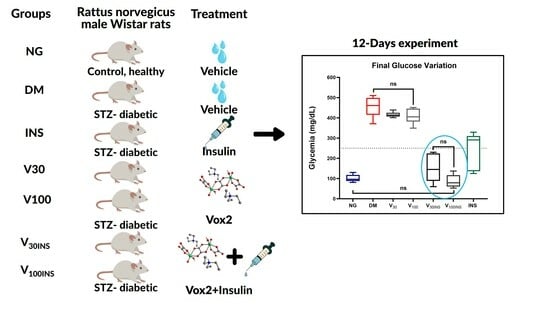
A Non-Toxic Binuclear Vanadium(IV) Complex as Insulin Adjuvant Improves the Glycemic Control in Streptozotocin-Induced Diabetic Rats
Abstract
1. Introduction
2. Results
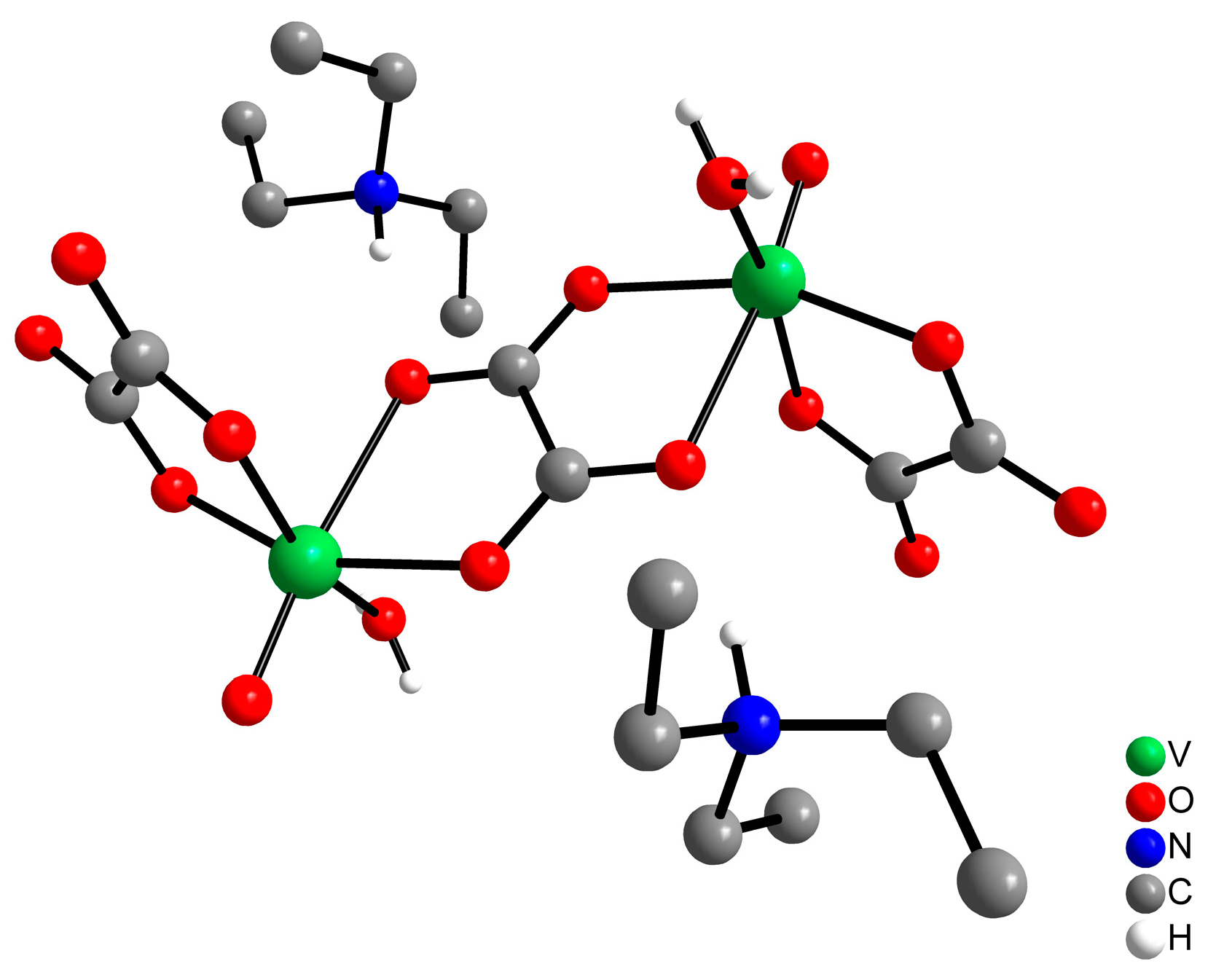
2.1. Preparation of the Oxidovanadium(IV) Complex
2.2. Vox2 Shows In Vitro Antioxidant Activity
2.3. Evaluation of the Acute Toxic Effects of the Vox2 Administration
2.4. Vanadium Compound Effects in Diabetes
2.5. General Clinical Observations and Biochemical Markers
3. Discussion
4. Materials and Methods
4.1. Synthesis of the Oxidovanadium(IV) Complex—Vox2
4.2. Protocols Followed for In Vivo Studies with Vox2 Complex on Wistar Rats
4.2.1. Monitoring and Analysis of Behavior
Open-Field Test
4.2.2. Vanadium Compound (Vox2) Effect in Diabetes
4.3. Organ Weights and Histopathology
4.4. Oxidative Stress Parameters
4.5. General Clinical Observations and Biochemical Markers
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation. Diabetes is “a pandemic of unprecedented magnitude” now affecting one in 10 adults worldwide. Diabetes Res. Clin. Pract. 2021, 181, 109133. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Unnikrishnan, R.; Pradeepa, R.; Joshi, S.R.; Mohan, V. Type 2 Diabetes: Demystifying the Global Epidemic. Diabetes 2017, 66, 1432–1442. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.B.; Florez, J.C. Genetics of diabetes mellitus and diabetes complications. Nat. Rev. Nephrol. 2020, 16, 377–390. [Google Scholar] [CrossRef] [PubMed]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Cusi, K.; Das, S.R.; Gibbons, C.H.; et al. Introduction and Methodology: Standards of Care in Diabetes—2023. Diabetes Care 2022, 46, S1–S4. [Google Scholar] [CrossRef] [PubMed]
- Skyler, J.S.; Bakris, G.L.; Bonifacio, E.; Darsow, T.; Eckel, R.H.; Groop, L.; Groop, P.H.; Handelsman, Y.; Insel, R.A.; Mathieu, C.; et al. Differentiation of Diabetes by Pathophysiology, Natural History, and Prognosis. Diabetes 2017, 66, 241–255. [Google Scholar] [CrossRef]
- Danne, T.; Pettus, J.; Giaccari, A.; Cariou, B.; Rodbard, H.; Weinzimer, S.A.; Bonnemaire, M.; Sawhney, S.; Stewart, J.; Wang, S.; et al. Sotagliflozin Added to Optimized Insulin Therapy Leads to Lower Rates of Clinically Relevant Hypoglycemic Events at Any HbA1c at 52 Weeks in Adults with Type 1 Diabetes. Diabetes Technol. Ther. 2019, 21, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Kerru, N.; Singh-Pillay, A.; Awolade, P.; Singh, P. Current anti-diabetic agents and their molecular targets: A review. Eur. J. Med. Chem. 2018, 152, 436–488. [Google Scholar] [CrossRef]
- Trevino, S.; Diaz, A.; Sanchez-Lara, E.; Sanchez-Gaytan, B.L.; Perez-Aguilar, J.M.; Gonzalez-Vergara, E. Vanadium in Biological Action: Chemical, Pharmacological Aspects, and Metabolic Implications in Diabetes Mellitus. Biol. Trace Elem. Res. 2019, 188, 68–98. [Google Scholar] [CrossRef]
- Amaral, L.M.P.F.; Moniz, T.; Silva, A.M.N.; Rangel, M. Vanadium Compounds with Antidiabetic Potential. Int. J. Mol. Sci. 2023, 24, 15675. [Google Scholar] [CrossRef] [PubMed]
- Domingues, N.; Pelletier, J.; Ostenson, C.G.; Castro, M.M. Therapeutic properties of VO(dmpp)2 as assessed by in vitro and in vivo studies in type 2 diabetic GK rats. J. Inorg. Biochem. 2014, 131, 115–122. [Google Scholar] [CrossRef]
- Trevino, S.; Diaz, A. Vanadium and insulin: Partners in metabolic regulation. J. Inorg. Biochem. 2020, 208, 111094. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, H.; Fujii, K.; Watanabe, H.; Tamura, H. Orally active and long-term acting insulin-mimetic vanadyl complex:bis(picolinato)oxovanadium (IV). Biochem. Biophys. Res. Commun. 1995, 214, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.J.; Li, L.; Yang, X.D.; Liu, W.P.; Yan, S.P.; Niu, Y.F.; Meng, Z.H. A new insulin-enhancing agent: [N,N’-bis(4-hydroxysalicylidene)-o-phenylene-diamine]oxovanadium(IV) and its permeability and cytotoxicity. Eur. J. Med. Chem. 2010, 45, 2327–2335. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.H.; Lichter, J.; LeBel, C.; Scaife, M.C.; McNeill, J.H.; Orvig, C. Vanadium treatment of type 2 diabetes: A view to the future. J. Inorg. Biochem. 