Comparison of the Efficacy of Two Routes of Administration of Human Amniotic Epithelial Cells in Cell Therapy of Acute Hepatic Insufficiency
Abstract
:1. Introduction
2. Results
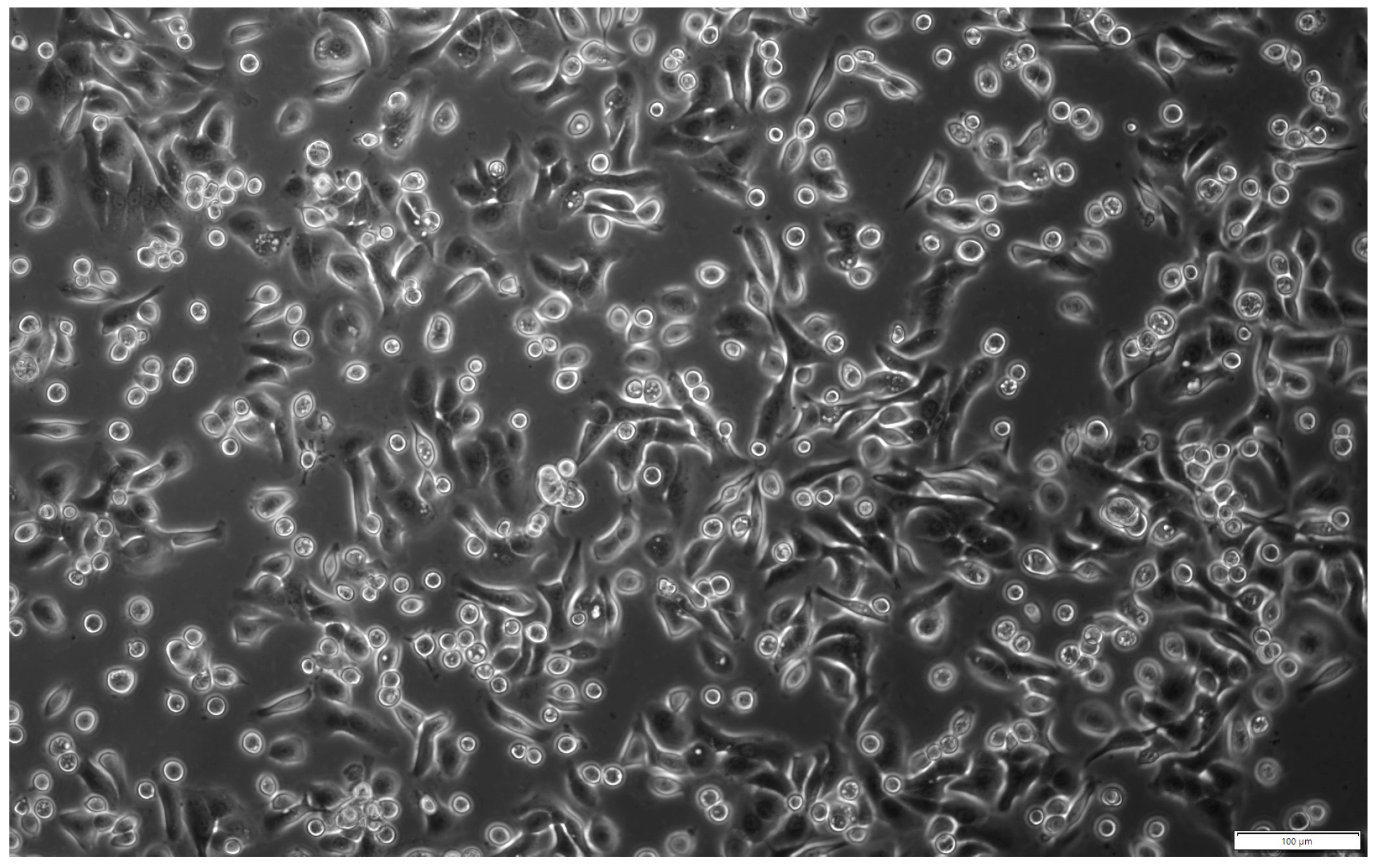
2.1. Characteristics of Isolated hAECs
2.2. Assessment of the Degree of Liver Damage
2.2.1. Assessment of Biochemical Parameters of Collected Blood
2.2.2. Evaluation of Histopathological Changes in the Liver
2.2.3. Assessment of Liver Cell Proliferation and Apoptosis
2.3. Identification of Transplanted Human Amniotic Cells in the Organs of Recipient Mice
3. Discussion
4. Materials and Methods
4.1. Experimental Model of Hepatotoxicity
4.2. Isolation of hAECs
4.3. Assessment of Viability and Phenotype of Isolated Amniotic Cells
4.4. Delivery of Isolated hAECs
4.5. Evaluation of the Liver Damage
4.5.1. Blood Parameters in the Recipient Mice
4.5.2. Histopathological Evaluation
4.5.3. Assessment of Liver Damage Based on Histological Staining with Haematoxylin and Eosin
4.5.4. Evaluation of Proliferative and Apoptotic Activity of Hepatic Cells
4.6. Identification of Transplanted Human Amniotic Cells in Recipient Mouse Tissues
4.6.1. Immunohistochemical Detection of hAECs
4.6.2. Identification of Human DNA from Transplanted hAECs
4.7. Statistical Analysis
5. Conclusions
- The biodistribution of transplanted hAECs varies depending on the route of administration and the degree of liver damage.
- After intravenous administration, most of the cells engraft in the lungs, while after intraperitoneal administration, they engraft primarily in the liver in both intoxicated and native mice.
- The increased presence of hAECs in the spleen is associated with progressive liver toxicity regardless of the route of administration—the greater the liver damage, the greater the number of hAECs in the spleen.
- After intravenous administration, a large number of the grafted hAECs in the lungs can have a therapeutic effect on the damaged liver.
- The therapeutic effect of intraperitoneally administered hAECs on histopathological and regenerative changes in the liver is very limited due to the low efficiency of cell engraftment in the damaged organ.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stravitz, R.T.; Lee, W.M. Acute Liver Failure. Lancet 2019, 394, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Wajtryt, O.; Zielonka, T.M. Ostra Niewydolność Wątroby Po Podaniu Amiodaronu. Folia Cardiol. 2019, 14, 242–246. [Google Scholar] [CrossRef]
- Czechowska, G.; Celiński, K.; Wójcicka, G. Liver Fibrosis Mechanisms: The Role of Stellate Cells, Oxidative and Nitrosative Stress. Postepy Hig. Med. Dosw. 2019, 73, 421–439. [Google Scholar] [CrossRef]
