Trametinib, a MEK1/2 Inhibitor, Protects Mice from Cisplatin- and Noise-Induced Hearing Loss
Abstract
:1. Introduction
2. Results
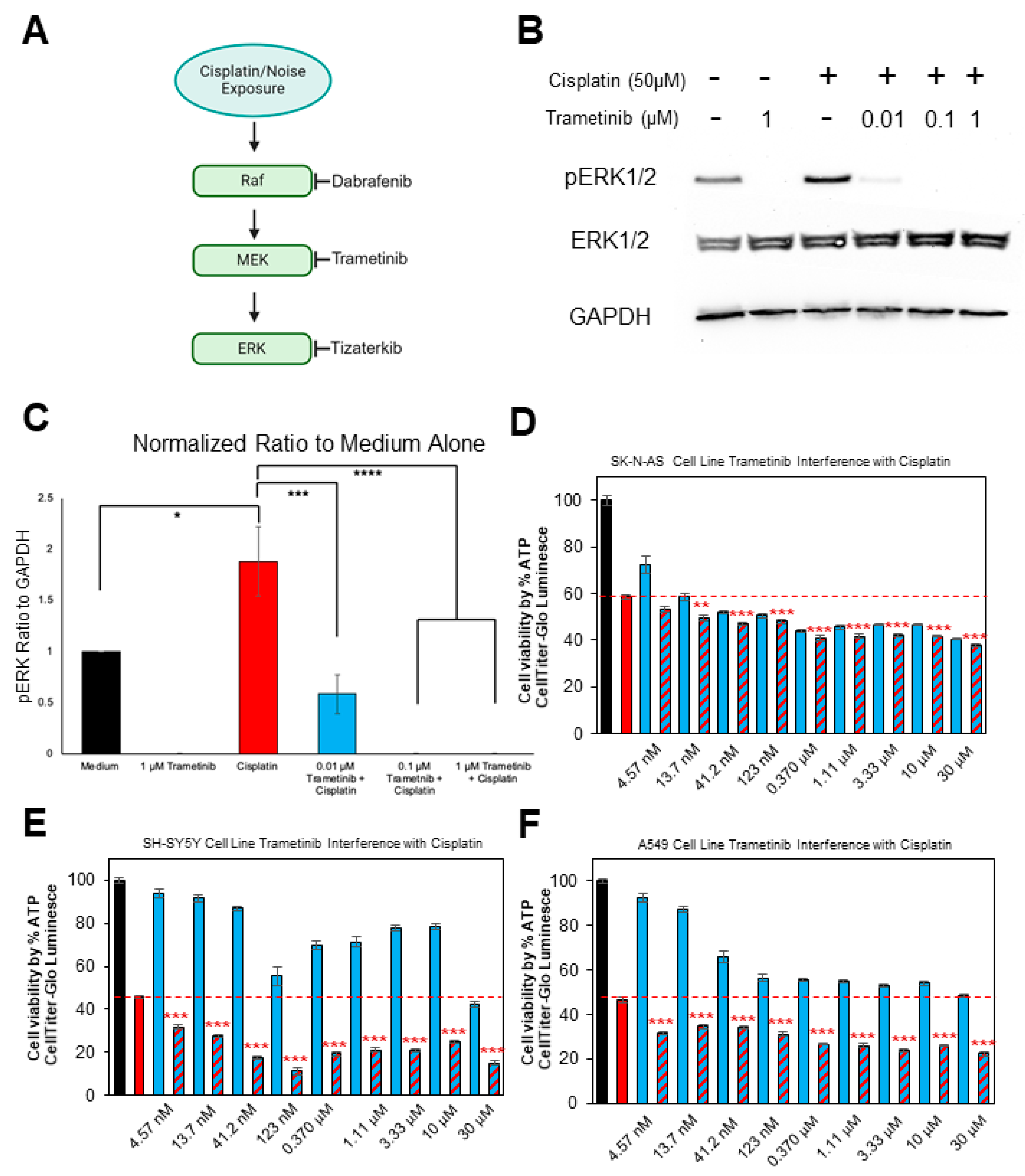
2.1. Trametinib Inhibits MAPK Activation in the HEI-OC1 Cell Line and Does Not Interfere with Cisplatin’s Tumor-Killing Ability in Multiple Cancer Cell Lines
2.2. Trametinib Protects from Cisplatin-Induced Hearing Loss in a Clinically Relevant Mouse Model
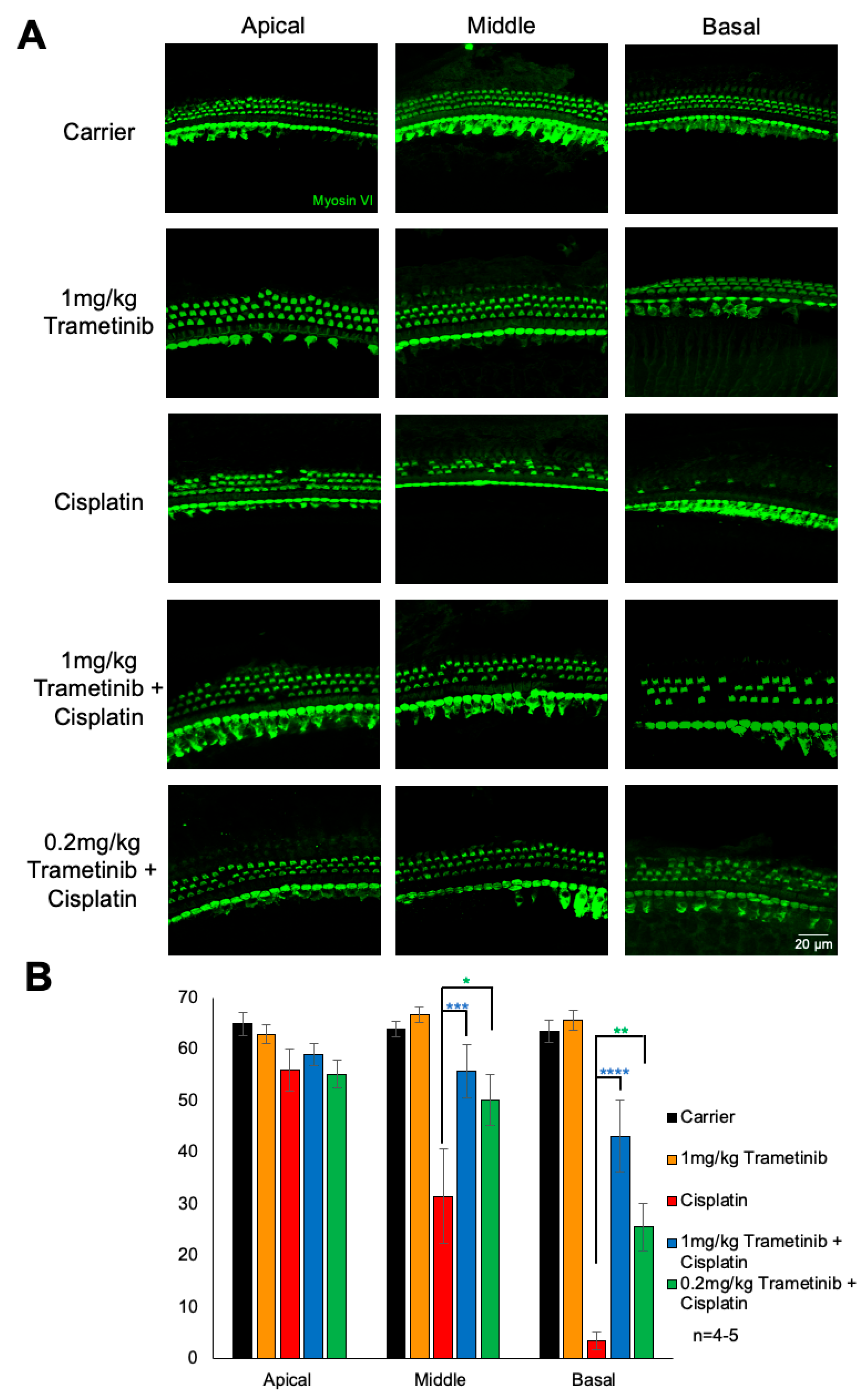
2.3. Trametinib Protects from Cisplatin-Induced Outer Hair Cell Loss
2.4. Trametinib Confers Slight Protection from Cisplatin-Induced Weight Loss but Co-Treatment of Higher Doses of Trametinib with Cisplatin Caused Mouse Death
2.5. Trametinib Protects from Noise-Induced Hearing Loss and Ribbon Synapse Loss in FVB Mice
3. Discussion
4. Materials and Methods
4.1. Study Approval
4.2. Mouse Models
4.3. HEI-OC1 Cell Line and Collection of Cell Lysates
4.4. Cancer Cell Lines and Cell Titer-Glo Assay
4.5. Immunoblotting
4.6. Multi-Cycle Cisplatin Treatment Model
4.7. Auditory Brainstem Response
4.8. Distortion Product Otoacoustic Emission
4.9. Noise Exposure
4.10. Tissue Preparation, Immunofluorescence, and OHC Counts
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2019 Hearing Loss Collaborators. Hearing Loss Prevalence and Years Lived with Disability, 1990–2019: Findings from the Global Burden of Disease Study 2019. Lancet 2021, 397, 996–1009. [Google Scholar] [CrossRef]
- D’Haese, P.S.C.; Van Rompaey, V.; De Bodt, M.; Van de Heyning, P. Severe Hearing Loss in the Aging Population Poses a Global Public Health Challenge. How Can We Better Realize the Benefits of Cochlear Implantation to Mitigate This Crisis? Front. Public Health 2019, 7, 227. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Yan, A.; Liu, K. What Is Noise-Induced Hearing Loss? Br. J. Hosp. Med. 2019, 80, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Kurabi, A.; Keithley, E.M.; Housley, G.D.; Ryan, A.F.; Wong, A.C.-Y. Cellular Mechanisms of Noise-Induced Hearing Loss. Hear. Res. 2017, 349, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Prayuenyong, P.; Baguley, D.M.; Kros, C.J.; Steyger, P.S. Preferential Cochleotoxicity of Cisplatin. Front. Neurosci. 2021, 15, 695268. [Google Scholar] [CrossRef] [PubMed]
- Sheth, S.; Mukherjea, D.; Rybak, L.P.; Ramkumar, V. Mechanisms of Cisplatin-Induced Ototoxicity and Otoprotection. Front. Cell Neurosci. 2017, 11, 338. [Google Scholar] [CrossRef] [PubMed]
