Nanoemulsified Essential Oil of Melaleuca leucadendron Leaves for Topical Application: In Vitro Photoprotective, Antioxidant and Anti-Melanoma Activities
Abstract
:1. Introduction
2. Results and Discussion
2.1. Yield of Essential Oil Extraction
2.2. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
2.3. Nanoemulsion
2.3.1. Zeta Potential, Hydrodynamic Diameter, Polydispersity Index and Centrifugation Test of the Nanoemulsions
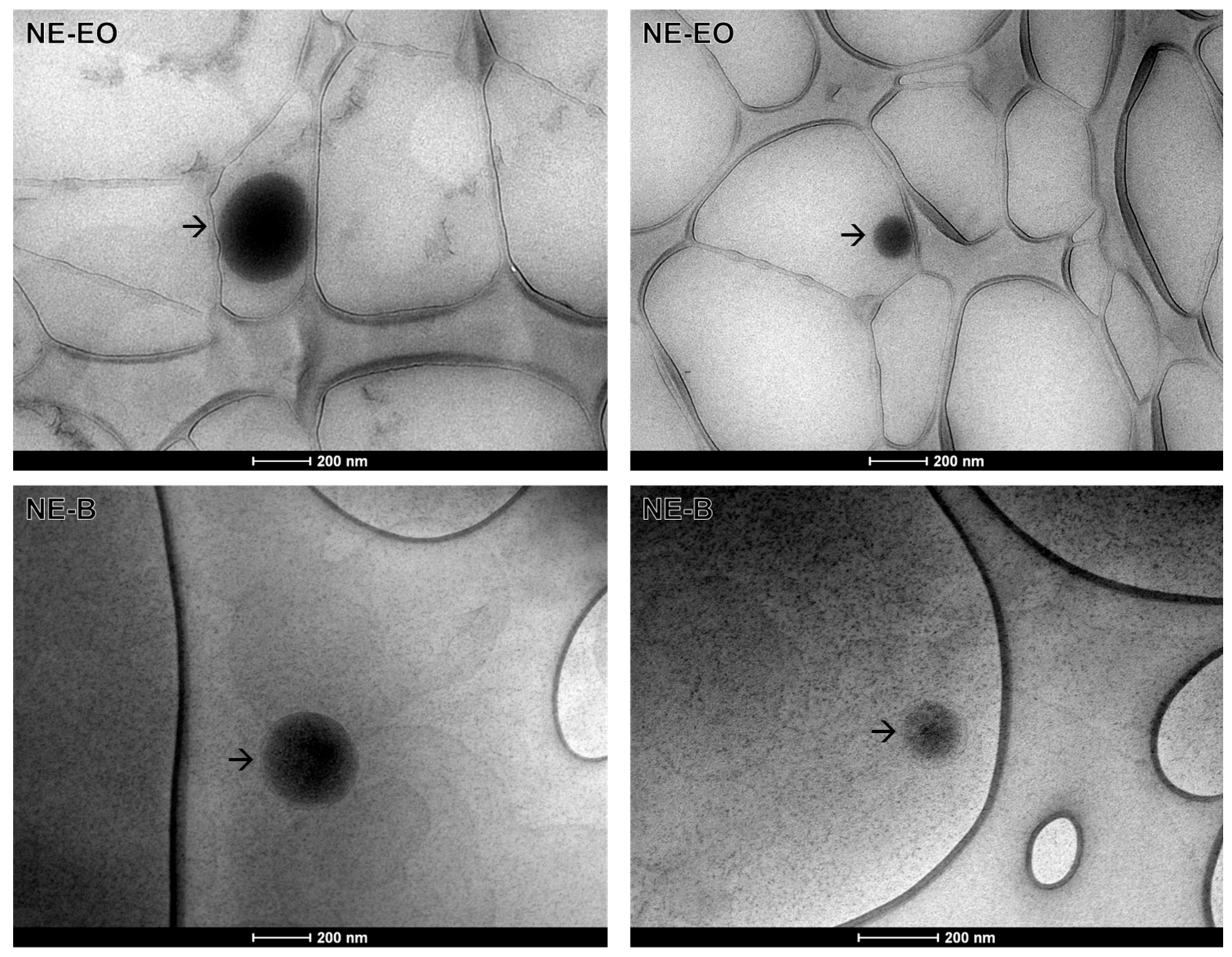
2.3.2. Transmission Electronic Microscopy
2.3.3. Rheological Behavior
2.4. Photoprotective Activity
2.5. Antioxidant Activity
2.6. Cytotoxicity
3. Materials and Methods
3.1. Plant Material
3.2. Extraction of the Essential oil from Melaleuca leucadendron Leaves
3.3. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
3.4. Nanoemulsion Development
3.4.1. Zeta Potential, Hydrodynamic Diameter and Polydispersity Index
3.4.2. Centrifugation Test
3.4.3. Transmission Electronic Microscopy
3.4.4. Rheological Analysis
3.5. Photoprotective Activity
3.6. Antioxidant Activity
3.6.1. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Inhibition
3.6.2. 2,2′-Azinobis(3-ethylbenzothiazoline-6-sulfonic Acid) (ABTS) Radical Inhibition
3.7. Cytotoxicity Assay
3.7.1. Cell Culture
3.7.2. Sulforhodamine B Method
3.7.3. Selectivity Index
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| UV | Ultraviolet |
| NE-EO | Nanoemulsion prepared with essential oil |
| NE-B | Nanoemulsion prepared without essential oil |
| EO | Essential oil of Melaleuca leucadendron leaves |
| DNA | Deoxyribonucleic acid |
| GC-MS | Gas chromatography–mass spectrometry |
| cP | Consistency index |
| n | Flow rate |
| SPF | Solar protection factor |
| FDA | Food and Drug Administration of the United States |
| C+ | Positive control |
| SisGen | Sistema Nacional de Gestão do Patrimônio Genético e do Conhecimento Tradicional Associado |
| TIC | Total ion chromatogram |
| PDI | Polydispersity index |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| ABTS | 2,2′-Azinobis(3-ethylbenzothiazoline-6-sulfonic acid) |
| ATCC | American Type Culture Collection |
| BCRJ | Banco de Células do Rio de Janeiro |
| FBS | Fetal Bovine Serum |
| SRB | Sulforhodamine B |
| TEM | Transmission electron microscopy |
| TIC | Total ion chromatogram |
| CCD | Charge-coupled device |
References
- Bolick, N.L.; Geller, A.C. Epidemiology of Melanoma. Hematol. Oncol. Clin. N. Am. 2021, 35, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. Am. Cancer Soc. J. 2015, 65, 22. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; de Vries, E.; Whiteman, D.C.; Jemal, A.; Bray, F.; Parkin, D.M.; Soerjomataram, I. Global burden of cutaneous melanoma attributable to ultraviolet radiation in 2012. Int. J. Cancer 2018, 143, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Singh, D.; Laversanne, M.; Vignat, J.; Vaccarella, S.; Meheus, F.; Cust, A.E.; de Vries, E.; Whiteman, D.C.; Bray, F. Global Burden of Cutaneous Melanoma in 2020 and Projections to 2040. JAMA Dermatol. 2022, 158, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Rabbie, R.; Ferguson, P.; Molina-Aguilar, C.; Adams, D.J.; Robles-Espinoza, C.D. Melanoma subtypes: Genomic profiles, prognostic molecular markers and therapeutic possibilities. J. Pathol. 2019, 247, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Whiteman, D.C.; Green, A.C.; Olsen, C.M. The Growing Burden of Invasive Melanoma: Projections of Incidence Rates and Numbers of New Cases in Six Susceptible Populations through 2031. J. Investig. Dermatol. 2016, 136, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- ACS. Cancer A-Z. Câncer de Pele Melanoma. Atlanta: American Cancer Society. 2023. Available online: https://www.cancer.org/cancer/types/melanoma-skin-cancer.html (accessed on 5 May 2024).
