The Influence of an Acute Administration of Cannabidiol or Rivastigmine, Alone and in Combination, on Scopolamine-Provoked Memory Impairment in the Passive Avoidance Test in Mice
Abstract
1. Introduction
2. Results
2.1. The Influence of an Acute Injection of CBD, Rivastigmine, or Scopolamine on Long-Term Memory in Mice in the PA Test
Acquisition, Consolidation, and Retrieval of Memory
- Long-term memory acquisition [F(9.87) = 24.21; p < 0.0001];
- Long-term memory consolidation [F(9.80) = 16.01; p < 0.0001];
- Long-term memory retrieval [F(9.82) = 16.00; p < 0.0001].
- Acquisition for memory—(p < 0.01 for CBD (30 mg/kg); p < 0.001 for rivastigmine (1 and 2.5 mg/kg));
- Consolidation for memory—(p < 0.01 for CBD (30 mg/kg); p < 0.01 for rivastigmine (1 mg/kg); p < 0.001 for rivastigmine (2.5 mg/kg));
- Retrieval for memory—(p < 0.001 for CBD (30 mg/kg); p < 0.05 for rivastigmine (1 mg/kg); (p < 0.001 for rivastigmine (2.5 mg/kg)).
- Acquisition of memory—(p < 0.05 for dose of 0.3 mg/kg and p < 0.001 for dose of 1 mg/kg);
- Consolidation of memory—(p < 0.01 for dose of 1 mg/kg);
- Retrieval of memory—(p < 0.01 for dose of 0.3 mg/kg and p < 0.001 for dose of 1 mg/kg).
2.2. The Influence of the Administration of CBD or/and Rivastigmine on the Memory Impairment Provoked by an Acute Administration of Scopolamine in the PA Test in Mice
Acquisition, Consolidation, and Retrieval of Memory
- Acquisition of memory—caused by pretreatment [F(3.69) = 17.85; p < 0.0001], as well as a statistically significant effect caused by interactions [F(3.69) = 3.471; p = 0.0207], but there are no statistically significant effects caused by treatment [F(1.69) = 0.007856; p = 0.9296].
- Consolidation of memory—caused by pretreatment [F(3.64) = 19.82; p < 0.0001], as well as a statistically significant effect caused by interactions [F(3.64) = 2.924; p = 0.0405], but there are no statistically significant effects caused by treatment [F(1.64) = 0.1437; p = 0.7059].
- Retrieval of memory—caused by pretreatment [F(3.60) = 20.75; p < 0.0001], but there are no statistically significant effects caused by treatment [F(1.60) = 0.3279; p = 0.5690] and no statistically significant effects caused by interactions [F(3.60) = 1.656; p = 0.1860].
- Acquisition of memory—scopolamine (p < 0.01);
- Consolidation of memory—scopolamine (p < 0.01);
- Retrieval of memory—scopolamine (p < 0.05).
- Acquisition of memory—(p < 0.001 for vehicle/rivastigmine (0.5 mg/kg)/scopolamine (1 mg/kg) group and for CBD (1 mg/kg)/vehicle/scopolamine (1 mg/kg) group);
- Consolidation of memory (p < 0.001 for vehicle/rivastigmine (0.5 mg/kg)/scopolamine (1 mg/kg) group and p < 0.05 for CBD (1 mg/kg)/vehicle/scopolamine (1 mg/kg) group);
- Retrieval of memory—(p < 0.001 for vehicle/rivastigmine (0.5 mg/kg)/scopolamine (1 mg/kg) group and p < 0.05 for CBD (1 mg/kg)/vehicle/scopolamine (1 mg/kg) group).
- Acquisition of memory—(p < 0.001 in comparison to the CBD (1 mg/kg)/vehicle/scopolamine (1 mg/kg)-treated group and p < 0.01 in comparison to the vehicle/rivastigmine (0.5 mg/kg)/scopolamine (1 mg/kg)-treated group);
- Consolidation of memory—(p < 0.001 in comparison to the CBD (1 mg/kg)/vehicle/scopolamine (1 mg/kg)-treated group and p < 0.05 in comparison to the vehicle/rivastigmine (0.5 mg/kg)/scopolamine (1 mg/kg)-treated group);
- Retrieval of memory—(p < 0.001 in comparison to the CBD (1 mg/kg)/vehicle/scopolamine (1 mg/kg)-treated group and p < 0.01 in comparison to the vehicle/rivastigmine (0.5 mg/kg)/scopolamine (1 mg/kg)-treated group (Figure 2A–C, appropriately)).