- Czekaj, P.; Król, M.; Limanówka, Ł.; Skubis-Sikora, A.; Kolanko, E.; Bogunia, E.; Hermyt, M.; Michalik, M.; Sikora, B.; Prusek, A.; et al. Dynamics of Acute Liver Injury in Experimental Models of Hepatotoxicity in the Context of Their Implementation in Preclinical Studies on Stem Cell Therapy. Front. Biosci. (Landmark Ed.) 2022, 27, 237. [Google Scholar] [CrossRef] [PubMed]
- Czekaj, P.; Król, M.; Limanówka, Ł.; Michalik, M.; Lorek, K.; Gramignoli, R. Assessment of Animal Experimental Models of Toxic Liver Injury in the Context of Their Potential Application as Preclinical Models for Cell Therapy. Eur. J. Pharmacol. 2019, 861, 172597. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, Z.; Jiang, J.; Wu, D.; Liu, X.; Xie, Z.; Chen, E.; Zhu, D.; Ye, C.; Zhang, X.; et al. Proteomic Signature of Acute Liver Failure: From Discovery and Verification in a Pig Model to Confirmation in Humans. Mol. Cell. Proteom. 2017, 16, 1188–1199. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho Batista Éboli, L.P.; Netto, A.A.S.; de Azevedo, R.A.; Lanzoni, V.P.; de Paula, T.S.; Goldenberg, A.; Gonzalez, A.M. Evaluating the Best Time to Intervene Acute Liver Failure in Rat Models Induced by D-Galactosamine. Acta Cir. Bras. 2016, 31, 783–792. [Google Scholar] [CrossRef]
- Decker, K.; Keppler, D. Galactosamine Hepatitis: Key Role of the Nucleotide Deficiency Period in the Pathogenesis of Cell Injury and Cell Death. Rev. Physiol. Biochem. Pharmacol. 1974, 71, 77–106. [Google Scholar] [CrossRef]
- Keppler, D.; Lesch, R.; Reutter, W.; Decker, K. Experimental Hepatitis Induced by D-Galactosamine. Exp. Mol. Pathol. 1968, 9, 279–290. [Google Scholar] [CrossRef]
- Yang, P.; Yuan, W.; Liu, J.; Li, J.; Tan, B.; Qiu, C.; Zhu, X.; Qiu, C. Biological Characterization of Human Amniotic Epithelial Cells in a Serum-Free System and Their Safety Evaluation. Acta Pharmacol. Sin. 2018, 39, 1305–1316. [Google Scholar] [CrossRef]
- Pratama, G.; Vaghjiani, V.; Tee, J.Y.; Liu, Y.H.; Chan, J.; Tan, C.; Murthi, P.; Gargett, C.; Manuelpillai, U. Changes in Culture Expanded Human Amniotic Epithelial Cells: Implications for Potential Therapeutic Applications. PLoS ONE 2011, 6, e26136. [Google Scholar] [CrossRef] [PubMed]
- Motedayyen, H.; Esmaeil, N.; Tajik, N.; Khadem, F.; Ghotloo, S.; Khani, B.; Rezaei, A. Method and Key Points for Isolation of Human Amniotic Epithelial Cells with High Yield, Viability and Purity. BMC Res. Notes 2017, 10, 552. [Google Scholar] [CrossRef] [PubMed]
- Gramignoli, R.; Srinivasan, R.C.; Kannisto, K.; Strom, S.C. Isolation of Human Amnion Epithelial Cells According to Current Good Manufacturing Procedures. Curr. Protoc. Stem Cell Biol. 2016, 2016, 1E.10.1–1E.10.13. [Google Scholar] [CrossRef]
