Click Chemistry in Polymersome Technology
Abstract
1. Introduction
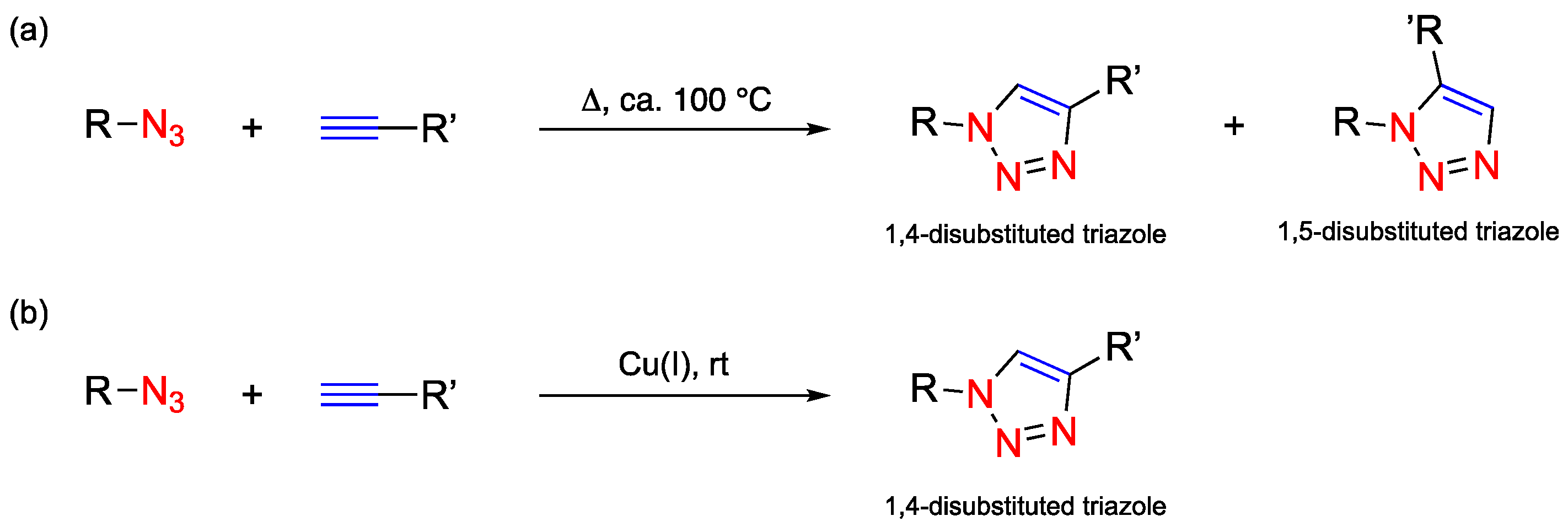
1.1. Click Chemistry
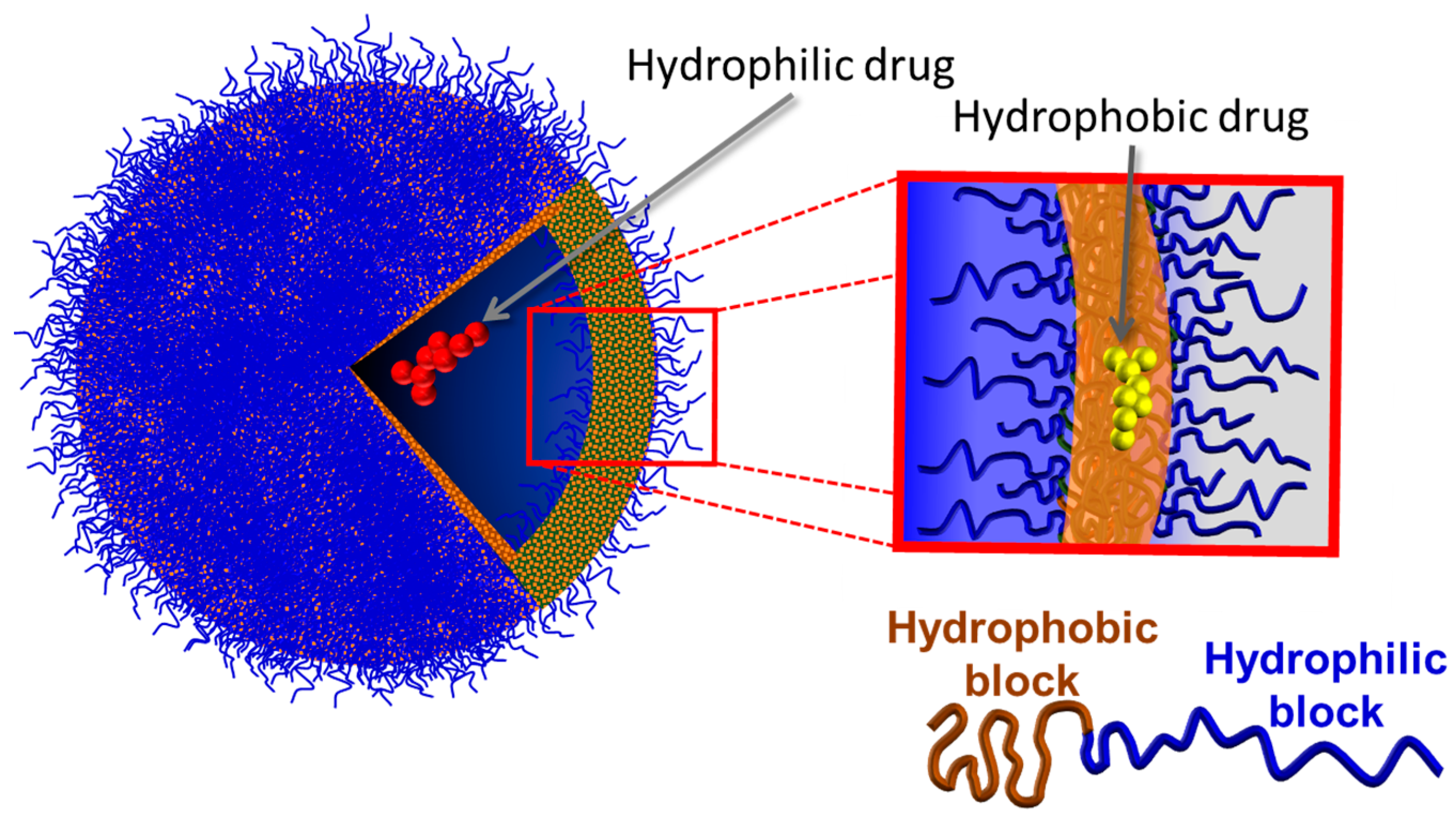
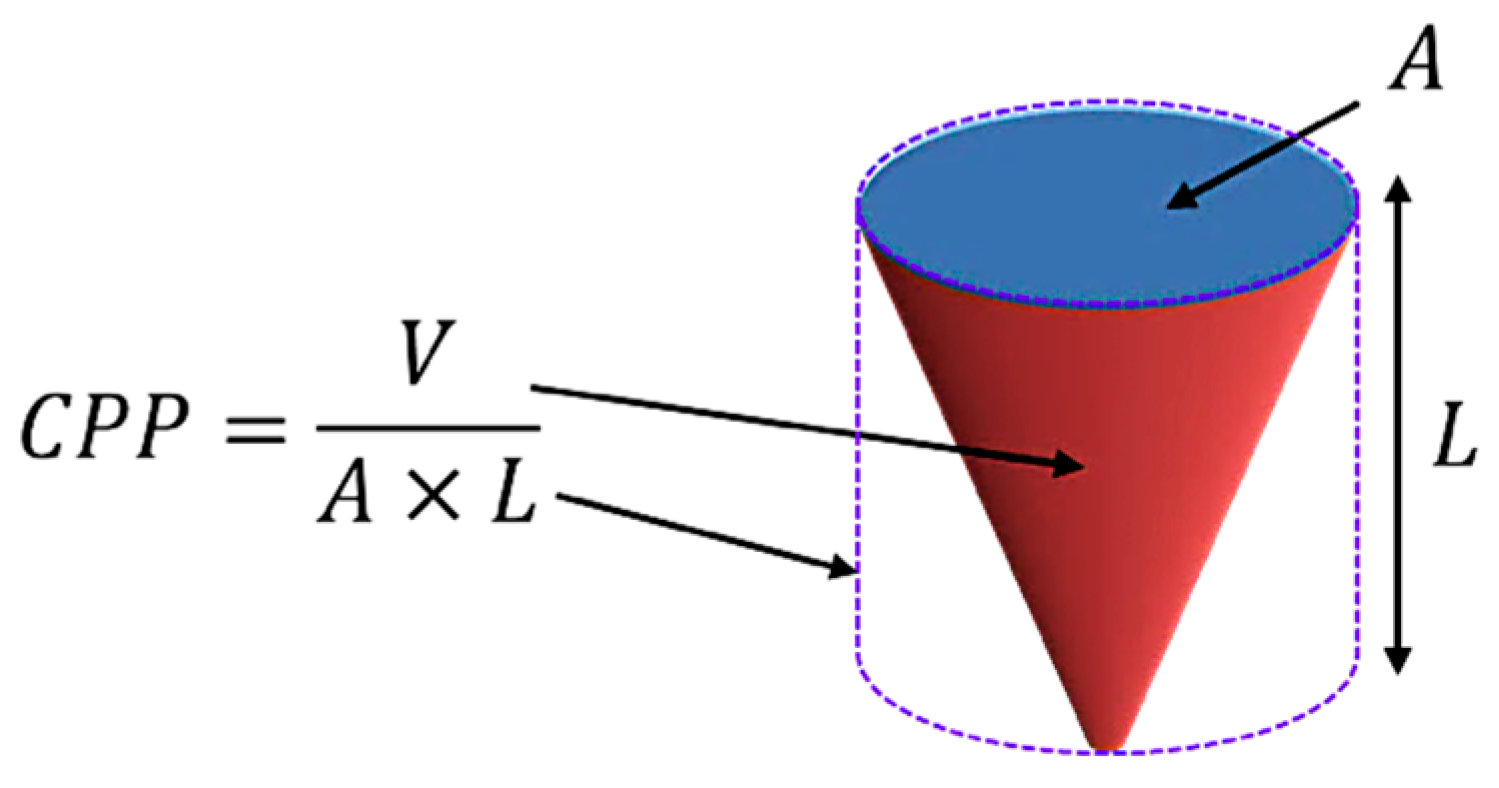
1.2. Polymersomes
2. Click Chemistry in Copolymer Synthesis
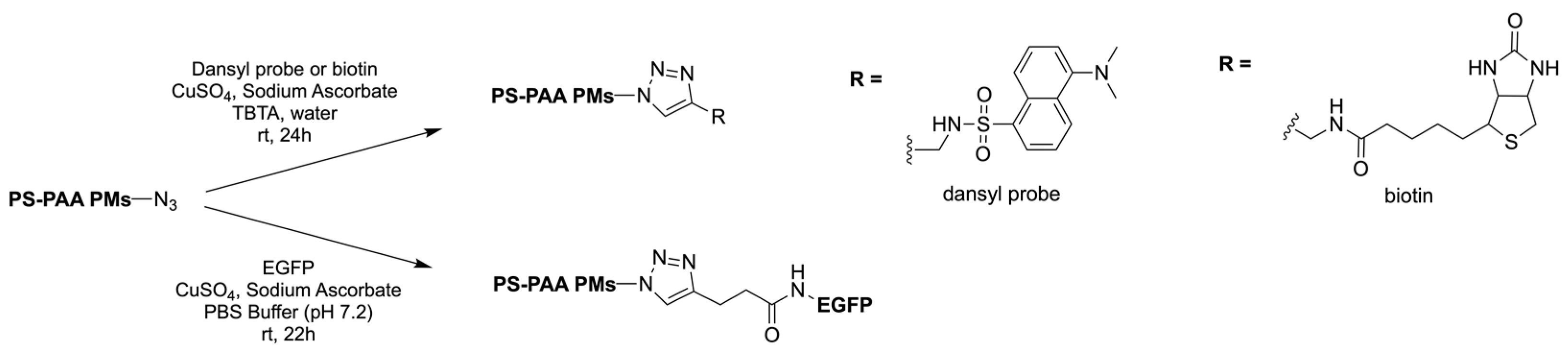
3. Click Chemistry in Polymersome Functionalisation
4. Structural Elucidation
4.1. Triazole and Azide–Alkyne Elucidation
4.2. Nanoparticle Assemble Elucidation
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The Nobel Prize in Chemistry 2022. 2022. Available online: https://www.nobelprize.org/prizes/chemistry/2022/press-release/ (accessed on 29 January 2024).