- Davis, L.E.; Shalin, S.C.; Tackett, A.J. Current state of melanoma diagnosis and treatment. Cancer Biol. Ther. 2019, 20, 1366–1379. [Google Scholar] [CrossRef]
- Ministério da Saúde. Diretrizes Diagnósticas e Terapêuticas—Melanoma Cutâneo. 2022. Available online: https://www.gov.br/conitec/pt-br/midias/consultas/relatorios/2022/20220516_ddt_melanoma_-pos-conitec.pdf (accessed on 5 May 2024).
- Arisi, M.; Zane, C.; Caravello, S.; Rovati, C.; Zanca, A.; Venturini, M.; Calzavara-Pinton, P. Sun Exposure and Melanoma, Certainties and Weaknesses of the Present Knowledge. Front. Med. 2018, 5, 235. [Google Scholar] [CrossRef]
- Larsson, P.; Andersson, E.; Johansson, U.; Öllinger, K.; Rosdahl, I. Ultraviolet A and B affect human melanocytes and keratinocytes differently. A study of oxidative alterations and apoptosis. Exp. Dermatol. 2005, 14, 117–123. [Google Scholar] [CrossRef]
- Downs, C.A.; Kramarsky-Winter, E.; Segal, R.; Fauth, J.; Knutson, S.; Bronstein, O.; Ciner, F.R.; Jeger, R.; Lichtenfeld, Y.; Woodley, C.M.; et al. Toxicopathological Effects of the Sunscreen UV Filter, Oxybenzone (Benzophenone-3), on Coral Planulae and Cultured Primary Cells and Its Environmental Contamination in Hawaii and the U.S. Virgin Islands. Arch. Environ. Contam. Toxicol. 2016, 70, 265–288. [Google Scholar] [CrossRef] [PubMed]
- Krishnaiah, D.; Sarbatly, R.; Nithyanandam, R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process. 2011, 89, 217–233. [Google Scholar] [CrossRef]
- Amparo, T.R.; da Silva, A.C.P.; Seibert, J.B.; Silva, D.d.S.d.; dos Santos, V.M.R.; Vieira, P.M.d.A.; Brandão, G.C.; de Souza, G.H.B.; Santos, B.A.M.C. In vitro and in silico investigation of the photoprotective and antioxidant potential of Protium spruceanum leaves and its main flavonoids. J. Photochem. Photobiol. A Chem. 2022, 431, 114037. [Google Scholar]
- da Silva, A.C.; Paiva, J.P.; Diniz, R.R.; dos Anjos, V.M.; Silva, A.B.S.M.; Pinto, A.V.; Dos Santos, E.P.; Leitão, A.C.; Cabral, L.M.; Rodrigues, C.R.; et al. Photoprotection assessment of olive (Olea europaea L.) leaves extract standardized to oleuropein: In vitro and in silico approach for improved sunscreens. J. Photochem. Photobiol. B 2019, 193, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; He, L.; Wang, W.; Wei, G.; Ma, L.; Liu, H.; Yao, L. Artemisia sieversiana Ehrhart ex Willd. Essential oil and its main component, chamazulene: Their photoprotective effect against uvb-induced cellular damage and potential as novel natural sunscreen aditives. ACS Sustain. Chem. Eng. 2023, 11, 17675–17686. [Google Scholar] [CrossRef]
- Pino, J.A.; Regalado, E.L.; Rodríguez, J.L.; Fernández, M.D. Phytochemical analysis and in vitro free-radical-scavenging activities of the essential oils from leaf and fruit of Melaleuca leucadendra L. Chem. Biodivers. 2010, 7, 2281–2288. [Google Scholar] [CrossRef] [PubMed]
- Di Martile, M.; Garzoli, S.; Sabatino, M.; Valentini, E.; D’Aguanno, S.; Ragno, R.; Del Bufalo, D. Antitumor effect of Melaleuca alternifolia essential oil and its main component terpinen-4-ol in combination with target therapy in melanoma models. Cell Death Discov. 2021, 7, 127. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, Y.; Li, M.; Mao, J.; Zhang, L.; Huang, R.; Jin, X.; Ye, L. Anti-tumor effect of α-pinene on human hepatoma cell lines through inducing G2/M cell cycle arrest. J. Pharmacol. Sci. 2015, 127, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Liu, S.; Ju, X.; Du, J.; Xu, B.; Yuan, H.; Qin, F.; Li, L. The antitumor effect of hinesol, extract from Atractylodes lancea (Thunb.) DC. by proliferation, inhibition, and apoptosis induction via MEK/ERK and NF-κB pathway in non-small cell lung cancer cell lines A549 and NCI-H1299. J. Cell. Biochem. 2019, 120, 18600–18607. [Google Scholar] [CrossRef]
