Exploring the Potential of Halotolerant Actinomycetes from Rann of Kutch, India: A Study on the Synthesis, Characterization, and Biomedical Applications of Silver Nanoparticles
Abstract
:1. Introduction
2. Results
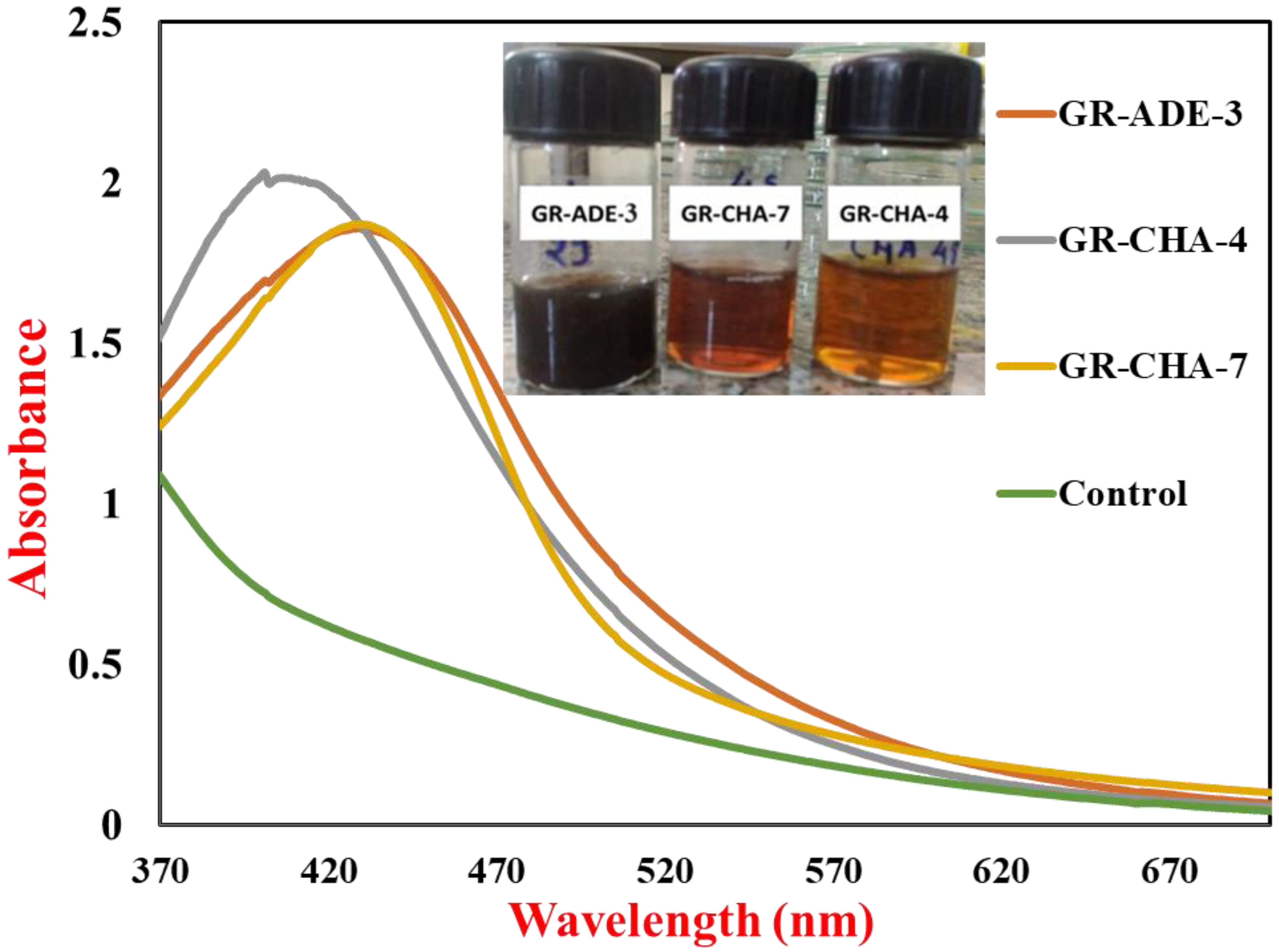
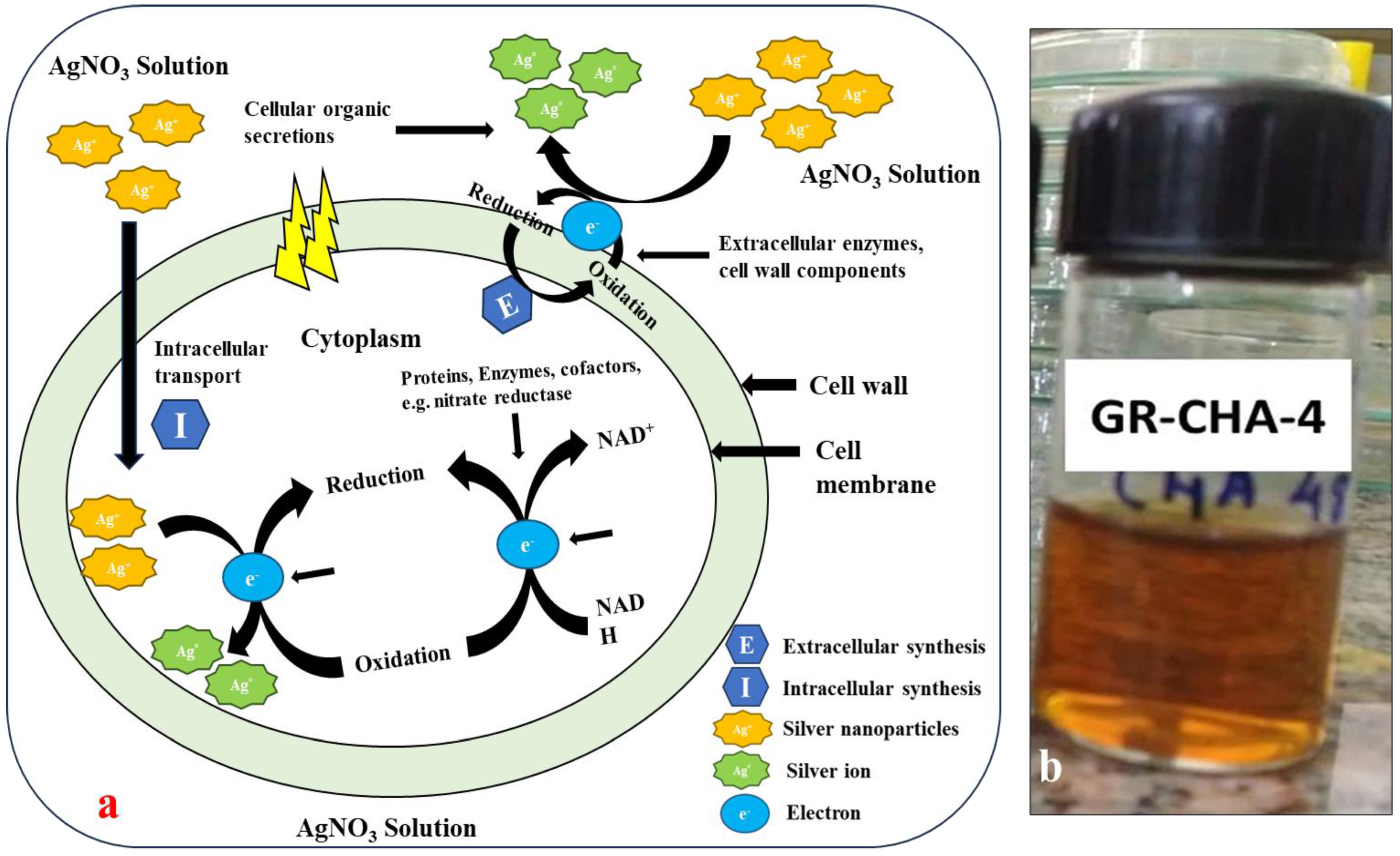
2.1. Extracellular Biosynthesis of AgNPs from Saline Desert Actinomycetes
2.2. UV-Vis Spectroscopic Analysis of AgNPs Synthesized by S. tendae (GR-CHA-4)
2.3. Effect of Reaction Parameters on AgNP Synthesis Using Isolate S. tendae (GR-CHA-4)
2.3.1. Medium Optimization for Nanoparticle Synthesis
2.3.2. Synthesis of AgNPs under Normal, Alkaline, Saline, and Saline + Alkaline Conditions
2.3.3. Effect of AgNO3 Concentration on Nanoparticle Synthesis
2.3.4. Effect of pH on AgNP Synthesis
2.3.5. Effect of Temperature on AgNP Synthesis
2.3.6. AgNP Synthesis under Optimized Conditions Using S. tendae (GR-CHA-4)
