Vesicular Drug Delivery Systems: Promising Approaches in Ocular Drug Delivery
Abstract
1. Introduction
2. Ocular Anatomy and Drug Administration
3. Ocular Drug Administration Routes
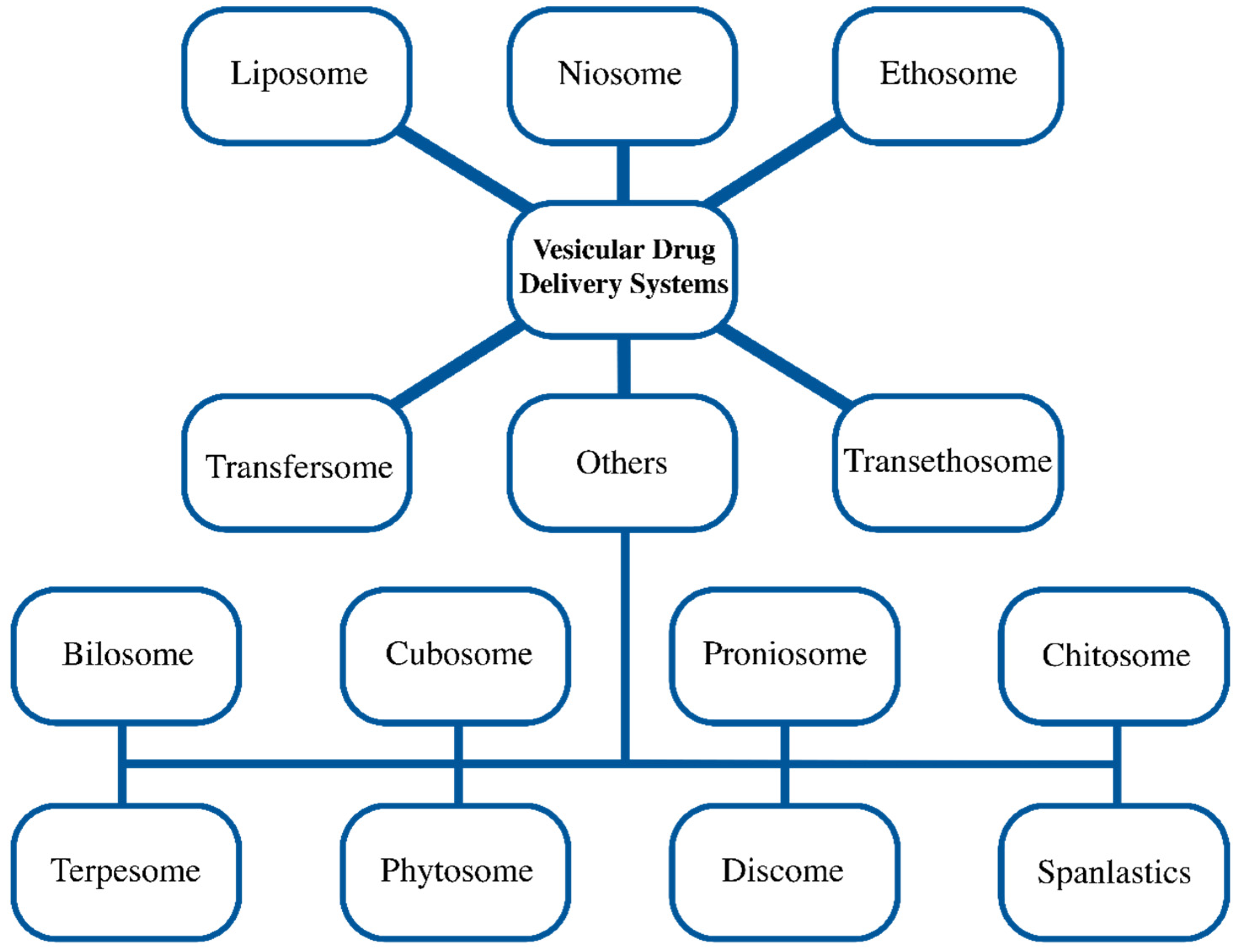
4. Vesicular Systems
4.1. Liposome
4.2. Niosome
4.3. Ethosome/Transethosome
4.4. Transfersome
4.5. Other Vesicular Systems
5. Functionalization of Vesicular Systems
6. Future Perspectives on Scientific and Commercial
- Liposomal commercial products are currently produced for different areas of use (liposomal drugs [135] liposomal cosmeceutical [136], etc.). For this reason, the industry is more familiar with liposome preparation techniques, precautions to be taken against possible problems, and quality control parameters.
- Both academic and industry knowledge of liposome technology facilitates technology transfer between units.
- Since there is less knowledge in other vesicular systems, it is more difficult to start industrial production and monitor the process. Although there are products of other vesicular systems in the cosmeceutical market [136], it takes a certain amount of time and effort to transfer this technology to the pharmaceutical industry.
- On the other hand, although the developed ocular drug delivery systems have achieved successful results in in vitro, ex vivo, and in vivo studies, obtaining ethics committee approvals for clinical studies is not an easy process. It is a long process for other vesicular systems to obtain the necessary permissions, complete the clinical trial processes, obtain regulatory approval, and be launched as a commercial product.
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shafiq, M.; Rafique, M.; Cui, Y.; Pan, L.; Do, C.-W.; Ho, E.A. An Insight on Ophthalmic Drug Delivery Systems: Focus on Polymeric Biomaterials-Based Carriers. J. Control. Release 2023, 362, 446–467. [Google Scholar] [CrossRef]
- Han, H.; Li, S.; Xu, M.; Zhong, Y.; Fan, W.; Xu, J.; Zhou, T.; Ji, J.; Ye, J.; Yao, K. Polymer- and Lipid-Based Nanocarriers for Ocular Drug Delivery: Current Status and Future Perspectives. Adv. Drug Deliv. Rev. 2023, 196, 114770. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Nayak, A.K.; Mallick, S. Lipid-Based Nanocarriers for Ocular Drug Delivery: An Updated Review. J Drug Deliv. Sci. Technol. 2022, 76, 103780. [Google Scholar] [CrossRef]
- Ameeduzzafar, A.; Ali, J.; Fazil, M.; Qumbar, M.; Khan, N.; Ali, A. Colloidal Drug Delivery System: Amplify the Ocular Delivery. Drug Deliv. 2016, 23, 710–726. [Google Scholar] [CrossRef]
- Abdelbary, G.; El-Gendy, N. Niosome-Encapsulated Gentamicin for Ophthalmic Controlled Delivery. AAPS PharmSciTech 2008, 9, 740–747. [Google Scholar] [CrossRef]
- Kaur, I.P.; Garg, A.; Singla, A.K.; Aggarwal, D. Vesicular Systems in Ocular Drug Delivery: An Overview. Int. J. Pharm. 2004, 269, 1–14. [Google Scholar] [CrossRef]
- Mosallam, S.; Albash, R.; Abdelbari, M.A. Advanced Vesicular Systems for Antifungal Drug Delivery. AAPS PharmSciTech 2022, 23, 206. [Google Scholar] [CrossRef]
- Dong, Y.; Dong, P.; Huang, D.; Mei, L.; Xia, Y.; Wang, Z.; Pan, X.; Li, G.; Wu, C. Fabrication and Characterization of Silk Fibroin-Coated Liposomes for Ocular Drug Delivery. Eur. J. Pharm. Biopharm. 2015, 91, 82–90. [Google Scholar] [CrossRef]
- Tan, G.; Yu, S.; Pan, H.; Li, J.; Liu, D.; Yuan, K.; Yang, X.; Pan, W. Bioadhesive Chitosan-Loaded Liposomes: A More Efficient and Higher Permeable Ocular Delivery Platform for Timolol Maleate. Int. J. Biol. Macromol. 2017, 94, 355–363. [Google Scholar] [CrossRef]
- Siafaka, P.I.; Özcan Bülbül, E.; Miliotou, A.N.; Karantas, I.D.; Okur, M.E.; Üstündağ Okur, N. Delivering Active Molecules to the Eye; the Concept of Electrospinning as Potent Tool for Drug Delivery Systems. J. Drug Deliv. Sci. Technol. 2023, 84, 104565. [Google Scholar] [CrossRef]
- Zhang, W.; Prausnitz, M.R.; Edwards, A. Model of Transient Drug Diffusion across Cornea. J. Control. Release 2004, 99, 241–258. [Google Scholar] [CrossRef]
- Cunha-Vaz, J.; Bernardes, R.; Lobo, C. Blood-Retinal Barrier. Eur. J. Ophthalmol. 2011, 21, 3–9. [Google Scholar] [CrossRef]
- Nosrati, H.; Ashrafi, K.; Alizadeh, Z.; Sanami, S.; Banitalebi-Dehkordi, M. Biopolymer-Based Scaffolds for Corneal Stromal Regeneration: A Review. Polym. Med. 2020, 50, 57–64. [Google Scholar] [CrossRef]
- Binkhathlan, Z.; Ali, R.; Alomrani, A.H.; Abul Kalam, M.; Alshamsan, A.; Lavasanifar, A. Role of Polymeric Micelles in Ocular Drug Delivery: An Overview of Decades of Research. Mol. Pharm. 2023, 20, 5359–5382. [Google Scholar] [CrossRef]
- Santos, A.; Altamirano-Vallejo, J.C.; Navarro-Partida, J.; González-De la Rosa, A.; Hsiao, J.H. Breaking down the Barrier: Topical Liposomes as Nanocarriers for Drug Delivery into the Posterior Segment of the Eyeball. Role Nov. Drug Deliv. Veh. Nanobiomed. 2020, 23, 86601. [Google Scholar]
- Summers Rada, J.A.; Shelton, S.; Norton, T.T. The Sclera and Myopia. Exp. Eye Res. 2006, 82, 185–200. [Google Scholar] [CrossRef]
- Nickla, D.L.; Wallman, J. The Multifunctional Choroid. Prog. Retin. Eye Res. 2010, 29, 144–168. [Google Scholar] [CrossRef]
- Yu, D.-Y.; Yu, P.K.; Cringle, S.J.; Kang, M.H.; Su, E.-N. Functional and Morphological Characteristics of the Retinal and Choroidal Vasculature. Prog. Retin. Eye Res. 2014, 40, 53–93. [Google Scholar] [CrossRef]
