Preventive and Therapeutic Effects of Crocetin in Rats with Heart Failure
Abstract
1. Introduction
2. Results
2.1. Preventive and Therapeutic Effects of CRA on Chronic Heart Failure Caused by Abdominal Aortic Coarctation
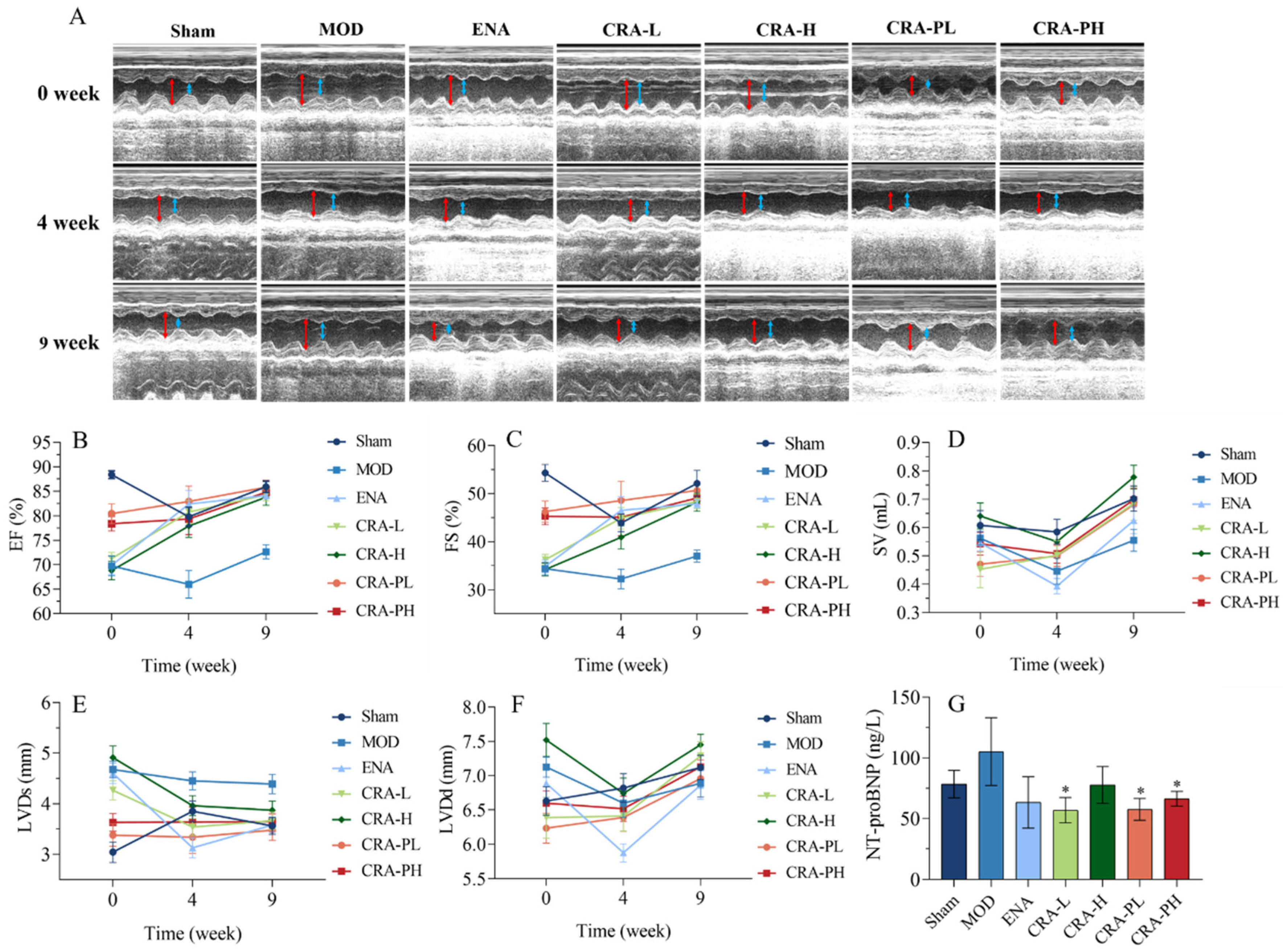
2.1.1. CRA Can Improve Cardiac Function in Rats with Chronic Heart Failure Caused by Abdominal Aortic Coarctation
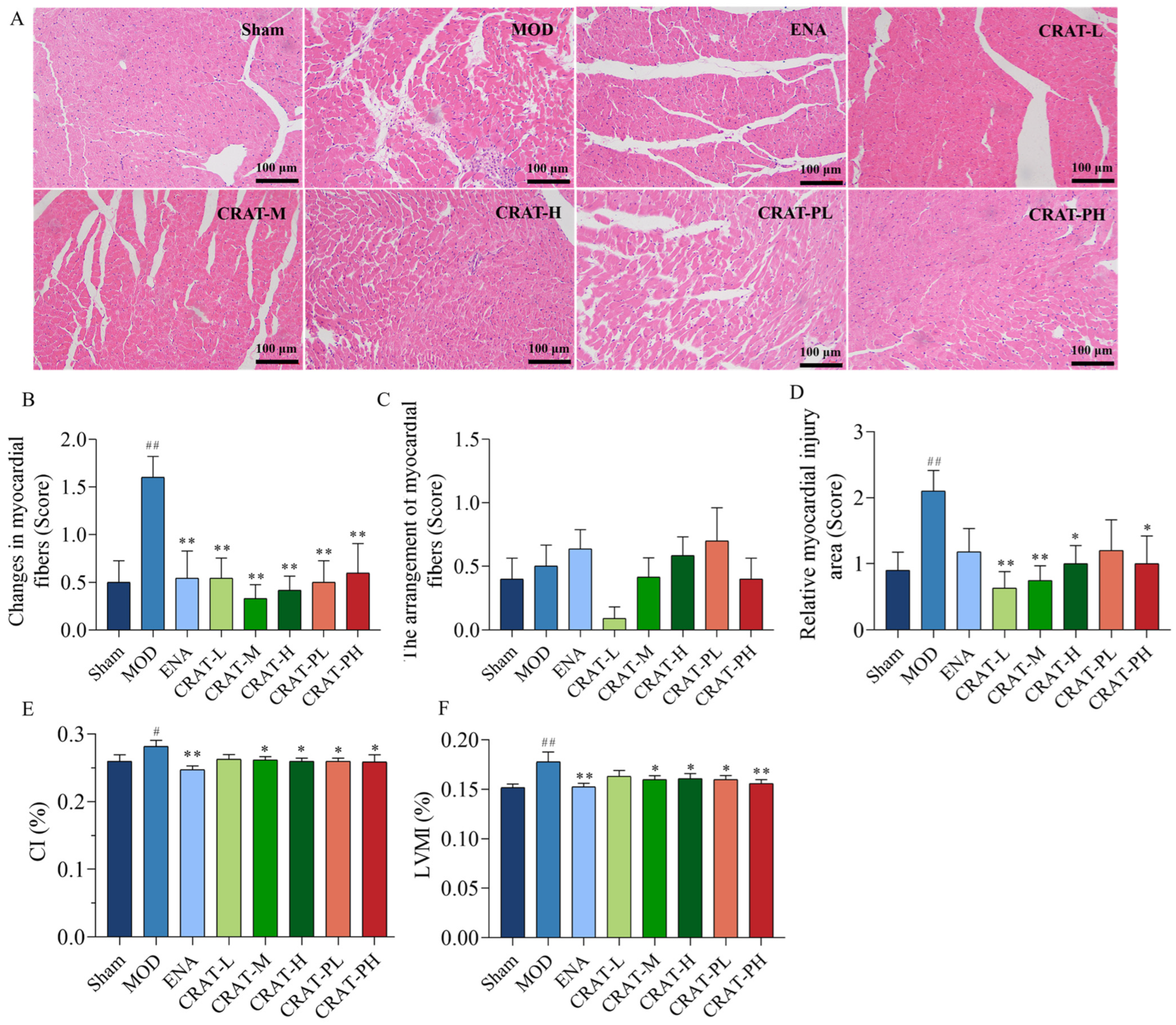
2.1.2. CRA Can Improve Myocardial Hypertrophy in Chronic Heart Failure Rats Caused by Abdominal Aortic Stenosis
2.1.3. CRA Can Reduce Myocardial Fibrosis in Abdominal Aortic Coarctation-Induced Chronic Heart Failure Rats
2.2. Preventive and Therapeutic Effects of CRAT on Heart Failure Rats Caused by Renal Hypertension
2.2.1. CRAT Can Reduce Blood Pressure
2.2.2. CRAT Can Improve Cardiac Function in Heart Failure Rats Caused by Renal Hypertension
2.2.3. CRAT May Reduce the Degree of Heart Failure by Inhibiting the Renin-Angiotensin System and Improving Lipid Metabolism
2.2.4. CRAT Can Improve Myocardial Hypertrophy and Pathological Damage Caused by Heart Failure Caused by Renal Hypertension
2.3. Preventive and Therapeutic Effects of CRAT on Chronic Preserved Heart Failure Rats Induced by Coronary Artery Ligation
2.3.1. CRAT Can Improve Cardiac Function in Heart Failure Rats with Chronic Preserved Heart Failure Caused by Coronary Artery Ligation
2.3.2. CRAT Can Improve Energy Metabolism and Lipid Metabolism
2.3.3. CRAT Can Improve Myocardial Hypertrophy Caused by Chronic Heart Failure Induced by Coronary Artery Ligation
2.3.4. CRAT Can Improve Myocardial Fibrosis and Inflammation in Heart Failure Rats with Retained Chronic Heart Failure Induced by Coronary Artery Ligation
2.3.5. CRAT Can Increase Myocardial Neovascularization
3. Discussion
4. Material and Methods
4.1. Drug and Reagents
4.2. Instruments
4.3. Animals
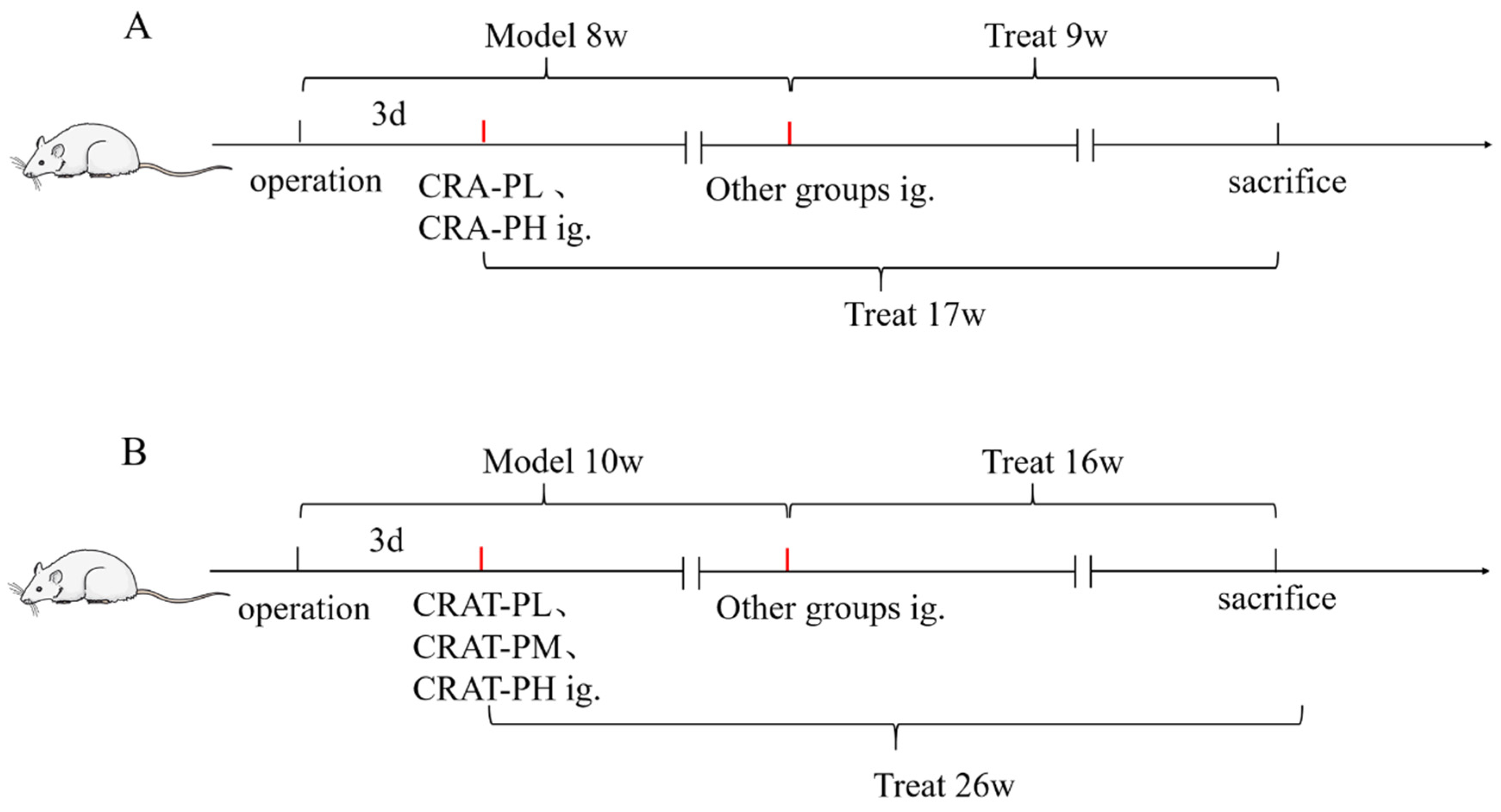
4.4. Study Design
4.4.1. Preserved Chronic Heart Failure Induced by Coarctation of the Abdominal Aorta in Rats
4.4.2. Preserved Chronic Heart Failure Induced by Renal Hypertension in Rats
4.4.3. Preserved Chronic Heart Failure Induced by Coronary Artery Ligation in Rats
4.5. Detection by Echocardiography
4.6. Blood Pressure
4.7. Measurement of Cardiac Hypertrophy
4.8. H&E
4.9. Masson’s Triple Stain
4.10. Immunohistochemical Stain