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar]
- Kondengadan, S.M.; Bansal, S.; Yang, C.; Liu, D.; Fultz, Z.; Wang, B. Click chemistry and drug delivery: A bird’s-eye view. Acta Pharm. Sin. B 2023, 13, 1990–2016. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, N.K.; Finn, M.G. Introduction-Click Chemistry. Chem. Rev. 2021, 121, 6697–6698. [Google Scholar] [CrossRef] [PubMed]
- Agard, N.J.; Baskin, J.M.; Prescher, J.A.; Lo, A.; Bertozzi, C.R. A Comparative Study of Bioorthogonal Reactions with Azides. ACS Chem. Biol. 2006, 1, 644–648. [Google Scholar] [CrossRef]
- Saxon, E.; Bertozzi, C.R. Cell Surface Engineering by a Modified Staudinger Reaction. Science 2000, 287, 2007–2010. [Google Scholar] [CrossRef] [PubMed]
- Sletten, E.M.; Bertozzi, C.R. From Mechanism to Mouse: A Tale of Two Bioorthogonal Reactions. Acc. Chem. Res. 2011, 44, 666–676. [Google Scholar] [PubMed]
- Le Droumaguet, B.; Velonia, K. Click chemistry: A powerful tool to create polymer-based macromolecular chimeras. Macromol. Rapid Commun. 2008, 29, 1073–1089. [Google Scholar] [CrossRef]
- Slavin, S.; Burns, J.; Haddleton, D.M.; Becer, C.R. Synthesis of glycopolymers via click reactions. Eur. Polym. J. 2011, 47, 435–446. [Google Scholar] [CrossRef]
- Takayama, Y.; Kusamori, K.; Nishikawa, M. Click chemistry as a tool for cell engineering and drug delivery. Molecules 2019, 24, 172. [Google Scholar] [CrossRef]
- Bartenstein, J.E.; Robertson, J.; Battaglia, G.; Briscoe, W.H. Stability of polymersomes prepared by size exclusion chromatography and extrusion. Colloids Surf. A Physicochem. Eng. Asp. 2016, 506, 739–746. [Google Scholar] [CrossRef]
- Wang, X.; Huang, B.; Liu, X.; Zhan, P. Discovery of bioactive molecules from CuAAC click-chemistry-based combinatorial libraries. Drug Discov. Today 2016, 21, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, M.K.; Sajadi, S.M.; Seidi, F.; Rabiee, N.; Fatahi, Y.; Rabiee, M.; Dominic, C.D.M.; Zarrintaj, P.; Formela, K.; Saeb, M.R.; et al. Clickable Polysaccharides for Biomedical Applications: A Comprehensive Review. Prog. Polym. Sci. 2022, 133, 101590. [Google Scholar] [CrossRef] [PubMed]
- Discher, B.M.; Won, Y.Y.; Ege, D.S.; Lee, J.C.M.; Bates, F.S.; Discher, D.E.; Hammer, D.A. Polymersomes: Tough vesicles made from diblock copolymers. Science 1999, 284, 1143–1146. [Google Scholar] [CrossRef] [PubMed]
- Chandrawati, R.; Caruso, F. Biomimetic liposome- and polymersome-based multicompartmentalized assemblies. Langmuir 2012, 28, 13798–13807. [Google Scholar] [CrossRef]
- Bermudez, H.; Brannan, A.K.; Hammer, D.A.; Bates, F.S.; Discher, D.E. Molecular weight dependence of polymersome membrane structure, elasticity, and stability. Macromolecules 2002, 35, 8203–8208. [Google Scholar] [CrossRef]
- Cevc, G.; Marsh, D. (Eds.) Phospholipid Bilayers Physical Principles and Models; John Wiley and Sons Ltd.: Warsaw, Poland, 1987. [Google Scholar]
- Discher, D.E.; Ahmed, F. Polymersomes. Annu. Rev. Biomed. Eng. 2006, 8, 323–341. [Google Scholar] [CrossRef]
- Aranda-Espinoza, H.; Bermudez, H.; Bates, F.S.; Discher, D.E. Electromechanical limits of polymersomes. Phys. Rev. Lett. 2001, 87, 208301-1–208301-4. [Google Scholar] [CrossRef]
- Discher, D.E.; Eisenberg, A. Polymer vesicles. Science 2002, 297, 967–973. [Google Scholar] [CrossRef]
- Photos, P.J.; Bacakova, L.; Discher, B.; Bates, F.S.; Discher, D.E. Polymer vesicles in vivo: Correlations with PEG molecular weight. J. Control. Release 2003, 90, 323–334. [Google Scholar] [CrossRef]
- Alves, A.; Silva, A.M.; Moreira, J.; Nunes, C.; Reis, S.; Pinto, M.; Cidade, H.; Rodrigues, F.; Ferreira, D.; Costa, P.C.; et al. Polymersomes for Sustained Delivery of a Chalcone Derivative Targeting Glioblastoma Cells. Brain Sci. 2024, 14, 82. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.; Silva, A.M.; Nunes, C.; Cravo, S.; Reis, S.; Pinto, M.; Sousa, E.; Rodrigues, F.; Ferreira, D.; Costa, P.C.; et al. The Synthesis and Characterization of a Delivery System Based on Polymersomes and a Xanthone with Inhibitory Activity in Glioblastoma. Life 2024, 14, 132. [Google Scholar] [CrossRef] [PubMed]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: Applications, advantages and disadvantages. Res. Pharm. Sci. 2018, 13, 288. [Google Scholar] [CrossRef] [PubMed]
- Kammari, R.; Das, N.G.; Das, S.K. Nanoparticulate Systems for Therapeutic and Diagnostic Applications. In Emerging Nanotechnologies for Diagnostics, Drug Delivery and Medical Devices; Elsevier: Amsterdam, The Netherlands, 2017; pp. 105–144. [Google Scholar] [CrossRef]