- Lee, J.; Rhee, Y.-S. Ophthalmic Dosage Forms for Drug Delivery to Posterior Segment. J. Pharm. Investig. 2022, 52, 161–173. [Google Scholar] [CrossRef]
- Wu, K.Y.; Ashkar, S.; Jain, S.; Marchand, M.; Tran, S.D. Breaking Barriers in Eye Treatment: Polymeric Nano-Based Drug-Delivery System for Anterior Segment Diseases and Glaucoma. Polymers 2023, 15, 1373. [Google Scholar] [CrossRef]
- Nagymihály, R.; Nemesh, Y.; Ardan, T.; Motlik, J.; Eidet, J.R.; Moe, M.C.; Bergersen, L.H.; Lytvynchuk, L.; Petrovski, G. The Retinal Pigment Epithelium: At the Forefront of the Blood-Retinal Barrier in Physiology and Disease. In Tissue Barriers in Disease, Injury and Regeneration; Elsevier: Amsterdam, The Netherlands, 2021; pp. 115–146. ISBN 9780128185612. [Google Scholar]
- Varela-Fernández, R.; Díaz-Tomé, V.; Luaces-Rodríguez, A.; Conde-Penedo, A.; García-Otero, X.; Luzardo-álvarez, A.; Fernández-Ferreiro, A.; Otero-Espinar, F.J. Drug Delivery to the Posterior Segment of the Eye: Biopharmaceutic and Pharmacokinetic Considerations. Pharmaceutics 2020, 12, 269. [Google Scholar] [CrossRef]
- del Amo, E.M.; Rimpelä, A.K.; Heikkinen, E.; Kari, O.K.; Ramsay, E.; Lajunen, T.; Schmitt, M.; Pelkonen, L.; Bhattacharya, M.; Richardson, D.; et al. Pharmacokinetic Aspects of Retinal Drug Delivery. Prog. Retin. Eye Res. 2017, 57, 134–185. [Google Scholar] [CrossRef]
- Choonara, Y.E.; Pillay, V.; Danckwerts, M.P.; Carmichael, T.R.; Du Toit, L.C. A Review of Implantable Intravitreal Drug Delivery Technologies for the Treatment of Posterior Segment Eye Diseases. J. Pharm. Sci. 2010, 99, 2219–2239. [Google Scholar] [CrossRef]
- Jiang, P.; Chaparro, F.J.; Cuddington, C.T.; Palmer, A.F.; Ohr, M.P.; Lannutti, J.J.; Swindle-Reilly, K.E. Injectable Biodegradable Bi-Layered Capsule for Sustained Delivery of Bevacizumab in Treating Wet Age-Related Macular Degeneration. J. Control. Release 2020, 320, 442–456. [Google Scholar] [CrossRef]
- Ryu, M.; Nakazawa, T.; Akagi, T.; Tanaka, T.; Watanabe, R.; Yasuda, M.; Himori, N.; Maruyama, K.; Yamashita, T.; Abe, T.; et al. Suppression of Phagocytic Cells in Retinal Disorders Using Amphiphilic Poly(γ-Glutamic Acid) Nanoparticles Containing Dexamethasone. J. Control. Release 2011, 151, 65–73. [Google Scholar] [CrossRef]
- Tan, G.; Liu, D.; Zhu, R.; Pan, H.; Li, J.; Pan, W. A Core-Shell Nanoplatform as a Nonviral Vector for Targeted Delivery of Genes to the Retina. Acta Biomater. 2021, 134, 605–620. [Google Scholar] [CrossRef]
- Bisht, R.; Jaiswal, J.K.; Chen, Y.-S.; Jin, J.; Rupenthal, I.D. Light-Responsive in Situ Forming Injectable Implants for Effective Drug Delivery to the Posterior Segment of the Eye. Expert Opin. Drug Deliv. 2016, 13, 953–962. [Google Scholar] [CrossRef]
- Naftali Ben Haim, L.; Moisseiev, E. Drug Delivery via the Suprachoroidal Space for the Treatment of Retinal Diseases. Pharmaceutics 2021, 13, 967. [Google Scholar] [CrossRef]
- Rai, U.D.J.P.; Young, S.A.; Thrimawithana, T.R.; Abdelkader, H.; Alani, A.W.G.; Pierscionek, B.; Alany, R.G. The Suprachoroidal Pathway: A New Drug Delivery Route to the Back of the Eye. Drug Discov. Today 2015, 20, 491–495. [Google Scholar] [CrossRef]
- Hancock, S.E.; Wan, C.R.; Fisher, N.E.; Andino, R.V.; Ciulla, T.A. Biomechanics of Suprachoroidal Drug Delivery: From Benchtop to Clinical Investigation in Ocular Therapies. Expert Opin. Drug Deliv. 2021, 18, 777–788. [Google Scholar] [CrossRef]
- Luo, L.J.; Lin, T.Y.; Yao, C.H.; Kuo, P.Y.; Matsusaki, M.; Harroun, S.G.; Huang, C.C.; Lai, J.Y. Dual-Functional Gelatin-Capped Silver Nanoparticles for Antibacterial and Antiangiogenic Treatment of Bacterial Keratitis. J. Colloid Interface Sci. 2019, 536, 112–126. [Google Scholar] [CrossRef]
- Kompella, U.B.; Hartman, R.R.; Patil, M.A. Extraocular, Periocular, and Intraocular Routes for Sustained Drug Delivery for Glaucoma. Prog. Retin. Eye Res. 2021, 82, 100901. [Google Scholar] [CrossRef]
- Awwad, S.; Henein, C.; Ibeanu, N.; Khaw, P.T.; Brocchini, S. Preclinical Challenges for Developing Long Acting Intravitreal Medicines. Eur. J. Pharm. Biopharm. 2020, 153, 130–149. [Google Scholar] [CrossRef]
- Liu, L.C.; Chen, Y.H.; Lu, D.W. Overview of Recent Advances in Nano-Based Ocular Drug Delivery. Int. J. Mol. Sci. 2023, 24, 15352. [Google Scholar] [CrossRef]
- Sabur, A.; Asad, M.; Ali, N. Lipid Based Delivery and Immuno-Stimulatory Systems: Master Tools to Combat Leishmaniasis. Cell Immunol. 2016, 309, 55–60. [Google Scholar] [CrossRef]
- Kapoor, B.; Gupta, R.; Gulati, M.; Singh, S.K.; Khursheed, R.; Gupta, M. The Why, Where, Who, How, and What of the Vesicular Delivery Systems. Adv. Colloid Interface Sci. 2019, 271, 101985. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, Preparation, and Applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Li, Q.; Weng, J.; Wong, S.N.; Thomas Lee, W.Y.; Chow, S.F. Nanoparticulate Drug Delivery to the Retina. Mol. Pharm. 2021, 18, 506–521. [Google Scholar] [CrossRef]
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth Liposomes: Review of the Basic Science, Rationale, and Clinical Applications, Existing and Potential. Int. J. Nanomed. 2006, 1, 297–315. [Google Scholar]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of Univalent Ions across the Lamellae of Swollen Phospholipids. J. Mol. Biol. 1965, 13, 238-IN27. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Samanta, A.; Mondal, S.; Roy, D.; Nayak, A.K. Design and Release Kinetics of Liposomes Containing Abiraterone Acetate for Treatment of Prostate Cancer. Sens. Int. 2021, 2, 100077. [Google Scholar] [CrossRef]