4.11. Quantification and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rao, Z.; Zhang, F.; Dong, Y.; Wei, Y. Research progress on “quality evaluation through morphological identification” and cause of quality formation in Gardeniae Fructus. Chin. Tradit. Herb. Drugs 2023, 54, 7. [Google Scholar]
- Han, Y.; Wen, J.; Zhou, T.; Fan, G. Chemical fingerprinting of Gardenia jasminoides Ellis by HPLC–DAD–ESI-MS combined with chemometrics methods. Food Chem. 2015, 188, 648–657. [Google Scholar] [CrossRef]
- Li, M.; Tian, X.; Li, X.; Mao, T.; Liu, T. Anti-fatigue activity of gardenia yellow pigment and Cistanche phenylethanol glycosides mixture in hypoxia. Food Biosci. 2021, 40, 100902. [Google Scholar] [CrossRef]
- Yamada, S.; Oshima, H.; Saito, I.; Hayakawa, J. Adoption of crocetin as an indicator compound for detection of gardenia yellow in food products. Food Hyg. Saf. Sci. 1996, 37, 372–377. [Google Scholar] [CrossRef][Green Version]
- Higashino, S.; Sasaki, Y.; Giddings, J.C.; Hyodo, K.; Sakata, S.F.; Matsuda, K.; Horikawa, Y.; Yamamoto, J. Crocetin, a Carotenoid from Gardenia jasminoides Ellis, Protects against Hypertension and Cerebral Thrombogenesis in Stroke-prone Spontaneously Hypertensive Rats. Phytother. Res. 2014, 28, 1315–1319. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ren, J.; Jin, N.; Liu, X.; Li, X. Is Crocin a Potential Anti-tumor Candidate Targeting Microtubules? Computational Insights from Molecular Docking and Dynamics Simulations. Front. Mol. Biosci. 2020, 7, 586970. [Google Scholar]
- Li, S.; Qu, Y.; Shen, X.Y.; Ouyang, T.; Fu, W.B.; Luo, T.; Wang, H.Q. Multiple Signal Pathways Involved in Crocetin-Induced Apoptosis in KYSE-150 Cells. Pharmacology 2019, 103, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Umigai, N.; Tanaka, J.; Tsuruma, K.; Shimazawa, M.; Hara, H. Crocetin, a carotenoid derivative, inhibits VEGF-induced angiogenesis via suppression of p38 phosphorylation. Curr. Neurovascular Res. 2012, 9, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.C.; Lu, Y.; Qian, Z.Y. Effects of crocetin on the matrix metalloproteinases in cardiac hypertrophy induced by norepinephrine in rats. J. Asian Nat. Prod. Res. 2006, 8, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Yi, F.F.; Bian, Z.Y.; Shen, D.F.; Yang, L.; Yan, L.; Tang, Q.Z.; Yang, X.C.; Li, H. Crocetin protects against cardiac hypertrophy by blocking MEK-ERK1/2 signalling pathway. J. Cell. Mol. Med. 2009, 13, 909–925. [Google Scholar] [CrossRef]
