A Holographic-Type Model in the Description of Polymer–Drug Delivery Processes
Abstract
:1. Introduction
1.1. Polymer-Based Drug Delivery Systems
1.2. Polymeric Systems as Nanocarriers
1.3. Polymer–Drug Delivery Systems (DDS)
1.4. Polymer–Drug Release Dynamics
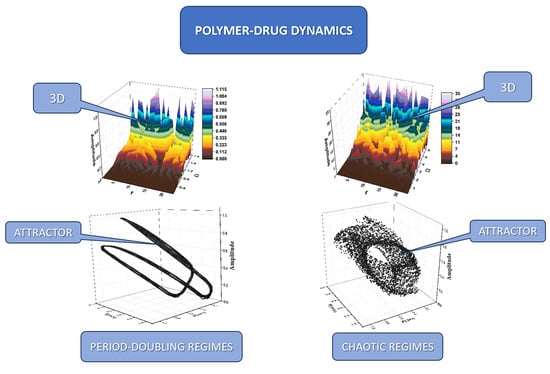
2. Results
3. Discussions
4. Methods
4.1. A Brief Recall of the Multifractal Schrödinger and Madelung Scenarios
4.2. Some Implications of the Multifractal Schrödinger Scenario
4.3. Synchronizations in Polymer–Drug Dynamics through a Hidden Symmetry
4.4. Simultaneities in Polymer-Drug Dynamics
4.5. Some Implications of the Multifractal Madelung Scenario in Drug Delivery Dynamics
5. Conclusions
- (i)
- A unitary model for describing disperse dynamics in polymer–drug systems has been suggested, considering that such a system can potentially be perceived as a multifractal mathematical object.
- (ii)
- Using this theory, the polymer–drug dynamics can be referred to by employing the Scale Relativity Theory in the monofractal or the multifractal case.
- (iii)
- This approach allows the characterization of polymer–drug dynamics through fractal/multifractal curves. Since these graphical representations display the self-similarity propriety, it can be stated that the description of these dynamics implies holographic-type behaviors; therefore, a holographic model for polymer–drug dynamics can be developed.
- (iv)
- The use of a holographic model for describing drug release dynamics can be reduced to two scenarios, namely a Schrödinger-type scenario and a Madelung-type scenario.
- (v)
- In the Schrödinger-type scenario, the synchronization of any polymer–drug structural unit’s dynamics implies the SL(2ℝ) group, while the simultaneity of the same dynamics through a Riccati-type gauge implies various release modes, “mimed” through several behaviors, such as period doubling (which can be associated to the swelling of the polymeric complex), damped oscillations regimes (which can be associated to surface release mechanisms), self-modulated regimes (which can be associated to in-depth release mechanisms), or chaotic regimes (which can be associated to polymer degrading release mechanisms).