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. The Compounds Which Were Tested
- CBD (1 mg/kg) (Tocris Bioscience a Bio-Techne Brand, Biotechne, Warsaw, Poland)—a CB receptor ligand;
- Rivastigmine (0.5 mg/kg) (Tocris Bioscience a Bio-Techne Brand, Biotechne, Warsaw, Poland)—an AChEI;
- Scopolamine (1 mg/kg) (Tocris Bioscience a Bio-Techne Brand, Biotechne, Warsaw, Poland)—a cholinergic muscarinic receptor antagonist.
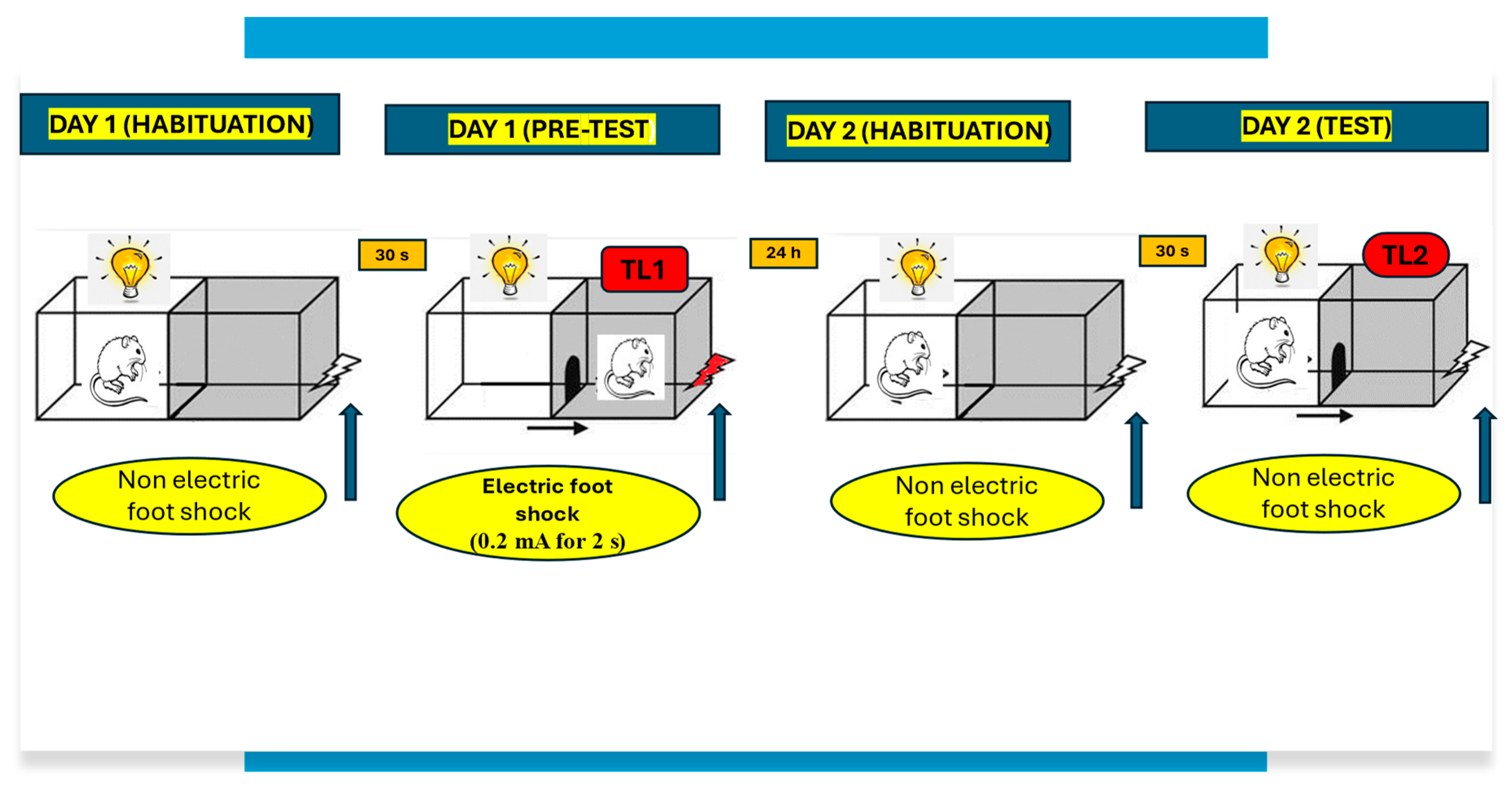
4.3. Experimental Procedure
- TL1—the time taken to enter the dark compartment during the training;
- TL2—the time taken to re-enter the dark compartment during the retention [55].