- Ochiai, T.; Shimeno, H.; Mishima, K.I.; Iwasaki, K.; Fujiwara, M.; Tanaka, H.; Shoyama, Y.; Toda, A.; Eyanagi, R.; Soeda, S. Protective effects of carotenoids from saffron on neuronal injury in vitro and in vivo. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2007, 1770, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.N.; Park, Y.M.; Jung, H.J.; Lee, J.Y.; Min, B.D.; Park, S.U.; Jung, W.S.; Cho, K.H.; Park, J.H.; Kang, I. Anti-inflammatory effects of crocin and crocetin in rat brain microglial cells. Eur. J. Pharmacol. 2010, 648, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Asdaq SM, B.; Inamdar, M.N. Potential of Crocus sativus (saffron) and its Constituent, Crocin, as Hypolipidemic and Antioxidant in Rats. Appl. Biochem. Biotechnol. 2009, 162, 358–372. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Gao, R.; Song, X.; Li, X.; Zhu, J. Cardio-protective and Anti-atherosclerosis Effect of Crocetin on Vitamin D3 and HFD-induced Atherosclerosis in Rats. J. Oleo Sci. 2021, 70, 1447–1459. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.A.; Abogresha, N.M.; Abdelkader, G.; Hassan, R.; Abdelaziz, E.Z.; Greish, S.M. Antioxidant and Anti-Inflammatory Effects of Crocin Ameliorate Doxorubicin-Induced Nephrotoxicity in Rats. Oxidative Med. Cell. Longev. 2021, 2021, 8841726. [Google Scholar] [CrossRef] [PubMed]
- Bastani, S.; Vahedian, V.; Rashidi, M.; Mir, A.; Mirzaei, S.; Alipourfard, I.; Pouremamali, F.; Nejabati, H.; Kadkhoda, J.; Maroufi, N.F.; et al. An evaluation on potential anti-oxidant and anti-inflammatory effects of Crocin. Biomed. Pharmacother. 2022, 153, 113297. [Google Scholar] [CrossRef] [PubMed]
- Ghadrdoost, B.; Vafaei, A.A.; Rashidy-Pour, A.; Hajisoltani, R.; Bandegi, A.R.; Motamedi, F.; Haghighi, S.; Sameni, H.R.; Pahlvan, S. Protective effects of saffron extract and its active constituent crocin against oxidative stress and spatial learning and memory deficits induced by chronic stress in rats. Eur. J. Pharmacol. 2011, 667, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Yuan, C.; Wang, L.; Chen, R.; Li, X.; Zhang, Y.; Liu, C.; Liu, X.; Liang, W.; Xing, Y.; et al. The Beneficial Effects of Saffron Extract on Potential Oxidative Stress in Cardiovascular Diseases. Oxidative Med. Cell. Longev. 