Abstract
Background: Epilepsy is defined by an excessive level of activity in the neurons and coordinated bursts of electrical activity, resulting in the occurrence of seizure episodes. The precise cause of epileptogenesis remains uncertain; nevertheless, the etiology of epilepsy may involve neuroinflammation, oxidative stress, and malfunction of the neurotransmitter system. Objective: The goal of this investigation was to assess barbaloin’s protective properties with respect to pentylenetetrazol (PTZ)-)-induced cognitive deficits in rats via antioxidative, anti-inflammatory, and neurotransmitter-modulating effects. Methods: Wistar rats were subjected to PTZ [40 mg/kg (i.p.)], which induced cognitive decline. Behavior assessment using a kindling score, open-field test (OFT), novel object recognition test (NORT), and assays for superoxide dismutase (SOD), reduced glutathione (GSH), catalase (CAT), malondialdehyde (MDA), acetylcholinesterase (AChE), caspase-3, nitric oxide (NO), interleukins-1β (IL-1β), tumor necrosis factor-α (TNF-α), IL-6, nuclear factor kappa-B (NF-κB), Bcl-2 and Bax, and neurotransmitter levels [GABA, DA, NE, and serotonin (5-HT)] were performed. Results: The treatment of rats with barbaloin resulted in behavior improvement and significant changes in the levels of GSH, SOD, CAT, MDA, AChE, NO, IL-6, IL-1β, TNF-α, NF-κB, caspase-3, Bcl-2, and Bax compared to the PTZ control group. Barbaloin treatment resulted in notable changes in neurotransmitter levels (GABA, NE, 5-HT, DA) compared to the PTZ group. Conclusions: The ongoing study has gathered evidence indicating that the injection of barbaloin has resulted in significant improvements in cognitive performance in rats. This is achieved by inhibiting oxidative stress, enhancing the activity of natural antioxidant enzymes, reducing cytokine levels, and increasing the levels of neurotransmitters in the brain. These results were detected in comparison to a PTZ control and can be attributed to the potent anti-inflammatory and antioxidant capabilities of barbaloin, which could be linked to its neuroprotective properties. Barbaloin may potentially increase cognitive decline and boost neuronal survival by altering the expression of Bax, caspase-3, Bcl-2.
1. Introduction
Epilepsy is a widespread neurological condition that impacts around 1% of the global population, with a significantly greater occurrence in poorer nations [1]. Epilepsy is defined by an excessive level of activity in the neurons and coordinated bursts of electrical activity, resulting in the occurrence of seizure episodes [2]. Multiple sources of evidence have shown that inflammatory markers enhance the excitability of neurons, increase the blood–brain barrier’s (BBR) permeability, activate glial cells, and induce neuronal death. Evidence demonstrates that the secretion of cytokines and chemokines by activated glial cells, specifically astrocytes and microglia, is highly significant in the development and advancement of neurological illnesses such as epilepsy [3,4,5]. The precise cause of epileptogenesis remains uncertain; nevertheless, the etiology of epilepsy may involve neuroinflammation, oxidative stress, and malfunction of the neurotransmitter system [6].
The transcription factor Nrf2 controls antioxidant function by inducing the expression of many antioxidant enzymes, like reduced glutathione (GSH) and superoxide dismutase (SOD)-related enzymes. Consequently, the Nrf2 molecule was specifically focused on during the creation of anti-epileptic medications [7]. Furthermore, the presence of neuroinflammation is indicated by the activation of neuroglial cells and the release of cytokines. This has been proposed to intensify epileptic convulsions. Reported cytokines such as nuclear factor kappa B (NF-κB), GFAP, tumor necrosis factor-α (TNF-α), and IL-6 have been shown to produce neuronal hyperexcitability, leading to the development of seizures [8,9].
Pentylenetetrazol (PTZ)-kindling is a long-term model of epilepsy, a condition marked by a progressive escalation of seizures. PTZ-induced kindling induces alterations occurring at the cellular and molecular level, which contribute to neural plasticity [10,11,12]. The induction of the kindling model is achieved with the repeated administration of PTZ as an antagonist of γ-aminobutyric acid type A (GABA-A) at sub-convulsive dosages [13,14]. Studies have demonstrated that the injection of PTZ leads to the death of neurons and the activation of glial cells in particular areas of the hippocampus [15]. Furthermore, the researchers employed PTZ kindling, a well-established paradigm commonly utilized to investigate the processes behind epileptogenesis, as well as the cognitive impairments resulting from seizures [16,17]. With repeated PTZ administration, each injection triggers a seizure, and the severity of the seizures gradually increases [18]. Preclinical and clinical research provide compelling data indicating that epilepsy may lead to neuroinflammation [19]. Excessive inflammatory processes are linked to impaired neuronal function, and proinflammatory cytokines contribute to the development of seizures by triggering the infiltration of white blood cells, disrupting the blood–brain barrier and boosting the oxidation of lipids [20,21].
Herbal medications provide promising and valuable reservoirs of medicinal compounds [22,23]. Barbaloin is an organic compound with bioactive properties that is derived from the Aloe vera L. plant. Barbaloin, like A. vera, has several pharmacological impacts such as anti-inflammatory, antimicrobial, and antioxidant effects [24,25,26]. Prior studies have confirmed that barbaloin triggers the overexpression of IL-6, tumor necrosis factor-α, and IL-1β by activating NF-κB [26,27,28,29]. Moreover, the impact was notably reduced when the PI3K/AKT signaling was obstructed. The previous results provide empirical evidence that substantiates the notion that barbaloin effectively reduces the generation of reactive oxygen species (ROS) in cells by impeding the phosphorylation process of PI3K and AKT. Consequently, this obstacle inhibits the stimulation of NF-κB [27,28]. The findings suggest that barbaloin possesses antidiabetic, antioxidant, neuroinflammatory, cytokine-inhibitory, and acetylcholinesterase (AChE)-inhibiting properties. These properties may contribute to its ability to protect against cognitive decline caused by STZ. Barbaloin may have potential clinical applications in the treatment of neurological and cognitive deficits in individuals with diabetes [28]. Barbaloin’s ability to improve cognitive function stems from its ability to inhibit oxidative stress and enhance the activity of endogenous antioxidant enzymes within the brain [30]. By minimizing the damage caused by oxidative stress, barbaloin can preserve the integrity of the brain’s structure and function, leading to improved cognitive performance.
There has been no research conducted on the impact of barbaloin in epilepsy animal models. The objective of the current study was to investigate the impact of barbaloin on seizure tendencies and cognitive decline in a PTZ-induced kindling paradigm. In addition, the concentration of inflammation, as well as caspase-3, Bcl-2-associated X protein (Bax), B-cell lymphoma 2 (Bcl-2) marker, and neurotransmitter content in the brain, were assessed in rats treated with barbaloin. The observed results were compared to a PTZ control and can be attributed to the potent anti-neuroinflammatory and oxidative stress decline capabilities of barbaloin, which may enhance its ability to protect against neuronal damage.
2. Results
2.1. Kindling Score
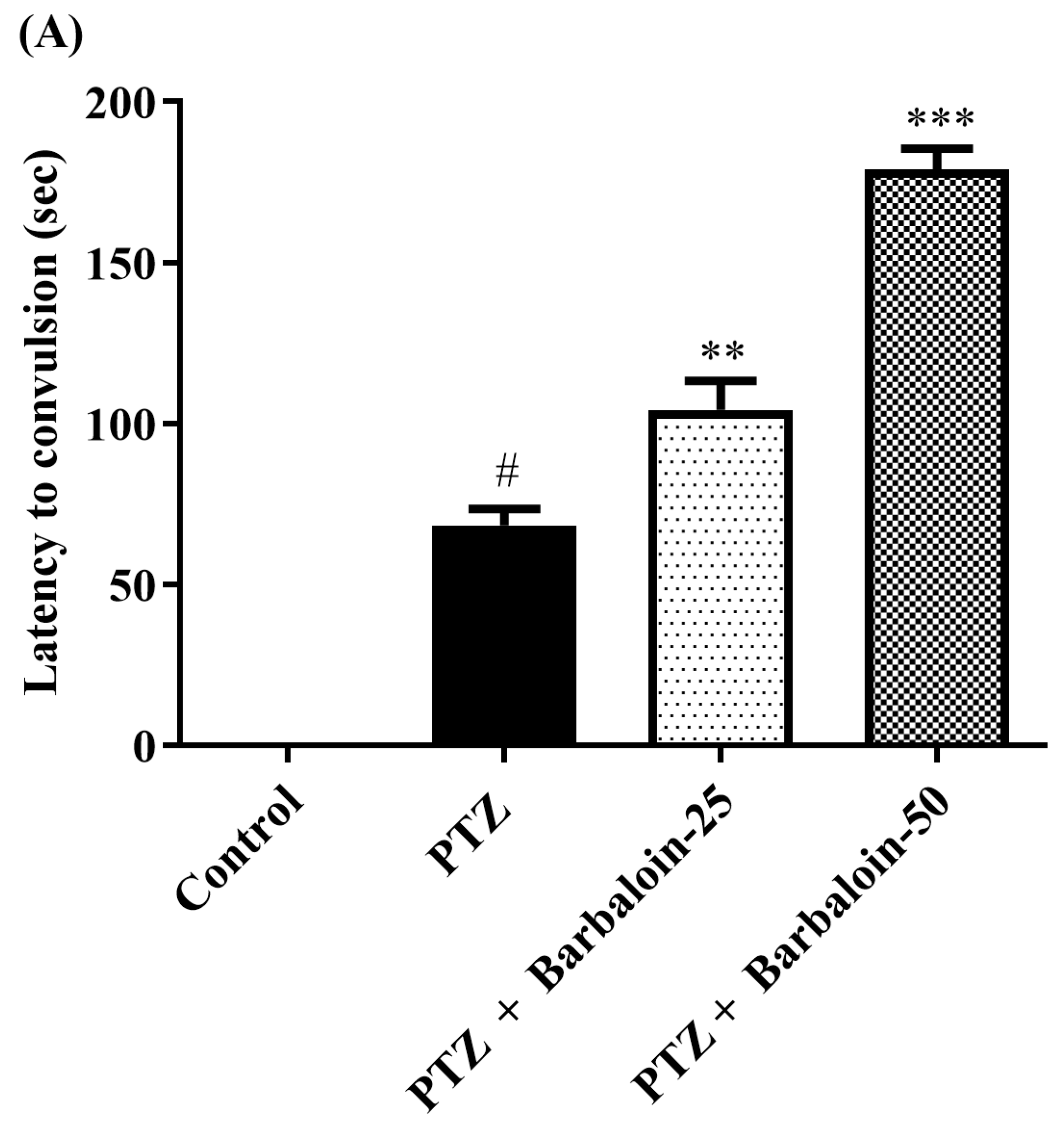
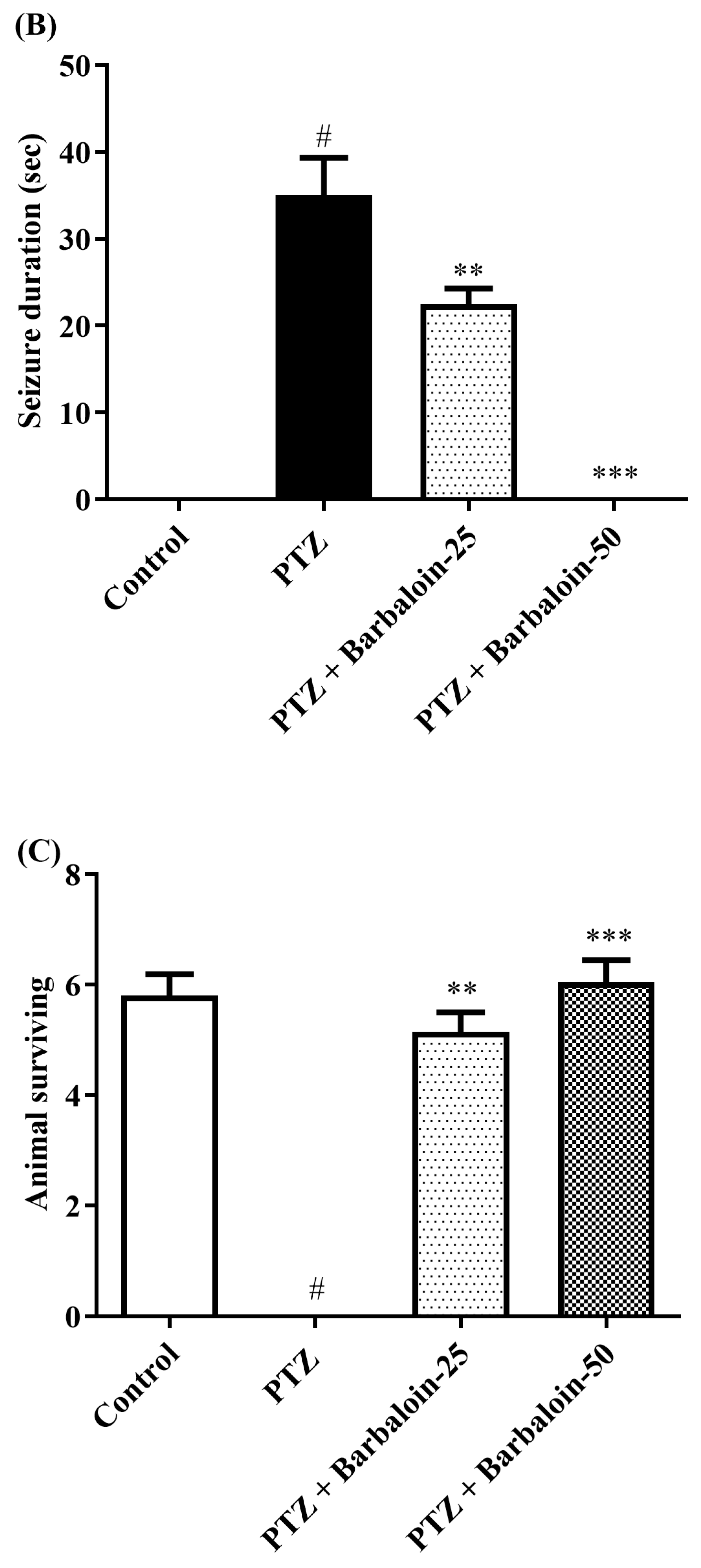
The administration of PTZ induced generalized tonic–clonic seizures (GTCS), which were assessed using the Racine scale. According to the data shown in Figure 1A–C, the administration of barbaloin for a period of 4 weeks significantly reduced the duration of seizures [F (3, 20) = 55.54, p < 0.0001]. The treated groups had a significantly prolonged latent time before the onset of seizures compared to the PTZ control rats [F (3, 20) = 148.7, p < 0.0001]. Furthermore, the mortality rate was significantly decreased in the treated groups compared to the PTZ control group [F (3, 20) = 76.09, p < 0.0001]. The findings indicate that barbaloin has anticonvulsant properties against PTZ-induced seizures.
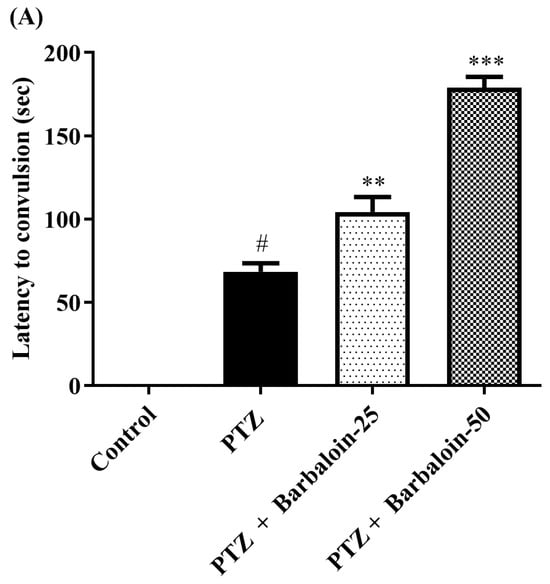
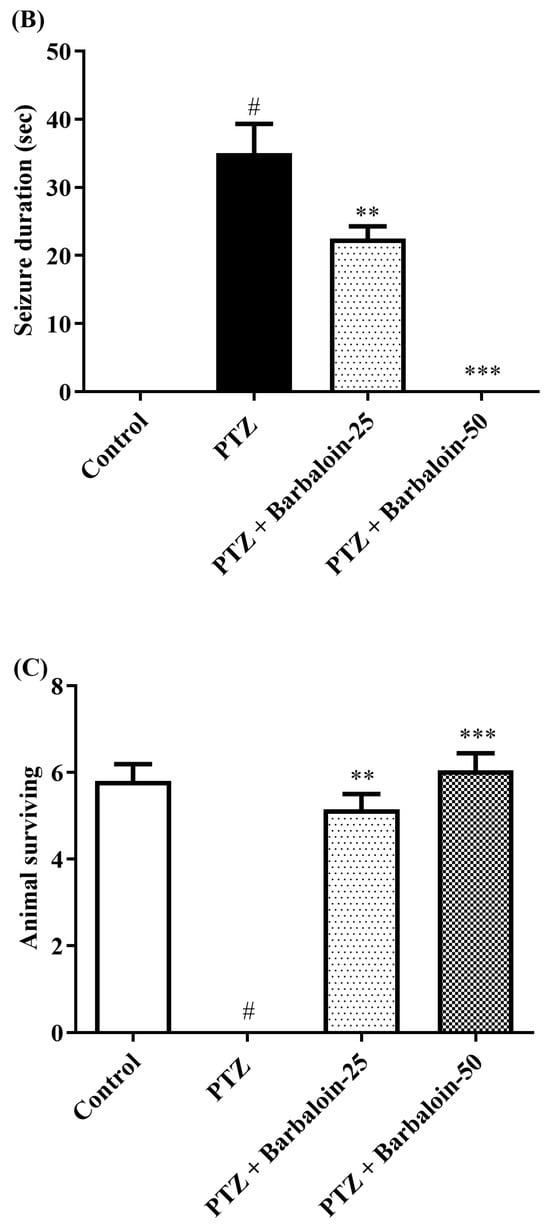
Figure 1.
