Pharmacokinetics and Pharmacodynamics of a Nanostructured Lipid Carrier Co-Encapsulating Artemether and miRNA for Mitigating Cerebral Malaria
Abstract
:1. Introduction
2. Results
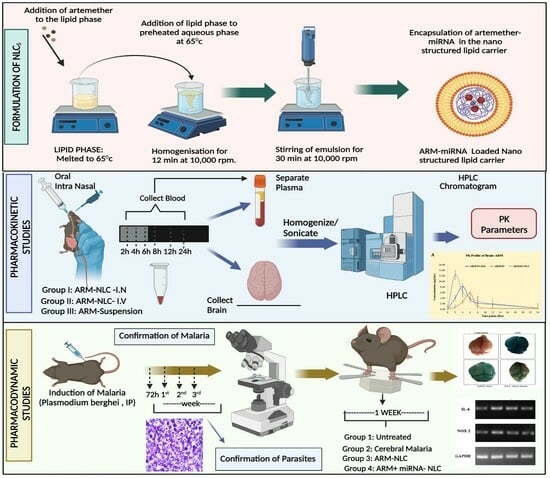
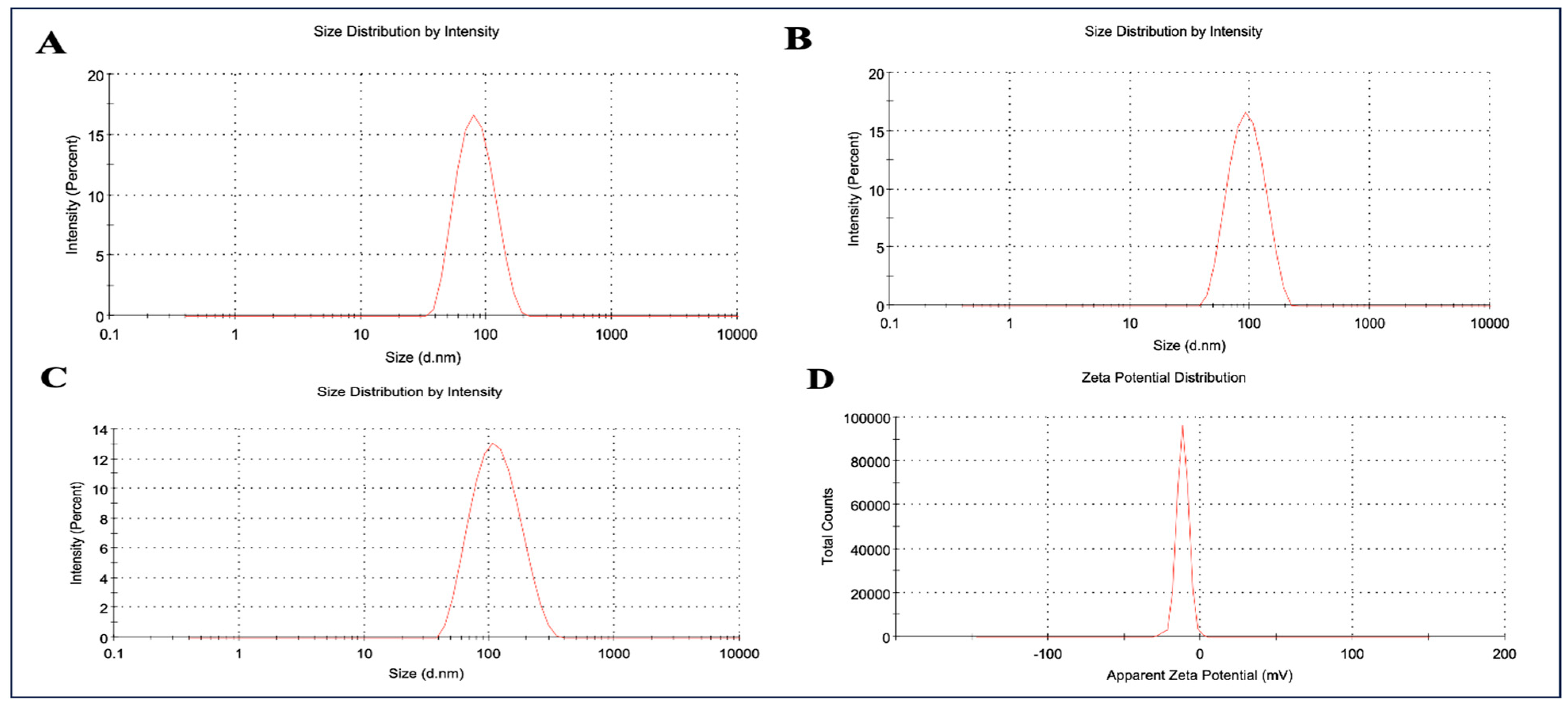
2.1. Formulation and Characterization of ARM+ miRNA-NLCs
2.2. Evaluation of Linearity of Plasma Samples by Employing RP-HPLC
2.3. Agarose Gel Electrophoresis
2.4. Cellular Absorption Evaluation
2.5. Pharmacokinetic Parameters
2.6. Estimation of Targeting Efficiency
2.7. Pharmacodynamic Evaluation: In Vivo Anti-Malarial Efficacy
2.7.1. Parasitaemia Evaluation
2.7.2. Histopathology Examinations
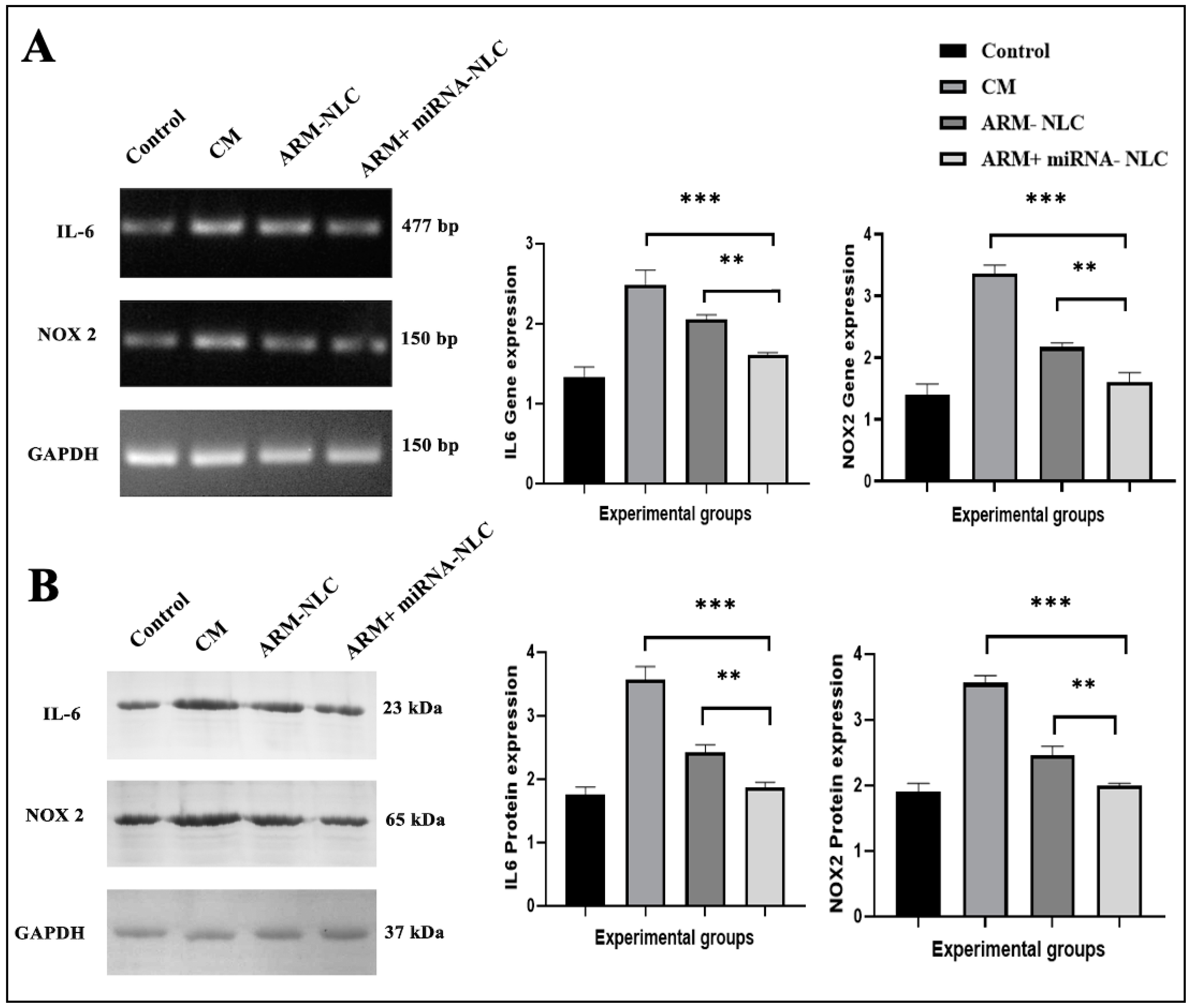
2.7.3. Molecular Interplay Driving Pathogenesis in Cerebral Malaria
Modulation of Gene Expression
Protein Expression Profiling
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. NLC Formulation
4.3. Characterization of ARM+ miRNA-NLCs
4.3.1. Particle Size and Zeta Potential Analyses for Nanostructured Lipid Carrier Systems
4.3.2. Encapsulation Efficiency (% EE)
4.4. miRNA Retardation Assay Using Agarose Gel Electrophoresis
4.5. Cellular Uptake Visualization through Confocal Laser Scanning Microscopy
4.6. RP-HPLC Specifications and Mobile Phase
4.7. Pharmacokinetic Parameters and Animal Husbandry
4.8. Assessment of Targeting Efficiency
4.9. In Vivo Assessment of Antimalarial Effectiveness in Mice Infected with Plasmodium berghei ANKA (PbA)
4.9.1. Evaluation of Parasitaemia in ECM
4.9.2. Histopathological Analysis: Brain Examination through Haematoxylin and Eosin (H&E) Staining
4.9.3. Evaluation of Brain Samples through Reverse Transcription Quantitative PCR (RT-qPCR) and Western Blot Analysis
4.9.4. Western Blot
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, X.; Wei, W.; Cheng, W.; Zhu, H.; Wang, W.; Dong, H.; Li, J. Cerebral Malaria Induced by Plasmodium Falciparum: Clinical Features, Pathogenesis, Diagnosis, and Treatment. Front. Cell. Infect. Microbiol. 2022, 12, 939532. [Google Scholar] [CrossRef]
- Egwu, C.O.; Augereau, J.-M.; Reybier, K.; Benoit-Vical, F. Reactive Oxygen Species as the Brainbox in Malaria Treatment. Antioxidants 2021, 10, 1872. [Google Scholar] [CrossRef]
- Amante, F.H.; Haque, A.; Stanley, A.C.; Rivera, F.d.L.; Randall, L.M.; Wilson, Y.A.; Yeo, G.; Pieper, C.; Crabb, B.S.; de Koning-Ward, T.F. Immune-Mediated Mechanisms of Parasite Tissue Sequestration during Experimental Cerebral Malaria. J. Immunol. 2010, 185, 3632–3642. [Google Scholar] [CrossRef]