- Meure, L.A.; Foster, N.R.; Dehghani, F. Conventional and Dense Gas Techniques for the Production of Liposomes: A Review. AAPS PharmSciTech 2008, 9, 798–809. [Google Scholar] [CrossRef]
- Agarwal, R.; Iezhitsa, I.; Agarwal, P.; Abdul Nasir, N.A.; Razali, N.; Alyautdin, R.; Ismail, N.M. Liposomes in Topical Ophthalmic Drug Delivery: An Update. Drug Deliv. 2016, 23, 1075–1091. [Google Scholar] [CrossRef]
- López-Cano, J.J.; González-Cela-Casamayor, M.A.; Andrés-Guerrero, V.; Herrero-Vanrell, R.; Molina-Martínez, I.T. Liposomes as Vehicles for Topical Ophthalmic Drug Delivery and Ocular Surface Protection. Expert Opin. Drug Deliv. 2021, 18, 819–847. [Google Scholar] [CrossRef]
- Lajunen, T.; Nurmi, R.; Kontturi, L.; Viitala, L.; Yliperttula, M.; Murtomäki, L.; Urtti, A. Light Activated Liposomes: Functionality and Prospects in Ocular Drug Delivery. J. Control. Release 2016, 244, 157–166. [Google Scholar] [CrossRef]
- Qiao, H.; Xu, Z.; Sun, M.; Fu, S.; Zhao, F.; Wang, D.; He, Z.; Zhai, Y.; Sun, J. Rebamipide Liposome as an Effective Ocular Delivery System for the Management of Dry Eye Disease. J. Drug Deliv. Sci. Technol. 2022, 75, 103654. [Google Scholar] [CrossRef]
- Campardelli, R.; Trucillo, P.; Reverchon, E. Supercritical Assisted Process for the Efficient Production of Liposomes Containing Antibiotics for Ocular Delivery. J. CO2 Util. 2018, 25, 235–241. [Google Scholar] [CrossRef]
- Moustafa, M.A.; Elnaggar, Y.S.R.; El-Refaie, W.M.; Abdallah, O.Y. Hyalugel-Integrated Liposomes as a Novel Ocular Nanosized Delivery System of Fluconazole with Promising Prolonged Effect. Int. J. Pharm. 2017, 534, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Karumanchi, D.K.; Skrypai, Y.; Thomas, A.; Gaillard, E.R. Rational Design of Liposomes for Sustained Release Drug Delivery of Bevacizumab to Treat Ocular Angiogenesis. J. Drug Deliv. Sci. Technol. 2018, 47, 275–282. [Google Scholar] [CrossRef]
- Chetoni, P.; Monti, D.; Tampucci, S.; Matteoli, B.; Ceccherini-Nelli, L.; Subissi, A.; Burgalassi, S. Liposomes as a Potential Ocular Delivery System of Distamycin A. Int. J. Pharm. 2015, 492, 120–126. [Google Scholar] [CrossRef]
- Zorzi, G.K.; Schuh, R.S.; Maschio, V.J.; Brazil, N.T.; Rott, M.B.; Teixeira, H.F. Box Behnken Design of SiRNA-Loaded Liposomes for the Treatment of a Murine Model of Ocular Keratitis Caused by Acanthamoeba. Colloids Surf. B Biointerfaces 2019, 173, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Chen, P.; Liu, X.; Lian, Y.; Xi, J.; Li, J.; Song, J.; Li, X. Trimethylated Chitosan-Coated Flexible Liposomes with Resveratrol for Topical Drug Delivery to Reduce Blue-Light-Induced Retinal Damage. Int. J. Biol. Macromol. 2023, 252, 126480. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tian, Q.; Sun, H.; Zhang, Y.; Yang, X.; Kaur, P.; Wang, R.; Fang, Y.; Yan, H.; Du, X.; et al. A Novel, Liposome-Loaded, Injectable Hydrogel for Enhanced Treatment of Choroidal Neovascularization by Sub-Tenon’s Injection. Mater. Today Nano 2022, 20, 100264. [Google Scholar] [CrossRef]
- Li, J.; Cheng, T.; Tian, Q.; Cheng, Y.; Zhao, L.; Zhang, X.; Qu, Y. A More Efficient Ocular Delivery System of Triamcinolone Acetonide as Eye Drop to the Posterior Segment of the Eye. Drug Deliv. 2019, 26, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Zhang, T.; Wu, Y.; Wang, X.; Liu, R.; Jin, X. MPEG-CS-Modified Flexible Liposomes-Reinforced Thermosensitive Sol-Gel Reversible Hydrogels for Ocular Delivery of Multiple Drugs with Enhanced Synergism. Colloids Surf. B Biointerfaces 2023, 231, 113560. [Google Scholar] [CrossRef] [PubMed]
- Shukr, M.H.; Ismail, S.; El-Hossary, G.G.; El-Shazly, A.H. Design and Evaluation of Mucoadhesive in Situ Liposomal Gel for Sustained Ocular Delivery of Travoprost Using Two Steps Factorial Design. J. Drug Deliv. Sci. Technol. 2021, 61, 102333. [Google Scholar] [CrossRef]
- Asasutjarit, R.; Managit, C.; Phanaksri, T.; Treesuppharat, W.; Fuongfuchat, A. Formulation Development and in Vitro Evaluation of Transferrin-Conjugated Liposomes as a Carrier of Ganciclovir Targeting the Retina. Int. J. Pharm. 2020, 577, 119084. [Google Scholar] [CrossRef]
- Londhe, V.Y.; Sharma, S. Formulation, Characterization, Optimization and in-Vivo Evaluation of Methazolamide Liposomal in-Situ Gel for Treating Glaucoma. J. Drug Deliv. Sci. Technol. 2022, 67, 102951. [Google Scholar] [CrossRef]
- Sanap, S.N.; Bisen, A.C.; Mishra, A.; Biswas, A.; Agrawal, S.; Yadav, K.S.; Krishna, A.; Chopra, S.; Mugale, M.N.; Bhatta, R.S. QbD Based Antifungal Drug-Loaded Ophthalmic Liposomal Formulation for the Management of Fungal Keratitis: In Vitro, Ex Vivo and in Vivo Pharmacokinetic Studies. J. Drug Deliv. Sci. Technol. 2022, 74, 103517. [Google Scholar] [CrossRef]
- Malakouti-Nejad, M.; Bardania, H.; Aliakbari, F.; Baradaran-Rafii, A.; Elahi, E.; Monti, D.; Morshedi, D. Formulation of Nanoliposome-Encapsulated Bevacizumab (Avastin): Statistical Optimization for Enhanced Drug Encapsulation and Properties Evaluation. Int. J. Pharm. 2020, 590, 119895. [Google Scholar] [CrossRef]
- Duman, G.; Yıldır, İ.; Macit, M.; Genç, E.; Sümer, E.; Kale, S.; Deniz, İ. Development and Evaluation of 3D-Printed Ocular Insert Containing Liposomal Moxifloxacin. J. Drug Deliv. Sci. Technol. 2024, 92, 105353. [Google Scholar] [CrossRef]
