Therapeutic Use of G4-Ligands in Cancer: State-of-the-Art and Future Perspectives
Abstract
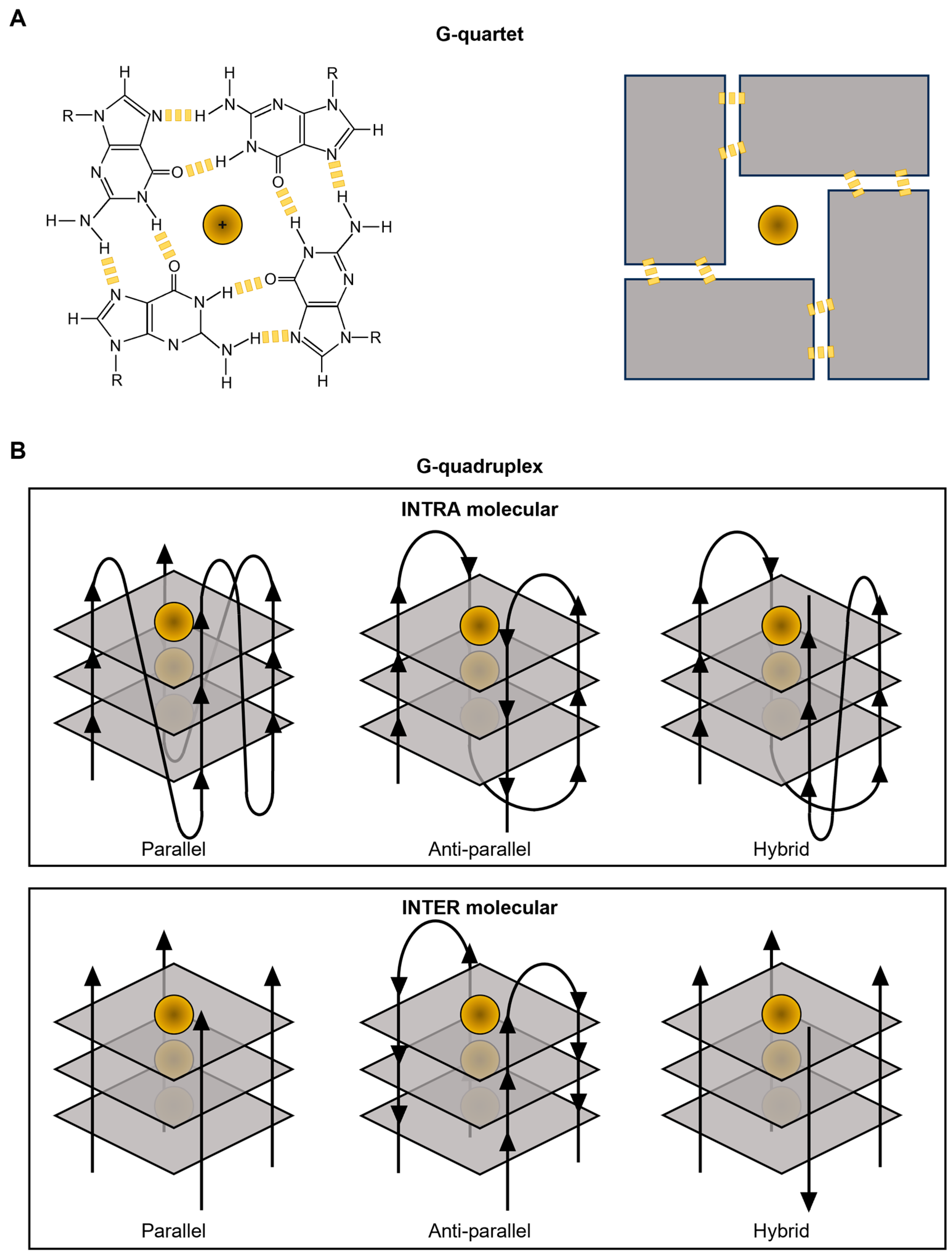
:1. G4s Are Non-Canonical Secondary Structures Spontaneously Originating from G-Rich Sequences of Nucleic Acids
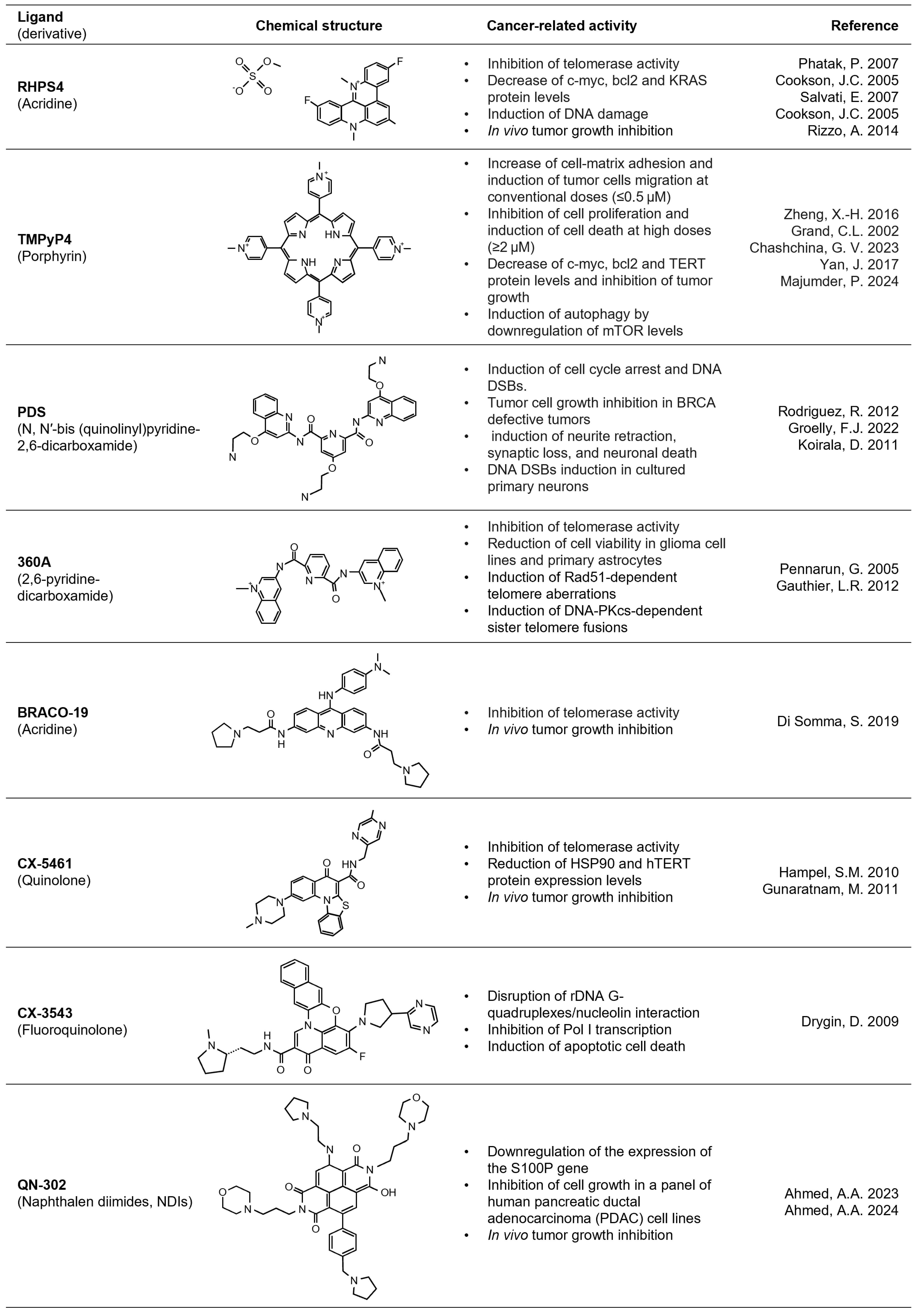
2. Therapeutic Relevance of G4 Structures in Cancer
3. Limitations of G4-Based Antitumoral Therapies
4. Optimization of G4 Ligands for Therapeutic Use in Cancer: Novel Strategies and Future Perspectives
5. Conclusive Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Watson, J.D.; Crick, F.H. Molecular Structure of Nucleic Acids; a Structure for Deoxyribose Nucleic Acid. Nature 1953, 171, 737–738. [Google Scholar] [CrossRef]
- Ghosh, A.; Bansal, M. A Glossary of DNA Structures from A to Z. Acta Crystallogr. Sect. D Biol. Crystallogr. 2003, 59, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Burge, S.; Parkinson, G.N.; Hazel, P.; Todd, A.K.; Neidle, S. Quadruplex DNA: Sequence, Topology and Structure. Nucleic Acids Res. 2006, 34, 5402–5415. [Google Scholar] [CrossRef]
- Biffi, G.; Tannahill, D.; McCafferty, J.; Balasubramanian, S. Quantitative Visualization of DNA G-Quadruplex Structures in Human Cells. Nat. Chem. 2013, 5, 182–186. [Google Scholar] [CrossRef]
- Umar, M.I.; Ji, D.; Chan, C.-Y.; Kwok, C.K. G-Quadruplex-Based Fluorescent Turn-On Ligands and Aptamers: From Development to Applications. Molecules 2019, 24, 2416. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Hoque, M.E.; Mao, H. Single-Molecule Investigations of G-Quadruplex. In G-Quadruplex Nucleic Acids. Methods in Molecular Biology; Humana: New York, NY, USA, 2019. [Google Scholar]
- Di Antonio, M.; Ponjavic, A.; Radzevičius, A.; Ranasinghe, R.T.; Catalano, M.; Zhang, X.; Shen, J.; Needham, L.-M.; Lee, S.F.; Klenerman, D.; et al. Single-Molecule Visualization of DNA G-Quadruplex Formation in Live Cells. Nat. Chem. 2020, 12, 832–837. [Google Scholar] [CrossRef]
- Tran, P.L.T.; Mergny, J.-L.; Alberti, P. Stability of Telomeric G-Quadruplexes. Nucleic Acids Res. 2011, 39, 3282–3294. [Google Scholar] [CrossRef]
- Bryan, T.M. G-Quadruplexes at Telomeres: Friend or Foe? Molecules 2020, 25, 3686. [Google Scholar] [CrossRef] [PubMed]
- Akincilar, S.C.; Unal, B.; Tergaonkar, V. Reactivation of Telomerase in Cancer. Cell. Mol. Life Sci. 2016, 73, 1659–1670. [Google Scholar] [CrossRef]
- Figueiredo, J.; Mergny, J.-L.; Cruz, C. G-Quadruplex Ligands in Cancer Therapy: Progress, Challenges, and Clinical Perspectives. Life Sci. 2024, 340, 122481. [Google Scholar] [CrossRef]