(A–C) Outcome of barbaloin on kindling score. Mean ± S.E.M (n = 6). One-way ANOVA followed by Tukey’s post hoc test (n = 6). # p < 0.001 vs. control, ** p < 0.001, *** p < 0.0001 vs. PTZ.
2.2. Open Field Test (OFT)
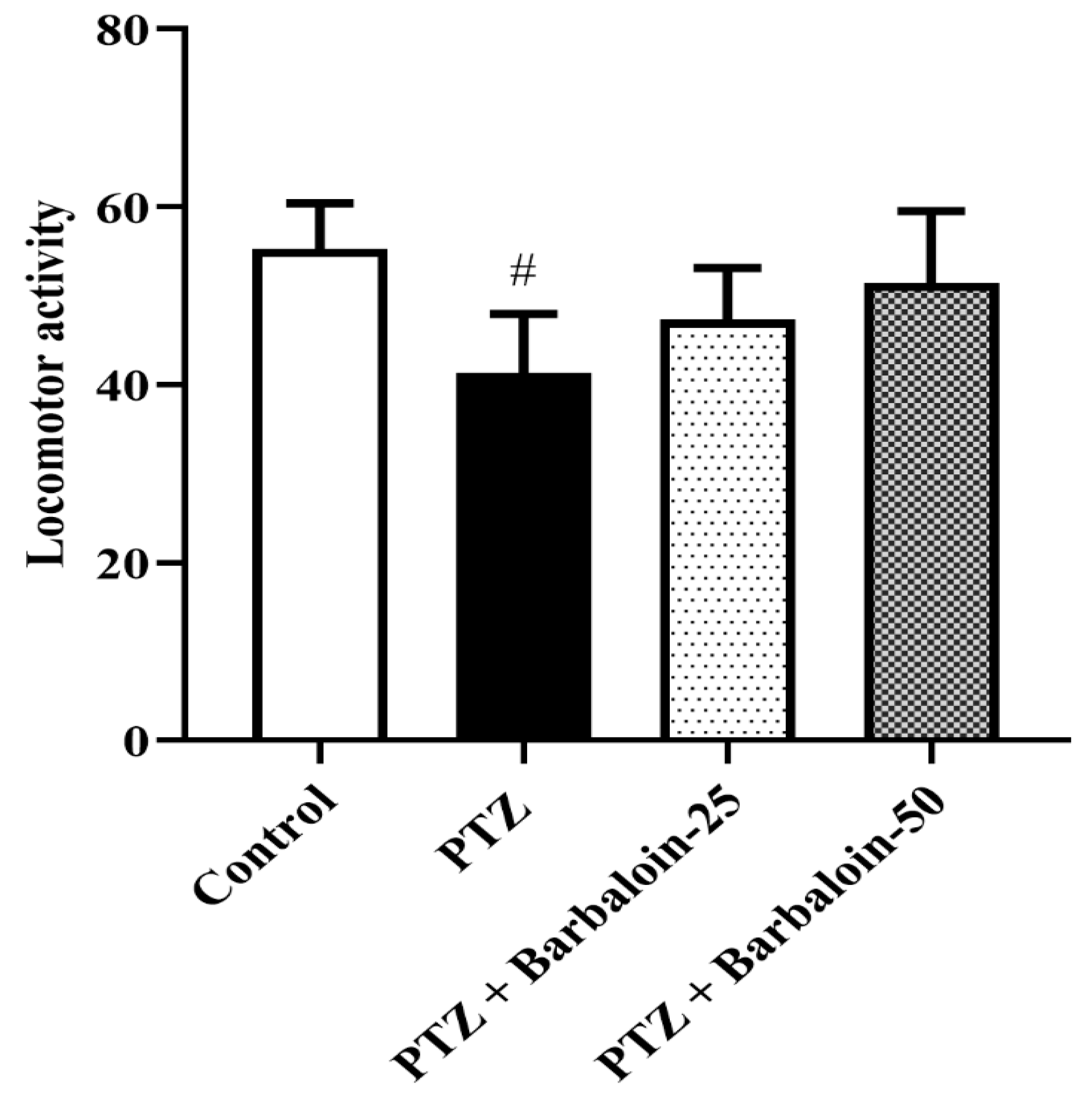
PTZ control rats had no significant effect on exploratory and locomotor in comparison to rats in normal control (p < 0.0001). Treatment with barbaloin [F (3, 20) = 0.8348, p = 0.4905] also had no significant effect compared to PTZ control rats (Figure 2).
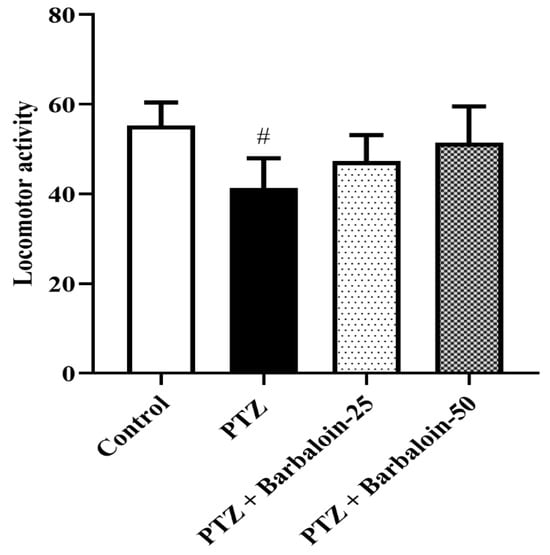
Figure 2.
The effect of barbaloin on OFT. Mean ± S.E.M. One-way ANOVA followed by Tukey’s post hoc test (n = 6). # p < 0.001 vs. control.
2.3. Novel Object Recognition Test (NORT)
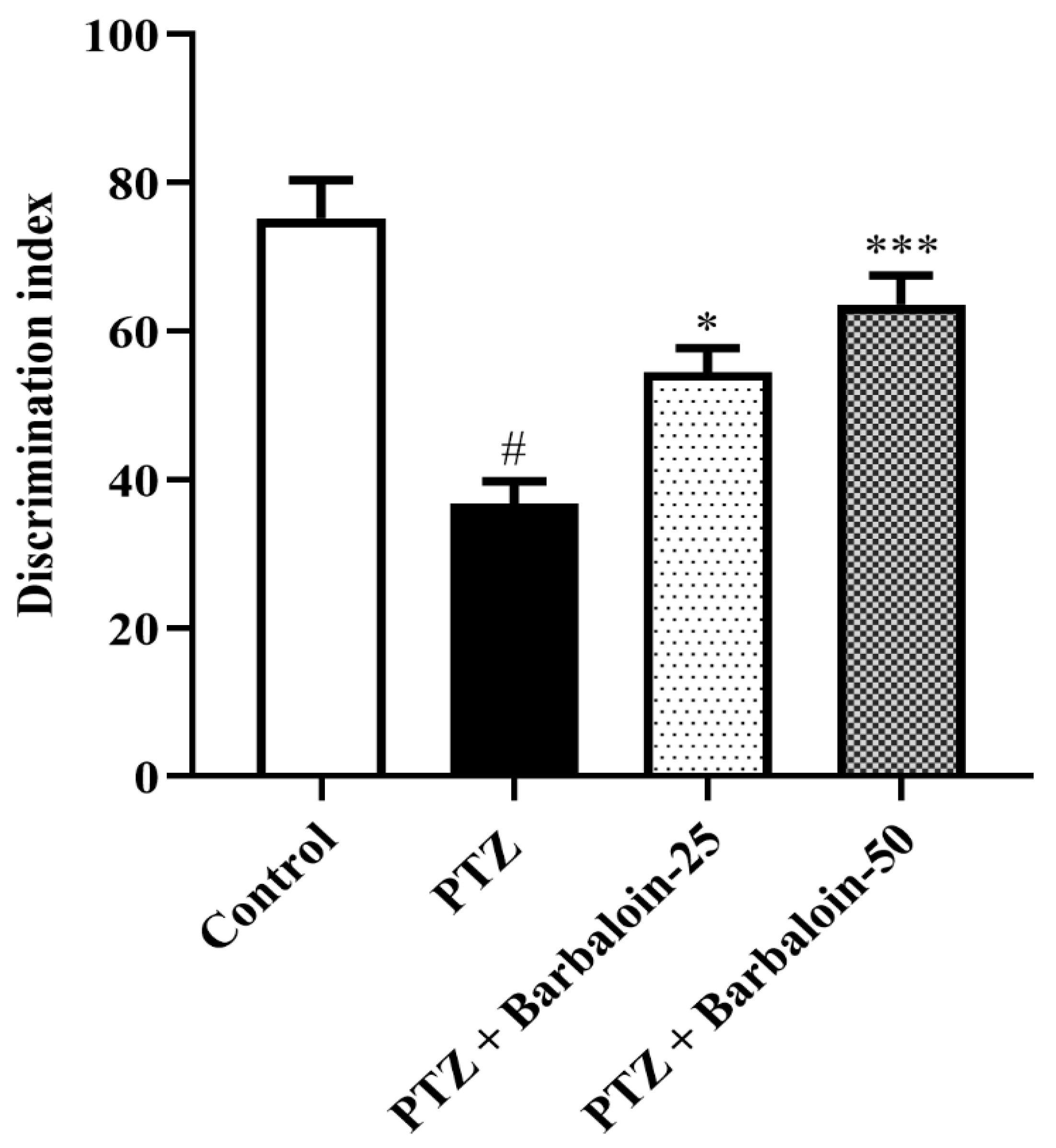
PTZ control rats displayed a significantly decreased discrimination index in comparison to normal control rats (p < 0.0001). Treatment with barbaloin enhanced the discrimination index [F (3, 20) = 16.87, p < 0.0001] compared to PTZ control rats (Figure 3).
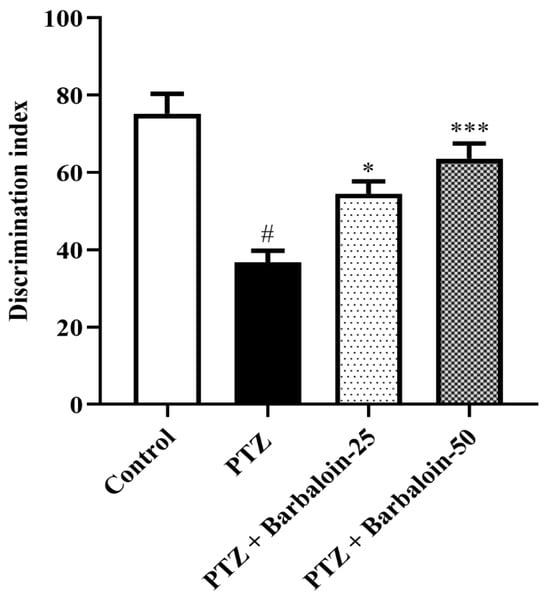
Figure 3.
The effect of barbaloin on NORT. Mean ± S.E.M. One-way ANOVA followed by Tukey’s post hoc test (n = 6). # p < 0.001 vs. control, * p < 0.05, *** p < 0.0001 vs. PTZ.
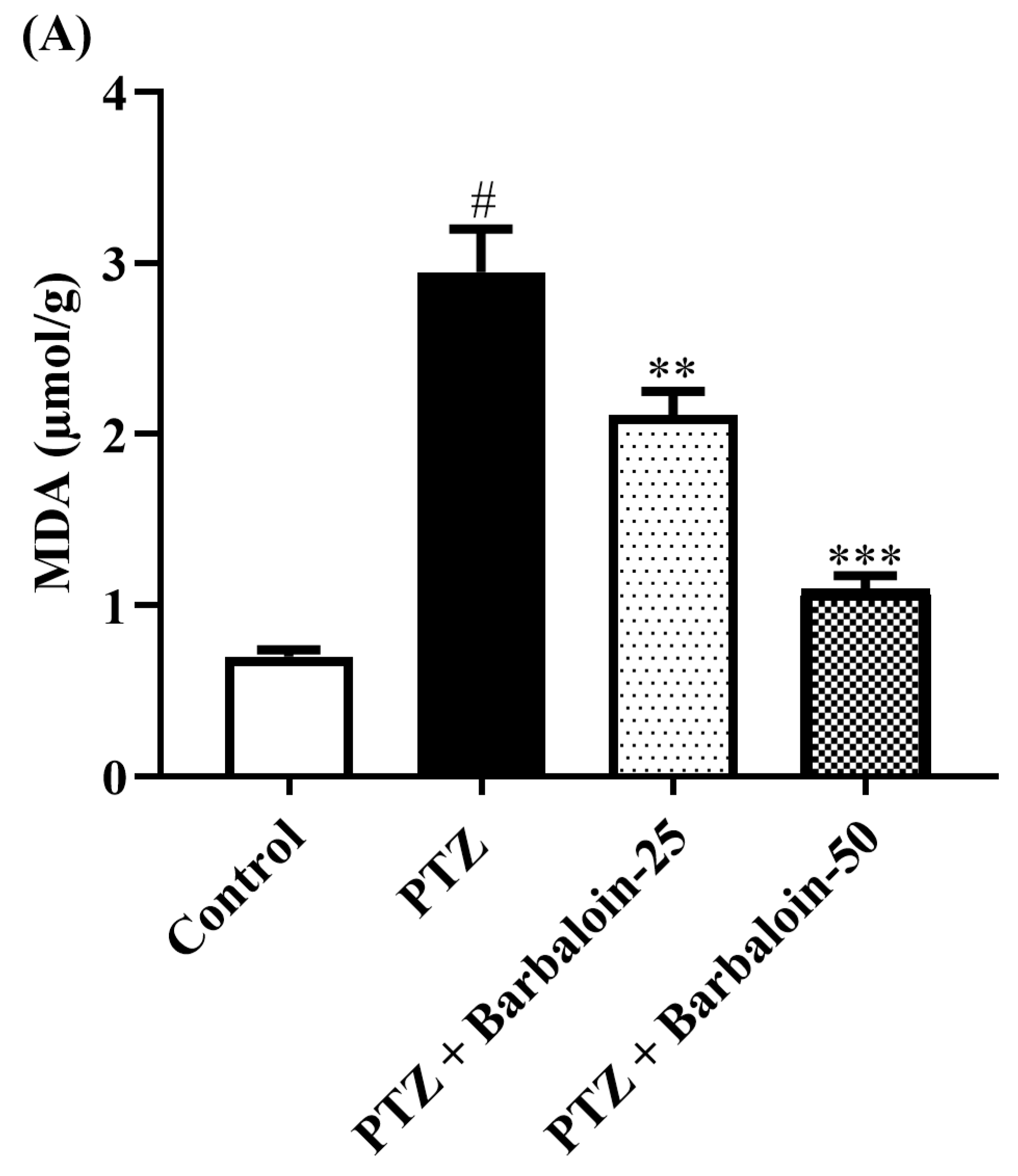
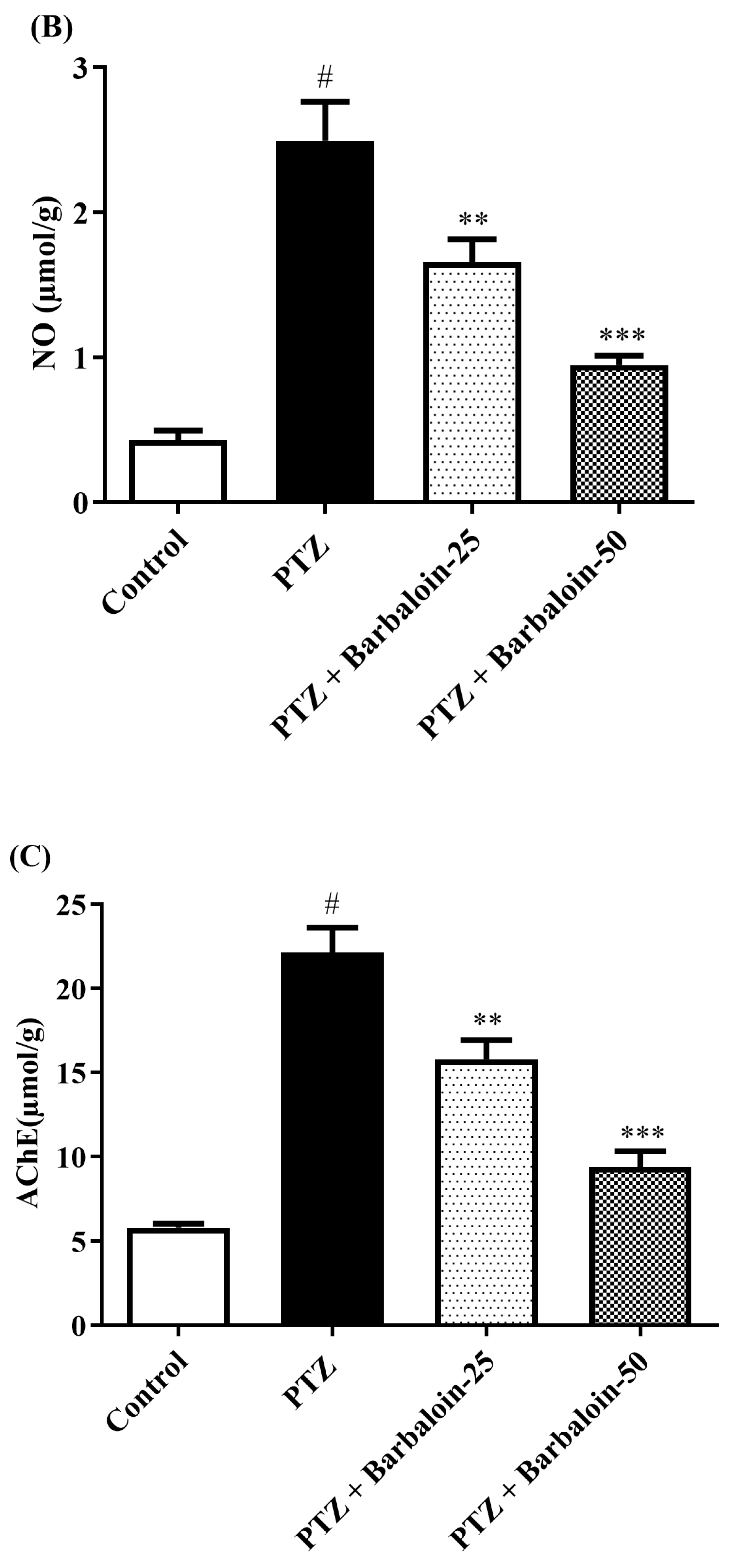
2.4. Malondialdehyde (MDA), Nitric Oxide (NO), AChE Estimation
PTZ control rats significantly enhanced brain MDA, NO, and AChE concentration in comparison to rats in normal control (p < 0.0001). Treatment with barbaloin lowered the MDA [F (3, 20) = 45.40, p < 0.0001], NO [F (3, 20) = 30.54, p < 0.0001], and AChE [F (3, 20) = 46.54, p < 0.0001] levels, respectively, compared to PTZ control rats (Figure 4A–C).