- Aibani, N.; Khan, T.N.; Callan, B. Liposome mimicking polymersomes; A comparative study of the merits of polymersomes in terms of formulation and stability. Int. J. Pharm. X 2020, 2, 100040. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Hamaguchi, T.; Ura, T.; Muro, K.; Yamada, Y.; Shimada, Y.; Shirao, K.; Okusaka, T.; Ueno, H.; Ikeda, M.; et al. Phase I clinical trial and pharmacokinetic evaluation of NK911, a micelle-encapsulated doxorubicin. Br. J. Cancer 2004, 91, 1775–1781. [Google Scholar] [CrossRef] [PubMed]
- Danson, S.; Ferry, D.; Alakhov, V.; Margison, J.; Kerr, D.; Jowle, D.; Brampton, M.; Halbert, G.; Ranson, M. Phase I dose escalation and pharmacokinetic study of pluronic polymer-bound doxorubicin (SP1049C) in patients with advanced cancer. Br. J. Cancer 2004, 90, 2085–2091. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-T.; Neerman, M.F.; Parrish, A.R.; Simanek, E.E. Cytotoxicity, Hemolysis, and Acute in Vivo Toxicity of Dendrimers Based on Melamine, Candidate Vehicles for Drug Delivery. J. Am. Chem. Soc. 2004, 126, 10044–10048. [Google Scholar] [CrossRef]
- Derfus, A.M.; Chan, W.C.W.; Bhatia, S.N. Probing the Cytotoxicity of Semiconductor Quantum Dots. Nano Lett. 2004, 4, 11–18. [Google Scholar] [CrossRef]
- Bose, S.; Khare, R.A.; Moldenaers, P. Assessing the strengths and weaknesses of various types of pre-treatments of carbon nanotubes on the properties of polymer/carbon nanotubes composites: A critical review. Polymer 2010, 51, 975–993. [Google Scholar] [CrossRef]
- Ferdous, Z.; Nemmar, A. Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef]
- Pham, Q.T.; Ngo, G.L.; Nguyen, X.A.; Nguyen, C.T.; Ledoux-Rak, I.; Lai, N.D. Direct Synthesis of Gold Nanoparticles in Polymer Matrix. Polymers 2022, 15, 16. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Saxena, M.; Rishi, N. An Overview of Recent Advances in Biomedical Applications of Click Chemistry. Bioconjugate Chem. 2021, 32, 1455–1471. [Google Scholar] [CrossRef] [PubMed]
- Matoori, S.; Leroux, J.-C. Twenty-five years of polymersomes: Lost in translation? Mater. Horiz. 2020, 7, 1297–1309. [Google Scholar] [CrossRef]
- Fonseca, M.; Jarak, I.; Victor, F.; Domingues, C.; Veiga, F.; Figueiras, A. Polymersomes as the Next Attractive Generation of Drug Delivery Systems: Definition, Synthesis and Applications. Materials 2024, 17, 319. [Google Scholar] [CrossRef] [PubMed]
- LoPresti, C.; Lomas, H.; Massignani, M.; Smart, T.; Battaglia, G. Polymersomes: Nature inspired nanometer sized compartments. J. Mater. Chem. 2009, 19, 3576–3590. [Google Scholar] [CrossRef]
- Guan, L.; Rizzello, L.; Battaglia, G. Polymersomes and their applications in cancer delivery and therapy. Nanomedicine 2015, 10, 2757–2780. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Prasher, P.; Aljabali, A.A.; Mishra, V.; Gandhi, H.; Kumar, S.; Mutalik, S.; Chellappan, D.K.; Tambuwala, M.M.; Dua, K.; et al. Emerging era of “somes”: Polymersomes as versatile drug delivery carrier for cancer diagnostics and therapy. Drug Deliv. Transl. Res. 2020, 10, 1171–1190. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Qiu, L. Polymersomes: Preparation and characterization. Methods Mol. Biol. 2019, 2000, 247–265. [Google Scholar] [CrossRef] [PubMed]
- Rideau, E.; Dimova, R.; Schwille, P.; Wurm, F.R.; Landfester, K. Liposomes and polymersomes: A comparative review towards cell mimicking. Chem. Soc. Rev. 2018, 47, 8572–8610. [Google Scholar] [CrossRef]
- Pallavi, P.; Harini, K.; Gowtham, P.; Girigoswami, K.; Girigoswami, A. Fabrication of Polymersomes: A Macromolecular Architecture in Nanotherapeutics. Chemistry 2022, 4, 1028–1043. [Google Scholar] [CrossRef]
- Toprakcioglul, C.; Dail, L.; Ansarifarl, M.A.; Stamm, M.; Motschmann, H. Equilibrium and dynamic aspects of end-attached diblock and triblock copolymer chains. Prog. Colloid. Polym. Sci. 1993, 91, 83–87. [Google Scholar] [CrossRef]
- Napoli, A.; Tirelli, N.; Wehrli, E.; Hubbell, J.A. Lyotropic behavior in water of amphiphilic ABA triblock copolymers based on poly(propylene sulfide) and poly(ethylene glycol). Langmuir 2002, 18, 8324–8329. [Google Scholar] [CrossRef]
- Stoenescu, R.; Graff, A.; Meier, W. Asymmetric ABC-triblock copolymer membranes induce a directed insertion of membrane proteins. Macromol. Biosci. 2004, 4, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Wittemann, A.; Azzam, T.; Eisenberg, A. Biocompatible polymer vesicles from biamphiphilic triblock copolymers and their interaction with bovine serum albumin. Langmuir 2007, 23, 2224–2230. [Google Scholar] [CrossRef] [PubMed]
