Effect of GLP-1 Receptor Agonist on Ischemia Reperfusion Injury in Rats with Metabolic Syndrome
Abstract
:1. Introduction
2. Results
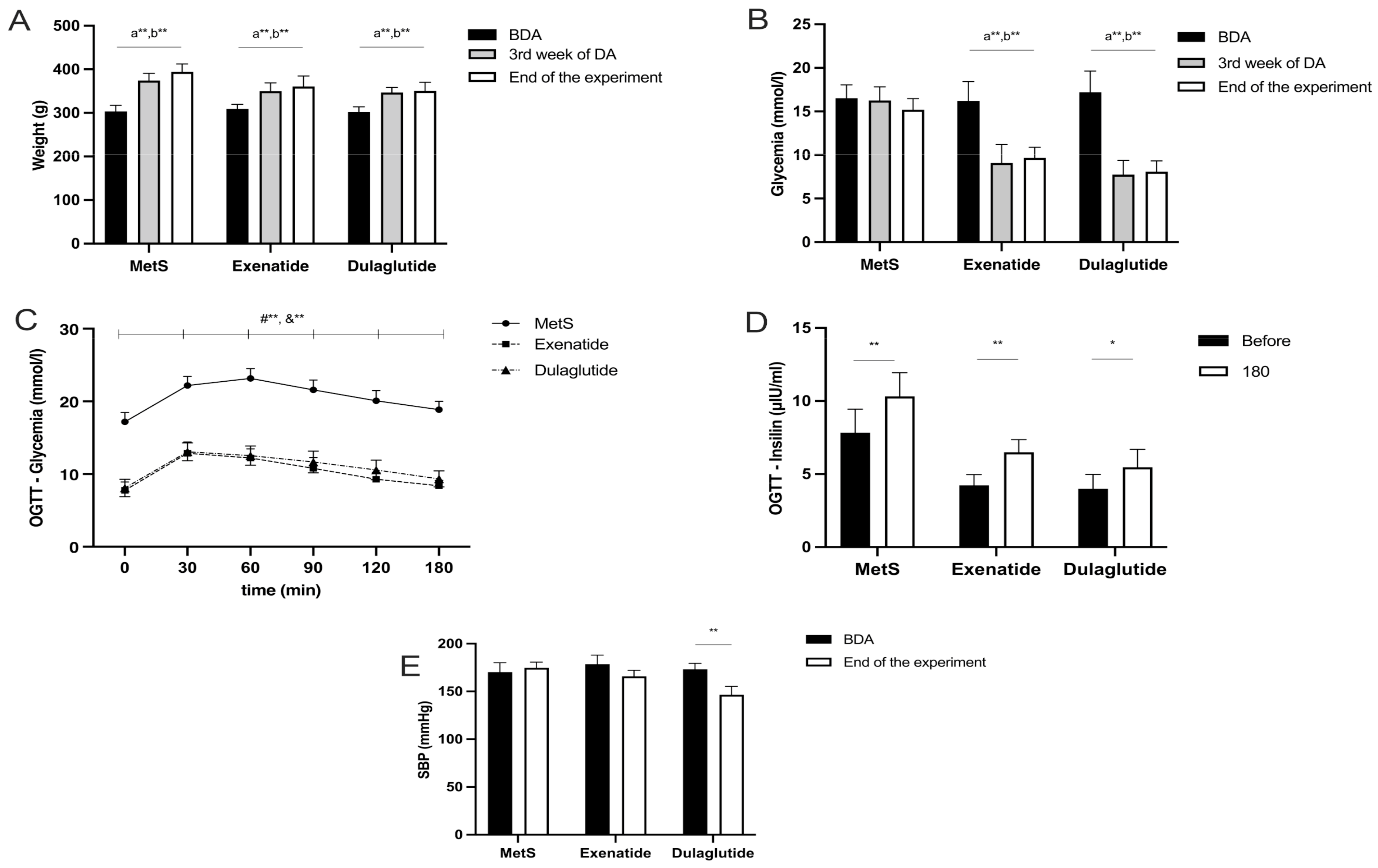
2.1. Effects of GLP-1RAs on Weight, Glycemia, Insulin Levels, and Systolic Blood Pressure
2.2. Effects of GLP-1RA on In Vivo Cardiac Function
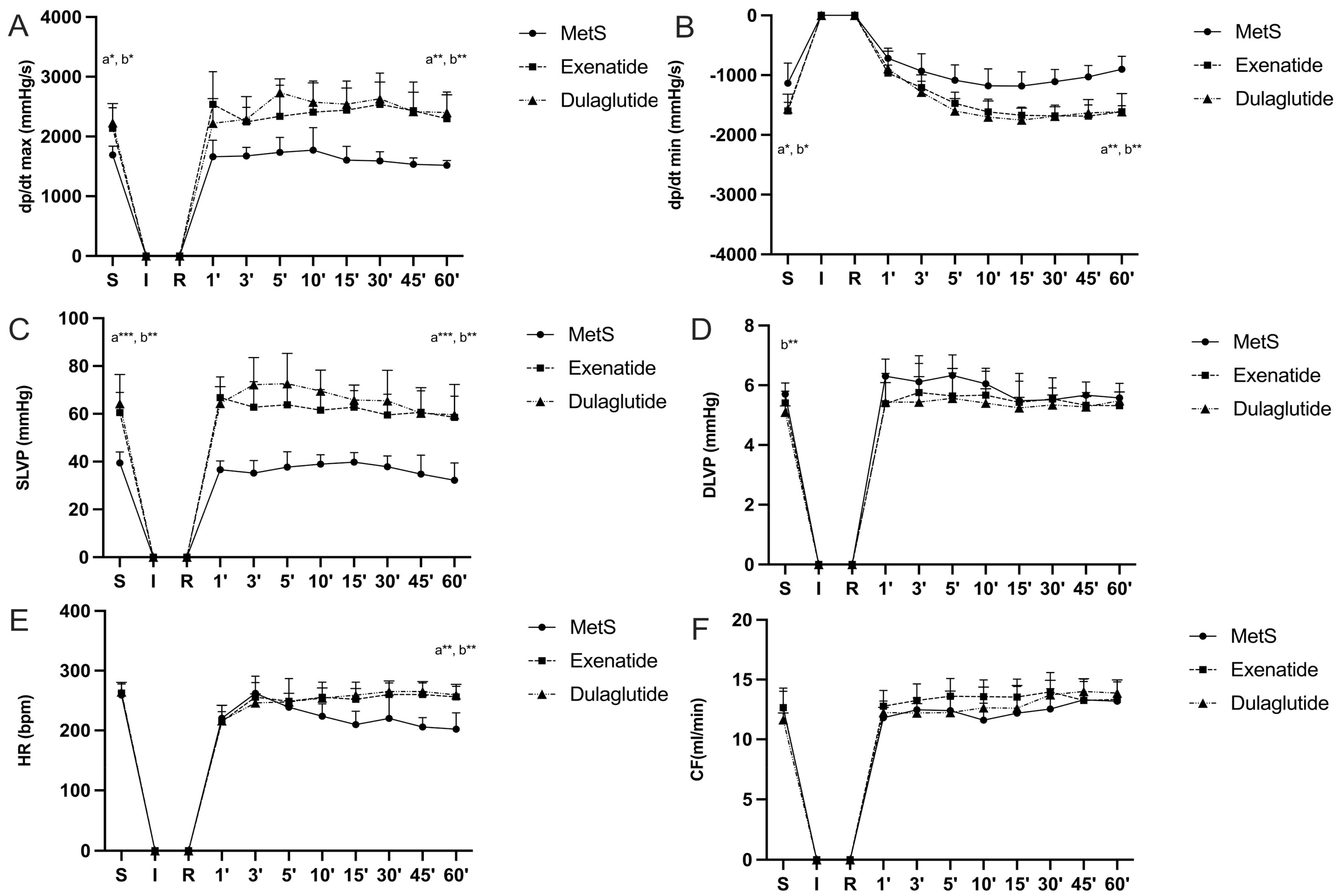