2.3.7. Particle Size Analysis (PSA) and Zeta Potential of Synthesized AgNPs
2.3.8. Fourier Transform Infrared (FT-IR) Spectroscopy of AgNPs for the Identification of Functional Groups
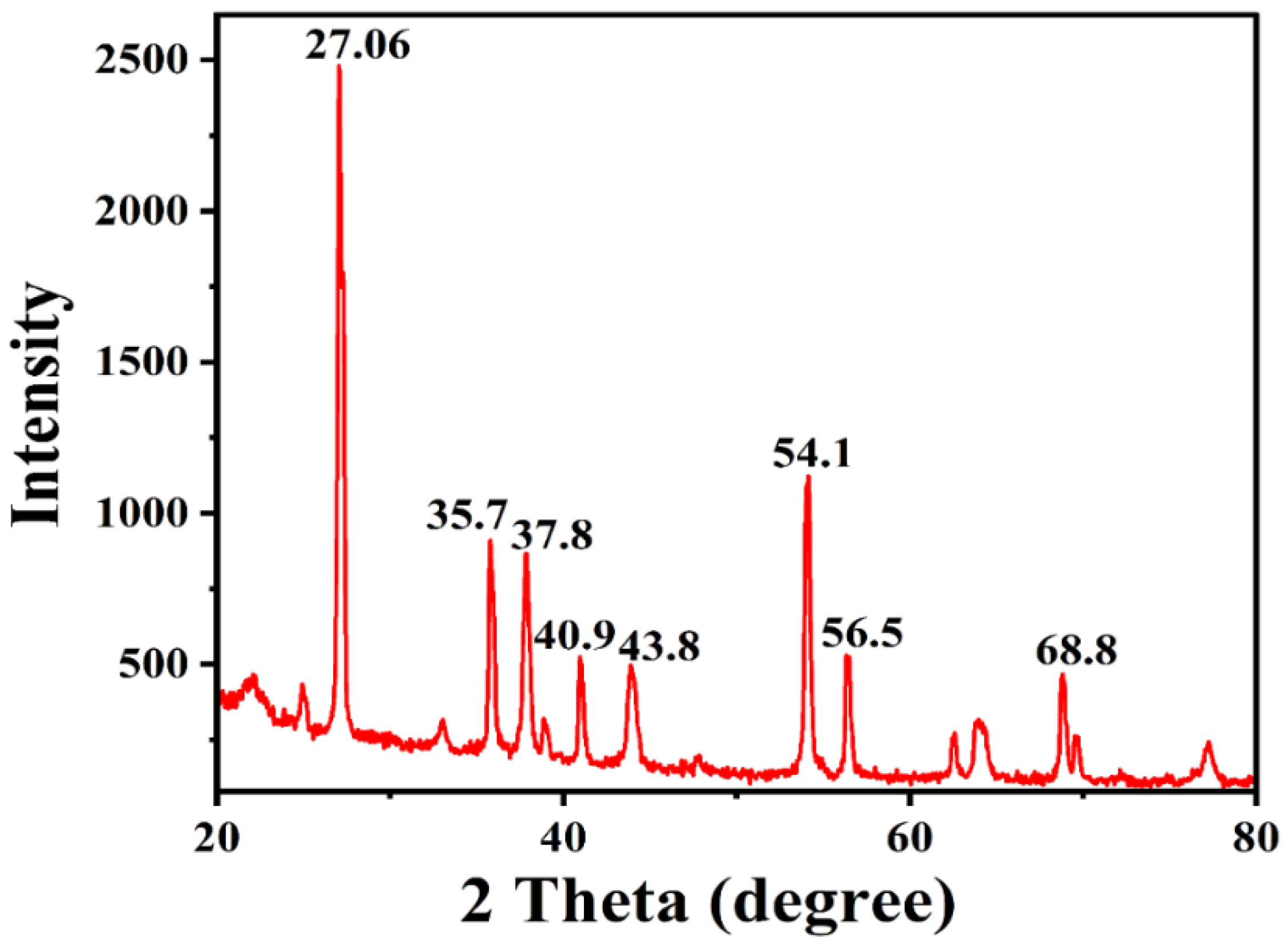
2.3.9. X-ray Diffraction (XRD) Analysis for Phase Identification
2.3.10. Morphological Analysis of AgNPs Synthesized by S. tendae by Using Scanning Electron Microscope (SEM)
2.3.11. Three-Dimensional Analysis of AgNPs Using an Atomic Force Microscope (AFM)
2.3.12. Antimicrobial Activity of AgNPs (MIC)
2.3.13. Synergistic Effect of AgNPs
2.3.14. AgNP Activity against MRSA
2.3.15. Anti-Biofilm Activity of AgNPs Synthesized with S. tendae
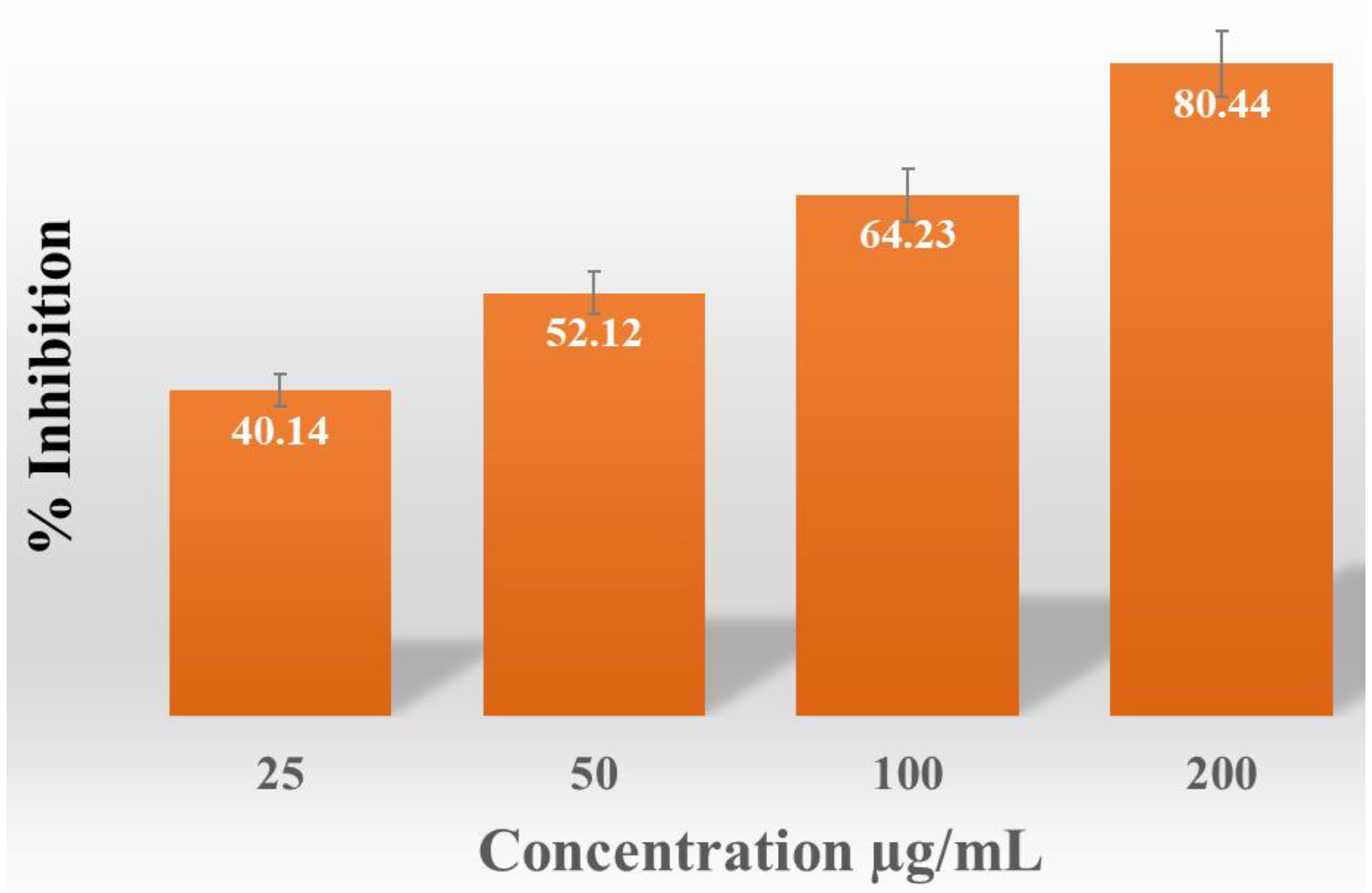
2.3.16. Anti-Oxidant Activity of AgNPs
2.3.17. Preparation of Anti-Microbial Cotton
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
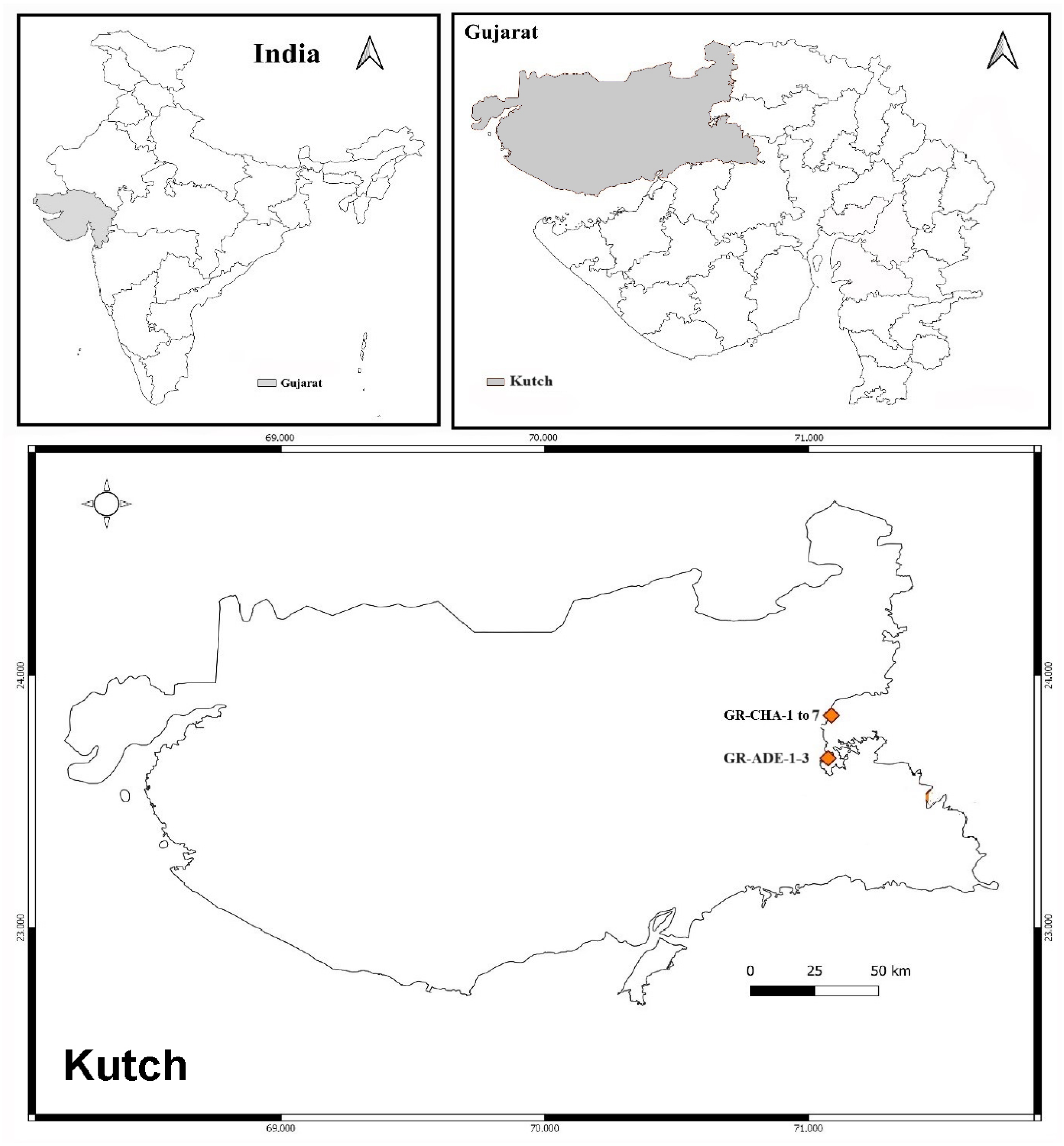
4.2.1. Sample Collection Site
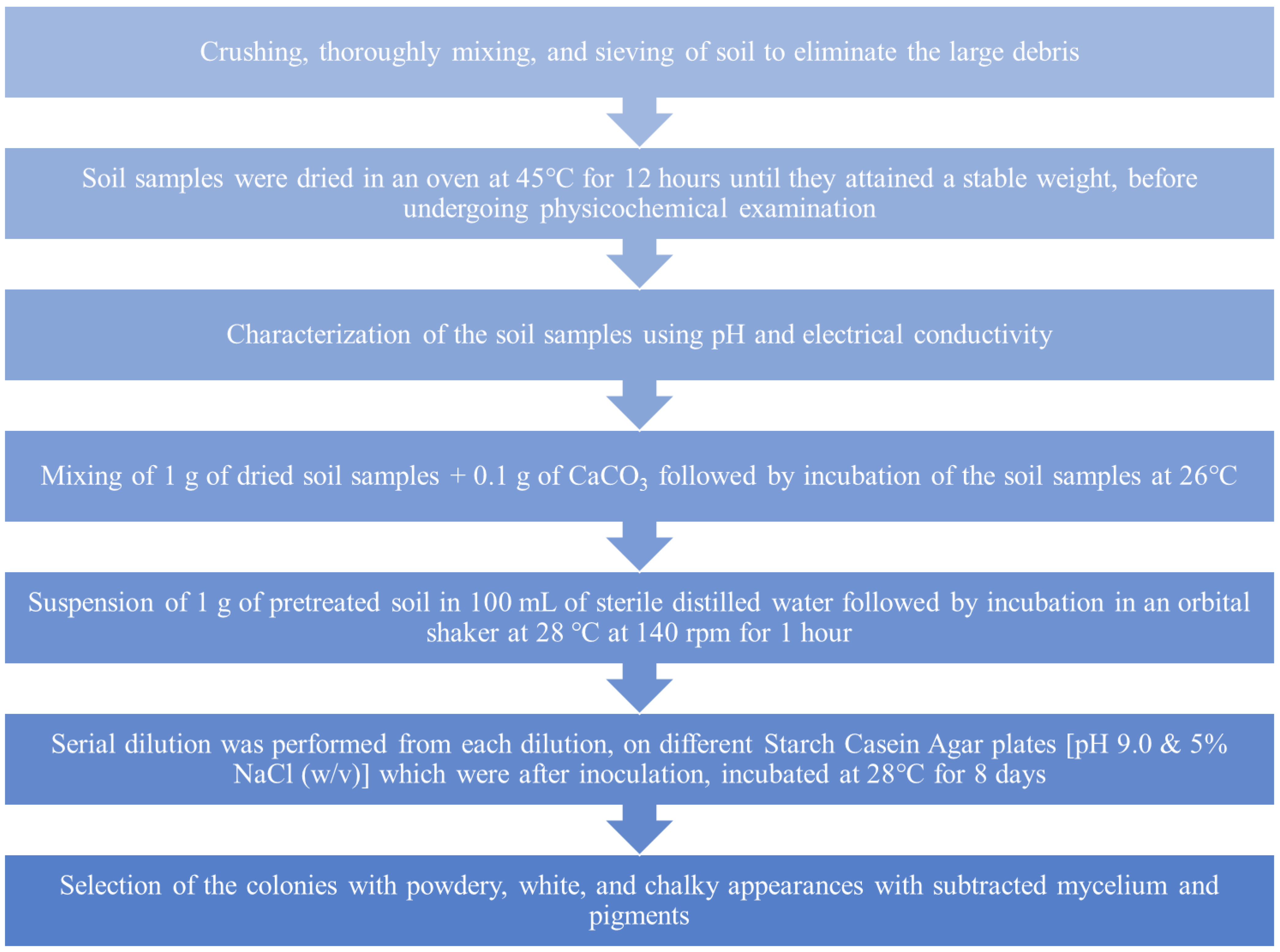
4.2.2. Enrichment and Isolation of Actinomycetes
4.2.3. Effect of Salt Concentration on the Growth of Isolate
4.2.4. Effect of pH on the Growth of Isolates
4.2.5. Screening for the Presence of Antagonistic Substances
4.2.6. Molecular Identification of the Actinomycete Isolates
4.2.7. Biosynthesis of AgNPs
4.2.8. Optimization of Physicochemical Parameters
Medium Optimization for AgNP Synthesis
Nanoparticle Synthesis under Normal, Alkaline, Saline, and Saline + Alkaline Conditions
Effect of AgNO3 Concentration on AgNP Synthesis
Effect of pH on AgNP Synthesis
Effect of Temperature on AgNP Synthesis
4.2.9. In Vitro Antimicrobial Activity of AgNPs
4.2.10. Synergistic Activity of AgNPs with Commercial Antibiotics
4.2.11. Activity against MRSA
4.2.12. Anti-Biofilm Activity
4.2.13. Antioxidant Activity of Synthesized AgNPs
- RSA = Radical scavenging activity;
- Abs control = absorbance of DPPH radical + ethanol;
- Abs sample = absorbance of DPPH radical + AgNPs.