- Torrente, M.C.; Tamblay, N.; Herrada, J.; Maass, J.C. Hearing Loss in School-Aged Children. Acta Oto-Laryngol. 2023, 143, 28–30. [Google Scholar] [CrossRef]
- Loughrey, D.G.; Kelly, M.E.; Kelley, G.A.; Brennan, S.; Lawlor, B.A. Association of Age-Related Hearing Loss With Cognitive Function, Cognitive Impairment, and Dementia: A Systematic Review and Meta-Analysis. JAMA Otolaryngol. Head Neck Surg. 2018, 144, 115–126. [Google Scholar] [CrossRef]
- Lin, F.R.; Pike, J.R.; Albert, M.S.; Arnold, M.; Burgard, S.; Chisolm, T.; Couper, D.; Deal, J.A.; Goman, A.M.; Glynn, N.W.; et al. Hearing Intervention versus Health Education Control to Reduce Cognitive Decline in Older Adults with Hearing Loss in the USA (ACHIEVE): A Multicentre, Randomised Controlled Trial. Lancet 2023, 402, 786–797. [Google Scholar] [CrossRef] [PubMed]
- Chadha, S.; Cieza, A. Promoting Global Action on Hearing Loss: World Hearing Day. Int. J. Audiol. 2017, 56, 145–147. [Google Scholar] [CrossRef]
- Dhillon, S. Sodium Thiosulfate: Pediatric First Approval. Pediatr. Drugs 2023, 25, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Freyer, D.R.; Orgel, E.; Knight, K.; Krailo, M. Special Considerations in the Design and Implementation of Pediatric Otoprotection Trials. J. Cancer Surviv. 2023, 17, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Brock, P.; Meijer, A.; Kogner, P.; Ansari, M.; Capra, M.; Geller, J.; van den Heuvel-Eibrink, M.; Knight, K.; Kruger, M.; Lindemulder, S.; et al. Sodium Thiosulfate as Cisplatin Otoprotectant in Children: The Challenge of When to Use It. Pediatr. Blood Cancer 2023, 70, e30248. [Google Scholar] [CrossRef] [PubMed]
- Schvartz-Leyzac, K.C.; Colesa, D.J.; Swiderski, D.L.; Raphael, Y.; Pfingst, B.E. Cochlear Health and Cochlear-Implant Function. J. Assoc. Res. Otolaryngol. 2023, 24, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Williger, B.; Lang, F.R. Managing Age-Related Hearing Loss: How to Use Hearing Aids Efficiently—A Mini-Review. Gerontology 2014, 60, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, K.; Spielbauer, K.; Rusheen, A.; Wang, L.; Baker, T.; Eyles, S.; Cunningham, L. Lovastatin Protects against Cisplatin-Induced Hearing Loss in Mice. Hear. Res. 2020, 389, 107905. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, K.A.; Allen, P.; Campbell, M.; Page, B.; Townes, T.; Li, C.-M.; Cheng, H.; Garrett, J.; Mulquin, M.; Clements, A.; et al. Atorvastatin Is Associated with Reduced Cisplatin-Induced Hearing Loss. J. Clin. Investig. 2021, 131, e142616. [Google Scholar] [CrossRef]