- Andoh, N.E.; Gyan, B.A. The Potential Roles of Glial Cells in the Neuropathogenesis of Cerebral Malaria. Front. Cell. Infect. Microbiol. 2021, 957, 741370. [Google Scholar] [CrossRef]
- Nyariki, J.N.; Ochola, L.A.; Jillani, N.E.; Nyamweya, N.O.; Amwayi, P.E.; Yole, D.S.; Azonvide, L.; Isaac, A.O. Oral administration of Coenzyme Q10 protects mice against oxidative stress and neuro-inflammation during experimental cerebral malaria. Parasitol. Int. 2019, 71, 106–120. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Y.; Du, W.; Liang, L.-J.; Wang, W.; Jin, X. Role of Glial Cell-Derived Oxidative Stress in Blood-Brain Barrier Damage after Acute Ischemic Stroke. Oxid. Med. Cell. Longev. 2022, 2022, 7762078. [Google Scholar] [CrossRef]
- Terashvili, M.; Pratt, P.F.; Gebremedhin, D.; Narayanan, J.; Harder, D.R. Reactive Oxygen Species Cerebral Autoregulation in Health and Disease. Pediatr. Clin. 2006, 53, 1029–1037. [Google Scholar] [CrossRef]
- Barua, S.; Kim, J.Y.; Yenari, M.A.; Lee, J.E. The Role of NOX Inhibitors in Neurodegenerative Diseases. IBRO Rep. 2019, 7, 59–69. [Google Scholar] [CrossRef]
- Percário, S.; Moreira, D.R.; Gomes, B.A.Q.; Ferreira, M.E.S.; Gonçalves, A.C.M.; Laurindo, P.S.O.C.; Vilhena, T.C.; Dolabela, M.F.; Green, M.D. Oxidative Stress in Malaria. Int. J. Mol. Sci. 2012, 13, 16346–16372. [Google Scholar] [CrossRef]
- Imai, T.; Iwawaki, T.; Akai, R.; Suzue, K.; Hirai, M.; Taniguchi, T.; Okada, H.; Hisaeda, H. Evaluating Experimental Cerebral Malaria Using Oxidative Stress Indicator OKD48 Mice. Int. J. Parasitol. 2014, 44, 681–685. [Google Scholar] [CrossRef]
- Tarafdar, A.; Pula, G. The Role of NADPH Oxidases and Oxidative Stress in Neurodegenerative Disorders. Int. J. Mol. Sci. 2018, 19, 3824. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Park, J.; Lee, J.E.; Yenari, M.A. NOX Inhibitors-a Promising Avenue for Ischemic Stroke. Exp. Neurobiol. 2017, 26, 195. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gan, Y.; Xu, G.; Hua, K.; Liu, D. Exosomes from MSCs Overexpressing MicroRNA-223-3p Attenuate Cerebral Ischemia through Inhibiting Microglial M1 Polarization Mediated Inflammation. Life Sci. 2020, 260, 118403. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Niu, P.; Zhao, Y.; Cheng, Y.; Chen, W.; Lin, L.; Lu, J.; Cheng, X.; Xu, Z. Impact of MiR-223-3p and MiR-2909 on Inflammatory Factors IL-6, IL-1ß, and TNF-α, and the TLR4/TLR2/NF-ΚB/STAT3 Signaling Pathway Induced by Lipopolysaccharide in Human Adipose Stem Cells. PLoS ONE 2019, 14, e0212063. [Google Scholar] [CrossRef] [PubMed]