4.2.14. Biosynthesis of AgNPs on Cotton Fabrics
4.3. Characterization of the Synthesized AgNPs
4.3.1. UV-Vis Spectral Analysis
4.3.2. Nanoparticle Tracking Analysis (NTA) and Zeta Potential of AgNPs
4.3.3. Fourier Transform Infra-Red (FT-IR) Analysis
4.3.4. SEM
4.3.5. X-ray Diffraction (XRD) Analysis
4.3.6. AFM
5. Conclusions
6. Future Prospects and Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Simeis, D.; Serra, S. Actinomycetes: A Never-Ending Source of Bioactive Compounds—An Overview on Antibiotics Production. Antibiotics 2021, 10, 483. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, G.; Suthindhiran, K. Diversity and Biotechnological Potential of Marine Actinomycetes from India. Indian J. Microbiol. 2022, 62, 475–493. [Google Scholar] [CrossRef]
- Selim, M.S.M.; Abdelhamid, S.A.; Mohamed, S.S. Secondary Metabolites and Biodiversity of Actinomycetes. J. Genet. Eng. Biotechnol. 2021, 19, 72. [Google Scholar] [CrossRef] [PubMed]
- Subathra Devi, C.; Merlyn Keziah, S.; Jemimah Naine, S.; Mohanasrinivasan, V. Actinomycetes: Microbiology to Systems Biology. In Actinobacteria; Springer: Singapore, 2022; pp. 1–35. [Google Scholar]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural Products in Drug Discovery: Advances and Opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Duan, C.; Yu, M.; Xu, J.; Li, B.-Y.; Zhao, Y.; Kankala, R.K. Overcoming Cancer Multi-Drug Resistance (MDR): Reasons, Mechanisms, Nanotherapeutic Solutions, and Challenges. Biomed. Pharmacother. 2023, 162, 114643. [Google Scholar] [CrossRef] [PubMed]
- Duncan, K.R.; Crüsemann, M.; Lechner, A.; Sarkar, A.; Li, J.; Ziemert, N.; Wang, M.; Bandeira, N.; Moore, B.S.; Dorrestein, P.C.; et al. Molecular Networking and Pattern-Based Genome Mining Improves Discovery of Biosynthetic Gene Clusters and Their Products from Salinispora Species. Chem. Biol. 2015, 22, 460–471. [Google Scholar] [CrossRef]
- Kim, H.; Kim, S.; Kim, M.; Lee, C.; Yang, I.; Nam, S.-J. Bioactive Natural Products from the Genus Salinospora: A Review. Arch. Pharm. Res. 2020, 43, 1230–1258. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Hu, Y.; Wang, Q.; Zhou, H.; Wang, Y.; Gan, M. Tetrocarcins N and O, Glycosidic Spirotetronates from a Marine-Derived Micromonospora Sp. Identified by PCR-Based Screening. RSC Adv. 2016, 6, 91773–91778. [Google Scholar] [CrossRef]
- Motallebirad, T.; Mardanshah, O.; Safarabadi, M.; Ghaffari, K.; Orouji, M.A.; Abedi, B.; Azadi, D. Screening, Molecular Identification, Population Diversity, and Antibiotic Susceptibility Pattern of Actinomycetes Species Isolated from Meat and Meat Products of Slaughterhouses, Restaurants, and Meat Stores of a Developing Country, Iran. Front. Microbiol. 2023, 14, 1134368. [Google Scholar] [CrossRef]
- Liu, K.; Jiang, Z.; Lalancette, R.A.; Tang, X.; Jäkle, F. Near-Infrared-Absorbing B–N Lewis Pair-Functionalized Anthracenes: Electronic Structure Tuning, Conformational Isomerism, and Applications in Photothermal Cancer Therapy. J. Am. Chem. Soc. 2022, 144, 18908–18917. [Google Scholar] [CrossRef]
- Kronheim, S.; Solomon, E.; Ho, L.; Glossop, M.; Davidson, A.R.; Maxwell, K.L. Complete Genomes and Comparative Analyses of Streptomyces Phages That Influence Secondary Metabolism and Sporulation. Sci. Rep. 2023, 13, 9820. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Liu, X.; Huang, Q.; Arai, T. Recent Progress of Magnetically Actuated DNA Micro/Nanorobots. Cyborg Bionic Syst. 2022, 2022, 9758460. [Google Scholar] [CrossRef] [PubMed]
- Alam, K.; Mazumder, A.; Sikdar, S.; Zhao, Y.-M.; Hao, J.; Song, C.; Wang, Y.; Sarkar, R.; Islam, S.; Zhang, Y.; et al. Streptomyces: The Biofactory of Secondary Metabolites. Front. Microbiol. 2022, 13, 968053. [Google Scholar] [CrossRef] [PubMed]
- Caicedo-Montoya, C.; Manzo-Ruiz, M.; Ríos-Estepa, R. Pan-Genome of the Genus Streptomyces and Prioritization of Biosynthetic Gene Clusters With Potential to Produce Antibiotic Compounds. Front. Microbiol. 2021, 12, 677558. [Google Scholar] [CrossRef] [PubMed]
- Parra, J.; Beaton, A.; Seipke, R.F.; Wilkinson, B.; Hutchings, M.I.; Duncan, K.R. Antibiotics from Rare Actinomycetes, beyond the Genus Streptomyces. Curr. Opin. Microbiol. 2023, 76, 102385. [Google Scholar] [CrossRef] [PubMed]
- Barbuto Ferraiuolo, S.; Cammarota, M.; Schiraldi, C.; Restaino, O.F. Streptomycetes as Platform for Biotechnological Production Processes of Drugs. Appl. Microbiol. Biotechnol. 2021, 105, 551–568. [Google Scholar] [CrossRef] [PubMed]
- Darby, E.M.; Trampari, E.; Siasat, P.; Gaya, M.S.; Alav, I.; Webber, M.A.; Blair, J.M.A. Molecular Mechanisms of Antibiotic Resistance Revisited. Nat. Rev. Microbiol. 2023, 21, 280–295. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Das, P.; Lv, X.; Shi, M.; Aa, J.; Wang, K.; Duan, L.; Gilbert, J.A.; Nie, Y.; Wu, X.-L. Effects of ‘Healthy’ Fecal Microbiota Transplantation against the Deterioration of Depression in Fawn-Hooded Rats. mSystems 2022, 7, e0021822. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Jiang, Z.; Akakuru, O.U.; Li, J.; Wu, A. Nanoscale Covalent Organic Frameworks: From Controlled Synthesis to Cancer Therapy. Chem. Commun. 2021, 57, 12417–12435. [Google Scholar] [CrossRef]
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial Antibiotic Resistance: The Most Critical Pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef]
- Liu, K.; Jiang, Z.; Zhao, F.; Wang, W.; Jäkle, F.; Wang, N.; Tang, X.; Yin, X.; Chen, P. Triarylboron-Doped Acenethiophenes as Organic Sonosensitizers for Highly Efficient Sonodynamic Therapy with Low Phototoxicity. Adv. Mater. 2022, 34, 202206594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shi, H.; Tan, X.; Jiang, Z.; Wang, P.; Qin, J. Ten-Gram-Scale Mechanochemical Synthesis of Ternary Lanthanum Coordination Polymers for Antibacterial and Antitumor Activities. Front. Chem. 2022, 1, 898324. [Google Scholar] [CrossRef] [PubMed]
- Pulingam, T.; Parumasivam, T.; Gazzali, A.M.; Sulaiman, A.M.; Chee, J.Y.; Lakshmanan, M.; Chin, C.F.; Sudesh, K. Antimicrobial Resistance: Prevalence, Economic Burden, Mechanisms of Resistance and Strategies to Overcome. Eur. J. Pharm. Sci. 2022, 170, 106103. [Google Scholar] [CrossRef]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Fu, R.; Zheng, Q.; Song, X.; Wu, J.; Ren, Y. Continuous Mode of Color and Functionality Construction for Cotton by Bacterial Pigment Based on Nano-Suspension System. Ind. Crops Prod. 2024, 214, 118510. [Google Scholar] [CrossRef]
- Wang, Y.; Zhai, W.; Cheng, S.; Li, J.; Zhang, H. Surface-Functionalized Design of Blood-Contacting Biomaterials for Preventing Coagulation and Promoting Hemostasis. Friction 2023, 11, 1371–1394. [Google Scholar] [CrossRef]
- Zhang, G.; Bai, J.; Zhai, Y.; Jia, J.; Zhao, Q.; Wang, W.; Hu, X. Microbial Diversity and Functions in Saline Soils: A Review from a Biogeochemical Perspective. J. Adv. Res. 2023, 59, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhao, C.; Wang, S.; Pan, Z.; Jiang, Z.; Tang, X. Multifunctional TiO2/C Nanosheets Derived from 3D Metal–Organic Frameworks for Mild-Temperature-Photothermal-Sonodynamic-Chemodynamic Therapy under Photoacoustic Image Guidance. J. Colloid. Interface Sci. 2022, 621, 360–373. [Google Scholar] [CrossRef]
- Jiang, Z.; Han, X.; Zhao, C.; Wang, S.; Tang, X. Recent Advance in Biological Responsive Nanomaterials for Biosensing and Molecular Imaging Application. Int. J. Mol. Sci. 2022, 23, 1923. [Google Scholar] [CrossRef]