- Hänsel-Hertsch, R.; Di Antonio, M.; Balasubramanian, S. DNA G-Quadruplexes in the Human Genome: Detection, Functions and Therapeutic Potential. Nat. Rev. Mol. Cell Biol. 2017, 18, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, A.; Roy, S.S.; Chowdhury, S. Non-Duplex G-Quadruplex DNA Structure: A Developing Story from Predicted Sequences to DNA Structure-Dependent Epigenetics and Beyond. Acc. Chem. Res. 2021, 54, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhou, J. G-Quadruplexes from Non-Coding RNAs. J. Mol. Med. 2023, 101, 621–635. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-Y.; Joshi, M.; Wang, A.; Myong, S. 5′UTR G-Quadruplex Structure Enhances Translation in Size Dependent Manner. Nat. Commun. 2024, 15, 3963. [Google Scholar] [CrossRef] [PubMed]
- Falabella, M.; Fernandez, R.J.; Johnson, F.B.; Kaufman, B.A. Potential Roles for G-Quadruplexes in Mitochondria. Curr. Med. Chem. 2019, 26, 2918–2932. [Google Scholar] [CrossRef] [PubMed]
- Varshney, D.; Cuesta, S.M.; Herdy, B.; Abdullah, U.B.; Tannahill, D.; Balasubramanian, S. RNA G-Quadruplex Structures Control Ribosomal Protein Production. Sci. Rep. 2021, 11, 22735. [Google Scholar] [CrossRef] [PubMed]
- Datta, A.; Pollock, K.J.; Kormuth, K.A.; Brosh, R.M., Jr. G-Quadruplex Assembly by Ribosomal DNA: Emerging Roles in Disease Pathogenesis and Cancer Biology. Cytogenet. Genome Res. 2021, 161, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Hänsel-Hertsch, R.; Beraldi, D.; Lensing, S.V.; Marsico, G.; Zyner, K.; Parry, A.; Di Antonio, M.; Pike, J.; Kimura, H.; Narita, M.; et al. G-Quadruplex Structures Mark Human Regulatory Chromatin. Nat. Genet. 2016, 48, 1267–1272. [Google Scholar] [CrossRef] [PubMed]
- Amato, R.; Valenzuela, M.; Berardinelli, F.; Salvati, E.; Maresca, C.; Leone, S.; Antoccia, A.; Sgura, A. G-Quadruplex Stabilization Fuels the ALT Pathway in ALT-Positive Osteosarcoma Cells. Genes 2020, 11, 304. [Google Scholar] [CrossRef]
- Grand, C.L.; Han, H.; Muñoz, R.M.; Weitman, S.; Von Hoff, D.D.; Hurley, L.H.; Bearss, D.J. The Cationic Porphyrin TMPyP4 Down-Regulates c-MYC and Human Telomerase Reverse Transcriptase Expression and Inhibits Tumor Growth In Vivo. Mol. Cancer Ther. 2002, 1, 565–573. [Google Scholar]
- Bidzinska, J.; Cimino-Reale, G.; Zaffaroni, N.; Folini, M. G-Quadruplex Structures in the Human Genome as Novel Therapeutic Targets. Molecules 2013, 18, 12368–12395. [Google Scholar] [CrossRef] [PubMed]