2009, 103, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Willsky, G.R.; Goldfine, A.B.; Kostyniak, P.J.; McNeill, J.H.; Yang, L.Q.; Khan, H.R.; Crans, D.C. Effect of vanadium(IV) compounds in the treatment of diabetes: In vivo and in vitro studies with vanadyl sulfate and bis(maltolato)oxovandium(IV). J. Inorg. Biochem. 2001, 85, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.M.; Pickering, R.M.; Lewith, G.T. A systematic review of vanadium oral supplements for glycaemic control in type 2 diabetes mellitus. QJM Int. J. Med. 2008, 101, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Berhan, A.; Habtewolde, A. Effects of Vanadium Compounds on Glycemic control In Type 2 Diabetes Mellitus: A Metaanalysis of Comparative Study on Rats. Int. J. Pharm. Sci. Res. 2012, 3, 3717–3724. [Google Scholar]
- Ghosh, S.K.; Saha, R.; Saha, B. Toxicity of inorganic vanadium compounds. Res. Chem. Intermed. 2015, 41, 4873–4897. [Google Scholar] [CrossRef]
- Srivastava, A.K. Anti-diabetic and toxic effects of vanadium compounds. Mol. Cell. Biochem. 2000, 206, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Domingo, J.L.; Gomez, M.; Sanchez, D.J.; Llobet, J.M.; Keen, C.L. Toxicology of vanadium compounds in diabetic rats: The action of chelating agents on vanadium accumulation. Mol. Cell. Biochem. 1995, 153, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, M.R.; Kale, P.P. Mini review–vanadium-induced neurotoxicity and possible targets. Neurol. Sci. 2020, 41, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Goldwaser, I.; Gefel, D.; Gershonov, E.; Fridkin, M.; Shechter, Y. Insulin-like effects of vanadium: Basic and clinical implications. J. Inorg. Biochem. 2000, 80, 21–25. [Google Scholar] [CrossRef]
- McNeill, J.H.; Yuen, V.G.; Hoveyda, H.R.; Orvig, C. Bis(maltolato)oxovanadium(IV) is a potent insulin mimic. J. Med. Chem. 1992, 35, 1489–1491. [Google Scholar] [CrossRef] [PubMed]
- Yuen, V.G.; Orvig, C.; McNeill, J.H. Glucose-lowering effects of a new organic vanadium complex, bis(maltolato)oxovanadium(IV). Can. J. Physiol. Pharmacol. 1993, 71, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Poucheret, P.; Verma, S.; Grynpas, M.D.; McNeill, J.H. Vanadium and diabetes. Mol. Cell. Biochem. 1998, 188, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.I.; Subramanian, S.P.; Kandaswamy, M. Evaluation of antioxidant efficacy of vanadium-3-hydroxyflavone complex in streptozotocin-diabetic rats. Chem. Biol. Interact. 2013, 204, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, B.; Ravi, K.; Narayanan, V.; Kandaswamy, M.; Subramanian, S. Protective effect of macrocyclic binuclear oxovanadium complex on oxidative stress in pancreas of streptozotocin induced diabetic rats. Chem. Biol. Interact. 2004, 149, 9–21. [Google Scholar] [CrossRef]
- Aureliano, M.; De Sousa-Coelho, A.L.; Dolan, C.C.; Roess, D.A.; Crans, D.C. Biological Consequences of Vanadium Effects on Formation of Reactive Oxygen Species and Lipid Peroxidation. Int. J. Mol. Sci. 2023, 24, 5382. [Google Scholar] [CrossRef]
- Ścibior, A.; Kurus, J. Vanadium and Oxidative Stress Markers—In Vivo Model: A Review. Curr. Med. Chem. 2019, 26, 5456–5500. [Google Scholar] [CrossRef] [PubMed]
- Ścibior, A.; Pietrzyk, Ł.; Plewa, Z.; Skiba, A. Vanadium: Risks and possible benefits in the light of a comprehensive overview of its pharmacotoxicological mechanisms and multi-applications with a summary of further research trends. J. Trace Elem. Med. Biol. 2020, 61, 126508. [Google Scholar] [CrossRef] [PubMed]
- Wilk, A.; Szypulska-Koziarska, D.; Wiszniewska, B. The toxicity of vanadium on gastrointestinal, urinary and reproductive system, and its influence on fertility and fetuses malformations. Postep. Hig. Med. Dosw. 2017, 71, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.H.; Orvig, C. Vanadium in diabetes: 100 years from Phase 0 to Phase I. J. Inorg. Biochem. 2006, 100, 1925–1935. [Google Scholar] [CrossRef]
