Testing of Anti-EMT, Anti-Inflammatory and Antibacterial Activities of 2′,4′-Dimethoxychalcone
Abstract
1. Introduction
2. Results
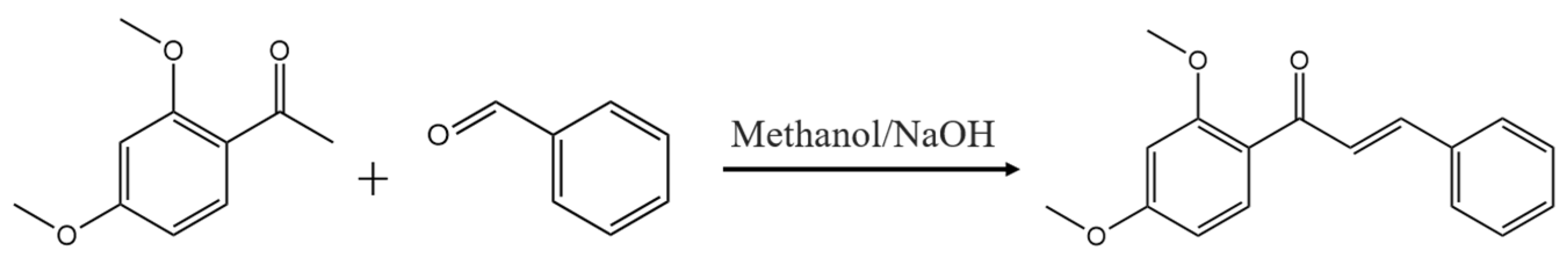
2.1. Chemistry
2.2. Assessment of Biology
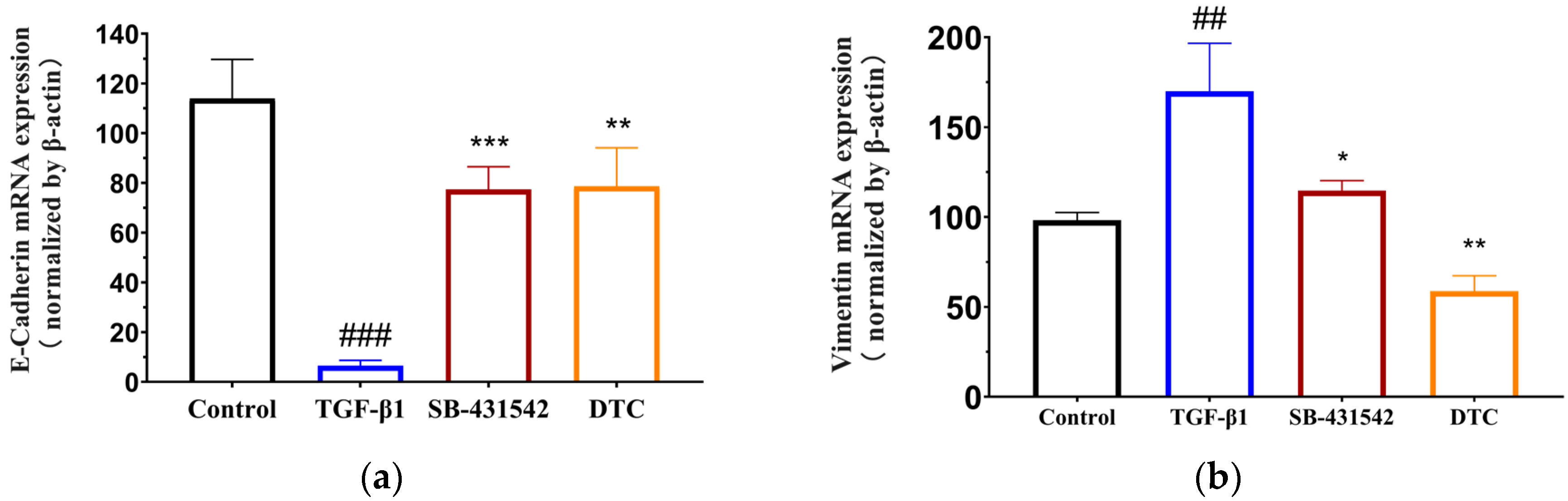
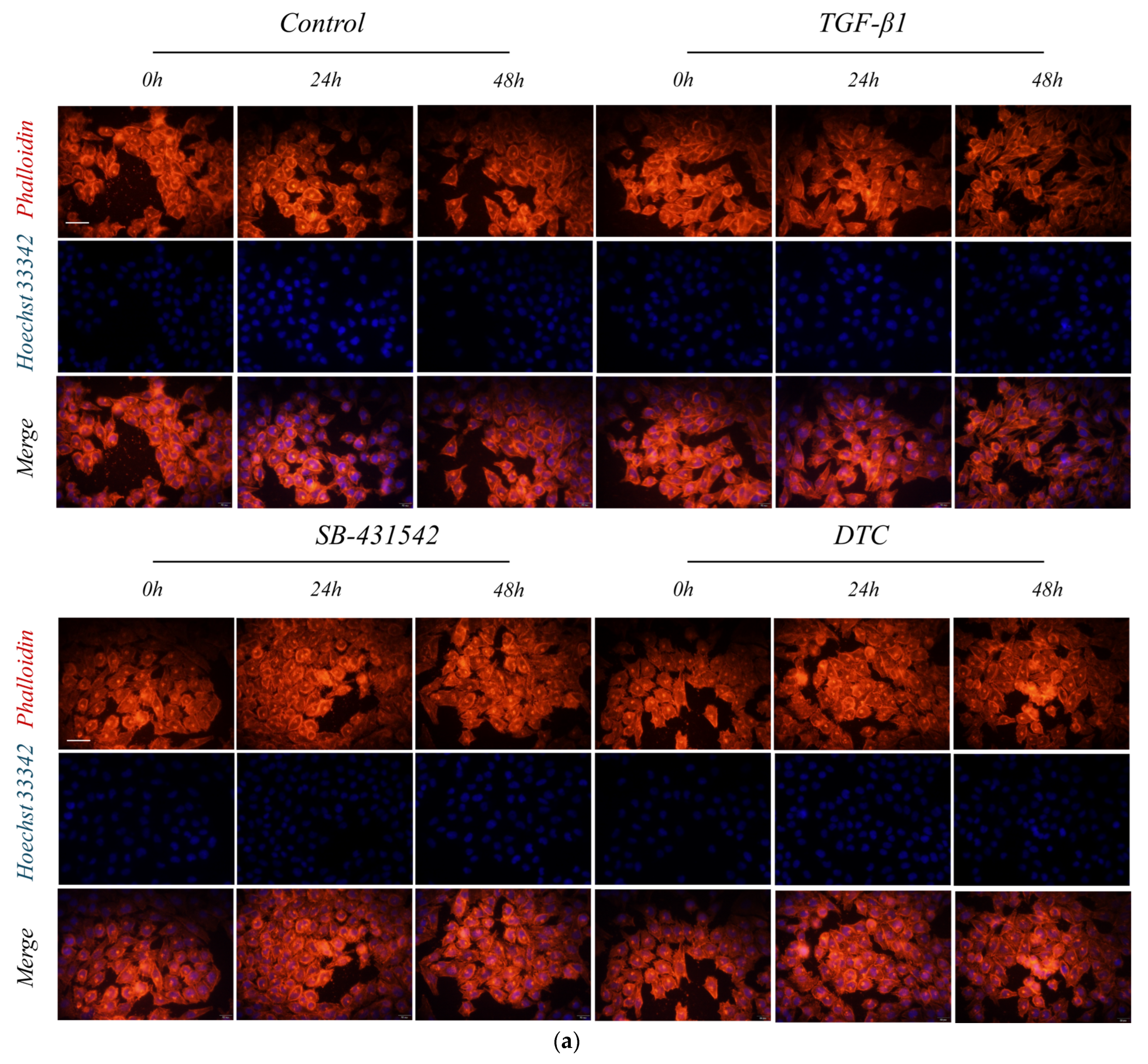
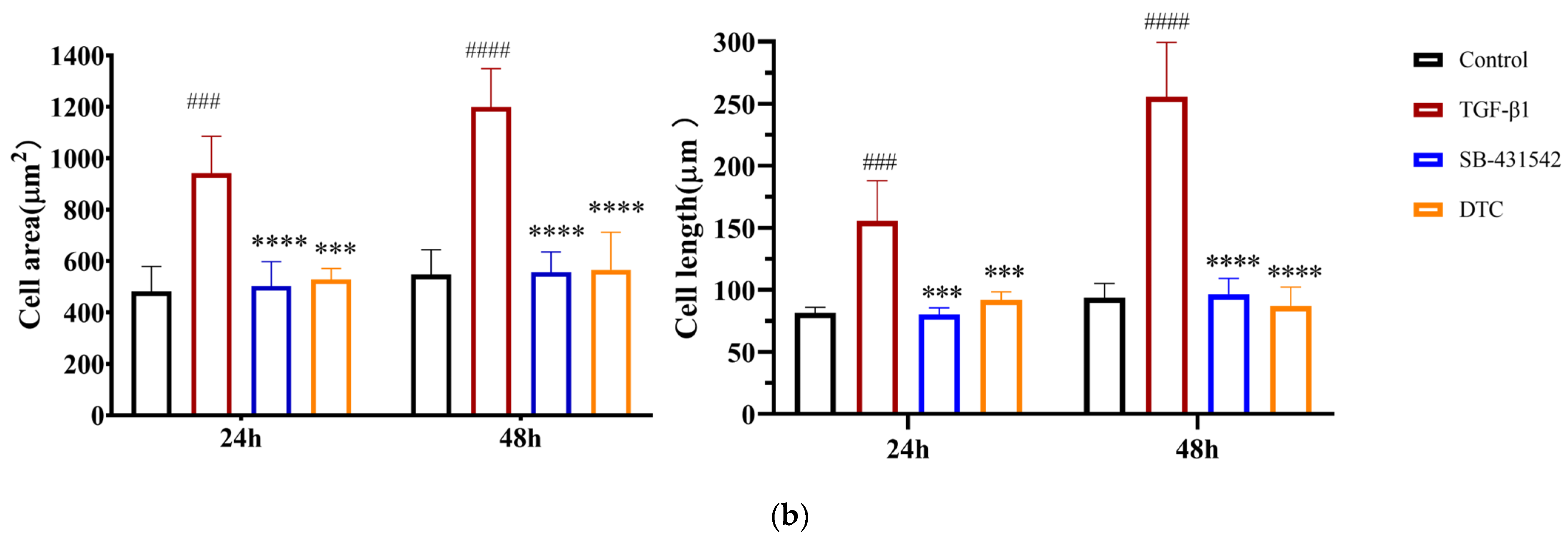
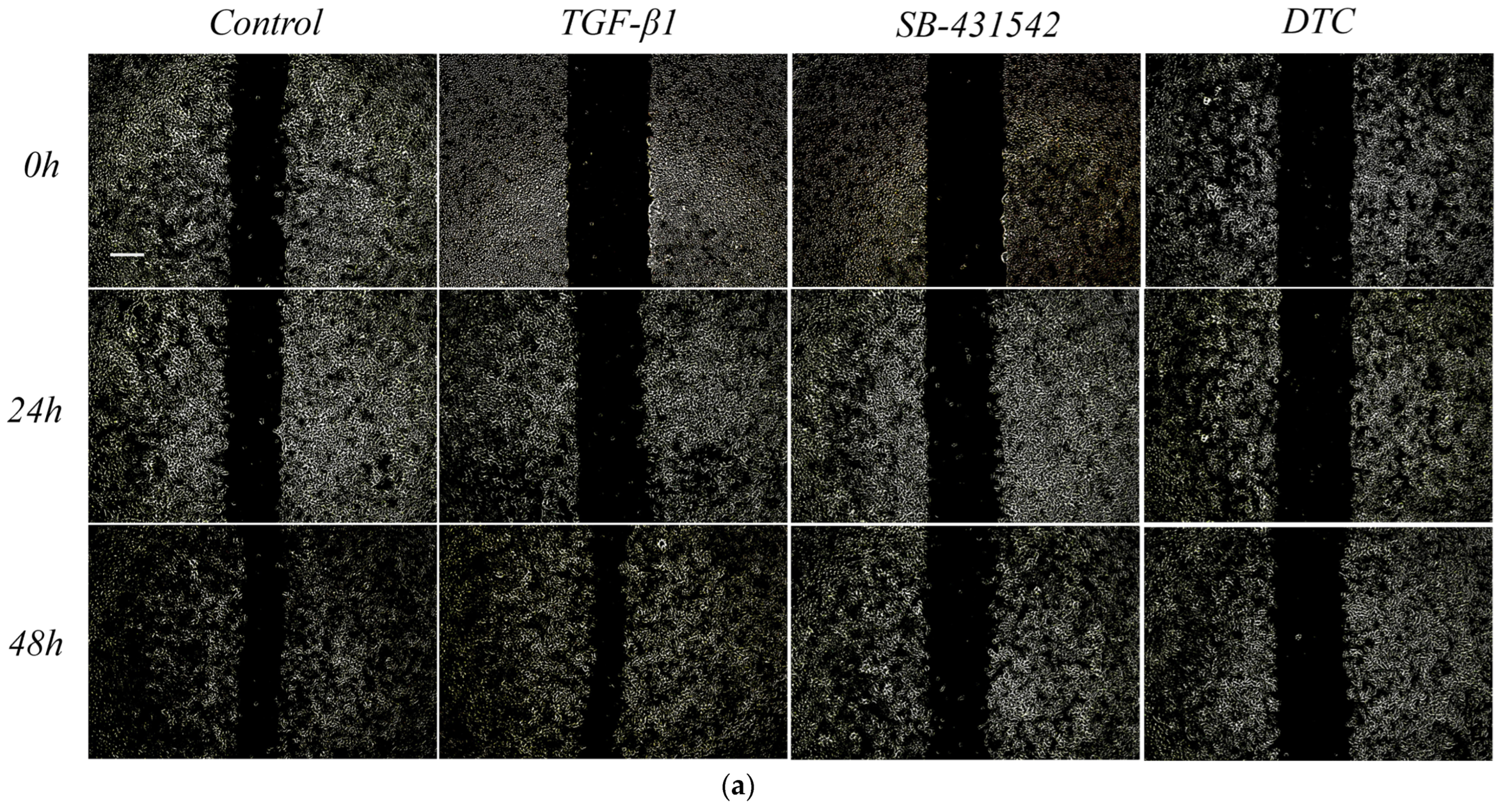
2.2.1. Effect of DTC on TGF-β1-Induced EMT in A549 Cells
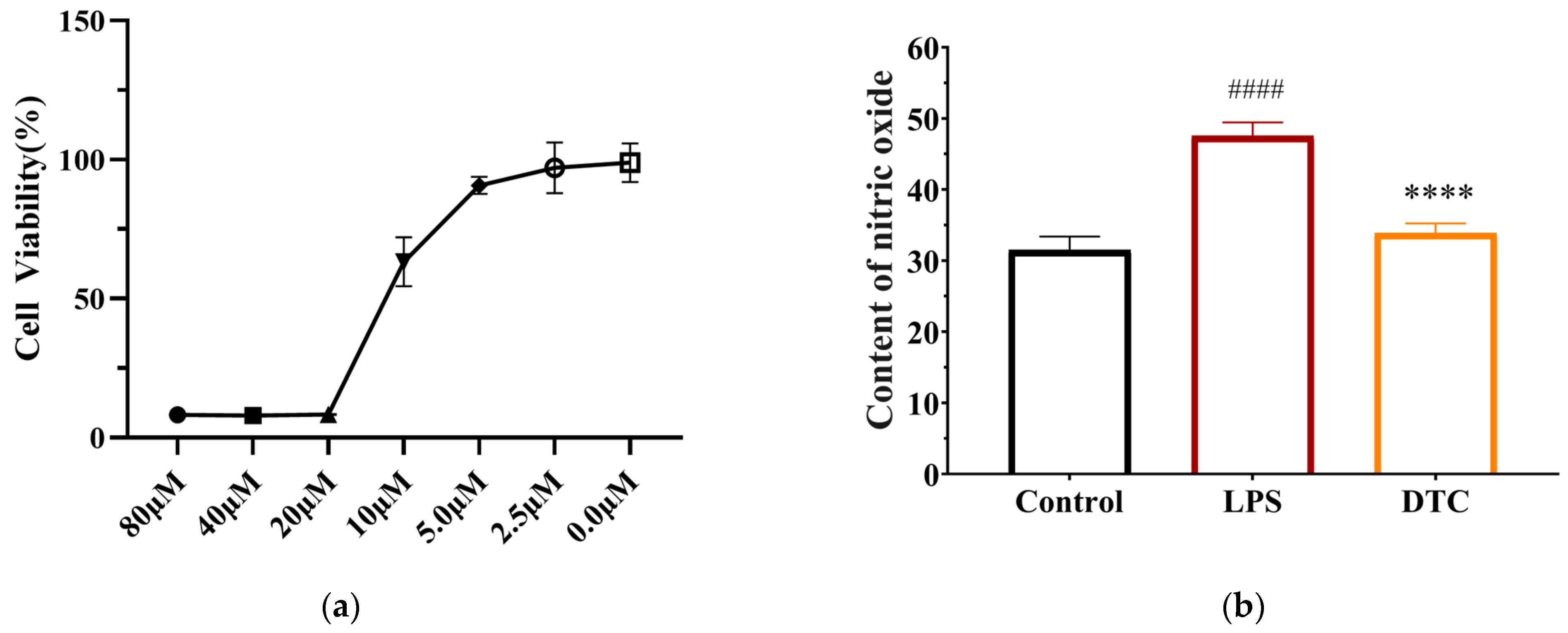
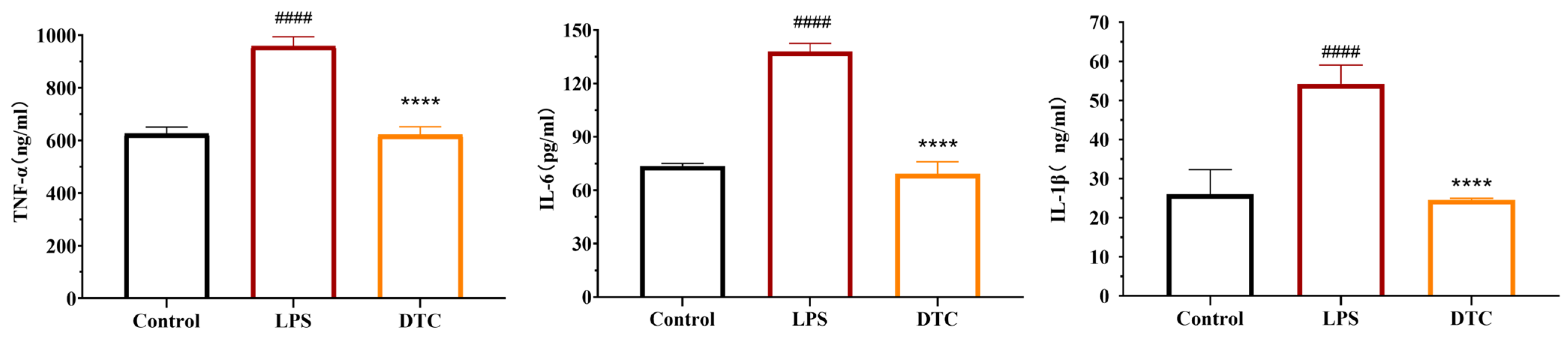
2.2.2. Effect of DTC on Inflammatory Factors in LPS-Stimulated RAW264.7 Cells
2.2.3. Bacteriostatic Activity of DTC
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.2. Biological Assays for Anti-EMT
4.2.1. Cell Culture and Drug Configuration
4.2.2. MTT Assay
4.2.3. qRT-PCR Assay
4.2.4. Cell Staining
4.2.5. Assay for Cell Scratches
4.3. Biological Assays for Anti-Inflammation
4.3.1. Cell Culture and Drug Configuration
4.3.2. MTT Assay
4.3.3. Effect of DTC on NO Content in RAW264.7 Cell Supernatant by Griess Method
4.3.4. Detection of TNF-α, IL-1β and IL-6 Levels in Cell Supernatants Using ELISA Kits
4.4. Assays for Antimicrobial Biologicals
4.4.1. Bacterial Activation and Culture
4.4.2. MIC and MBC of DTC