2.3. Effects of GLP-1RAs on Cardiodynamic Parameters
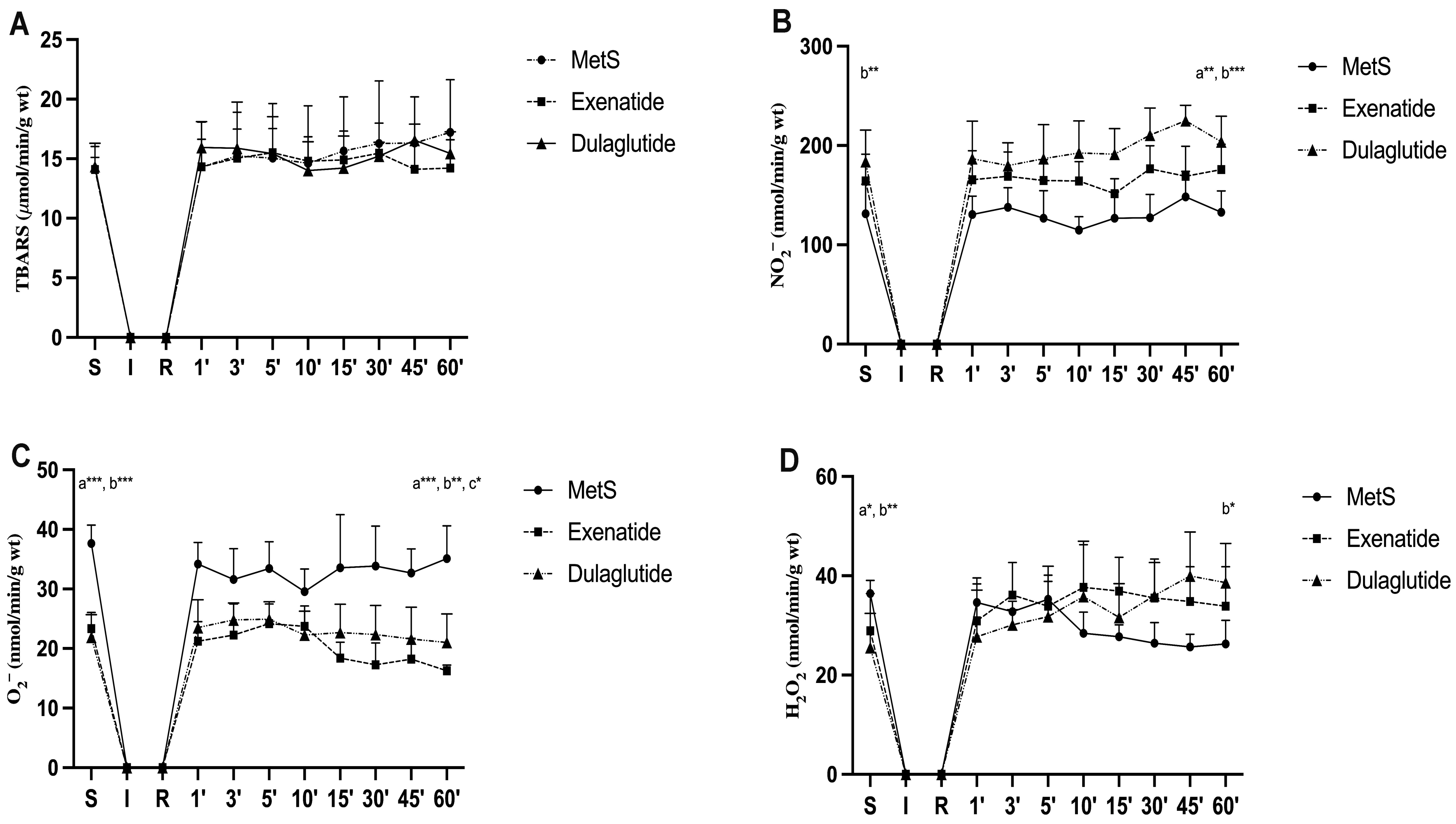
2.4. Effects of GLP-1RAs on Oxidative Stress
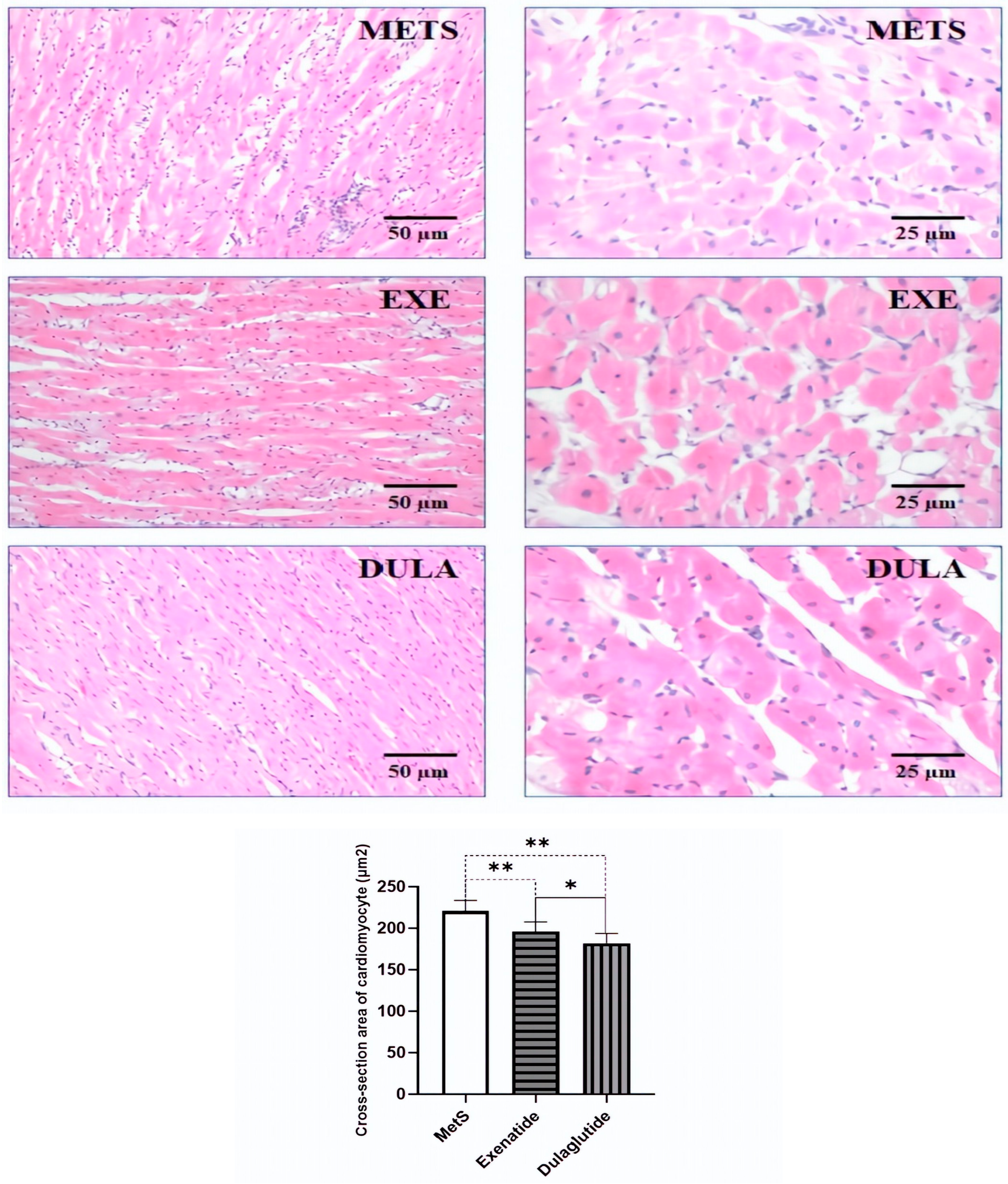
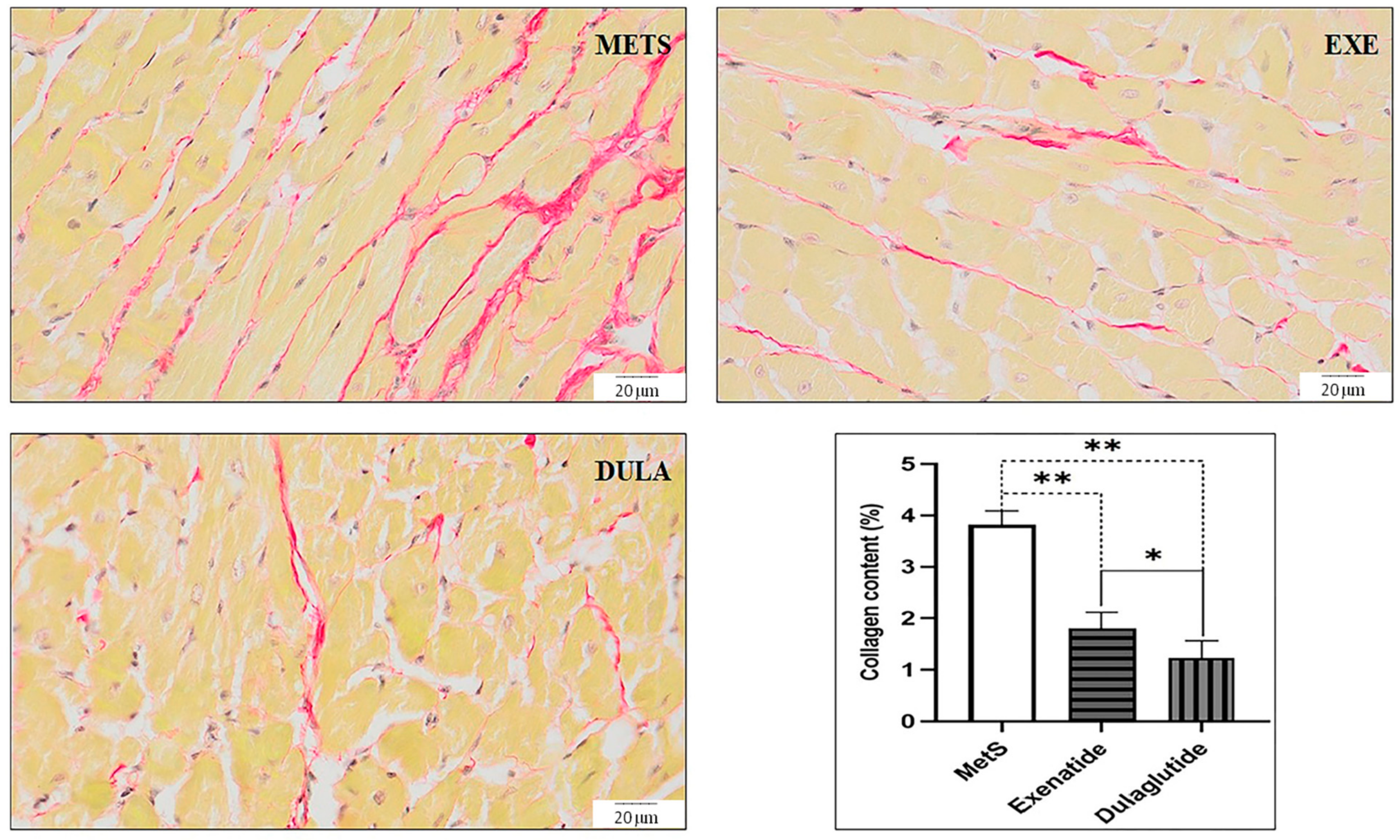
2.5. Effects of GLP-1RAs on Heart Morphology
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Design
- MetS group—control group, rats treated with saline subcutaneously (NaCl 0.9%) in a dose of 0.5 mL per day for 6 weeks;
- Exenatide group—rats treated with exenatide subcutaneously in a dose of 5 µg/kg per day for 6 weeks [65];
- Dulaglutide group—rats treated with dulaglutide subcutaneously in a dose of 0.6 mg/kg/twice a week for 6 weeks [66].
