Anti-Inflammatory Properties of Cannabidiol and Beta-Caryophyllene Alone or Combined in an In Vitro Inflammation Model
Abstract
:1. Introduction
2. Results
2.1. Effects of Cannabidiol and Beta-Caryophyllene on Cell Viability in Human Keratinocyte (HaCaT) Cells
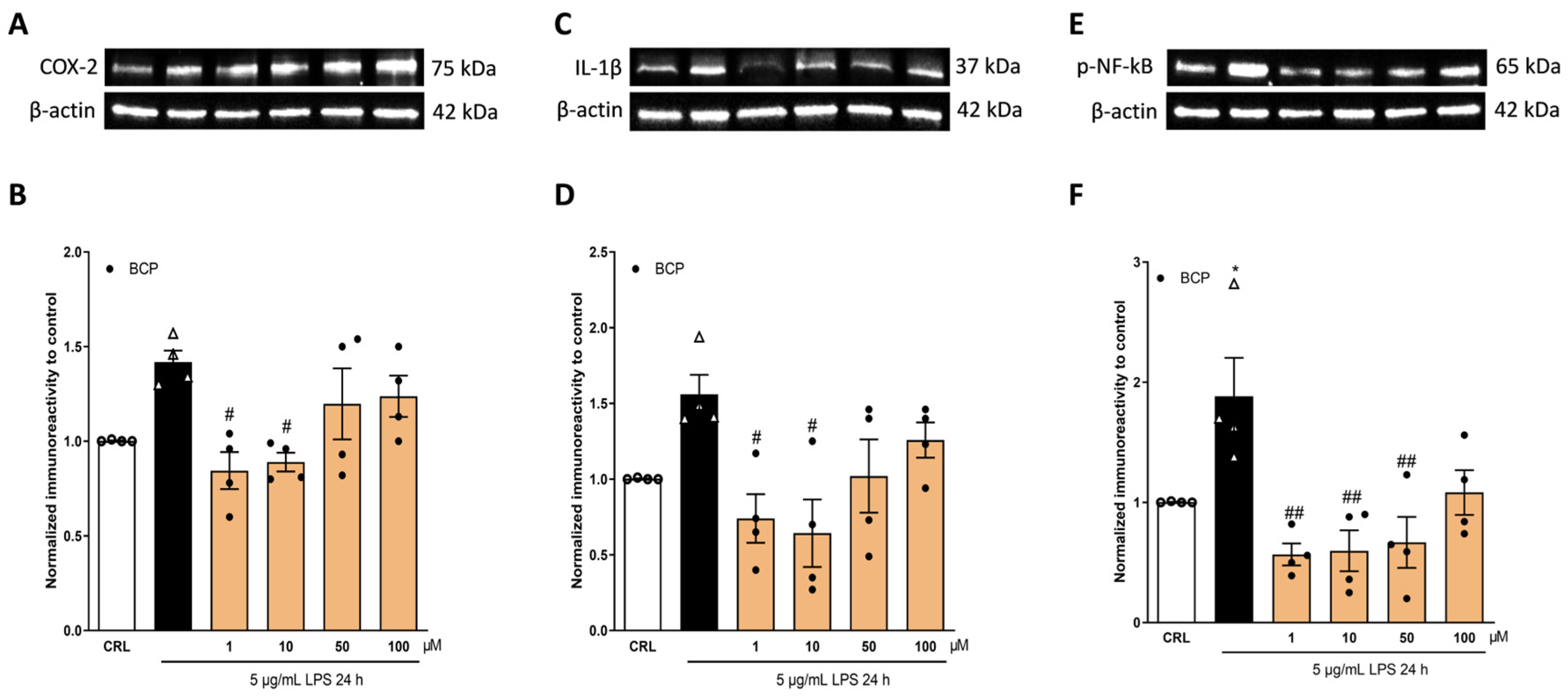
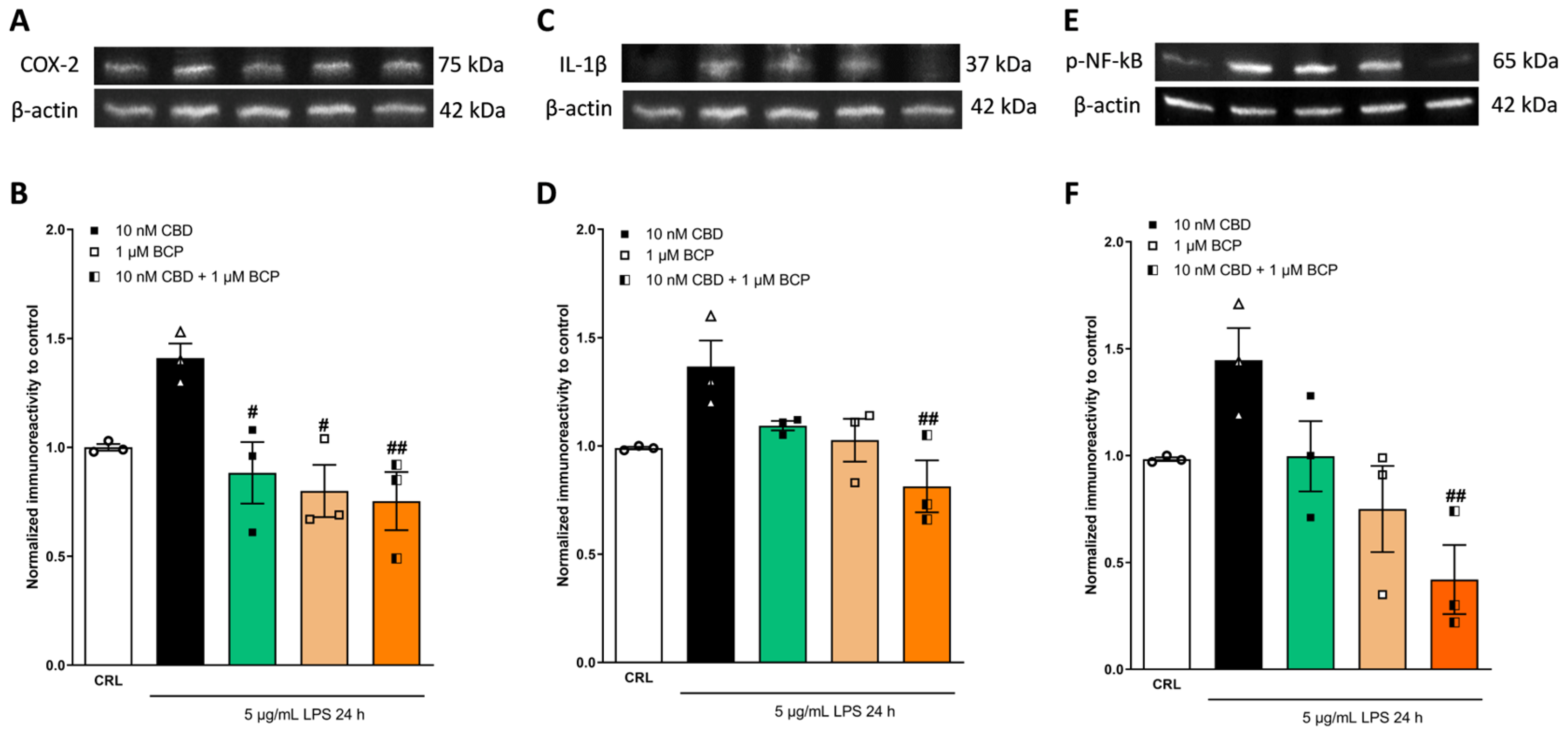
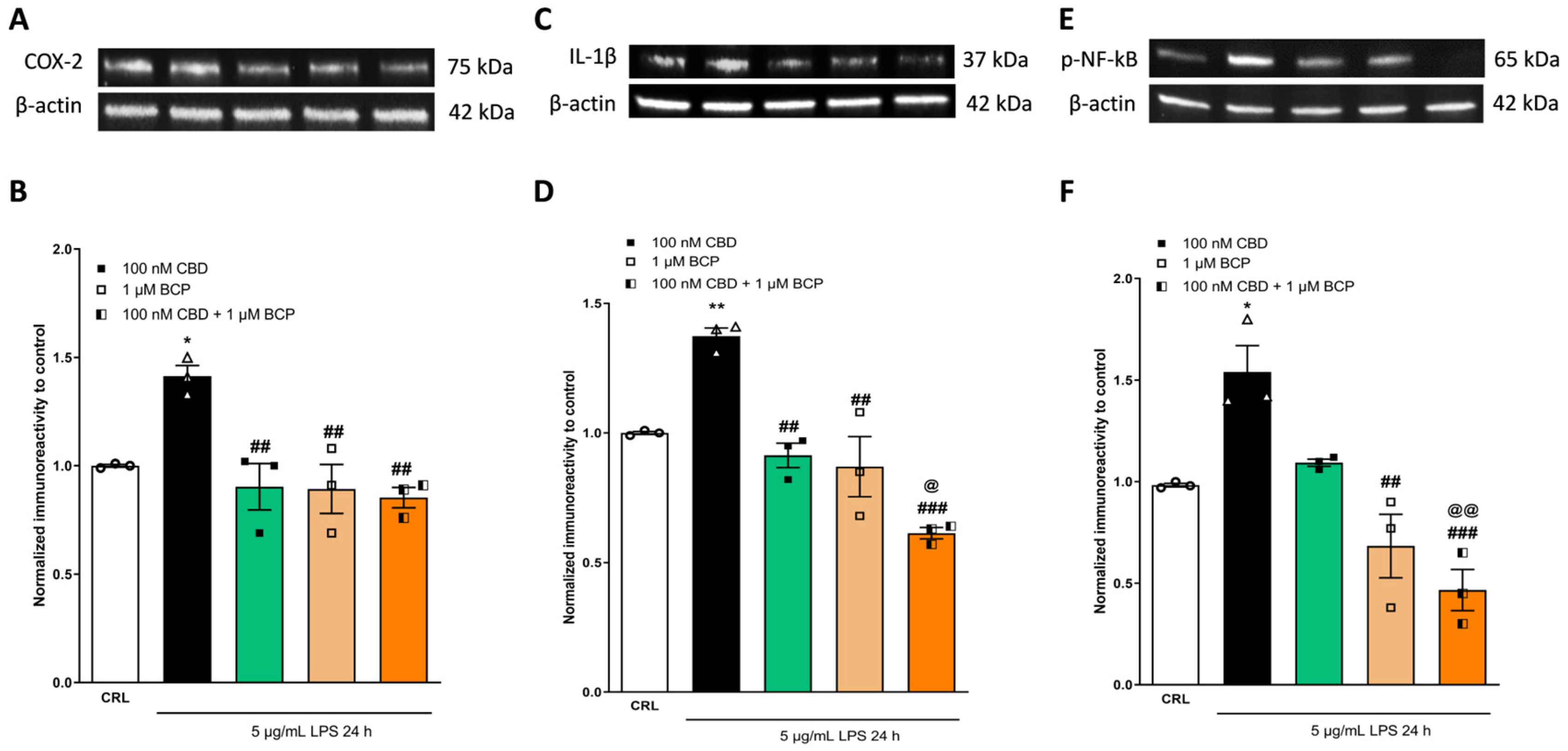
2.2. The Anti-Inflammatory Effects of Cannabidiol and Beta-Caryophyllene on Lipopolysaccharide (LPS)-Stimulated Human Keratinocyte (HaCaT) Cells
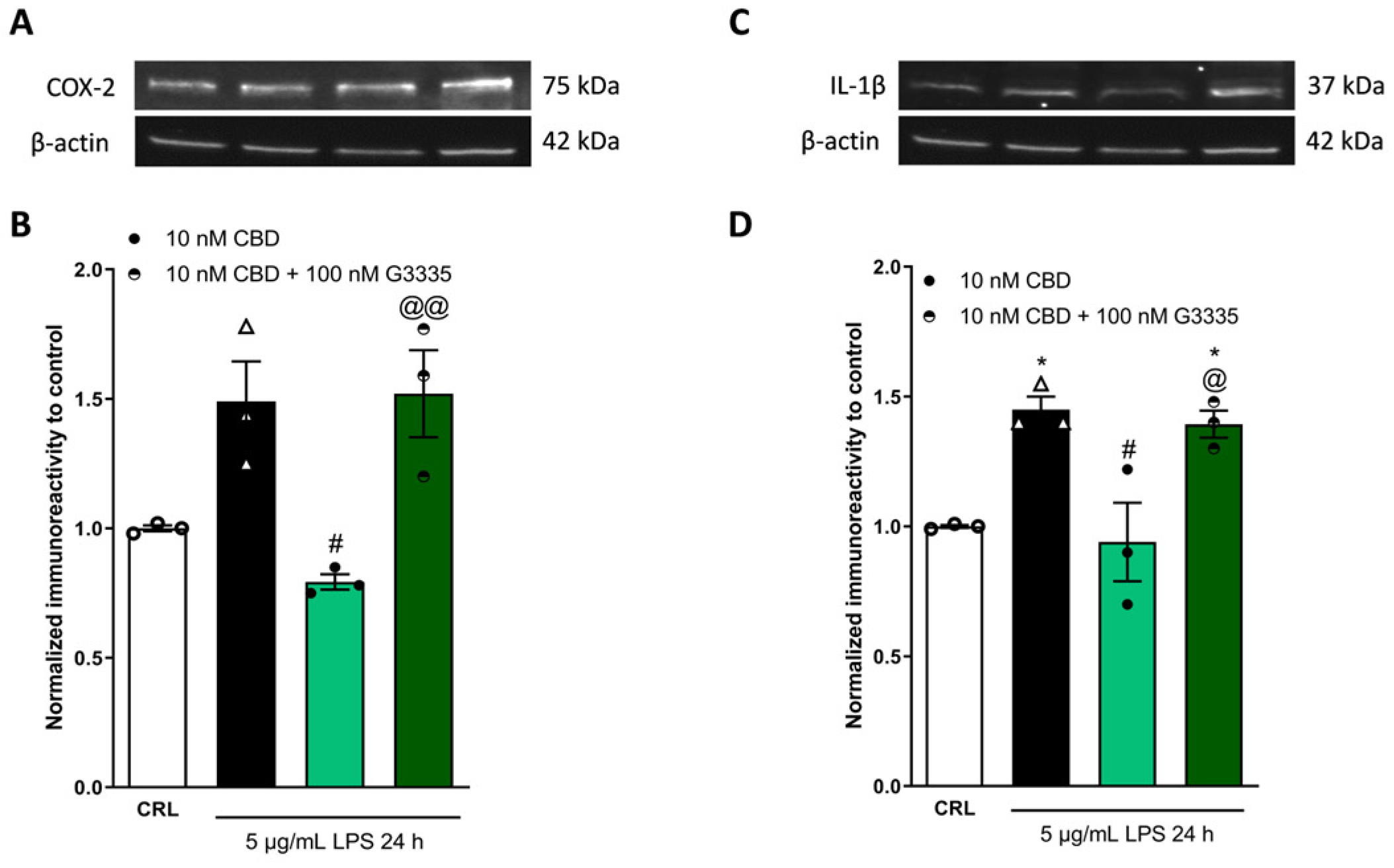
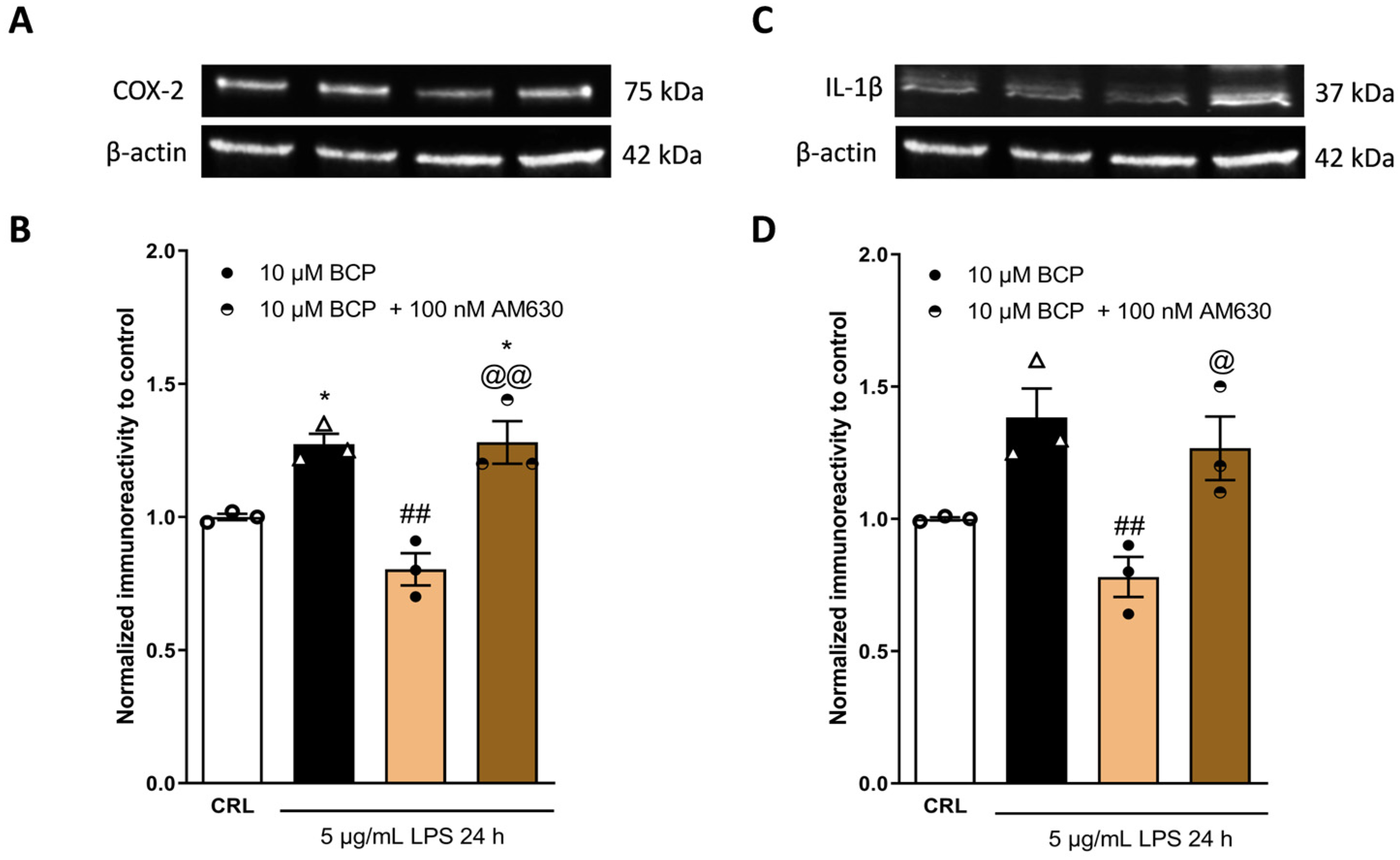
2.3. The Anti-Inflammatory Effects of Cannabidiol and Beta-Caryophyllene on Peroxisome Proliferator-Activated Receptors (PPARγ) and Cannabinoid Type 2 (CB2) Receptors
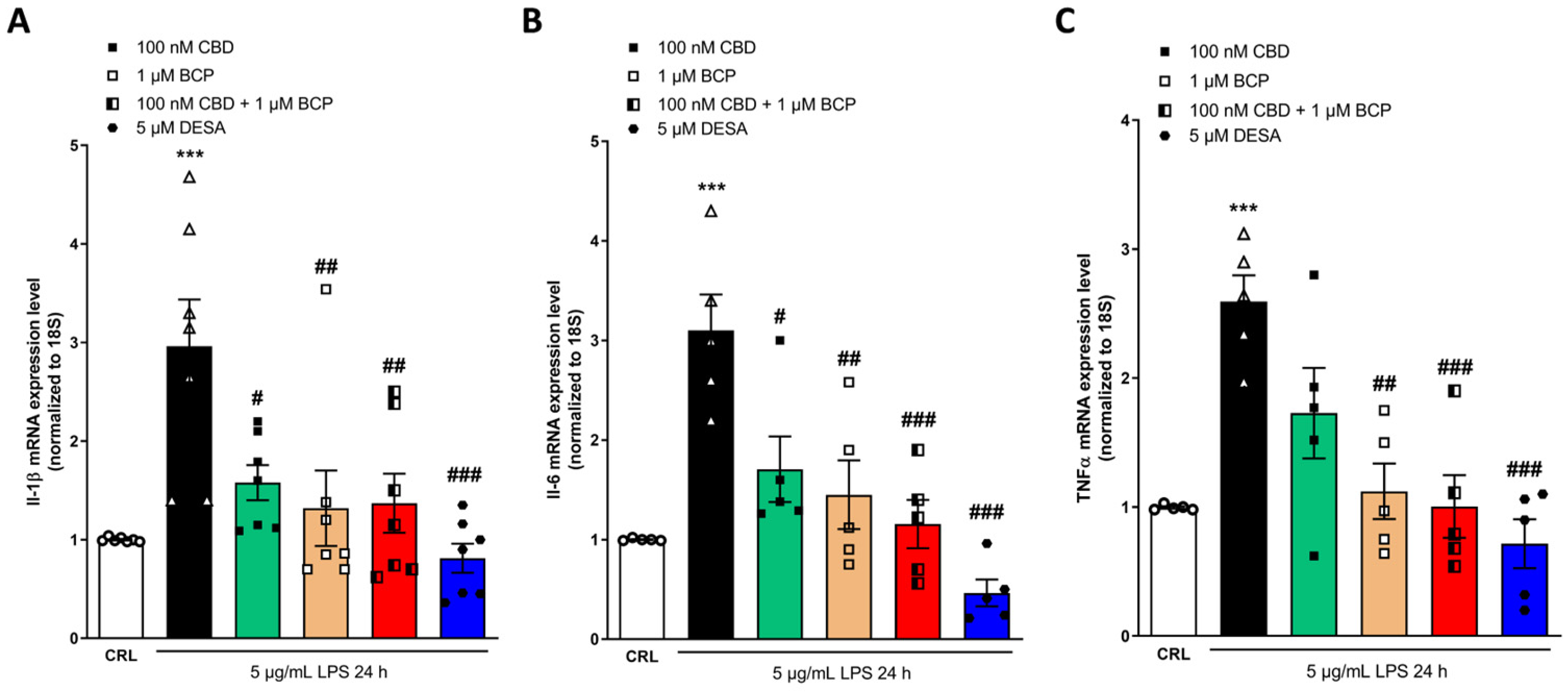
2.4. Investigation of the Additive Effects
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Line and Culture Conditions
4.3. Analysis of In Vitro Cytotoxicity
4.4. 3-(4,5-dimethylthiazol-2-yl)-25-diphenyltetrazolium bromide (MTT) Assay
4.5. Lactate Dehydrogenase (LDH) Assay
4.6. Western Blotting (WB)
4.7. Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zaid, N.A.M.; Sekar, M.; Bonam, S.R.; Gan, S.H.; Lum, P.T.; Begum, M.Y.; Rani, N.N.I.M.; Vaijanathappa, J.; Wu, Y.S.; Subramaniyan, V.; et al. Promising Natural Products in New Drug Design, Development, and Therapy for Skin Disorders: An Overview of Scientific Evidence and Understanding Their Mechanism of Action. Drug Des. Dev. Ther. 2022, 16, 23–66. [Google Scholar] [CrossRef] [PubMed]
- McKenna, M.; McDougall, J.J. Cannabinoid control of neurogenic inflammation. Br. J. Pharmacol. 2020, 177, 4386–4399. [Google Scholar] [CrossRef] [PubMed]