- Majumder, P.; Shukla, C.; Arya, A.; Sharma, S.; Datta, B. G-Quadruplexes in MTOR and Induction of Autophagy. Sci. Rep. 2024, 14, 2525. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Di Antonio, M.; McKinney, S.; Mathew, V.; Ho, B.; O’Neil, N.J.; Dos Santos, N.; Silvester, J.; Wei, V.; Garcia, J.; et al. CX-5461 Is a DNA G-Quadruplex Stabilizer with Selective Lethality in BRCA1/2 Deficient Tumours. Nat. Commun. 2017, 8, 14432. [Google Scholar] [CrossRef] [PubMed]
- McLuckie, K.I.E.; Di Antonio, M.; Zecchini, H.; Xian, J.; Caldas, C.; Krippendorff, B.-F.; Tannahill, D.; Lowe, C.; Balasubramanian, S. G-Quadruplex DNA as a Molecular Target for Induced Synthetic Lethality in Cancer Cells. J. Am. Chem. Soc. 2013, 135, 9640–9643. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, M.; Cho, T.; Álvarez-Quilón, A.; Li, K.; Schellenberg, M.J.; Zimmermann, M.; Hustedt, N.; Rossi, S.E.; Adam, S.; Melo, H.; et al. A Genetic Map of the Response to DNA Damage in Human Cells. Cell 2020, 182, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Bossaert, M.; Pipier, A.; Riou, J.-F.; Noirot, C.; Nguyên, L.-T.; Serre, R.-F.; Bouchez, O.; Defrancq, E.; Calsou, P.; Britton, S.; et al. Transcription-Associated Topoisomerase 2α (TOP2A) Activity Is a Major Effector of Cytotoxicity Induced by G-Quadruplex Ligands. elife 2021, 10, e65184. [Google Scholar] [CrossRef] [PubMed]
- Phatak, P.; Cookson, J.C.; Dai, F.; Smith, V.; Gartenhaus, R.B.; Stevens, M.F.G.; Burger, A.M. Telomere Uncapping by the G-Quadruplex Ligand RHPS4 Inhibits Clonogenic Tumour Cell Growth In Vitro and In Vivo Consistent with a Cancer Stem Cell Targeting Mechanism. Br. J. Cancer 2007, 96, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- Cookson, J.C.; Dai, F.; Smith, V.; Heald, R.A.; Laughton, C.A.; Stevens, M.F.G.; Burger, A.M. Pharmacodynamics of the G-Quadruplex-Stabilizing Telomerase Inhibitor 3,11-Difluoro-6,8,13-Trimethyl-8H-Quino[4,3,2-Kl]Acridinium Methosulfate (RHPS4) in Vitro: Activity in Human Tumor Cells Correlates with Telomere Length and Can Be Enhanced, or Antagonized, with Cytotoxic Agents. Mol. Pharmacol. 2005, 68, 1551–1558. [Google Scholar] [CrossRef]
- Salvati, E.; Leonetti, C.; Rizzo, A.; Scarsella, M.; Mottolese, M.; Galati, R.; Sperduti, I.; Stevens, M.F.G.; D’Incalci, M.; Blasco, M.; et al. Telomere Damage Induced by the G-Quadruplex Ligand RHPS4 Has an Antitumor Effect. J. Clin. Investig. 2007, 117, 3236–3247. [Google Scholar] [CrossRef]