4.2. Physiological Parameters (Weight, Glycemia, and Insulin Values during the Oral Glucose Tolerance Test
4.3. In Vivo and Ex Vivo Examination of Cardiac Function
- The maximum rate of pressure development in the left ventricle (dp/dt max);
- The minimum rate of pressure development in the left ventricle (dp/dt min);
- The systolic left ventricular pressure (SLVP);
- The diastolic left ventricular pressure (DLVP);
- The heart rate (HR).
4.4. Redox Status
- The index of lipid peroxidation, measured as thiobarbituric acid reactive substances (TBARS);
- The level of nitrite (NO2−);
- The level of superoxide anion radical (O2−);
- The level of hydrogen peroxide (H2O2).
4.4.1. TBARS Determination
4.4.2. Nitrite Determination
4.4.3. Superoxide Anion Radical Determination
4.4.4. Hydrogen Peroxide Determination
4.5. Histological Analysis
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hausenloy, D.J.; Bøtker, H.E.; Ferdinandy, P.; Heusch, G.; Ng, G.A.; Redington, A.; Garcia-Dorado, D. Cardiac Innervation in Acute Myocardial Ischaemia/Reperfusion Injury and Cardioprotection. Cardiovasc. Res. 2019, 115, 1167–1177. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, K.; Khalaji, A.; Behnoush, A.H.; Soleimani, H.; Mehrban, S.; Amirsardari, Z.; Najafi, K.; Fathian Sabet, M.; Hosseini Mohammadi, N.S.; Shojaei, S.; et al. The Association between Metabolic Syndrome and Major Adverse Cardiac and Cerebrovascular Events in Patients with Acute Coronary Syndrome Undergoing Percutaneous Coronary Intervention. Sci. Rep. 2024, 14, 697. [Google Scholar] [CrossRef] [PubMed]
- Dhalla, N.S.; Shah, A.K.; Adameova, A.; Bartekova, M. Role of Oxidative Stress in Cardiac Dysfunction and Subcellular Defects Due to Ischemia-Reperfusion Injury. Biomedicines 2022, 10, 1473. [Google Scholar] [CrossRef] [PubMed]
- Algoet, M.; Janssens, S.; Himmelreich, U.; Gsell, W.; Pusovnik, M.; Van Den Eynde, J.; Oosterlinck, W. Myocardial Ischemia-Reperfusion Injury and the Influence of Inflammation. Trends Cardiovasc. Med. 2023, 33, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Caccioppo, A.; Franchin, L.; Grosso, A.; Angelini, F.; D’Ascenzo, F.; Brizzi, M.F. Ischemia Reperfusion Injury: Mechanisms of Damage/Protection and Novel Strategies for Cardiac Recovery/Regeneration. Int. J. Mol. Sci. 2019, 20, 5024. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.; Steenbergen, C. Ion Transport and Energetics During Cell Death and Protection. Physiology 2008, 23, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, A.; Babiker, F. Discrepancy in Calcium Release from the Sarcoplasmic Reticulum and Intracellular Acidic Stores for the Protection of the Heart against Ischemia/Reperfusion Injury. J. Physiol. Biochem. 2016, 72, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Marin, W.; Marin, D.; Ao, X.; Liu, Y. Mitochondria as a Therapeutic Target for Cardiac Ischemia-reperfusion Injury (Review). Int. J. Mol. Med. 2020, 47, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Marber, M.S.; Yellon, D.M. Hypoxic Preconditioning of Ischaemic Myocardium. Cardiovasc. Res. 1992, 26, 556–557. [Google Scholar] [CrossRef] [PubMed]
- De Miranda, D.C.; De Oliveira Faria, G.; Hermidorff, M.M.; Dos Santos Silva, F.C.; De Assis, L.V.M.; Isoldi, M.C. Pre- and Post-Conditioning of the Heart: An Overview of Cardioprotective Signaling Pathways. Curr. Vasc. Pharmacol. 2021, 19, 499–524. [Google Scholar] [CrossRef] [PubMed]
- Sobot, N.M.; Sobot, T.S.; Jeremic, J.N.; Bolevich, S.B.; Bolevich, S.S.; Mitrovic, S.L.; Fisenko, V.P.; Inic, S.G.; Samanovic, A.D.M.; Rankovic, M.R.; et al. Minocycline as Heart Conditioning Agent in Experimental Type 2 Diabetes Mellitus—An Antibacterial Drug in Heart Protection. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2022, 395, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Rankovic, M.; Jakovljevic, V.; Bradic, J.; Jakovljevic, B.; Zivkovic, V.; Srejovic, I.; Bolevich, S.; Milosavljevic, I.; Jeremic, J.; Ravic, M.; et al. Effects of High Intensity Interval vs. Endurance Training on Cardiac Parameters in Ischemia/Reperfusion of Male Rats: Focus on Oxidative Stress. Front. Physiol. 2021, 12, 534127. [Google Scholar] [CrossRef] [PubMed]
- Gadzieva, L.; Bradic, J.; Milosavljevic, I.; Zivkovic, V.; Srejovic, I.; Jakovljevic, V.; Bolevich, S.; Bolevich, S.; Jeremic, N.; Alisultanovich-Omarov, I.; et al. Creatine Phosphate Administration in Cardiac Ischemia–Reperfusion Injury in Rats: Focus on Differences between Preconditioning, Perconditioning, and Postconditioning Protocols. Can. J. Physiol. Pharmacol. 2022, 100, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Liu, S.; Sun, Y.; Chen, C.; Yang, S.; Lin, M.; Long, J.; Yao, J.; Lin, Y.; Yi, F.; et al. Targeting Oxidative Stress as a Preventive and Therapeutic Approach for Cardiovascular Disease. J. Transl. Med. 2023, 21, 519. [Google Scholar] [CrossRef] [PubMed]