- Walther, A.; Müller, A.H.E. Janus particles. Soft Matter 2008, 4, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Sommerdijk, N.A.J.M.; Holder, S.J.; Hiorns, R.C.; Jones, R.G.; Nolte, R.J.M. Self-assembled structures from an amphiphilic multiblock copolymer containing rigid semiconductor segments. Macromolecules 2000, 33, 8289–8294. [Google Scholar] [CrossRef]
- Brannan, A.K.; Bates, F.S. ABCA tetrablock copolymer vesicles. Macromolecules 2004, 37, 8816–8819. [Google Scholar] [CrossRef]
- Opsteen, J.A.; van Hest, J.C.M. Modular synthesis of block copolymers via cycloaddition of terminal azide and alkyne functionalized polymers. Chem. Commun. 2005, 1, 57. [Google Scholar] [CrossRef] [PubMed]
- Van Dongen, S.F.M.; Nallani, M.; Schoffelen, S.; Cornelissen, J.J.L.M.; Nolte, R.J.M.; van Hest, J.C.M. A block copolymer for functionalisation of polymersome surfaces. Macromol. Rapid Commun. 2008, 29, 321–325. [Google Scholar] [CrossRef]
- Van Dongen, S.F.M.; Nallani, M.; Cornelissen, J.J.L.M.; Nolte, R.J.M.; van Hest, J.C.M. A Three-Enzyme Cascade Reaction through Positional Assembly of Enzymesin a Polymersome Nanoreactor. Chem. Eur. J. 2009, 15, 1107–1114. [Google Scholar] [CrossRef]
- Binder, W.H.; Sachsenhofer, R. Polymersome/silica capsules by ’click’-chemistry. Macromol. Rapid Commun. 2008, 29, 1097–1103. [Google Scholar] [CrossRef]
- Kumar Upadhyay, K.; Le Meins, J.F.; Misra, A.; Voisin, P.; Bouchaud, V.; Ibarboure, E.; Schatz, C.; Lecommandoux, S. Biomimetic doxorubicin loaded polymersomes from hyaluronan-block- poly(γ-benzyl glutamate) copolymers. Biomacromolecules 2009, 10, 2802–2808. [Google Scholar] [CrossRef] [PubMed]
- Shahriari, M.; Taghdisi, S.M.; Abnous, K.; Ramezani, M.; Alibolandi, M. Self-targeted polymersomal co-formulation of doxorubicin, camptothecin and FOXM1 aptamer for efficient treatment of non-small cell lung cancer. J. Control. Release 2021, 335, 369–388. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Kang, H.C.; Huh, K.M.; Bae, Y.H. Biocompatible, pH-sensitive AB2 miktoarm polymer-based polymersomes: Preparation, characterization, and acidic pH-activated nanostructural transformation. J. Mater. Chem. 2012, 22, 19168–19178. [Google Scholar] [CrossRef] [PubMed]
- Gaitzsch, J.; Chudasama, V.; Morecroft, E.; Messager, L.; Battaglia, G. Synthesis of an Amphiphilic Miktoarm Star Terpolymer for Self-Assembly into Patchy Polymersomes. ACS Macro Lett. 2016, 5, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Khoee, S.; Hashemi, A.; Molavipordanjani, S. Synthesis and characterization of IUdR loaded PEG/PCL/PEG polymersome in mixed DCM/DMF solvent: Experimental and molecular dynamics insights into the role of solvent composition and star architecture in drug dispersion and diffusion. Eur. J. Pharm. Sci. 2018, 114, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rijpkema, S.J.; Langens, S.G.H.A.; van der Kolk, M.R.; Gavriel, K.; Toebes, B.J.; Wilson, D.A. Modular Approach to the Functionalization of Polymersomes. Biomacromolecules 2020, 21, 1853–1864. [Google Scholar] [CrossRef] [PubMed]
- Malinge, J.; Allain, C.; Galmiche, L.; Miomandre, F.; Audebert, P. Preparation, Photophysical, Electrochemical, and Sensing Properties of Luminescent Tetrazine-Doped Silica Nanoparticles. Chem. Mater. 2011, 23, 4599–4605. [Google Scholar] [CrossRef]
- Barker, I.A.; Hall, D.J.; Hansell, C.F.; du Prez, F.E.; O’Reilly, R.K.; Dove, A.P. Tetrazine-Norbornene Click Reactions to Functionalize Degradable Polymers Derived from Lactide. Macromol. Rapid Commun. 2011, 32, 1362–1366. [Google Scholar] [CrossRef] [PubMed]
- Blackman, M.L.; Royzen, M.; Fox, J.M. Tetrazine Ligation: Fast Bioconjugation Based on Inverse-Electron-Demand Diels−Alder Reactivity. J. Am. Chem. Soc. 2008, 130, 13518–13519. [Google Scholar] [CrossRef] [PubMed]
- Penelas, M.J.; Soler-Illia, G.J.A.A.; Levi, V.; Bordoni, A.V.; Wolosiuk, A. Click-based thiol-ene photografting of COOH groups to SiO2 nanoparticles: Strategies comparison. Colloids Surf. A Physicochem. Eng. Asp. 2019, 562, 61–70. [Google Scholar] [CrossRef]
- Ruizendaal, L.; Pujari, S.P.; Gevaerts, V.; Paulusse, J.M.J.; Zuilhof, H. Biofunctional Silicon Nanoparticles by Means of Thiol-Ene Click Chemistry. Chem. Asian J. 2011, 6, 2776–2786. [Google Scholar] [CrossRef] [PubMed]
- Hoyle, C.E.; Bowman, C.N. Thiol-Ene Click Chemistry. Angew. Chem. Int. Ed. 2010, 49, 1540–1573. [Google Scholar] [CrossRef] [PubMed]
- Toebes, B.J.; Abdelmohsen, L.K.E.A.; Wilson, D.A. Enzyme-driven biodegradable nanomotor based on tubular-shaped polymeric vesicles. Polym. Chem. 2018, 9, 3190–3194. [Google Scholar] [CrossRef]
- Von Maltzahn, G.; Ren, Y.; Park, J.-H.; Min, D.-H.; Kotamraju, V.R.; Jayakumar, J.; Fogal, V.; Sailor, M.J.; Ruoslahti, E.; Bhatia, S.N. In Vivo Tumor Cell Targeting with “Click” Nanoparticles. Bioconjug. Chem. 2008, 19, 1570–1578. [Google Scholar] [CrossRef] [PubMed]