- Salem, S.S.; Mekky, A.E. Biogenic Nanomaterials: Synthesis, Characterization, and Applications. In Biogenic Nanomaterials for Environmental Sustainability: Principles, Practices, and Opportunities. Environmental Science and Engineering; Springer: Cham, Switzerland, 2024; pp. 13–43. [Google Scholar]
- Yadav, V.K.; Choudhary, N.; Ali, D.; Alarifi, S.; Dwivedi, V.; Patel, A.; Fulekar, M.H. A Novel and Economical Approach for the Fusarium Oxysporum Mediated Myco-Synthesis of Mesoporous Floral-Shaped Silica Nanoparticles from Coal Fly Ash Waste. Part. Part. Syst. Charact. 2024, 2400007. [Google Scholar] [CrossRef]
- Habibullah, G.; Viktorova, J.; Ruml, T. Current Strategies for Noble Metal Nanoparticle Synthesis. Nanoscale Res. Lett. 2021, 16, 47. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, N.; Chaudhari, J.; Mochi, V.; Patel, P.; Ali, D.; Alarifi, S.; Sahoo, D.K.; Patel, A.; Kumar Yadav, V. Phytonanofabrication of Copper Oxide by Albizia Saman and Their Potential as an Antimicrobial Agent and Remediation of Congo Red Dye from Wastewater. Water 2023, 15, 3787. [Google Scholar] [CrossRef]
- Rabiee, N.; Ahmadi, S.; Akhavan, O.; Luque, R. Silver and Gold Nanoparticles for Antimicrobial Purposes against Multi-Drug Resistance Bacteria. Materials 2022, 15, 1799. [Google Scholar] [CrossRef]
- Kaushal, A.; Khurana, I.; Yadav, P.; Allawadhi, P.; Banothu, A.K.; Neeradi, D.; Thalugula, S.; Barani, P.J.; Naik, R.R.; Navik, U.; et al. Advances in Therapeutic Applications of Silver Nanoparticles. Chem. Biol. Interact. 2023, 382, 110590. [Google Scholar] [CrossRef]
- Ibrahim, E.; Fouad, H.; Zhang, M.; Zhang, Y.; Qiu, W.; Yan, C.; Li, B.; Mo, J.; Chen, J. Biosynthesis of Silver Nanoparticles Using Endophytic Bacteria and Their Role in Inhibition of Rice Pathogenic Bacteria and Plant Growth Promotion. RSC Adv. 2019, 9, 29293–29299. [Google Scholar] [CrossRef]
- Nasr Azadani, F.; Madani, M.; Karimi, J.; Sepahvand, S. Green Synthesis of Silver Nanoparticles by Fusarium Oxysporum and Its Function Against Aspergillus and Fusarium Fungi. Indian J. Microbiol. 2024, 64, 213–224. [Google Scholar] [CrossRef] [PubMed]
- El Ouardy, K.; Lbouhmadi, R.; Attaoui, H.; Mouzaki, M.; Mouine, H.; Lemkhente, Z.; Mir, Y. Biosynthesis and Characterization of Silver Nanoparticles Produced by Parachlorella Kessleri and Cyclotella Spp., and the Evaluation of Their Antibacterial Activity. Int. J. Mol. Sci. 2023, 24, 10599. [Google Scholar] [CrossRef]
- Golinska, P.; Wypij, M.; Ingle, A.P.A.; Gupta, I.; Dahm, H.; Rai, M. Biogenic Synthesis of Metal Nanoparticles from Actinomycetes: Biomedical Applications and Cytotoxicity. Appl. Microbiol. Biotechnol. 2014, 98, 8083–8097. [Google Scholar] [CrossRef] [PubMed]
- Paterlini, P.; Rodríguez, C.; Ledesma, A.; Pereyra, J.; Dávila Costa, J.S.; Álvarez, A.; Romero, C.M. Characterization of Biosynthesized Silver Nanoparticles from Streptomyces Aqueous Extract and Evaluation of Surface-Capping Proteins Involved in the Process. Nano-Struct. Nano-Objects 2021, 26, 100755. [Google Scholar] [CrossRef]
- Subbaiya, R.; Selvam, M.M.; Sundar, K. Biological Synthesis of Silver Nanorods from Nocardia Mediterranei -5016 and Its Antitumor Activity against Non-Small Cell Lung Carcinoma Cell Line. Int. J. Pharmtech Res. 2015, 8, 298–307. [Google Scholar]
- Oza, G.; Pandey, S.; Gupta, A.; Kesarkar, R.; Sharon, M.; Ambernath, W. Biosynthetic Reduction of Gold Ions to Gold Nanoparticles by Nocardia Farcinica. J. Microbiol. Biotech. 2012, 2, 511–515. [Google Scholar]
- Manivasagan, P.; Alam, M.S.; Kang, K.-H.; Kwak, M.; Kim, S.-K. Extracellular Synthesis of Gold Bionanoparticles by Nocardiopsis Sp. and Evaluation of Its Antimicrobial, Antioxidant and Cytotoxic Activities. Bioprocess Biosyst. Eng. 2015, 38, 1167–1177. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Pandit, C.; Gacem, A.; Alqahtani, M.S.; Bilal, M.; Islam, S.; Hossain, M.d.J.; Jameel, M. Biologically Derived Gold Nanoparticles and Their Applications. Bioinorg. Chem. Appl. 2022, 2022, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Tehri, N.; Gahlaut, A.; Hooda, V. Actinomycetes Mediated Synthesis, Characterization, and Applications of Metallic Nanoparticles. Inorg. Nano-Met. Chem. 2021, 51, 1386–1395. [Google Scholar] [CrossRef]
- Wypij, M.; Golinska, P.; Dahm, H.; Rai, M. Actinobacterial-mediated Synthesis of Silver Nanoparticles and Their Activity against Pathogenic Bacteria. IET Nanobiotechnol. 2017, 11, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Otari, S.V.; Patil, R.M.; Ghosh, S.J.; Thorat, N.D.; Pawar, S.H. Intracellular Synthesis of Silver Nanoparticle by Actinobacteria and Its Antimicrobial Activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 136, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Abou El-Enain, I.M.; Elqady, E.M.; El-said, E.; Salem, H.H.A.; Badr, N.F.; Abd-Allah, G.E.; Rezk, M.M. Biosynthesized Silver Nanoparticles (Ag NPs) from Isolated Actinomycetes Strains and Their Impact on the Black Cutworm, Agrotis Ipsilon. Pestic. Biochem. Physiol. 2023, 194, 105492. [Google Scholar] [CrossRef]
- Asif, M.; Yasmin, R.; Asif, R.; Ambreen, A.; Mustafa, M.; Umbreen, S. Green Synthesis of Silver Nanoparticles (AgNPs), Structural Characterization, and Their Antibacterial Potential. Dose-Response 2022, 20, 155932582210887. [Google Scholar] [CrossRef]
- Kaur, H.; Kaur, H.; Sharma, A. Study of SPR Peak Shifting of Silver Nanoparticles with Change in Surrounding Medium. Mater. Today Proc. 2021, 37, 3574–3576. [Google Scholar] [CrossRef]
- Ejaz, U.; Afzal, M.; Mazhar, M.; Riaz, M.; Ahmed, N.; Rizg, W.Y.; Alahmadi, A.A.; Badr, M.Y.; Mushtaq, R.Y.; Yean, C.Y. Characterization, Synthesis, and Biological Activities of Silver Nanoparticles Produced via Green Synthesis Method Using Thymus Vulgaris Aqueous Extract. Int. J. Nanomed. 2024, 19, 453–469. [Google Scholar] [CrossRef]
- Jini, D.; Sharmila, S.; Anitha, A.; Pandian, M.; Rajapaksha, R.M.H. In Vitro and in Silico Studies of Silver Nanoparticles (AgNPs) from Allium Sativum against Diabetes. Sci. Rep. 2022, 12, 22109. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.; Gao, K.; Yang, J.; Li, T.; Gong, W.; Sun, Q.; Wang, Y. Prevalence of Multidrug-Resistant Pathogens Causing Neonatal Early and Late Onset Sepsis, a Retrospective Study from the Tertiary Referral Children’s Hospital. Infect. Drug Resist. 2023, 16, 4213–4225. [Google Scholar] [CrossRef] [PubMed]
- Kalimuthu, K.; Suresh Babu, R.; Venkataraman, D.; Bilal, M.; Gurunathan, S. Biosynthesis of Silver Nanocrystals by Bacillus Licheniformis. Colloids Surf. B Biointerfaces 2008, 65, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.; Sinha, S.N. Isolation and Characterization of Phosphate Solubilizing Bacterium Pseudomonas Aeruginosa KUPSB12 with Antibacterial Potential from River Ganga, India. Ann. Agrar. Sci. 2017, 15, 130–136. [Google Scholar] [CrossRef]
- Koilparambil, D.; Liya, C.; Smitha, V.; Shaikmoideen, M. Green Synthesis of Silver Nanoparticles by Escherichia Coli: Analysis of Antibacterial Activity. J. Water Environ. Nanotechnol. 2016, 1, 63–74. [Google Scholar] [CrossRef]
- Saravanan, M.; Vemu, A.K.; Barik, S.K. Rapid Biosynthesis of Silver Nanoparticles from Bacillus Megaterium (NCIM 2326) and Their Antibacterial Activity on Multi Drug Resistant Clinical Pathogens. Colloids Surf. B Biointerfaces 2011, 88, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Rathod, D.; Golinska, P.; Wypij, M.; Dahm, H.; Rai, M. A New Report of Nocardiopsis Valliformis Strain OT1 from Alkaline Lonar Crater of India and Its Use in Synthesis of Silver Nanoparticles with Special Reference to Evaluation of Antibacterial Activity and Cytotoxicity. Med. Microbiol. Immunol. 2016, 205, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elnaby, H.M.; Abo-Elala, G.M.; Abdel-Raouf, U.M.; Hamed, M.M. Antibacterial and Anticancer Activity of Extracellular Synthesized Silver Nanoparticles from Marine Streptomyces Rochei MHM13. Egypt. J. Aquat. Res. 2016, 42, 301–312. [Google Scholar] [CrossRef]