2021, 2021, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mitic, V.T.; Stojanovic, D.R.; Ilic, M.Z.D.; Stojanovic, M.M.; Bojanic, V.V. Cardiac Remodeling Biomarkers as Potential Circulating Markers of Left Ventricular Hypertrophy in Heart Failure with Preserved Ejection Fraction. Tohoku J. Exp. Med. 2020, 250, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Mann, D.L.; Bristow, M.R. Mechanisms and models in heart failure: The biomechanical model and beyond. Circulation 2005, 112, E75. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Newby, L.K.; Arnold, S.V.; Bittner, V.; Brewer, L.C.; Demeter, S.H.; Dixon, D.L.; Fearon, W.F.; Hess, B.; Johnson, H.M.; et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA Guideline for the Management of Patients With Chronic Coronary Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2023, 148, 9–119. [Google Scholar] [CrossRef] [PubMed]
- Koop, A.M.C.; Bossers, G.P.L.; Ploegstra, M.J.; Hagdorn, Q.A.J.; Berger, R.M.F.; Silljé, H.H.W.; Bartelds, B. Metabolic Remodeling in the Pressure-Loaded Right Ventricle: Shifts in Glucose and Fatty Acid Metabolism—A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2019, 8, e012086. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Xu, Q. Role of myocardial energy metabolism therapy in the development of chronic heart failure. Chin. J. Clin. 2024, 52, 15–19. [Google Scholar]
- Zhou, S.; Jiang, Z.Y.; Zhong, G.Q. Mitophagy and heart failure. Chin. J. Pathophysiol. 2020, 36, 924–929+935. [Google Scholar]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef] [PubMed]
- Al-Mohammad, A.; Mant, J. The diagnosis and management of chronic heart failure: Review following the publication of the NICE guidelines. Heart (Br. Card. Soc.) 2011, 97, 411–416. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. DAPA-HF Trial Committees and Investigators, Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. New Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Dai, Y.; Liu, W.; Wang, N.; Wang, Z. Astragaloside IV enhances taxol chemosensitivity of breast cancer via caveolin-1-targeting oxidant damage. J. Cell. Physiol. 2019, 234, 4277–4290. [Google Scholar] [CrossRef] [PubMed]