- Sakurai, H.; Kojima, Y.; Yoshikawa, Y.; Kawabe, K.; Yasui, H. Antidiabetic vanadium(IV) and zinc(II) complexes. Coord. Chem. Rev. 2002, 226, 187–198. [Google Scholar] [CrossRef]
- Crans, D.C. Chemistry and insulin-like properties of vanadium(IV) and vanadium(V) compounds. J. Inorg. Biochem. 2000, 80, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.H.; Chiles, J.; Yuen, V.G.; Tse, J.; McNeill, J.H.; Orvig, C. Comparison of anti-hyperglycemic effect amongst vanadium, molybdenum and other metal maltol complexes. J. Inorg. Biochem. 2004, 98, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Monga, V.; Thompson, K.H.; Yuen, V.G.; Sharma, V.; Patrick, B.O.; McNeill, J.H.; Orvig, C. Vanadium complexes with mixed O,S anionic ligands derived from maltol: Synthesis, characterization, and biological studies. Inorg. Chem. 2005, 44, 2678–2688. [Google Scholar] [CrossRef] [PubMed]
- Yuen, V.G.; Caravan, P.; Gelmini, L.; Glover, N.; McNeill, J.H.; Setyawati, I.A.; Zhou, Y.; Orvig, C. Glucose-lowering properties of vanadium compounds: Comparison of coordination complexes with maltol or kojic acid as ligands. J. Inorg. Biochem. 1997, 68, 109–116. [Google Scholar] [CrossRef]
- Fukui, K.; Fujisawa, Y.; OhyaNishiguchi, H.; Kamada, H.; Sakurai, H. In vivo coordination structural changes of a potent insulin-mimetic agent, bis(picolinato)oxovanadium(IV), studied by electron spin-echo envelope modulation spectroscopy. J. Inorg. Biochem. 1999, 77, 215–224. [Google Scholar] [CrossRef]
- Crans, D.C.; Mahroof-Tahir, M.; Johnson, M.D.; Wilkins, P.C.; Yang, L.; Robbins, K.; Johnson, A.; Alfano, J.A.; Godzala, M.E.; Austin, L.T.; et al. Vanadium(IV) and vanadium(V) complexes of dipicolinic acid and derivatives. Synthesis, X-ray structure, solution state properties: And effects in rats with STZ-induced diabetes. Inorg. Chim. Acta 2003, 356, 365–378. [Google Scholar] [CrossRef]
- Gätjens, J.; Meier, B.; Adachi, Y.; Sakurai, H.; Rehder, D. Characterization and Insulin-Mimetic Potential of Oxidovanadium(IV) Complexes Derived from Monoesters and -carboxylates of 2,5-Dipicolinic Acid. Eur. J. Inorg. Chem. 2006, 2006, 3575–3585. [Google Scholar] [CrossRef]
- Gätjens, J.; Meier, B.; Kiss, T.; Nagy, E.M.; Buglyó, P.; Sakurai, H.; Kawabe, K.; Rehder, D. A New Family of Insulin-Mimetic Vanadium Complexes Derived from 5-Carboalkoxypicolinates. Chem. A Eur. J. 2003, 9, 4924–4935. [Google Scholar] [CrossRef] [PubMed]
- Woo, L.C.; Yuen, V.G.; Thompson, K.H.; McNeill, J.H.; Orvig, C. Vanadyl-biguanide complexes as potential synergistic insulin mimics. J. Inorg. Biochem. 1999, 76, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.S.; Cryer, K.; Zhang, B.; Dutta, S.K.; Eaton, S.S.; Anderson, O.P.; Miller, S.M.; Reul, B.A.; Brichard, S.M.; Crans, D.C. Chemistry and insulin-mimetic properties of bis(acetylacetonate)oxovanadium(IV) and derivatives. Inorg. Chem. 2000, 39, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Kiersztan, A.; Modzelewska, A.; Jarzyna, R.; Jagielska, E.; Bryla, J. Inhibition of gluconeogenesis by vanadium and metformin in kidney-cortex tubules isolated from control and diabetic rabbits. Biochem. Pharmacol. 2002, 63, 1371–1382. [Google Scholar] [CrossRef]
- Kawabe, K.; Sasagawa, T.; Yoshikawa, Y.; Ichimura, A.; Kumekawa, K.; Yanagihara, N.; Takino, T.; Sakurai, H.; Kojima, Y. Synthesis, structure analysis, solution chemistry, and in vitro insulinomimetic activity of novel oxovanadium(IV) complexes with tripodal ligands containing an imidazole group derived from amino acids. JBIC J. Biol. Inorg. Chem. 2003, 8, 893–906. [Google Scholar] [CrossRef]
- Baptistella, G.B.; Manica, G.C.M.; de Souza, S.W.; Santana, F.S.; Fachini, L.G.; Hughes, D.L.; de Sá, E.L.; Picheth, G.; Soares, J.F.; Rego, F.G.M.; et al. An oxalate-bridged oxidovanadium(IV) binuclear complex that improves the in vitro cell uptake of a fluorescent glucose analog. Polyhedron 2021, 198, 115071. [Google Scholar] [CrossRef]
- Walsh, R.N.; Cummins, R.A. The open-field test: A critical review. Psychol. Bull. 1976, 83, 482–504. [Google Scholar] [CrossRef]