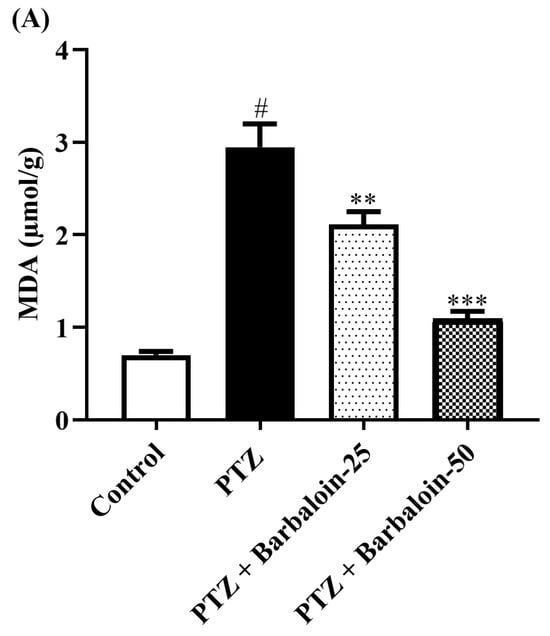
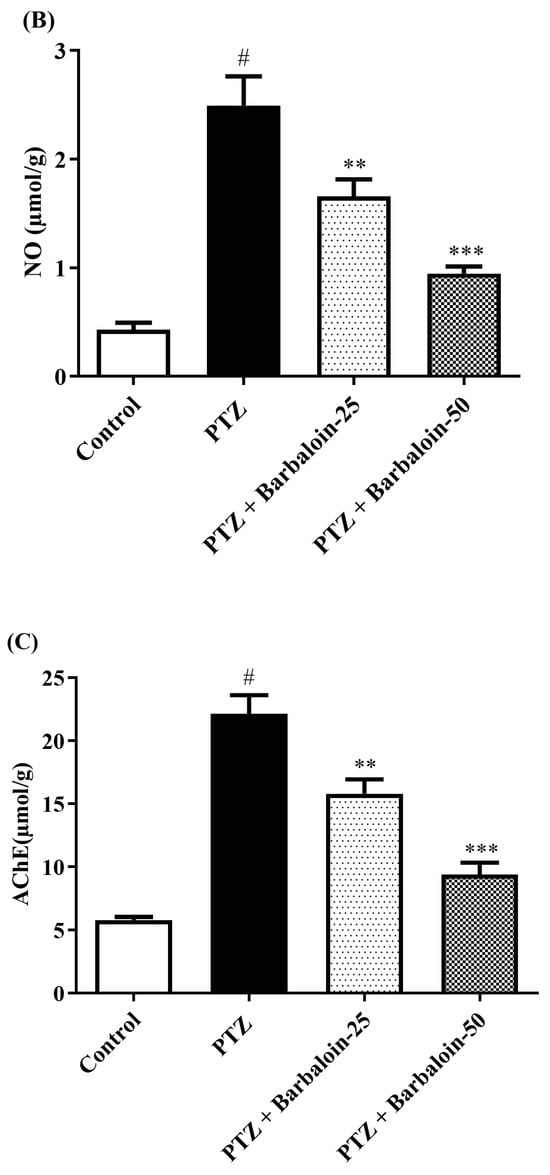
Figure 4.
(A–C) The effect of barbaloin on MDA, NO, and AChE levels. Mean ± S.E.M. One-way ANOVA followed by Tukey’s post hoc test (n = 6). # p < 0.001 vs. control, ** p < 0.001, *** p < 0.0001 vs. PTZ.
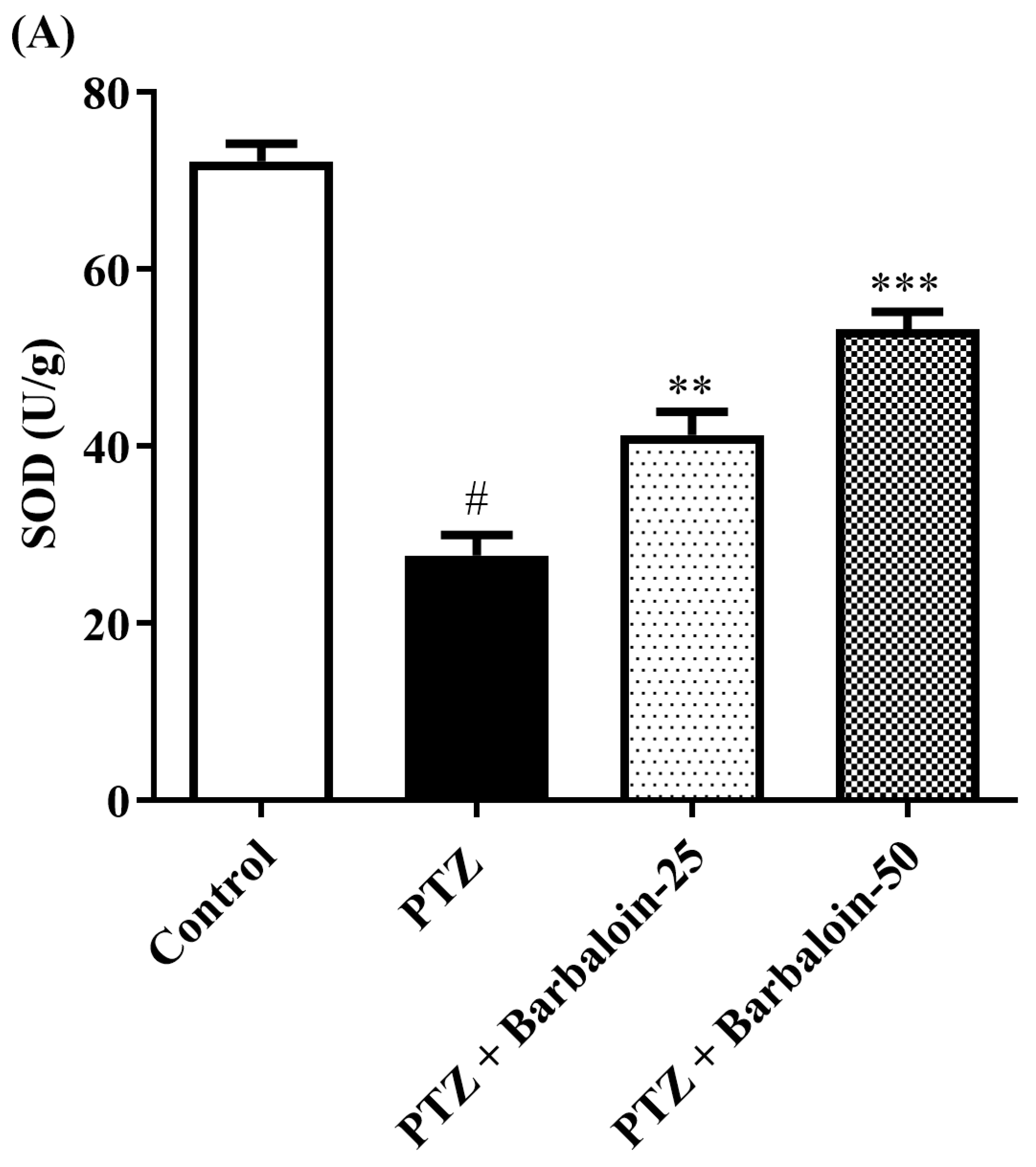
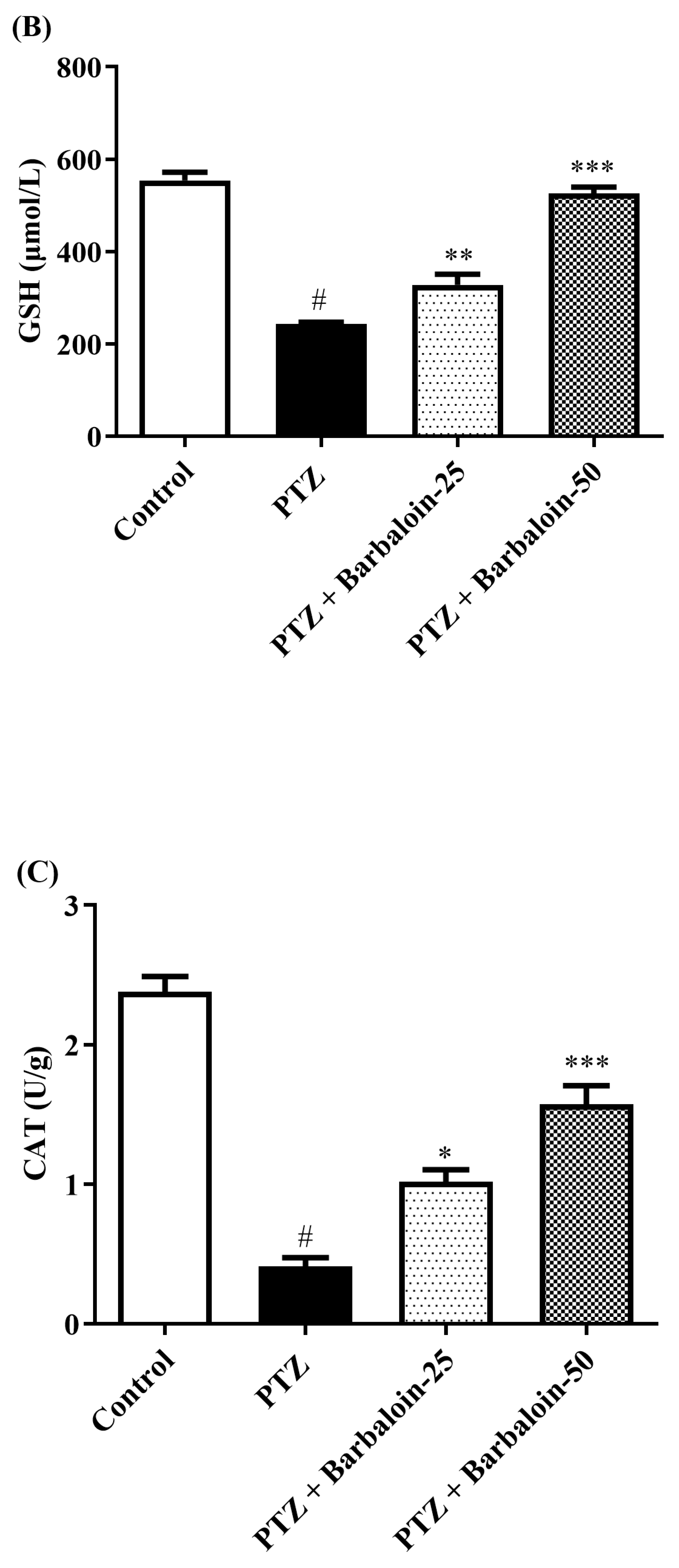
2.5. SOD, GSH, Catalase (CAT) Estimation
PTZ control rats significantly decreased brain SOD, GSH, and CAT concentration in comparison to rats in normal control (p < 0.0001). Treatment with barbaloin enhanced brain SOD [F (3, 20) = 70.84, p < 0.0001], GSH [F (3, 20) = 86.95, p < 0.0001], and CAT [F (3, 20) = 68.84, p < 0.0001] levels, respectively, compared to PTZ control rats (Figure 5A–C).