- Bu, L.; Dai, O.; Zhou, F.; Liu, F.; Chen, J.F.; Peng, C.; Xiong, L. Traditional Chinese medicine formulas, extracts, and compounds promote angiogenesis. Biomed. Pharmacother. 2020, 132, 110855. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Tsenovoy, P.L.; Thompson, E.A.; Falck, J.R.; Touchon, R.; Sodhi, K.; Rezzani, R.; Shapiro, J.I.; Abraham, N.G. Agonists of epoxyeicosatrienoic acids reduce infarct size and ameliorate cardiac dysfunction via activation of HO-1 and Wnt1 canonical pathway. Prostaglandins Other Lipid Mediat. 2015, 116–117, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, K.; Liu, M.; Su, J.; Qin, X.; Wang, X.; Zhang, J.; Li, S.; Fan, G. An herbal preparation ameliorates heart failure with preserved ejection fraction by alleviating microvascular endothelial inflammation and activating NO-cGMP-PKG pathway. Phytomedicine 2021, 91, 153633. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Q.; Li, C.; Lu, L.; Zhang, Q.; Zhu, R.; Wang, W. A Review of Chinese Herbal Medicine for the Treatment of Chronic Heart Failure. Curr. Pharm. Des. 2018, 23, 5115–5124. [Google Scholar] [CrossRef]
- Wang, S.M.; Ye, L.F.; Wang, L.H. Traditional Chinese medicine enhances myocardial metabolism during heart failure. Biomed. Pharmacother. 2022, 146, 112538. [Google Scholar]
- Song, L.; Kang, C.; Sun, Y.; Huang, W.; Liu, W.; Qian, Z. Crocetin Inhibits Lipopolysaccharide-Induced Inflammatory Response in Human Umbilical Vein Endothelial Cells. Cell. Physiol. Biochem. 2016, 40, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Manabe, H.; Wang, Y.; Yoshimura, R.; Cai, Y.; Fitzgerald, M.; Clarke, R.; Lee, K.S. Metabolic Reflow as a Therapy for Ischemic Brain Injury. Acta Neurochir. Suppl. 2011, 110 Pt 2, 87–91. [Google Scholar] [PubMed]
- Chang, G.; Chen, Y.; Zhang, H.; Zhou, W. Trans sodium crocetinate alleviates ischemia/reperfusion-induced myocardial oxidative stress and apoptosis via the SIRT3/FOXO3a/SOD2 signaling pathway. Int. Immunopharmacol. 2019, 71, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.C.; Qian, Z.Y. Effect of crocetin on cardiac hypertrophy induced by overloading pressure in rats. Yao Xue Xue Bao 2004, 39, 172–175. [Google Scholar] [PubMed]
- Gu, D.; Zhou, J. The relationship between peripheral blood soluble ST2, BNP levels, cardiac function, and prognosis in patients with heart failure. Am. J. Transl. Res. 2023, 15, 2878–2884. [Google Scholar] [PubMed]