- Ahmad, K. Insulin sources and types: A review of insulin in terms of its mode on diabetes mellitus. J. Tradit. Chin. Med. 2014, 34, 234–237. [Google Scholar] [CrossRef]
- Pessoa, J.C.; Etcheverry, S.; Gambino, D. Vanadium compounds in medicine. Coord. Chem. Rev. 2015, 301, 24–48. [Google Scholar] [CrossRef] [PubMed]
- Reul, B.A.; Amin, S.S.; Buchet, J.P.; Ongemba, L.N.; Crans, D.C.; Brichard, S.M. Effects of vanadium complexes with organic ligands on glucose metabolism: A comparison study in diabetic rats. Br. J. Pharmacol. 1999, 126, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-González, C.; López-Chaves, C.; Trenzado, C.E.; Aranda, P.; López-Jurado, M.; Gómez-Aracena, J.; Montes-Bayón, M.; Sanz-Medel, A.; Llopis, J. Changes in Iron Metabolism and Oxidative Status in STZ-Induced Diabetic Rats Treated with Bis(maltolato) Oxovanadium (IV) as an Antidiabetic Agent. Sci. World J. 2014, 2014, 706074. [Google Scholar] [CrossRef] [PubMed]
- Crans, D.C.; Henry, L.; Cardiff, G.; Posner, B.I. Developing Vanadium as an Antidiabetic or Anticancer Drug: A Clinical and Historical Perspective. Met. Ions Life Sci. 2019, 19, 203–230. [Google Scholar] [CrossRef]
- Gould, T.D.; Dao, D.T.; Kovacsics, C.E. The Open Field Test. In Mood and Anxiety Related Phenotypes in Mice: Characterization Using Behavioral Tests; Gould, T.D., Ed.; Humana Press: Totowa, NJ, USA, 2009; pp. 1–20. [Google Scholar]
- Orvig, C.; Caravan, P.; Gelmini, L.; Glover, N.; Herring, F.G.; Li, H.; McNeill, J.H.; Rettig, S.J.; Setyawati, I.A. Reaction chemistry of BMOV, bis(maltolato)oxovanadium(IV), a potent insulin mimetic agent. J. Am. Chem. Soc. 1995, 117, 12759–12770. [Google Scholar] [CrossRef]
- Aureliano, M.; Gumerova, N.I.; Sciortino, G.; Garribba, E.; McLauchlan, C.C.; Rompel, A.; Crans, D.C. Polyoxidovanadates’ interactions with proteins: An overview. Coord. Chem. Rev. 2022, 454, 214344. [Google Scholar] [CrossRef]
- Posner, B.I.; Faure, R.; Burgess, J.W.; Bevan, A.P.; Lachance, D.; Zhang-Sun, G.; Fantus, I.G.; Ng, J.B.; Hall, D.A.; Lum, B.S.; et al. Peroxovanadium compounds. A new class of potent phosphotyrosine phosphatase inhibitors which are insulin mimetics. J. Biol. Chem. 1994, 269, 4596–4604. [Google Scholar] [CrossRef]
- Ramanadham, S.; Mongold, J.J.; Brownsey, R.W.; Cros, G.H.; McNeill, J.H. Oral vanadyl sulfate in treatment of diabetes mellitus in rats. Am. J. Physiol. Heart Circ. Physiol. 1989, 257, H904–H911. [Google Scholar] [CrossRef]
- De Nigro, T.P.; Manica, G.C.M.; de Souza, S.W.; Jesus, C.H.A.; Bottini, R.C.R.; Missina, J.M.; Valdameri, G.; Nunes, G.G.; da Cunha, J.M.; Picheth, G.; et al. Heteroleptic oxidovanadium(IV)-malate complex improves glucose uptake in HepG2 and enhances insulin action in streptozotocin-induced diabetic rats. BioMetals 2022, 35, 903–919. [Google Scholar] [CrossRef]
- Cusi, K.; Cukier, S.; DeFronzo, R.A.; Torres, M.; Puchulu, F.M.; Redondo, J.C. Vanadyl sulfate improves hepatic and muscle insulin sensitivity in type 2 diabetes. J. Clin. Endocrinol. Metab. 2001, 86, 1410–1417. [Google Scholar] [CrossRef]
- Lima, L.M.A.; da Silva, A.; Batista, E.K.; Postal, K.; Kostenkova, K.; Fenton, A.; Crans, D.C.; Silva, W.E.; Belian, M.F.; Lira, E.C. The antihyperglycemic and hypolipidemic activities of a sulfur-oxidovanadium(IV) complex. J. Inorg. Biochem. 2023, 241, 112127. [Google Scholar] [CrossRef] [PubMed]
- Willsky, G.R.; Chi, L.H.; Godzala, M., 3rd; Kostyniak, P.J.; Smee, J.J.; Trujillo, A.M.; Alfano, J.A.; Ding, W.; Hu, Z.; Crans, D.C. Anti-diabetic effects of a series of vanadium dipicolinate complexes in rats with streptozotocin-induced diabetes. Coord. Chem. Rev. 2011, 255, 2258–2269. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Elberg, G.; Crans, D.C.; Shechter, Y. Evidence for the distinct vanadyl(+4)-dependent activating system for manifesting insulin-like effects. Biochemistry 1996, 35, 8314–8318. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Chen, D.; Zhang, F.; Willsky, G.R.; Crans, D.C.; Ding, W. Effects of vanadium (III, IV, V)-chlorodipicolinate on glycolysis and antioxidant status in the liver of STZ-induced diabetic rats. J. Inorg. Biochem. 2014, 136, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Dimo, T.; Rakotonirina, S.V.; Tan, P.V.; Azay, J.; Dongo, E.; Kamtchouing, P.; Cros, G. Effect of Sclerocarya birrea (Anacardiaceae) stem bark methylene chloride/methanol extract on streptozotocin-diabetic rats. J. Ethnopharmacol. 2007, 110, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Diab, M.A.; Hassan, B.; El-Mezayen, H.A. Possible Therapeutic Role of Novel Vanadium Complexes in Diabetes MellitusAnimal Models. Sci. J. Damietta Fac. Sci. 2018, 8, 84–92. [Google Scholar] [CrossRef]