- Lim, R.; Malhotra, A.; Tan, J.; Chan, S.T.; Lau, S.; Zhu, D.; Mockler, J.C.; Wallace, E.M. First-In-Human Administration of Allogeneic Amnion Cells in Premature Infants with Bronchopulmonary Dysplasia: A Safety Study. Stem Cells Transl. Med. 2018, 7, 628–635. [Google Scholar] [CrossRef]
- Keung, C.; Nguyen, T.C.; Lim, R.; Gerstenmaier, A.; Sievert, W.; Moore, G.T. Local Fistula Injection of Allogeneic Human Amnion Epithelial Cells Is Safe and Well Tolerated in Patients with Refractory Complex Perianal Crohn’s Disease: A Phase I Open Label Study with Long-Term Follow Up. eBioMedicine 2023, 98, 104879. [Google Scholar] [CrossRef]
- Qiu, C.; Ge, Z.; Cui, W.; Yu, L.; Li, J. Human Amniotic Epithelial Stem Cells: A Promising Seed Cell for Clinical Applications. Int. J. Mol. Sci. 2020, 21, 7730. [Google Scholar] [CrossRef]
- Shukla, S.; Mittal, S.K.; Foulsham, W.; Elbasiony, E.; Singhania, D.; Sahu, S.K.; Chauhan, S.K. Therapeutic Efficacy of Different Routes of Mesenchymal Stem Cell Administration in Corneal Injury. Ocul. Surf. 2019, 17, 729–736. [Google Scholar] [CrossRef]
- Srinivasan, R.C.; Kannisto, K.; Strom, S.C.; Gramignoli, R. Evaluation of Different Routes of Administration and Biodistribution of Human Amnion Epithelial Cells in Mice. Cytotherapy 2018, 21, 113–124. [Google Scholar] [CrossRef]
- Packthongsuk, K.; Rathbun, T.; Troyer, D.; Davis, D.L. Porcine Wharton’s Jelly Cells Distribute throughout the Body after Intraperitoneal Injection. Stem Cell Res. Ther. 2018, 9, 38. [Google Scholar] [CrossRef]
- Nguyen, M.P.; Jain, V.; Iansante, V.; Mitry, R.R.; Filippi, C.; Dhawan, A. Clinical Application of Hepatocyte Transplantation: Current Status, Applicability, Limitations, and Future Outlook. Expert Rev. Gastroenterol. Hepatol. 2020, 14, 185–196. [Google Scholar] [CrossRef]
- Miki, T. Clinical Hepatocyte Transplantation. Gastroenterol. Hepatol. Engl. Ed. 2019, 42, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Donato, M.T.; Bolonio, M.; Cabezas, E.; Pelechá, M.; Pareja, E.; Domènech, A.; Castell, J.V.; Gómez-Lechón, M.J.; Tolosa, L. Improved In Vivo Efficacy of Clinical-Grade Cryopreserved Human Hepatocytes in Mice with Acute Liver Failure. Cytotherapy 2019, 22, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Ganai, A.A.; Husain, M. Genistein Attenuates D-GalN Induced Liver Fibrosis/Chronic Liver Damage in Rats by Blocking the TGF-β/Smad Signaling Pathways. Chem. Biol. Interact. 2017, 261, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Ganai, A.A.; Ganaie, I.A.; Verma, N.; Farooqi, H. Regression of Fibrosis/Cirrhosis by Glycine Propionyl-l-Carnitine Treatment in D-Galactosamine Induced Chronic Liver Damage. Chem. Biol. Interact. 2016, 260, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Obayashi, Y.; Arisaka, H.; Yoshida, S.; Mori, M.; Takahashi, M. The Protection Mechanism of Proline from D-Galactosamine Hepatitis Involves the Early Activation of ROS-Eliminating Pathway in the Liver. SpringerPlus 2015, 4, 199. [Google Scholar] [CrossRef]