- (vi)
- In the Madelung-type scenario, the same processes that are “functional” in the Schrödinger-type scenario (synchronization and simultaneity) imply dynamics described through multifractal diffusion equations. By these equations, Fickian, non-Fickian, or Fickian–non-Fickian transitory behaviors can be substantiated in drug release dynamics.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cortes, J.; Perez-García, J.M.; Llombart-Cussac, A.; Curigliano, G.; El Saghir, N.S.; Cardoso, F.; Barrios, C.H.; Wagle, S.; Roman, J.; Harbeck, N. Enhancing global access to cancer medicines. CA Cancer J. Clin. 2020, 70, 105–124. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Kathuria, H.; Momin, M. Clinical therapies and nano drug delivery systems for urinary bladder cancer. Pharmacol. Ther. 2021, 226, 107871. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liang, T.; Ma, Q. Layer-by-Layer assembled nano-drug delivery systems for cancer treatment. Drug Deliv. 2021, 28, 655–669. [Google Scholar] [CrossRef] [PubMed]
- Sinha, V.R.; Khosla, L. Bioabsorbable polymers for implantable therapeutic systems. Drug Dev. Ind. Pharm. 1998, 24, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.K.; Kim, S.W. Recent advances in polymeric drug delivery systems. Biomater. Res. 2020, 24, 12. [Google Scholar] [CrossRef] [PubMed]
- Cacciatore, I.; Ciulla, M.; Fornasari, E.; Marinelli, L.; Di Stefano, A. Solid lipid nanoparticles as a drug delivery system for the treatment of neurodegenerative diseases. Expert Opin. Drug Deliv. 2016, 13, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, W.; Gao, Y.; Tong, H.; Chen, Z.; Shi, J.; Santos, H.A.; Xia, B. Near-infrared light and magnetic field dual-responsive porous silicon-based nanocarriers to overcome multidrug resistance in breast cancer cells with enhanced efficiency. J. Mater. Chem. B 2020, 8, 546–557. [Google Scholar] [CrossRef]
- Karaman, D.Ş.; Kaasalainen, M.; Kettiger, H.; Rosenholm, J.M. Opportunities and Challenges of Silicon-based Nanoparticles for Drug Delivery and Imaging. Charact. Pharm. Nano Microsyst. 2021, 9, 291–337. [Google Scholar]
- Liu, X.; Cheng, Y.; Mu, Y.; Zhang, Z.; Tian, D.; Liu, Y.; Hu, X.; Wen, T. Diverse drug delivery systems for the enhancement of cancer immunotherapy: An overview. Front. Immunol. 2024, 15, 1328145. [Google Scholar] [CrossRef]
- Iakimova, T.M.; Bubley, A.A.; Boychenko, O.P.; Guk, D.A.; Vaneev, A.N.; Prusov, A.N.; Erofeev, A.S.; Gorelkin, P.V.; Krasnovskaya, O.O.; Klyachko, N.L.; et al. Liposomal form of 2-alkylthioimidazolone-based copper complexes for combined cancer therapy. Nanomedicine 2023, 18, 2105–2123. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Chen, Y.; Zhu, L.; You, L.; Tong, H.; Meng, H.; Sheng, J.; Jin, J. Harnessing Nanotechnology: Emerging Strategies for Multiple Myeloma Therapy. Biomolecules 2024, 14, 83. [Google Scholar] [CrossRef] [PubMed]
- Kumbhar, P.R.; Kumar, P.; Lasure, A.; Velayutham, R.; Mandal, D. An updated landscape on nanotechnology-based drug delivery, immunotherapy, vaccinations, imaging, and biomarker detections for cancers: Recent trends and future directions with clinical success. Discov. Nano 2023, 18, 156. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, S.; Ma, P.; Jiang, Y.; Cheng, K.; Yu, Y.; Jiang, N.; Miao, H.; Tang, Q.; Liu, F.; et al. Drug conjugate-based anticancer therapy—Current status and perspectives. Cancer Lett. 2023, 552, 215969. [Google Scholar] [CrossRef] [PubMed]