- Patel, R.; Shervington, A.; Pariente, J.A.; Martinez-Burgos, M.A.; Salido, G.M.; Adeghate, E.; Singh, J. Mechanism of Exocrine Pancreatic Insufficiency in Streptozotocin-Induced Type 1 Diabetes Mellitus. Ann. N. Y. Acad. Sci. 2006, 1084, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Seaborn, C.D.; Mitchell, E.D.; Stoecker, B.J. Vanadium and ascorbate effects on 3-hydroxy-3-methylglutaryl coenzyme A reductase, cholesterol and tissue minerals in guinea pigs fed low-chromium diets. Magnes. Trace Elem. 1991, 10, 327–338. [Google Scholar] [PubMed]
- Young, N.L.; Lopez, D.; McNamara, D. Contributions of Absorbed Dietary Cholesterol and Cholesterol Synthesized in Small Intestine to Hypercholesterolemia in Diabetic Rats. Diabetes 1988, 37, 1151–1156. [Google Scholar] [CrossRef]
- Verch, R.L.; Wallach, S.; Taylor, R.; Agrawal, R. Pancreatic exocrine function and cyclic nucleotides in the diabetic rat. J. Am. Coll. Nutr. 1984, 3, 61–67. [Google Scholar] [CrossRef]
- Sankaran, H.; Iwamoto, Y.; Korc, M.; Williams, J.A.; Goldfine, I.D. Insulin action in pancreatic acini from streptozotocin-treated rats. II. Binding of 125I-insulin to receptors. Am. J. Physiol. 1981, 240, G63–G68. [Google Scholar] [CrossRef]
- Aureliano, M.; Crans, D.C. Decavanadate (V10 O28 6-) and oxovanadates: Oxometalates with many biological activities. J. Inorg. Biochem. 2009, 103, 536–546. [Google Scholar] [CrossRef]
- Toxicological Profile for Vanadium; Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profiles: Atlanta, GA, USA, 2012.
- Li, M.; Smee, J.J.; Ding, W.; Crans, D.C. Anti-diabetic effects of sodium 4-amino-2,6-dipicolinatodioxovanadium(V) dihydrate in streptozotocin-induced diabetic rats. J. Inorg. Biochem. 2009, 103, 585–589. [Google Scholar] [CrossRef]
- Li, M.; Ding, W.; Smee, J.J.; Baruah, B.; Willsky, G.R.; Crans, D.C. Anti-diabetic effects of vanadium(III, IV, V)-chlorodipicolinate complexes in streptozotocin-induced diabetic rats. BioMetals 2009, 22, 895–905. [Google Scholar] [CrossRef]
- Li, M.; Ding, W.; Baruah, B.; Crans, D.C.; Wang, R. Inhibition of protein tyrosine phosphatase 1B and alkaline phosphatase by bis(maltolato)oxovanadium (IV). J. Inorg. Biochem. 2008, 102, 1846–1853. [Google Scholar] [CrossRef] [PubMed]
- Gylling, H.; Tuominen, J.A.; Koivisto, V.A.; Miettinen, T.A. Cholesterol Metabolism in Type 1 Diabetes. Diabetes 2004, 53, 2217–2222. [Google Scholar] [CrossRef] [PubMed]
- Punitha, I.R.; Rajendran, K.; Shirwaikar, A.; Shirwaikar, A. Alcoholic Stem Extract of Coscinium fenestratum Regulates Carbohydrate Metabolism and Improves Antioxidant Status in Streptozotocin–Nicotinamide Induced Diabetic Rats. Evid. Based Complement. Altern. Med. 2005, 2, 825271. [Google Scholar] [CrossRef]
- Ramachandran, B.; Kandaswamy, M.; Narayanan, V.; Subramanian, S. Insulin mimetic effects of macrocyclic binuclear oxovanadium complexes on streptozotocin-induced experimental diabetes in rats. Diabetes Obes. Metab. 2003, 5, 455–461. [Google Scholar] [CrossRef]
- Morita, T.; Imagawa, T.; Kanagawa, A.; Ueki, H. Sodium orthovanadate increases phospholipase A2 activity in isolated rat fat pads: A role of phospholipase A2 in the vanadate-stimulated release of lipoprotein lipase activity. Biol. Pharm. Bull. 1995, 18, 347–349. [Google Scholar] [CrossRef]
- Brichard, S.M.; Ongemba, L.N.; Girard, J.; Henquin, J.C. Tissue-specific correction of lipogenic enzyme gene expression in diabetic rats given vanadate. Diabetologia 1994, 37, 1065–1072. [Google Scholar] [CrossRef]