- Jiao, P.; Wang, X.-P.; Luoreng, Z.-M.; Yang, J.; Jia, L.; Ma, Y.; Wei, D.-W. MiR-223: An Effective Regulator of Immune Cell Differentiation and Inflammation. Int. J. Biol. Sci. 2021, 17, 2308. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Wu, Q.; Wang, Z.; Che, Y.; Zheng, S.; Chen, Y.; Zhong, X.; Shi, F. MiR-223: An Immune Regulator in Infectious Disorders. Front. Immunol. 2021, 12, 781815. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tan, M.; Xiang, Q.; Zhou, Z.; Yan, H. Thrombin-Activated Platelet-Derived Exosomes Regulate Endothelial Cell Expression of ICAM-1 via MicroRNA-223 during the Thrombosis-Inflammation Response. Thromb. Res. 2017, 154, 96–105. [Google Scholar] [CrossRef] [PubMed]
- LaMonte, G.; Philip, N.; Reardon, J.; Lacsina, J.R.; Majoros, W.; Chapman, L.; Thornburg, C.D.; Telen, M.J.; Ohler, U.; Nicchitta, C.V. Translocation of Sickle Cell Erythrocyte MicroRNAs into Plasmodium Falciparum Inhibits Parasite Translation and Contributes to Malaria Resistance. Cell Host Microbe 2012, 12, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Olliaro, P.L.; Taylor, W.R.J. Antimalarial Compounds: From Bench to Bedside. J. Exp. Biol. 2003, 206, 3753–3759. [Google Scholar] [CrossRef]
- O’Neill, P.M.; Barton, V.E.; Ward, S.A. The Molecular Mechanism of Action of Artemisinin—The Debate Continues. Molecules 2010, 15, 1705–1721. [Google Scholar] [CrossRef]
- Gopalakrishnan, A.M.; Kumar, N. Antimalarial Action of Artesunate Involves DNA Damage Mediated by Reactive Oxygen Species. Antimicrob. Agents Chemother. 2015, 59, 317–325. [Google Scholar] [CrossRef]
- Badshah, S.L.; Ullah, A.; Ahmad, N.; Almarhoon, Z.M.; Mabkhot, Y. Increasing the Strength and Production of Artemisinin and Its Derivatives. Molecules 2018, 23, 100. [Google Scholar] [CrossRef] [PubMed]
- Okorji, U.P.; Velagapudi, R.; El-Bakoush, A.; Fiebich, B.L.; Olajide, O.A. Antimalarial Drug Artemether Inhibits Neuroinflammation in BV2 Microglia Through Nrf2-Dependent Mechanisms. Mol. Neurobiol. 2016, 53, 6426–6443. [Google Scholar] [CrossRef] [PubMed]