- Oláh, A.; Szabó-Papp, J.; Soeberdt, M.; Knie, U.; Dähnhardt-Pfeiffer, S.; Abels, C.; Bíró, T. Echinacea purpurea-derived alkylamides exhibit potent anti-inflammatory effects and alleviate clinical symptoms of atopic eczema. J. Dermatol. Sci. 2017, 88, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.M.; Gomes, A.L.; Vilas Boas, I.; Marto, J.; Ribeiro, H.M. Cannabis-Based Products for the Treatment of Skin Inflammatory Diseases: A Timely Review. Pharmaceuticals 2022, 15, 210. [Google Scholar] [CrossRef] [PubMed]
- Elhendawy, M.A.; Wanas, A.S.; Radwan, M.M.; Azzaz, N.A.; Toson, E.S.; Elsohly, M.A. Chemical and Biological Studies of Cannabis sativa Roots. Med. Cannabis Cannabinoids 2019, 1, 104–111. [Google Scholar] [CrossRef]
- Campos, A.C.; Fogaça, M.V.; Sonego, A.B.; Guimarães, F.S. Cannabidiol, neuroprotection and neuropsychiatric disorders. Pharmacol. Res. 2016, 112, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Sangiovanni, E.; Fumagalli, M.; Pacchetti, B.; Piazza, S.; Magnavacca, A.; Khalilpour, S.; Melzi, G.; Martinelli, G.; Dell’Agli, M. Cannabis sativa L. extract and cannabidiol inhibit in vitro mediators of skin inflammation and wound injury. Phytother. Res. 2019, 33, 2083–2093. [Google Scholar] [CrossRef]
- Sommano, S.R.; Chittasupho, C.; Ruksiriwanich, W.; Jantrawut, P. The Cannabis Terpenes. Molecules 2020, 25, 5792. [Google Scholar] [CrossRef] [PubMed]
- Sudeep, H.V.; Venkatakrishna, K.; Amritharaj; Gouthamchandra, K.; Reethi, B.; Naveen, P.; Lingaraju, H.B.; Shyamprasad, K. A standardized black pepper seed extract containing β-caryophyllene improves cognitive function in scopolamine-induced amnesia model mice via regulation of brain-derived neurotrophic factor and MAPK proteins. J. Food Biochem. 2021, 45, e13994. [Google Scholar] [CrossRef]