- Kennedy, C.L.; Shuster, B.; Amanipour, R.; Milon, B.; Patel, P.; Elkon, R.; Hertzano, R. Metformin Protects Against Noise-Induced Hearing Loss in Male Mice. Otol. Neurotol. 2023, 44, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-C.; Chung, C.-H.; Lu, C.-H.; Chien, W.-C. Metformin Decreases the Risk of Sudden Sensorineural Hearing Loss in Patients with Diabetes Mellitus: A 14-Year Follow-up Study. Diabetes Vasc. Dis. Res. 2019, 16, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Fukushima, K.; Omichi, R.; Kariya, S.; Nishizaki, K. Time Courses of Changes in Phospho- and Total-MAP Kinases in the Cochlea after Intense Noise Exposure. PLoS ONE 2013, 8, e58775. [Google Scholar] [CrossRef]
- Alagramam, K.N.; Stepanyan, R.; Jamesdaniel, S.; Chen, D.H.-C.; Davis, R.R. Noise Exposure Immediately Activates Cochlear Mitogen-Activated Protein Kinase Signaling. Noise Health 2014, 16, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Herranen, A.; Ikäheimo, K.; Virkkala, J.; Pirvola, U. The Stress Response in the Non-Sensory Cells of the Cochlea Under Pathological Conditions—Possible Role in Mediating Noise Vulnerability. J. Assoc. Res. Otolaryngol. 2018, 19, 637–652. [Google Scholar] [CrossRef] [PubMed]
- Lahne, M.; Gale, J.E. Damage-Induced Activation of ERK1/2 in Cochlear Supporting Cells Is a Hair Cell Death-Promoting Signal That Depends on Extracellular ATP and Calcium. J. Neurosci. 2008, 28, 4918–4928. [Google Scholar] [CrossRef] [PubMed]
- Ingersoll, M.A.; Lutze, R.D.; Pushpan, C.K.; Kelmann, R.G.; Liu, H.; May, M.T.; Hunter, W.J.; He, D.Z.; Teitz, T. Dabrafenib Protects from Cisplatin-Induced Hearing Loss in a Clinically Relevant Mouse Model. J. Clin. Investig. 2023, 8, e171140. [Google Scholar] [CrossRef] [PubMed]
- Ingersoll, M.A.; Malloy, E.A.; Caster, L.E.; Holland, E.M.; Xu, Z.; Zallocchi, M.; Currier, D.; Liu, H.; He, D.Z.Z.; Min, J.; et al. BRAF Inhibition Protects against Hearing Loss in Mice. Sci. Adv. 2020, 6, eabd0561. [Google Scholar] [CrossRef] [PubMed]
- Ingersoll, M.A.; Lutze, R.D.; Kelmann, R.G.; Kresock, D.F.; Marsh, J.D.; Quevedo, R.V.; Zuo, J.; Teitz, T. KSR1 Knockout Mouse Model Demonstrates MAPK Pathway’s Key Role in Cisplatin- and Noise-Induced Hearing Loss. J. Neurosci. 2024, 44, e2174232024. [Google Scholar] [CrossRef] [PubMed]
- Lutze, R.D.; Ingersoll, M.A.; Thotam, A.; Joseph, A.; Fernandes, J.; Teitz, T. ERK1/2 Inhibition Alleviates Noise-Induced Hearing Loss while Tempering Down the Immune Response. bioRxiv 2023. [Google Scholar] [CrossRef] [PubMed]