- Antunes, A.S.; Gouveia, A.P.; Diogo, G.M.; Taylor, J.G.; Sousa, L.R.D.; Amparo, T.R.; Perasoli, F.B.; Santos, O.D.D.; Cazati, T.; Vieira, P.M.; et al. In vitro Photoprotective Evaluation and Development of Novel Nanoemulsion with Chromone Derivative. J. Braz. Chem. Soc. 2021, 32, 1813–1821. [Google Scholar] [CrossRef]
- Forgiarini, A.; Esquena, J.; González, C.; Solans, C. Formation of Nano-emulsions by Low-Energy Emulsification Methods at Constant Temperature. Langmuir 2001, 17, 2076–2083. [Google Scholar] [CrossRef]
- Donsì, F.; Ferrari, G. Essential oil nanoemulsions as antimicrobial agents in food. J. Biotechnol. 2016, 233, 106–120. [Google Scholar] [CrossRef]
- Monge-Fuentes, V.; Muehlmann, L.A.; Longo, J.P.F.; Silva, J.R.; Fascineli, M.L.; de Souza, P.; Faria, F.; Degterev, I.A.; Rodriguez, A.; Carneiro, F.P.; et al. Photodynamic therapy mediated by acai oil (Euterpe oleracea Martius) in nanoemulsion: A potential treatment for melanoma. J. Photochem. Photobiol. B 2017, 166, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Noosidum, A.; Chareonviriyaphap, T.; Chandrapatya, A. Synergistic repellent and irritant effect of combined essential oils on Aedes aegypti (L.) mosquitoes. J. Vector Ecol. 2014, 39, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.A.; Sankeshwari, R.M.; Ankola, A.V.; Dodamani, S.; Tendulkar, S.; Jalihal, S.; Khot, A.J.P.; Varghese, A.S.; Chavan, P.; Shah, M.A.; et al. Antimicrobial Efficacy of Essential Oils and Their Combination Against Microorganisms Associated With Postradiation Therapy in Patients With Head and Neck Cancer: An In Vitro Study. Cureus 2023, 15, e40768. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; McClements, D.J. Overview of Nanoemulsion Properties: Stability, Rheology, and Appearance. Nanoemulsions 2018, 29, 21–49. [Google Scholar]
- Bautista-Silva, J.P.; Seibert, J.B.; Amparo, T.R.; Rodrigues, I.V.; Teixeira, L.F.M.; Souza, G.H.B.; dos Santos, O.D.H. Melaleuca leucadendra Essential Oil Promotes Loss of Cell Membrane and Wall Integrity and Inhibits Bacterial Growth: An In Silico and In Vitro Approach. Curr. Microbiol. 2020, 77, 2181–2191. [Google Scholar] [CrossRef] [PubMed]
- Song, J.E.; Kim, J.M.; Lee, N.H.; Yang, J.Y.; Lee, H.S. Acaricidal and Insecticidal Activities of Essential Oils against a Stored-Food Mite and Stored-Grain Insects. J. Food Prot. 2016, 79, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Seibert, J.B.S.; Rodrigues, I.V.; Carneiro, S.P.; Amparo, T.R.; Lanza, J.S.; Frézard, F.J.G.; de Souza, G.H.B.; dos Santos, O.D.H. Seasonality study of essential oil from leaves of Cymbopogon densiflorus and nanoemulsion development with antioxidant activity. Flavour Fragr. J. 2018, 34, 5–14. [Google Scholar] [CrossRef]
- Schaffazick, S.R.; Guterres, S.S.; Freitas, L.L.; Pohlmann, A.R. Caracterização e estabilidade físico-química de sistemas poliméricos nanoparticulados para administração de fármacos. Química Nova 2003, 26, 12. [Google Scholar] [CrossRef]
- Surender, V.; Deepika, M. Solid lipid nanoparticles: A comprehensive review. J. Chem. Pharm. Res. 2016, 8, 102–114. [Google Scholar]