- Cookson, J.C.; Heald, R.A.; Stevens, M.F.G. Antitumor Polycyclic Acridines. 17. Synthesis and Pharmaceutical Profiles of Pentacyclic Acridinium Salts Designed To Destabilize Telomeric Integrity. J. Med. Chem. 2005, 48, 7198–7207. [Google Scholar] [CrossRef]
- Rizzo, A.; Iachettini, S.; Zizza, P.; Cingolani, C.; Porru, M.; Artuso, S.; Stevens, M.; Hummersone, M.; Biroccio, A.; Salvati, E.; et al. Identification of Novel RHPS4-Derivative Ligands with Improved Toxicological Profiles and Telomere-Targeting Activities. J. Exp. Clin. Cancer Res. 2014, 33, 81. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.-H.; Nie, X.; Liu, H.-Y.; Fang, Y.-M.; Zhao, Y.; Xia, L.-X. TMPyP4 Promotes Cancer Cell Migration at Low Doses, but Induces Cell Death at High Doses. Sci. Rep. 2016, 6, 26592. [Google Scholar] [CrossRef] [PubMed]
- Chashchina, G.V.; Tevonyan, L.L.; Beniaminov, A.D.; Kaluzhny, D.N. Taq-Polymerase Stop Assay to Determine Target Selectivity of G4 Ligands in Native Promoter Sequences of MYC, TERT, and KIT Oncogenes. Pharmaceuticals 2023, 16, 544. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zhao, D.; Dong, L.; Pan, S.; Hao, F.; Guan, Y. A Novel G-Quadruplex Motif in the Human MET Promoter Region. Biosci. Rep. 2017, 37, BSR20171128. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.; Miller, K.M.; Forment, J.V.; Bradshaw, C.R.; Nikan, M.; Britton, S.; Oelschlaegel, T.; Xhemalce, B.; Balasubramanian, S.; Jackson, S.P. Small-Molecule–Induced DNA Damage Identifies Alternative DNA Structures in Human Genes. Nat. Chem. Biol. 2012, 8, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Groelly, F.J.; Porru, M.; Zimmer, J.; Benainous, H.; De Visser, Y.; Kosova, A.A.; Di Vito, S.; Serra, V.; Ryan, A.; Leonetti, C.; et al. Anti-tumoural Activity of the G-quadruplex Ligand Pyridostatin against BRCA1/2-deficient Tumours. EMBO Mol. Med. 2022, 14, e14501. [Google Scholar] [CrossRef] [PubMed]
- Koirala, D.; Dhakal, S.; Ashbridge, B.; Sannohe, Y.; Rodriguez, R.; Sugiyama, H.; Balasubramanian, S.; Mao, H. A Single-Molecule Platform for Investigation of Interactions between G-Quadruplexes and Small-Molecule Ligands. Nat. Chem. 2011, 3, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Pennarun, G.; Granotier, C.; Gauthier, L.R.; Gomez, D.; Hoffschir, F.; Mandine, E.; Riou, J.-F.; Mergny, J.-L.; Mailliet, P.; Boussin, F.D. Apoptosis Related to Telomere Instability and Cell Cycle Alterations in Human Glioma Cells Treated by New Highly Selective G-Quadruplex Ligands. Oncogene 2005, 24, 2917–2928. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, L.R.; Granotier, C.; Hoffschir, F.; Etienne, O.; Ayouaz, A.; Desmaze, C.; Mailliet, P.; Biard, D.S.; Boussin, F.D. Rad51 and DNA-PKcs Are Involved in the Generation of Specific Telomere Aberrations Induced by the Quadruplex Ligand 360A That Impair Mitotic Cell Progression and Lead to Cell Death. Cell. Mol. Life Sci. 2012, 69, 629–640. [Google Scholar] [CrossRef]
- Di Somma, S.; Amato, J.; Iaccarino, N.; Pagano, B.; Randazzo, A.; Portella, G.; Malfitano, A.M. G-Quadruplex Binders Induce Immunogenic Cell Death Markers in Aggressive Breast Cancer Cells. Cancers 2019, 11, 1797. [Google Scholar] [CrossRef]
- Hampel, S.M.; Sidibe, A.; Gunaratnam, M.; Riou, J.-F.; Neidle, S. Tetrasubstituted Naphthalene Diimide Ligands with Selectivity for Telomeric G-Quadruplexes and Cancer Cells. Bioorg. Med. Chem. Lett. 2010, 20, 6459–6463. [Google Scholar] [CrossRef] [PubMed]
- Gunaratnam, M.; de la Fuente, M.; Hampel, S.M.; Todd, A.K.; Reszka, A.P.; Schätzlein, A.; Neidle, S. Targeting Pancreatic Cancer with a G-Quadruplex Ligand. Bioorg. Med. Chem. 2011, 19, 7151–7157. [Google Scholar] [CrossRef] [PubMed]
- Drygin, D.; Siddiqui-Jain, A.; O’Brien, S.; Schwaebe, M.; Lin, A.; Bliesath, J.; Ho, C.B.; Proffitt, C.; Trent, K.; Whitten, J.P.; et al. Anticancer Activity of CX-3543: A Direct Inhibitor of RRNA Biogenesis. Cancer Res. 2009, 69, 7653–7661. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Greenhalf, W.; Palmer, D.H.; Williams, N.; Worthington, J.; Arshad, T.; Haider, S.; Alexandrou, E.; Guneri, D.; Waller, Z.A.E.; et al. The Potent G-Quadruplex-Binding Compound QN-302 Downregulates S100P Gene Expression in Cells and in an In Vivo Model of Pancreatic Cancer. Molecules 2023, 28, 2452. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.A.; Chen, S.; Roman-Escorza, M.; Angell, R.; Oxenford, S.; McConville, M.; Barton, N.; Sunose, M.; Neidle, D.; Haider, S.; et al. Structure–Activity Relationships for the G-Quadruplex-Targeting Experimental Drug QN-302 and Two Analogues Probed with Comparative Transcriptome Profiling and Molecular Modeling. Sci. Rep. 2024, 14, 3447. [Google Scholar] [CrossRef] [PubMed]
- Manaia, M.N.; Chiorcea-Paquim, A.-M. Cationic Porphyrin TMPyP4 Redox Behaviour and Interaction with Nucleic Acids: Towards a New Methodology for Screening Porphyrin-Based Anticancer Drugs. Electrochim. Acta 2023, 462, 142749. [Google Scholar] [CrossRef]
