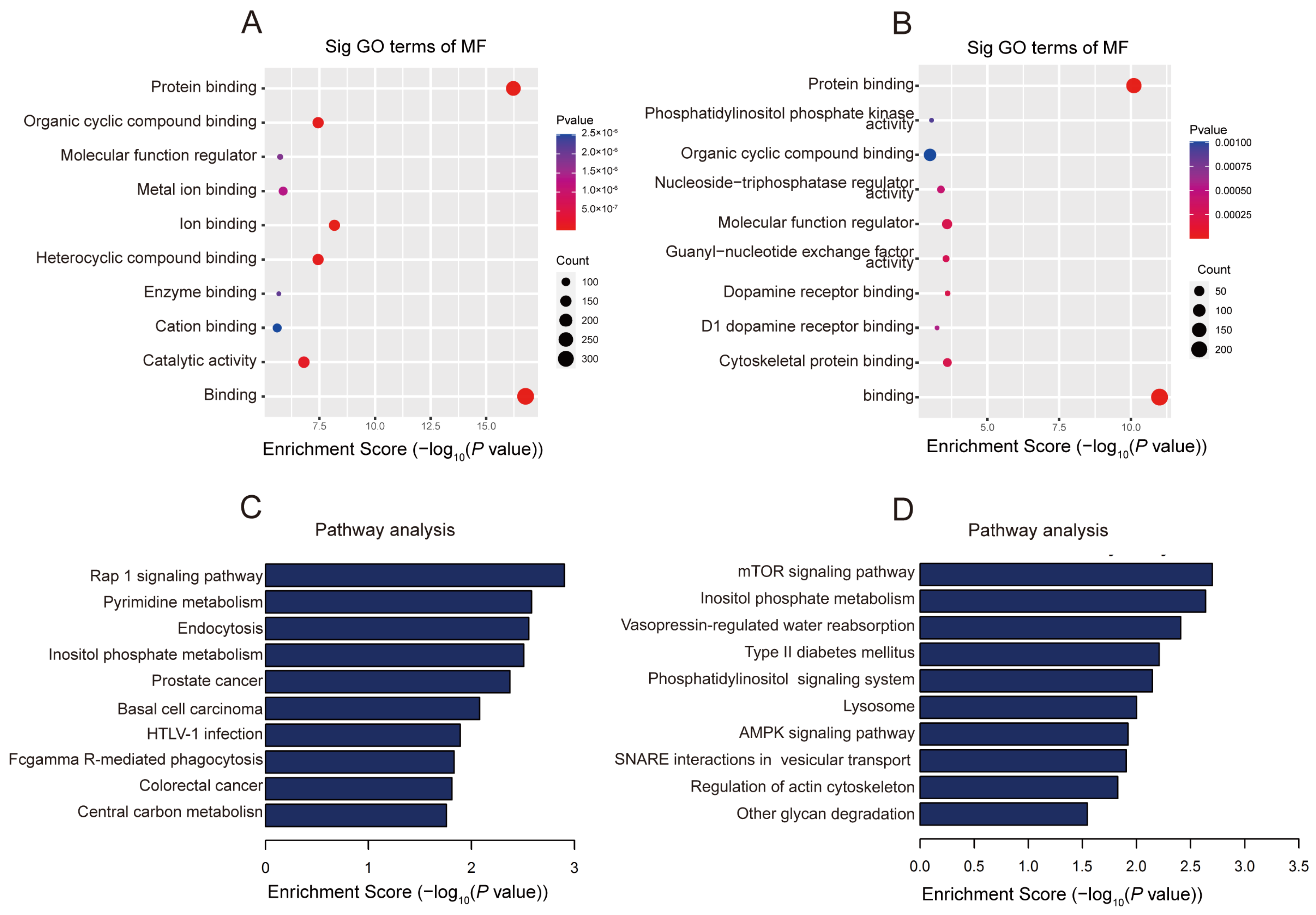
2.3. The Biological Information behind Differentially Methylated lncRNAs
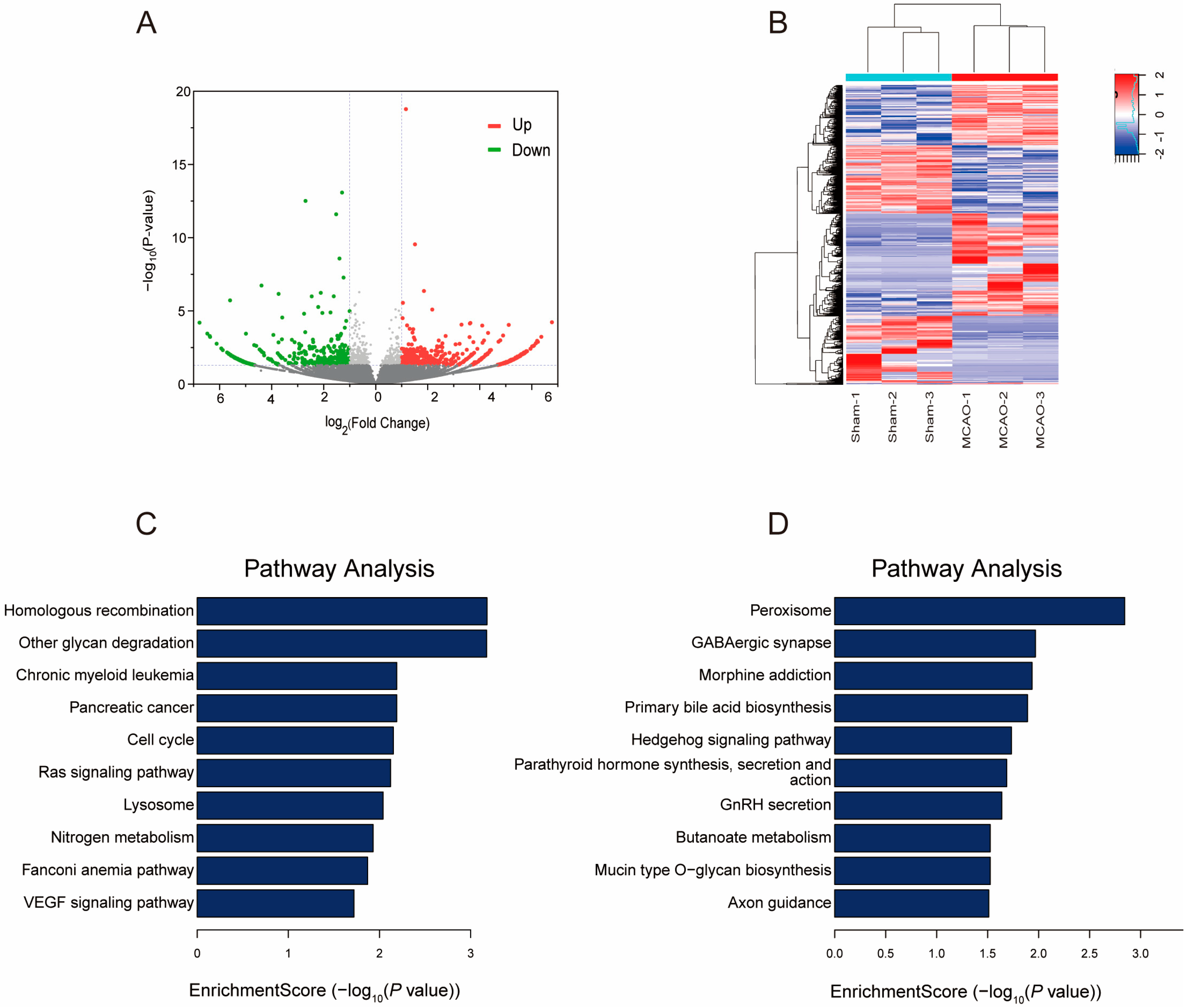
2.4. Gene Expression Profile following MCAO
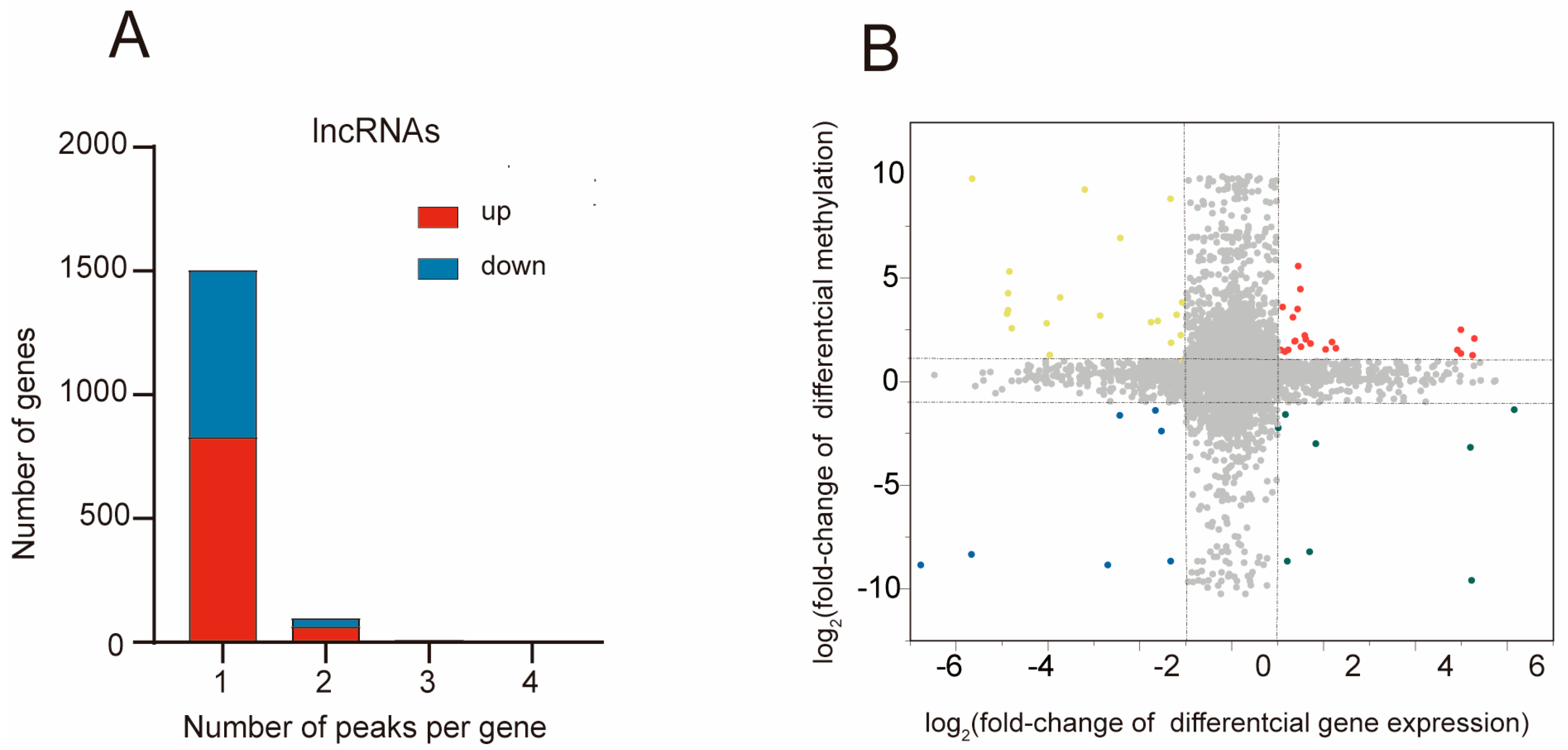
2.5. Combined Analysis of m5C Methylation and Gene Expression after MCAO
3. Discussion
4. Methods
4.1. MCAO Models Construction
4.2. TTC (2,3,5-Triphenyltetrazolium chloride) Staining
4.3. Evaluation of Neurological Deficit in Mice by Longa Biological Score
4.4. Nissl Staining
4.5. Immunofluorescence Staining
4.6. Extraction of RNA and Preparation of RNA-Seq Library
4.7. Preparation of Methylated RNA Immunoprecipitation Sequencing (MeRIP-Seq) Library
4.8. Data Processing and Bioinformatics Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murray, C.J.L.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2197–2223. [Google Scholar] [CrossRef]
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics—2022 Update: A Report From the American Heart Association. Circulation 2022, 145, E153–E639. [Google Scholar] [CrossRef]
- Kuriakose, D.; Xiao, Z. Pathophysiology and Treatment of Stroke: Present Status and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 7609. [Google Scholar] [CrossRef]
- Chamorro, Á.; Dirnagl, U.; Urra, X.; Planas, A.M. Neuroprotection in acute stroke: Targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016, 15, 869–881. [Google Scholar] [CrossRef]
- Shen, M.H.; Zhang, C.B.; Zhang, J.H.; Li, P.F. Electroacupuncture Attenuates Cerebral Ischemia and Reperfusion Injury in Middle Cerebral Artery Occlusion of Rat via Modulation of Apoptosis, Inflammation, Oxidative Stress, and Excitotoxicity. Evid. Based Complement. Altern. Med. 2016, 2016, 9438650. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.W.; Shin, B.S.; Ma, H.; Van Hoecke, M.; Brennan, A.M.; Yenari, M.A.; Swanson, R.A. Glucose and NADPH oxidase drive neuronal superoxide formation in stroke. Ann. Neurol. 2008, 64, 654–663. [Google Scholar] [CrossRef]
- Testai, F.D.; Aiyagari, V. Acute Hemorrhagic Stroke Pathophysiology and Medical Interventions: Blood Pressure Control, Management of Anticoagulant-Associated Brain Hemorrhage and General Management Principles. Neurol. Clin. 2008, 26, 963–985. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Guo, S.; Chen, H.; Peng, J.-J.; Jia, M.-M.; Li, N.-S.; Zhang, X.-J.; Yang, J.; Luo, X.-J.; Peng, J. Combination of Emricasan with Ponatinib Synergistically Reduces Ischemia/Reperfusion Injury in Rat Brain Through Simultaneous Prevention of Apoptosis and Necroptosis. Transl. Stroke Res. 2017, 9, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Song, M.; Qu, J.; Liu, G.-H. Epigenetic Modifications in Cardiovascular Aging and Diseases. Circ. Res. 2018, 123, 773–786. [Google Scholar] [CrossRef]