- Morad, H.O.J.; Luqman, S.; Pinto, L.G.; Cunningham, K.P.; Vilar, B.; Clayton, G.; Shankar-Hari, M.; McNaughton, P.A. Artemisinin Inhibits Neutrophil and Macrophage Chemotaxis, Cytokine Production and NET Release. Sci. Rep. 2022, 12, 11078. [Google Scholar] [CrossRef]
- Huda, S.N.; Shahab, T.; Ali, S.M.; Afzal, K.; Khan, H.M. A Comparative Clinical Trial of Artemether and Quinine in Children with Severe Malaria. Indian Pediatr. 2003, 40, 939–945. [Google Scholar]
- Garg, A.; Tomar, D.S.; Bhalala, K.; Wahajuddin, M. Development and Investigation of Artemether Loaded Binary Solid Lipid Nanoparticles: Physicochemical Characterization and In-Situ Single-Pass Intestinal Permeability. J. Drug Deliv. Sci. Technol. 2020, 60, 102072. [Google Scholar] [CrossRef]
- Lochhead, J.J.; Davis, T.P. Perivascular and Perineural Pathways Involved in Brain Delivery and Distribution of Drugs after Intranasal Administration. Pharmaceutics 2019, 11, 598. [Google Scholar] [CrossRef]
- Saeedi, M.; Eslamifar, M.; Khezri, K.; Dizaj, S.M. Applications of Nanotechnology in Drug Delivery to the Central Nervous System. Biomed. Pharmacother. 2019, 111, 666–675. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.d.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Mulvihill, J.J.; Cunnane, E.M.; Ross, A.M.; Duskey, J.T.; Tosi, G.; Grabrucker, A.M. Drug Delivery across the Blood–Brain Barrier: Recent Advances in the Use of Nanocarriers. Nanomedicine 2020, 15, 205–214. [Google Scholar] [CrossRef]
- Niu, X.; Chen, J.; Gao, J. Nanocarriers as a Powerful Vehicle to Overcome Blood-Brain Barrier in Treating Neurodegenerative Diseases: Focus on Recent Advances. Asian J. Pharm. Sci. 2019, 14, 480–496. [Google Scholar] [CrossRef]
- Kumar, A.; Pandey, A.N.; Jain, S.K. Nasal-Nanotechnology: Revolution for Efficient Therapeutics Delivery. Drug Deliv. 2016, 23, 671–683. [Google Scholar] [CrossRef]
- Hora, R.; Kapoor, P.; Thind, K.K.; Mishra, P.C. Cerebral Malaria–Clinical Manifestations and Pathogenesis. Metab. Brain Dis. 2016, 31, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Shabir, G.A. Validation of High-Performance Liquid Chromatography Methods for Pharmaceutical Analysis. J. Chromatogr. A 2003, 987, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Voirin, A.-C.; Perek, N.; Roche, F. Inflammatory Stress Induced by a Combination of Cytokines (IL-6, IL-17, TNF-α) Leads to a Loss of Integrity on BEnd.3 Endothelial Cells in Vitro BBB Model. Brain Res. 2020, 1730, 146647. [Google Scholar] [CrossRef] [PubMed]