- Chen, S.; Hanning, S.; Falconer, J.; Locke, M.; Wen, J. Recent Advances in Non-Ionic Surfactant Vesicles (Niosomes): Fabrication, Characterization, Pharmaceutical and Cosmetic Applications. Eur. J. Pharm. Biopharm. 2019, 144, 18–39. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.T.; Jaafar-Maalej, C.; Charcosset, C.; Fessi, H. Liposome and Niosome Preparation Using a Membrane Contactor for Scale-Up. Colloids Surf. B Biointerfaces 2012, 94, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.K.; Jindal, N. Formulation of Tyloxapol Niosomes for Encapsulation, Stabilization and Dissolution of Anti-Tubercular Drugs. Colloids Surf. B Biointerfaces 2013, 101, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Pando, D.; Gutiérrez, G.; Coca, J.; Pazos, C. Preparation and Characterization of Niosomes Containing Resveratrol. J. Food Eng. 2013, 117, 227–234. [Google Scholar] [CrossRef]
- Waddad, A.Y.; Abbad, S.; Yu, F.; Munyendo, W.L.L.; Wang, J.; Lv, H.; Zhou, J. Formulation, Characterization and Pharmacokinetics of Morin Hydrate Niosomes Prepared from Various Non-Ionic Surfactants. Int. J. Pharm. 2013, 456, 446–458. [Google Scholar] [CrossRef] [PubMed]
- Marianecci, C.; Di Marzio, L.; Rinaldi, F.; Celia, C.; Paolino, D.; Alhaique, F.; Esposito, S.; Carafa, M. Niosomes from 80s to Present: The State of the Art. Adv. Colloid Interface Sci. 2014, 205, 187–206. [Google Scholar] [CrossRef] [PubMed]
- Baldino, L.; Reverchon, E. Continuous Supercritical CO2 Assisted Process for the Production of Nano-Niosomes Loaded with a Second-Generation Antibiotic for Ocular Therapy. J. Supercrit. Fluids 2022, 188, 105673. [Google Scholar] [CrossRef]
- Santo, I.E.; Campardelli, R.; Albuquerque, E.C.; de Melo, S.V.; Della Porta, G.; Reverchon, E. Liposomes Preparation Using a Supercritical Fluid Assisted Continuous Process. Chem. Eng. J. 2014, 249, 153–159. [Google Scholar] [CrossRef]
- Gugleva, V.; Titeva, S.; Rangelov, S.; Momekova, D. Design and in Vitro Evaluation of Doxycycline Hyclate Niosomes as a Potential Ocular Delivery System. Int. J. Pharm. 2019, 567, 118431. [Google Scholar] [CrossRef]
- Abdelkader, H.; Ismail, S.; Kamal, A.; Alany, R.G. Design and Evaluation of Controlled-Release Niosomes and Discomes for Naltrexone Hydrochloride Ocular Delivery. J. Pharm. Sci. 2011, 100, 1833–1846. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, H.; Ismail, S.; Hussein, A.; Wu, Z.; Al-Kassas, R.; Alany, R.G. Conjunctival and Corneal Tolerability Assessment of Ocular Naltrexone Niosomes and Their Ingredients on the Hen’s Egg Chorioallantoic Membrane and Excised Bovine Cornea Models. Int. J. Pharm. 2012, 432, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Alany, R.G.; Rades, T.; Nicoll, J.; Tucker, I.G.; Davies, N.M. W/O Microemulsions for Ocular Delivery: Evaluation of Ocular Irritation and Precorneal Retention. J. Control. Release 2006, 111, 145–152. [Google Scholar] [CrossRef] [PubMed]
- El-Haddad, M.E.; Hussien, A.A.; Saeed, H.M.; Farid, R.M. Down Regulation of Inflammatory Cytokines by the Bioactive Resveratrol-Loaded Chitoniosomes in Induced Ocular Inflammation Model. J. Drug Deliv. Sci. Technol. 2021, 66, 102787. [Google Scholar] [CrossRef]
- Allam, A.; Elsabahy, M.; El Badry, M.; Eleraky, N.E. Betaxolol-loaded Niosomes Integrated within PH-sensitive in Situ Forming Gel for Management of Glaucoma. Int. J. Pharm. 2021, 598, 120380. [Google Scholar] [CrossRef] [PubMed]
- Uner, B.; Ozdemir, S.; Nur Pilevne, S.; Cenk Celebi, A.R. Timolol-Loaded Ethosomes for Ophthalmic Delivery: Reduction of High Intraocular Pressure in Vivo. Int. J. Pharm. 2023, 640, 123021. [Google Scholar] [CrossRef] [PubMed]
- Mbah, C.C.; Builders, P.F.; Attama, A.A. Nanovesicular Carriers as Alternative Drug Delivery Systems: Ethosomes in Focus. Expert Opin. Drug Deliv. 2014, 11, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Pathak, K. Nanosized Ethanolic Vesicles Loaded with Econazole Nitrate for the Treatment of Deep Fungal Infections through Topical Gel Formulation. Nanomedicine 2012, 8, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Sushma, M.V.; Sankaranarayanan, S.A.; Bantal, V.; Pemmaraju, D.B.; Rengan, A.K. Ethosomal Nanoformulations for Combinational Photothermal Therapy of Fungal Keratitis. Adv. Ther. 2023, 6, 2200331. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Alzahrani, M.M.; Sirwi, A.; Alhakamy, N.A. The Antifungal and Ocular Permeation of Ketoconazole from Ophthalmic Formulations Containing Trans-Ethosomes Nanoparticles. Pharmaceutics 2021, 13, 151. [Google Scholar] [CrossRef]
- Rao, Y.; Zheng, F.; Zhang, X.; Gao, J.; Liang, W. In Vitro Percutaneous Permeation and Skin Accumulation of Finasteride Using Vesicular Ethosomal Carriers. AAPS PharmSciTech 2008, 9, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.N.; Mishra, V.; Rawat, A.; Dubey, P.; Mahor, S.; Jain, S.; Chatterji, D.P.; Vyas, S.P. Non-Invasive Vaccine Delivery in Transfersomes, Niosomes and Liposomes: A Comparative Study. Int. J. Pharm. 2005, 293, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.S.; Fang, X.Q.; Wang, L.L.; Zhang, Y.J. Preparation and Quality Assessment of Itraconazole Transfersomes. Int. J. Pharm. 2012, 436, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Mazyed, E.A.; Abdelaziz, A.E. Fabrication of Transgelosomes for Enhancing the Ocular Delivery of Acetazolamide: Statistical Optimization, in Vitro Characterization, and in Vivo Study. Pharmaceutics 2020, 12, 465. [Google Scholar] [CrossRef] [PubMed]