- Caturano, A.; D’Angelo, M.; Mormone, A.; Russo, V.; Mollica, M.P.; Salvatore, T.; Galiero, R.; Rinaldi, L.; Vetrano, E.; Marfella, R.; et al. Oxidative Stress in Type 2 Diabetes: Impacts from Pathogenesis to Lifestyle Modifications. Curr. Issues Mol. Biol. 2023, 45, 6651–6666. [Google Scholar] [CrossRef] [PubMed]
- Bertoccini, L.; Baroni, M.G. GLP-1 Receptor Agonists and SGLT2 Inhibitors for the Treatment of Type 2 Diabetes: New Insights and Opportunities for Cardiovascular Protection. In Diabetes: From Research to Clinical Practice; Islam, M.S., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2020; Volume 1307, pp. 193–212. ISBN 978-3-030-51088-6. [Google Scholar]
- Alharbi, S.H. Anti-Inflammatory Role of Glucagon-like Peptide 1 Receptor Agonists and Its Clinical Implications. Ther. Adv. Endocrinol. 2024, 15, 20420188231222367. [Google Scholar] [CrossRef]
- Ussher, J.R.; Drucker, D.J. Glucagon-like Peptide 1 Receptor Agonists: Cardiovascular Benefits and Mechanisms of Action. Nat. Rev. Cardiol. 2023, 20, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Durak, A.; Turan, B. Liraglutide Provides Cardioprotection through the Recovery of Mitochondrial Dysfunction and Oxidative Stress in Aging Hearts. J. Physiol. Biochem. 2023, 79, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Ismaeil, A.; Babiker, F.; Al-Sabah, S. Discrepancy between the Actions of Glucagon-like Peptide-1 Receptor Ligands in the Protection of the Heart against Ischemia Reperfusion Injury. Pharmaceuticals 2022, 15, 720. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J. GLP-1 Physiology Informs the Pharmacotherapy of Obesity. Mol. Metab. 2022, 57, 101351. [Google Scholar] [CrossRef]
- Drucker, D.J. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef] [PubMed]
- Bednarz, K.; Kowalczyk, K.; Cwynar, M.; Czapla, D.; Czarkowski, W.; Kmita, D.; Nowak, A.; Madej, P. The Role of Glp-1 Receptor Agonists in Insulin Resistance with Concomitant Obesity Treatment in Polycystic Ovary Syndrome. Int. J. Mol. Sci. 2022, 23, 4334. [Google Scholar] [CrossRef] [PubMed]
- Bouzas, C.; Pastor, R.; Garcia, S.; Monserrat-Mesquida, M.; Martínez-González, M.Á.; Salas-Salvadó, J.; Corella, D.; Goday, A.; Martínez, J.A.; Alonso-Gómez, Á.M.; et al. Comparative Effects of Glucagon-like Peptide-1 Receptors Agonists, 4-Dipeptidyl Peptidase Inhibitors, and Metformin on Metabolic Syndrome. Biomed. Pharmacother. 2023, 161, 114561. [Google Scholar] [CrossRef] [PubMed]
- Ferdinand, K.C.; White, W.B.; Calhoun, D.A.; Lonn, E.M.; Sager, P.T.; Brunelle, R.; Jiang, H.H.; Threlkeld, R.J.; Robertson, K.E.; Geiger, M.J. Effects of the Once-Weekly Glucagon-Like Peptide-1 Receptor Agonist Dulaglutide on Ambulatory Blood Pressure and Heart Rate in Patients With Type 2 Diabetes Mellitus. Hypertension 2014, 64, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Ludvik, B.; Frías, J.P.; Tinahones, F.J.; Wainstein, J.; Jiang, H.; Robertson, K.E.; García-Pérez, L.-E.; Woodward, D.B.; Milicevic, Z. Dulaglutide as Add-on Therapy to SGLT2 Inhibitors in Patients with Inadequately Controlled Type 2 Diabetes (AWARD-10): A 24-Week, Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Diabetes Endocrinol. 2018, 6, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; MacConell, L.; Zhuang, D.; Kothare, P.A.; Trautmann, M.; Fineman, M.; Taylor, K. Effects of Once-Weekly Dosing of a Long-Acting Release Formulation of Exenatide on Glucose Control and Body Weight in Subjects with Type 2 Diabetes. Diabetes Care 2007, 30, 1487–1493. [Google Scholar] [CrossRef]
- Berra, C.; Manfrini, R.; Regazzoli, D.; Radaelli, M.G.; Disoteo, O.; Sommese, C.; Fiorina, P.; Ambrosio, G.; Folli, F. Blood Pressure Control in Type 2 Diabetes Mellitus with Arterial Hypertension. The Important Ancillary Role of SGLT2-Inhibitors and GLP1-Receptor Agonists. Pharmacol. Res. 2020, 160, 105052. [Google Scholar] [CrossRef]
- Kim, M.; Platt, M.J.; Shibasaki, T.; Quaggin, S.E.; Backx, P.H.; Seino, S.; Simpson, J.A.; Drucker, D.J. GLP-1 Receptor Activation and Epac2 Link Atrial Natriuretic Peptide Secretion to Control of Blood Pressure. Nat. Med. 2013, 19, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Silva, J.C.; Tavares, C.A.M.; Girardi, A.C.C. The Blood Pressure Lowering Effects of Glucagon-like Peptide-1 Receptor Agonists: A Mini-Review of the Potential Mechanisms. Curr. Opin. Pharmacol. 2023, 69, 102355. [Google Scholar] [CrossRef]
- Ferreira, J.P.; Saraiva, F.; Sharma, A.; Vasques-Nóvoa, F.; Angélico-Gonçalves, A.; Leite, A.R.; Borges-Canha, M.; Carvalho, D.; Packer, M.; Zannad, F.; et al. Glucagon-like Peptide 1 Receptor Agonists in Patients with Type 2 Diabetes with and without Chronic Heart Failure: A Meta-analysis of Randomized Placebo-controlled Outcome Trials. Diabetes Obes. Metab. 2023, 25, 1495–1502. [Google Scholar] [CrossRef]