- Drucker, C.; Rabe, B.; Chalaris, A.; Schulz, E.; Scheller, J.; Rose-John, S. Interleukin-6 Trans-Signaling Regulates Glycogen Consumption after d-Galactosamine-Induced Liver Damage. J. Interferon Cytokine Res. 2009, 29, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, T.; Shinohara, T. Pathological Study of Chronic D-Galactosamine Induced Hepatitis in Mice by Administration of Adjuvants an Animal Model of the Chronic Active Hepatitis. Gastroenterol. Jpn. 1981, 16, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, X.; Wang, L.; Xu, J.; Wen, J. Hypoglycemia on Admission in Patients with Acute on Chronic Liver Failure: A Retrospective Cohort Analyzing the Current Situation, Risk Factors, and Associations with Prognosis. Ann. Palliat. Med. 2023, 12, 163–170. [Google Scholar] [CrossRef]
- Shah, N.J.; Royer, A.; John, S. Acute Liver Failure. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Ramanathan, R.; Rupert, S.; Selvaraj, S.; Satyanesan, J.; Vennila, R.; Rajagopal, S. Role of Human Wharton’s Jelly Derived Mesenchymal Stem Cells (WJ-MSCs) for Rescue of D-Galactosamine Induced Acute Liver Injury in Mice. J. Clin. Exp. Hepatol. 2017, 7, 205–214. [Google Scholar] [CrossRef]
- Chung, H.; Kim, H.J.; Jang, K.S.; Kim, M.; Yang, J.; Kang, K.S.; Kim, H.L.; Yoon, B.I.; Lee, M.O.; Lee, B.H.; et al. Comprehensive Analysis of Differential Gene Expression Profiles on D-Galactosamine-Induced Acute Mouse Liver Injury and Regeneration. Toxicology 2006, 227, 136–144. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Miki, T.; Mitamura, K.; Ross, M.A.; Stolz, D.B.; Strom, S.C. Identification of Stem Cell Marker-Positive Cells by Immunofluorescence in Term Human Amnion. J. Reprod. Immunol. 2007, 75, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zheng, Q.; Jin, L. The Role of B7 Family Molecules in Maternal–Fetal Immunity. Front. Immunol. 2020, 11, 458. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.S.; Zhou, L.; Sagayaraj, A.; Jumat, N.H.B.; Choolani, M.; Chan, J.K.Y.; Biswas, A.; Wong, P.C.; Lim, S.G.; Dan, Y.Y. Hepatic Differentiation of Human Amniotic Epithelial Cells and In Vivo Therapeutic Effect on Animal Model of Cirrhosis. J. Gastroenterol. Hepatol. 2015, 30, 1673–1682. [Google Scholar] [CrossRef] [PubMed]
- Maymó, J.L.; Riedel, R.; Pérez-Pérez, A.; Magatti, M.; Maskin, B.; Dueñas, J.L.; Parolini, O.; Sánchez-Margalet, V.; Varone, C.L. Proliferation and Survival of Human Amniotic Epithelial Cells during Their Hepatic Differentiation. PLoS ONE 2018, 13, e191489. [Google Scholar] [CrossRef] [PubMed]