- Janga, K.Y.; Tatke, A.; Dudhipala, N.; Balguri, S.P.; Ibrahim, M.M.; Maria, D.N.; Jablonski, M.M.; Majumdar, S. Gellan Gum Based Sol-to-Gel Transforming System of Natamycin Transfersomes Improves Topical Ocular Delivery. J. Pharmacol. Exp. Ther. 2019, 370, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, A.M.; Salama, A.; Kassem, A.A. Development of Acetazolamide Loaded Bilosomes for Improved Ocular Delivery: Preparation, Characterization and in Vivo Evaluation. J. Drug Deliv. Sci. Technol. 2020, 59, 101910. [Google Scholar] [CrossRef]
- Janga, K.Y.; Tatke, A.; Balguri, S.P.; Lamichanne, S.P.; Ibrahim, M.M.; Maria, D.N.; Jablonski, M.M.; Majumdar, S. Ion-Sensitive in Situ Hydrogels of Natamycin Bilosomes for Enhanced and Prolonged Ocular Pharmacotherapy: In Vitro Permeability, Cytotoxicity and in Vivo Evaluation. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Abdelbary, A.A.; Abd-Elsalam, W.H.; Al-mahallawi, A.M. Fabrication of Novel Ultradeformable Bilosomes for Enhanced Ocular Delivery of Terconazole: In Vitro Characterization, Ex Vivo Permeation and in Vivo Safety Assessment. Int. J. Pharm. 2016, 513, 688–696. [Google Scholar] [CrossRef]
- Said, M.; Aboelwafa, A.A.; Elshafeey, A.H.; Elsayed, I. Central Composite Optimization of Ocular Mucoadhesive Cubosomes for Enhanced Bioavailability and Controlled Delivery of Voriconazole. J. Drug Deliv. Sci. Technol. 2021, 61, 102075. [Google Scholar] [CrossRef]
- Teba, H.E.; Khalil, I.A.; El Sorogy, H.M. Novel Cubosome Based System for Ocular Delivery of Acetazolamide. Drug Deliv. 2021, 28, 2177–2186. [Google Scholar] [CrossRef]
- Bessone, C.D.V.; Akhlaghi, S.P.; Tártara, L.I.; Quinteros, D.A.; Loh, W.; Allemandi, D.A. Latanoprost-Loaded Phytantriol Cubosomes for the Treatment of Glaucoma. Eur. J. Pharm. Sci. 2021, 160, 105748. [Google Scholar] [CrossRef] [PubMed]
- El-Emam, G.A.; Girgis, G.N.S.; El Sokkary, M.M.A.; El-Azeem Soliman, O.A.; Abd El Gawad, A.E.G.H. Ocular Inserts of Voriconazole-Loaded Proniosomal Gels: Formulation, Evaluation and Microbiological Studies. Int. J. Nanomed. 2020, 15, 7825–7840. [Google Scholar] [CrossRef] [PubMed]
- Khalil, R.M.; Abdelbary, G.A.; Basha, M.; Awad, G.E.A.; El-Hashemy, H.A. Design and Evaluation of Proniosomes as a Carrier for Ocular Delivery of Lomefloxacin HCl. J. Liposome Res. 2017, 27, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Fouda, N.H.; Abdelrehim, R.T.; Hegazy, D.A.; Habib, B.A. Sustained Ocular Delivery of Dorzolamide-HCL via Proniosomal Gel Formulation: In-Vitro Characterization, Statistical Optimization, and in-Vivo Pharmacodynamic Evaluation in Rabbits. Drug Deliv. 2018, 25, 1340–1349. [Google Scholar] [CrossRef] [PubMed]
- Aboali, F.A.; Habib, D.A.; Elbedaiwy, H.M.; Farid, R.M. Curcumin-Loaded Proniosomal Gel as a Biofreindly Alternative for Treatment of Ocular Inflammation: In-Vitro and in-Vivo Assessment. Int. J. Pharm. 2020, 589, 119835. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.; Alruwaili, N.K.; Imam, S.S.; Alsaidan, O.A.; Alharbi, K.S.; Yasir, M.; Elmowafy, M.; Ansari, M.J.; Salahuddin, M.; Alshehri, S. Formulation of Carteolol Chitosomes for Ocular Delivery: Formulation Optimization, Ex-Vivo Permeation, and Ocular Toxicity Examination. Cutan. Ocul. Toxicol. 2021, 40, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Ameeduzzafar; Alruwaili, N.K.; Imam, S.S.; Alotaibi, N.H.; Alhakamy, N.A.; Alharbi, K.S.; Alshehri, S.; Afzal, M.; Alenezi, S.K.; Bukhari, S.N.A. Formulation of Chitosan Polymeric Vesicles of Ciprofloxacin for Ocular Delivery: Box-Behnken Optimization, In Vitro Characterization, HET-CAM Irritation, and Antimicrobial Assessment. AAPS PharmSciTech 2020, 21, 167. [Google Scholar] [CrossRef] [PubMed]
- Albash, R.; Al-Mahallawi, A.M.; Hassan, M.; Alaa-Eldin, A.A. Development and Optimization of Terpene-Enriched Vesicles (Terpesomes) for Effective Ocular Delivery of Fenticonazole Nitrate: In Vitro Characterization and in Vivo Assessment. Int. J. Nanomed. 2021, 16, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Albash, R.; Abdellatif, M.M.; Hassan, M.; Badawi, N.M. Tailoring Terpesomes and Leciplex for the Effective Ocular Conveyance of Moxifloxacin Hydrochloride (Comparative Assessment): In-Vitro, Ex-Vivo, and in-Vivo Evaluation. Int. J. Nanomed. 2021, 16, 5247–5263. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, H.; Longman, M.R.; Alany, R.G.; Pierscionek, B. Phytosome-Hyaluronic Acid Systems for Ocular Delivery of L-Carnosine. Int. J. Nanomed. 2016, 11, 2815–2827. [Google Scholar] [CrossRef]
- Abdelkader, H.; Wu, Z.; Al-Kassas, R.; Alany, R.G. Niosomes and Discomes for Ocular Delivery of Naltrexone Hydrochloride: Morphological, Rheological, Spreading Properties and Photo-Protective Effects. Int. J. Pharm. 2012, 433, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Abdelbari, M.A.; El-Mancy, S.S.; Elshafeey, A.H.; Abdelbary, A.A. Implementing Spanlastics for Improving the Ocular Delivery of Clotrimazole: In Vitro Characterization, Ex Vivo Permeability, Microbiological Assessment and In Vivo Safety Study. Int. J. Nanomed. 2021, 16, 6249–6261. [Google Scholar] [CrossRef] [PubMed]
- Pandey, N.; Kapoor, D.N.; Singh, S.K.; Sharma, P.; Mohanta, P.; Pandey, N.K.; Singh, S.K.; Sarvi, Y. Development of surfactant-based nanocarrier system for delivery of an antifungal drug. J. Pharm. Res. 2017, 11, 1153–1158. [Google Scholar]