- Debets, M.F.; van der Doelen, C.W.J.; Rutjes, F.P.J.T.; van Delft, F.L. Azide: A Unique Dipole for Metal-Free Bioorthogonal Ligations. ChemBioChem 2010, 11, 1168–1184. [Google Scholar] [CrossRef] [PubMed]
- Castillo, R.R.; Hernández-Escobar, D.; Gómez-Graña, S.; Vallet-Regí, M. Reversible Nanogate System for Mesoporous Silica Nanoparticles Based on Diels-Alder Adducts. Chem. A Eur. J. 2018, 24, 6992–7001. [Google Scholar] [CrossRef] [PubMed]
- Jarre, G.; Liang, Y.; Betz, P.; Lang, D.; Krueger, A. Playing the surface game—Diels–Alder reactions on diamond nanoparticles. Chem. Commun. 2011, 47, 544–546. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Wosnick, J.H.; Ho, K.; Keating, A.; Shoichet, M.S. Immuno-Polymeric Nanoparticles by Diels–Alder Chemistry. Angew. Chem. Int. Ed. 2007, 46, 6126–6131. [Google Scholar] [CrossRef]
- Pawar, P.V.; Gohil, S.V.; Jain, J.P.; Kumar, N. Functionalized polymersomes for biomedical applications. Polym. Chem. 2013, 4, 3160–3176. [Google Scholar] [CrossRef]
- Pangburn, T.O.; Bates, F.S.; Kokkoli, E. Polymersomes functionalized via “click” chemistry with the fibronectin mimetic peptides PR-b and GRGDSP for targeted delivery to cells with different levels of α5β1 expression. Soft Matter 2012, 8, 4449–4461. [Google Scholar] [CrossRef]
- Van Oers, M.C.M.; Abdelmohsen, L.K.E.A.; Rutjes, F.P.J.T.; van Hest, J.C.M. Aqueous asymmetric cyclopropanation reactions in polymersome membranes. Chem. Commun. 2014, 50, 4040–4043. [Google Scholar] [CrossRef]
- Van Oers, M.C.M.; Veldmate, W.S.; van Hest, J.C.M.; Rutjes, F.P.J.T. Aqueous asymmetric aldol reactions in polymersome membranes. Polym. Chem. 2015, 6, 5358–5361. [Google Scholar] [CrossRef]
- Eissa, A.M.; Smith, M.J.P.; Kubilis, A.; Mosely, J.A.; Cameron, N.R. Polymersome-forming amphiphilic glycosylated polymers: Synthesis and characterization. J. Polym. Sci. A Polym. Chem. 2013, 51, 5184–5193. [Google Scholar] [CrossRef]
- Opsteen, J.A.; Brinkhuis, R.P.; Teeuwen, R.L.M.; Löwik, D.W.P.M.; van Hest, J.C.M. “Clickable” polymersomes. Chem. Commun. 2007, 30, 3136–3138. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Martin, A.L.; Gillies, E.R. Multivalent polymer vesicles via surface functionalization. Chem. Commun. 2007, 48, 5217–5219. [Google Scholar] [CrossRef]
- Amos, R.C.; Nazemi, A.; Bonduelle, C.V.; Gillies, E.R. Tuning polymersome surfaces: Functionalization with dendritic groups. Soft Matter 2012, 8, 5947–5958. [Google Scholar] [CrossRef]
- Nazemi, A.; Gillies, E.R. Dendritic surface functionalization of nanomaterials- controlling properties and functions for biomedical applications. Braz. J. Pharm. Sci. 2013, 49, 15–32. [Google Scholar] [CrossRef]
- Rein, C.; Nissen, S.; Grzelakowski, M.; Meldal, M. Click-chemistry of polymersomes on nanoporous polymeric surfaces. J. Polym. Sci. A Polym. Chem. 2016, 54, 2032–2039. [Google Scholar] [CrossRef]
- Karandish, F.; Mamnoon, B.; Feng, L.; Haldar, M.K.; Xia, L.; Gange, K.N.; You, S.; Choi, Y.; Sarkar, K.; Mallik, S. Nucleus-Targeted, Echogenic Polymersomes for Delivering a Cancer Stemness Inhibitor to Pancreatic Cancer Cells. Biomacromolecules 2018, 19, 4122–4132. [Google Scholar] [CrossRef]
- Zhang, Q.; Ren, H.; Baker, G.L. Synthesis and click chemistry of a new class of biodegradable polylactide towards tunable thermo-responsive biomaterials. Polym. Chem. 2015, 6, 1275–1285. [Google Scholar] [CrossRef]
- Han, Y.; Yuan, L.; Li, G.; Huang, L.; Qin, T.; Chu, F.; Tang, C. Renewable polymers from lignin via copper-free thermal click chemistry. Polymer 2016, 83, 92–100. [Google Scholar] [CrossRef]
- Li, C.; Finn, M.G. Click chemistry in materials synthesis. II. Acid-swellable crosslinked polymers made by copper-catalyzed azide-alkyne cycloaddition. J. Polym. Sci. A Polym. Chem. 2006, 44, 5513–5518. [Google Scholar] [CrossRef]
- Lang, A.S.; Neubig, A.; Sommer, M.; Thelakkat, M. NMRP versus “Click” Chemistry for the Synthesis of Semiconductor Polymers Carrying Pendant Perylene Bisimides. Macromolecules 2010, 43, 7001–7010. [Google Scholar] [CrossRef]
- Gakiya-Teruya, M.; Palomino-Marcelo, L.; Pierce, S.; Angeles-Boza, A.M.; Krishna, V.; Rodriguez-Reyes, J.C.F. Enhanced antimicrobial activity of silver nanoparticles conjugated with synthetic peptide by click chemistry. J. Nano. Res. 2020, 22, 90. [Google Scholar] [CrossRef]
- Zou, L.; Shi, Y.; Cao, X.; Gan, W.; Wang, X.; Graff, R.W.; Hu, D.; Gao, H. Synthesis of acid-degradable hyperbranched polymers by chain-growth CuAAC polymerization of an AB 3 monomer. Polym. Chem. 2016, 7, 5512–5517. [Google Scholar] [CrossRef]