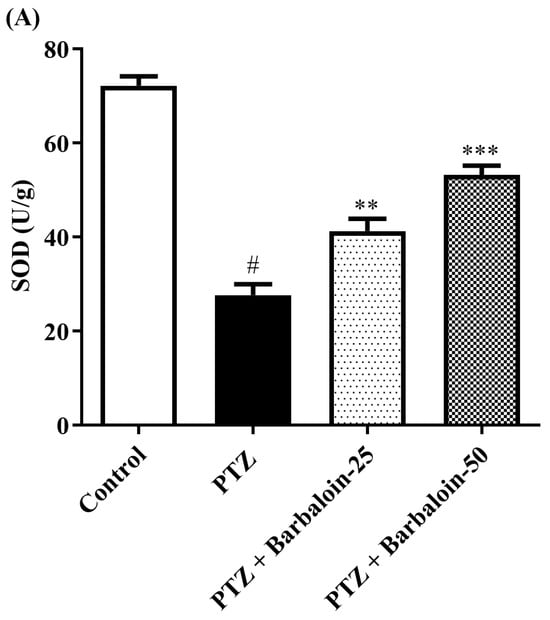
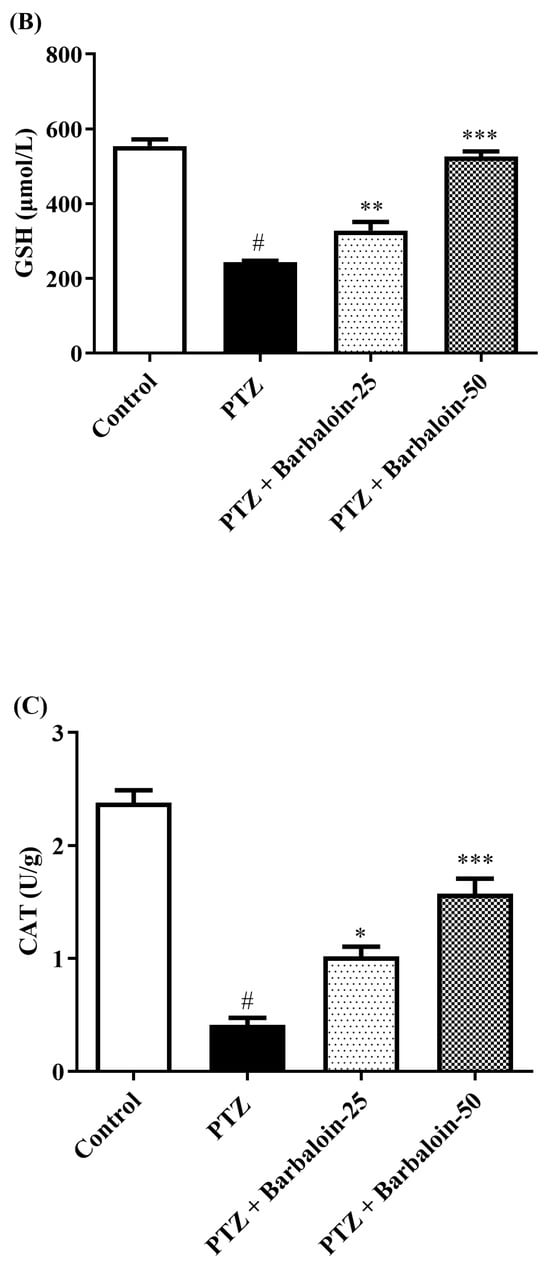
Figure 5.
(A–C) The effect of barbaloin on SOD, GSH, and CAT levels. Mean ± S.E.M. One-way ANOVA followed by Tukey’s post hoc test (n = 6). # p < 0.001 vs. control, * p < 0.05, ** p < 0.001, *** p < 0.0001 vs. PTZ.
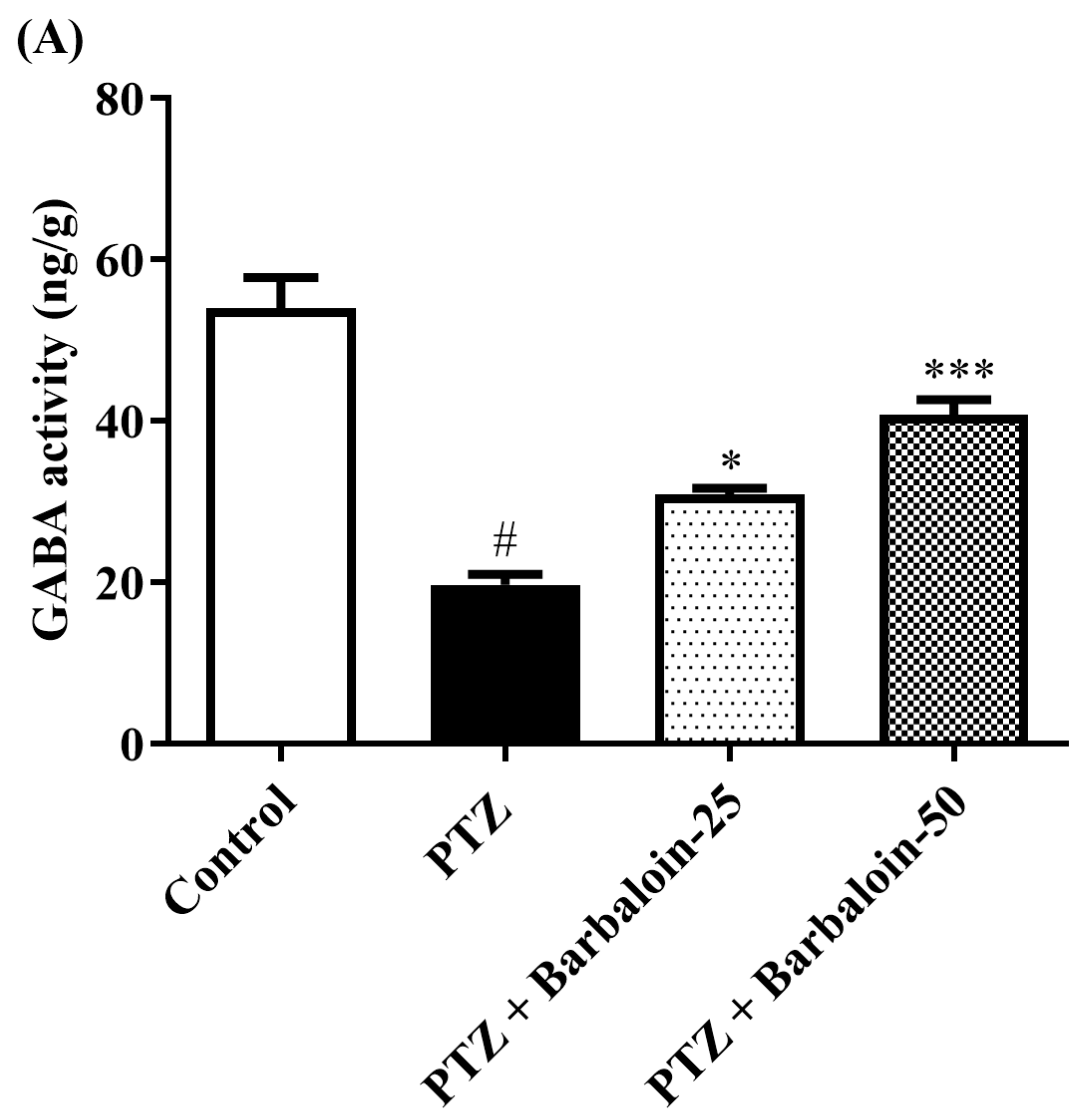
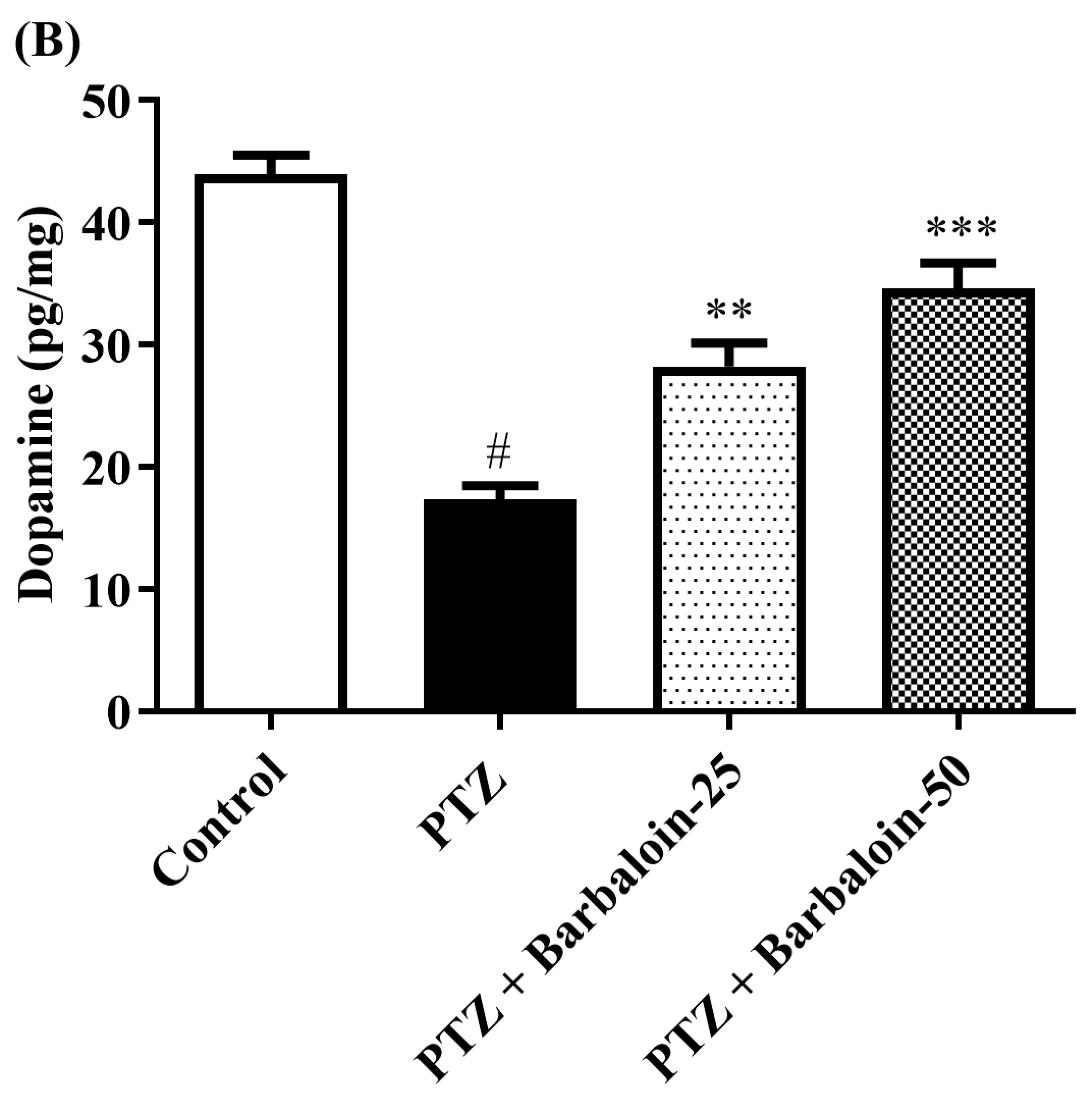
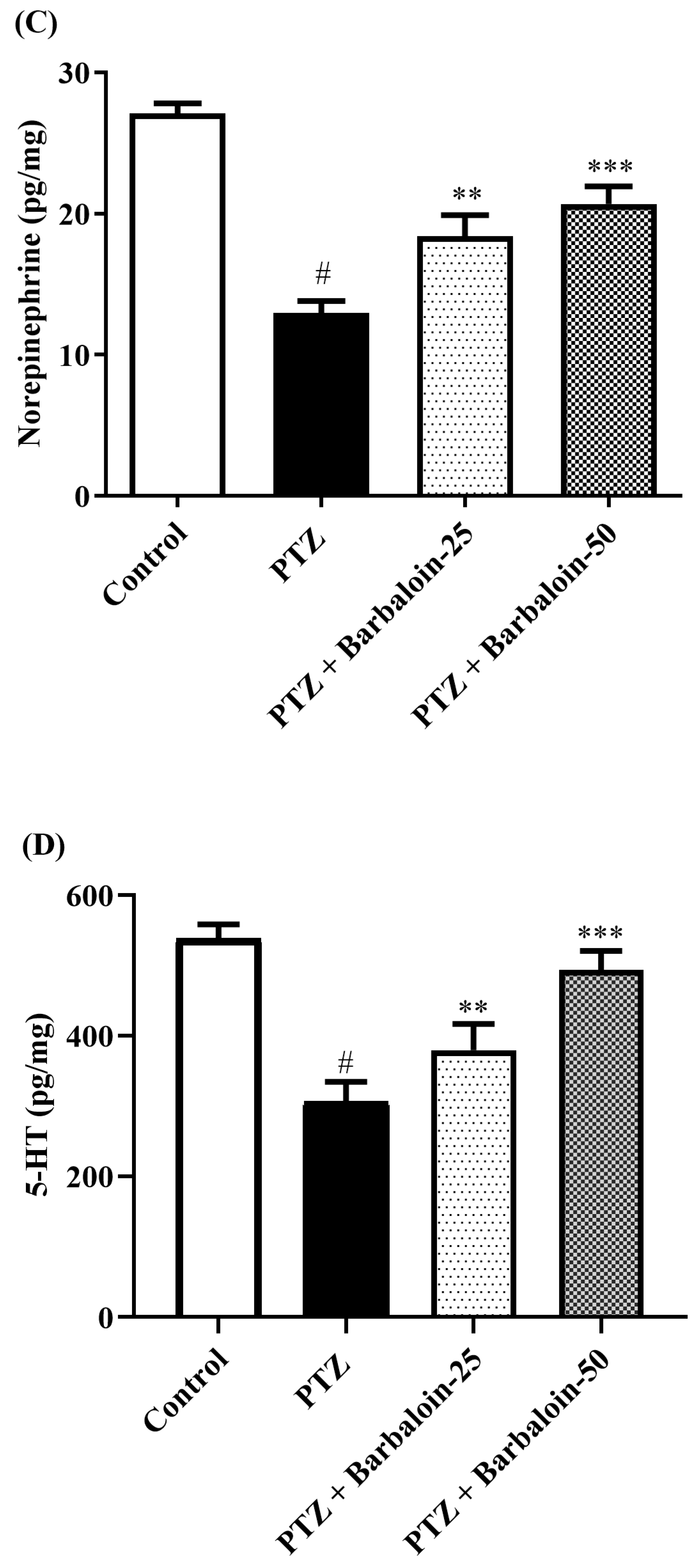
2.6. Brain GABA, Dopamine (DA), Norepinephrine (NE), and 5-Hydroxytryptamine (5-HT) Contents
Under comparable circumstances, PTZ control rats exhibited a significant decrease in GABA, DA, NE, and 5-HT levels in the brain compared to the normal control (p < 0.0001). Treatment with barbaloin enhanced the GABA [F (3, 20) = 41.59, p < 0.0001], DA [F (3, 20) = 42.39, p < 0.0001], NE [F (3, 20) = 26.74, p < 0.0001], and 5-HT [F (3, 20) = 79.84, p < 0.0001] contents in comparison to PTZ control rats (Figure 6A–D).
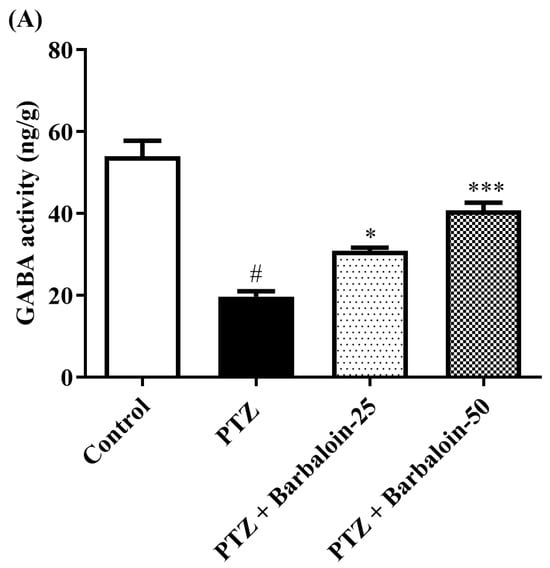
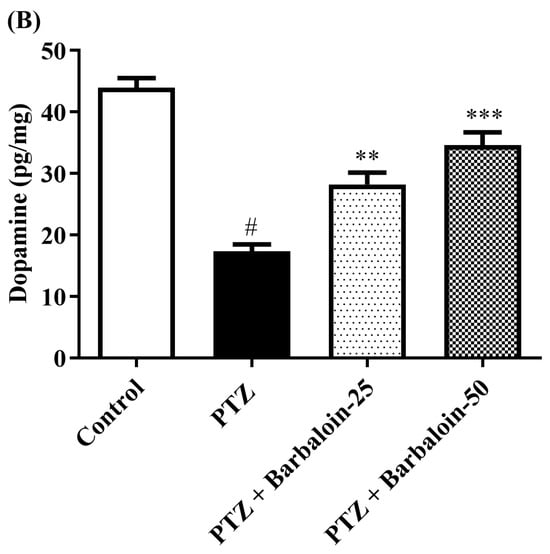
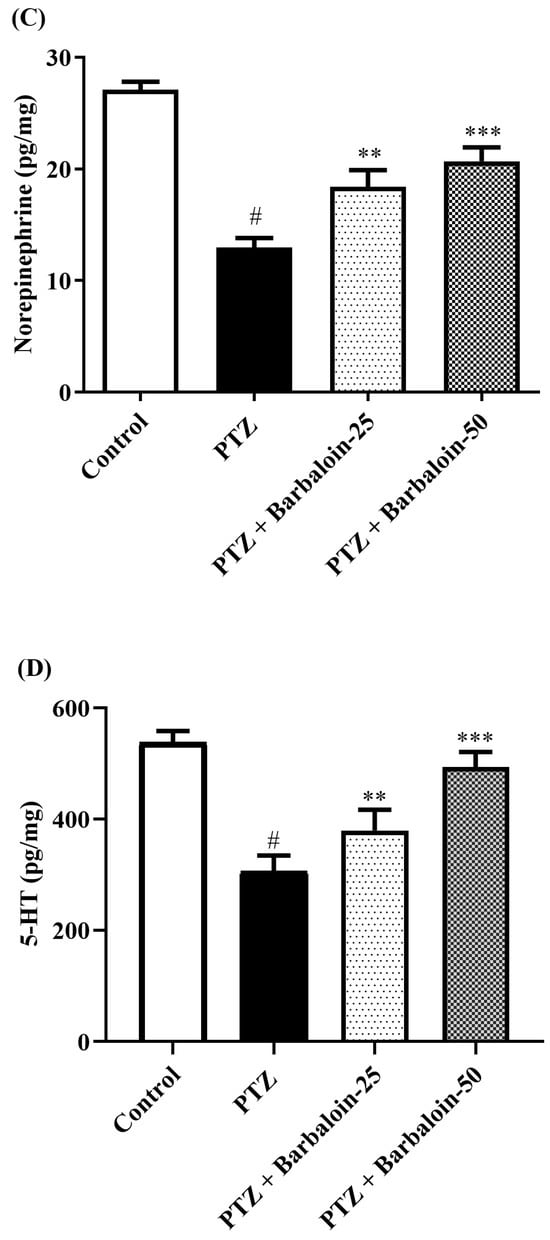
Figure 6.