- Aziz, D.; Mohamed, S.; Tayel, S.; Makhlouf, A. Flexosomes as a Promising Nanoplatform for Enhancing Tolnaftate Ocular Delivery: Formulation, In Vitro Characterization, Statistical Optimization, Ex Vivo and Microbial In Vivo Studies. Int. J. Pharm. 2023, 646, 123471. [Google Scholar] [CrossRef] [PubMed]
- Omran, S.; Elnaggar, Y.S.R.; Abdallah, O.Y. Controlled Release, Chitosan-Tethered Luteolin Phytocubosomes; Formulation Optimization to in-Vivo Antiglaucoma and Anti-Inflammatory Ocular Evaluation. Int. J. Biol. Macromol. 2024, 254, 127930. [Google Scholar] [CrossRef] [PubMed]
- Omran, S.; Elnaggar, Y.S.R.; Abdallah, O.Y. Carrageenan Tethered Ion Sensitive Smart Nanogel Containing Oleophytocubosomes for Improved Ocular Luteolin Delivery. Int. J. Pharm. 2023, 646, 123482. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Chen, M.; Xu, P.; Yang, Z. Nanostructured Cubosomes as a Platform for Oral Drug Delivery. Curr. Pharm. Biotechnol. 2015, 16, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Azmi, I.D.M.; Østergaard, J.; Stürup, S.; Gammelgaard, B.; Urtti, A.; Moghimi, S.M.; Yaghmur, A. Cisplatin Encapsulation Generates Morphologically Different Multicompartments in the Internal Nanostructures of Nonlamellar Liquid-Crystalline Self-Assemblies. Langmuir 2018, 34, 6570–6581. [Google Scholar] [CrossRef] [PubMed]
- Garg, G.; Saraf, S.; Saraf, S. Cubosomes: An Overview. Biol. Pharm. Bull. 2007, 30, 350–353. [Google Scholar] [CrossRef]
- Wadhwa, S.; Paliwal, R.; Rai Paliwal, S.; Vyas, S.P. Chitosan and Its Role in Ocular Therapeutics. Mini-Rev. Med. Chem. 2009, 9, 1639–1647. [Google Scholar] [CrossRef]
- Shaveta, S.; Singh, J.; Afzal, M.; Kaur, R.; Imam, S.S.; Alruwaili, N.K.; Alharbi, K.S.; Alotaibi, N.H.; Alshammari, M.S.; Kazmi, I.; et al. Development of Solid Lipid Nanoparticle as Carrier of Pioglitazone for Amplification of Oral Efficacy: Formulation Design Optimization, in-Vitro Characterization and in-Vivo Biological Evaluation. J. Drug Deliv. Sci. Technol. 2020, 57, 101674. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; Feo, V. De Essential Oils and Antifungal Activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Li, Y.; Huang, Y.; Zhou, C.; Lin, J.; Wang, Y.; Cui, F.; Zhou, S.; Jia, M.; Ye, S.; et al. Phytosomes Loaded with Mitomycin C–Soybean Phosphatidylcholine Complex Developed for Drug Delivery. Mol. Pharm. 2013, 10, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Freag, M.S.; Elnaggar, Y.S.; Abdallah, O.Y. Lyophilized Phytosomal Nanocarriers as Platforms for Enhanced Diosmin Delivery: Optimization and Ex Vivo Permeation. Int. J. Nanomed. 2013, 8, 2385. [Google Scholar] [CrossRef]
- Kakkar, S.; Kaur, I.P. Spanlastics—A Novel Nanovesicular Carrier System for Ocular Delivery. Int. J. Pharm. 2011, 413, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Yang, J.; Zhang, H.; Gan, L. Cationized Hyaluronic Acid Coated Spanlastics for Cyclosporine A Ocular Delivery: Prolonged Ocular Retention, Enhanced Corneal Permeation and Improved Tear Production. Int. J. Pharm. 2019, 565, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Klibanov, A.L.; Maruyama, K.; Torchilin, V.P.; Huang, L. Amphipathic Polyethyleneglycols Effectively Prolong the Circulation Time of Liposomes. FEBS Lett. 1990, 268, 235–237. [Google Scholar] [CrossRef]
- Blume, G.; Cevc, G. Molecular Mechanism of the Lipid Vesicle Longevity in Vivo. Biochim. Biophys. Acta (BBA) Biomembr. 1993, 1146, 157–168. [Google Scholar] [CrossRef]
- Farooq, M.A.; Trevaskis, N.L. TPGS Decorated Liposomes as Multifunctional Nano-Delivery Systems. Pharm. Res. 2023, 40, 245–263. [Google Scholar] [CrossRef]
- Godse, R.; Singh, K.; Shrivastava, A.; Shinde, U. Polymeric Nanoparticulate Systems: A Potential Approach for Ocular Drug Delivery. In Nano-Biomaterials for Ophthalmic Drug Delivery; Springer International Publishing: Cham, Switzerland, 2016; pp. 351–387. [Google Scholar]
- Gaudana, R.; Ananthula, H.K.; Parenky, A.; Mitra, A.K. Ocular Drug Delivery. AAPS J. 2010, 12, 348–360. [Google Scholar] [CrossRef]
- Jin, Q.; Li, H.; Jin, Z.; Huang, L.; Wang, F.; Zhou, Y.; Liu, Y.; Jiang, C.; Oswald, J.; Wu, J.; et al. TPGS Modified Nanoliposomes as an Effective Ocular Delivery System to Treat Glaucoma. Int. J. Pharm. 2018, 553, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Durgun, M.E.; Güngör, S.; Özsoy, Y. Micelles: Promising Ocular Drug Carriers for Anterior and Posterior Segment Diseases. J. Ocul. Pharmacol. Ther. 2020, 36, 323–341. [Google Scholar] [CrossRef] [PubMed]
- U.S. National Library of Medicine PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov (accessed on 20 February 2024).
- Macha, S.; Hughes, P.; Mitra, A. Overview of Ocular Drug Delivery. In Ophthalmic Drug Delivery Systems, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2003; pp. 1–12. [Google Scholar]
- NovaLipo. Available online: https://novaxpharma.com/navilipo (accessed on 18 March 2024).
- VisuEVO®. Available online: https://visushop.co.uk/product/visuevo-10ml-bottle/ (accessed on 18 March 2024).
- Eye Logic. Available online: https://www.savant-health.com/products/eye-logic-liposomal-eye-spray (accessed on 18 March 2024).
- Perfect Liposomal Eye Spray. Available online: https://www.mpge.de/index.php/perfect-aqua-plus.html (accessed on 18 March 2024).