- Das, N.; Prabhu, P. Emerging Avenues for the Management of Cerebral Malaria. J. Pharm. Pharmacol. 2022, 74, 800–811. [Google Scholar] [CrossRef]
- Zafar, A.; Alsaidan, O.A.; Alruwaili, N.K.; Imam, S.S.; Yasir, M.; Alharbi, K.S.; Singh, L.; Ahmed, M.M. Formulation of Intranasal Surface Engineered Nanostructured Lipid Carriers of Rotigotine: Full Factorial Design Optimization, in Vitro Characterization, and Pharmacokinetic Evaluation. Int. J. Pharm. 2022, 627, 122232. [Google Scholar] [CrossRef]
- Vanka, R.; Kuppusamy, G.; Praveen Kumar, S.; Baruah, U.K.; Karri, V.V.S.R.; Pandey, V.; Babu, P.P. Ameliorating the in Vivo Antimalarial Efficacy of Artemether Using Nanostructured Lipid Carriers. J. Microencapsul. 2018, 35, 121–136. [Google Scholar] [CrossRef]
- Zhao, X.; Tang, D.; Yang, T.; Wang, C. Facile Preparation of Biocompatible Nanostructured Lipid Carrier with Ultra-Small Size as a Tumor-Penetration Delivery System. Colloids Surf. B Biointerfaces 2018, 170, 355–363. [Google Scholar] [CrossRef]
- Parashar, D.; NP, A.; RSR, M. Development of Artemether and Lumefantrine Co-Loaded Nanostructured Lipid Carriers: Physicochemical Characterization and in Vivo Antimalarial Activity. Drug Deliv. 2016, 23, 123–129. [Google Scholar] [CrossRef]
- Jarzębski, M.; Siejak, P.; Sawerski, A.; Stasiak, M.; Ratajczak, K.; Masewicz, Ł.; Polewski, K.; Fathordoobady, F.; Guo, Y.; Singh, A.P. Nanoparticles Size Determination by Dynamic Light Scattering in Real (Non-Standard) Conditions Regulators-Design, Tests and Applications. In Proceedings of the Practical Aspects of Chemical Engineering: Selected Contributions from PAIC 2019; Springer: Berlin/Heidelberg, Germany, 2020; pp. 122–131. [Google Scholar]
- Peltonen, L.; Aitta, J.; Hyvönen, S.; Karjalainen, M.; Hirvonen, J. Improved Entrapment Efficiency of Hydrophilic Drug Substance during Nanoprecipitation of Poly (I) Lactide Nanoparticles. Aaps Pharmscitech 2004, 5, 115–120. [Google Scholar] [PubMed]
- Li, D.; Yang, Z.; Luo, Y.; Zhao, X.; Tian, M.; Kang, P. Delivery of MiR335-5p-Pendant Tetrahedron DNA Nanostructures Using an Injectable Heparin Lithium Hydrogel for Challenging Bone Defects in Steroid-Associated Osteonecrosis. Adv. Healthc. Mater. 2022, 11, 2101412. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Kim, S.A.; Yang, Y.G.; Yoo, H.; Lim, S.