(A–D) The effect of barbaloin on GABA, DA, NE, and 5-HT levels. Mean ± S.E.M. One-way ANOVA followed by Tukey’s post hoc test (n = 6). # p < 0.001 vs. control, * p < 0.05, ** p < 0.001, *** p < 0.0001 vs. PTZ.
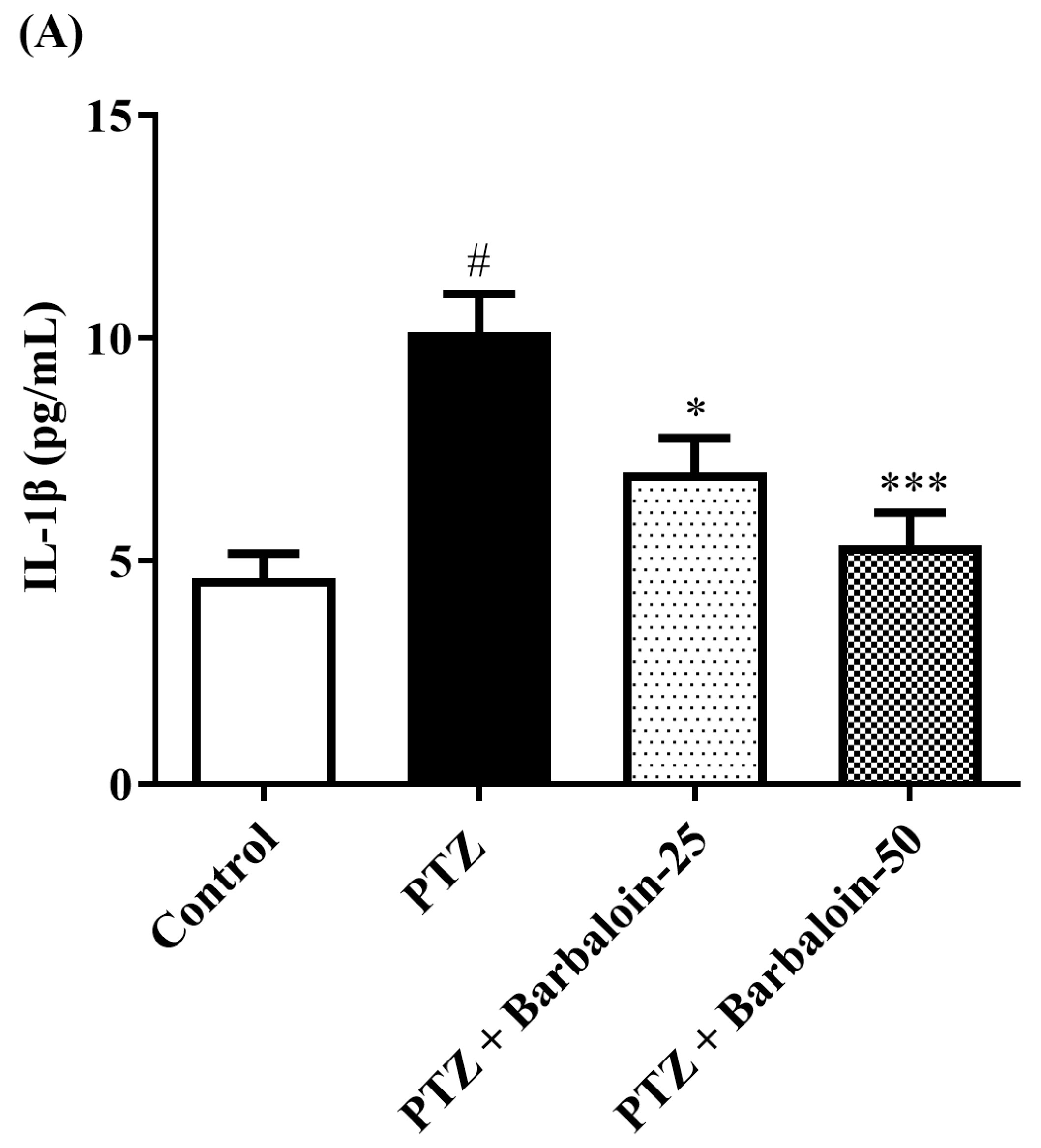
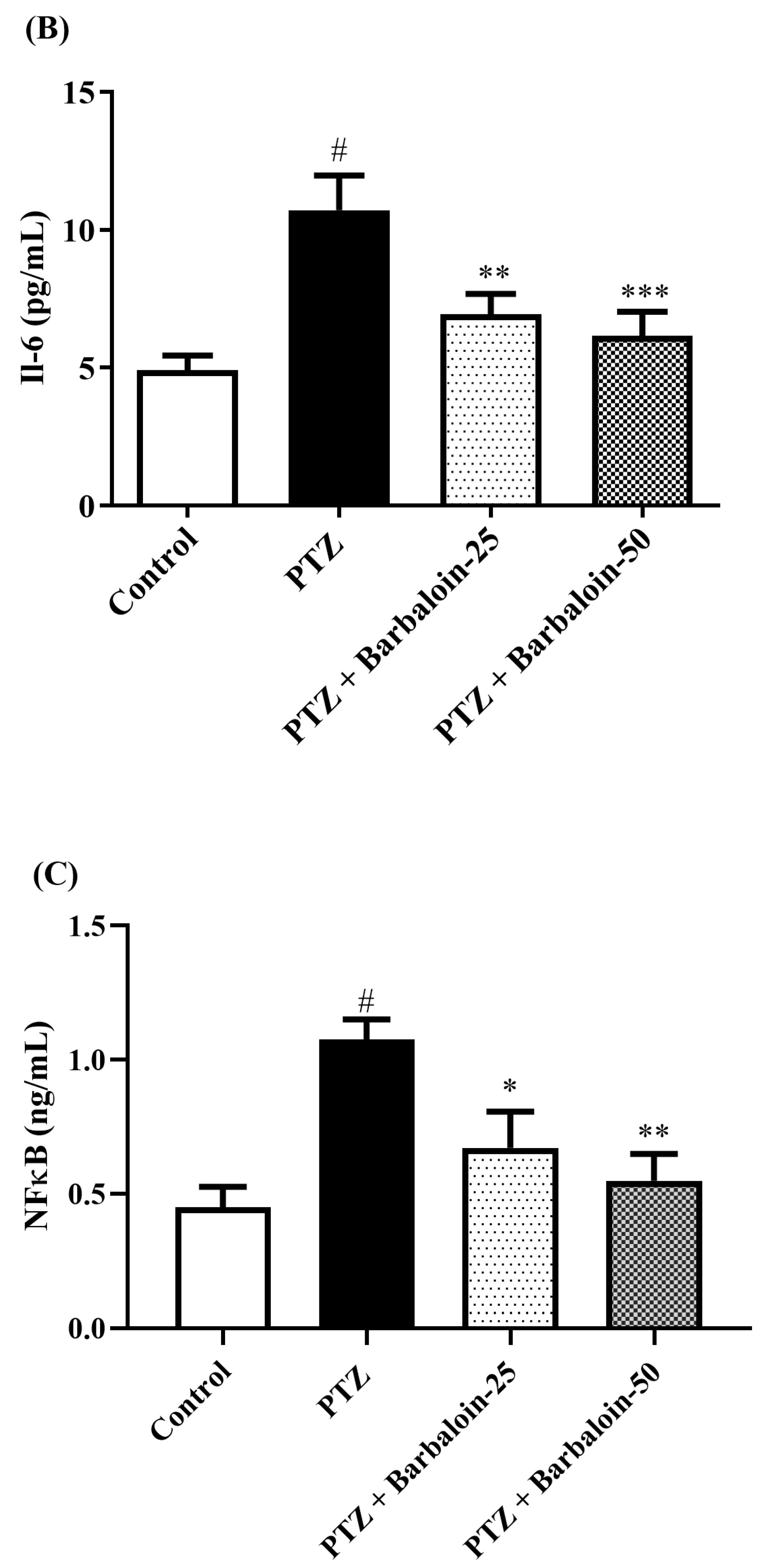
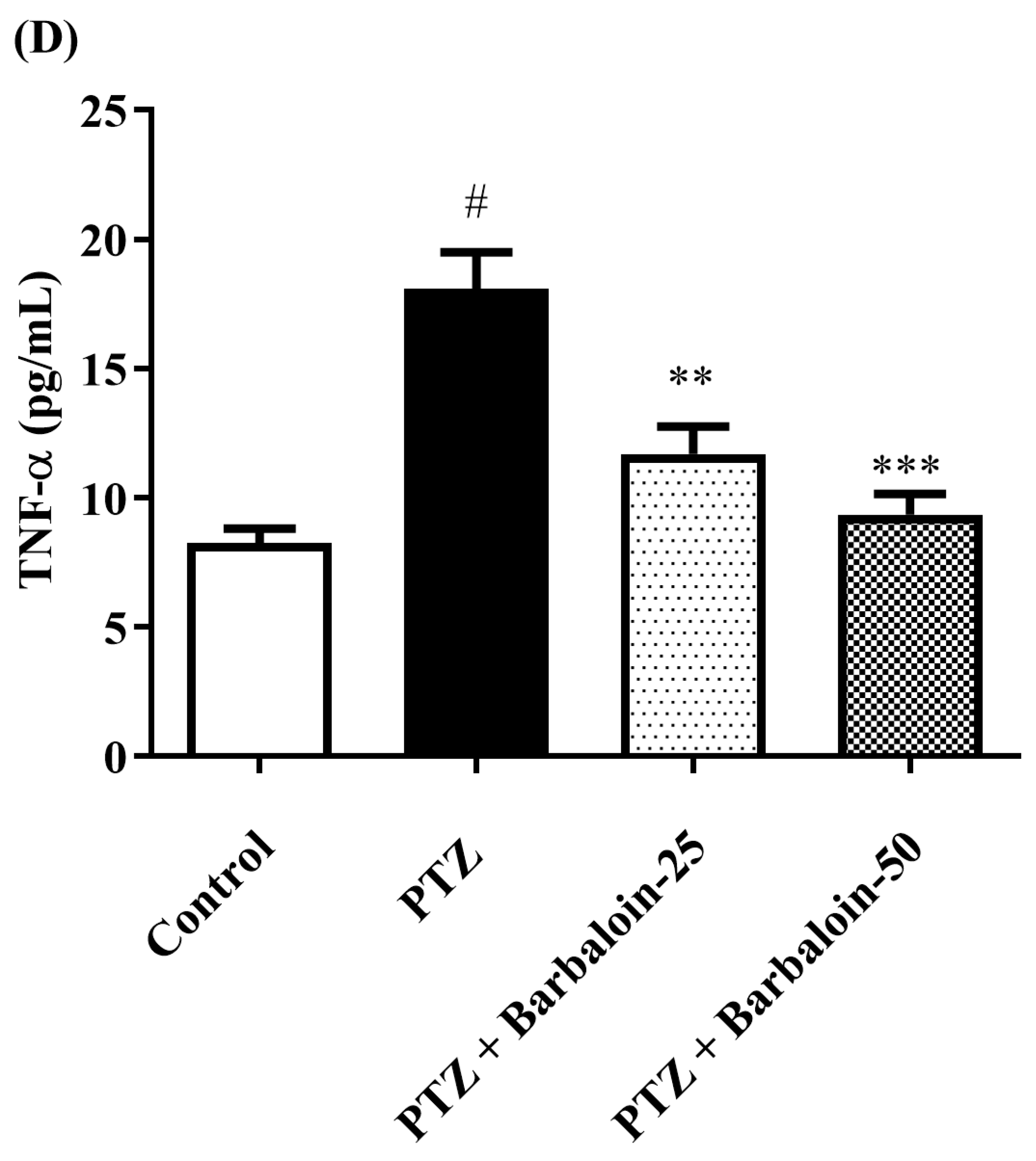
2.7. Estimation of Cytokines
Cytokines IL-6, IL-1β, NF-κB, and TNF-α were significantly increased in the PTZ control in comparison to rats in normal control (p < 0.0001). Administration of barbaloin seemingly declines the IL-1β [F (3, 20) = 10.99, p = 0.0002], IL-6 [F (3, 20) = 7.912, p = 0.0011], NF-κB [F (3, 20) = 7.573, p = 0.0014], and TNF-α [F (3, 20) = 18.38, p < 0.0001] levels in comparison to PTZ control. Figure 7A–D display the outcomes acquired from the IL-1β, IL-6, NF-κB, and TNF-α examination.
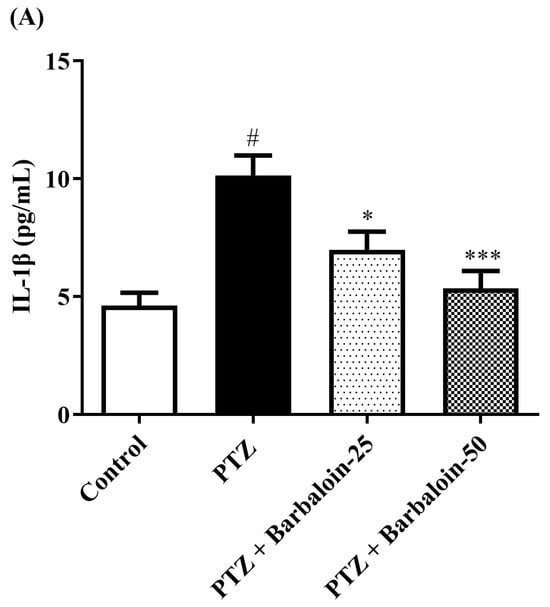
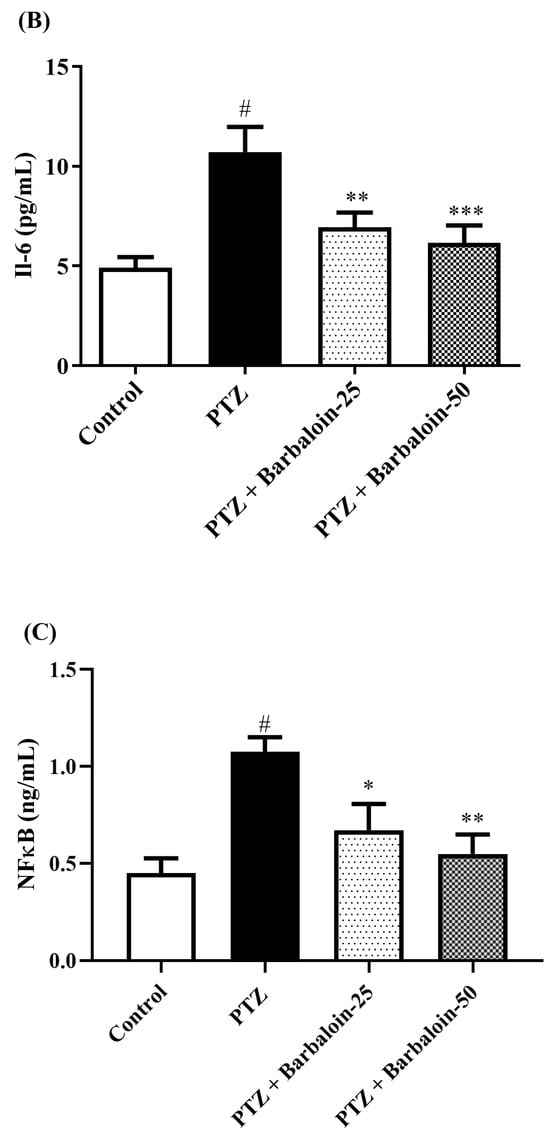
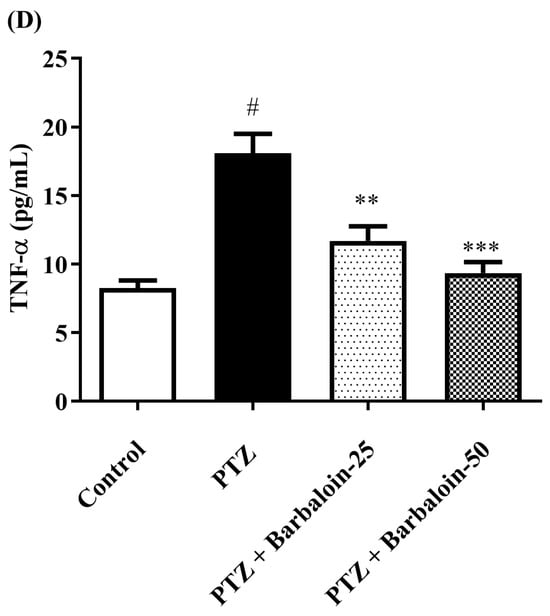
Figure 7.