4.4. Treatment
4.5. Statistical Analysis
5. Conclusions
- Single, acute administration of CBD (1 mg/kg; ip) significantly affected changes in scopolamine-induced disturbances in memory acquisition and consolidation.
- Single, acute administration of rivastigmine (0.5 mg/kg; ip) significantly affected changes in scopolamine-induced disturbances in memory acquisition.
- Co-administration of non-effective, in the PA test, doses of CBD (1 mg/kg; ip) and rivastigmine (0.5 mg/kg; ip) attenuated, more significantly than single administration of the used drugs, memory impairment provoked by scopolamine (1 mg/kg; ip) injection in the PA test in mice.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alzheimer’s Association. 2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 2022, 18, 700–789. [Google Scholar] [CrossRef]
- Peng, Y.; Jin, H.; Xue, Y.H.; Chen, Q.; Yao, S.Y.; Du, M.Q.; Liu, S. Current and future therapeutic strategies for Alzheimer’s disease: An overview of drug development bottlenecks. Front. Aging Neurosci. 2023, 5, 1206572. [Google Scholar] [CrossRef]
- Alexander, N.; Alexander, D.C.; Barkhof, F.; Denaxas, S. Identifying and evaluating clinical subtypes of Alzheimer’s disease in care electronic health records using unsupervised machine learning. BMC Med. Inform. Decis. Mak. 2021, 21, 343. [Google Scholar] [CrossRef]
- Meftah, S.; Gan, J. Alzheimer’s disease as a synaptopathy: Evidence for dysfunction of synapses during disease progression. Front. Synaptic. Neurosci. 2023, 15, 1129036. [Google Scholar] [CrossRef]
- Russo, E.B. Cannabis Therapeutics and the Future of Neurology. Front. Integrat. Neurosci. 2018, 12, 51. [Google Scholar] [CrossRef]
- Bedse, G.; Di Domenico, F.; Serviddio, G.; Cassano, T. Aberrant insulin signaling in Alzheimer’s disease: Current knowledge. Front. Neurosci. 2015, 9, 204. [Google Scholar] [CrossRef]
- Geldenhuys, W.J.; Van der Schyf, C.J. Role of serotonin in Alzheimer’s disease: A new therapeutic target? CNS Drugs 2011, 25, 765–781. [Google Scholar] [CrossRef]
- Alexander, S.P.H. Therapeutic potential of cannabis-related drugs. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 64, 157–166. [Google Scholar] [CrossRef]
- Ahmed, A.; van der Marck, M.A.; van den Elsen, G.; Olde Rikkert, M. Cannabinoids in late-onset Alzheimer’s disease. Clin. Pharm. Therap. 2015, 97, 597–606. [Google Scholar] [CrossRef]
- Aso, E.; Sánchez-Pla, A.; Vegas-Lozano, E.; Maldonado, R.; Ferrer, I. Cannabis-based medicine reduces multiple pathological processes in AβPP/PS1 mice. J. Alzheimers Dis. 2015, 43, 977–991. [Google Scholar] [CrossRef]
- Kruk-Slomka, M.; Dzik, A.; Biala, G. The Influence of CB2-Receptor Ligands on the Memory-Related Responses in Connection with Cholinergic Pathways in Mice in the Passive Avoidance Test. Molecules 2022, 27, 4252. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, K.L.C.; Dos Santos Alcântara, M.G.; de Aquino, T.M.; da Silva-Júnior, E.F. Cannabinoid pharmacology and its therapeutic uses in Alzheimer’s disease. Neural. Regen. Res. 2021, 16, 990–991. [Google Scholar] [PubMed]