- Moore, R.L.; Yelamarty, R.V.; Misawa, H.; Scaduto, R.C., Jr.; Pawlush, D.G.; Elensky, M.; Cheung, J.Y. Altered Ca2+ dynamics in single cardiac myocytes from renovascular hypertensive rats. Am. J. Physiol. 1991, 260 Pt 1, C327–C337. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.P.; Jia, Y.Q.; Zhu, B.L. BNP and NT-proBNP as Diagnostic Biomarkers for Cardiac Dysfunction in Both Clinical and Forensic Medicine. Int. J. Mol. Sci. 2019, 20, 1820. [Google Scholar] [CrossRef] [PubMed]
- Radwan, H.; Selem, A.; Ghazal, K. Reply to: N-terminal pro brain natriuretic peptide in coronary artery disease. J. Saudi Heart Assoc. 2015, 27, 225. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Higaki, J.; Aoki, M.; Morishita, R.; Kida, I.; Taniyama, Y.; Tomita, N.; Yamamoto, K.; Moriguchi, A.; Kaneda, Y.; Ogihara, T. In vivo evidence of the importance of cardiac angiotensin-converting enzyme in the pathogenesis of cardiac hypertrophy. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 428–434. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gradman, A.H.; Wilson, J.T. Hypertension and diastolic heart failure. Curr. Cardiol. Rep. 2009, 11, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Brilla, C.G.; Funck, R.C.; Rupp, H. Lisinopril-Mediated Regression of Myocardial Fibrosis in Patients with Hypertensive Heart Disease. Circulation 2000, 102, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
- Gyöngyösi, M.; Winkler, J.; Ramos, I.; Do, Q.T.; Firat, H.; McDonald, K.; González, A.; Thum, T.; Díez, J.; Jaisser, F.; et al. Myocardial fibrosis: Biomedical research from bench to bedside. Eur. J. Heart Fail. 2017, 19, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Demaison, L. Oxidative Stress and Obesity- and Type 2 Diabetes-Induced Heart Failure. Antioxidants 2020, 9, 653. [Google Scholar] [CrossRef]
- Balestrino, M. Role of Creatine in the Heart: Health and Disease. Nutrients 2021, 13, 1215. [Google Scholar] [CrossRef] [PubMed]
- Conrad, N.; Judge, A.; Tran, J.; Mohseni, H.; Hedgecott, D.; Crespillo, A.P.; Allison, M.; Hemingway, H.; Cleland, J.G.; McMurray, J.J.V.; et al. Temporal trends and patterns in heart failure incidence: A population-based study of 4 million individuals. Lancet 2018, 391, 572–580. [Google Scholar] [CrossRef]
- Dunlay, S.M.; Roger, V.L. Understanding the Epidemic of Heart Failure: Past, Present, and Future. Curr. Heart Fail. Rep. 2014, 11, 404–415. [Google Scholar] [CrossRef]
- Nair, N. Epidemiology and pathogenesis of heart failure with preserved ejection fraction. Rev. Cardiovasc. Med. 2020, 21, 531–540. [Google Scholar]