-J.; Mok, H. Long Chain MicroRNA Conjugates in Calcium Phosphate Nanoparticles for Efficient Formulation and Delivery. Arch. Pharm. Res. 2015, 38, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, A.; Kumar, S.; Nguyen, A.; Brouillard, A.; Kulkarni, A. Lipid-Based Phagocytosis Nanoenhancer for Macrophage Immunotherapy. Nanoscale 2020, 12, 1875–1885. [Google Scholar] [CrossRef]
- Shrivastava, A.; Issarani, R.; Nagori, B.P. Stability Indicating High-Performance Liquid Chromatography Method for the Estimation of Artemether in Capsule Dosage Forms. J. Young Pharm. 2010, 2, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Johno, I.; Nakamura, T.; Kitazawa, S. An Estimation of Pharmacokinetic Parameters for Each Dosing at Unequal Doses and Dosing Intervals. Ther. Drug Monit. 1988, 10, 296–298. [Google Scholar] [CrossRef] [PubMed]
- Kozlovskaya, L.; Abou-Kaoud, M.; Stepensky, D. Quantitative Analysis of Drug Delivery to the Brain via Nasal Route. J. Control. Release 2014, 189, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Li, Y.; Ji, W.; Zhao, R.; Lu, Z.; Shen, J.; Wu, Y.; Wang, J.; Hao, Q.; Wang, J. Intranasal Administration of Self-Oriented Nanocarriers Based on Therapeutic Exosomes for Synergistic Treatment of Parkinson’s Disease. ACS Nano 2022, 16, 869–884. [Google Scholar] [CrossRef] [PubMed]
- Hearn, J.; Rayment, N.; Landon, D.N.; Katz, D.R.; de Souza, J.B. Immunopathology of Cerebral Malaria: Morphological Evidence of Parasite Sequestration in Murine Brain Microvasculature. Infect. Immun. 2000, 68, 5364–5376. [Google Scholar] [CrossRef]
- Alturkistani, H.A.; Tashkandi, F.M.; Mohammedsaleh, Z.M. Histological Stains: A Literature Review and Case Study. Glob. J. Health Sci. 2016, 8, 72. [Google Scholar] [CrossRef]
- Chacko, A.; Ittiyavirah, S.P. Neuroprotective Effect of against Aluminium-Induced Gracilaria Corticata Neurotoxicity in the Hippocampus and Cerebral Cortex of Rat Brain: Biochemical and Histological Approach. Asian J. Pharm. Pharmacol. 2019, 5, 604–613. [Google Scholar] [CrossRef]
- Martins, Y.C.; Freeman, B.D.; Ndunge, O.B.A.; Weiss, L.M.; Tanowitz, H.B.; Desruisseaux, M.S. Endothelin-1 Treatment Induces an Experimental Cerebral Malaria–Like Syndrome in C57BL/6 Mice Infected with Plasmodium Berghei NK65. Am. J. Pathol. 2016, 186, 2957–2969. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Hou, X.; Qi, N.; Li, C.; Luo, Y.; Hu, D.; Li, Y.; Liao, W. An Optimized Method to Obtain High-Quality RNA from Different Tissues in Lilium Davidii Var. Unicolor. Sci. Rep. 2022, 12, 2825. [Google Scholar] [CrossRef] [PubMed]