- Chen, J.; Yang, T.; Yu, H.; Sun, K.; Shi, Y.; Song, W.; Bai, Y.; Wang, X.; Lou, K.; Song, Y.; et al. A functional variant in the 3′-UTR of angiopoietin-1 might reduce stroke risk by interfering with the binding efficiency of microRNA. Hum. Mol. Genet. 2010, 19, 2524–2533. [Google Scholar] [CrossRef] [PubMed]
- Udali, S.; Guarini, P.; Moruzzi, S.; Choi, S.-W.; Friso, S. Cardiovascular epigenetics: From DNA methylation to microRNAs. Mol. Asp. Med. 2013, 34, 883–901. [Google Scholar] [CrossRef] [PubMed]
- Bertogliat, M.J.; Morris-Blanco, K.C.; Vemuganti, R. Epigenetic mechanisms of neurodegenerative diseases and acute brain injury. Neurochem. Int. 2020, 133, 104642. [Google Scholar] [CrossRef] [PubMed]
- Taj, S.H.; Kho, W.; Riou, A.; Wiedermann, D.; Hoehn, M. MiRNA-124 induces neuroprotection and functional improvement after focal cerebral ischemia. Biomaterials 2016, 91, 151–165. [Google Scholar]
- Hashimoto, R.; Hough, C.; Nakazawa, T.; Yamamoto, T.; Chuang, D. Lithium protection against glutamate excitotoxicity in rat cerebral cortical neurons: Involvement of NMDA receptor inhibition possibly by decreasing NR2B tyrosine phosphorylation. J. Neurochem. 2002, 80, 589–597. [Google Scholar] [CrossRef]
- Leng, Y.; Chuang, D.-M. Endogenous α-Synuclein Is Induced by Valproic Acid through Histone Deacetylase Inhibition and Participates in Neuroprotection against Glutamate-Induced Excitotoxicity. J. Neurosci. 2006, 26, 7502–7512. [Google Scholar] [CrossRef]
- Baltan, S.; Murphy, S.P.; Danilov, C.A.; Bachleda, A.; Morrison, R.S. Histone Deacetylase Inhibitors Preserve White Matter Structure and Function during Ischemia by Conserving ATP and Reducing Excitotoxicity. J. Neurosci. 2011, 31, 3990–3999. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, M.; Zhang, Q.; Yu, X.; Sun, Z.; He, Y.; Guo, W. Role of Main RNA Methylation in Hepatocellular Carcinoma: N6-Methyladenosine, 5-Methylcytosine, and N1-Methyladenosine. Front. Cell Dev. Biol. 2021, 9, 767668. [Google Scholar] [CrossRef]
- Trixl, L.; Lusser, A. The dynamic RNA modification 5-methylcytosine and its emerging role as an epitranscriptomic mark. Wiley Interdiscip. Rev. RNA 2019, 10, e1510. [Google Scholar] [CrossRef]
- García-Vílchez, R.; Sevilla, A.; Blanco, S. Post-transcriptional regulation by cytosine-5 methylation of RNA. Biochim. et Biophys. Acta Gene Regul. Mech. 2019, 1862, 240–252. [Google Scholar] [CrossRef]