- So, H.; Kim, H.; Lee, J.-H.; Park, C.; Kim, Y.; Kim, E.; Kim, J.-K.; Yun, K.-J.; Lee, K.-M.; Lee, H.-Y.; et al. Cisplatin Cytotoxicity of Auditory Cells Requires Secretions of Proinflammatory Cytokines via Activation of ERK and NF-κB. J. Assoc. Res. Otolaryngol. 2007, 8, 338–355. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Shi, S.; Ren, T.; Zhang, Y.; Guo, P.; Wang, J.; Wang, W. U0126 Pretreatment Inhibits Cisplatin-Induced Apoptosis and Autophagy in HEI-OC1 Cells and Cochlear Hair Cells. Toxicol. Appl. Pharmacol. 2021, 415, 115447. [Google Scholar] [CrossRef] [PubMed]
- Kolch, W. Meaningful Relationships: The Regulation of the Ras/Raf/MEK/ERK Pathway by Protein Interactions. Biochem. J. 2000, 351, 289–305. [Google Scholar] [CrossRef] [PubMed]
- Braicu, C.; Buse, M.; Busuioc, C.; Drula, R.; Gulei, D.; Raduly, L.; Rusu, A.; Irimie, A.; Atanasov, A.G.; Slaby, O.; et al. A Comprehensive Review on MAPK: A Promising Therapeutic Target in Cancer. Cancers 2019, 11, 1618. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-J.; Pan, W.-W.; Liu, S.-B.; Shen, Z.-F.; Xu, Y.; Hu, L.-L. ERK/MAPK Signalling Pathway and Tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Cagnol, S.; Chambard, J.-C. ERK and Cell Death: Mechanisms of ERK-Induced Cell Death—Apoptosis, Autophagy and Senescence. FEBS J. 2010, 277, 2–21. [Google Scholar] [CrossRef] [PubMed]
- Gouda, M.A.; Subbiah, V. Expanding the Benefit: Dabrafenib/Trametinib as Tissue-Agnostic Therapy for BRAF V600E–Positive Adult and Pediatric Solid Tumors. Am. Soc. Clin. Oncol. Educ. Book 2023, 43, e404770. [Google Scholar] [CrossRef]
- Dummer, R.; Hauschild, A.; Santinami, M.; Atkinson, V.; Mandalà, M.; Kirkwood, J.M.; Chiarion Sileni, V.; Larkin, J.; Nyakas, M.; Dutriaux, C.; et al. Five-Year Analysis of Adjuvant Dabrafenib plus Trametinib in Stage III Melanoma. N. Engl. J. Med. 2020, 383, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Odogwu, L.; Mathieu, L.; Blumenthal, G.; Larkins, E.; Goldberg, K.B.; Griffin, N.; Bijwaard, K.; Lee, E.Y.; Philip, R.; Jiang, X.; et al. FDA Approval Summary: Dabrafenib and Trametinib for the Treatment of Metastatic Non-Small Cell Lung Cancers Harboring BRAF V600E Mutations. Oncologist 2018, 23, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Grob, J.J.; Stroyakovskiy, D.; Karaszewska, B.; Hauschild, A.; Levchenko, E.; Chiarion