(A–D) The effect of barbaloin on IL-1β, IL-6, NF-κB, and TNF-α levels. Mean ± S.E.M. One-way ANOVA followed by Tukey’s post hoc test (n = 6). # p < 0.001 vs. control, * p < 0.05, ** p < 0.001, *** p < 0.0001 vs. PTZ.
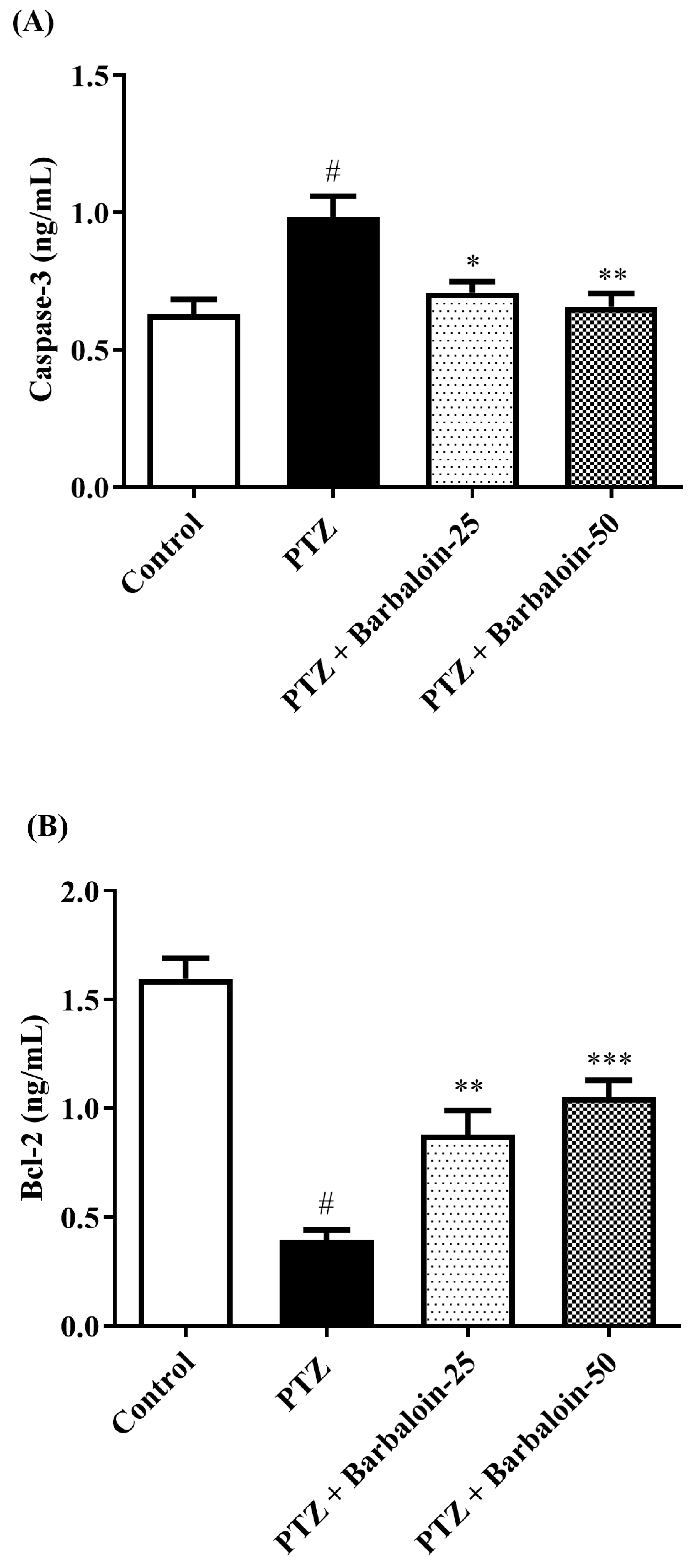
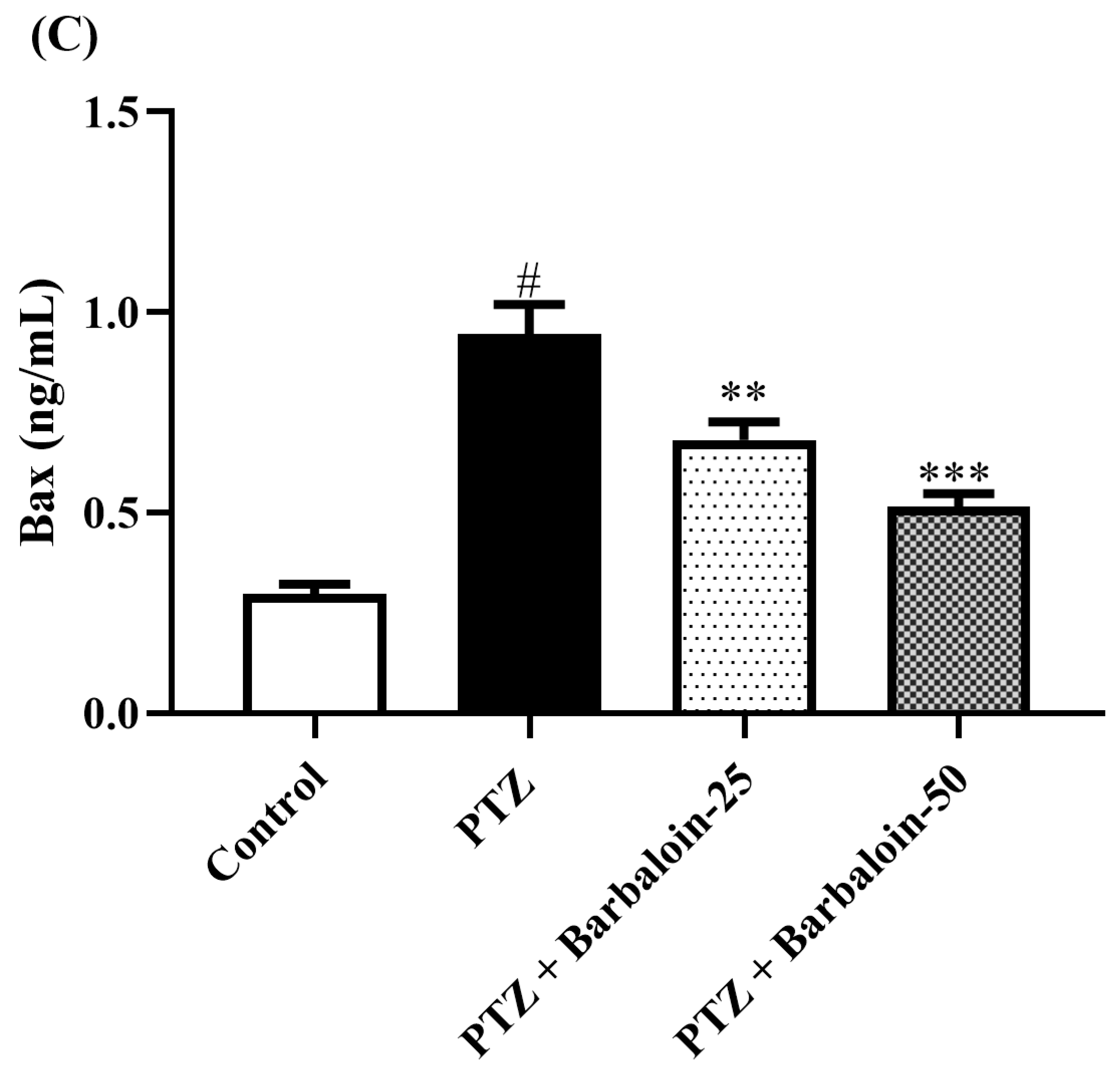
2.8. Caspase-3 Bcl-2 and Bax Contents
PTZ control rats considerably upregulated brain caspase-3 and Bax and downregulated Bcl-2 expression in comparison to rats in normal control (p < 0.0001). Barbaloin-treatment downregulated Bax [F (3, 20) = 32.04, p < 0.0001] and caspase-3 [F (3, 20) = 8.379, p = 0.0008] and upregulated the Bcl-2 [F (3, 20) = 33.65, p < 0.0001] level, respectively, in comparison to PTZ control rats (Figure 8A–C).
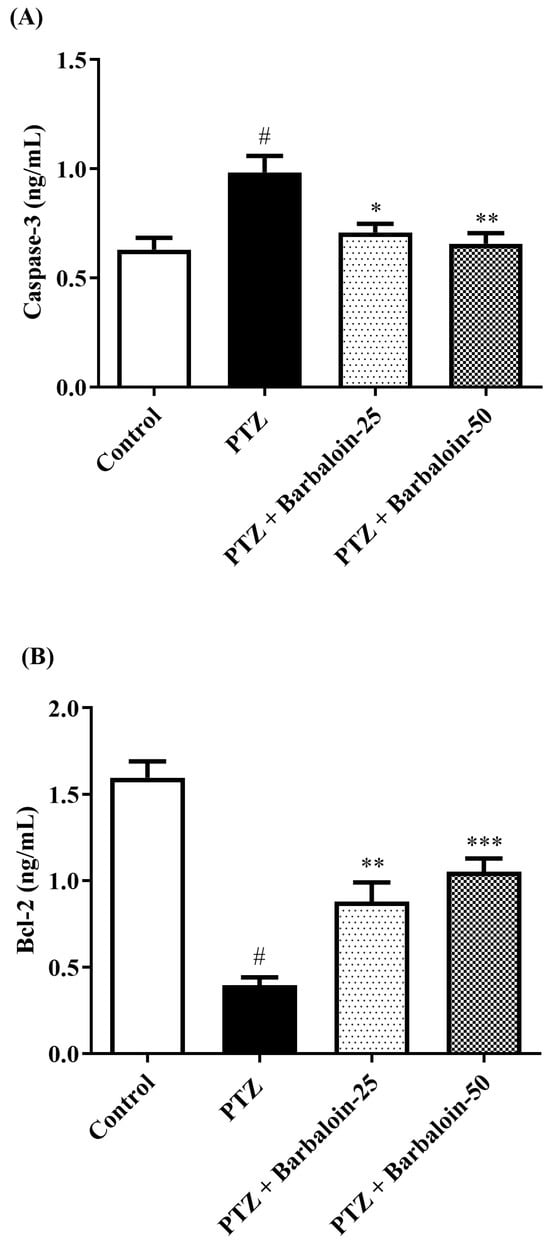
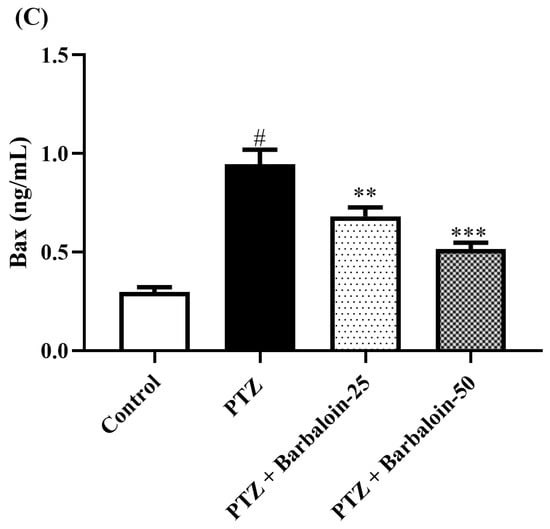
Figure 8.
(A–C) The effect of barbaloin on caspase-3, Bcl-2, and Bax levels. Mean ± S.E.M. One-way ANOVA followed by Tukey’s post hoc test (n = 6). # p < 0.001 vs. control, * p < 0.05, ** p < 0.001, *** p < 0.0001 vs. PTZ.
3. Discussion
This study aimed to assess the potential neuroprotective impact of barbaloin in rats with PTZ-induced kindling, which leads to cognitive decline. The ingestion of PTZ was observed to impede these benefits. This was achieved by examining changes in kindling score, open field test, NORT, oxidative stress levels, cytokine levels, and the expression of proteins caspase-3, Bcl-2, and Bax. The results indicate that the administration of barbaloin maintained the kindling score, as well as in the levels of NO, GSH, MDA, CAT, AChE, SOD, TNF-α, Bcl-2, IL-6, Bax, IL-1β, NF-κB, and caspase-3. Furthermore, the barbaloin also led to significant enhancements in the level of neurotransmitters such as 5-HT, DA, NE, and GABA.
The experiment demonstrated that the PTZ-treated animals interacted more similarly with both familiar and novel objects and were unable to recall the familiar object, as seen by the drop in the discrimination index in the NORT, whereas treatment with barbaloin enhanced the discrimination index. According to studies, the hippocampus is important for memory related to object recognition. Anterograde memory will alter modestly and consistently if this structure is compromised [31]. In OFT, the PTZ-induced and barbaloin treatment group had no significant effect on locomotor and exploratory activity. These findings suggest that the PTZ and barbaloin treatment may not have a direct impact on locomotor and exploratory activity.
Oxidative stress is well recognized as one of the primary and essential factors that lead to recurrent seizure occurrence. Excitotoxicity results in the release of free radicals and ROS. Consequently, lipids, proteins, and DNA are oxidized, leading to changes in membrane permeability, protein function, and gene expression [32]. These alterations can make neurons more susceptible to degeneration or mortality [32,33]. According to this study, PTZ kindling leads to a reduction in GSH and elevation in MDA levels. This might happen either via the direct removal of ROS or by promoting the formation of GSH [34,35]. Moreover, PTZ has the ability to universally elevate the concentration of NO throughout the whole brain [36]. In addition, our findings align with these prior studies. This concept is reinforced by the evidence that some medically prescribed antiepileptic medicines (AEDs) decrease ROS during seizures [37], whilst numerous others enhance oxidative harm [38,39]. Therefore, it may be inferred that the use of antioxidants as a supplementary treatment with AEDs can be advantageous in the control of epilepsy, as previously proven [40]. Our investigation found a reduced concentration of GSH, CAT, and SOD, consistent with previous findings suggesting that PTZ treatment causes oxidative stress [41,42]. The current study suggests that rats treated with PTZ exhibit decreased activities of GSH, SOD, and CAT, together with increased levels of MDA and NO, which are indicative of lipid peroxidation. The barbaloin led to a significant increase in the activities of SOD, GSH, and CAT, while simultaneously reducing the quantity of MDA and nitric oxide. Oxidative stress is caused by free radicals, which can cause cell and tissue damage. Barbaloin’s ability to improve cognitive function stems from its ability to inhibit oxidative stress and enhance the activity of endogenous antioxidant enzymes within the brain [30]. By minimizing the damage caused by oxidative stress, barbaloin can preserve the integrity of the brain’s structure and function, leading to improved behavioral cognitive performance.
The AChE is a crucial enzyme that catalyzes the hydrolysis of ACh, hence terminating cholinergic signaling. Thus, AChE is regarded as a significant therapeutic target, and many AChE-reversible antagonists are now employed in clinical practice to increase memory in patients suffering from neurological conditions and epilepsy [43,44,45]. In this work, AChE concentration was enhanced in the PTZ-injected rats. However, the administration of barbaloin to rats resulted in a significant decrease in AChE levels compared to animals stimulated with PTZ.