- Mannino, F.; Pallio, G.; Corsaro, R.; Minutoli, L.; Altavilla, D.; Vermiglio, G.; Allegra, A.; Eid, A.; Bitto, A.; Squadrito, F.; et al. Beta-Caryophyllene Exhibits Anti-Proliferative Effects through Apoptosis Induction and Cell Cycle Modulation in Multiple Myeloma Cells. Cancers 2021, 13, 5741. [Google Scholar] [CrossRef]
- Yovas, A.; Manjusha, W.A.; Ponnian, S.M.P. β-caryophyllene modulates B-cell lymphoma gene-2 family genes and inhibits the intrinsic pathway of apoptosis in isoproterenol-induced myocardial infarcted rats; A molecular mechanism. Eur. J. Pharmacol. 2022, 932, 175181. [Google Scholar] [CrossRef]
- Picciolo, G.; Pallio, G.; Altavilla, D.; Vaccaro, M.; Oteri, G.; Irrera, N.; Squadrito, F. β-Caryophyllene Reduces the Inflammatory Phenotype of Periodontal Cells by Targeting CB2 Receptors. Biomedicines 2020, 8, 164. [Google Scholar] [CrossRef] [PubMed]
- Baswan, S.M.; Klosner, A.E.; Glynn, K.; Rajgopal, A.; Malik, K.; Yim, S.; Stern, N. Therapeutic Potential of Cannabidiol (CBD) for Skin Health and Disorders. Clin. Cosmet. Investig. Dermatol. 2020, 13, 927–942. [Google Scholar] [CrossRef]
- Yim, S.; Lee, J.; Jo, H.; Scholten, J.; Willingham, R.; Nicoll, J.; Baswan, S.M. Chrysanthemum Morifolium Extract And Ascorbic Acid-2-Glucoside (AA2G) Blend Inhibits UVA-Induced Delayed Cyclobutane Pyrimidine Dimer (CPD) Production In Melanocytes. Clin. Cosmet. Investig. Dermatol. 2019, 12, 823–832. [Google Scholar] [CrossRef]
- Bickers, D.R.; Athar, M. Oxidative stress in the pathogenesis of skin disease. J. Invest. Dermatol. 2006, 126, 2565–2575. [Google Scholar] [CrossRef]
- Tang, J.; Diao, P.; Shu, X.; Li, L.; Xiong, L. Quercetin and Quercitrin Attenuates the Inflammatory Response and Oxidative Stress in LPS-Induced RAW264.7 Cells: In Vitro Assessment and a Theoretical Model. BioMed Res. Int. 2019, 2019, 7039802. [Google Scholar] [CrossRef] [PubMed]
- Piipponen, M.; Li, D.; Landén, N.X. The Immune Functions of Keratinocytes in Skin Wound Healing. Int. J. Mol. Sci. 2020, 21, 8790. [Google Scholar] [CrossRef] [PubMed]