- Amort, T.; Rieder, D.; Wille, A.; Khokhlova-Cubberley, D.; Riml, C.; Trixl, L.; Jia, X.-Y.; Micura, R.; Lusser, A. Distinct 5-methylcytosine profiles in poly(A) RNA from mouse embryonic stem cells and brain. Genome Biol. 2017, 18, 1. [Google Scholar] [CrossRef]
- Flores, J.V.; Cordero-Espinoza, L.; Oeztuerk-Winder, F.; Andersson-Rolf, A.; Selmi, T.; Blanco, S.; Tailor, J.; Dietmann, S.; Frye, M. Cytosine-5 RNA Methylation Regulates Neural Stem Cell Differentiation and Motility. Stem Cell Rep. 2017, 8, 112–124. [Google Scholar] [CrossRef]
- Cui, X.; Liang, Z.; Shen, L.; Zhang, Q.; Bao, S.; Geng, Y.; Zhang, B.; Leo, V.; Vardy, L.A.; Lu, T.; et al. 5-Methylcytosine RNA Methylation in Arabidopsis Thaliana. Mol. Plant 2017, 10, 1387–1399. [Google Scholar] [CrossRef]
- Squires, J.E.; Patel, H.R.; Nousch, M.; Sibbritt, T.D.; Humphreys, D.T.; Parker, B.J.; Suter, C.M.; Preiss, T. Widespread occurrence of 5-methylcytosine in human coding and non-coding RNA. Nucleic Acids Res. 2012, 40, 5023–5033. [Google Scholar] [CrossRef]
- Deng, W.; Zhang, Y.; Cai, J.; Zhang, J.; Liu, X.; Yin, J. LncRNA-ANRIL promotes gastric cancer progression by enhancing NF-kB signaling. Exp. Biol. Med. 2019, 244, 953–959. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, S.; Zhang, Y.; Liu, R.; Chen, M. Roles of m5C RNA Modification Patterns in Biochemical Recurrence and Tumor Microenvironment Characterization of Prostate Adenocarcinoma. Front. Immunol. 2022, 13, 869759. [Google Scholar] [CrossRef]
- Sun, Z.; Xue, S.; Zhang, M.; Xu, H.; Hu, X.; Chen, S.; Liu, Y.; Guo, M.; Cui, H. Aberrant NSUN2-mediated m5C modification of H19 lncRNA is associated with poor differentiation of hepatocellular carcinoma. Oncogene 2020, 39, 6906–6919. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Huang, H.; Lv, J.; Chen, Z.; Lu, C.; Jiang, T.; Xu, P.; Li, Y.; Wang, S.; Li, B.; et al. m5C-methylated lncRNA NR_033928 promotes gastric cancer proliferation by stabilizing GLS mRNA to promote glutamine metabolism reprogramming. Cell Death Dis. 2023, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Cusenza, V.Y.; Tameni, A.; Neri, A.; Frazzi, R. The lncRNA epigenetics: The significance of m6A and m5C lncRNA modifications in cancer. Front. Oncol. 2023, 13, 1063636. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Liu, J.; Zhao, L.; Wu, P.; Chen, G.; Chen, Q.; Shen, P.; Yang, T.; Fan, S.; Xiao, B.; et al. Prognostic Risk Model and Tumor Immune Environment Modulation of m5C-Related LncRNAs in Pancreatic Ductal Adenocarcinoma. Front. Immunol. 2021, 12, 800268. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.-H.; Choi, I.-A.; Lee, J. The Role of DNA Methylation in Stroke Recovery. Int. J. Mol. Sci. 2022, 23, 10373. [Google Scholar] [CrossRef]