- Goldfine, A.B.; Simonson, D.C.; Folli, F.; Patti, M.E.; Kahn, C.R. Metabolic effects of sodium metavanadate in humans with insulin-dependent and noninsulin-dependent diabetes mellitus in vivo and in vitro studies. J. Clin. Endocrinol. Metab. 1995, 80, 3311–3320. [Google Scholar] [CrossRef]
- Ramachandran, B.; Subramanian, S. Amelioration of diabetic dyslipidemia by macrocyclic binuclear oxovanadium complex on streptozotocin induced diabetic rats. Mol. Cell. Biochem. 2005, 272, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Metelo, A.; Arias-Ramos, N.; López-Larrubia, P.; Castro, M. Metabolic effects of VO(dmpp) 2—An ex vivo 1 H-HRMAS NMR study to unveil its pharmacological properties. New J. Chem. 2019, 43, 17841–17849. [Google Scholar] [CrossRef]
- Yuen, V.G.; Orvig, C.; McNeill, J.H. Comparison of the glucose-lowering properties of vanadyl sulfate and bis(maltolato)oxovanadium(IV) following acute and chronic administration. Can. J. Physiol. Pharmacol. 1995, 73, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Cam, M.C.; Faun, J.; McNeill, J.H. Concentration-dependent glucose-lowering effects of oral vanadyl are maintained following treatment withdrawal in streptozotocin-diabetic rats. Metab. Clin. Exp. 1995, 44, 332–339. [Google Scholar] [CrossRef]
- Evans, J.L.; Goldfine, I.D.; Maddux, B.A.; Grodsky, G.M. Oxidative Stress and Stress-Activated Signaling Pathways: A Unifying Hypothesis of Type 2 Diabetes. Endocr. Rev. 2002, 23, 599–622. [Google Scholar] [CrossRef]
- Maritim, A.C.; Sanders, R.A.; Watkins, J.B., III. Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 2003, 17, 24–38. [Google Scholar] [CrossRef]
- Genet, S.; Kale, R.K.; Baquer, N.Z. Alterations in antioxidant enzymes and oxidative damage in experimental diabetic rat tissues: Effect of vanadate and fenugreek (Trigonella foenum graecum). Mol. Cell. Biochem. 2002, 236, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Kurt, O.; Ozden, T.Y.; Ozsoy, N.; Tunali, S.; Can, A.; Akev, N.; Yanardag, R. Influence of vanadium supplementation on oxidative stress factors in the muscle of STZ-diabetic rats. BioMetals 2011, 24, 943–949. [Google Scholar] [CrossRef]
- Treviño, S.; Velázquez-Vázquez, D.; Sánchez-Lara, E.; Diaz-Fonseca, A.; Flores-Hernandez, J.Á.; Pérez-Benítez, A.; Brambila-Colombres, E.; González-Vergara, E. Metforminium Decavanadate as a Potential Metallopharmaceutical Drug for the Treatment of Diabetes Mellitus. Oxidative Med. Cell. Longev. 2016, 2016, 6058705. [Google Scholar] [CrossRef]
- Doi, K.; Sawada, F.; Toda, G.; Yamachika, S.; Seto, S.; Urata, Y.; Ihara, Y.; Sakata, N.; Taniguchi, N.; Kondo, T.; et al. Alteration of antioxidants during the progression of heart disease in streptozotocin-induced diabetic rats. Free Radic. Res. 2001, 34, 251–261. [Google Scholar] [CrossRef]
- Xu, Z.; Patel, K.P.; Lou, M.F.; Rozanski, G.J. Up-regulation of K(+) channels in diabetic rat ventricular myocytes by insulin and glutathione. Cardiovasc. Res. 2002, 53, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, X.; Li, Y.L.; Shao, C.H.; Bidasee, K.R.; Rozanski, G.J. Insulin regulation of glutathione and contractile phenotype in diabetic rat ventricular myocytes. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H1619–H1629. [Google Scholar] [CrossRef] [PubMed]
- Desco, M.-C.; Asensi, M.; Márquez, R.; Martínez-Valls, J.; Vento, M.x.; Pallardó, F.V.; Sastre, J.; Viña, J. Xanthine Oxidase Is Involved in Free Radical Production in Type 1 Diabetes: Protection by Allopurinol. Diabetes 2002, 51, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Robertson, R.P.; Harmon, J.; Tran, P.O.; Tanaka, Y.; Takahashi, H. Glucose Toxicity in β-Cells: Type 2 Diabetes, Good Radicals Gone Bad, and the Glutathione Connection. Diabetes 2003, 52, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich-Reiterer, E.E.; Rosenbauer, J.; Bechtold-Dalla Pozza, S.; Hofer, S.E.; Schober, E.; Holl, R.W. Predictors of increasing BMI during the course of diabetes in children and adolescents with type 1 diabetes: Data from the German/Austrian DPV multicentre survey. Arch. Dis. Child. 