4.5. Analysis of Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jayachandran, J.; Srinivasan, H.; Mani, K.P. Molecular mechanism involved in epithelial to mesenchymal transition. Arch. Biochem. Biophys. 2021, 710, 108984. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-Y.; Chiang, Y.-F.; Huang, J.-S.; Huang, T.-C.; Shih, Y.-H.; Wang, K.-L.; Ali, M.; Hong, Y.-H.; Shieh, T.-M.; Hsia, S.-M. Isoliquiritigenin Reverses Epithelial-Mesenchymal Transition Through Modulation of the TGF-β/Smad Signaling Pathway in Endometrial Cancer. Cancers 2021, 13, 1236. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.; Chaudhary, R.; Rajput, S.; Agarwal, V.; Kaushik, A.S.; Srivastava, S.; Srivastava, S.; Singh, R.; Aziz, I.; Singh, S.; et al. Butein ameliorates chronic stress induced atherosclerosis via targeting anti-inflammatory, anti-fibrotic and BDNF pathways. Physiol. Behav. 2023, 267, 114207. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Liu, Y.; Li, T.; Liu, X.; Hao, Z.; Ding, S.; Panichayupakaranant, P.; Zhu, K.; Shen, J. Plant Natural Flavonoids Against Multidrug Resistant Pathogens. Adv. Sci. 2021, 8, e2100749. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Li, J.; Chen, X.; Fu, X.; Sun, S.; Wu, Q. Chalcone Derivatives: Role in Anticancer Therapy. Biomolecules 2021, 11, 894. [Google Scholar] [CrossRef] [PubMed]