Sileni, V.; Schachter, J.; Garbe, C.; Bondarenko, I.; et al. Five-Year Outcomes with Dabrafenib plus Trametinib in Metastatic Melanoma. N. Engl. J. Med. 2019, 381, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Harilal, S.; Gupta, S.V.; Jose, J.; Thomas Parambi, D.G.; Uddin, M.S.; Shah, M.A.; Mathew, B. Exploring the New Horizons of Drug Repurposing: A Vital Tool for Turning Hard Work into Smart Work. Eur. J. Med. Chem. 2019, 182, 111602. [Google Scholar] [CrossRef] [PubMed]
- Falchook, G.S.; Lewis, K.D.; Infante, J.R.; Gordon, M.S.; Vogelzang, N.J.; DeMarini, D.J.; Sun, P.; Moy, C.; Szabo, S.A.; Roadcap, L.T.; et al. Activity of the MEK Inhibitor Trametinib (GSK1120212) in Advanced Melanoma in a Phase I, Dose-Escalation Trial. Lancet Oncol. 2012, 13, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Gilmartin, A.G.; Bleam, M.R.; Groy, A.; Moss, K.G.; Minthorn, E.A.; Kulkarni, S.G.; Rominger, C.M.; Erskine, S.; Fisher, K.E.; Yang, J.; et al. GSK1120212 (JTP-74057) Is an Inhibitor of MEK Activity and Activation with Favorable Pharmacokinetic Properties for Sustained in Vivo Pathway Inhibition. Clin. Cancer Res. 2011, 17, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- King, A.J.; Arnone, M.R.; Bleam, M.R.; Moss, K.G.; Yang, J.; Fedorowicz, K.E.; Smitheman, K.N.; Erhardt, J.A.; Hughes-Earle, A.; Kane-Carson, L.S.; et al. Dabrafenib; Preclinical Characterization, Increased Efficacy When Combined with Trametinib, While BRAF/MEK Tool Combination Reduced Skin Lesions. PLoS ONE 2013, 8, e67583. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, K.; Wafa, T.; Fitzgerald, T.; Cunningham, L. An Optimized, Clinically Relevant Mouse Model of Cisplatin-Induced Ototoxicity. Hear. Res. 2019, 375, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Ryals, M.M.; Van den Bruele, A.B.; Fitzgerald, T.S.; Cunningham, L.L. Sound Preconditioning Therapy Inhibits Ototoxic Hearing Loss in Mice. J. Clin. Investig. 2013, 123, 4945–4949. [Google Scholar] [CrossRef] [PubMed]
- Breglio, A.M.; Rusheen, A.E.; Shide, E.D.; Fernandez, K.A.; Spielbauer, K.K.; McLachlin, K.M.; Hall, M.D.; Amable, L.; Cunningham, L.L. Cisplatin Is Retained in the Cochlea Indefinitely Following Chemotherapy. Nat. Commun. 2017, 8, 1654. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, J.J. Chapter 30—Auditory Brainstem Response. In Handbook of Clinical Neurology; Levin, K.H., Chauvel, P., Eds.; Clinical Neurophysiology: Basis and Technical Aspects; Elsevier: Amsterdam, The Netherlands, 2019; Volume 160, pp. 451–464. [Google Scholar]