- Alhomrani, M.; Correia, J.; Zavou, M.; Leaw, B.; Kuk, N.; Xu, R.; Saad, M.I.; Hodge, A.; Greening, D.W.; Lim, R.; et al. The Human Amnion Epithelial Cell Secretome Decreases Hepatic Fibrosis in Mice with Chronic Liver Fibrosis. Front. Pharmacol. 2017, 8, 748. [Google Scholar] [CrossRef]
- Liu, J.; Hu, X.; Chen, J.; Li, X.; Wang, L.; Wang, B.; Peng, W.; Yang, C.; Li, Z.; Chen, Y.; et al. Pericentral Hepatocytes Produce Insulin-like Growth Factor-2 to Promote Liver Regeneration during Selected Injuries in Mice. Hepatology 2017, 66, 2002–2015. [Google Scholar] [CrossRef] [PubMed]
- Andrewartha, N.; Yeoh, G. Human Amnion Epithelial Cell Therapy for Chronic Liver Disease. Stem Cells Int. 2019, 2019, 8106482. [Google Scholar] [CrossRef]
- Boone, L.; Meyer, D.; Cusick, P.; Ennulat, D.; Provencher Bolliger, A.; Everds, N.; Meador, V.; Elliott, G.; Honor, D.; Bounous, D.; et al. Selection and Interpretation of Clinical Pathology Indicators of Hepatic Injury in Preclinical Studies. Vet. Clin. Pathol. 2005, 34, 182–188. [Google Scholar] [CrossRef]
- Aulbach, A.D.; Amuzie, C.J. Biomarkers in Nonclinical Drug Development, 2nd ed.; Elsevier Inc., Academic Press: Boston, MA, USA, 2017; pp. 447–471. [Google Scholar]
- Liu, W.; Zhang, L.; Xuan, K.; Hu, C.; Li, L.; Zhang, Y.; Jin, F.; Jin, Y. Alkaline Phosphatase Controls Lineage Switching of Mesenchymal Stem Cells by Regulating the Lrp6/Gsk3β Complex in Hypophosphatasia. Theranostics 2018, 8, 5575–5592. [Google Scholar] [CrossRef]
- Wang, M.; Liang, C.; Hu, H.; Zhou, L.; Xu, B.; Wang, X.; Han, Y.; Nie, Y.; Jia, S.; Liang, J.; et al. Intraperitoneal Injection (IP), Intravenous Injection (IV) or Anal Injection (AI)? Best Way for Mesenchymal Stem Cells Transplantation for Colitis. Sci. Rep. 2016, 6, 30696. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.V.; Brabb, T.; Pekow, C.; Vasbinder, M.A. Administration of Substances to Laboratory Animals: Routes of Administration and Factors to Consider. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 600–613. [Google Scholar] [PubMed]
- Al Shoyaib, A.; Archie, S.R.; Karamyan, V.T. Intraperitoneal Route of Drug Administration: Should It Be Used in Experimental Animal Studies? Pharm. Res. 2019, 37, 12. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Fan, X.; Zhang, L.; Shi, G.; Aili, M.; Lu, X.; Jiang, T.; Zhang, Y. Bone Mesenchymal Stem Cell Transplantation via Four Routes for the Treatment of Acute Liver Failure in Rats. Int. J. Mol. Med. 2014, 34, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Vanaclocha, F. The Prometastatic Microenvironment of the Liver. Cancer Microenviron. 2008, 1, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Diaz, M.; Quiñones-Vico, M.I.; de la Torre, R.S.; Montero-Vílchez, T.; Sierra-Sánchez, A.; Molina-Leyva, A.; Arias-Santiago, S. Biodistribution of Mesenchymal Stromal Cells after Administration in Animal Models and Humans: A Systematic Review. J. Clin. Med. 2021, 10, 2925. [Google Scholar] [CrossRef] [PubMed]