- Shah, K.S.; Xu, H.; Matsouaka, R.A.; Bhatt, D.L.; Heidenreich, P.A.; Hernandez, A.F.; Devore, A.D.; Yancy, C.W.; Fonarow, G.C. Heart Failure With Preserved, Borderline, and Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2017, 70, 2476–2486. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.; Butler, J.; Yang, X.; Xie, P.; Guo, D.; Wei, T.; Yu, J.; Wu, Z.; Gao, Y.; et al. Contemporary Epidemiology, Management, and Outcomes of Patients Hospitalized for Heart Failure in China: Results from the China Heart Failure (China-HF) Registry. J. Card. Fail. 2017, 23, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.; Wang, X.; Chen, Z.; Zhang, L.; Zhang, Y.; Wei, B.; Zheng, C.; Kang, Y.; Jiang, L.; Zhu, Z.; et al. Prevalence of heart failure and left ventricular dysfunction in China: The China Hypertension Survey, 2012–2015. Eur. J. Heart Fail. 2019, 21, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Colapietro, A.; Mancini, A.; D’Alessandro, A.M.; Festuccia, C. Crocetin and Crocin from Saffron in Cancer Chemotherapy and Chemoprevention. Anti-Cancer Agents Med. Chem. 2019, 19, 38–47. [Google Scholar] [CrossRef]
- Hashemi, S.A.; Bathaie, S.Z.; Mohagheghi, M.A. Crocetin and crocin decreased cholesterol and triglyceride content of both breast cancer tumors and cell lines. Avicenna J. Phytomed. 2019, 10, 384–397. [Google Scholar]
- Yang, M.; Mao, G.; Ouyang, L.; Shi, C.; Hu, P.; Huang, S. Crocetin alleviates myocardial ischemia/reperfusion injury by regulating inflammation and the unfolded protein response. Mol. Med. Rep. 2019, 21, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, C.; Ye, T.; Chen, X.; Liu, X.; Chen, X.; Sun, Y.; Qu, C.; Liang, J.; Shi, S.; et al. Pinocembrin ameliorates arrhythmias in rats with chronic ischaemic heart failure. Ann. Med. 2021, 53, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.N.; Lu, P.P.; Yan, S.Y.; Guo, X.T.; Ma, J.; Guo, C.X.; Ma, L.H. Xinfuli granule alleviates metabolic remodeling through inhibition of endoplasmic reticulum stress and mitochondrial injury in heart failure. J. Ethnopharmacol. 2023, 303, 115782. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Yang, S.; Deng, J.; Wu, Q.; Zang, W.J. Amelioration of circadian disruption and calcium-handling protein defects by choline alleviates cardiac remodeling in abdominal aorta coarctation rats. Lab. Investig. 2021, 101, 878–896. [Google Scholar] [CrossRef] [PubMed]