- Xie, S.; Zhang, M.; Shi, W.; Xing, Y.; Huang, Y.; Fang, W.; Liu, S.; Chen, M.; Zhang, T.; Chen, S.; et al. Long-Term Activation of Glucagon-like Peptide-1 Receptor by Dulaglutide Prevents Diabetic Heart Failure and Metabolic Remodeling in Type 2 Diabetes. JAHA 2022, 11, e026728. [Google Scholar] [CrossRef] [PubMed]
- Durak, A.; Akkus, E.; Canpolat, A.G.; Tuncay, E.; Corapcioglu, D.; Turan, B. Glucagon-like Peptide-1 Receptor Agonist Treatment of High Carbohydrate Intake-induced Metabolic Syndrome Provides Pleiotropic Effects on Cardiac Dysfunction through Alleviations in Electrical and Intracellular Ca 2+ Abnormalities and Mitochondrial Dysfunction. Clin. Exp. Pharmacol. Physiol. 2022, 49, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Ha, S.J.; Woo, J.-S.; Lee, G.-J.; Lee, S.-R.; Kim, J.W.; Park, H.K.; Kim, W. Exenatide Prevents Morphological and Structural Changes of Mitochondria Following Ischaemia-Reperfusion Injury. Heart Lung Circ. 2017, 26, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Timmers, L.; Henriques, J.P.S.; De Kleijn, D.P.V.; DeVries, J.H.; Kemperman, H.; Steendijk, P.; Verlaan, C.W.J.; Kerver, M.; Piek, J.J.; Doevendans, P.A.; et al. Exenatide Reduces Infarct Size and Improves Cardiac Function in a Porcine Model of Ischemia and Reperfusion Injury. J. Am. Coll. Cardiol. 2009, 53, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Sokos, G.G.; Nikolaidis, L.A.; Mankad, S.; Elahi, D.; Shannon, R.P. Glucagon-Like Peptide-1 Infusion Improves Left Ventricular Ejection Fraction and Functional Status in Patients With Chronic Heart Failure. J. Card. Fail. 2006, 12, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Li, Y.; Xu, M.; Zhao, X.; Chen, M. Effects of Dulaglutide on Endothelial Progenitor Cells and Arterial Elasticity in Patients with Type 2 Diabetes Mellitus. Cardiovasc. Diabetol. 2022, 21, 200. [Google Scholar] [CrossRef] [PubMed]
- Tate, M.; Robinson, E.; Green, B.D.; McDermott, B.J.; Grieve, D.J. Exendin-4 Attenuates Adverse Cardiac Remodelling in Streptozocin-Induced Diabetes via Specific Actions on Infiltrating Macrophages. Basic Res. Cardiol. 2016, 111, 1. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.E.; Holst, J.J.; Jeppesen, P.B.; Kissow, H. GLP-1 and Intestinal Diseases. Biomedicines 2021, 9, 383. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, Y.; Ye, Y.; Bajaj, M. Myocardial Protection Against Ischemia-Reperfusion Injury by GLP-1: Molecular Mechanisms. Metab. Syndr. Relat. Disord. 2012, 10, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Ban, K.; Noyan-Ashraf, M.H.; Hoefer, J.; Bolz, S.-S.; Drucker, D.J.; Husain, M. Cardioprotective and Vasodilatory Actions of Glucagon-Like Peptide 1 Receptor Are Mediated Through Both Glucagon-Like Peptide 1 Receptor–Dependent and –Independent Pathways. Circulation 2008, 117, 2340–2350. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, R.T.; Ambery, P.; Jermutus, L.; Murray, A.J. Glucagon and Exenatide Improve Contractile Recovery Following Ischaemia/Reperfusion in the Isolated Perfused Rat Heart. Physiol. Rep. 2023, 11, e15597. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Meier, J.J. GLP-1 Receptor Agonists in the Treatment of Type 2 Diabetes—State-of-the-Art. Mol. Metab. 2021, 46, 101102. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, Y.; Li, Y.; Zhao, X.; Zhou, W.; Loghin, C.; Tham, L.S.; Cui, X.; Cui, Y.; Wang, W. Pharmacokinetics, Pharmacodynamics, and Safety of Dulaglutide After Single or Multiple Doses in Chinese Healthy Subjects and Patients with T2DM: A Randomized, Placebo-Controlled, Phase I Study. Adv. Ther. 2022, 39, 488–503. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.; Zhang, D.; Liu, J.; Zhang, P.; Ye, L.; Lu, K.; Duan, Q.; Zheng, A.; Qin, S. Exenatide Protects against Hypoxia/Reoxygenation-Induced Apoptosis by Improving Mitochondrial Function in H9c2 Cells. Exp. Biol. Med. 2014, 239, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Monji, A.; Mitsui, T.; Bando, Y.K.; Aoyama, M.; Shigeta, T.; Murohara, T. Glucagon-like Peptide-1 Receptor Activation Reverses Cardiac Remodeling via Normalizing Cardiac Steatosis and Oxidative Stress in Type 2 Diabetes. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H295–H304. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Tong, G.; Fan, H.; Zhen, C.; Zeng, L.; Xue, L.; Chen, J.; Sun, Z.; He, P. Exendin-4 Alleviates Myocardial Ischemia Reperfusion Injury by Enhancing Autophagy through Promoting Nuclear Translocation of TFEB. Exp. Cell Res. 2023, 423, 113469. [Google Scholar] [CrossRef] [PubMed]