- Junyaprasert, V.B.; Thummarati, P. Innovative Design of Targeted Nanoparticles: Polymer-Drug Conjugates for Enhanced Cancer Therapy. Pharmaceutics 2023, 15, 2216. [Google Scholar] [CrossRef] [PubMed]
- Puccetti, M.; Pariano, M.; Schoubben, A.; Giovagnoli, S.; Ricci, M. Biologics, Theranostics, and Personalized Medicine in Drug Delivery Systems. Pharmacol. Res. 2024, 201, 107086. [Google Scholar] [CrossRef] [PubMed]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for drug delivery systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173. [Google Scholar] [CrossRef]
- Liechty, W.B.; Peppas, N.A. Expert opinion: Responsive polymer nanoparticles in cancer therapy. Eur. J. Pharm. Biopharm. 2012, 80, 241–246. [Google Scholar] [CrossRef]
- Thakor, P.; Bhavana, V.; Sharma, R.; Srivastava, S.; Singh, S.B.; Mehra, N.K. Polymer-drug conjugates: Recent advances and future perspectives. Drug Discov. Today 2020, 25, 1718–1726. [Google Scholar] [CrossRef]
- Fathi, M.; Barar, J. Perspective highlights on biodegradable polymeric nanosystems for targeted therapy of solid tumors. Bioimpacts 2017, 7, 49–57. [Google Scholar] [CrossRef]
- Maranescu, B.; Visa, A. Applications of Metal-Organic Frameworks as Drug Delivery Systems. Int. J. Mol. Sci. 2022, 23, 4458. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, H.; Rasool, A.; Wang, H.; Manzoor, R.; Zhang, G. Polymeric nanoparticles (PNPs) for oral delivery of insulin. J. Nanobiotechnology 2024, 22, 1. [Google Scholar] [CrossRef] [PubMed]
- Filippousi, M.; Turner, S.; Leus, K.; Siafaka, P.I.; Tseligka, E.D.; Vandichel, M.; Nanaki, S.G.; Vizirianakis, I.S.; Bikiaris, D.N.; Van Der Voort, P.; et al. Biocompatible Zr-based nanoscale MOFs coated with modified poly(ε-caprolactone) as anticancer drug carriers. Int. J. Pharm. 2016, 509, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Dahri, M.; Abolmaali, S.S.; Maleki, R.; Najafi, H.; Abedanzadeh, M.; Tamaddon, A.M. Nanoscale aggregation of doxorubicin-short peptide conjugates for enzyme-responsive delivery with various MOF carriers: In-silico steps towards smart cancer chemotherapy. Comput. Biol. Med. 2022, 144, 105386. [Google Scholar] [CrossRef] [PubMed]
- Karimi Alavijeh, R.; Akhbari, K. Improvement of curcumin loading into a nanoporous functionalized poor hydrolytic stable metal-organic framework for high anticancer activity against human gastric cancer AGS cells. Colloids Surf. B Biointerfaces 2022, 212, 112340. [Google Scholar] [CrossRef] [PubMed]
- Gupta, C.; Naik, I.; Menon, M.; Ambre, P.; Coutinho, E. A Review on Exploring the Opportunities of Polymer Drug Conjugated Systems for Targeted Cancer Treatment. Curr. Drug Deliv. 2022, 20, 8–30. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.H.; De, R.; Lee, K.T. Emerging strategies to fabricate polymeric nanocarriers for enhanced drug delivery across blood-brain barrier: An overview. Adv. Colloid Interface Sci. 2023, 320, 103008. [Google Scholar] [CrossRef] [PubMed]
- Wen, R.; Umeano, A.C.; Chen, P.; Farooqi, A.A. Polymer-Based Drug Delivery Systems for Cancer. Crit. Rev. Ther. Drug Carr. Syst. 2018, 35, 521–553. [Google Scholar] [CrossRef] [PubMed]