- Tears Again. Available online: https://biorevive.com/collections/tearsagain (accessed on 18 March 2024).
- Occuvers Hyaluron. Available online: https://www.innomedis.com/our-products/eye-sprays/ocuvers-spray-hyaluron (accessed on 18 March 2024).
- Occuvers Lipostamin. Available online: https://www.innomedis.com/our-products/eye-sprays/ocuvers-spray-lipostamin (accessed on 18 March 2024).
- Retaine Liposome. Available online: https://www.ocusoft.com/retaine-liposome-spray-15ml-2 (accessed on 18 March 2024).
- FDA Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations. Available online: https://www.accessdata.fda.gov/scripts/cder/ob/search_product.cfm (accessed on 26 August 2020).
- Vaishampayan, P.; Rane, M.M. Herbal Nanocosmecuticals: A Review on Cosmeceutical Innovation. J. Cosmet. Dermatol. 2022, 21, 5464–5483. [Google Scholar] [CrossRef] [PubMed]

| Active Ingredient | Therapeutic Effect | Lipids/Surfactants | Preparation Method | Result | Ref. |
|---|---|---|---|---|---|
| Rebamipide (RBM) | Dry eye | Hydrogenated soybean phospholipids (HSPC) and high purity cholesterol | Remote loading technique | Enhanced retention time at the corneal surface and improved drug penetration. Compared with suspension, Mucosta®, the longer retention time at the cornea allows liposomes to maintain a high concentration in the cornea and aqueous humor for a long time. | [47] |
| Ampicillin and ofloxacin | Stop ocular post-surgery infections | Soybean L-α PC from egg yolk (PC, 60% purity) while the rest was composed of a mixture of similar double-tailored phospholipids | Supercritical Assisted Liposome formation (SuperLip) | Ofloxacin and ampicillin exhibited encapsulation efficiencies of up to 97% and 99%, respectively. Compared to conventional techniques, a new selection of SuperLip process ensures higher encapsulation efficiency. High PC/H2O ratios produced higher EE thanks to longer water droplets flying time in the formation vessel and better lipid coverage. | [48] |
| Fluconazole (FLZ) | Fungal keratitis treatment | Lipoid S100 (PC from soybean)/Tween 80, Transcutol HP, and Caproyl 90 | TFH | Enhanced corneal permeability. Ex vivo cumulative corneal permeation of FLZ after 6 h from HYS7, was 2.99 and 4.18 folds higher than conventional liposomes and FLZ suspension, respectively. In vivo corneal permeation of HYS7 showed sustained effect of FLZ reaching 24 h. | [49] |
| Ibuprofen | Corestenoma induced by cataract removal surgery | Purified soybean lecithin (PC S100, P94%, PC, approximately 70% linoleic acid, 8% lineolic acid, 5% oleic acid, 13% palmitic acid and 4% stearic acid residues) and stearylamine (SA) | Ethanol injection method | More sustained drug release behavior, and the release rates reduced as the SF concentration increased. | [8] |
| Timolol maleate (TML) | Glaucoma | Soybean phosphatidylcholine (SPC) and cholesterol | Ammonium sulfate gradient coupled with pH-gradient method | CH coating enabled sustained retention in the precornea, providing sustained action to increase drug permeability and bioavailability. | [9] |
| Bevacizumab | Ocular angiogenesis | 1,2-dipalmitoyl-sn- glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)- 2000] (ammonium salt) (DPPE-PEG2000) and 1,2-dipalmitoyl-sn-gly- cero-3-phospho-(1′-rac-glycerol) (sodium salt) (DPPG), DOPE, cholesterol/PEG200 | Modified lipid hydration and extrusion methods | Slow release and sustained anti-VEGF activity. | [50] |
| Distamycin A (DA) | Acyclovir-resistant HSV keratitis | Phosphatidylcholine (PC), Cholesterol | REV | Enhanced the bioavailability. The in vivo investigations showed the high bioavailability of DA in tear fluid that at the same time allowed an appreciable uptake of drug into the cornea up to concentration values able to produce the inhibition of viral replication (IC50) and without any evidence of transcorneal permeation. | [51] |
| siRNA | Acanthamoeba keratitis | 1,2-di-(9E-octadecenoyl)-sn-glycero-3- phosphoethanolamine (DOPE) (Lipoid, GER), 1,2-dioleoylsn-glycero-3- trimethylammonium propane (DOTAP) (Lipoid, GER), and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (ammonium salt) (DSPE-PEG) (Lipoid, GER) | TFH | 60% complete regression in corneal damage, without lymphocytic infiltrate. | [52] |
| Resveratrol | Blue-light-induced retinal damage | Cholesterol, Egg yolk phospholipid (EYPC) | Ethanol injection method | Trimethylated chitosan (TMC-coated) liposomes more easily penetrated the fundus than the bare flexible liposomes and aided in the enrichment of TMC-Lipo in the retina. | [53] |
| Sunitinib and Acriflavine | Choroidal neovascularization (CNV) | Lecithin, cholesterol/DSEP-PEG2000 and DSEP-PEG2000-cRGD | Ethanol injection method | Longer retention time in the target area, significant anti-CNV effect. | [54] |
| Triamcinolone acetonide | Macular edema | Soybean (PC), Coumarin6(C6) and cholesterol | Calcium acetate gradient method | High entrapment efficiency, exhibited a sustained release profile, and showed excellent physical stability. | [55] |
| Astragaloside IV (AS-IV) and tetramethylpyrazine (TMP) | AMD | Egg yolk lecithin and cholesterol/Poloxamers (P407 and P188), Propylene glycol, Gelucire44/14 and mPEG-CS | Ethanol injection method | Enhance the ocular bioavailability of AS-IV and TMP, which is the enhanced synergism of well-permeable liposome and slow-releasing hydrogel. | [56] |
| Travoprost (TRAVO) | Glaucoma and ocular hypertension | Soya bean lecithin, Cholesterol/Gellan gum and carbopol 934 | TFH | Non-irritant, higher concentration of TRAVO in aqueous humor. | [57] |
| Ganciclovir (GCV) | Cytomegalovirus (CMV) retinitis | Cholesterol, 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), DSPE-PEG, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[maleimide (polyethylene glycol)-2000] (DSPEPEG-Mal) | REV | In vitro cytotoxicity test showed that formulations were safe for the ARPE-19 cells with percentage cell viability of 80–100% and they could inhibit the expression of CMV glycoprotein B after infection effectively. | [58] |
| Methazolamide (MTA) | Glaucoma | PC, Cholesterol | TFH | Longer precorneal residence time and ability to withstand drug release, better patient acceptance. | [59] |
| Fluconazole (FLZ) | Fungal keratitis | Phospholipon 90G (P-90G) and Cholesterol | TFH | Increased residence time, higher ex vivo permeation, no hemolysis, and ocular irritation was observed in a preclinical study. | [60] |
| Bevacizumab | Ocular angiogenesis | DPPC (1,2-dipalimitoyl-Sn-glycero-3-phosphocholine) and cholesterol | TFH | Improved the therapeutic application, and patient compliance thanks to small size, the ability of penetration through the cellular barrier, and its safety. The results showed the stability of BVZ after encapsulation in the nanoliposome | [61] |