- Morena, M.; Campolongo, P. The endocannabinoid system: An emotional buffer in the modulation of memory function. Neurobiol. Learn. Mem. 2014, 112, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Lim, C.S. Understanding the Modulatory Effects of Cannabidiol on Alzheimer’s Disease. Brain Sci. 2021, 11, 1211. [Google Scholar] [CrossRef] [PubMed]
- Renner, U.D.; Oertel, R.; Kirch, W. Pharmacokinetics and pharmacodynamics of clinical use of scopolamine. Ther. Drug Monit. 2005, 27, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Yoon, W.; Choi, H.; Kim, J.; Park, J.; Kang, M.; Bae, S.; Lee, Y.; Choi, Y.; Kim, K.; Jung, Y.S.; et al. Comparison of scopolamine-induced cognitive impairment responses in three different ICR stocks. Lab. Anim. Res. 2018, 34, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Khoury, R.; Rajamanickam, J.; Grossberg, G.T. An update on the safety of current therapies for Alzheimer’s disease: Focus on rivastigmine. Ther. Adv. Drug. Saf. 2017, 9, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Bejar, C.; Wang, R.H.; Weinstock, M. Effect of rivastigmine on scopolamine-induced memory impairment in rats. Eur. J. Pharmacol. 1999, 383, 231–240. [Google Scholar] [CrossRef]
- Feldman, H.H.; Lane, R. Study 304 Group. Rivastigmine: A placebo controlled trial of twice daily and three times daily regimens in patients with Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2007, 78, 1056–1063. [Google Scholar] [CrossRef]
- Doraiswamy, P.M.; Krishnan, K.R.; Anand, R.; Sohn, H.; Danyluk, J.; Hartman, R.D.; Veach, J. Long-term effects of rivastigmine in moderately severe Alzheimer’s disease: Does early initiation of therapy offer sustained benefits? Prog. Neuropsychopharmacol. Biol. Psychiatry 2002, 26, 705–712. [Google Scholar] [CrossRef]
- Birks, J.S.; Chong, L.Y.; Grimley, E.J. Rivastigmine for Alzheimer’s disease. Cochrane Database Syst Rev. 2015, 9, CD00119. [Google Scholar]
- Cheng, D.; Low, J.K.; Logge, W.; Garner, B.; Karl, T. Chronic cannabidiol treatment improves social and object recognition in double transgenic APPswe/PS1∆E9 mice. Psychopharmacology 2014, 231, 3009–3017. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Cilio, M.R.; Cross, H.; Fernandez-Ruiz, J.; French, J.; Hill, C.; Katz, R.; Di Marzo, V.; Jutras-Aswad, D.; Notcutt, W.G.; et al. Cannabidiol: Pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia 2014, 55, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Hotz, J.; Fehlmann, B.; Papassotiropoulos, A.; de Quervain, D.J.F.; Schicktanz, N.S. Cannabidiol enhances verbal episodic memory in healthy young participants: A randomized clinical trial. J. Psychiatry Res. 2021, 143, 327–333. [Google Scholar] [CrossRef]