- Valinluck, V.; Tsai, H.H.; Rogstad, D.K.; Burdzy, A.; Bird, A.; Sowers, L.C. Oxidative damage to methyl-CpG sequences inhibits the binding of the methyl-CpG binding domain (MBD) of methyl-CpG binding protein 2 (MeCP2). Nucleic Acids Res. 2004, 32, 4100–4108. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.; Siotto, M.; Germanotta, M.; Mastrorosa, A.; Papadopoulou, D.; Aprile, I. Association Study of SLC6A4 (5-HTTLPR) Polymorphism and Its Promoter Methylation with Rehabilitation Outcome in Patients with Subacute Stroke. Genes 2021, 12, 579. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Tárraga, C.; Lazcano, U.; Jiménez-Conde, J.; Ois, A.; Cuadrado-Godia, E.; Giralt-Steinhauer, E.; Rodríguez-Campello, A.; Gomez-Gonzalez, A.; Avellaneda-Gómez, C.; Vivanco-Hidalgo, R.M.; et al. Biological age is a novel biomarker to predict stroke recurrence. J. Neurol. 2021, 268, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Tárraga, C.; Mola-Caminal, M.; Giralt-Steinhauer, E.; Ois, A.; Rodríguez-Campello, A.; Cuadrado-Godia, E.; Gómez-González, A.; Vivanco-Hidalgo, R.M.; Fernández-Cadenas, I.; Cullell, N.; et al. Biological age is better than chronological as predictor of 3-month outcome in ischemic stroke. Neurology 2017, 89, 830–836. [Google Scholar] [CrossRef]
- Ørom, U.A.; Derrien, T.; Beringer, M.; Gumireddy, K.; Gardini, A.; Bussotti, G.; Lai, F.; Zytnicki, M.; Notredame, C.; Huang, Q.; et al. Long Noncoding RNAs with Enhancer-like Function in Human Cells. Cell 2010, 143, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Bridges, M.C.; Daulagala, A.C.; Kourtidis, A. LNCcation: lncRNA localization and function. J. Cell Biol. 2021, 220, 9045. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wei, D.; Cai, D.; Chen, S.; Li, S.; Chen, W. Altered Long Non-Coding RNA Transcriptomic Profiles in Ischemic Stroke. Hum. Gene Ther. 2018, 29, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wu, Z.; Wu, P.; Zuo, X.; Yu, N.; Qin, Y.; Xu, Q. LncRNA-N1LR Enhances Neuroprotection Against Ischemic Stroke Probably by Inhibiting p53 Phosphorylation. Mol. Neurobiol. 2017, 54, 7670–7685. [Google Scholar] [CrossRef]
- Wang, J.; Cao, B.; Han, D.; Sun, M.; Feng, J. Long Non-coding RNA H19 Induces Cerebral Ischemia Reperfusion Injury via Activation of Autophagy. Aging Dis. 2017, 8, 71–84. [Google Scholar] [CrossRef]
- Zhan, R.; Xu, K.; Pan, J.; Xu, Q.; Xu, S.; Shen, J. Long noncoding RNA MEG3 mediated angiogenesis after cerebral infarction through regulating p53/NOX4 axis. Biochem. Biophys. Res. Commun. 2017, 490, 700–706. [Google Scholar] [CrossRef]