- Tadros, T.; Izquierdo, P.; Esquena, J.; Solans, C. Formation and stability of nano-emulsions. Adv. Colloid Interface Sci. 2004, 108–109, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yue, Y.; Liu, G.; Li, Y.; Zhang, J.; Gong, Q.; Yan, Z.; Duan, M. Preparation and characterization of a lecithin nanoemulsion as a topical delivery system. Nanoscale Res. Lett. 2009, 5, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Seibert, J.B.; Bautista-Silva, J.P.; Amparo, T.R.; Petit, A.; Pervier, P.; dos Santos Almeida, J.C.; Azevedo, M.C.; Silveira, B.M.; Brandão, G.C.; de Souza, G.H.B.; et al. Development of propolis nanoemulsion with antioxidant and antimicrobial activity for use as a potential natural preservative. Food Chem. 2019, 287, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Coelho, E.A.; Ribeiro, T.G.; Chávez-Fumagalli, M.; Valadares, D.G.; França, J.R.; Rodrigues, L.B.; Duarte, M.C.; Lage, P.S.; Andrade, P.H.R.; Lage, D.P.; et al. Novel targeting using nanoparticles: An approach to the development of an effective anti-leishmanial drug-delivery system. Int. J. Nanomed. 2014, 9, 877–890. [Google Scholar] [CrossRef] [PubMed]
- Marques Borges, G.S.; Oliveira Ferencs, M.; Mello Gomide Loures, C.; Abdel-Salam, M.A.; Gontijo Evangelista, F.C.; Sales, C.C.; Reis da Silva, P.H.; de Oliveira, R.B.; Malachias, Â.; Yoshida, M.I.; et al. Novel self-nanoemulsifying drug-delivery system enhances antileukemic properties of all-trans retinoic acid. Nanomedicine 2020, 15, 1471–1486. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.A. Rheology of Fluid, Semisolid, and Solid Foods: Principles and Applications, 3rd ed.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Mehrnia, M.A.; Jafari, S.M.; Makhmal-Zadeh, B.S.; Maghsoudlou, Y. Rheological and release properties of double nano-emulsions containing crocin prepared with Angum gum, Arabic gum and whey protein. Food Hydrocoll. 2017, 66, 259–267. [Google Scholar] [CrossRef]
- Vliet, T.V. Rheology and Fracture Mechanics of Foods; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Costa, C.M. Caracterização reológica de fluidos complexos. Rev. Bras. Iniciação Científica 2017, 4, 3–28. [Google Scholar]
- Hao, Z.; Chen, Z.; Chang, M.; Meng, J.; Liu, J.; Feng, C. Rheological properties and gel characteristics of polysaccharides from fruit-bodies of Sparassis crispa. Int. J. Food Prop. 2018, 21, 2283–2295. [Google Scholar] [CrossRef]
- Freire, I.S. Reologia Escoamento e Deformação da Matéria; Instituto Brasileiro de Informação em Ciência e Tecnologia: Brasília, Brazil, 2012. [Google Scholar]
- Moravkova, T.; Filip, P. The Influence of Emulsifier on Rheological and Sensory Properties of Cosmetic Lotions. Adv. Mater. Sci. Eng. 2013, 2013, 168503. [Google Scholar] [CrossRef]
- Dănilă, E.M.; Zenovia, K.; Mădălina, G.A.G.; Violeta, M. Formulation and characterization of some oil in water cosmetic emulsions based on collagen hydrolysate and vegetable oils mixtures. J. Pure Appl. Chem. 2019, 91, 1493–1507. [Google Scholar] [CrossRef]
- Mohania, D.; Chandel, S.; Kumar, P.; Verma, V.; Digvijay, K.; Tripathi, D.; Choudhury, K.; Mitten, S.K.; Shah, D. Ultraviolet Radiations: Skin Defense-Damage Mechanism. Adv. Exp. Med. Biol. 2017, 996, 71–87. [Google Scholar] [PubMed]
- Paszkowska-Szczur, K.; Scott, R.; Serrano-Fernandez, P.; Mirecka, A.; Gapska, P.; Górski, B.; Cybulski, C.; Maleszka, R.; Sulikowski, M.; Nagay, L.; et al. Xeroderma pigmentosum genes and melanoma risk. Int. J. Cancer 2013, 133, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- FDA. Sunscreen: How to Help Protect Your Skin from the Sun. Available online: https://www.fda.gov/drugs/understanding-over-counter-medicines/sunscreen-how-help-protect-your-skin-un#:~:text=Use%20broad%20spectrum%20sunscreens%20with,and%20out%20of%20the%20water (accessed on 9 April 2024).