- Kita-Tokarczyk, K.; Grumelard, J.; Haefele, T.; Meier, W. Block copolymer vesicles—Using concepts from polymer chemistry to mimic biomembranes. Polymer 2005, 46, 3540–3563. [Google Scholar] [CrossRef]
- Lee, J.S.; Feijen, J. Polymersomes for drug delivery: Design, formation and characterization. J. Control. Release 2012, 161, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, B.; Karanam, V.; Chellan, V.R.; Siram, K.; Natarajan, T.S.; Gregory, M. Polymersomes as an effective drug delivery system for glioma—A review. J. Drug Target. 2014, 22, 469–477. [Google Scholar] [CrossRef]
- Striegel, A.M.; Yau, W.W.; Kirkland, J.J.; Bly, D.D. Modern Size-Exclusion Liquid Chromatography; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar] [CrossRef]
- Chang, R. Physical Chemistry for the Biosciences 2005. Available online: https://books.google.pt/books?id=PNH1fHj5Tw0C&printsec=frontcover&redir_esc=y#v=onepage&q&f=false (accessed on 3 June 2024).
- Skoog, D.; Holler, F.; Crouch, S. Principles of Instrumental Analysis 1985. Available online: https://books.google.pt/books/about/Principles_of_Instrumental_Analysis.html?id=D13EDQAAQBAJ&redir_esc=y (accessed on 3 June 2024).
- Lathe, G.H.; Ruthven, C.R. The Separation of Substances on the Basis of their Molecular Weights, Using Columns of Starch and Water. Biochem. J. 1955, 60, xxxiv. Available online: https://pubmed.ncbi.nlm.nih.gov/13249976/ (accessed on 3 June 2024).

| DDS | Disadvantages Compared to PMs |
|---|---|
| Liposomes | The thickness of the liposome (3–5 nm) provides less stability, and less retention of content [16]. |
| Solid Liquid Nanoparticles | These particles have some disadvantages, such as the rapid loss of large quantities of drugs and the lack of controlled drug release [25,26]. |
| Microemulsions | Less stable—they can be affected by temperature, pH, and other environmental factors and have lower encapsulation efficiencies [27]. |
| Micelles | Reduced stability in the bloodstream, since the critical micellar concentration (CMC) can be reduced by blood dilution and the encapsulated drugs can leak out, minimizing drug circulation [28,29]. |
| Dendrimers | Showed cytotoxicity [30]. |
| Quantum Dots | Cytotoxicity of small semiconductor particles [31]. |
| Carbon nanotubes | The process of production is expensive and lacks solubility in aqueous media [32]. |
| Silver nanoparticles | Toxic effects on cells and organisms [33]. |
| Golden nanoparticles | The methods used for the synthesis are expensive and can also use toxic ingredients. This makes it difficult to implement this recent technology in all the places where it could be useful [34]. |
| Packing Formation | CPP | Interface Formed |
|---|---|---|
 | <1/3 (spherical) 1/3–1/2 (cylindric micelles) |  |
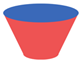 | 1/2–1 (Flexible lamellae, vesicles, polymersomes) |  |
 | ≈1 (Planar lamellae) |  |
 | >1 (Inverted structures) |  |
| Reactions Used for Azide–Alkyl Functional Group Introduction | ||||
|---|---|---|---|---|
| Entry | Transfer Azide–Alkyl | End Group | Reaction Conditions | Ref. |
| 1 |  | 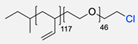 | Argon atmosphere, DMF, 65 °C Undisclosed yield | [74] |
| 2 | 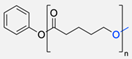 | (i) TsCl, Et3N, DCM, rt (ii) NaN3, DMF, rt Undisclosed yield | [55,56] | |
| 3 | 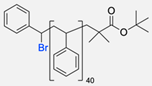 | DMF, rt 89% yield | [52] | |
| 4 |  | DMF, rt 66% yield | [75,76] | |
| 5 | 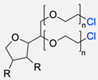 | DMF, 120 °C (4 h) 99% yield | [59] | |
| 6 |  | H2O, 80 °C (24 h) 80–90% yield | [77] | |
| 7 | 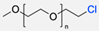 | N2 atmosphere, DMF, rt (24 h) 85% yield | [57] | |
| 8 | 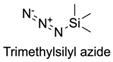 | 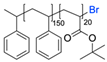 | TBAF, THF, rt Undisclosed yield | [78] |
| 9 | 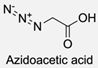 |  | DCC, DMAP, DPTS, DCM, rt 95% yield | [79,80,81] |
| 10 | 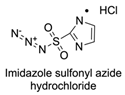 |  | K2CO3, [Cu(II)SO4] *, H2O, rt Undisclosed yield | [82] |
| 11 | 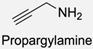 | 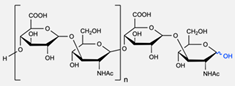 | NaBH3CN, Acetate buffer, 50 °C (5 days) Quantitative yield | [55,56] |
| 12 | 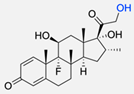 | (i) MsCl, N2 atmosphere, Pyridine, rt (ii) N2 atmosphere, DMF, 65 °C (2 h) 62% yield | [83] | |
| 13 | 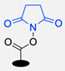 | NaHCO3, rt (1.5 h) Undisclosed yield | [82] | |
| 14 | 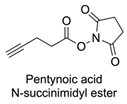 | 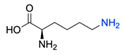 | DCM, rt (2 h) 97% yield | [78] |