- Shiraki, A.; Oyama, J.; Komoda, H.; Asaka, M.; Komatsu, A.; Sakuma, M.; Kodama, K.; Sakamoto, Y.; Kotooka, N.; Hirase, T.; et al. The Glucagon-like Peptide 1 Analog Liraglutide Reduces TNF-α-Induced Oxidative Stress and Inflammation in Endothelial Cells. Atherosclerosis 2012, 221, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Bajic, Z.; Sobot, T.; Uletilovic, S.; Mandic-Kovacevic, N.; Cvjetkovic, T.; Malicevic, U.; Djukanovic, D.; Duran, M.; Vesic, N.; Avram, S.; et al. Cardioprotective Effects of Liraglutide Pretreatment on Isoprenaline-Induced Myocardial Injury in Rats. Can. J. Physiol. Pharmacol. 2023, 101, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, L.A.; Mankad, S.; Sokos, G.G.; Miske, G.; Shah, A.; Elahi, D.; Shannon, R.P. Effects of Glucagon-Like Peptide-1 in Patients With Acute Myocardial Infarction and Left Ventricular Dysfunction After Successful Reperfusion. Circulation 2004, 109, 962–965. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, N.; Han, Y.; Xu, J.; Xu, Z. Dulaglutide Alleviates LPS-Induced Injury in Cardiomyocytes. ACS Omega 2021, 6, 8271–8278. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.-D.; Huang, H.-F.; Yang, Q.; Chen, X.-Q. Liraglutide Improves Myocardial Fibrosis after Myocardial Infarction through Inhibition of CTGF by Activating cAMP in Mice. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 4648–4656. [Google Scholar] [CrossRef] [PubMed]
- Banks, T.E.; Rajapaksha, M.; Zhang, L.-H.; Bai, F.; Wang, N.-P.; Zhao, Z.-Q. Suppression of Angiotensin II-Activated NOX4/NADPH Oxidase and Mitochondrial Dysfunction by Preserving Glucagon-like Peptide-1 Attenuates Myocardial Fibrosis and Hypertension. Eur. J. Pharmacol. 2022, 927, 175048. [Google Scholar] [CrossRef] [PubMed]
- Wójcicka, G.; Pradiuch, A.; Fornal, E.; Stachniuk, A.; Korolczuk, A.; Marzec-Kotarska, B.; Nikolaichuk, H.; Czechowska, G.; Kozub, A.; Trzpil, A.; et al. The Effect of Exenatide (a GLP-1 Analogue) and Sitagliptin (a DPP-4 Inhibitor) on Asymmetric Dimethylarginine (ADMA) Metabolism and Selected Biomarkers of Cardiac Fibrosis in Rats with Fructose-Induced Metabolic Syndrome. Biochem. Pharmacol. 2023, 214, 115637. [Google Scholar] [CrossRef] [PubMed]
- Eid, R.A.; Alharbi, S.A.; El-kott, A.F.; Eleawa, S.M.; Zaki, M.S.A.; El-Sayed, F.; Eldeen, M.A.; Aldera, H.; Al-Shudiefat, A.A.-R.S. Exendin-4 Ameliorates Cardiac Remodeling in Experimentally Induced Myocardial Infarction in Rats by Inhibiting PARP1/NF-κB Axis in A SIRT1-Dependent Mechanism. Cardiovasc. Toxicol. 2020, 20, 401–418. [Google Scholar] [CrossRef]
- Wright, E.J.; Hodson, N.W.; Sherratt, M.J.; Kassem, M.; Lewis, A.L.; Wallrapp, C.; Malik, N.; Holt, C.M. Combined MSC and GLP-1 Therapy Modulates Collagen Remodeling and Apoptosis Following Myocardial Infarction. Stem Cells Int. 2016, 2016, 7357096. [Google Scholar] [CrossRef] [PubMed]
- Siraj, M.A.; Mundil, D.; Beca, S.; Momen, A.; Shikatani, E.A.; Afroze, T.; Sun, X.; Liu, Y.; Ghaffari, S.; Lee, W.; et al. Cardioprotective GLP-1 Metabolite Prevents Ischemic Cardiac Injury by Inhibiting Mitochondrial Trifunctional Protein-α. J. Clin. Investig. 2020, 130, 1392–1404. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Mangmool, S.; Parichatikanond, W. Multifaceted Roles of GLP-1 and Its Analogs: A Review on Molecular Mechanisms with a Cardiotherapeutic Perspective. Pharmaceuticals 2023, 16, 836. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, H.E.; Abdelhady, M.A.; Abdel Aal, S.M.; Elrashidy, R.A. Dulaglutide Mitigates High Dietary Fructose-Induced Renal Fibrosis in Rats through Suppressing Epithelial-Mesenchymal Transition Mediated by GSK-3β/TGF-Β1/Smad3 Signaling Pathways. Life Sci. 2022, 309, 120999. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, M.; Kanemoto, S.; Leshnower, B.G.; Albone, E.F.; Hinmon, R.; Plappert, T.; Gorman, J.H.; Gorman, R.C. Single Dose GLP-1-Tf Ameliorates Myocardial Ischemia/Reperfusion Injury. J. Surg. Res. 2011, 165, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Visniauskas, B.; Kilanowski-Doroh, I.; Ogola, B.O.; Mcnally, A.B.; Horton, A.C.; Imulinde Sugi, A.; Lindsey, S.H. Estrogen-Mediated Mechanisms in Hypertension and Other Cardiovascular Diseases. J. Hum. Hypertens. 2022, 37, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, G.D.; Birnbaum, I.; Ye, Y.; Birnbaum, Y. Statin-Induced Cardioprotection Against Ischemia-Reperfusion Injury: Potential Drug-Drug Interactions. Lesson to Be Learnt by Translating Results from Animal Models to the Clinical Settings. Cardiovasc. Drugs Ther. 2015, 29, 461–467. [Google Scholar] [CrossRef]
- Bułdak, Ł.; Machnik, G.; Skudrzyk, E.; Bołdys, A.; Maligłówka, M.; Kosowski, M.; Basiak, M.; Bułdak, R.J.; Okopień, B. Exenatide Prevents Statin-Related LDL Receptor Increase and Improves Insulin Secretion in Pancreatic Beta Cells (1.1E7) in a Protein Kinase A-Dependent Manner. J. Appl. Biomed. 2022, 20, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Jeremic, J.N.; Jakovljevic, V.L.; Zivkovic, V.I.; Srejovic, I.M.; Bradic, J.V.; Milosavljevic, I.M.; Mitrovic, S.L.; Jovicic, N.U.; Bolevich, S.B.; Svistunov, A.A.; et al. Garlic Derived Diallyl Trisulfide in Experimental Metabolic Syndrome: Metabolic Effects and Cardioprotective Role. Int. J. Mol. Sci. 2020, 21, 9100. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Jiang, L.; Feng, B.; He, N.; Zhang, Y.; Ye, H. Protective Effects of Glucagon-like Peptide-1 on Cardiac Remodeling by Inhibiting Oxidative Stress through Mammalian Target of Rapamycin Complex 1/P70 Ribosomal Protein S6 Kinase Pathway in Diabetes Mellitus. J. Diabetes Investig. 2020, 11, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Shamardl, H.A.M.A.; Ibrahim, N.A.; Merzeban, D.H.; Elamir, A.M.; Golam, R.M.; Elsayed, A.M. Resveratrol and Dulaglutide Ameliorate Adiposity and Liver Dysfunction in Rats with Diet-Induced Metabolic Syndrome: Role of SIRT-1 / Adipokines / PPARγ and IGF-1. DARU J. Pharm. Sci. 2023, 31, 13–27. [Google Scholar] [CrossRef]