- Lohani, A.; Mishra, A.K.; Verma, A. Cosmeceutical potential of geranium and calendula essential oil: Determination of antioxidant activity and in vitro sun protection factor. J. Cosmet. Dermatol. 2019, 18, 550–557. [Google Scholar] [CrossRef]
- Arianto, A.; Cella, G.; Bangun, H. Preparation and Evaluation of Sunscreen Nanoemulsions with Synergistic Efficacy on SPF by Combination of Soybean Oil, Avobenzone, and Octyl Methoxycinnamate. Open Access Maced. J. Med. Sci. 2019, 7, 2751–2756. [Google Scholar] [CrossRef] [PubMed]
- Baccarin, T.; Mitjans, M.; Ramos, D.; Lemos-Senna, E.; Vinardell, M.P. Photoprotection by Punica granatum seed oil nanoemulsion entrapping polyphenol-rich ethyl acetate fraction against UVB-induced DNA damage in human keratinocyte (HaCaT) cell line. J. Photochem. Photobiol. B 2015, 153, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Sample, A.; He, Y.Y. Mechanisms and prevention of UV-induced melanoma. Photodermatol. Photoimmunol. Photomed. 2018, 34, 13–24. [Google Scholar] [CrossRef]
- Yin, Y.; Li, W.; Son, Y.-O.; Sun, L.; Lu, J.; Kim, D.; Wang, X.; Yao, H.; Wang, L.; Pratheeshkumar, P.; et al. Quercitrin Protects Skin from UVB-induced Oxidative Damage. Toxicol. Appl. Pharmacol. 2013, 269, 89–99. [Google Scholar] [CrossRef]
- Ankur, J.; Khan, M.H. Evaluation of antioxidant activity of essential oils of some Indian medicinal plants by DPPH, FRAP and ABTS assay. J. Pharm. Negat. Results 2022, 6, 2602–2607. [Google Scholar] [CrossRef]
- Shahbazi, Y. Antioxidant, antibacterial, and antifungal properties of nanoemulsion of clove essential oil. Nanomed. Res. J. 2019, 4, 204–208. [Google Scholar]
- Ramadan, M.M.; Ali, M.M.; Ghanem, K.Z.; El-Ghorab, A.H. Essential oils from Egyptian aromatic plants as antioxidant and novel anticancer agents in human cancer cell lines. Grasas Aceites 2015, 66, e080. [Google Scholar]
- Huang, X.W.; Feng, Y.C.; Huang, Y.; Li, H.L. Chemical composition, antioxidant and the possible use as skin-care ingredient of clove oil (Syzygium aromaticum (L.) Merr. & Perry) and citronella oil (Cymbopogon goeringii) from China. J. Essent. Oil Res. 2013, 25, 315–323. [Google Scholar]
- Teles, A.M.; Silva-Silva, J.V.; Fernandes, J.M.P.; Abreu-Silva, A.L.; Calabrese, K.D.S.; Mendes Filho, N.E.; Mouchrek, A.N.; Almeida-Souza, F. GC-MS Characterization of Antibacterial, Antioxidant, and Antitrypanosomal Activity of Syzygium aromaticum Essential Oil and Eugenol. Evid.-Based Complement. Altern. Med. 2021, 2021, 6663255. [Google Scholar] [CrossRef] [PubMed]
- Irahal, I.N.; Guenaou, I.; Lahlou, F.A.; Hmimid, F.; Bourhim, N. Syzygium aromaticum bud (clove) essential oil is a novel and safe aldose reductase inhibitor: In silico, in vitro, and in vivo evidence. Hormones 2022, 21, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, İ. Antioxidant activity of eugenol: A structure-activity relationship study. J. Med. Food 2011, 14, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Burits, M.; Asres, K.; Bucar, F. The antioxidant activity of the essential oils of Artemisia afra, Artemisia abyssinica and Juniperus procera. Phytother. Res. 2001, 15, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Ruberto, G.; Baratta, M.T. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem. 2000, 69, 167–174. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, Y.; Guo, L.; Jiang, H.; Ji, Q. In Vitro Evaluation of Antioxidant and Antimicrobial Activities of Melaleuca alternifolia Essential Oil. BioMed Res. Int. 2018, 2018, 2396109. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.D.; Kaur, I.; Kaur, R.; Chauhan, A. Essential Oil from Melaleuca alternifolia: Aromatic Profiling, Phytochemical Analysis and Assessment of Diverse Biological Activities. Russ. Agric. Sci. 2023, 49, 558–574. [Google Scholar] [CrossRef]