- Young, A.; Ng, M. Otoacoustic Emissions. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Wu, P.-K.; Park, J.-I. MEK1/2 Inhibitors: Molecular Activity and Resistance Mechanisms. Semin. Oncol. 2015, 42, 849–862. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S. Cisplatin: The First Metal Based Anticancer Drug. Bioorg. Chem. 2019, 88, 102925. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Tchounwou, P.B. Cisplatin in Cancer Therapy: Molecular Mechanisms of Action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Teitz, T.; Fang, J.; Goktug, A.N.; Bonga, J.D.; Diao, S.; Hazlitt, R.A.; Iconaru, L.; Morfouace, M.; Currier, D.; Zhou, Y.; et al. CDK2 Inhibitors as Candidate Therapeutics for Cisplatin- and Noise-Induced Hearing Loss. J. Exp. Med. 2018, 215, 1187–1203. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Jacob, S. A Simple Practice Guide for Dose Conversion between Animals and Human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Kobel, M.; Le Prell, C.G.; Liu, J.; Hawks, J.W.; Bao, J. Noise-Induced Cochlear Synaptopathy: Past Findings and Future Studies. Hear. Res. 2017, 349, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Kujawa, S.G.; Liberman, M.C. Synaptopathy in the Noise-Exposed and Aging Cochlea: Primary Neural Degeneration in Acquired Sensorineural Hearing Loss. Hear. Res. 2015, 330, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Paquette, S.T.; Gilels, F.; White, P.M. Noise Exposure Modulates Cochlear Inner Hair Cell Ribbon Volumes, Correlating with Changes in Auditory Measures in the FVB/nJ Mouse. Sci. Rep. 2016, 6, 25056. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.K.; Yeo, J.-S.; Park, C.Y.; Na, H.; Lim, J.A.; Lee, J.-E.; Hong, S.W.; Park, S.-S.; Lim, D.G.; Kwak, K.H. Inhibition of Reactive Oxygen Species Downregulates the MAPK Pathway in Rat Spinal Cord after Limb Ischemia Reperfusion Injury. Int. J. Surg. 2015, 22, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Cho, H.-J.; Sagong, B.; Kim, S.-J.; Lee, J.-T.; So, H.-S.; Lee, I.-K.; Kim, U.-K.; Lee, K.-Y.; Choo, Y.-S. Alpha-Lipoic Acid Protects against Cisplatin-Induced Ototoxicity via the Regulation of MAPKs and Proinflammatory Cytokines. Biochem. Biophys. Res. Commun. 2014, 449, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.; Cheong, Y.-K.; Kim, N.-H.; Chung, H.-T.; Kang, D.G.; Pae, H.-O. Mitogen-Activated Protein Kinases and Reactive Oxygen Species: How Can ROS Activate MAPK Pathways? J. Signal Transduct. 2011, 2011, 792639. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhu, X.; Huang, C.; Chen, J.; Shu, S.; Chen, G.; Xu, Y.; Hu, Y. ERK Inhibition Reduces Neuronal Death and Ameliorates Inflammatory Responses in Forebrain-Specific Ppp2cα Knockout Mice. FASEB J. 2022, 36, e22515. [Google Scholar] [CrossRef]