- Kruk-Slomka, M.; Biala, G. CB1 receptors in the formation of the different phases of memory-related processes in the inhibitory avoidance test in mice. Behav. Brain Res. 2016, 301, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Kruk-Slomka, M.; Biala, G. Cannabidiol Attenuates MK-801-Induced Cognitive Symptoms of Schizophrenia in the Passive Avoidance Test in Mice. Molecules 2021, 26, 5977. [Google Scholar] [CrossRef] [PubMed]
- Niloy, N.; Hediyal, T.A.; Vichitra, C.; Sonali, S.; Chidambaram, S.B.; Gorantla, V.R.; Mahalakshmi, A.M. Effect of Cannabis on Memory Consolidation, Learning and Retrieval and Its Current Legal Status in India: A Review. Biomolecules. 2023, 13, 162. [Google Scholar] [CrossRef] [PubMed]
- Schubart, C.D.; Sommer, I.E.; van Gastel, W.A.; Goetgebuer, R.L.; Kahn, R.S.; Boks, M.P. Cannabis with high cannabidiol content is associated with fewer psychotic experiences. Schizophr. Res. 2011, 130, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, S.; Kolishetti, N.; Arias, A.Y.; Vashist, A.; Nair, M. Cannabidiol for neurodegenerative disorders: A comprehensive review. Front. Pharmacol. 2022, 13, 989717. [Google Scholar] [CrossRef]
- Cooray, R.; Gupta, V.; Suphioglu, C. Current Aspects of the Endocannabinoid System and Targeted THC and CBD Phytocannabinoids as Potential Therapeutics for Parkinson’s and Alzheimer’s Diseases: A Review. Mol. Neurobiol. 2020, 57, 4878–4890. [Google Scholar] [CrossRef]
- Drożak, P.; Skrobas, U.; Drożak, M. Cannabidiol in the Treatment and Prevention of Alzheimer’s Disease—A Comprehensive Overview of in Vitro and in Vivo Studies. J. Educ. Health Sport. 2022, 12, 834–845. [Google Scholar] [CrossRef]
- Voicu, V.; Brehar, F.-M.; Toader, C.; Covache-Busuioc, R.-A.; Corlatescu, A.D.; Bordeianu, A.; Costin, H.P.; Bratu, B.-G.; Glavan, L.-A.; Ciurea, A.V. Cannabinoids in Medicine: A Multifaceted Exploration of Types, Therapeutic Applications, and Emerging Opportunities in Neurodegenerative Diseases and Cancer Therapy. Biomolecules 2023, 13, 1388. [Google Scholar] [CrossRef]
- Cheng, D.; Spiro, A.S.; Jenner, A.M.; Garner, B.; Karl, T. Long-term cannabidiol treatment prevents the development of social recognition memory deficits in Alzheimer’s disease transgenic mice. J. Alzheimers Dis. 2014, 42, 1383–1396. [Google Scholar] [CrossRef]
- Coles, M.; Watt, G.; Kreilaus, F.; Karl, T. Medium-dose chronic cannabidiol treatment reverses object recognition memory deficits of APP Swe /PS1ΔE9 transgenic female mice. Front. Pharmacol. 2020, 11, 587604. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Scuderi, C.; Savani, C.; Steardo, L., Jr.; De Filippis, D.; Cottone, P.; Iuvone, T.; Cuomo, V.; Steardo, L. Cannabidiol in vivo blunts beta-amyloid induced neuroinflammation by suppressing IL-1beta and iNOS expression. Br. J. Pharmacol. 2007, 151, 1272–1279. [Google Scholar] [CrossRef]