- Bohnen, N.I.; Charron, M.; Reyes, J.; Rubinstein, W.; Strom, S.C.; Swanson, D.; Towbin, R. Use of Indium-111-Labeled Hepatocytes to Determine the Biodistribution of Transplanted Hepatocytes through Portal Vein Infusion. Clin. Nucl. Med. 2000, 25, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Nitzsche, F.; Müller, C.; Lukomska, B.; Jolkkonen, J.; Deten, A.; Boltze, J. Concise Review: MSC Adhesion Cascade—Insights into Homing and Transendothelial Migration. Stem Cells 2017, 35, 1446–1460. [Google Scholar] [CrossRef] [PubMed]
- Park, B.N.; Lim, T.S.; Yoon, J.K.; An, Y.S. In Vivo Tracking of Intravenously Injected Mesenchymal Stem Cells in an Alzheimer’s Animal Model. Cell Transplant. 2018, 27, 1203–1209. [Google Scholar] [CrossRef]
- Eggenhofer, E.; Benseler, V.; Kroemer, A.; Popp, F.C.; Geissler, E.K.; Schlitt, H.J.; Baan, C.C.; Dahlke, M.H.; Hoogduijn, M.J. Mesenchymal Stem Cells Are Short-Lived and Do Not Migrate beyond the Lungs after Intravenous Infusion. Front. Immunol. 2012, 3, 297. [Google Scholar] [CrossRef]
- Nguyen, P.K.; Riegler, J.; Wu, J.C. Stem Cell Imaging: From Bench to Bedside. Cell Stem Cell 2014, 14, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Gholamrezanezhad, A.; Mirpour, S.; Bagheri, M.; Mohamadnejad, M.; Alimoghaddam, K.; Abdolahzadeh, L.; Saghari, M.; Malekzadeh, R. In Vivo Tracking of 111In-Oxine Labeled Mesenchymal Stem Cells Following Infusion in Patients with Advanced Cirrhosis. Nucl. Med. Biol. 2011, 38, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Gao, F.; Lin, J.; Lu, L.; Xu, H.; Xu, G.T. Conditioned Medium of Human Amniotic Epithelial Cells Alleviates Experimental Allergic Conjunctivitis Mainly by IL-1ra and IL-10. Front. Immunol. 2021, 12, 774601. [Google Scholar] [CrossRef] [PubMed]
- Gramignoli, R. Therapeutic Use of Human Amnion-Derived Products: Cell-Based Therapy for Liver Disease. Curr. Pathobiol. Rep. 2016, 4, 157–167. [Google Scholar] [CrossRef]
- Jafari, A.; Niknejad, H.; Rezaei-Tavirani, M.; Sarrami-Forooshani, R.; Gilanchi, S.; Jafari, Z. Antiproliferative and Apoptotic Effects of Conditioned Medium Released from Human Amniotic Epithelial Stem Cells on Breast and Cervical Cancer Cells. Int. J. Immunopathol. Pharmacol. 2023, 37, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dong, V.; Nanchal, R.; Karvellas, C.J. Pathophysiology of Acute Liver Failure. Nutr. Clin. Pract. 2020, 35, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ding, H.; Zhou, J.; Xia, S.; Shi, X.; Ren, H. Transplantation of Mesenchymal Stem Cells Attenuates Acute Liver Failure in Mice via an Interleukin-4-Dependent Switch to the M2 Macrophage Anti-Inflammatory Phenotype. J. Clin. Transl. Hepatol. 2022, 10, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Zare, H.; Jamshidi, S.; Dehghan, M.M.; Saheli, M.; Piryaei, A. Bone Marrow or Adipose Tissue Mesenchymal Stem Cells: Comparison of the Therapeutic Potentials in Mice Model of Acute Liver Failure. J. Cell. Biochem. 2018, 119, 5834–5842. [Google Scholar] [CrossRef]