- Howells, D.W.; Porritt, M.J.; Rewell, S.S.; O’Collins, V.; Sena, E.S.; van der Worp, H.B.; Traystman, R.J.; Macleod, M.R. Different Strokes for Different Folks: The Rich Diversity of Animal Models of Focal Cerebral Ischemia. J. Cereb. Blood Flow Metab. 2010, 30, 1412–1431. [Google Scholar] [CrossRef]
- Hossmann, K.A. Cerebral ischemia: Models, methods and outcomes. Neuropharmacology 2008, 55, 257–270. [Google Scholar] [CrossRef]
- Mehta, S.L.; Kim, T.; Vemuganti, R. Long Noncoding RNA FosDT Promotes Ischemic Brain Injury by Interacting with REST-Associated Chromatin-Modifying Proteins. J. Neurosci. 2015, 35, 16443–16449. [Google Scholar] [CrossRef]
- Ruscher, K.; Kuric, E.; Wieloch, T. Levodopa Treatment Improves Functional Recovery After Experimental Stroke. Stroke 2012, 43, 507–513. [Google Scholar] [CrossRef]
- Guo, J.; Tuo, Q.Z.; Lei, P. Iron, ferroptosis, and ischemic stroke. J. Neurochem. 2023, 165, 487–520. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cai, Q.; Liang, R.; Zhang, D.; Liu, X.; Zhang, M.; Xiong, Y.; Xu, M.; Liu, Q.; Li, P.; et al. Copper homeostasis and copper-induced cell death in the pathogenesis of cardiovascular disease and therapeutic strategies. Cell Death Dis. 2023, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Iida, T.; Furuta, A.; Nakabeppu, Y.; Iwaki, T. Defense mechanism to oxidative DNA damage in glial cells. Neuropathology 2004, 24, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Simcox, J.; Lamming, D.W. The central moTOR of metabolism. Dev. Cell 2022, 57, 691–706. [Google Scholar] [CrossRef] [PubMed]
- Chou, N.; Mochizuki, H.; Nakao, A.; Mizuno, Y.; Urabe, T. Dual role of Fcgamma receptor in transient focal cerebral ischemia in mice. Stroke 2004, 35, 958–963. [Google Scholar]
- Kluk, M.J.; Hla, T. Signaling of sphingosine-1-phosphate via the S1P/EDG-family of G-protein-coupled receptors. Biochim. Biophys. Acta 2002, 1582, 72–80. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.; Xu, Y.; Ruan, W.; Wang, H.; Zhang, Y.; Saavedra, J.M.; Zhang, L.; Huang, Z.; Pang, T. A Dual AMPK/Nrf2 Activator Reduces Brain Inflammation After Stroke by Enhancing Microglia M2 Polarization. Antioxid. Redox Signal. 2018, 28, 141–163. [Google Scholar] [CrossRef] [PubMed]