- Jakoviljevic, V.; Milic, P.; Bradic, J.; Jeremic, J.; Zivkovic, V.; Srejovic, I.; Nikolic Turnic, T.; Milosavljevic, I.; Jeremic, N.; Bolevich, S.; et al. Standardized Aronia Melanocarpa Extract as Novel Supplement against Metabolic Syndrome: A Rat Model. Int. J. Mol. Sci. 2018, 20, 6. [Google Scholar] [CrossRef] [PubMed]
- Stypmann, J.; Engelen, M.A.; Troatz, C.; Rothenburger, M.; Eckardt, L.; Tiemann, K. Echocardiographic Assessment of Global Left Ventricular Function in Mice. Lab. Anim. 2009, 43, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Stojic, I.; Srejovic, I.; Zivkovic, V.; Jeremic, N.; Djuric, M.; Stevanovic, A.; Milanovic, T.; Djuric, D.; Jakovljevic, V. The Effects of Verapamil and Its Combinations with Glutamate and Glycine on Cardiodynamics, Coronary Flow and Oxidative Stress in Isolated Rat Heart. J. Physiol. Biochem. 2017, 73, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Ristic, P.; Savic, M.; Bolevich, S.; Bolevich, S.; Orlova, A.; Mikhaleva, A.; Kartashova, A.; Yavlieva, K.; Nikolic Turnic, T.; Pindovic, B.; et al. Examining the Effects of Hyperbaric Oxygen Therapy on the Cardiovascular System and Oxidative Stress in Insulin-Treated and Non-Treated Diabetic Rats. Animals 2023, 13, 2847. [Google Scholar] [CrossRef]
- Sretenovic, J.; Zivkovic, V.; Srejovic, I.; Pantovic, S.; Jovic, J.J.; Nikolic, M.; Turnic, T.N.; Savic, M.; Jevdjevic, M.; Milosavljevic, Z.; et al. Nandrolone Decanoate and Swimming Affects Cardiodynamic and Morphometric Parameters in the Isolated Rat Heart. Life 2022, 12, 1242. [Google Scholar] [CrossRef] [PubMed]
- Draginic, N.D.; Jakovljevic, V.L.; Jeremic, J.N.; Srejovic, I.M.; Andjic, M.M.; Rankovic, M.R.; Sretenovic, J.Z.; Zivkovic, V.I.; Ljujic, B.T.; Mitrovic, S.L.; et al. Melissa officinalis L. Supplementation Provides Cardioprotection in a Rat Model of Experimental Autoimmune Myocarditis. Oxidative Med. Cell. Longev. 2022, 2022, 1344946. [Google Scholar] [CrossRef] [PubMed]
- Draginic, N.; Milosavljevic, I.; Andjic, M.; Jeremic, J.; Nikolic, M.; Sretenovic, J.; Kocovic, A.; Srejovic, I.; Zivkovic, V.; Bolevich, S.; et al. Short-Term Administration of Lemon Balm Extract Ameliorates Myocardial Ischemia/Reperfusion Injury: Focus on Oxidative Stress. Pharmaceuticals 2022, 15, 840. [Google Scholar] [CrossRef] [PubMed]


| MetS | Exenatide | Dulaglutide | |
|---|---|---|---|
| IVSd (cm) | 0.332 ± 0.079 | 0.140 ± 0.031∞ | 0.140 ± 0.020# |
| LVIDd (cm) | 0.675 ± 0.050 | 0.754 ± 0.024 | 0.676 ± 0.071 |
| LVPWd (cm) | 0.178 ± 0.008 | 0.157 ± 0.039∞ | 0.140 ± 0.005#§ |
| IVSs (cm) | 0.214 ± 0.019 | 0.173 ± 0.029∞ | 0.147 ± 0.023#§ |
| LVIDs (cm) | 0.485 ± 0.056 | 0.415 ± 0.018∞ | 0.341 ± 0.039# |
| LVPWs (cm) | 0.216 ± 0.021 | 0.184 ± 0.019∞ | 0.170 ± 0.041# |
| FS (%) | 42.075 ± 3.767 | 41.367 ± 9.136 | 47.725 ± 5.291 |
| EF (%) | 78.586 ± 4.086 | 81.312 ± 1.283 | 85.248 ± 3.301#§ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravic, M.; Srejovic, I.; Novakovic, J.; Andjic, M.; Sretenovic, J.; Muric, M.; Nikolic, M.; Bolevich, S.; Alekseevich Kasabov, K.; Petrovich Fisenko, V.; et al. Effect of GLP-1 Receptor Agonist on Ischemia Reperfusion Injury in Rats with Metabolic Syndrome. Pharmaceuticals 2024, 17, 525. https://doi.org/10.3390/ph17040525
Ravic M, Srejovic I, Novakovic J, Andjic M, Sretenovic J, Muric M, Nikolic M, Bolevich S, Alekseevich Kasabov K, Petrovich Fisenko V, et al. Effect of GLP-1 Receptor Agonist on Ischemia Reperfusion Injury in Rats with Metabolic Syndrome. Pharmaceuticals. 2024; 17(4):525. https://doi.org/10.3390/ph17040525
Chicago/Turabian StyleRavic, Marko, Ivan Srejovic, Jovana Novakovic, Marijana Andjic, Jasmina Sretenovic, Maja Muric, Marina Nikolic, Sergey Bolevich, Kirill Alekseevich Kasabov, Vladimir Petrovich Fisenko, and et al. 2024. "Effect of GLP-1 Receptor Agonist on Ischemia Reperfusion Injury in Rats with Metabolic Syndrome" Pharmaceuticals 17, no. 4: 525. https://doi.org/10.3390/ph17040525
APA StyleRavic, M., Srejovic, I., Novakovic, J., Andjic, M., Sretenovic, J., Muric, M., Nikolic, M., Bolevich, S., Alekseevich Kasabov, K., Petrovich Fisenko, V., Stojanovic, A., & Jakovljevic, V. (2024). Effect of GLP-1 Receptor Agonist on Ischemia Reperfusion Injury in Rats with Metabolic Syndrome. Pharmaceuticals, 17(4), 525. https://doi.org/10.3390/ph17040525