Seizure activity is linked to a diverse array of localized metabolic alterations that impact different neurotransmitters, including monoamines and amino acids [46]. The present investigation revealed that PTZ led to a decline in GABA, NE, DA, and 5-HT concentration in the hippocampal area. These findings align with the earlier investigations conducted by Visweswari et al. (2010) [47,48,49]. Monoamines are crucial in the epileptogenesis process, which involves the formation and advancement of epilepsy. An observed decrease in 5-HT level was shown to be linked to a decline in its synaptosomal absorption, the tryptophan hydroxylase activity inhibition, and a decline in tryptophan concentration in the epilepsy model [50,51]. Conversely, the reduction in DA levels observed in individuals with epilepsy can be linked to the heightened activity of monoamine oxidase and the diminished reuptake process [52]. In addition, NE functions as a neuromodulator with anticonvulsant properties. The reduction in NE levels in individuals with epilepsy is attributed to the decrease in the density of α1 receptors in the brain. This drop may possibly be attributed to the decline in the activity of dopamine-β-hydroxylase (DBH), which is the enzyme that limits the rate of norepinephrine production [53]. During the present research, it was shown that treatment with barbaloin effectively reversed the changes in levels of DA, NE, 5-HT, and GABA.
The synthesis of pro-inflammatory indicators, such as IL-6, TNF-α, and IL-1β, is caused by the activation of NF-κB signal transduction through the creation of ROS [54,55]. IL-1 induces the upregulation of the gene expression of the enzyme cyclooxygenase-2 (Cox-2), which is responsible for converting arachidonic acid into prostaglandins. Prostaglandin is a substance that comes before prostacyclin and is involved in inflammatory reactions. It activates astrocytes to generate glutamate, which leads to increased neuroexcitability linked with seizures [56]. The findings demonstrated that the PTZ injection resulted in a neuroinflammatory reaction, as indicated by a substantial rise in the protein concentration of cytokines (IL-6, TNF-α, NF-κB, and IL-1β) in the cerebral cortex [57]. Furthermore, the injection of PTZ resulted in a considerable rise in GFAP, which suggests the activation of astrocytes and subsequent inflammatory and apoptotic reactions in the brain [58]. Our data show that administering barbaloin has a considerable inhibitory effect on the raised levels of IL-6, TNF-α, IL-1β, and NF-κB in PTZ-induced kindling rats. These substances are known as inflammatory markers.
Preclinical investigations have shown that both transient and oxidative stress and neuronal death may arise from recurrent seizures, which entail molecular-level changes in the hippocampus [59,60]. The collapse of neurons can be attributed to the stimulation of pro-apoptotic proteins, including Bax and caspases 3. The findings from the current study showed that the administration of PTZ led to an enhanced concentration of caspase-3 and Bax and a lower concentration of the Bcl-2. However, these aberrations were reversed and returned to normal levels following treatment with barbaloin. Based on this evidence, barbaloin may inhibit PTZ-induced cognitive decline and changes in rats via modification of cytokines, oxidative stress, and protein expression Bcl-2, caspases-3, and Bax. PTZ-induced rats exhibited cognitive impairment, as demonstrated by alterations in kindling score, OFT, and NORT. Treatment with barbaloin resulted in significant improvements in cognitive function compared to the PTZ control groups. This suggests that barbaloin has the potential to help with cognitive deficits associated with epilepsy. The administration of PTZ led to changes in oxidative stress markers such as GSH, MDA, CAT, NO, and SOD. However, barbaloin treatment substantially restored these markers, indicating its antioxidative properties and its ability to counteract oxidative damage in the brain. In addition, PTZ-induced neuroinflammation was characterized by elevated levels of NF-κB, IL-1β, TNF-α, and IL-6. But when barbaloin was administered, the levels of these pro-inflammatory cytokines were significantly reduced. This suggests that barbaloin has anti-inflammatory effects that may help mitigate neuroinflammation associated with epilepsy. Moreover, barbaloin treatment caused significant changes in neurotransmitter levels such as GABA, 5-HT, and DA compared to PTZ-induced controls. These changes indicate that barbaloin may exert its neuroprotective effects, at least in part, through the modulation of neurotransmitter systems implicated in epilepsy pathogenesis. Lastly, barbaloin altered the expression of apoptotic markers, including caspase-3, Bcl-2, and Bax, suggesting its potential role in regulating apoptotic pathways and promoting neuronal survival in epileptic conditions. This work employed a minimal number of animals, and in future investigations, histopathology and Western blotting will be necessary to confirm this mechanism.
4. Materials and Methods
4.1. Animals
Thirty Wistar rats, with a weight range of 275–300 g, were acquired from T. G. Lab, India for this investigation. The participants were housed in a regulated setting, ensuring 22 ± 0.5 °C an interior temperature, and subjected to 12 h alternating periods of light and darkness. They had full access to food and drink. The experimental approach was approved in accordance with the ARRIVE guidelines (LNCP/IAEC/23/005).
4.2. Drugs
Barbaloin (purity: ≥97%) was obtained from Yucca Enterprises, India, and PTZ (purity: ≥98.0%) was procured from Sigma Aldrich, USA. All the chemicals used in this study were of excellent grade. The quantification of IL-1β, Bcl-2, IL-6, NF-κB, caspase-3, TNF-α and Bax was performed using the ELISA kit acquired from MSW Pharma, India.
4.3. Epileptic Seizure Scoring and a Rat Model Induced by PTZ
To induce epileptic episodes, we employed the methodology described in the study conducted by Hansen et al [61]. To initiate a dosage of 40 mg/kg, PTZ was administered intraperitoneally (i.p.) once every forty-eight hours for seventeen days until the animal displayed complete motor convulsions. Following each injection of PTZ, each rat was monitored for a period of 30 min to measure the time it took for an epileptic seizure to occur, the length of the episode, and the stage of the seizure based on the Racine scale, with some modification [51], as follows:
Level 0: No response;
Level 1: Eye and face twitching;
Level 2: Axially passing convulsive waves across the body;
Level 3: Myoclonic jerks of the body;
Level 4: Move to the side position, clonic-tonic seizures (CTS); and
Level 5: Turn over and lie on the back, GTCS, or mortality. To be completely kindled, a seizure number had to hit level 4 or 5 on three separate trials.
4.4. Research Design
The animals were selected randomly and then divided into four groups, with each group consisting of six animals and treated every 48 h as follows: Group I (Normal control) was administered the saline; Group II (PTZ control) PTZ was administered intraperitoneally 40 mg/kg; [62] and Group III and Group IV were administered PTZ + barbaloin 25 mg/kg and PTZ + barbaloin 50 mg/kg, respectively, for seventeen days, followed by PTZ (40 mg/kg) for another seventeen days.
At the end of the investigation, cervical dislocation was used to sacrifice the rats. The brain was excised and cleaned with a cold 0.9% NaCl solution, and then their hippocampus areas were dissected so that biochemical data could be evaluated. The tissue from the brain regions was thoroughly combined (10% weight/volume) in a buffer solution containing 0.01 M sodium phosphate (pH 7.4) and 1.15% potassium chloride per gram of tissue, ensuring a low temperature; it was then separated and, thereafter, kept at a temperature of 4 °C for enzymatic assay.
4.5. Behavioral Tests
4.5.1. OFT
A study was conducted to ascertain the long-term impact of stress on mice. The OFT test is meant to examine the impact of PTZ on the motivational activity of animals on the final day of the trials. The unit comprises a rectangular box of 80 × 80 × 50 cm, with the floor divided into squares of equal size measuring 25 × 16 × 16 cm. The mice were placed in the central part of an open field and allowed to freely explore for a duration of 3 min. During this test, two criteria were measured, namely, the rate of locomotion, which is the number of times the mice crossed one of the four-paw grid lines. The animals were housed in the testing facility for a minimum of 2 h before the test commenced. The OFT technique was carried out in a soundproof chamber without any human involvement. Room cleaning was conducted using a 5% ethanol–water solution to eliminate any bias in behavioral testing caused by the stench left by previously used rats. A study of the motivated behavior of the mice was conducted randomly by two unbiased observers. The resulting data were statistically analyzed to assess the reliability of the interobserver test [63].
4.5.2. NORT
To evaluate recognition, a novel object recognition task is used memory. In this task, rodents explore unfamiliar objects within their environment based on their innate curiosity. The purpose of this test is to determine whether a mouse is able to distinguish between familiar objects and novel objects. To start, each mouse was habituated to a 30 × 30 × 15 cm plexiglass box for 5 min. In the acquisition phase, the mice explored two identical objects for 5 min after 15 min. For mice, the objects were heavy and tall enough that they could neither move them nor climb over them. A 5-min interval was followed by mice being presented with similar objects, but with a novel or unknown object replacing one familiar object. After 5 min, the animals were allowed to explore the objects again. A discrimination index was calculated as follows: (time exploring new object − time exploring familiar object)/(time exploring new object + time exploring familiar object) [64,65].
4.6. Oxidative Stress Estimation
The quantification of MDA was conducted using the thiobarbituric acid technique as outlined by Ohkawa et al. in 1979 [66]. The concentration of NO in the brain supernatant was measured using the methodology established by Koracevic et al. [67]. The GSH concentration was measured using the method reported by Jollow et al. [68]. The enzymatic activity of the antioxidants CAT and SOD were determined using the methods previously described by Yousef et al. (2020) [69].
4.7. AChE Estimation
An approach similar to the one explained by Ellman (1961) was used to measure the level of AChE, expressed as μmol per min per mg of protein [70,71].
4.8. Neurotransmitter Levels
The amounts of neurotransmitters such as serotonin (5-HT), DA, and GABA were determined using high-performance liquid chromatography (HPLC).
4.9. Biological Inflammation
An ELISA kit was used to assess the levels of cytokines, namely, IL-1β, IL-6, NF-κB, TNF-α, caspase-3, Bcl-2, and Bax. The levels of IL-1β, Bcl-2, TNF-α, caspase-3, Bax, and IL-6 indicators were assessed in pg/mL, whereas the level of NF-κB was evaluated in ng/mL [72].
4.10. Analysis of Statistics
The data from the exams were evaluated using GraphPad software (8.0.2), developed by GraphPad Software Inc., California, United States. The ultimate outcomes were thereafter presented as the average value accompanied by the SEM. The results were evaluated using one-way analysis of variance (ANOVA) for the MWM test, followed by Bonferroni’s post hoc test. In addition, a one-way analysis of variance (ANOVA) was performed using Tukey’s test.
5. Conclusions
Barbaloin, a plant-derived natural product, has been discovered to alleviate cognitive impairments caused by PTZ. This effect is achieved by reducing oxidative stress triggered by the NF-κβ pathway, neuroinflammatory cytokines, and the caspases-3, Bcl-2, and Bax pathways. These findings suggest that barbaloin holds promise as a phytotherapeutic agent for treating cognitive impairments induced by PTZ in rats. Further investigation is necessary to confirm its potential neuroprotective effects in various cognitive dysfunction models.
Author Contributions
Conceptualization, M.A.; Methodology, A.E.A.; Software, A.E.A. and K.S.A.; Validation, A.E.A. and K.S.A.; Formal analysis, K.S.A.; Investigation, N.G.; Resources, N.G.; Data curation, N.G. and N.S.; Writing—original draft, M.A. and I.K.; Writing—review & editing, M.A., S.K.A. and I.K.; Supervision, N.S.; Project administration, N.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The experimental approach was approved by Institutional Animals Ethics Committee of Laxmi Narain College, Bhopal, India in accordance with the ARRIVE guidelines (LNCP/IAEC/23/005).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mohamed, H.K.; Eltony, S.A. Effect of acute pentylenetetrazol injection induced epileptic seizures on rat dentate gyrus at different postnatal ages. Anat. Cell Biol. 2020, 53, 84–94. [Google Scholar] [CrossRef]
- Boison, D.; Steinhäuser, C. Epilepsy and astrocyte energy metabolism. Glia 2018, 66, 1235–1243. [Google Scholar] [CrossRef]
- Cerri, C.; Caleo, M.; Bozzi, Y. Chemokines as new inflammatory players in the pathogenesis of epilepsy. Epilepsy Res. 2017, 136, 77–83. [Google Scholar] [CrossRef]
- Gales, J.M.; Prayson, R.A. Chronic inflammation in refractory hippocampal sclerosis-related temporal lobe epilepsy. Ann. Diagn. Pathol. 2017, 30, 12–16. [Google Scholar] [CrossRef]
- Salgado, P.R.R.; da Fonsêca, D.V.; de Melo, C.G.F.; Leite, F.C.; Alves, A.F.; Ferreira, P.B.; Piuvezam, M.R.; de Sousa, D.P.; de Almeida, R.N. Comparison of behavioral, neuroprotective, and proinflammatory cytokine modulating effects exercised by (+)-cis-EC and (-)-cis-EC stereoisomers in a PTZ-induced kindling test in mice. Fundam. Clin. Pharmacol. 2018, 32, 507–515. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Wu, Y.; Li, T.; Wang, W. Salidroside shows anticonvulsant and neuroprotective effects by activating the Nrf2-ARE pathway in a pentylenetetrazol-kindling epileptic model. Brain Res. Bull. 2020, 164, 14–20. [Google Scholar] [CrossRef]
- Carmona-Aparicio, L.; Pérez-Cruz, C.; Zavala-Tecuapetla, C.; Granados-Rojas, L.; Rivera-Espinosa, L.; Montesinos-Correa, H.; Hernández-Damián, J.; Pedraza-Chaverri, J.; Sampieri, A., 3rd; Coballase-Urrutia, E.; et al. Overview of Nrf2 as Therapeutic Target in Epilepsy. Int. J. Mol. Sci. 2015, 16, 18348–18367. [Google Scholar] [CrossRef]
- Shimada, T.; Takemiya, T.; Sugiura, H.; Yamagata, K. Role of inflammatory mediators in the pathogenesis of epilepsy. Mediat. Inflamm. 2014, 2014, 901902. [Google Scholar] [CrossRef]
- Hashemian, M.; Anissian, D.; Ghasemi-Kasman, M.; Akbari, A.; Khalili-Fomeshi, M.; Ghasemi, S.; Ahmadi, F.; Moghadamnia, A.A.; Ebrahimpour, A. Curcumin-loaded chitosan-alginate-STPP nanoparticles ameliorate memory deficits and reduce glial activation in pentylenetetrazol-induced kindling model of epilepsy. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 79, 462–471. [Google Scholar] [CrossRef]