- Kuperkar, K.; Atanase, L.I.; Bahadur, A.; Crivei, I.C.; Bahadur, P. Degradable Polymeric Bio(nano)materials and Their Biomedical Applications: A Comprehensive Overview and Recent Updates. Polymers 2024, 16, 206. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Azar, A.T. (Ed.) Modeling and Control of Drug Delivery Systems; Academic Press: Cambridge, MA, USA, 2021. [Google Scholar]
- Shariatinia, Z. Carboxymethyl chitosan: Properties and biomedical applications. Int. J. Biol. Macromol. 2018, 120 Pt B, 1406–1419. [Google Scholar] [CrossRef] [PubMed]
- Avramović, N.; Mandić, B.; Savić-Radojević, A.; Simić, T. Polymeric Nanocarriers of Drug Delivery Systems in Cancer Therapy. Pharmaceutics 2020, 12, 298. [Google Scholar] [CrossRef] [PubMed]
- Zielinska, A.; Skwarek, E.; Zaleska, A.; Gazda, M.; Hupka, J. Preparation of silver nanoparticles with controlled particle size. Procedia Chem. 2009, 1, 1560–1566. [Google Scholar] [CrossRef]
- Garcês, A.; Amaral, M.; Lobo, J.S.; Silva, A.C. Formulations based on solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for cutaneous use: A review. Eur. J. Pharm. Sci. 2018, 112, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; He, J.; Li, Q.; Gao, L.; Hu, J.; Zeng, R.; Qin, J.; Wang, S.X.; Wang, Q. Self-healing of electrical damage in polymers using superparamagnetic nanoparticles. Nat. Nanotechnol. 2019, 14, 151–155. [Google Scholar] [CrossRef]
- He, C.; Cheng, J.; Zhang, X.; Douthwaite, M.; Pattisson, S.; Hao, Z. Recent advances in the catalytic oxidation of volatile organic compounds: A review based on pollutant sorts and sources. Chem. Rev. 2019, 119, 4471–4568. [Google Scholar] [CrossRef]
- Chouaib, R.; Sarieddine, R.; Gali-Muhtasib, H. Nanoparticles as Drug Delivery Systems for Cancer Treatment: Applications in Targeted Therapy and Personalized Medicine. In Nanoparticle Drug Delivery Systems for Cancer Treatment; CRC Press: Boca Raton, FL, USA, 2020; pp. 1–22. [Google Scholar]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of nanoparticle toxicity on their physical and chemical properties. Nanoscale Res. Lett. 2018, 13, 1–21. [Google Scholar] [CrossRef]
- Xu, C.; Qiao, L.; Guo, Y.; Ma, L.; Cheng, Y. Preparation, characteristics and antioxidant activity of polysaccharides and protein scapped selenium nanoparticles synthesized by Lactobacillus casei ATCC 393. Carbohydr. Polym. 2018, 195, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Kister, T.; Monego, D.; Mulvaney, P.; Widmer-Cooper, A.; Kraus, T. Colloidal stability of apolar nanoparticles: The role of particle size and ligand shell structure. ACS Nano 2018, 12, 5969–5977. [Google Scholar] [CrossRef]
- Quinson, J.; Inaba, M.; Neumann, S.; Swane, A.A.; Bucher, J.; Simonsen, S.B.; Theil Kuhn, L.; Kirkensgaard, J.J.; Jensen, K.M.; Oezaslan, M. Investigating particle size effects in catalysis by applying a size-controlled and surfactant-free synthesis of colloidal nanoparticles in alkaline ethylene glycol: Case study of the oxygen reduction reaction on Pt. ACS Catal. 2018, 8, 6627–6635. [Google Scholar] [CrossRef]