- Esposito, G.; Scuderi, C.; Valenza, M.; Togna, G.I.; Latina, V.; De Filippis, D.; Cipriano, M.; Carratù, M.R.; Iuvone, T.; Steardo, L. Cannabidiol reduces Aβ-induced neuroinflammation and promotes hippocampal neurogenesis through PPARγ involvement. PLoS ONE 2011, 6, e28668. [Google Scholar] [CrossRef] [PubMed]
- Hao, F.; Feng, Y. Cannabidiol (CBD) enhanced the hippocampal immune response and autophagy of APP/PS1 Alzheimer’s mice uncovered by RNA-seq. Life Sci. 2021, 264, 118624. [Google Scholar] [CrossRef] [PubMed]
- Khodadadi, H.; Salles, É.L.; Jarrahi, A.; Costigliola, V.; Khan, M.B.; Yu, J.C.; Morgan, J.C.; Hess, D.C.; Vaibhav, K.; Dhandapani, K.M.; et al. Cannabidiol ameliorates cognitive function via regulation of IL-33 and TREM2 upregulation in a murine model of alzheimer’s disease. J. Alzheimers Dis. 2021, 80, 973–977. [Google Scholar] [CrossRef] [PubMed]
- Martín-Moreno, A.M.; Reigada, D.; Ramírez, B.G.; Mechoulam, R.; Innamorato, N.; Cuadrado, A.; Morgan, J.C.; Hess, D.C.; Vaibhav, K.; Dhandapani, K.M.; et al. Cannabidiol and other cannabinoids reduce microglial activation in vitro and in vivo: Relevance to alzheimer’s disease. Mol. Pharmacol. 2011, 79, 964. [Google Scholar] [CrossRef] [PubMed]
- Watt, G.; Shang, K.; Zieba, J.; Olaya, J.; Li, H.; Garner, B.; Karl, T. Chronic treatment with 50 mg/kg cannabidiol improves cognition and moderately reduces Aβ40 levels in 12-month-old male AβPPswe/PS1ΔE9 transgenic mice. J. Alzheimers Dis. 2020, 74, 937–950. [Google Scholar] [CrossRef]
- Kreilaus, F.; Przybyla, M.; Ittner, L.; Karl, T. Cannabidiol (CBD) treatment improves spatial memory in 14-month-old female TAU58/2 transgenic mice. Behav. Brain Res. 2022, 425, 113812. [Google Scholar] [CrossRef] [PubMed]
- Fagherazzi, E.V.; Garcia, V.A.; Maurmann, N.; Bervanger, T.; Halmenschlager, L.H.; Busato, S.B.; Hallak, J.E.; Zuardi, A.Z.; Crippa, J.A.; Schröder, N. Memory-rescuing effects of cannabidiol in an animal model of cognitive impairment relevant to neurodegenerative disorders. Psychopharmacology. 2012, 219, 1133–1140. [Google Scholar] [CrossRef]
- Karl, T.; Garner, B.; Cheng, D. The therapeutic potential of the phytocannabinoid cannabidiol for Alzheimer’s disease. Behav. Pharmacol. 2017, 28, 142–160. [Google Scholar] [CrossRef]
- Fadda, P.; Robinson, L.; Fratta, W.; Pertwee, R.G.; Riedel, G. Differential effects of THC- and CBD-rich cannabis-extracts on working memory in rats. Neuropahrmacology. 2004, 47, 1170–1179. [Google Scholar] [CrossRef]
- Fadda, P.; Robinson, L.; Fratta, W.; Pertwee, R.G.; Riedel, G. Scopolamine and MK801-induced working memory deficits in rats are not reversed by CBD-rich cannabis extracts. Behav. Brain Res. 2006, 168, 307–311. [Google Scholar] [CrossRef]
- Peres, F.F.; Diana, M.C.; Levin, R.; Suiama, M.A.; Almeida, V.; Vendramini, A.M.; Santos, C.M.; Zuardi, A.W.; Hallak, J.E.C.; Crippa, J.A.; et al. Cannabidiol Administered During Peri-Adolescence Prevents Behavioral Abnormalities in an Animal Model of Schizophrenia. Front. Pharmacol. 2018, 9, 901. [Google Scholar] [CrossRef]