2014, 99, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Rosenbauer, J.; Dost, A.; Karges, B.; Hungele, A.; Stahl, A.; Bächle, C.; Gerstl, E.M.; Kastendieck, C.; Hofer, S.E.; Holl, R.W. Improved metabolic control in children and adolescents with type 1 diabetes: A trend analysis using prospective multicenter data from Germany and Austria. Diabetes Care 2012, 35, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Schwandt, A.; Hermann, J.M.; Rosenbauer, J.; Boettcher, C.; Dunstheimer, D.; Grulich-Henn, J.; Kuss, O.; Rami-Merhar, B.; Vogel, C.; Holl, R.W. Longitudinal Trajectories of Metabolic Control from Childhood to Young Adulthood in Type 1 Diabetes from a Large German/Austrian Registry: A Group-Based Modeling Approach. Diabetes Care 2017, 40, 309–316. [Google Scholar] [CrossRef]
- Kueh, M.T.W.; Chew, N.W.S.; Al-Ozairi, E.; le Roux, C.W. The emergence of obesity in type 1 diabetes. Int. J. Obes. 2024, 48, 289–301. [Google Scholar] [CrossRef]
- MacRae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef]
- Takeda, N.; Hasegawa, S.; Morita, M.; Matsunaga, T. Pica in rats is analogous to emesis: An animal model in emesis research. Pharmacol. Biochem. Behav. 1993, 45, 817–821. [Google Scholar] [CrossRef] [PubMed]
- Meotti, F.C.; Luiz, A.P.; Pizzolatti, M.G.; Kassuya, C.A.L.; Calixto, J.B.; Santos, A.R.S. Analysis of the Antinociceptive Effect of the Flavonoid Myricitrin: Evidence for a Role of the l-Arginine-Nitric Oxide and Protein Kinase C Pathways. J. Pharmacol. Exp. Ther. 2006, 316, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Santiago, R.M.; Barbieiro, J.; Lima, M.M.; Dombrowski, P.A.; Andreatini, R.; Vital, M.A. Depressive-like behaviors alterations induced by intranigral MPTP, 6-OHDA, LPS and rotenone models of Parkinson’s disease are predominantly associated with serotonin and dopamine. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2010, 34, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, K.; Ramarao, P. Animal models in type 2 diabetes research: An overview. Indian J. Med. Res. 2007, 125, 451–472. [Google Scholar] [PubMed]
- Thulé, P.M.; Liu, J.M. Regulated hepatic insulin gene therapy of STZ-diabetic rats. Gene Ther. 2000, 7, 1744–1752. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Knodell, R.G.; Ishak, K.G.; Black, W.C.; Chen, T.S.; Craig, R.; Kaplowitz, N.; Kiernan, T.W.; Wollman, J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology 1981, 1, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.-A.; Wu, A.-B.; Chen, C.-Y. The influence of different treatments on the free radical scavenging activity of burdock and variations of its active components. Food Chem. 2004, 86, 479–484. [Google Scholar] [CrossRef]
- Urrea-Victoria, V.; Santos, J.; Torres, P.; Chow, F.; Santos, D. Ensaio Antioxidante em Microplaca do Poder de Redução do Ferro (FRAP) para Extratos de Algas; Instituto de Biociências, Universidade de São Paulo: São Paulo, Brazil, 2016. [Google Scholar]
- Jiang, Z.Y.; Woollard, A.C.; Wolff, S.P. Lipid hydroperoxide measurement by oxidation of Fe2+ in the presence of xylenol orange. Comparison with the TBA assay and an iodometric method. Lipids 1991, 26, 853–856. [Google Scholar] [CrossRef] [PubMed]
- Sedlak, J.; Lindsay, R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]


| Parameters | NG | DM | V30 | V100 | INS | V30INS | V100INS | p |
|---|---|---|---|---|---|---|---|---|
| Lipidic profile | ||||||||
| Total Cholesterol, mg/dL | 41.7 ± 9.8 | 65.7 ± 8.3 a | 71.0 ± 30.8 a | 61.8 ± 9.1 a | 52.6 ± 9.6 | 50.7 ± 9.3 | 58.8 ± 10.2 | <0.001 |
| Triglycerides, mg/dL | 99.5 ± 39.7 | 188.8 ± 99.7 | 99.0 ± 46.2 | 84.0 ± 17.2 | 133.0 ± 60.4 | 155.3 ± 97.1 | 123.6 ± 66.9 | 0.118 |
| HDL-C, mg/dL | 34.0 ± 6.6 | 51.1 ± 10.3 a | 46.5 ± 9.4 | 47.5 ± 6.8 a | 42.4 ± 8.6 | 37.8 ± 4.0 | 41.4 ± 7.3 | 0.005 |
| nHDL, mg/dL | 7.7 ± 3.2 | 15.4 ± 4.6 a | 13.4 ± 3.3 | 14.3 ± 6.8 | 10.2 ± 3.1 | 11.2 ± 3.7 | 17.4 ± 5.6 a | 0.010 |
| Kidney function | ||||||||
| Urea, mg/dL | 35.4 ± 3.9 | 53.3 ± 6.8 a | 51.5 ± 7.7 a | 50.8 ± 4.6 a | 56.7 ± 8.6 a | 57.4 ± 4.7 a | 47.4 ± 13.3 | <0.001 |