| 15 | 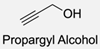 | 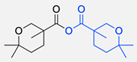 | DMAP, DOWEX H+, MeOH, Pyridine 95% yield | [79,80,81] |
| 16 | 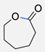 | Sn(Oct)2, 100 °C (18 h) Undisclosed yield | [59] | |
| 17 | 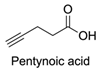 |  | EDC, DMAP, DCM, rt 84% yield | [52,53] |
| 18 |  | DIPEA, HBTU, DMF, rt Undisclosed yield | [74] | |
| 19 | 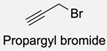 |  | NaH, THF, N2 atmosphere, rt (2 h)–70 °C (6 h) 88% yield | [77] |
| 20 | 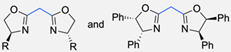 | BuLi, THF, −10 °C (3 h) 66% yield | [75] | |
| 21 | 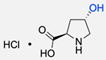 | NaH, THF, 0 °C (2 h)–rt (18 h) 57% yield | [76] | |
| Hydrophilic Block Polymer | Hydrophobic Block Polymer | Ligand | Main Achievements | Ref. |
|---|---|---|---|---|
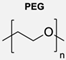
 |  | Enzymes: CalB, GOx, and HRP | Functionalised polymersomes increased the local concentration of enzymes, leading to higher reaction rates, making it possible to remove catalytical enzyme species in one single step. | [52,53] |
 |  | Peptides GRGDSP and PR_b | Polymersomes functionalised with the peptides were more effective in delivering doxorubicin to colon cancer cells than “naked” polymersomes. The functionalisation allowed for precise targeting, which is crucial for minimising off-target effects and maximising therapeutic efficacy. | [74] |
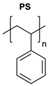
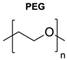 |  | Copper-bis(oxazoline) complexes | The hydrophobic layer of polymersomes allowed for the immobilisation of the metal complex, making the reaction possible to occur in an aqueous media instead of an organic solvent. | [75] |
 | 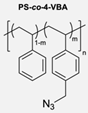 | L-Proline catalyst | The hydrophobic layer of polymersomes allowed for the immobilisation of the catalyst, making the reaction possible to occur in an aqueous media instead of an organic solvent and therefore improving the yield, diastereoselectivity, and enantioselectivity. | [76] |
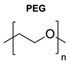 |  | Sugars: Fucose and Glucose | The functionalisation of the polymersomes with D-glucoside allowed for better binding and affinity to their lectins (carbohydrate-binding proteins), proving to be a valuable strategy for targeted drug delivery. | [77] |
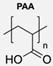 | 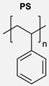 | Fluorescent dansyl probe, biotin ligands, and EGFP | This work proved that functionalisation of the outer membrane of polymersomes is possible. | [78] |
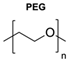 |  | Dendrons | The dendritic architecture allowed for the conjugation of multiple functional groups, such as chromophores and biologically relevant ligands, increasing the versatility of polymersomes. | [79] |
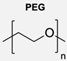 | 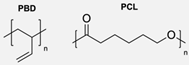 | Dendrons | Surface functionalisation of polymersomes with dendritic groups offered a valuable framework for controlling their biological properties without significantly affecting their physical characteristics, such as size and stability. | [80] |
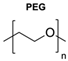 | 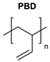 | Dendrons | With this study, an ideal percentage for azide polymer in polymersome vesicles was determined. Also, it was determined that the presence of the dendron group did not alter the polymersome morphology. | [81] |
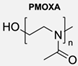 | 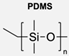 | Dyes and PAN membranes | Immobilisation of polymersomes on a planar solid structure was shown to be possible for production, usage, and handling. | [82] |
 |  | Dexamethasone | Dexamethasone-functionalised polymersomes proved to be more effective than “naked” particles in pancreatic cancer cells. | [83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saraiva, N.M.; Alves, A.; Costa, P.C.; Correia-da-Silva, M. Click Chemistry in Polymersome Technology. Pharmaceuticals 2024, 17, 747. https://doi.org/10.3390/ph17060747
Saraiva NM, Alves A, Costa PC, Correia-da-Silva M. Click Chemistry in Polymersome Technology. Pharmaceuticals. 2024; 17(6):747. https://doi.org/10.3390/ph17060747
Chicago/Turabian StyleSaraiva, Nuno M., Ana Alves, Paulo C. Costa, and Marta Correia-da-Silva. 2024. "Click Chemistry in Polymersome Technology" Pharmaceuticals 17, no. 6: 747. https://doi.org/10.3390/ph17060747
APA StyleSaraiva, N. M., Alves, A., Costa, P. C., & Correia-da-Silva, M. (2024). Click Chemistry in Polymersome Technology. Pharmaceuticals, 17(6), 747. https://doi.org/10.3390/ph17060747