- Chandran, N.; Bayal, M.; Pilankatta, R.; Nair, S.S. Tuning of Surface Plasmon Resonance (SPR) in Metallic Nanoparticles for Their Applications in SERS. In Nanomaterials for Luminescent Devices, Sensors, and Bio-imaging Applications. Progress in Optical Science and Photonics; Springer: Singapore, 2021; pp. 39–66. [Google Scholar]
- Selwal, N.; Rahayu, F.; Herwati, A.; Latifah, E.; Supriyono; Suhara, C.; Kade Suastika, I.B.; Mahayu, W.M.; Wani, A.K. Enhancing Secondary Metabolite Production in Plants: Exploring Traditional and Modern Strategies. J. Agric. Food Res. 2023, 14, 100702. [Google Scholar] [CrossRef]
- Singh, V.; Haque, S.; Niwas, R.; Srivastava, A.; Pasupuleti, M.; Tripathi, C.K.M. Strategies for Fermentation Medium Optimization: An In-Depth Review. Front. Microbiol. 2017, 7, 02087. [Google Scholar] [CrossRef]
- Waghmare, S.S.; Deshmukh, M.A.; Sadowski, Z. Biosynthesis, Optimization, Purification and Characterization of Gold Nanoparticles. Afr. J. Microbiol. Res. 2014, 8, 138–146. [Google Scholar] [CrossRef]
- Składanowski, M.; Wypij, M.; Laskowski, D.; Golińska, P.; Dahm, H.; Rai, M. Silver and Gold Nanoparticles Synthesized from Streptomyces Sp. Isolated from Acid Forest Soil with Special Reference to Its Antibacterial Activity against Pathogens. J. Clust. Sci. 2017, 28, 59–79. [Google Scholar] [CrossRef]
- Khodashenas, B.; Ghorbani, H.R. Synthesis of Silver Nanoparticles with Different Shapes. Arab. J. Chem. 2019, 12, 1823–1838. [Google Scholar] [CrossRef]
- Shaikh, W.A.; Chakraborty, S.; Owens, G.; Islam, R.U. A Review of the Phytochemical Mediated Synthesis of AgNP (Silver Nanoparticle): The Wonder Particle of the Past Decade. Appl. Nanosci. 2021, 11, 2625–2660. [Google Scholar] [CrossRef]
- Alqahtani, M.A.; Al Othman, M.R.; Mohammed, A.E. Bio Fabrication of Silver Nanoparticles with Antibacterial and Cytotoxic Abilities Using Lichens. Sci. Rep. 2020, 10, 16781. [Google Scholar] [CrossRef]
- Giri, A.K.; Jena, B.; Biswal, B.; Pradhan, A.K.; Arakha, M.; Acharya, S.; Acharya, L. Green Synthesis and Characterization of Silver Nanoparticles Using Eugenia Roxburghii DC. Extract and Activity against Biofilm-Producing Bacteria. Sci. Rep. 2022, 12, 8383. [Google Scholar] [CrossRef]
- Shiraz, M.; Imtiaz, H.; Azam, A.; Hayat, S. Phytogenic Nanoparticles: Synthesis, Characterization, and Their Roles in Physiology and Biochemistry of Plants. BioMetals 2023, 37, 23–70. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, G.; Wei, W. Simulation of Nanoparticles Interacting with a Cell Membrane: Probing the Structural Basis and Potential Biomedical Application. NPG Asia Mater. 2021, 13, 52. [Google Scholar] [CrossRef]
- Sowani, H.; Mohite, P.; Damale, S.; Kulkarni, M.; Zinjarde, S. Carotenoid Stabilized Gold and Silver Nanoparticles Derived from the Actinomycete Gordonia Amicalis HS-11 as Effective Free Radical Scavengers. Enzym. Microb. Technol. 2016, 95, 164–173. [Google Scholar] [CrossRef]
- Wypij, M.; Czarnecka, J.; Świecimska, M.; Dahm, H.; Rai, M.; Golinska, P. Synthesis, Characterization and Evaluation of Antimicrobial and Cytotoxic Activities of Biogenic Silver Nanoparticles Synthesized from Streptomyces Xinghaiensis OF1 Strain. World J. Microbiol. Biotechnol. 2018, 34, 23. [Google Scholar] [CrossRef]
- Shalaby, E.A. Algae-Mediated Silver Nanoparticles: Synthesis, Properties, and Biological Activities. In Green Synthesis of Silver Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2022; pp. 525–545. [Google Scholar]
- Kiran Manam, V.; Murugesan, S. Biological Synthesis of Silver Nanoparticles from Marine Alga Copomenia Sinuosa and Its Invitro Ant-Diabetic Efficacy Biological Synthesis of Silver Nanoparticles from Marine Alga Colpomenia Sinuosa and Its in Vitro Anti-Diabetic Activity. Am. J. Bio-Pharmacol. Biochem. Life Sci. 2014, 3, 01–07. [Google Scholar]
- Baygar, T.; Ugur, A. Biosynthesis of Silver Nanoparticles by Streptomyces Griseorubens Isolated from Soil and Their Antioxidant Activity. IET Nanobiotechnol. 2017, 11, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Vijayabharathi, R.; Sathya, A.; Gopalakrishnan, S. Extracellular Biosynthesis of Silver Nanoparticles Using Streptomyces Griseoplanus SAI-25 and Its Antifungal Activity against Macrophomina Phaseolina, the Charcoal Rot Pathogen of Sorghum. Biocatal. Agric. Biotechnol. 2018, 14, 166–171. [Google Scholar] [CrossRef]
- Anasane, N.; Golińska, P.; Wypij, M.; Rathod, D.; Dahm, H.; Rai, M. Acidophilic Actinobacteria Synthesised Silver Nanoparticles Showed Remarkable Activity against Fungi-causing Superficial Mycoses in Humans. Mycoses 2016, 59, 157–166. [Google Scholar] [CrossRef]
- Potara, M.; Bawaskar, M.; Simon, T.; Gaikwad, S.; Licarete, E.; Ingle, A.; Banciu, M.; Vulpoi, A.; Astilean, S.; Rai, M. Biosynthesized Silver Nanoparticles Performing as Biogenic SERS-Nanotags for Investigation of C26 Colon Carcinoma Cells. Colloids Surf. B Biointerfaces 2015, 133, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elhady, H.M.; Ashor, M.A.; Hazem, A.; Saleh, F.M.; Selim, S.; El Nahhas, N.; Abdel-Hafez, S.H.; Sayed, S.; Hassan, E.A. Biosynthesis and Characterization of Extracellular Silver Nanoparticles from Streptomyces Aizuneusis: Antimicrobial, Anti Larval, and Anticancer Activities. Molecules 2021, 27, 212. [Google Scholar] [CrossRef] [PubMed]
- Marathe, K.; Naik, J.; Maheshwari, V. Biogenic Synthesis of Silver Nanoparticles Using Streptomyces Spp. and Their Antifungal Activity Against Fusarium Verticillioides. J. Clust. Sci. 2021, 32, 1299–1309. [Google Scholar] [CrossRef]
- Devaraj, P.; Kumari, P.; Aarti, C.; Renganathan, A. Synthesis and Characterization of Silver Nanoparticles Using Cannonball Leaves and Their Cytotoxic Activity against MCF-7 Cell Line. J. Nanotechnol. 2013, 2013, 1–5. [Google Scholar] [CrossRef]
- Urnukhsaikhan, E.; Bold, B.-E.; Gunbileg, A.; Sukhbaatar, N.; Mishig-Ochir, T. Antibacterial Activity and Characteristics of Silver Nanoparticles Biosynthesized from Carduus Crispus. Sci. Rep. 2021, 11, 21047. [Google Scholar] [CrossRef] [PubMed]
- Hayes, H.C.; Luk, L.Y.P.; Tsai, Y.-H. Approaches for Peptide and Protein Cyclisation. Org. Biomol. Chem. 2021, 19, 3983–4001. [Google Scholar] [CrossRef]
- Sahoo, S.; Shivani, K.; Padhy, A.A.; Kumari, V.; Mishra, P. Principles, Methods, and Applications of Protein Folding Inside Cells. In Protein Folding Dynamics and Stability; Springer: Singapore, 2023; pp. 251–284. [Google Scholar]
- Muthusamy, S.; Vaishnavi, R.; Neelakannan, M.; Kannan, D.; Silambarasan, T.; Immanuel, G. Investigation on Characterization and Biomedical Properties of Silver Nanoparticles Synthesized by an Actinobacterium Streptomyces Olivaceus (MSU3). Biocatal. Agric. Biotechnol. 2019, 17, 151–159. [Google Scholar] [CrossRef]
- Jain, A.S.; Pawar, P.S.; Sarkar, A.; Junnuthula, V.; Dyawanapelly, S. Bionanofactories for Green Synthesis of Silver Nanoparticles: Toward Antimicrobial Applications. Int. J. Mol. Sci. 2021, 22, 11993. [Google Scholar] [CrossRef] [PubMed]