- Iansante, V.; Mitry, R.R.; Filippi, C.; Fitzpatrick, E.; Dhawan, A. Human Hepatocyte Transplantation for Liver Disease: Current Status and Future Perspectives. Pediatr. Res. 2018, 83, 232–240. [Google Scholar] [CrossRef]
- Gilbert, J.C.; Stein, J.E.; Takeda, T. Quantitation of Transplanted Hepatic Mass Necessary Gunn Rat Model of Hyperbilirubinemia. J. Pediatr. Surg. 1991, 27, 298–301. [Google Scholar]








| Intoxication/Cell Delivery | Time after Injection | Route of hAEC Administration | ALAT | ASAT | ALP | TP | Glucose |
|---|---|---|---|---|---|---|---|
| D-GaIN | 6 h | intraperitoneally | 9% ↑ | 18% ↑ | 58% a↑ | 18% a↓ | 89% a↓ |
| 24 h | 81% a↑ | 81% a↑ | 56% a↑ | 1% ↓ | 329% a↓ | ||
| 72 h | 76% a↑ | 46% a↑ | 41% a↑ | 7% ↓ | 170% a↓ | ||
| hAEC | 3 h | intraperitoneally | 37% ↑ | 8% ↑ | 59% a↑ | 0% | 51% a↓ |
| intravenously | 61% a↑ | 82% a↑ | 52% ↑ | 2% ↓ | 32% ↓ | ||
| 21 h | intraperitoneally | 9% ↑ | 12% ↑ | 63% a↑ | 2% ↓ | 24% a↓ | |
| intravenously | 56%↑ | 55% a↑ | 49% a↑ | 3% ↓ | 37% ↓ | ||
| 69 h | intraperitoneally | 6% ↑ | 13% ↓ | 62% a↑ | 7% ↑ | 85% a↓ | |
| intravenously | 11%↓ | 38% ↑ | 21% ↑ | 3% ↑ | 41% ↓ | ||
| hAEC + D-GaIN | 3/6 h | intraperitoneally | 27% ↑ | 6% ↑ | 7% ↑ | 10% b↑ | 0% |
| intravenously | 20% ↑ | 39% ↑ | 13% ↓ | 9% ↑ | 38% ↓ | ||
| 21/24 h | intraperitoneally | 41% ↑ | 16% ↑ | 6% ↓ | 7% ↓ | 6% ↓ | |
| intravenously | 31% ↓ | 29% ↑ | 3% ↓ | 0% | 34% ↑ | ||
| 69/72 h | intraperitoneally | 65% ↑ | 40% ↑ | 15% ↑ | 6% ↑ | 11% ↑ | |
| intravenously | 443% b↓ | 89% ↓ | 38% ↓ | 15% b↑ | 36% b↑ |
| Organ | Route of hAEC Administration | Untreated Mouse | Treated Mouse | ||||
|---|---|---|---|---|---|---|---|
| Time [h] | |||||||
| 3 | 21 | 69 | 3 | 21 | 69 | ||
| Lung | Intraperitoneally | 2 | 0 b | 5 b,d | 0 | 0 | 0 |
| Intravenously | 138,397 a | 23,581 a,b | 19,400 a,b | 23,496 a | 77,361 a | 11,450 a | |
| Spleen | Intraperitoneally | 332 | 0 b | 92 | 69 | 788 c,d | 361 c,f |
| Intravenously | 0 | 5 | 0 | 0 | 71 a | 55 a | |
| Liver | Intraperitoneally | 449 | 125 | 343 | 261 | 139 | 552 e |
| Intravenously | 189 | 0 b | 59 a | 22 a | 0 a | 0 a | |
| Blood | Intraperitoneally | 0 | 11 b | 8 b | 22 | 33 | 0 |
| Intravenously | 18 a | 0 | 0 | 905 a | 0 a,c | 0 | |
| Group | Subgroups | Route of Administration | Injection | |
|---|---|---|---|---|
| Control group | I | 72O | Intraperitoneally | NaCl |