- Sathiyaseelan, A.; Zhang, X.; Wang, M.-H. Aumentando os efeitos antioxidantes, antibacterianos e cicatrizantes de feridas do óleo de Melaleuca alternifolia, microencapsulando-o em microesferas de quitosana-alginato de sódio. Nutrientes 2023, 15, 1319. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Sousa, L.R.D.; Azevedo, M.L.S.; Rocha, D.F.; Andrade, Â.L.; Amparo, T.R.; dos Santos, O.D.H.; Seibert, J.; Pereira, L.; Vieira, P.; Carneiro, C.; et al. Trypanocidal Activity and Increased Solubility of Benznidazole Incorporated in PEG 4000 and Its Derivatives. J. Braz. Chem. Soc. 2021, 32, 11. [Google Scholar] [CrossRef]
- Sousa, L.R.D.; Amparo, T.R.; Souza, G.H.B.d.; Ferraz, A.T.; Fonseca, K.d.S.; Azevedo, A.S.d.; Nascimento, A.M.d.; Andrade, Â.L.; Seibert, J.B.; Valverde, T.M.; et al. Anti-Trypanosoma cruzi Potential of Vestitol Isolated from Lyophilized Red Propolis. Molecules 2023, 28, 7812. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Zhou, C.L.; Chen, F.P.; Han, D.; Wang, C.Y.; Li, J.X.; Chi, Z.; Liu, C.G. Development of a carboxymethyl chitosan functionalized nanoemulsion formulation for increasing aqueous solubility, stability and skin permeability of astaxanthin using low-energy method. J. Microencapsul. 2017, 34, 707–721. [Google Scholar] [CrossRef]
- Asmawi, A.A.; Salim, N.; Abdulmalek, E.; Abdul Rahman, M.B. Size-Controlled Preparation of Docetaxel- and Curcumin-Loaded Nanoemulsions for Potential Pulmonary Delivery. Pharmaceutics 2023, 15, 652. [Google Scholar] [CrossRef] [PubMed]
- Gledovic, A.; Janosevic Lezaic, A.; Krstonosic, V.; Djokovic, J.; Nikolic, I.; Bajuk-Bogdanovic, D.; Stankovic, J.A.; Randjelovic, D.; Savic, S.M.; Filipovic, M.; et al. Low-energy nanoemulsions as carriers for red raspberry seed oil: Formulation approach based on Raman spectroscopy and textural analysis, physicochemical properties, stability and in vitro antioxidant/ biological activity. PLoS ONE 2020, 15, e0230993. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.C.; Hung, C.F.; Chen, B.H. Preparation of coffee oil-algae oil-based nanoemulsions and the study of their inhibition effect on UVA-induced skin damage in mice and melanoma cell growth. Int. J. Nanomed. 2017, 12, 6559–6580. [Google Scholar] [CrossRef] [PubMed]
- Tagne, J.-B.; Srikanth, K.; Robert, J.N. Nanoemulsion preparations of the anticancer drug dacarbazine significantly increase its efficacy in a xenograft mouse melanoma model. Mol. Pharm. 2008, 5, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Almeida, W.A.S.; Antunes, A.S.; Penido, R.G.; Correa, H.S.G.; do Nascimento, A.M.; Andrade, Â.L.; Santos, V.R.; Cazati, T.; Amparo, T.R.; de Souza, G.H.B.; et al. Photoprotective activity and increase of SPF in sunscreen formulation using lyophilized red propolis extracts from Alagoas. Rev. Bras. Farmacogn. 2019, 23, 373–380. [Google Scholar] [CrossRef]