- Stagg, C.J.; Bachtiar, V.; Johansen-Berg, H. The Role of GABA in Human Motor Learning. Curr. Biol. 2011, 21, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.G.; Zhang, L.; Jiang, Q.; Zhang, R.; Davies, K.; Powers, C.; van Bruggen, N.; Chopp, M. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J. Clin. Investig. 2000, 106, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Shan, Y.; Lin, Y.; Liao, S.; Zhang, B.; Zeng, Q. Neutralization of interleukin-9 ameliorates experimental stroke by repairing the blood-brain barrier via down-regulation of astrocyte-derived vascular endothelial growth factor-A. FASEB J. 2019, 33, 4376–4387. [Google Scholar] [CrossRef] [PubMed]
- Bu, F.; Min, J.-W.; Munshi, Y.; Lai, Y.-J.; Qi, L.; Urayama, A.; McCullough, L.D.; Li, J. Activation of endothelial ras-related C3 botulinum toxin substrate 1 (Rac1) improves post-stroke recovery and angiogenesis via activating Pak1 in mice. Exp. Neurol. 2019, 322, 113059. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.E.; Keita, J.A.; Narayan, L.; Brady, S.M.; Frederick, R.; Carlson, S. The combination of dimethoxycurcumin with DNA methylation inhibitor enhances gene re-expression of promoter-methylated genes and antagonizes their cytotoxic effect. Epigenetics 2016, 11, 740–749. [Google Scholar] [CrossRef]
- Jones, P.A.; Baylin, S.B. The fundamental role of epigenetic events in cancer. Nat. Rev. Genet. 2002, 3, 415–428. [Google Scholar] [CrossRef]
- Durdevic, Z.; Mobin, M.B.; Hanna, K.; Lyko, F.; Schaefer, M. The RNA Methyltransferase Dnmt2 Is Required for Efficient Dicer-2-Dependent siRNA Pathway Activity in Drosophila. Cell Rep. 2013, 4, 931–937. [Google Scholar] [CrossRef]
- Blanco, S.; Dietmann, S.; Flores, J.V.; Hussain, S.; Kutter, C.; Humphreys, P.; Lukk, M.; Lombard, P.; Treps, L.; Popis, M.; et al. Aberrant methylation of tRNAs links cellular stress to neuro-developmental disorders. EMBO J. 2014, 33, 2020–2039. [Google Scholar] [CrossRef] [PubMed]
- Rai, K.; Chidester, S.; Zavala, C.V.; Manos, E.J.; James, S.R.; Karpf, A.R.; Jones, D.A.; Cairns, B.R. Dnmt2 functions in the cytoplasm to promote liver, brain, and retina development in zebrafish. Genes Dev. 2007, 21, 261–266. [Google Scholar] [CrossRef]
- Gao, Y.; Fang, J. RNA 5-methylcytosine modification and its emerging role as an epitranscriptomic mark. RNA Biol. 2021, 18, 117–127. [Google Scholar] [CrossRef]
- Khan, M.A.; Rafiq, M.A.; Noor, A.; Hussain, S.; Flores, J.V.; Rupp, V. Mutation in NSUN2, which encodes an RNA methyltransferase, causes autosomal-recessive intellectual disability. Am. J. Hum. Genet. 2012, 90, 856–863. [Google Scholar] [CrossRef]
- Endres, M.; Meisel, A.; Biniszkiewicz, D.; Namura, S.; Prass, K.; Ruscher, K.; Lipski, A.; Jaenisch, R.; Moskowitz, M.A.; Dirnagl, U. DNA Methyltransferase Contributes to Delayed Ischemic Brain Injury. J. Neurosci. 2000, 20, 3175–3181. [Google Scholar] [CrossRef]