- Marques, B.C.; Santos, M.B.; Anselmo, D.B.; Monteiro, D.A.; Gomes, E.; Saiki, M.F.; Rahal, P.; Rosalen, P.L.; Sardi, J.C.O.; Regasini, L.O. Methoxychalcones: Effect of Methoxyl Group on the Antifungal, Antibacterial and Antiproliferative Activities. Med. Chem. 2020, 16, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Warsito, W.; Murlistyarini, S.; Suratmo, S.; Azzahra, V.O.; Sucahyo, A. Molecular Docking Compounds of Cinnamaldehyde Derivatives as Anticancer Agents. Asian Pac. J. Cancer Prev. APJCP 2021, 22, 2409–2419. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Xiong, L.; Xie, X.; Tang, H.; Huang, R.; Peng, C. Isoliquiritigenin Derivative Regulates miR-374a/BAX Axis to Suppress Triple-Negative Breast Cancer Tumorigenesis and Development. Front. Pharmacol. 2020, 11, 378. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A Privileged Structure in Medicinal Chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.H. Discovery of 2′,4′-dimethoxychalcone as a Hsp90 inhibitor and its effect on iressa-resistant non-small cell lung cancer (NSCLC). Arch. Pharmacal Res. 2015, 38, 1783–1788. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Luo, F.; Wu, X.; Fan, B.; Yang, M.; Zhong, W.; Guan, D.; Wang, F.; Wang, Q. Anti-Inflammatory Effects and Mechanisms of Dandelion in RAW264.7 Macrophages and Zebrafish Larvae. Front. Pharmacol. 2022, 13, 906927. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Antin, P.; Berx, G.; Blanpain, C.; Brabletz, T.; Bronner, M.; Campbell, K.; Cano, A.; Casanova, J.; Christofori, G.; et al. Guidelines and definitions for research on epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2020, 21, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Li, F.-Z.; Cai, P.-C.; Song, L.-J.; Zhou, L.-L.; Zhang, Q.; Rao, S.-S.; Xia, Y.; Xiang, F.; Xin, J.-B.; Greer, P.-A.; et al. Crosstalk between calpain activation and TGF-β1 augments collagen-I synthesis in pulmonary fibrosis. Biochim. Biophys. Acta 2015, 1852, 1796–1804. [Google Scholar] [CrossRef] [PubMed]
- Oghbaei, F.; Zarezadeh, R.; Jafari-Gharabaghlou, D.; Ranjbar, M.; Nouri, M.; Fattahi, A.; Imakawa, K. Epithelial-mesenchymal transition process during embryo implantation. Cell Tissue Res. 2022, 388, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Marconi, G.D.; Fonticoli, L.; Rajan, T.S.; Pierdomenico, S.D.; Trubiani, O.; Pizzicannella, J.; Diomede, F. Epithelial-Mesenchymal