- Kanagala, P.; Cheng, A.S.H.; Singh, A.; Khan, J.N.; Gulsin, G.S.; Patel, P.; Gupta, P.; Arnold, J.R.; Squire, I.B.; Ng, L.L.; et al. Relationship between Focal and Diffuse Fibrosis Assessed by CMR and Clinical Outcomes in Heart Failure with Preserved Ejection Fraction. JACC Cardiovasc. Imaging 2019, 12, 2291–2301. [Google Scholar] [CrossRef] [PubMed]
- Tuleta, I.; Frangogiannis, N.G. Fibrosis of the diabetic heart: Clinical significance, molecular mechanisms, and therapeutic opportunities. Adv. Drug Deliv. Rev. 2021, 176, 113904. [Google Scholar] [CrossRef] [PubMed]
- Su, M.Y.; Lin, L.Y.; Tseng, Y.H.; Chang, C.C.; Wu, C.K.; Lin, J.L.; Tseng, W.Y. CMR-verified diffuse myocardial fibrosis is associated with diastolic dysfunction in HFpEF. JACC Cardiovasc. Imaging 2014, 7, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Riehle, C.; Bauersachs, J. Small animal models of heart failure. Cardiovasc. Res. 2019, 115, 1838–1849. [Google Scholar] [CrossRef] [PubMed]
- Janssen, P.M.L.; Elnakish, M.T. Modeling heart failure in animal models for novel drug discovery and development. Expert Opin. Drug Discov. 2019, 14, 355–363. [Google Scholar] [CrossRef] [PubMed]







| The Extent of Lesion Damage | Score |
|---|---|
| 0–25% | 1 |
| 26–50% | 2 |
| 51–75% | 3 |
| 76–100% | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, R.; Li, S.; Mo, Q.; Chen, X.; Liang, Y.; Hu, T.; Hu, H.; He, B.; Li, R.; Kou, J.; et al. Preventive and Therapeutic Effects of Crocetin in Rats with Heart Failure. Pharmaceuticals 2024, 17, 496. https://doi.org/10.3390/ph17040496
Ma R, Li S, Mo Q, Chen X, Liang Y, Hu T, Hu H, He B, Li R, Kou J, et al. Preventive and Therapeutic Effects of Crocetin in Rats with Heart Failure. Pharmaceuticals. 2024; 17(4):496. https://doi.org/10.3390/ph17040496
Chicago/Turabian StyleMa, Renqiang, Sijia Li, Qingmei Mo, Xiaojuan Chen, Yan Liang, Tao Hu, Hui Hu, Bao He, Renshi Li, Junping Kou, and et al. 2024. "Preventive and Therapeutic Effects of Crocetin in Rats with Heart Failure" Pharmaceuticals 17, no. 4: 496. https://doi.org/10.3390/ph17040496
APA StyleMa, R., Li, S., Mo, Q., Chen, X., Liang, Y., Hu, T., Hu, H., He, B., Li, R., Kou, J., & Yu, B. (2024). Preventive and Therapeutic Effects of Crocetin in Rats with Heart Failure. Pharmaceuticals, 17(4), 496. https://doi.org/10.3390/ph17040496