- Mansur, J.S.; Breder, M.N.R.; Mansur, M.C.A.; Azulay, R.D. Determination of sun protection factor by spectrophotometry. An. Bras. Dermatol. 1986, 61, 121–124. [Google Scholar]
- Sousa, C.M.D.M.; Silva, H.R.; Ayres, M.C.C.; Costa, C.L.S.D.; Araújo, D.S.; Cavalcante, L.C.D.; Barros, E.D.S.; Araújo, P.B.D.M.; Brandão, M.S.; Chaves, M.H. Fenóis totais e atividade antioxidante de cinco plantas medicinais. Química Nova 2007, 30, 351–355. [Google Scholar] [CrossRef]
- Li, X.; Wu, X.; Huang, L. Correlation between Antioxidant Activities and Phenolic Contents of Radix Angelicae Sinensis (Danggui). Molecules 2009, 14, 5349–5361. [Google Scholar] [CrossRef] [PubMed]



| No. | RT (min) | Compound | Class | Area (%) | Similarity |
|---|---|---|---|---|---|
| 1 | 5.78 | α-pinene | Monoterpene | 8.19 | 95 |
| 2 | 6.84 | β-pinene | Monoterpene | 2.96 | 95 |
| 3 | 8.02 | (+)-2-Carene | Monoterpene | 0.51 | 93 |
| 4 | 8.60 | β-terpineol | Monoterpene | 17.09 | 89 |
| 5 | 8.66 | Eucalyptol | Monoterpene | 2.88 | 89 |
| 6 | 9.34 | γ-terpinene | Monoterpene | 1.43 | 95 |
| 7 | 10.29 | Cyclohexene, 4-methyl-3-(1-methylethylidene)- | Monoterpene | 0.60 | 93 |
| 8 | 10.68 | Linalool | Monoterpene | 1.03 | 95 |
| 9 | 12.27 | Isopulegol | Monoterpene | 0.54 | 96 |
| 10 | 13.42 | Terpinen-4-ol | Monoterpene | 1.88 | 94 |
| 11 | 14.02 | α-terpineol | Monoterpene | 6.65 | 94 |
| 12 | 21.80 | Caryophyllene | Sesquiterpene | 2.22 | 94 |
| 13 | 22.90 | α-caryophyllene | Sesquiterpene | 0.62 | 95 |
| 14 | 23.14 | Aromadendrene | Sesquiterpene | 0.74 | 94 |
| 15 | 23.97 | β-selinene | Sesquiterpene | 0.83 | 94 |
| 16 | 24.26 | Ledene | Sesquiterpene | 1.33 | 91 |
| 17 | 25.10 | δ-cadinene | Sesquiterpene | 0.60 | 90 |
| 18 | 26.55 | Palustrol | Sesquiterpene | 1.05 | 94 |
| 19 | 27.04 | Caryophyllene epoxide | Sesquiterpene | 2.23 | 93 |
| 20 | 27.83 | Hinesol | Sesquiterpene | 28.74 | 88 |
| 21 | 27.97 | Viridiflorol | Sesquiterpene | 4.43 | 92 |
| 22 | 28.81 | α-cadinol | Sesquiterpene | 1.00 | 90 |
| 23 | 29.12 | β-selinenol | Sesquiterpene | 0.76 | 92 |
| 24 | 29.21 | Selinenol | Sesquiterpene | 1.23 | 85 |
| Period (Days) | Zeta Potential (mV) | |
|---|---|---|
| NE-EO | NE-B | |
| 1 | −18.0 ± 1.06 aA | −19.9 ± 1.78 aA |
| 7 | −23.0 ± 0.92 bA | −15.6 ± 1.50 bB |
| 14 | −15.1 ± 0.60 cA | −19.1 ± 1.83 aA |
| 21 | −20.2 ± 0.30 aA | −21.4 ± 1.69 aA |
| 28 | −15.4 ± 0.30 cA | −19.5 ± 0.62 aB |
| Period (Days) | Size (nm) | PDI | ||
|---|---|---|---|---|
| NE-EO | NE-B | NE-EO | NE-B | |
| 1 | 179.5 ± 1.852 aA | 138.2 ± 0.833 aB | 0.23 ± 0.010 aA | 0.15 ± 0.011 aB |
| 7 | 184.0 ± 2.676 aA | 137.6 ± 0.954 aB | 0.23 ± 0.031 aA | 0.17 ± 0.004 aA |
| 14 | 181.1 ± 0.950 aA | 135.6 ± 0.458 aB | 0.25 ± 0.029 aA | 0.17 ± 0.011 aB |
| 21 | 188.6 ± 14.330 aA | 136.1 ± 0.208 aB | 0.27 ± 0.048 aA | 0.18 ± 0.005 bA |
| 28 | 180.2 ± 1.305 aA | 137.3 ± 1.185 aB | 0.25 ± 0.014 aA | 0.18 ± 0.004 bB |
| Period (Days) | Consistency Index (cP) | Flow Rate (n) | Confidence (%) | |||
|---|---|---|---|---|---|---|
| NE-EO | NE-B | NE-EO | NE-B | NE-EO | NE-B | |
| 1 | 240.70 ± 13.308 aA | 12.63 ± 1.115 aB | 0.60 ± 0.006 aA | 0.86 ± 0.015 aB | 92.6 ± 0.808 | 97.1 ± 1.015 |
| 7 | 259.66 ± 28.854 aA | 18.90 ± 0.916 bB | 0.60 ± 0.012 aA | 0.87 ± 0.030 aB | 96.7 ± 0.404 | 97.7 ± 0.305 |