| Creatinine, mg/dL | 0.12 ± 0.04 | 0.07 ± 0.04 | 0.09 ± 0.10 | 0.11 ± 0.07 | 0.14 ± 0.04 | 0.06 ± 0.04 | 0.13 ± 0.04 | 0.237 |
| Uric acid, mg/dL | 0.4 ± 0.3 | 0.3 ± 0.2 | 0.8 ± 1.0 | 0.8 ± 0.6 | 0.8 ± 0.4 | 0.7 ± 1.0 | 0.6 ± 0.2 | 0.790 |
| Nutritional profile | ||||||||
| Total Protein, g/dL | 6.2 ± 0.6 | 6.1 ± 0.5 | 6.1 ± 0.8 | 5.6 ± 0.7 | 6.2 ± 1.0 | 5.8 ± 0.6 | 6.2 ± 0.6 | 0.703 |
| Albumin, g/dL | 2.8 ± 0.2 | 2.7 ± 0.2 | 2.6 ± 0.1 | 2.4 ± 0.4 | 2.8 ± 0.4 | 2.6 ± 0.3 | 2.7 ± 0.2 | 0.388 |
| Liver function | ||||||||
| AST, U/L | 150 ± 91 | 165 ± 66 | 127 ± 63 | 175 ± 75 | 191 ± 74 | 200 ± 128 | 200 ± 58 | 0.626 |
| ALT, U/L | 71 ± 15 | 74 ± 30 | 78 ± 33 | 90 ± 12 | 82 ± 13 | 82 ± 23 | 85 ± 7 | 0.675 |
| ALP, U/L | 265 ± 62 | 465 ± 29 | 440 ± 111 | 260 ± 60 | 472 ± 189 | 445 ± 273 | 151 ± 60 b | <0.001 |
| LDH, U/L | 1449 ± 677 | 1607 ± 619 | 1131 ± 850 | 1654 ± 481 | 1634 ± 640 | 1496 ± 686 | 1755 ± 593 | 0.687 |
| Pancreatic function | ||||||||
| Amylase, U/L | 478 ± 44 | 277 ± 50 a | 239 ± 92 a | 299 ± 62 a | 389 ± 102 | 400 ± 127 | 452 ± 101 b | <0.001 |
| Oxidative stress Liver | ||||||||
| GSH, µg/g tissue | 1628 ± 750 | 1755 ± 1098 | 1834 ± 1030 | 1927 ± 1196 | 1808 ± 954 | 2533 ± 1446 | 1609 ± 1139 | 0.726 |
| LPO, nmol/mg protein | 8.64 ± 2.4 | 10.5 ± 5.9 | 12.71 ± 8.5 | 6.76 ± 1.4 | 8.05 ± 0.9 | 8.72 ± 5.0 | 8.95 ± 1.8 | 0.345 |
| Tissue protein, mg/mL | 12.8 ± 2.8 | 13.8 ± 1.7 | 12.2 ± 3.4 | 10.7 ± 2.8 | 12.9 ± 1.9 | 11.8 ± 2.1 | 12.3 ± 1.4 | 0.319 |
| Pancreas | ||||||||
| GSH, µg/g tissue | 841 ± 290 | 557 ± 433 | 328 ± 159 | 505 ± 269 | 683 ± 344 | 1417 ± 551 b | 1382 ± 658 b | <0.001 |
| Organ weights | ||||||||
| Liver, g | 3.50 ± 0.3 | 3.79 ± 0.9 | 3.11 ± 0.6 | 3.33 ± 0.4 | 3.60 ± 1.0 | 3.60 ± 0.8 | 3.17 ± 1.1 | 0.643 |
| Kidneys, g | 1.25 ± 0.2 | 1.35 ± 0.2 | 1.39 ± 0.2 | 1.16 ± 0.1 | 1.28 ± 0.1 | 1.34 ± 0.2 | 1.18 ± 0.2 | 0.523 |
| Adrenals, g | 0.10 ± 0.05 | 0.06 ± 0.02 | 0.07 ± 0.06 | 0.16 ± 0.11 | 0.08 ± 0.06 | 0.06 ± 0.04 | 0.09 ± 0.05 | 0.051 |
| Nutritional parameters | ||||||||
| Food intake (g/day) | 26.0 ± 2.7 | 33.9 ± 4.4 a | 34.4 ± 5.4 a | 31.7 ± 3.1 a,b | 30.8 ± 3.4 a,b | 31.1 ± 3.4 a,b | 30.8 ± 3.8 a,b | <0.001 |
| Water (mL/day) | 49.8 ± 5.4 | 119.6 ± 9.5 a | 120.3 ± 9.6 a | 118.7 ± 9.1 a | 103.9 ± 8.3 a,b | 109.0 ± 8.3 a,b | 108.3 ± 9.1 a,b | <0.001 |
| Animals | ||||||||
| weight (g) | 284 ± 18 | 249 ± 28 | 238 ± 32 a | 234 ± 21 a | 247 ± 24 | 238 ± 20 a | 247 ± 29 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, M.S.; Baptistella, G.B.; Nunes, G.G.; Ferreira, M.V.; Cunha, J.M.; Oliveira, K.M.d.; Acco, A.; Lopes, M.L.C.; Couto Alves, A.; Valdameri, G.; et al. A Non-Toxic Binuclear Vanadium(IV) Complex as Insulin Adjuvant Improves the Glycemic Control in Streptozotocin-Induced Diabetic Rats. Pharmaceuticals 2024, 17, 486. https://doi.org/10.3390/ph17040486
Lopes MS, Baptistella GB, Nunes GG, Ferreira MV, Cunha JM, Oliveira KMd, Acco A, Lopes MLC, Couto Alves A, Valdameri G, et al. A Non-Toxic Binuclear Vanadium(IV) Complex as Insulin Adjuvant Improves the Glycemic Control in Streptozotocin-Induced Diabetic Rats. Pharmaceuticals. 2024; 17(4):486. https://doi.org/10.3390/ph17040486
Chicago/Turabian StyleLopes, Mateus S., Gabriel B. Baptistella, Giovana G. Nunes, Matheus V. Ferreira, Joice Maria Cunha, Kauê Marcel de Oliveira, Alexandra Acco, Maria Luiza C. Lopes, Alexessander Couto Alves, Glaucio Valdameri, and et al. 2024. "A Non-Toxic Binuclear Vanadium(IV) Complex as Insulin Adjuvant Improves the Glycemic Control in Streptozotocin-Induced Diabetic Rats" Pharmaceuticals 17, no. 4: 486. https://doi.org/10.3390/ph17040486
APA StyleLopes, M. S., Baptistella, G. B., Nunes, G. G., Ferreira, M. V., Cunha, J. M., Oliveira, K. M. d., Acco, A., Lopes, M. L. C., Couto Alves, A., Valdameri, G., Moure, V. R., Picheth, G., Manica, G. C. M., & Rego, F. G. M. (2024). A Non-Toxic Binuclear Vanadium(IV) Complex as Insulin Adjuvant Improves the Glycemic Control in Streptozotocin-Induced Diabetic Rats. Pharmaceuticals, 17(4), 486. https://doi.org/10.3390/ph17040486