- Franke, H.; Kittner, H. Morphological alterations of neurons and astrocytes and changes in emotional behavior in pentylenetetrazol-kindled rats. Pharmacol. Biochem. Behav. 2001, 70, 291–303. [Google Scholar] [CrossRef]
- Mortazavi, F.; Ericson, M.; Story, D.; Hulce, V.D.; Dunbar, G.L. Spatial learning deficits and emotional impairments in pentylenetetrazole-kindled rats. Epilepsy Behav. 2005, 7, 629–638. [Google Scholar] [CrossRef]
- Zhu, X.; Dong, J.; Han, B.; Huang, R.; Zhang, A.; Xia, Z.; Chang, H.; Chao, J.; Yao, H. Neuronal Nitric Oxide Synthase Contributes to PTZ Kindling-Induced Cognitive Impairment and Depressive-Like Behavior. Front. Behav. Neurosci. 2017, 11, 203. [Google Scholar] [CrossRef]
- Dhir, A. Pentylenetetrazol (PTZ) kindling model of epilepsy. Curr. Protoc. Neurosci. 2012, 58, 9–37. [Google Scholar] [CrossRef]
- Ahmadian, S.R.; Ghasemi-Kasman, M.; Pouramir, M.; Sadeghi, F. Arbutin attenuates cognitive impairment and inflammatory response in pentylenetetrazol-induced kindling model of epilepsy. Neuropharmacology 2019, 146, 117–127. [Google Scholar] [CrossRef]
- Kaur, H.; Patro, I.; Tikoo, K.; Sandhir, R. Curcumin attenuates inflammatory response and cognitive deficits in experimental model of chronic epilepsy. Neurochem. Int. 2015, 89, 40–50. [Google Scholar] [CrossRef]
- Choudhary, K.M.; Mishra, A.; Poroikov, V.V.; Goel, R.K. Ameliorative effect of Curcumin on seizure severity, depression like behavior, learning and memory deficit in post-pentylenetetrazole-kindled mice. Eur. J. Pharmacol. 2013, 704, 33–40. [Google Scholar] [CrossRef]
- Safar, M.M.; Shahin, N.N.; Mohamed, A.F.; Abdelkader, N.F. Suppression of BACE1 and amyloidogenic/RAGE axis by sitagliptin ameliorates PTZ kindling-induced cognitive deficits in rats. Chem. Biol. Interact. 2020, 328, 109144. [Google Scholar] [CrossRef]
- Shimada, T.; Yamagata, K. Pentylenetetrazole-Induced Kindling Mouse Model. J. Vis. Exp. 2018, 136, e56573. [Google Scholar] [CrossRef]
- Pracucci, E.; Pillai, V.; Lamers, D.; Parra, R.; Landi, S. Neuroinflammation: A Signature or a Cause of Epilepsy? Int. J. Mol. Sci. 2021, 22, 6981. [Google Scholar] [CrossRef]
- Utech, M.; Mennigen, R.; Bruewer, M. Endocytosis and recycling of tight junction proteins in inflammation. J. Biomed. Biotechnol. 2010, 2010, 484987. [Google Scholar] [CrossRef]
- Javaid, S.; Alqahtani, F.; Ashraf, W.; Anjum, S.M.M.; Rasool, M.F.; Ahmad, T.; Alasmari, F.; Alasmari, A.F.; Alqarni, S.A.; Imran, I. Tiagabine suppresses pentylenetetrazole-induced seizures in mice and improves behavioral and cognitive parameters by modulating BDNF/TrkB expression and neuroinflammatory markers. Biomed. Pharmacother. 2023, 160, 114406. [Google Scholar] [CrossRef]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Yang, F. Barbaloin Treatment Contributes to the Rebalance of Glucose and Lipid Homeostasis of Gestational Diabetes Mellitus Mice. Dose Response 2020, 18, 1559325820984910. [Google Scholar] [CrossRef]
- El-Shemy, H.A.; Aboul-Soud, M.A.; Nassr-Allah, A.A.; Aboul-Enein, K.M.; Kabash, A.; Yagi, A. Antitumor properties and modulation of antioxidant enzymes’ activity by Aloe vera leaf active principles isolated via supercritical carbon dioxide extraction. Curr. Med. Chem. 2010, 17, 129–138. [Google Scholar] [CrossRef]
- Patel, D.K.; Patel, K.; Tahilyani, V. Barbaloin: A concise report of its pharmacological and analytical aspects. Asian Pac. J. Trop. Biomed. 2012, 2, 835–838. [Google Scholar] [CrossRef]
- Gai, L.; Chu, L.; Xia, R.; Chen, Q.; Sun, X. Barbaloin Attenuates Mucosal Damage in Experimental Models of Rat Colitis by Regulating Inflammation and the AMPK Signaling Pathway. Med. Sci. Monit. 2019, 25, 10045–10056. [Google Scholar] [CrossRef]
- Jiang, K.; Guo, S.; Yang, C.; Yang, J.; Chen, Y.; Shaukat, A.; Zhao, G.; Wu, H.; Deng, G. Barbaloin protects against lipopolysaccharide (LPS)-induced acute lung injury by inhibiting the ROS-mediated PI3K/AKT/NF-κB pathway. Int. Immunopharmacol. 2018, 64, 140–150. [Google Scholar] [CrossRef]
- Omer, A.B.; Afzal, O.; Altamimi, A.S.A.; Patil, S.; AlGhamdi, S.A.; Alghamdi, A.M.; Alzarea, S.I.; Almalki, W.H.; Kazmi, I. Neuroprotective Effect of Barbaloin on Streptozotocin-Induced Cognitive Dysfunction in Rats via Inhibiting Cholinergic and Neuroinflammatory Cytokines Pathway-TNF-α/IL-1β/IL-6/NF-κB. ACS Omega 2023, 8, 8110–8118. [Google Scholar] [CrossRef]
- Kazmi, I.; Afzal, M.; Imam, F.; Alzarea, S.I.; Patil, S.; Mhaiskar, A.; Shah, U.; Almalki, W.H. Barbaloin’s Chemical Intervention in Aluminum Chloride Induced Cognitive Deficits and Changes in Rats through Modulation of Oxidative Stress, Cytokines, and BDNF Expression. ACS Omega 2024, 9, 6976–6985. [Google Scholar] [CrossRef]
- Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Holsinger, R.M.D. Oxidative Stress and Antioxidants in Neurodegenerative Disorders. Antioxidants 2023, 12, 517. [Google Scholar] [CrossRef]
- Antunes, M.; Biala, G. The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cogn. Process 2012, 13, 93–110. [Google Scholar] [CrossRef]
- Chehaibi, K.; Trabelsi, I.; Mahdouani, K.; Slimane, M.N. Correlation of Oxidative Stress Parameters and Inflammatory Markers in Ischemic Stroke Patients. J. Stroke Cerebrovasc. Dis. 2016, 25, 2585–2593. [Google Scholar] [CrossRef]
- Shin, E.J.; Jeong, J.H.; Chung, Y.H.; Kim, W.K.; Ko, K.H.; Bach, J.H.; Hong, J.S.; Yoneda, Y.; Kim, H.C. Role of oxidative stress in epileptic seizures. Neurochem. Int. 2011, 59, 122–137. [Google Scholar] [CrossRef]
- Kandeda, A.K.; Taiwe, G.S.; Moto, F.C.O.; Ngoupaye, G.T.; Nkantchoua, G.C.N.; Njapdounke, J.S.K.; Omam, J.P.O.; Pale, S.; Kouemou, N.; Ngo Bum, E. Antiepileptogenic and Neuroprotective Effects of Pergularia daemia on Pilocarpine Model of Epilepsy. Front. Pharmacol. 2017, 8, 440. [Google Scholar] [CrossRef]
- Kavaye Kandeda, A.; Okomolo Moto, F.C.; Mbomo Ayissi, R.E.; Omam Omam, J.P.; Ojong, L.; Ngo Bum, E. Pergularia daemia hydro-ethanolic extract protects against pentylenetetrazole kindling-induced seizures, oxidative stress, and neuroinflammation in mice. J. Ethnopharmacol. 2021, 279, 114338. [Google Scholar] [CrossRef]
- Rauca, C.; Zerbe, R.; Jantze, H. Formation of free hydroxyl radicals after pentylenetetrazol-induced seizure and kindling. Brain Res. 1999, 847, 347–351. [Google Scholar] [CrossRef]
- Ueda, Y.; Doi, T.; Takaki, M.; Nagatomo, K.; Nakajima, A.; Willmore, L.J. Levetiracetam enhances endogenous antioxidant in the hippocampus of rats: In vivo evaluation by brain microdialysis combined with ESR spectroscopy. Brain Res. 2009, 1266, 1–7. [Google Scholar] [CrossRef]
- Verrotti, A.; Scardapane, A.; Franzoni, E.; Manco, R.; Chiarelli, F. Increased oxidative stress in epileptic children treated with valproic acid. Epilepsy Res. 2008, 78, 171–177. [Google Scholar] [CrossRef]
- Karikas, G.A.; Schulpis, K.H.; Bartzeliotou, A.; Regoutas, S.; Thanopoulou, C.; Papaevangelou, V.; Giannoulia-Karantana, A.; Papassotiriou, I.; Fytou-Pallikari, A. Early effects of sodium valproate monotherapy on serum paraoxonase/arylesterase activities. Scand. J. Clin. Lab. Investig. 2009, 69, 31–35. [Google Scholar] [CrossRef]
- Uma Devi, P.; Pillai, K.K.; Vohora, D. Modulation of pentylenetetrazole-induced seizures and oxidative stress parameters by sodium valproate in the absence and presence of N-acetylcysteine. Fundam. Clin. Pharmacol. 2006, 20, 247–253. [Google Scholar] [CrossRef]
- Méndez-Armenta, M.; Nava-Ruíz, C.; Juárez-Rebollar, D.; Rodríguez-Martínez, E.; Gómez, P.Y. Oxidative stress associated with neuronal apoptosis in experimental models of epilepsy. Oxid. Med. Cell Longev. 2014, 2014, 293689. [Google Scholar] [CrossRef]
- Goel, R.; Saxena, P. Pycnogenol Protects against Pentylenetetrazole-Induced Oxidative Stress and Seizures in Mice. Curr. Clin. Pharmacol. 2019, 14, 68–75. [Google Scholar] [CrossRef]
- Farlow, M.R. Do cholinesterase inhibitors slow progression of Alzheimer’s disease? Int. J. Clin. Pract. Suppl. 2002, 127, 37–44. [Google Scholar]
- Giacobini, E. Cholinesterases: New roles in brain function and in Alzheimer’s disease. Neurochem. Res. 2003, 28, 515–522. [Google Scholar] [CrossRef]
- Alachkar, A.; Azimullah, S.; Lotfy, M.; Adeghate, E.; Ojha, S.K.; Beiram, R.; Łażewska, D.; Kieć-Kononowicz, K.; Sadek, B. Antagonism of Histamine H3 receptors Alleviates Pentylenetetrazole-Induced Kindling and Associated Memory Deficits by Mitigating Oxidative Stress, Central Neurotransmitters, and c-Fos Protein Expression in Rats. Molecules 2020, 25, 1575. [Google Scholar] [CrossRef]
- Uddin, M.S.; Mamun, A.A.; Kabir, M.T.; Nasrullah, M.; Wahid, F.; Begum, M.M. Neurochemistry of neurochemicals: Messengers of brain functions. J. Intellect. Disabil. Diagn. Treat. 2018, 5, 137–151. [Google Scholar] [CrossRef]
- Chimakurthy, J.; Talasila, M. Effects of curcumin on pentylenetetrazole-induced anxiety-like behaviors and associated changes in cognition and monoamine levels. Psychol. Neurosci. 2010, 3, 238–244. [Google Scholar] [CrossRef]
- Visweswari, G.; Prasad, K.S.; Chetan, P.S.; Lokanatha, V.; Rajendra, W. Evaluation of the anticonvulsant effect of Centella asiatica (gotu kola) in pentylenetetrazol-induced seizures with respect to cholinergic neurotransmission. Epilepsy Behav. 2010, 17, 332–335. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.; Arafa, N.; El-Khadragy, M.; Kassab, R. The neuroprotective role of Nigella sativa extract on ciprofloxacin and pentylenetetrazole treated rats. Afr. J. Pharm. Pharmacol. 2013, 7, 660–1670. [Google Scholar] [CrossRef]
- Tchekalarova, J.; Pechlivanova, D.; Atanasova, T.; Markova, P.; Lozanov, V.; Stoynev, A. Diurnal variations in depression-like behavior of Wistar and spontaneously hypertensive rats in the kainate model of temporal lobe epilepsy. Epilepsy Behav. 2011, 20, 277–285. [Google Scholar] [CrossRef]
- Essawy, A.E.; El-Sayed, S.A.; Tousson, E.; Abd El-Gawad, H.S.; Alhasani, R.H.; Abd Elkader, H.A.E. Anti-kindling effect of Ginkgo biloba leaf extract and L-carnitine in the pentylenetetrazol model of epilepsy. Environ. Sci. Pollut. Res. Int. 2022, 29, 48573–48587. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, M.; Sadeghian, A.; Roohi, N.; Shojaei, A.; Mirnajafi-Zadeh, J. Epilepsy and dopaminergic system. Physiol. Pharmacol. 2017, 21, 1–14. [Google Scholar]
- Yuan, X.; Fu, Z.; Ji, P.; Guo, L.; Al-Ghamdy, A.O.; Alkandiri, A.; Habotta, O.A.; Abdel Moneim, A.E.; Kassab, R.B. Selenium Nanoparticles Pre-Treatment Reverse Behavioral, Oxidative Damage, Neuronal Loss and Neurochemical Alterations in Pentylenetetrazole-Induced Epileptic Seizures in Mice. Int. J. Nanomed. 2020, 15, 6339–6353. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, T.; Dong, K. Effect of formononetin from Trifolium pratense L. on oxidative stress, energy metabolism and inflammatory response after cerebral ischemia-reperfusion injury in mice. Food Sci. Technol. 2021, 42, e57821. [Google Scholar] [CrossRef]
- Font-Nieves, M.; Sans-Fons, M.G.; Gorina, R.; Bonfill-Teixidor, E.; Salas-Pérdomo, A.; Márquez-Kisinousky, L.; Santalucia, T.; Planas, A.M. Induction of COX-2 enzyme and down-regulation of COX-1 expression by lipopolysaccharide (LPS) control prostaglandin E2 production in astrocytes. J. Biol. Chem. 2012, 287, 6454–6468. [Google Scholar] [CrossRef] [PubMed]
- Othman, M.S.; Obeidat, S.T.; Aleid, G.M.; Al-Bagawi, A.H.; Fareid, M.A.; Hameed, R.A.; Mohamed, K.M.; Abdelfattah, M.S.; Fehaid, A.; Hussein, M.M.; et al. Green Synthetized Selenium Nanoparticles Using Syzygium aromaticum (Clove) Extract Reduce Pentylenetetrazol-Induced Epilepsy and Associated Cortical Damage in Rats. Appl. Sci. 2023, 13, 1050. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Liang, J.; Yan, J.X.; Ye, Y.C.; Wang, J.J.; Chen, C.; Sun, H.T.; Chen, F.; Tu, Y.; Li, X.H. TBHQ improved neurological recovery after traumatic brain injury by inhibiting the overactivation of astrocytes. Brain Res. 2020, 1739, 146818. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Sun, A.Y. Oxidative mechanisms involved in kainate-induced cytotoxicity in cortical neurons. Neurochem. Res. 1994, 19, 1557–1564. [Google Scholar] [CrossRef]
- Henshall, D.C.; Clark, R.S.; Adelson, P.D.; Chen, M.; Watkins, S.C.; Simon, R.P. Alterations in bcl-2 and caspase gene family protein expression in human temporal lobe epilepsy. Neurology 2000, 55, 250–257. [Google Scholar] [CrossRef]
- Hansen, N.; Widman, G.; Witt, J.A.; Wagner, J.; Becker, A.J.; Elger, C.E.; Helmstaedter, C. Seizure control and cognitive improvement via immunotherapy in late onset epilepsy patients with paraneoplastic versus GAD65 autoantibody-associated limbic encephalitis. Epilepsy Behav. 2016, 65, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Waggas, A.M.; Al-Hasani, R.H. Neurophysiological study on possible protective and therapeutic effects of Sidr (Zizyphus spina-christi L.) leaf extract in male albino rats treated with pentylenetetrazol. Saudi J. Biol. Sci. 2010, 17, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Singh, L.; Pandey, R.; Singh, P.; Ali, M.; Kaushik, R.; Soni, P. Neuroprotective Effect of Chlorogenic Acid against Pentylenetetrazol Induced Kindled Epilepsy in Mice. Int. J. Drug Deliv. Technol. 2023, 13, 1030–1036. [Google Scholar] [CrossRef]
- Gáll, Z.; Kelemen, K.; Tolokán, A.; Zolcseak, I.; Sável, I.; Bod, R.; Ferencz, E.; Vancea, S.; Urkon, M.; Kolcsár, M. Anticonvulsant Action and Long-Term Effects of Chronic Cannabidiol Treatment in the Rat Pentylenetetrazole-Kindling Model of Epilepsy. Biomedicines 2022, 10, 1811. [Google Scholar] [CrossRef]
- Dix, S.L.; Aggleton, J.P. Extending the spontaneous preference test of recognition: Evidence of object-location and object-context recognition. Behav. Brain Res. 1999, 99, 191–200. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Koracevic, D.; Koracevic, G.; Djordjevic, V.; Andrejevic, S.; Cosic, V. Method for the measurement of antioxidant activity in human fluids. J. Clin. Pathol. 2001, 54, 356–361. [Google Scholar] [CrossRef]
- Jollow, D.J.; Mitchell, J.R.; Zampaglione, N.; Gillette, J.R. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 1974, 11, 151–169. [Google Scholar] [CrossRef]
- Yousef, M.I.; Abdou, H.M.; Abd Elkader, H.-T.A.E.A.; Hussein, H.K.; Abou Samra, W.E.M. Neuroprotective Potential of Spirulina Platensis Against Aluminium Chloride-Induced Neural Degeneration. Curr. Top. Nutraceutical Res. 2020, 18, 310. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Djeuzong, E.; Kandeda, A.K.; Djiogue, S.; Stéphanie, L.; Nguedia, D.; Ngueguim, F.; Djientcheu, J.P.; Kouamouo, J.; Dimo, T. Antiamnesic and Neuroprotective Effects of an Aqueous Extract of Ziziphus jujuba Mill. (Rhamnaceae) on Scopolamine-Induced Cognitive Impairments in Rats. Evid. Based Complement. Altern. Med. 2021, 2021, 5577163. [Google Scholar] [CrossRef]
- Shahid Nadeem, M.; Khan, J.A.; Al-Abbasi, F.A.; AlGhamdi, S.A.; Alghamdi, A.M.; Sayyed, N.; Gupta, G.; Kazmi, I. Protective Effect of Hirsutidin against Rotenone-Induced Parkinsonism via Inhibition of Caspase-3/Interleukins-6 and 1β. ACS Omega 2023, 8, 13016–13025. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).