- Mondal, N.K.; Behera, J.; Kelly, K.E.; George, A.K.; Tyagi, P.K.; Tyagi, N. Tetrahydrocurcumin epigenetically mitigates mitochondrial dysfunction in brain vasculature during ischemic stroke. Neurochem. Int. 2019, 122, 120–138. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.; He, Y.; Xin, N.; Sun, M.; Chen, L.; Lin, L.; Li, J.; Kong, J.; Jin, P.; Xu, X. Altering 5-hydroxymethylcytosine modification impacts ischemic brain injury. Hum. Mol. Genet. 2015, 24, 5855–5866. [Google Scholar] [CrossRef] [PubMed]
- Scheidtmann, K.; Fries, W.; Müller, F.; Koenig, E. Effect of levodopa in combination with physiotherapy on functional motor recovery after stroke: A prospective, randomised, double-blind study. Lancet 2001, 358, 787–790. [Google Scholar] [CrossRef]
- Johansson, B.B.; Belichenko, P.V. Neuronal Plasticity and Dendritic Spines: Effect of Environmental Enrichment on Intact and Postischemic Rat Brain. J. Cereb. Blood Flow Metab. 2002, 22, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Ruscher, K.; Johannesson, E.; Brugiere, E.; Erickson, A.; Rickhag, M.; Wieloch, T. Enriched Environment Reduces Apolipoprotein E (ApoE) in Reactive Astrocytes and Attenuates Inflammation of the Peri-Infarct Tissue after Experimental Stroke. J. Cereb. Blood Flow Metab. 2009, 29, 1796–1805. [Google Scholar] [CrossRef]
- Kuric, E.; Wieloch, T.; Ruscher, K. Dopamine receptor activation increases glial cell line-derived neurotrophic factor in experimental stroke. Exp. Neurol. 2013, 247, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Chong, Z.Z.; Shang, Y.C.; Zhang, L.; Wang, S.; Maiese, K. Mammalian target of rapamycin: Hitting the bull’s-eye for neurological disorders. Oxidative Med. Cell. Longev. 2010, 3, 374–391. [Google Scholar] [CrossRef] [PubMed]
- Blicher, J.U.; Near, J.; Næss-Schmidt, E.; Stagg, C.J.; Johansen-Berg, H.; Nielsen, J.F.; Østergaard, L.; Ho, Y.-C.L. GABA Levels Are Decreased After Stroke and GABA Changes During Rehabilitation Correlate with Motor Improvement. Neurorehabilit. Neural Repair 2015, 29, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, Y.; Zhao, Y.; Xu, X.; Lu, W.; Li, Y.; Bian, F.; Xiang, L.; Zhou, L. Activation of the Epac/Rap1 signaling pathway alleviates blood-brain barrier disruption and brain damage following cerebral ischemia/reperfusion injury. Int. Immunopharmacol. 2023, 117, 110014. [Google Scholar] [CrossRef] [PubMed]
| LncRNA | Chromosome | Peak Start | Peak End | p-Value | Log2 (Fold Change) | Methylated Regulation |
|---|---|---|---|---|---|---|
| ENSMUST00000176434 | chr17 | 24,516,301 | 24,516,599 | 3 × 10−9 | 9.789697 | up |