- Caster, J.M.; Stephanie, K.Y.; Patel, A.N.; Newman, N.J.; Lee, Z.J.; Warner, S.B.; Wagner, K.T.; Roche, K.C.; Tian, X.; Min, Y. Effect of particle size on the biodistribution, toxicity, and efficacy of drug-loaded polymeric nanoparticles in chemoradiotherapy. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1673–1683. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Holden, J.A.; Heath, D.E.; O’Brien-Simpson, N.M.; O’Connor, A.J. Engineering highly effective antimicrobial selenium nanoparticles through control of particle size. Nanoscale 2019, 11, 14937–14951. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yuen, M.C.; White, E.L.; Boley, J.W.; Deng, B.; Cheng, G.J.; Kramer-Bottiglio, R. Laser sintering of liquid metal nanoparticles for scalable manufacturing of soft and flexible electronics. ACS Appl. Mater. Interfaces 2018, 10, 28232–28241. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-D.; Huang, L. Pharmacokinetics and biodistribution of nanoparticles. Mol. Pharm. 2008, 5, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Gao, H. The impact of protein corona on the behavior and targeting capability of nanoparticle-based delivery system. Int. J. Pharm. 2018, 552, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Anchordoquy, T.J.; Barenholz, Y.; Boraschi, D.; Chorny, M.; Decuzzi, P.; Dobrovolskaia, M.A.; Farhangrazi, Z.S.; Farrell, D.; Gabizon, A.; Ghandehari, H.; et al. Mechanisms and Barriers in Cancer Nanomedicine: Addressing Challenges, Looking for Solutions. ACS Nano 2017, 11, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Liang, Q.; Wang, F. Mechanisms that control the adsorption–desorption behavior of phosphate on magnetite nanoparticles: The role of particle size and surface chemistry characteristics. RSC Adv. 2020, 10, 2378–2388. [Google Scholar] [CrossRef]
- Parveen, S.; Arjmand, F.; Tabassum, S. Clinical developments of antitumor polymertherapeutics. RSC Adv. 2019, 9, 24699–24721. [Google Scholar] [CrossRef] [PubMed]
- Calzoni, E.; Cesaretti, A.; Polchi, A.; Di Michele, A.; Tancini, B.; Emiliani, C. Biocompatible Polymer Nanoparticles for Drug Delivery Applications in Cancer and Neurodegenerative Disorder Therapies. J. Funct. Biomater. 2019, 10, 4. [Google Scholar] [CrossRef]
- Bordat, A.; Boissenot, T.; Nicolas, J.; Tsapis, N. Thermoresponsive polymer nanocarriers for biomedical applications. Adv. Drug Del. Rev. 2019, 138, 167–192. [Google Scholar] [CrossRef]
- Allen, T.M. Ligand-targeted therapeutics in anticancer therapy. Nat. Rev. Cancer 2002, 2, 750–763. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V. Antibody-modified liposomes for cancer chemotherapy. Expert Opin. Drug Deliv. 2008, 5, 1003–1025. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.W.; Allen, T.M. The use of single chain Fv as targeting agents for immunoliposomes: An update on immunoliposomal drugs for cancer treatment. Expert Opin. Drug Deliv. 2010, 7, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Farokhzad, O.C.; Cheng, J.; Teply, B.A.; Sherifi, I.; Jon, S.; Kanto, P.W.; Richie, J.P.; Langer, R. Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc. Natl. Acad. Sci. USA 2006, 103, 6315–6320. [Google Scholar] [CrossRef] [PubMed]
- Oba, M.; Vachutinsky, Y.; Miyata, K.; Kano, M.R.; Ikeda, S.; Nishiyama, N.; Itaka, K.; Miyazono, K.; Koyama, H.; Kataoka, K. Antiangiogenic gene therapy of solid tumor bysystemic injection of polyplex micelles loading plasmid DNA encoding soluble flt-1. Mol. Pharm. 2010, 7, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Miura, Y.; Takenaka, T.; Toh, K.; Wu, S.; Nishihara, H.; Kano, M.R.; Ino, Y.; Nomoto, T.; Matsumoto, Y.; Koyama, H.; et al. Cyclic RGD-linked polymeric micelles for targeted delivery of platinum anticancer drugs to glioblastoma through the blood-brain tumor barrier. ACS Nano 2013, 7, 8583–8592. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, S.; Zhang, F.; Yu, J.; Zhang, X.; Yang, G.; Liu, X. pH-sensitive biomaterials for drug delivery. Molecules 2020, 25, 5649. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Cell penetrating peptide-modified pharmaceuticalnanocarriers for intracellular drug and gene delivery. Biopolymers 2008, 90, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Glassman, P.M.; Muzykantov, V.R. Pharmacokinetic and Pharmacodynamic Properties of Drug Delivery Systems. J. Pharmacol. Exp. Ther. 2019, 370, 570–580. [Google Scholar] [CrossRef]
- Nunes, D.; Loureiro, J.A.; Pereira, M.C. Drug Delivery Systems as a Strategy to Improve the Efficacy of FDA-Approved Alzheimer’s Drugs. Pharmaceutics 2022, 14, 2296. [Google Scholar] [CrossRef]
- Wen, H.; Jung, H.; Li, X. Drug Delivery Approaches in Addressing Clinical Pharmacology-Related Issues: Opportunities and Challenges. AAPS J. 2015, 17, 1327–1340. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Karp, J.M.; Langer, R.; Joshi, N. The Future of Drug Delivery. Chem. Mater. 2023, 35, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Onugwu, A.L.; Nwagwu, C.S.; Onugwu, O.S.; Echezona, A.C.; Agbo, C.P.; Ihim, S.A.; Emeh, P.; Nnamani, P.O.; Attama, A.A.; Khutoryanskiy, V.V. Nanotechnology based drug delivery systems for the treatment of anterior segment eye diseases. J. Control Release 2023, 354, 465–488. [Google Scholar] [CrossRef] [PubMed]
- Sahu, T.; Ratre, Y.K.; Chauhan, S.; Bhaskar, L.V.K.S.; Nair, M.P.; Verma, H.K. Nanotechnology based drug delivery system: Current strategies emerging therapeutic potential for medical science. J. Drug Deliv. Sci. Technol. 2021, 63, 102487. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zou, M.; Xu, Y.; Lin, P.; Lei, C.; Xia, X. Nano Drug Delivery System for Tumor Immunotherapy: Next-Generation Therapeutics. Front. Oncol. 2022, 12, 864301. [Google Scholar] [CrossRef]
- Hardenia, A.; Maheshwari, N.; Hardenia, S.S.; Dwivedi, S.K.; Maheshwari, R.; Tekade, R.K. Scientific rationale for designing controlled drug delivery systems. In Basic Fundamentals of Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–28. [Google Scholar]
- Manzari, M.T.; Shamay, Y.; Kiguchi, H.; Rosen, N.; Scaltriti, M.; Heller, D.A. Targeted drug delivery strategies for precision medicines. Nat. Rev. Mater. 2021, 6, 351–370. [Google Scholar] [CrossRef] [PubMed]
- Raijada, D.; Wac, K.; Greisen, E.; Rantanen, J.; Genina, N. Integration of personalized drug delivery systems into digital health. Adv. Drug Deliv. Rev. 2021, 176, 113857. [Google Scholar] [CrossRef]
- Coelho, J.F.; Ferreira, P.C.; Alves, P.; Cordeiro, R.; Fonseca, A.C.; Góis, J.R.; Gil, M.H. Drug delivery systems: Advanced technologies potentially applicable in personalized treatments. EPMA J. 2010, 1, 164–209. [Google Scholar] [CrossRef]
- Nikam, A.N.; More, M.P.; Pandey, A.P.; Patil, P.O.; Patil, A.G.; Deshmukh, P.K. Design and development of thiolated graphene oxide nanosheets for brain tumor targeting. Int. J. Polym. Mater. 2019, 69, 611–621. [Google Scholar] [CrossRef]
- Li, J.; Xiao, S.; Xu, Y.; Zuo, S.; Zha, Z.; Ke, W.; He, C.; Ge, Z. Smart Asymmetric Vesicles with Triggered Availability of Inner Cell-Penetrating Shells for Specific Intracellular Drug Delivery. ACS Appl. Mater. Interfaces 2017, 9, 17727–17735. [Google Scholar] [CrossRef] [PubMed]