- Dalmau, N.; Andrieu-Abadie, N.; Tauler, R.; Bedia, C. Phenotypic and lipidomic characterization of primary human epidermal keratinocytes exposed to simulated solar UV radiation. J. Dermatol. Sci. 2018, 92, 97–105. [Google Scholar] [CrossRef]
- Lebre, M.C.; Van Der Aar, A.M.G.; Van Baarsen, L.; Van Capel, T.M.M.; Schuitemaker, J.H.N.; Kapsenberg, M.L.; De Jong, E.C. Human keratinocytes express functional Toll-like receptor 3, 4, 5, and 9. J. Invest. Dermatol. 2007, 127, 331–341. [Google Scholar] [CrossRef]
- Barker, J.N.W.N.; Griffiths, C.E.M.; Nickoloff, B.J.; Mitra, R.S.; Dixit, V.M.; Nickoloff, B.J. Keratinocytes as initiators of inflammation. Lancet 1991, 337, 211–214. [Google Scholar] [CrossRef]
- Maldonado, R.F.; Sá-Correia, I.; Valvano, M.A. Lipopolysaccharide modification in Gram-negative bacteria during chronic infection. FEMS Microbiol. Rev. 2016, 40, 480–493. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Kim, B.; Kim, H.-E.; Sun, Q.; Shi, S.; Ma, G.; Kim, Y.; Kim, O.; Kim, O.-J. The Protective Role of Feruloylserotonin in LPS-Induced HaCaT Cells. Molecules 2019, 24, 3064. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wu, X.; Huang, S.; Wang, J.; Niu, S.; Chen, M.; Zhang, G.; Cai, S.; Wu, J.; Hong, B. Phenolic Metabolites from a Deep-Sea-Derived Fungus Aspergillus puniceus A2 and Their Nrf2-Dependent Anti-Inflammatory Effects. Mar. Drugs 2022, 20, 575. [Google Scholar] [CrossRef]
- Lee, J.L.; Mukhtar, H.; Bickers, D.R.; Kopelovich, L.; Athar, M. Cyclooxygenases in the skin: Pharmacological and toxicological implications. Toxicol. Appl. Pharmacol. 2003, 192, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Benamar, K. IUPHAR review—Preclinical models of neuropathic pain: Evaluating multifunctional properties of natural cannabinoid receptors ligands. Pharmacol. Res. 2024, 199, 107013. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X.; Yang, Y.; Quan, Q.; Huo, T.; Yang, S.; Ju, R.; An, Q. Comparison of the in vitro Anti-Inflammatory Effect of Cannabidiol to Dexamethasone. Clin. Cosmet. Investig. Dermatol. 2022, 15, 1959–1967. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.S.; Yeo, H.; Jung, E.; Ou, S.; Lee, Y.H.; Lim, Y.; Shin, S.Y. β-Caryophyllene Ameliorates 2,4-Dinitrochlorobenzene-Induced Atopic Dermatitis through the Downregulation of Mitogen-Activated Protein Kinase/EGR1/TSLP Signaling Axis. Int. J. Mol. Sci. 2022, 23, 14861. [Google Scholar] [CrossRef] [PubMed]
- Blanton, H.; Yin, L.; Duong, J.; Benamar, K. Cannabidiol and Beta-Caryophyllene in Combination: A Therapeutic Functional Interaction. Int. J. Mol. Sci. 2022, 23, 15470. [Google Scholar] [CrossRef] [PubMed]
- Pasquariello, N.; Oddi, S.; Malaponti, M.; Maccarrone, M. Regulation of gene transcription and keratinocyte differentiation by anandamide. Vitam. Horm. 2009, 81, 441–467. [Google Scholar]
- Pertwee, R.G. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199. [Google Scholar] [CrossRef]
- Landucci, E.; Mazzantini, C.; Lana, D.; Davolio, P.L.; Giovannini, M.G.; Pellegrini-Giampietro, D.E. Neuroprotective Effects of Cannabidiol but Not Δ9-Tetrahydrocannabinol in Rat Hippocampal Slices Exposed to Oxygen-Glucose Deprivation: Studies with Cannabis Extracts and Selected Cannabinoids. Int. J. Mol. Sci. 2021, 22, 9773. [Google Scholar] [CrossRef]
- Landucci, E.; Mazzantini, C.; Lana, D.; Calvani, M.; Magni, G.; Giovannini, M.G.; Pellegrini-Giampietro, D.E. Cannabidiol inhibits microglia activation and mitigates neuronal damage induced by kainate in an in-vitro seizure model. Neurobiol. Dis. 2022, 174, 105895. [Google Scholar] [CrossRef]