- Taetz, S.; Baldes, C.; Mürdter, T.E.; Kleideiter, E.; Piotrowska, K.; Bock, U.; Haltner-Ukomadu, E.; Mueller, J.; Huwer, H.; Schaefer, U.F.; et al. Biopharmaceutical Characterization of the Telomerase Inhibitor BRACO19. Pharm. Res. 2006, 23, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-J.; Wu, Y.-L.; Tanaka, Y.; Zhang, W. Small Molecules Targeting C-Myc Oncogene: Promising Anti-Cancer Therapeutics. Int. J. Biol. Sci. 2014, 10, 1084. [Google Scholar] [CrossRef]
- Sanchez-Martin, V.; Soriano, M.; Garcia-Salcedo, J.A. Quadruplex Ligands in Cancer Therapy. Cancers 2021, 13, 3156. [Google Scholar] [CrossRef]
- Sullivan, H.-J.; Chen, B.; Wu, C. Molecular Dynamics Study on the Binding of an Anticancer DNA G-Quadruplex Stabilizer, CX-5461, to Human Telomeric, c-KIT1, and c-Myc G-Quadruplexes and a DNA Duplex. J. Chem. Inf. Model. 2020, 60, 5203–5224. [Google Scholar] [CrossRef]
- Drygin, D.; Lin, A.; Bliesath, J.; Ho, C.B.; O’Brien, S.E.; Proffitt, C.; Omori, M.; Haddach, M.; Schwaebe, M.K.; Siddiqui-Jain, A.; et al. Targeting RNA Polymerase I with an Oral Small Molecule CX-5461 Inhibits Ribosomal RNA Synthesis and Solid Tumor Growth. Cancer Res. 2011, 71, 1418–1430. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, J.; Tacconi, E.M.C.; Folio, C.; Badie, S.; Porru, M.; Klare, K.; Tumiati, M.; Markkanen, E.; Halder, S.; Ryan, A.; et al. Targeting BRCA1 and BRCA2 Deficiencies with G-Quadruplex-Interacting Compounds. Mol. Cell 2016, 61, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.A.; Angell, R.; Oxenford, S.; Worthington, J.; Williams, N.; Barton, N.; Fowler, T.G.; O’Flynn, D.E.; Sunose, M.; McConville, M.; et al. Asymmetrically Substituted Quadruplex-Binding Naphthalene Diimide Showing Potent Activity in Pancreatic Cancer Models. ACS Med. Chem. Lett. 2020, 11, 1634–1644. [Google Scholar] [CrossRef] [PubMed]
- Biver, T. Discriminating between Parallel, Anti-Parallel and Hybrid G-Quadruplexes: Mechanistic Details on Their Binding to Small Molecules. Molecules 2022, 27, 4165. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Dickerhoff, J.; Sakai, S.; Yang, D. DNA G-Quadruplex in Human Telomeres and Oncogene Promoters: Structures, Functions, and Small Molecule Targeting. Acc. Chem. Res. 2022, 55, 2628–2646. [Google Scholar] [CrossRef] [PubMed]
- Kench, T.; Rakers, V.; Bouzada, D.; Gomez-González, J.; Robinson, J.; Kuimova, M.K.; Vázquez López, M.; Vázquez, M.E.; Vilar, R. Dimeric Metal-Salphen Complexes Which Target Multimeric G-Quadruplex DNA. Bioconjug. Chem. 2023, 34, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, S.; Razzaq, M.; Parveen, N.; Ghosh, A.; Kim, K.K. The Effect of Hairpin Loop on the Structure and Gene Expression Activity of the Long-Loop G-Quadruplex. Nucleic Acids Res. 2021, 49, 10689–10706. [Google Scholar] [CrossRef]