Transition (EMT): The Type-2 EMT in Wound Healing, Tissue Regeneration and Organ Fibrosis. Cells 2021, 10, 1587. [Google Scholar] [CrossRef] [PubMed]
- Brabletz, S.; Schuhwerk, H.; Brabletz, T.; Stemmler, M.P. Dynamic EMT: A multi-tool for tumor progression. EMBO J. 2021, 40, e108647. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Chen, J.; Ren, G.; Zhang, Y.; Tan, X.; Yang, L. Punicalagin Prevents Inflammation in LPS-Induced RAW264.7 Macrophages by Inhibiting FoxO3a/Autophagy Signaling Pathway. Nutrients 2019, 11, 2794. [Google Scholar] [CrossRef] [PubMed]
- Henry, E.J.; Bird, S.J.; Gowland, P.; Collins, M.; Cassella, J.P. Ferrocenyl chalcone derivatives as possible antimicrobial agents. J. Antibiot. 2020, 73, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Koudokpon, H.; Armstrong, N.; Dougnon, T.V.; Fah, L.; Hounsa, E.; Bankolé, H.S.; Loko, F.; Chabrière, E.; Rolain, J.M. Antibacterial Activity of Chalcone and Dihydrochalcone Compounds from Uvaria chamae Roots against Multidrug-Resistant Bacteria. BioMed Res. Int. 2018, 2018, 1453173. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.P.R.; Basty, C.; Boopathi, S.; Dhivya, L.S.; Alarjani, K.M.; Gawwad, M.R.; Hager, R.; Kathiravan, M.K.; Arockiaraj, J. Furan-based Chalcone Annihilates the Multi-Drug-Resistant Pseudomonas aeruginosa and Protects Zebra Fish Against its Infection. J. Microbiol. 2024, 62, 75–89. [Google Scholar] [CrossRef] [PubMed]

| Compound | Structure | Antagonist IC10 (μM) |
|---|---|---|
| DTC | 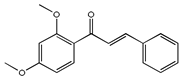 | 23.89 |
| Strains | MIC (μM) | MBC (μM) |
|---|---|---|
| S. aureus | 12.5 | 15 |
| P. aeruginosa | -- | -- |
| P. vulgaris | 15 | 20 |
| E. coli | -- | -- |
| MRSA | 15 | 20 |
| C. albicans | 10 | 12.5 |
| G. vaginalis | -- | -- |
| S. agalactiae | -- | -- |
| E. faecalis | -- | -- |
| B. subtilis | -- | -- |
| Gene Name | Primer Sequence (from 5′ to 3′) | |
|---|---|---|
| E-Cadherin | Forward | 5-GAGTGCCAACTGGACCATTCAGTA-3′ |
| Reverse | 5′-CACAGTCACACACGCTGACCTCTA-3′ | |
| Vimentin | Forward | 5′-TGACATTGAGATTGCCACCTACAG-3′ |
| Reverse | 5′-TCAACCGTCTTAATCAGAAGTGTCC-3′ | |
| β-actin | Forward | 5′-TGACGTGGACATCCGCAAAG-3′ |
| Reverse | 5′-CTGGAAGGTGGACAGCGAGG-3′ |
| Name | Source |
|---|---|
| S. aureus (ATCC: 23235) | Courtesy of Department of Microbiology, Shandong University of Traditional Chinese Medicine |