- Chida, T.; Miura, Y.; Cabral, H.; Nomoto, T.; Kataoka, K.; Nishiyama, N. Epirubicin-loaded polymeric micelles effectively treat axillary lymph nodes metastasis of breast cancer through selective accumulation and pH-triggered drug release. J. Control Release 2018, 292, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.; Fukushima, S.; Harada, A.; Kataoka, K. Design of environment-sensitive supramolecular assemblies for intracellular drug delivery: Polymeric micelles that are responsive to intracellular pH change. Angew. Chem. Int. Ed. Engl. 2003, 42, 4640–4643. [Google Scholar] [CrossRef] [PubMed]
- Varshosaz, J.; Taymouri, S.; Hassanzadeh, F.; Javanmard, S.H.; Rostami, M. Folated synperonic-cholesteryl hemisuccinate polymeric micelles for the targeted delivery of docetaxel in melanoma. Biomed. Res. Int. 2015, 2015, 746093. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, J.; Tian, D.; Sun, L.; Wang, X.; Tian, M. Theranostic combinatorial drug-loaded coated cubosomes for enhanced targeting and efficacy against cancer cells. Cell Death Dis. 2020, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Mandelbrot, B.B. The Fractal Geometry of Nature; W. H. Freeman and Co.: San Fracisco, CA, USA, 1982. [Google Scholar]
- Cristescu, C.P. Nonlinear Dynamics and Chaos. Theoretical Fundaments and Applications; Romanian Academy Publishing House: Bucharest, Romania, 2008. [Google Scholar]
- Iurciuc, C.E.; Popa, M.; Atanase, L.I.; Popa, O.; Ochiuz, L.; Postolache, P.; Ghizdovat, V.; Irimiciuc, S.A.; Agop, M.; Volovat, C.; et al. Multi-fractal modeling of curcumin release mechanism from polymeric nanomicelles. Drug Deliv. 2022, 29, 2883–2896. [Google Scholar]
- Andreica, B.I.; Anisiei, A.; Iftime, M.M.; Ababei, R.V.; Ochiuz, L.; Vasincu, D.; Vasilache, I.A.; Volovat, C.; Boboc, D.; Poroch, V.; et al. Theoretical—Experimental Approach of Chitosan/Quaternized Chitosan Nanofibers’ Behavior in Wound Exudate Media. Pharmaceutics 2023, 15, 2722. [Google Scholar] [CrossRef]
- Himiniuc, L.M.; Toma, B.F.; Popovici, R.; Grigore, A.M.; Hamod, A.; Volovat, C.; Volovat, S.; Nica, I.; Vasincu, D.; Agop, M.; et al. Update on the use of nanocarriers and drug delivery systems and future directions in cervical cancer. J. Immunol. Res. 2022, 2022, 1636908. [Google Scholar] [CrossRef]
- Nottale, L. Scale Relativity and Fractal Space-Time: A New Approach to Unifying Relativity and Quantum Mechanics; Imperial College Press: London, UK, 2011. [Google Scholar]
- Merches, I.; Agop, M. Differentiability and Fractality in Dynamics of Physical Systems; World Scientific: Hackensack, NJ, USA, 2016. [Google Scholar]
- Agop, M.; Paun, V.P. On the New Perspectives of the Fractal Theory. Fundaments and Applications; Romanian Academy Publishing House: Bucharest, Romania, 2017. [Google Scholar]
- Gan, W.C.; Shu, F.W. Holography as deep learning. Int. J. Mod. Phys. D 2017, 26, 1743020. [Google Scholar] [CrossRef]
- Rivenson, Y.; Wu, Y.; Ozcan, A. Deep learning in holography and coherent imaging. Light Sci. Appl. 2019, 8, 85. [Google Scholar] [CrossRef] [PubMed]
- Shimobaba, T.; Blinder, D.; Birnbaum, T.; Hoshi, I.; Shiomi, H.; Schelkens, P.; Ito, T. Deep-learning computational holography: A review. Front. Photonics 2022, 3, 8. [Google Scholar] [CrossRef]
- Abdurashitov, A.S.; Proshin, P.I.; Tuchin, V.V.; Sukhorukov, G.B. Integrated binary hologram to monitor cargo release from a drug-eluting film. Light Adv. Manuf. 2022, 3, 417–426. [Google Scholar] [CrossRef]