| II | 72Z | Intravenously | ||
| hAEC injection group | III | 3O | Intraperitoneally | hAEC |
| 21O | ||||
| 69O | ||||
| IV | 3Z | Intravenously | ||
| 21Z | ||||
| 69Z | ||||
| D-GaIN injection group | 6G | Intraperitoneally | D-GaIN | |
| V | 24G | |||
| 72G | ||||
| D-GaIN and hAEC injection group | VI | 6GO | D-GaIN intraperitoneally; hAEC intraperitoneally | D-GaIN and hAEC after 3 h |
| 24GO | ||||
| 72GO | ||||
| VII | 6GZ | D-GaIN intraperitoneally; hAEC intravenously | ||
| 24GZ | ||||
| 72GZ | ||||
| Scale | Hepatitis Exponents | Scale | Exponents of Liver Parenchymal Injury | Scale | Presence of Acidophilic Apoptotic Bodies |
|---|---|---|---|---|---|
| 1 | No or minimal inflammation; absent or single infiltration in zone 1 of the hepatic stroma; or single infiltration of the parenchyma | 1 | No foci of damage/single foci of damage; parenchymal oedema | 1 | None |
| 2 | Infiltration in zone 1 of the hepatic stroma occupying <50% of the examined area | 2 | At least one outbreak of damage | 2 | Singular |
| 3 | Infiltration in zone 1 of the hepatic stroma occupying >50% of the examined area | 3 | Multiple foci of damage; necrosis; disruption of parenchymal architecture | 3 | Multiple |
| Scale | Evaluation |
|---|---|
| 1 | No protein expression |
| 2 | Protein present in <10 cells examined |
| 3 | Protein present in 10–25 cells tested |
| 4 | Protein present in 26–50 cells tested |
| 5 | Protein present in >50 cells tested |
| Gene | Name | Sequence | Product Length | Tm [°C] |
|---|---|---|---|---|
| CB | Cytochrome b | F: 5′-CCCATACATTGGGACAGACC-3′ R: 5′-GACGGATCGGAGAATTGTGT-3′ | 394 | 82.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wieczorek, P.; Czekaj, P.; Król, M.; Bogunia, E.; Hermyt, M.; Kolanko, E.; Toczek, J.; Skubis-Sikora, A.; Grajoszek, A.; Stojko, R. Comparison of the Efficacy of Two Routes of Administration of Human Amniotic Epithelial Cells in Cell Therapy of Acute Hepatic Insufficiency. Pharmaceuticals 2024, 17, 476. https://doi.org/10.3390/ph17040476
Wieczorek P, Czekaj P, Król M, Bogunia E, Hermyt M, Kolanko E, Toczek J, Skubis-Sikora A, Grajoszek A, Stojko R. Comparison of the Efficacy of Two Routes of Administration of Human Amniotic Epithelial Cells in Cell Therapy of Acute Hepatic Insufficiency. Pharmaceuticals. 2024; 17(4):476. https://doi.org/10.3390/ph17040476
Chicago/Turabian StyleWieczorek, Patrycja, Piotr Czekaj, Mateusz Król, Edyta Bogunia, Mateusz Hermyt, Emanuel Kolanko, Jakub Toczek, Aleksandra Skubis-Sikora, Aniela Grajoszek, and Rafał Stojko. 2024. "Comparison of the Efficacy of Two Routes of Administration of Human Amniotic Epithelial Cells in Cell Therapy of Acute Hepatic Insufficiency" Pharmaceuticals 17, no. 4: 476. https://doi.org/10.3390/ph17040476