- Ramírez, B.G.; Blázquez, C.; Pulgar, T.G.; Guzmán, M.; Ceballos, M.L. Prevention of alzheimer’s disease pathology by cannabinoids: Neuroprotection mediated by blockade of microglial activation. J. Neurosci. 2005, 25, 1904–1913. [Google Scholar] [CrossRef]
- Kumar, P.B.R.; Kumar, A.P.; Jose, J.A.; Prabitha, P.; Yuvaraj, S.; Chipurupalli, S.; Jeyarani, V.; Manisha, C.; Banerjee, S.; Jeyabalan, J.B.; et al. Minutes of PPAR-γ agonism and neuroprotection. Neurochem. Int. 2020, 140, 104814. [Google Scholar] [CrossRef]
- Giuliano, C.; Francavilla, M.; Ongari, G.; Petese, A.; Ghezzi, C.; Rossini, N.; Blandini, F.; Cerri, S. Neuroprotective and symptomatic effects of cannabidiol in an animal model of Parkinson’s disease. Int. J. Mol. Sci. 2021, 22, 8920. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.B.; Burnett, A.; Hall, B.; Parker, K.K. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem. Res. 2005, 30, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Balerio, G.N.; Aso, E.; Maldonado, R. Role of the cannabinoid system in the effects induced by nicotine on anxiety-like behaviour in mice. Psychopharmacology 2006, 184, 504–513. [Google Scholar] [CrossRef]
- Castañé, A.; Berrendero, F.; Maldonado, R. The role of the cannabinoid system in nicotine addiction. Pharmacol. Biochem. Behav. 2005, 81, 381–386. [Google Scholar] [CrossRef]
- Valjent, E.; Mitchell, J.M.; Besson, M.J.; Caboche, J.; Maldonado, R.; Le Fur, G.; Soubrie, P. Behavioral and biochemical evidence for interactions between delta-9-tetrahydrocannabinol and nicotine. Br. J. Pharmacol. 2002, 135, 564–578. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, Z.; Zuo, J.; Wu, C.; Zha, L.; Xu, Y.; Wang, S.; Shi, J.; Liu, X.H.; Zhang, J.; et al. Novel cannabidiol−carbamate hybrids as selective BuChE inhibitors: Docking-based fragment reassembly for the development of potential therapeutic agents against Alzheimer’s disease. Eur. J. Med. Chem. 2021, 223, 113735. [Google Scholar] [CrossRef]
- Chimakurthy, J.; Talasila, M. Effects of curcumin on pentylenetetrazole-induced anxiety-like behaviors and associated changes in cognition and monoamine levels. Psychol. Neurosci. 2010, 3, 239–244. [Google Scholar] [CrossRef]
- Kruk-Slomka, M.; Budzynska, B.; Biala, G. Involvement of cholinergic receptors in the different stages of memory measured in the modified elevated plus maze test in mice. Pharmacol. Rep. 2012, 64, 1066–1080. [Google Scholar] [CrossRef]
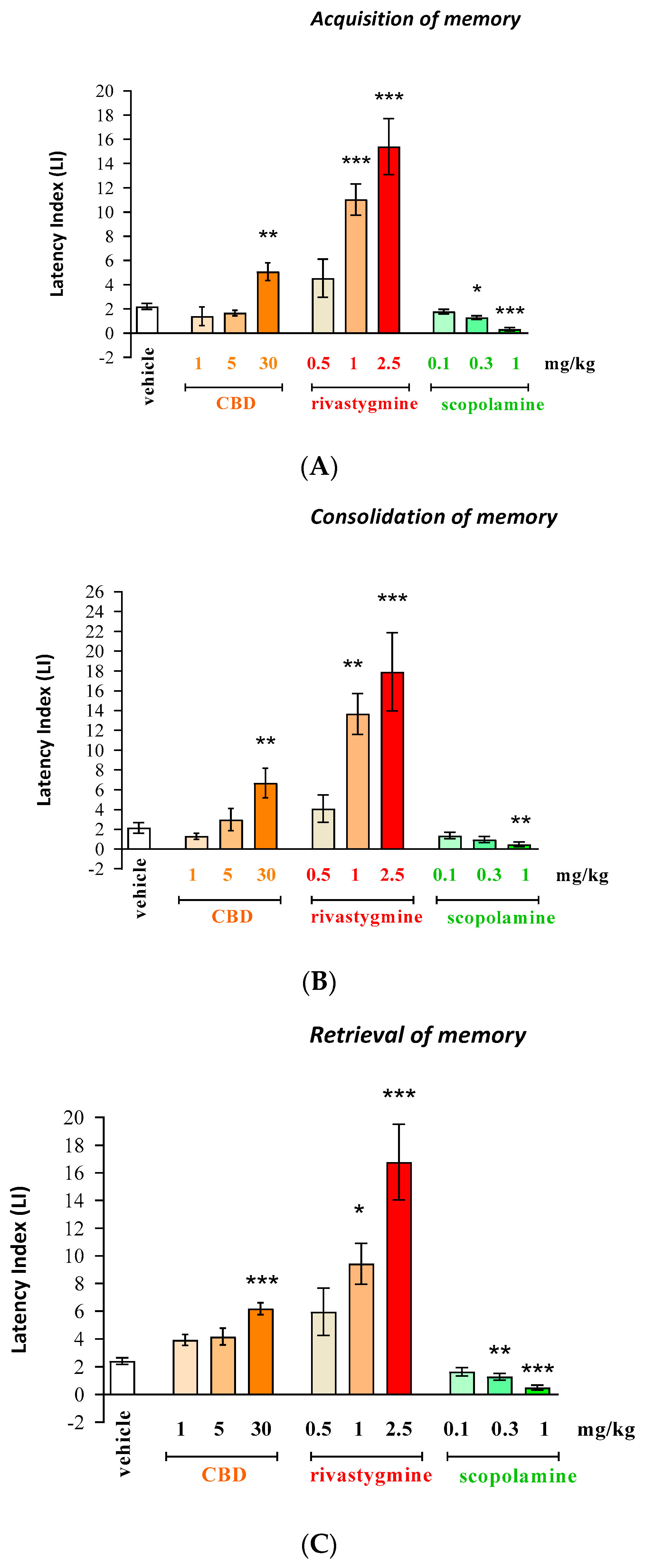





| (A) | ||||||||||
| VEH | CBD 1 | CBD 5 | CBD 30 | RIV 0.5 | RIV 1 | RIV 2.5 | SCOP 0.1 | SCOP 0.3 | SCOP 1 | |
| Number of Mice | 11 | 12 | 7 | 8 | 8 | 8 | 8 | 8 | 8 | 10 |
| Mininmum | 0.98 | −0.69 | 1.04 | 1.57 | 0.41 | 4.39 | 4.95 | 0.95 | 0.95 | −0.11 |