- Manikprabhu, D.; Lingappa, K. Microwave Assisted Rapid and Green Synthesis of Silver Nanoparticles Using a Pigment Produced by Streptomyces Coelicolor Klmp33. Bioinorg. Chem. Appl. 2013, 2013, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Pallavi, S.S.; Rudayni, H.A.; Bepari, A.; Niazi, S.K.; Nayaka, S. Green Synthesis of Silver Nanoparticles Using Streptomyces Hirsutus Strain SNPGA-8 and Their Characterization, Antimicrobial Activity, and Anticancer Activity against Human Lung Carcinoma Cell Line A549. Saudi J. Biol. Sci. 2022, 29, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Ghany, M.N.; Hamdi, S.A.; Korany, S.M.; Elbaz, R.M.; Emam, A.N.; Farahat, M.G. Biogenic Silver Nanoparticles Produced by Soil Rare Actinomycetes and Their Significant Effect on Aspergillus-Derived Mycotoxins. Microorganisms 2023, 11, 1006. [Google Scholar] [CrossRef]
- Nayaka, S.; Chakraborty, B.; Bhat, M.P.; Nagaraja, S.K.; Airodagi, D.; Swamy, P.S.; Rudrappa, M.; Hiremath, H.; Basavarajappa, D.S.; Kanakannanavar, B. Biosynthesis, Characterization, and in Vitro Assessment on Cytotoxicity of Actinomycete-Synthesized Silver Nanoparticles on Allium Cepa Root Tip Cells. Beni Suef Univ. J. Basic. Appl. Sci. 2020, 9, 51. [Google Scholar] [CrossRef]
- Subbaiya, R.; Saravanan, M.; Priya, A.R.; Shankar, K.R.; Selvam, M.; Ovais, M.; Balajee, R.; Barabadi, H. Biomimetic Synthesis of Silver Nanoparticles from Streptomyces Atrovirens and Their Potential Anticancer Activity against Human Breast Cancer Cells. IET Nanobiotechnol. 2017, 11, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.; Arora, M.; Sati, P.C.; Chhoker, S.; Katyal, S.C.; Kumar, M. Structural, Vibrational, Optical, Magnetic and Dielectric Properties of Bi1−x Bax FeO3 Nanoparticles. Ceram. Int. 2013, 39, 6399–6405. [Google Scholar] [CrossRef]
- Sadhasivam, S.; Shanmugam, P.; Yun, K. Biosynthesis of Silver Nanoparticles by Streptomyces Hygroscopicus and Antimicrobial Activity against Medically Important Pathogenic Microorganisms. Colloids Surf. B Biointerfaces 2010, 81, 358–362. [Google Scholar] [CrossRef]
- Iniyan, A.M.; Kannan, R.R.; Joseph, F.-J.R.S.; Mary, T.R.J.; Rajasekar, M.; Sumy, P.C.; Rabel, A.M.; Ramachandran, D.; Vincent, S.G.P. In Vivo Safety Evaluation of Antibacterial Silver Chloride Nanoparticles from Streptomyces Exfoliatus ICN25 in Zebrafish Embryos. Microb. Pathog. 2017, 112, 76–82. [Google Scholar] [CrossRef]
- Sivasankar, P.; Seedevi, P.; Poongodi, S.; Sivakumar, M.; Murugan, T.; Sivakumar, L.; Sivakumar, K.; Balasubramanian, T. Characterization, Antimicrobial and Antioxidant Property of Exopolysaccharide Mediated Silver Nanoparticles Synthesized by Streptomyces Violaceus MM72. Carbohydr. Polym. 2018, 181, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Railean-Plugaru, V.; Pomastowski, P.; Wypij, M.; Szultka-Mlynska, M.; Rafinska, K.; Golinska, P.; Dahm, H.; Buszewski, B. Study of Silver Nanoparticles Synthesized by Acidophilic Strain of Actinobacteria Isolated from the of Picea Sitchensis Forest Soil. J. Appl. Microbiol. 2016, 120, 1250–1263. [Google Scholar] [CrossRef]
- Shahid, M.; Naeem-Ullah, U.; Khan, W.S.; Saeed, S.; Razzaq, K. Biocidal Activity of Green Synthesized Silver Nanoformulation by Azadirachta Indica Extract a Biorational Approach against Notorious Cotton Pest Whitefly, Bemisia Tabaci (Homoptera; Aleyrodidae). Int. J. Trop. Insect Sci. 2022, 42, 2443–2454. [Google Scholar] [CrossRef]
- Matras, E.; Gorczyca, A.; Przemieniecki, S.W.; Oćwieja, M. Surface Properties-Dependent Antifungal Activity of Silver Nanoparticles. Sci. Rep. 2022, 12, 18046. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Silver Nanoparticles: Mechanism of Action and Probable Bio-Application. J. Funct. Biomater. 2020, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Li, Y.; Tjong, S. Bactericidal and Cytotoxic Properties of Silver Nanoparticles. Int. J. Mol. Sci. 2019, 20, 449. [Google Scholar] [CrossRef]
- Aremu, O.S.; Qwebani-Ogunleye, T.; Katata-Seru, L.; Mkhize, Z.; Trant, J.F. Synergistic Broad-Spectrum Antibacterial Activity of Hypoxis Hemerocallidea-Derived Silver Nanoparticles and Streptomycin against Respiratory Pathobionts. Sci. Rep. 2021, 11, 15222. [Google Scholar] [CrossRef] [PubMed]
- Al-Ansari, M.; Alkubaisi, N.; Vijayaragavan, P.; Murugan, K. Antimicrobial Potential of Streptomyces Sp. to the Gram Positive and Gram Negative Pathogens. J. Infect. Public Health 2019, 12, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Gao, Z.; Chen, X.; Wang, C.; Song, J.; Xu, J.; Liu, X.; Qian, Y.; Suo, H. New Strategies and Mechanisms for Targeting Streptococcus Mutans Biofilm Formation to Prevent Dental Caries: A Review. Microbiol. Res. 2024, 278, 127526. [Google Scholar] [CrossRef]
- Buszewski, B.; Railean-Plugaru, V.; Pomastowski, P.; Rafińska, K.; Szultka-Mlynska, M.; Golinska, P.; Wypij, M.; Laskowski, D.; Dahm, H. Antimicrobial Activity of Biosilver Nanoparticles Produced by a Novel Streptacidiphilus Durhamensis Strain. J. Microbiol. Immunol. Infect. 2018, 51, 45–54. [Google Scholar] [CrossRef] [PubMed]
- El-Sayyad, G.S.; Mosallam, F.M.; El-Batal, A.I. One-Pot Green Synthesis of Magnesium Oxide Nanoparticles Using Penicillium Chrysogenum Melanin Pigment and Gamma Rays with Antimicrobial Activity against Multidrug-Resistant Microbes. Adv. Powder Technol. 2018, 29, 2616–2625. [Google Scholar] [CrossRef]
- Rasool, U.; Hemalatha, S. Marine Endophytic Actinomycetes Assisted Synthesis of Copper Nanoparticles (CuNPs): Characterization and Antibacterial Efficacy against Human Pathogens. Mater. Lett. 2017, 194, 176–180. [Google Scholar] [CrossRef]
- Kalishwaralal, K.; BarathManiKanth, S.; Pandian, S.R.K.; Deepak, V.; Gurunathan, S. Silver Nanoparticles Impede the Biofilm Formation by Pseudomonas Aeruginosa and Staphylococcus Epidermidis. Colloids Surf. B Biointerfaces 2010, 79, 340–344. [Google Scholar] [CrossRef] [PubMed]
- AboElmaaty, S.A.; Shati, A.A.; Alfaifi, M.Y.; Elbehairi, S.E.I.; Sheraba, N.S.; Hassan, M.G.; Badawy, M.S.E.M.; Ghareeb, A.; Hamed, A.A.; Gabr, E.Z. Biofilm Inhibitory Activity of Actinomycete-Synthesized AgNPs with Low Cytotoxic Effect: Experimental and In Silico Study. Microorganisms 2022, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Abdelgawad, A.M.; El-Naggar, M.E.; Rojas, O.J. Antibacterial Activity of Silver Nanoparticles Synthesized In-Situ by Solution Spraying onto Cellulose. Carbohydr. Polym. 2016, 147, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Basak, S.; Ali, S.W. Synthesis of Copper Nanoparticles on Cellulosic Fabrics and Evaluation of Their Multifunctional Performances. Cellulose 2022, 29, 7973–7988. [Google Scholar] [CrossRef]
- Kim, I.; Viswanathan, K.; Kasi, G.; Thanakkasaranee, S.; Sadeghi, K.; Seo, J. ZnO Nanostructures in Active Antibacterial Food Packaging: Preparation Methods, Antimicrobial Mechanisms, Safety Issues, Future Prospects, and Challenges. Food Rev. Int. 2022, 38, 537–565. [Google Scholar] [CrossRef]
- Kalia, A.; Kaur, M.; Shami, A.; Jawandha, S.K.; Alghuthaymi, M.A.; Thakur, A.; Abd-Elsalam, K.A. Nettle-Leaf Extract Derived ZnO/CuO Nanoparticle-Biopolymer-Based Antioxidant and Antimicrobial Nanocomposite Packaging Films and Their Impact on Extending the Post-Harvest Shelf Life of Guava Fruit. Biomolecules 2021, 11, 224. [Google Scholar] [CrossRef]
- Huynh, N.T.N.; Pham, N.T.; Van Tran, C.; Nguyen, D.T.; Dinh, M.T.N.; Nguyen, V.T.; Nguyen, M.H.; Van Ngo, H.; Ho, A.N.; Cheng, J.; et al. Screening for Antimycobacterial Activity of Actinomycetes Collected in Vietnam—Isolation and Activity of Metabolites from Streptomyces Alboniger (A121). Nat. Prod. Commun. 2024, 19, 1934578X231224994. [Google Scholar] [CrossRef]
- Sharma, A.; Dev, K.; Sourirajan, A.; Choudhary, M. Isolation and Characterization of Salt-Tolerant Bacteria with Plant Growth-Promoting Activities from Saline Agricultural Fields of Haryana, India. J. Genet. Eng. Biotechnol. 2021, 19, 99. [Google Scholar] [CrossRef] [PubMed]