- Askari, V.R.; Shafiee-Nick, R. The protective effects of β-caryophyllene on LPS-induced primary microglia M 1/M 2 imbalance: A mechanistic evaluation. Life Sci. 2019, 219, 40–73. [Google Scholar] [CrossRef]
- Askari, V.R.; Shafiee-Nick, R. Promising neuroprotective effects of β-caryophyllene against LPS-induced oligodendrocyte toxicity: A mechanistic study. Biochem. Pharmacol. 2019, 159, 154–171. [Google Scholar] [CrossRef]
- Gertsch, J.; Leonti, M.; Raduner, S.; Racz, I.; Chen, J.Z.; Xie, X.Q.; Altmann, K.H.; Karsak, M.; Zimmer, A. Beta-caryophyllene is a dietary cannabinoid. Proc. Natl. Acad. Sci. USA 2008, 105, 9099–9104. [Google Scholar] [CrossRef]
- Yokubaitis, C.G.; Jessani, H.N.; Li, H.; Amodea, A.K.; Ward, S.J. Effects of Cannabidiol and Beta-Caryophyllene Alone or in Combination in a Mouse Model of Permanent Ischemia. Int. J. Mol. Sci. 2021, 22, 2866. [Google Scholar] [CrossRef]
- Alonso, C.; Satta, V.; Hernández-Fisac, I.; Fernández-Ruiz, J.; Sagredo, O. Disease-modifying effects of cannabidiol, β-caryophyllene and their combination in Syn1-Cre/Scn1aWT/A1783V mice, a preclinical model of Dravet syndrome. Neuropharmacology 2023, 237, 109602. [Google Scholar] [CrossRef]
- Dawud, H.; Abu Ammar, A.A. Rapidly Dissolving Microneedles for the Delivery of Steroid-Loaded Nanoparticles Intended for the Treatment of Inflammatory Skin Diseases. Pharmaceutics 2023, 15, 526. [Google Scholar] [CrossRef]
- Johnson, D.B.; Lopez, M.J.; Kelley, B. Dexamethasone. 2023 May 2. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Pellati, F.; Borgonetti, V.; Brighenti, V.; Biagi, M.; Benvenuti, S.; Corsi, L. Cannabis sativa L. and Nonpsychoactive Cannabinoids: Their Chemistry and Role against Oxidative Stress, Inflammation, and Cancer. BioMed Res. Int. 2018, 2018, 1691428. [Google Scholar] [CrossRef]