| P. aeruginosa (ATCC: 27853) | Courtesy of Department of Microbiology, Shandong University of Traditional Chinese Medicine |
| P. vulgaris (ATCC: 49132) | Courtesy of Department of Microbiology, Shandong University of Traditional Chinese Medicine |
| E. coli (ATCC: 25922) | Courtesy of Department of Microbiology, Shandong University of Traditional Chinese Medicine |
| MRSA (ATCC: BAA-172) | Courtesy of Department of Microbiology, Shandong University of Traditional Chinese Medicine |
| C. albicans (ATCC: 18804) | Courtesy of Department of Microbiology, Shandong University of Traditional Chinese Medicine |
| G. vaginalis (ATCC: 49145) | Courtesy of Department of Microbiology, Shandong University of Traditional Chinese Medicine |
| S. agalactiae (ATCC: 13813) | Courtesy of Department of Microbiology, Shandong University of Traditional Chinese Medicine |
| E. faecalis (ATCC: 700802) | Courtesy of Department of Microbiology, Shandong University of Traditional Chinese Medicine |
| B. subtilis (ATCC: 6051) | Courtesy of Department of Microbiology, Shandong University of Traditional Chinese Medicine |
| Name | Source |
|---|---|
| Sabouraud Dextrose Broth Medium | Qingdao Hi-Tech Industrial Park Haibo Biotechnology Co. (Qingdao, China) |