- Schoder, D.; Schmalwieser, A.; Schauberger, G.; Hoorfar, J.; Kuhn, M.; Wagner, M. Novel Approach for Assessing Performance of PCR Cyclers Used for Diagnostic Testing. J. Clin. Microbiol. 2005, 43, 2724–2728. [Google Scholar] [CrossRef]
- Lackner, P.; Hametner, C.; Beer, R.; Burger, C.; Broessner, G.; Helbok, R.; Speth, C.; Schmutzhard, E. Complement Factors C1q, C3 and C5 in Brain and Serum of Mice with Cerebral Malaria. Malar. J. 2008, 7, 207. [Google Scholar] [CrossRef]



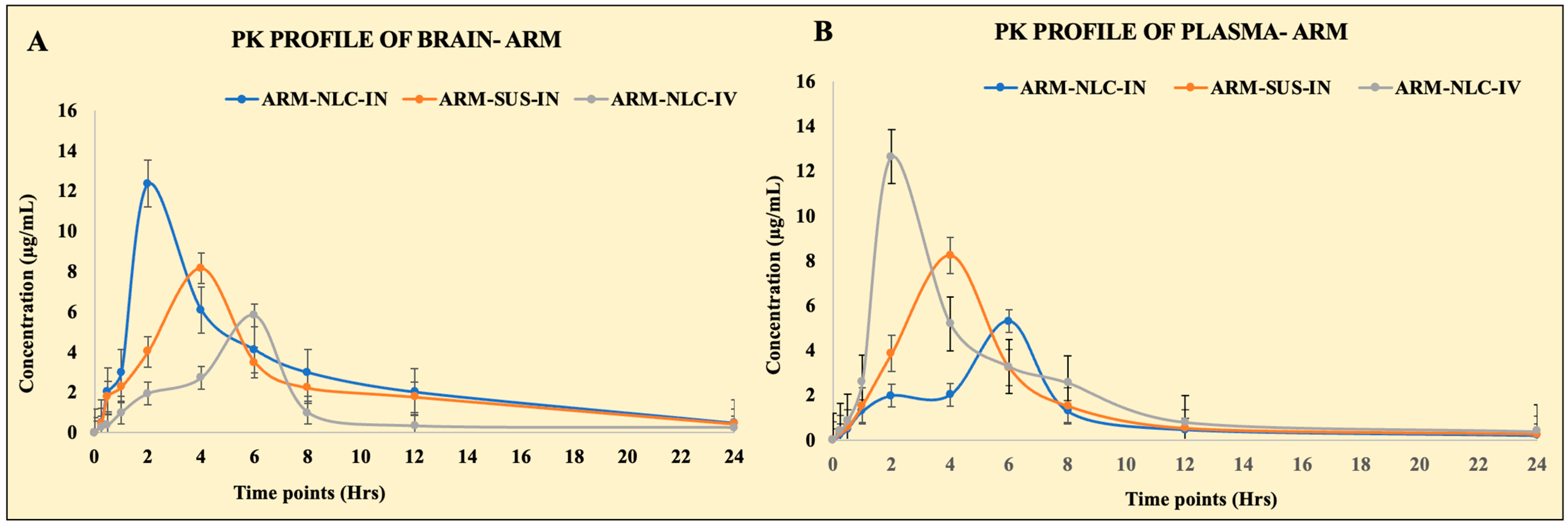



| Pharmacokinetic Parameter | Route of Administration and Nature of Formulation | |||||
|---|---|---|---|---|---|---|
| NLCs Loaded with ARM (IN) | ARM Suspension (IN) | NLCs Loaded with ARM (IV) | ||||
| Brain | Plasma | Brain | Plasma | Brain | Plasma | |
| Cmax (µg/mL) | 12.36 ± 0.18 | 5.30 ± 0.51 | 8.16 ± 1.01 | 8.24 ± 0.96 | 5.82 ± 0.45 | 12.64 ± 0.95 |
| Tmax (h) | 2 | 6 | 4 | 4 | 6 | 2 |
| AUC0–24h (µg·h/mL) | 72.88 ± 5.50 | 27.32 ± 3.79 | 54.38 ± 4.59 | 40.08 ± 9.45 | 28.13 ± 8.16 | 58.45 ± 7.55 |
| AUC0–∞ (µg·h/mL) | 78.23 ± 6.72 | 29.08 ± 6.59 | 58.96 ± 8.99 | 42.62 ± 8.57 | 30.60 ± 11.35 | 62.32 ± 11.45 |
| AUMC0–24 (µg·h2/mL) | 488.12 ± 26.54 | 177.92 ± 16.46 | 403.04 ± 10.88 | 226.36 ± 23.01 | 176.76 ± 11.62 | 323.92 ± 12.77 |
| AUMC0–∞ (µg·h2/mL) | 1054.85 ± 21.56 | 348.27 ± 16.46 | 906.30 ± 19.76 | 647.93 ± 23.01 | 393.50 ± 18.55 | 652.81 ± 12.77 |
| Kel (h−1) | 0.10 ± 0.08 | 0.09 ± 0.06 | 0.089 ± 0.06 | 0.10 ± 0.09 | 0.086 ± 0.12 | 0.11 ± 0.13 |
| T1/2 | 8.01 | 5.98 | 7.73 | 6.45 | 6.69 | 7.12 |
| MRT0–∞ | 17.88 | 10.47 | 12.85 | 11.97 | 11.58 | 15.19 |
| Relative bioavailability | 132.95 ± 10.56 | |||||
| Absolute bioavailability | 256.77 ± 14.80 | |||||
| Group Name | Administration Route | Description | Dosage |
|---|---|---|---|
| ARM suspension (free drug) | IN | ARM dispersed freely in PBS solution was administered through IN route to the mice. | 5 mg/kg |
| ARM-NLCs | IN | Mice received IN administration of ARM-loaded NLCs. | 5 mg/kg |
| ARM-NLCs | Intravenous (IV) | Mice were administered ARM-loaded NLCs intravenously. | 5 mg/kg |
| Group and Substance | No. of Animals | Description | Treatment |
|---|---|---|---|
| Control | 6 | No malaria infection | No treatment |