| 14 | 322.40 ± 12.322 bA | 25.33 ± 1.436 cB | 0.61 ± 0.006 aA | 0.87 ± 0.010 aB | 92.4 ± 0.404 | 97.1 ± 0.289 |
| 21 | 343.33 ± 39.172 bA | 42.23 ± 1.616 dB | 0.61 ± 0.026 aA | 0.83 ± 0.006 aB | 90.5 ± 1.137 | 98.0 ± 0.737 |
| 28 | 268.20 ± 5.415 aA | 85.00 ± 32.568 eB | 0.64 ± 0.020 aA | 0.75 ± 0,052 bA | 89.6 ± 0.472 | 97.3 ± 0.557 |
| Concentration | Solar Protection Factor (SPF) |
|---|---|
| EO | |
| 100 μg/mL | 0.1350 ± 0.0273 |
| 200 μg/mL | 0.1964 ± 0.0144 |
| 300 μg/mL | 0.2658 ± 0.0125 |
| 400 μg/mL | 0.3423 ± 0.0265 |
| 500 μg/mL | 0.4213 ± 0.0432 |
| Concentration | Solar Protection Factor (SPF) | |
|---|---|---|
| NE-EO | NE-B | |
| 1% v/v | 0.2892 (28.92 A) ± 0.0039 | 0.1194 (11.94 A) ± 0.0019 |
| 2% v/v | 0.6334 (31.67 B) ± 0.0025 | 0.2421 (12.10 B) ± 0.0384 |
| 3% v/v | 0.9532 (30.82 C) ± 0.0236 | 0.4946 (15.99 C) ± 0.0097 |
| Sample | DPPH | ABTS |
|---|---|---|
| IC50 (µg/mL) | IC50 (µg/mL) | |
| EO | 277.38 ± 22.12 | 40.72 ± 5.70 |
| NE-EO | >10,000.00 (>200.00 A) | 265.13 (5.30 A) ± 12.94 |
| NE-B | >10,000.00 | 408.33 ± 13.37 |
| C+ | 1.17 ± 0.28 | 0.24 ± 0.02 |
| Skin Cell Line | Sample | |||
|---|---|---|---|---|
| EO | NE-EO | NE-B | ||
| L-929 CC50 (µg/mL) | 24 h | 81.10 ± 4.42 | 2124.33 (42.49 C) ± 433.21 | 3311.00 ± 429.85 |
| 48 h | 63.40 ± 1.85 | 846.33 (16.93 C) ± 112.29 | 2030.10 ± 177.66 | |
| B16-F10 CC50 (µg/mL) | 24 h | 60.61 ± 2.16 | 945.30 (18.91 C) ± 82.14 | 3008.67 ± 40.08 |
| 48 h | 44.64 ± 2.00 | 566.87 (11.34 C) ± 31.03 | 2003.67 ± 111.38 | |
| SI A | 24 h | 1.34 | 2.25 | 1.10 |
| 48 h | 1.42 | 1.49 | 1.01 | |
| NGM CC50 (µg/mL) | 24 h | 155.2 ± 29.15 | 1067.0 (21.34 C) ± 24.23 | 2091.0 ± 66.19 |
| 48 h | 129.6 ± 27.78 | 508.5 (10.17 C) ± 13.31 | 1406.0 ± 25.87 | |
| MeWo CC50 (µg/mL) | 24 h | 58.5 ± 13.03 | 404.1 (8.10 C) ± 11.63 | 1027.0 ± 11.43 |
| 48 h | 50.46 ± 19.33 | 391.71 (7.83 C) ± 13.75 | 919.8 ± 13.86 | |
| SI B | 24 h | 2.65 | 2.64 | 2.04 |
| 48 h | 2.57 | 1.30 | 1.53 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, L.R.D.; Santos, M.L.d.C.; Sampaio, L.S.; Faustino, C.G.; Guigueno, M.L.L.; Freitas, K.M.; Lopes, M.T.P.; Mota, G.C.F.; dos Santos, V.M.R.; Seibert, J.B.; et al. Nanoemulsified Essential Oil of Melaleuca leucadendron Leaves for Topical Application: In Vitro Photoprotective, Antioxidant and Anti-Melanoma Activities. Pharmaceuticals 2024, 17, 721. https://doi.org/10.3390/ph17060721
Sousa LRD, Santos MLdC, Sampaio LS, Faustino CG, Guigueno MLL, Freitas KM, Lopes MTP, Mota GCF, dos Santos VMR, Seibert JB, et al. Nanoemulsified Essential Oil of Melaleuca leucadendron Leaves for Topical Application: In Vitro Photoprotective, Antioxidant and Anti-Melanoma Activities. Pharmaceuticals. 2024; 17(6):721. https://doi.org/10.3390/ph17060721
Chicago/Turabian StyleSousa, Lucas Resende Dutra, Maria Luiza da Costa Santos, Larissa Silva Sampaio, Clarisse Gaëlle Faustino, Mérine Lauriane Loïce Guigueno, Kátia Michelle Freitas, Miriam Teresa Paz Lopes, Gabriela Cristina Ferreira Mota, Viviane Martins Rebello dos Santos, Janaína Brandão Seibert, and et al. 2024. "Nanoemulsified Essential Oil of Melaleuca leucadendron Leaves for Topical Application: In Vitro Photoprotective, Antioxidant and Anti-Melanoma Activities" Pharmaceuticals 17, no. 6: 721. https://doi.org/10.3390/ph17060721