- Tortolani, D.; Di Meo, C.; Standoli, S.; Ciaramellano, F.; Kadhim, S.; Hsu, E.; Rapino, C.; Maccarrone, M. Rare Phytocannabinoids Exert Anti-Inflammatory Effects on Human Keratinocytes via the Endocannabinoid System and MAPK Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 2721. [Google Scholar] [CrossRef]
- Lim, H.S.; Jin, S.E.; Kim, O.S.; Shin, H.K.; Jeong, S.J. Alantolactone from Saussurea lappa Exerts Antiinflammatory Effects by Inhibiting Chemokine Production and STAT1 Phosphorylation in TNF-α and IFN-γ-induced in HaCaT cells. Phytother. Res. 2015, 29, 1088–1096. [Google Scholar] [CrossRef]
- Zamansky, M.; Zehavi, N.; Sintov, A.C.; Ben-Shabat, S. The Fundamental Role of Lipids in Polymeric Nanoparticles: Dermal Delivery and Anti-Inflammatory Activity of Cannabidiol. Molecules 2023, 28, 1774. [Google Scholar] [CrossRef]
- Juknat, A.; Kozela, E.; Kaushansky, N.; Mechoulam, R.; Vogel, Z. Anti-inflammatory effects of the cannabidiol derivative dimethylheptyl-cannabidiol—Studies in BV-2 microglia and encephalitogenic T cells. J. Basic Clin. Physiol. Pharmacol. 2016, 27, 289–296. [Google Scholar] [CrossRef]
- Scandiffio, R.; Geddo, F.; Cottone, E.; Querio, G.; Antoniotti, S.; Pia Gallo, M.; Maffei, M.E.; Bovolin, P. Protective Effects of (E)-β-Caryophyllene (BCP) in Chronic Inflammation. Nutrients 2020, 12, 3273. [Google Scholar] [CrossRef]
- Iannotti, F.A.; Vitale, R.M. The Endocannabinoid System and PPARs: Focus on Their Signalling Crosstalk, Action and Transcriptional Regulation. Cells 2021, 10, 586. [Google Scholar] [CrossRef]
- Bort, A.; Alvarado-Vazquez, P.A.; Moracho-Vilrriales, C.; Virga, K.G.; Gumina, G.; Romero-Sandoval, A.; Asbill, S. Effects of JWH015 in cytokine secretion in primary human keratinocytes and fibroblasts and its suitability for topical/transdermal delivery. Mol. Pain 2017, 13, 174480691668822. [Google Scholar] [CrossRef]