| Moxifloxacin | Bacterial keratitis | Soy lecithin | Probe sonication | Improved particle size and homogeneity, 3D printed ocular inserts have high content uniformity and stability and controlled release. | [62] |
| Delivery Systems | Active Ingredient | Therapeutic Effect | Lipids/Surfactants | Preparation Method | Result | Ref. |
|---|---|---|---|---|---|---|
| Bilosome | Acetazolamide (ACZ) | Glaucoma | Cholesterol/Span 60 | TFH | Improved the bioavailability, reduced systemic absorption, and decreased the necessity for frequent administration, leading to enhanced patient compliance. | [87] |
| Natamycin (NT) | Fungal keratitis and other fungal infections | Cholesterol/Span 60 | TFH | Improved tear/corneal surface contact time and corneal permeability. | [88] | |
| Terconazole | Ocular fungal infections | Cholesterol/Span 60 | Ethanol injection method | Ultradeformable bilosomes (UBs) contain an edge activator that imparts extra elasticity to the vesicles and consequently hypothesized to result in improved corneal permeation. | [89] | |
| Cubosome | Voriconazole | Ocular fungal infections | DL-ά-Monoolein (MO), Pluronic F127 (F127) | Melt dispersion emulsification method | High mucoadhesive properties and enhanced precorneal residence time. | [90] |
| Acetazolamide (ACZ) | Glaucoma | Glyceryl monooleate)(GMO)/Poloxamer 407) (P407) | Emulsification technique | Increased corneal permeability of ACZ. | [91] | |
| Latanoprost | Glaucoma | Phytantriol (3,7,11,15-tetramethyl-1,2,3-hexadecanetriol)/Pluronic F127 | Bottom-up (BU) and top-down (TD) method | Demonstrated slow and sustained in vitro releasing profile of latanoprost. | [92] | |
| Proniosome | Voriconazole | Fungal keratitis | Cholesterol, Span 60 (sorbitan monostearate), Span 80 (sorbitan monooleate) and pluronic F 127 (polox- amer 407) | Coacervation-phase method | Reduce the frequency of dosing intervals and improve patient compliance. | [93] |
| Lomefloxacin HCl | Bacterial conjunctivitis | Cholesterol/Brij 35 (Polyoxyethylene (23) lauryl ether), Brij 72, Brij 98 Span 20, Span 40 Span 60, Tween 40, Tween 60, Tween 80 | Coacervation-phase method | Enhances the retention of the drug maintaining a high local effect in the cornea, control the drug. release and the expected high stability on storage | [94] | |
| Dorzolamide HCI | Glaucoma | L-a-lecithin from soya bean, Span 40, cholesterol | Coacervation-phase method | Higher reduction in IOP, significantly sustaining and increasing Dorz bioavailability compared to Trusopt® eye drops. | [95] | |
| Curcumin | Ocular inflammation | Span 60, and Cholesterol, Lecithin, Tween 80 | Coacervation-phase method | Curcumin is a natural biofreindly alternative to an anti-inflammatory drug with fewer side effects. Selected proniosomal gel showed enhanced permeability 3.22-fold and 1.76-fold higher than curcumin dispersion and its lyophilized form, respectively. | [96] | |
| Chitosome | Carteolol | Glaucoma | Cholesterol, span 60 | TFH | Chitosan-coated carteolol niosome exhibited sustained in vitro drug release and enhanced chitosan permeation than chitosan solution. | [97] |
| Ciprofloxacin | Bacterial conjunctivitis | Cholesterol, span 60 | TFH | Enhanced the ocular retention time. The permeation study showed 1.79-fold enhancements in corneal permeation compared with marketed ciprofloxacin eye drop. HET-CAM study showed 0 scores (no irritation). | [98] | |
| Terpesome | Fenticonazole nitrate | Ocular fungal infection | L-α phosphatidylcho- line (from egg yolk source) | TFH | High ocular retention, the in vivo study showed higher ocular retention of the optimized fenticonazole nitrate-loaded terpesomes relative to the drug suspension. | [99] |
| Moxifloxacin hydrochloride | Bacterial keratitis | L-α phosphatidylcho- line (from egg yolk source) | TFH | Enhanced ocular drug conveyance | [100] | |
| Phytosome | L-carnosine | Relief of dry eye conditions and for promoting healing after cataract and other refractive surgeries | Lipoid s 75 | Solvent evaporation method | Enhanced corneal permeation | [101] |
| Discome | Naltrexone hydrochloride (NTX) | Diabetic keratopathy | Span 60, cholesterol | Reverse-phase evaporation (REV) method | Enhanced corneal uptake of the hydrophilic drug (NTX) and protected the encapsulated NTX from photo-oxidation compared with conventional NTX aqueous solutions. | [102] |
| Spanlastics | Clotrimazole | Ocular fungal infection | Tween 80, Kolliphor RH40 and Pluronic F127 | Ethanol injection method | Optimum corneal permeability and elasticity. | [103] |
| Miconazole nitrate | Ocular fungal infection | Cholesterol, tween 80, span 60 | Ethanol injection method | Enhances the ocular permeability and bioavailability | [104] | |
| Flexosome | Tolnaftate (TOL) | Ocular fungal infection | L-a-phosphatidylcholine from egg yolk and Tween 80 | Ethanol injection method | Showed high encapsulation efficiency, small particle size, and spherical morphology, enhanced corneal permeation and antifungal activity. | [105] |
| Phytocubosome | Luteolin (LU) | Glaucoma and ocular inflammation | Glyceryl monooleate (GMO), Poloxamer 407, Phospholipid S100 (PL) | Hydrotrope technique | CH-coated phytocubosomes possessed improved transcorneal permeation, stronger anti-glaucomal action than uncoated phytocubosome, cubosome and suspension. | [106] |
| Oleophytocubosome | Luteolin (LU) | Glaucoma and ocular inflammation | Glyceryl monooleate (GMO), Poloxamer 407, Phospholipid S100 (PL) | Hydrotrope technique | Carrageenan-based ion-sensitive in situ gel (ISG) loaded with oleophytocubosome increases luteolin solubility and ocular bioavailability | [107] |
| Vesicular System | Commercial Name | Dosage Form | Disease | Company | References |
|---|---|---|---|---|---|
| Liposome | NaviLipo® | Drop | Dry eye syndrome | Novax Pharma | [127] |
| VisuEvo® | Visufarma | [128] | |||
| Eye Logic | Spray | Savant Health | [129] | ||
| Perfect Liposomal | MPG&E | [130] | |||
| Tears Again | BioRevive | [131] | |||
| Occuvers Hyaluron | Innomedis | [132] | |||
| Occuvers Lipostamin | [133] | ||||
| Retaine Liposome | OcuSoft | [134] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batur, E.; Özdemir, S.; Durgun, M.E.; Özsoy, Y. Vesicular Drug Delivery Systems: Promising Approaches in Ocular Drug Delivery. Pharmaceuticals 2024, 17, 511. https://doi.org/10.3390/ph17040511
Batur E, Özdemir S, Durgun ME, Özsoy Y. Vesicular Drug Delivery Systems: Promising Approaches in Ocular Drug Delivery. Pharmaceuticals. 2024; 17(4):511. https://doi.org/10.3390/ph17040511
Chicago/Turabian StyleBatur, Eslim, Samet Özdemir, Meltem Ezgi Durgun, and Yıldız Özsoy. 2024. "Vesicular Drug Delivery Systems: Promising Approaches in Ocular Drug Delivery" Pharmaceuticals 17, no. 4: 511. https://doi.org/10.3390/ph17040511
APA StyleBatur, E., Özdemir, S., Durgun, M. E., & Özsoy, Y. (2024). Vesicular Drug Delivery Systems: Promising Approaches in Ocular Drug Delivery. Pharmaceuticals, 17(4), 511. https://doi.org/10.3390/ph17040511