- Monzack, E.L.; Cunningham, L.L. Lead Roles for Supporting Actors: Critical Functions of Inner Ear Supporting Cells. Hear. Res. 2013, 303, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, M.; Corfas, G.; Liberman, M.C. Influence of Supporting Cells on Neuronal Degeneration After Hair Cell Loss. J. Assoc. Res. Otolaryngol. 2005, 6, 136–147. [Google Scholar] [CrossRef]
- Waissbluth, S.; Maass, J.C.; Sanchez, H.A.; Martínez, A.D. Supporting Cells and Their Potential Roles in Cisplatin-Induced Ototoxicity. Front. Neurosci. 2022, 16, 867034. [Google Scholar] [CrossRef]
- Hakuba, N.; Koga, K.; Gyo, K.; Usami, S.; Tanaka, K. Exacerbation of Noise-Induced Hearing Loss in Mice Lacking the Glutamate Transporter GLAST. J. Neurosci. 2000, 20, 8750–8753. [Google Scholar] [CrossRef]
- Mao, H.; Chen, Y. Noise-Induced Hearing Loss: Updates on Molecular Targets and Potential Interventions. Neural Plast. 2021, 2021, 4784385. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Fang, J.; Gao, J.; Yu, Y.; Lagarde, M.M.; Zuo, J. Normal Hearing Sensitivity at Low-to-Middle Frequencies with 34% Prestin-Charge Density. PLoS ONE 2012, 7, e45453. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lutze, R.D.; Ingersoll, M.A.; Kelmann, R.G.; Teitz, T. Trametinib, a MEK1/2 Inhibitor, Protects Mice from Cisplatin- and Noise-Induced Hearing Loss. Pharmaceuticals 2024, 17, 735. https://doi.org/10.3390/ph17060735
Lutze RD, Ingersoll MA, Kelmann RG, Teitz T. Trametinib, a MEK1/2 Inhibitor, Protects Mice from Cisplatin- and Noise-Induced Hearing Loss. Pharmaceuticals. 2024; 17(6):735. https://doi.org/10.3390/ph17060735
Chicago/Turabian StyleLutze, Richard D., Matthew A. Ingersoll, Regina G. Kelmann, and Tal Teitz. 2024. "Trametinib, a MEK1/2 Inhibitor, Protects Mice from Cisplatin- and Noise-Induced Hearing Loss" Pharmaceuticals 17, no. 6: 735. https://doi.org/10.3390/ph17060735
APA StyleLutze, R. D., Ingersoll, M. A., Kelmann, R. G., & Teitz, T. (2024). Trametinib, a MEK1/2 Inhibitor, Protects Mice from Cisplatin- and Noise-Induced Hearing Loss. Pharmaceuticals, 17(6), 735. https://doi.org/10.3390/ph17060735