| CM | 8 | Female mice infected intraperitoneally with 1 × 106 Plasmodium berghei ANKA (PbA) of parasitic blood stages diluted in 200 µL chilled sterile 1x PBS pH 7.4. | No treatment |
| ARM-NLCs | 8 | Animals with PbA infection exhibiting neurological symptoms were considered for the experiment. | ARM-NLCs were administered intranasally at a dosage of 5 mg/kg per day for a period of 7 days in a 40 µL volume. |
| ARM+ miRNA-NLCs | 8 | The infected mice showing behavioural symptoms were chosen. | ARM-miRNA-NLCs were administered intranasally at a dosage of 5 mg/kg of the drug and 10 nmol of the miRNA daily for a duration of 7 days. |
| NCBI Reference Sequence | Sequence (5′->3′) | Length | Product Length | Annealing Temperature (°C) | |
|---|---|---|---|---|---|
| IL-6 | |||||
| Forward | Mus musculus IL6 Cybb X54542.1 | TTGCCTTCTTGGGACTGATGC | 21 | 187 | 55.8 |
| Reverse | TTGGAAATTGGGGTAGGAAGGA | 22 | |||
| NOX2 | |||||
| Forward | Mus musculus Nox2 (Cybb) Fj168469.1 | TGGAAACCCTCCTATGACTTG | 24 | 216 | 57.5 |
| Reverse | AACTTGGATACCTTGGGGCAC | 24 | |||
| GAPDH | |||||
| Forward | NM_008084.3 | GTGTGAACGGATTTGGCCGTATTG | 24 | 146 | 58.8 |
| Reverse | TTTGCCGTGAGTGGAGTCATACTG | 24 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goli, V.V.N.; Tatineni, S.; Hani, U.; Ghazwani, M.; Talath, S.; Sridhar, S.B.; Alhamhoom, Y.; Fatima, F.; Osmani, R.A.M.; Shivaswamy, U.; et al. Pharmacokinetics and Pharmacodynamics of a Nanostructured Lipid Carrier Co-Encapsulating Artemether and miRNA for Mitigating Cerebral Malaria. Pharmaceuticals 2024, 17, 466. https://doi.org/10.3390/ph17040466
Goli VVN, Tatineni S, Hani U, Ghazwani M, Talath S, Sridhar SB, Alhamhoom Y, Fatima F, Osmani RAM, Shivaswamy U, et al. Pharmacokinetics and Pharmacodynamics of a Nanostructured Lipid Carrier Co-Encapsulating Artemether and miRNA for Mitigating Cerebral Malaria. Pharmaceuticals. 2024; 17(4):466. https://doi.org/10.3390/ph17040466
Chicago/Turabian StyleGoli, Veera Venkata Nishanth, Spandana Tatineni, Umme Hani, Mohammed Ghazwani, Sirajunisa Talath, Sathvik Belagodu Sridhar, Yahya Alhamhoom, Farhat Fatima, Riyaz Ali M. Osmani, Umamaheshwari Shivaswamy, and et al. 2024. "Pharmacokinetics and Pharmacodynamics of a Nanostructured Lipid Carrier Co-Encapsulating Artemether and miRNA for Mitigating Cerebral Malaria" Pharmaceuticals 17, no. 4: 466. https://doi.org/10.3390/ph17040466