- Racchi, I.; Scaramuzza, N.; Hidalgo, A.; Berni, E. Combined Effect of Water Activity and PH on the Growth of Food-Related Ascospore-Forming Molds. Ann. Microbiol. 2020, 70, 69. [Google Scholar] [CrossRef]
- El Karkouri, A.; Assou, S.A.; El Hassouni, M. Isolation and Screening of Actinomycetes Producing Antimicrobial Substances from an Extreme Moroccan Biotope. Pan Afr. Med. J. 2019, 33, 329. [Google Scholar] [CrossRef] [PubMed]
- Goodfellow, M.; Fiedler, H.-P. A Guide to Successful Bioprospecting: Informed by Actinobacterial Systematics. Antonie Van. Leeuwenhoek 2010, 98, 119–142. [Google Scholar] [CrossRef] [PubMed]
- Bizuye, A.; Gedamu, L.; Bii, C.; Gatebe, E.; Maina, N. Molecular-Based Identification of Actinomycetes Species That Synthesize Antibacterial Silver Nanoparticles. Int. J. Microbiol. 2020, 2020, 1–17. [Google Scholar] [CrossRef]
- Pathalam, G.; Rajendran, H.A.D.; Appadurai, D.R.; Gandhi, M.R.; Michael, G.P.; Savarimuthu, I.; Naif, A.A.-D. Isolation and Molecular Characterization of Actinomycetes with Antimicrobial and Mosquito Larvicidal Properties. Beni Suef Univ. J. Basic. Appl. Sci. 2017, 6, 209–217. [Google Scholar] [CrossRef]
- Veelken, M.; Pape, H. Production of Tylosin and Nikkomycin by Immobilized Streptomyces Cells. Eur. J. Appl. Microbiol. Biotechnol. 1982, 15, 206–210. [Google Scholar] [CrossRef]
- Zhang, Y.; Douglas, G.B.; Kaksonen, A.H.; Cui, L.; Ye, Z. Microbial Reduction of Nitrate in the Presence of Zero-Valent Iron. Sci. Total Environ. 2019, 646, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Hamedi, S.; Ghaseminezhad, M.; Shokrollahzadeh, S.; Shojaosadati, S.A. Controlled Biosynthesis of Silver Nanoparticles Using Nitrate Reductase Enzyme Induction of Filamentous Fungus and Their Antibacterial Evaluation. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1588–1596. [Google Scholar] [CrossRef]
- Mechouche, M.S.; Merouane, F.; Messaad, C.E.H.; Golzadeh, N.; Vasseghian, Y.; Berkani, M. Biosynthesis, Characterization, and Evaluation of Antibacterial and Photocatalytic Methylene Blue Dye Degradation Activities of Silver Nanoparticles from Streptomyces Tuirus Strain. Envron. Res. 2022, 204, 112360. [Google Scholar] [CrossRef]
- Damavandi, M.S.; Shojaei, H.; Esfahani, B.N. The Anticancer and Antibacterial Potential of Bioactive Secondary Metabolites Derived From Bacterial Endophytes in Association with Artemisia Absinthium. Sci. Rep. 2023, 13, 18473. [Google Scholar] [CrossRef]
- Charousová, I.; Medo, J.; Hleba, L.; Javoreková, S. Streptomyces Globosus DK15 and Streptomyces Ederensis ST13 as New Producers of Factumycin and Tetrangomycin Antibiotics. Braz. J. Microbiol. 2018, 49, 816–822. [Google Scholar] [CrossRef]
- Oliveira, A.; Cunha, M. de L.R. Comparison of Methods for the Detection of Biofilm Production in Coagulase-Negative Staphylococci. BMC Res. Notes 2010, 3, 260. [Google Scholar] [CrossRef]
- Chen, H.; Wubbolts, R.W.; Haagsman, H.P.; Veldhuizen, E.J.A. Inhibition and Eradication of Pseudomonas Aeruginosa Biofilms by Host Defence Peptides. Sci. Rep. 2018, 8, 10446. [Google Scholar] [CrossRef]
- Wei, G.-X.; Campagna, A.N.; Bobek, L.A. Effect of MUC7 Peptides on the Growth of Bacteria and on Streptococcus Mutans Biofilm. J. Antimicrob. Chemother. 2006, 57, 1100–1109. [Google Scholar] [CrossRef]
- Losada-Barreiro, S.; Sezgin-Bayindir, Z.; Paiva-Martins, F.; Bravo-Díaz, C. Biochemistry of Antioxidants: Mechanisms and Pharmaceutical Applications. Biomedicines 2022, 10, 3051. [Google Scholar] [CrossRef]
- Shilpa, S.A.; Pavithra, A.J.; Hikku, G.S.; Jeyasubramanian, K.; Veluswamy, P.; Ikeda, H. Imparting Efficient Antibacterial Activity to Cotton Fabrics by Coating with Green Synthesized Nano-Ag/PMMA Composite. Bionanoscience 2023, 13, 2180–2194. [Google Scholar] [CrossRef]
- Ibrahim, H.M.M.; Hassan, M.S. Characterization and Antimicrobial Properties of Cotton Fabric Loaded with Green Synthesized Silver Nanoparticles. Carbohydr. Polym. 2016, 151, 841–850. [Google Scholar] [CrossRef]





| S. No. | Tested Microorganism | MIC of AgNPs (µg/mL) | MBC of AgNP (µg/mL) | MIC of Antibiotic (µg/mL) | ||
|---|---|---|---|---|---|---|
| AMP | K | TE | ||||
| 1 | B. subtilis MTCC 441 | 128 | 128 | 128 | 8 | 4 |
| 2 | P. aeruginosa MTCC 1688 | 8 | 16 | 512 | 12 | 64 |
| 3 | S. aureus MTCC 737 | 256 | 256 | 128 | 3 | 64 |
| 4 | E. coli MTCC 1687 | 32 | 64 | 512 | 128 | 64 |
| AMB | FLU | |||||
| 5 | C. albicans MTCC 183 | 32 | 64 | 8 | 256 | |
| 6 | A. niger MTCC 1344 | 64 | 64 | 4 | 512 | |
| S. No. | Tested Microorganism | AgNPs + Ampicillin (µg/mL) | FIC | AgNPs +Tetracycline (µg/mL) | FIC | AgNPs+ Amphotericin B (µg/mL) | FIC |
|---|---|---|---|---|---|---|---|
| 1 | Bacillus subtilis (MTCC 441) | 64 + 64 | 1 | 16 + 0.5 | 0.25 | ||
| 2 | P. aeruginosa (MTCC 1688) | 2 + 128 | 0.5 | 1 + 8 | 0.25 | ||
| 3 | S. aureus (MTCC 737) | 128 + 64 | 1 | 64 + 16 | 0.5 | ||
| 4 | E. coli (MTCC 1687) | 8 + 128 | 0.5 | 4 + 8 | 0.375 | ||
| 5 | C. albicans (MTCC 183) | 16 + 4 | 1 | ||||
| 6 | A. niger (MTCC 1344) | 32 + 2 | 1 | ||||
| FIC index | Interpretation | ||||||
| ≤0.5 | Synergistic | ||||||
| >0.5–1.0 | Additive or non-synergistic | ||||||
| 1.0–4.0 | In different | ||||||
| >4 | Antagonistic | ||||||
| Tested Microbes | Percentage Inhibition | ||||||
|---|---|---|---|---|---|---|---|
| AgNPs Dose (µg/mL) | AMP | AgNPs + AMP | |||||
| 10 | 20 | 30 | 40 | 50 | 50 | 30 µg/mL+ 30 µg/mL | |
| P. aeruginosa | 15 | 22 | 30 | 46 | 60 | 35 | 98 |
| S. pneumoniae | 12 | 20 | 25 | 32 | 44 | 28 | 83 |
| Condition | pH | NaCl (%, w/v) |
|---|---|---|
| Normal | 7.0 | 0.0 |
| Alkaline | 9.0 | 0.0 |
| Saline | 7.0 | 5.0 |
| Saline + alkaline | 9.0 | 5.0 |
| S. No. | EzTaxon Database Suggested Species | Selected Species | Hit Strain Name | Similarity (%) |
|---|---|---|---|---|
| 1 | S. tendae | S. tendae | ATCC 19812(T) | 98.46 |
| 2 | Streptomyces violaceorubidus | LMG 20319(T) | 98.46 | |
| 3 | Streptomyces gougerotii | NBRC 3198(T) | 98.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dayma, P.; Choudhary, N.; Ali, D.; Alarifi, S.; Dudhagara, P.; Luhana, K.; Yadav, V.K.; Patel, A.; Patel, R. Exploring the Potential of Halotolerant Actinomycetes from Rann of Kutch, India: A Study on the Synthesis, Characterization, and Biomedical Applications of Silver Nanoparticles. Pharmaceuticals 2024, 17, 743. https://doi.org/10.3390/ph17060743
Dayma P, Choudhary N, Ali D, Alarifi S, Dudhagara P, Luhana K, Yadav VK, Patel A, Patel R. Exploring the Potential of Halotolerant Actinomycetes from Rann of Kutch, India: A Study on the Synthesis, Characterization, and Biomedical Applications of Silver Nanoparticles. Pharmaceuticals. 2024; 17(6):743. https://doi.org/10.3390/ph17060743
Chicago/Turabian StyleDayma, Paras, Nisha Choudhary, Daoud Ali, Saud Alarifi, Pravin Dudhagara, Kuldeep Luhana, Virendra Kumar Yadav, Ashish Patel, and Rajesh Patel. 2024. "Exploring the Potential of Halotolerant Actinomycetes from Rann of Kutch, India: A Study on the Synthesis, Characterization, and Biomedical Applications of Silver Nanoparticles" Pharmaceuticals 17, no. 6: 743. https://doi.org/10.3390/ph17060743
APA StyleDayma, P., Choudhary, N., Ali, D., Alarifi, S., Dudhagara, P., Luhana, K., Yadav, V. K., Patel, A., & Patel, R. (2024). Exploring the Potential of Halotolerant Actinomycetes from Rann of Kutch, India: A Study on the Synthesis, Characterization, and Biomedical Applications of Silver Nanoparticles. Pharmaceuticals, 17(6), 743. https://doi.org/10.3390/ph17060743