- Cadoni, E.; De Paepe, L.; Manicardi, A.; Madder, A. Beyond Small Molecules: Targeting G-Quadruplex Structures with Oligonucleotides and Their Analogues. Nucleic Acids Res. 2021, 49, 6638–6659. [Google Scholar] [CrossRef] [PubMed]
- Cadoni, E.; De Paepe, L.; Colpaert, G.; Tack, R.; Waegeman, D.; Manicardi, A.; Madder, A. A Red Light-Triggered Chemical Tool for Sequence-Specific Alkylation of G-Quadruplex and I-Motif DNA. Nucleic Acids Res. 2023, 51, 4112–4125. [Google Scholar] [CrossRef]
- Nele, V.; Campani, V.; Alia Moosavian, S.; De Rosa, G. Lipid Nanoparticles for RNA Delivery: Self-Assembling vs Driven-Assembling Strategies. Adv. Drug Deliv. Rev. 2024, 208, 115291. [Google Scholar] [CrossRef]
- Nele, V.; D’Aria, F.; Campani, V.; Silvestri, T.; Biondi, M.; Giancola, C.; De Rosa, G. Unravelling the Role of Lipid Composition on Liposome-Protein Interactions. J. Liposome Res. 2024, 34, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Nayak, P.; Bentivoglio, V.; Varani, M.; Signore, A. Three-Dimensional In Vitro Tumor Spheroid Models for Evaluation of Anticancer Therapy: Recent Updates. Cancers 2023, 15, 4846. [Google Scholar] [CrossRef] [PubMed]
- Foglizzo, V.; Cocco, E.; Marchiò, S. Advanced Cellular Models for Preclinical Drug Testing: From 2D Cultures to Organ-on-a-Chip Technology. Cancers 2022, 14, 4846. [Google Scholar] [CrossRef] [PubMed]
- Decarli, M.C.; Mizukami, A.; Azoubel, R.A.; Neto, P.I.; Mota, C.; Moraes, Â.M.; Silva, J.V.L.; Moroni, L. Static Systems to Obtain 3D Spheroid Cell Models: A Cost Analysis Comparing the Implementation of Four Types of Microwell Array Inserts. Biochem. Eng. J. 2022, 182, 108414. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iachettini, S.; Biroccio, A.; Zizza, P. Therapeutic Use of G4-Ligands in Cancer: State-of-the-Art and Future Perspectives. Pharmaceuticals 2024, 17, 771. https://doi.org/10.3390/ph17060771
Iachettini S, Biroccio A, Zizza P. Therapeutic Use of G4-Ligands in Cancer: State-of-the-Art and Future Perspectives. Pharmaceuticals. 2024; 17(6):771. https://doi.org/10.3390/ph17060771
Chicago/Turabian StyleIachettini, Sara, Annamaria Biroccio, and Pasquale Zizza. 2024. "Therapeutic Use of G4-Ligands in Cancer: State-of-the-Art and Future Perspectives" Pharmaceuticals 17, no. 6: 771. https://doi.org/10.3390/ph17060771