- Ray, A.; Li, S.; Segura, T.; Ozean, A. High-throughput holographic monitoring of nanoparticle degradation for drug delivery applications. In Proceedings of the 2018 Conference on Lasers and Electro-Optics (CLEO), San Jose, CA, USA, 13–18 May 2018; pp. 1–2. [Google Scholar]
- Gavriluţ, A.; Mercheş, I.; Agop, M. Atomicity through Fractal Measure Theory; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar]
- Mihăileanu, N. Complements of Geometry: Analytical, Projective and Differential; Editura Didactică şi Pedagogică: Bucharest, Romania, 1972. (In Romanian) [Google Scholar]
- Mazilu, N.; Agop, M. Skyrmions, a Great Finishing Touch to Classical Newtonian Philosophy; Nova Science Publishers Inc.: New York, NY, USA, 2012. [Google Scholar]
- Baroni, S.; Argenziano, M.; La Cava, F.; Soster, M.; Garello, F.; Lembo, D.; Cavalli, R.; Terreno, E. Hard-Shelled Glycol Chitosan Nanoparticles for Dual MRI/US Detection of Drug Delivery/Release: A Proof-of-Concept Study. Nanomaterials 2023, 13, 2227. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.J.; Lin, F.H.; Tsai, H.M.; Lin, C.F.; Chin, H.C.; Wong, J.M.; Shieh, M.J. Alginate-folic acid-modified chitosan nanoparticles for photodynamic detection of intestinal neoplasms. Biomaterials 2011, 32, 2174–2182. [Google Scholar] [CrossRef] [PubMed]
- Puluhulawa, L.E.; Joni, I.M.; Elamin, K.M.; Mohammed, A.F.A.; Muchtaridi, M.; Wathoni, N. Chitosan-Hyaluronic Acid Nanoparticles for Active Targeting in Cancer Therapy. Polymers 2022, 14, 3410. [Google Scholar] [CrossRef] [PubMed]
- Croy, S.R.; Kwon, G.S. Polymeric micelles for drug delivery. Curr. Pharm. Des. 2006, 12, 4669–4684. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Lee, J.K.; Jeong, J.; Choy, J.H. Toxicity evaluation of inorganic nanoparticles: Considerations and challenges. Mol. Cell. Toxicol. 2013, 9, 205–210. [Google Scholar] [CrossRef]
- Peng, F.; Setyawati, M.I.; Tee, J.K.; Ding, X.; Wang, J.; Nga, M.E.; Ho, H.K.; Leong, D.T. Nanoparticles promote in vivo breast cancer cell intravasation and extravasation by inducing endothelial leakiness. Nat. Nanotechnol. 2019, 14, 279–286. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nica, I.; Volovat, C.; Boboc, D.; Popa, O.; Ochiuz, L.; Vasincu, D.; Ghizdovat, V.; Agop, M.; Volovat, C.C.; Lupascu Ursulescu, C.; et al. A Holographic-Type Model in the Description of Polymer–Drug Delivery Processes. Pharmaceuticals 2024, 17, 541. https://doi.org/10.3390/ph17040541
Nica I, Volovat C, Boboc D, Popa O, Ochiuz L, Vasincu D, Ghizdovat V, Agop M, Volovat CC, Lupascu Ursulescu C, et al. A Holographic-Type Model in the Description of Polymer–Drug Delivery Processes. Pharmaceuticals. 2024; 17(4):541. https://doi.org/10.3390/ph17040541
Chicago/Turabian StyleNica, Irina, Constantin Volovat, Diana Boboc, Ovidiu Popa, Lacramioara Ochiuz, Decebal Vasincu, Vlad Ghizdovat, Maricel Agop, Cristian Constantin Volovat, Corina Lupascu Ursulescu, and et al. 2024. "A Holographic-Type Model in the Description of Polymer–Drug Delivery Processes" Pharmaceuticals 17, no. 4: 541. https://doi.org/10.3390/ph17040541
APA StyleNica, I., Volovat, C., Boboc, D., Popa, O., Ochiuz, L., Vasincu, D., Ghizdovat, V., Agop, M., Volovat, C. C., Lupascu Ursulescu, C., Lungulescu, C. V., & Volovat, S. R. (2024). A Holographic-Type Model in the Description of Polymer–Drug Delivery Processes. Pharmaceuticals, 17(4), 541. https://doi.org/10.3390/ph17040541