| ENSMUST00000133764 | chr4 | 42,164,721 | 42,164,736 | 3.66 × 10−9 | 9.843136 | up |
| ENSMUST00000130394 | chr11 | 69,601,781 | 69,602,080 | 8.93 × 10−9 | 9.890872 | up |
| ENSMUST00000123459 | chr11 | 3,533,741 | 3,534,000 | 3.44 × 10−9 | 9.960581 | up |
| ENSMUST00000004377 | chr6 | 124,732,221 | 124,732,440 | 8.64 × 10−9 | 9.964196 | up |
| ENSMUST00000089622 | chr6 | 83,441,761 | 83,442,105 | 3.47 × 10−9 | −10.6774 | down |
| ENSMUST00000141371 | chr3 | 88,542,675 | 88,542,781 | 4.29 × 10−9 | −10.5481 | down |
| ENSMUST00000069298 | chr19 | 42,779,821 | 42,780,060 | 3.78 × 10−9 | −10.5284 | down |
| ENSMUST00000184280 | chr7 | 16,825,789 | 16,825,850 | 1.05 × 10−8 | −10.3767 | down |
| ENSMUST00000149651 | chr6 | 119,215,341 | 119,215,660 | 1.33 × 10−8 | −10.2427 | down |
| LncRNA | Chromosome | Gene Start | Gene End | Log2 (Fold Change) | p-Value | Regulation |
|---|---|---|---|---|---|---|
| ENSMUST00000127704 | chr3 | 116,326,038 | 116,424,032 | 6.7759264 | 5.964 × 10−9 | up |
| ENSMUST00000159482 | chr6 | 29,473,161 | 29,484,144 | 6.3668936 | 0.000591 | up |
| AK076596 | chr14 | 95,882,943 | 95,884,433 | 6.2356941 | 0.0013011 | up |
| ENSMUST00000117051 | chr2 | 175,038,120 | 175,041,036 | 6.2093761 | 0.0011026 | up |
| ENSMUST00000121563 | chr5 | 88,739,246 | 88,739,729 | 6.1554138 | 0.0016276 | up |
| ENSMUST00000140938 | chr1 | 10,595,365 | 10,719,905 | −6.771754 | 6.317 × 10−9 | down |
| ENSMUST00000134513 | chr2 | 157,561,008 | 157,561,855 | −6.472668 | 0.0003559 | down |
| NR_029577 | chr9 | 108,568,318 | 108,568,392 | −6.38121 | 0.0005161 | down |
| ENSMUST00000182466 | chr19 | 24,692,399 | 24,693,370 | −6.367063 | 0.0005963 | down |
| AK041456 | chr6 | 31,795,668 | 31,798,497 | −6.11473 | 0.001745 | down |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Gao, J.; Xiong, D.; Zhao, Y. Transcriptome-Wide 5-Methylcytosine Profiling of lncRNAs in the Mouse Cerebral Ischemia Model. Pharmaceuticals 2024, 17, 384. https://doi.org/10.3390/ph17030384
Zhang C, Gao J, Xiong D, Zhao Y. Transcriptome-Wide 5-Methylcytosine Profiling of lncRNAs in the Mouse Cerebral Ischemia Model. Pharmaceuticals. 2024; 17(3):384. https://doi.org/10.3390/ph17030384
Chicago/Turabian StyleZhang, Chao, Junpeng Gao, Dan Xiong, and Yan Zhao. 2024. "Transcriptome-Wide 5-Methylcytosine Profiling of lncRNAs in the Mouse Cerebral Ischemia Model" Pharmaceuticals 17, no. 3: 384. https://doi.org/10.3390/ph17030384
APA StyleZhang, C., Gao, J., Xiong, D., & Zhao, Y. (2024). Transcriptome-Wide 5-Methylcytosine Profiling of lncRNAs in the Mouse Cerebral Ischemia Model. Pharmaceuticals, 17(3), 384. https://doi.org/10.3390/ph17030384