- Pistis, M.; Melis, M. From surface to nuclear receptors: The endocannabinoid family extends its assets. Curr. Med. Chem. 2010, 17, 1450–1467. [Google Scholar] [CrossRef]
- Russo, E.B. Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011, 163, 1344–1364. [Google Scholar] [CrossRef]
- Landucci, E.; Mazzantini, C.; Calvani, M.; Pellegrini-Giampietro, D.E.; Bergonzi, M.C. Evaluation of Conventional and Hyaluronic Acid-Coated Thymoquinone Liposomes in an In Vitro Model of Dry Eye. Pharmaceutics 2023, 15, 578. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzantini, C.; El Bourji, Z.; Parisio, C.; Davolio, P.L.; Cocchi, A.; Pellegrini-Giampietro, D.E.; Landucci, E. Anti-Inflammatory Properties of Cannabidiol and Beta-Caryophyllene Alone or Combined in an In Vitro Inflammation Model. Pharmaceuticals 2024, 17, 467. https://doi.org/10.3390/ph17040467
Mazzantini C, El Bourji Z, Parisio C, Davolio PL, Cocchi A, Pellegrini-Giampietro DE, Landucci E. Anti-Inflammatory Properties of Cannabidiol and Beta-Caryophyllene Alone or Combined in an In Vitro Inflammation Model. Pharmaceuticals. 2024; 17(4):467. https://doi.org/10.3390/ph17040467
Chicago/Turabian StyleMazzantini, Costanza, Zahraa El Bourji, Carmen Parisio, Pier Luigi Davolio, Arianna Cocchi, Domenico E. Pellegrini-Giampietro, and Elisa Landucci. 2024. "Anti-Inflammatory Properties of Cannabidiol and Beta-Caryophyllene Alone or Combined in an In Vitro Inflammation Model" Pharmaceuticals 17, no. 4: 467. https://doi.org/10.3390/ph17040467
APA StyleMazzantini, C., El Bourji, Z., Parisio, C., Davolio, P. L., Cocchi, A., Pellegrini-Giampietro, D. E., & Landucci, E. (2024). Anti-Inflammatory Properties of Cannabidiol and Beta-Caryophyllene Alone or Combined in an In Vitro Inflammation Model. Pharmaceuticals, 17(4), 467. https://doi.org/10.3390/ph17040467