| Columbia Broth | Shandong Top Biological Engineering Co. (Yantai, China) |
| MH | Qingdao Hi-Tech Industrial Park Haibo Biotechnology Co. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, P.; Xu, M.; Gong, K.; Lu, K.; Ruan, C.; Yu, X.; Zhu, J.; Guan, H.; Zhu, Q. Testing of Anti-EMT, Anti-Inflammatory and Antibacterial Activities of 2′,4′-Dimethoxychalcone. Pharmaceuticals 2024, 17, 653. https://doi.org/10.3390/ph17050653
Zhao P, Xu M, Gong K, Lu K, Ruan C, Yu X, Zhu J, Guan H, Zhu Q. Testing of Anti-EMT, Anti-Inflammatory and Antibacterial Activities of 2′,4′-Dimethoxychalcone. Pharmaceuticals. 2024; 17(5):653. https://doi.org/10.3390/ph17050653
Chicago/Turabian StyleZhao, Peiling, Mengzhen Xu, Kai Gong, Kaihui Lu, Chen Ruan, Xin Yu, Jiang Zhu, Haixing Guan, and Qingjun Zhu. 2024. "Testing of Anti-EMT, Anti-Inflammatory and Antibacterial Activities of 2′,4′-Dimethoxychalcone" Pharmaceuticals 17, no. 5: 653. https://doi.org/10.3390/ph17050653
APA StyleZhao, P., Xu, M., Gong, K., Lu, K., Ruan, C., Yu, X., Zhu, J., Guan, H., & Zhu, Q. (2024). Testing of Anti-EMT, Anti-Inflammatory and Antibacterial Activities of 2′,4′-Dimethoxychalcone. Pharmaceuticals, 17(5), 653. https://doi.org/10.3390/ph17050653