| Maximum | 3.27 | 8.64 | 2.52 | 7.60 | 12.79 | 15.95 | 22.96 | 2.26 | 1.97 | 0.95 |
| Range | 2.29 | 9.33 | 1.47 | 6.03 | 12.38 | 11.55 | 18.01 | 1.31 | 1.02 | 1.06 |
| Mean | 2.20 | 1.40 | 1.67 | 5.07 | 4.54 | 11.03 | 15.40 | 1.78 | 1.29 | 0.31 |
| Std. Deviation | 0.81 | 2.66 | 0.60 | 2.06 | 4.45 | 3.65 | 6.58 | 0.56 | 0.43 | 0.46 |
| Std. Error of Mean | 0.24 | 0.77 | 0.23 | 0.73 | 1.57 | 1.29 | 2.33 | 0.20 | 0.15 | 0.14 |
| (B) | ||||||||||
| VEH | CBD 1 | CBD 5 | CBD 30 | RIV 0.5 | RIV 1 | RIV 2.5 | SCOP 0.1 | SCOP 0.3 | SCOP 1 | |
| Number of Mice | 7 | 9 | 7 | 7 | 10 | 8 | 7 | 8 | 8 | 12 |
| Mininmum | 0.07 | −0.59 | 0.67 | 1.50 | −0.80 | 5.00 | 4.83 | 0.05 | −0.36 | −0.77 |
| Maximum | 3.69 | 2.67 | 6.47 | 11.30 | 12.64 | 20.43 | 32.33 | 2.47 | 2.05 | 1.63 |
| Range | 3.62 | 3.26 | 5.81 | 9.80 | 13.44 | 15.43 | 27.50 | 2.42 | 2.41 | 2.40 |
| Mean | 3.15 | 1.30 | 2.99 | 6.67 | 4.09 | 13.66 | 17.91 | 1.36 | 0.96 | 0.49 |
| Std. Deviation | 1.40 | 0.91 | 2.51 | 3.93 | 4.34 | 5.85 | 10.44 | 0.95 | 0.89 | 0.81 |
| Std. Error of Mean | 0.53 | 0.30 | 1.12 | 1.49 | 1.37 | 2.07 | 3.95 | 0.34 | 0.32 | 0.23 |
| (C) | ||||||||||
| VEH | CBD 1 | CBD 5 | CBD 30 | RIV 0.5 | RIV 1 | RIV 2.5 | SCOP 0.1 | SCOP 0.3 | SCOP 1 | |
| Number of Mice | 8 | 8 | 8 | 8 | 9 | 10 | 8 | 8 | 8 | 8 |
| Mininmum | 1.52 | 2.78 | 2.13 | 4.32 | 1.41 | 1.31 | 5.25 | 0.81 | 0.39 | −0.66 |
| Maximum | 3.72 | 6.00 | 7.16 | 7.46 | 15.29 | 14.79 | 25.27 | 3.11 | 2.15 | 0.95 |
| Range | 2.20 | 3.22 | 5.02 | 3.14 | 13.87 | 13.48 | 20.02 | 2.30 | 1.76 | 1.61 |
| Mean | 2.42 | 3.92 | 4.16 | 6.19 | 5.96 | 9.43 | 16.78 | 1.65 | 1.28 | 0.49 |
| Std. Deviation | 0.65 | 1.15 | 1.74 | 1.20 | 5.16 | 4.70 | 7.75 | 0.85 | 0.70 | 0.50 |
| Std. Error of Mean | 0.23 | 0.41 | 0.61 | 0.43 | 1.72 | 1.49 | 2.74 | 0.30 | 0.25 | 0.18 |
| (A) | ||||||||
| VEH/VEH/VEH | CBD/VEH/VEH | RIV/VEH/VEH | CBD/RIV/VEH | VEH/VEH/SCOP | CBD/VEH/SCOP | VEH/RIV/SCOP | CBD/RIV/SCOP | |
| Number of Mice | 10 | 10 | 9 | 8 | 12 | 10 | 8 | 10 |
| Mininmum | 0.98 | −0.53 | 1.41 | 1.43 | −0.95 | 1.29 | 0.45 | 4 |
| Maximum | 3.27 | 8.64 | 12.64 | 10.06 | 1.35 | 4.83 | 6.46 | 10.63 |
| Range | 2.29 | 9.16 | 11.22 | 8.63 | 2.29 | 3.55 | 6.00 | 6.63 |
| Mean | 2.10 | 1.43 | 4.99 | 5.34 | 0.22 | 2.85 | 3.29 | 7.32 |
| Std. Deviation | 0.77 | 2.80 | 3.68 | 3.97 | 0.68 | 1.37 | 1.95 | 2.25 |
| Std. Error of Mean | 0.24 | 0.89 | 1.23 | 1.40 | 0.20 | 0.43 | 0.69 | 0.71 |
| (B) | ||||||||
| VEH/VEH/VEH | CBD/VEH/VEH | RIV/VEH/VEH | CBD/RIV/VEH | VEH/VEH/SCOP | CBD/VEH/SCOP | VEH/RIV/SCOP | CBD/RIV/SCOP | |
| Number of Mice | 10 | 8 | 9 | 8 | 10 | 9 | 9 | 9 |
| Mininmum | 1.04 | 0.43 | 2.04 | 1.43 | −0.95 | 0.55 | 2.92 | 1.13 |
| Maximum | 3.27 | 4.52 | 9.00 | 10.06 | 0.95 | 4.57 | 6.47 | 11.42 |
| Range | 2.23 | 4.09 | 6.96 | 8.63 | 1.91 | 4.03 | 3.54 | 10.28 |
| Mean | 2.33 | 2.13 | 3.99 | 5.34 | 0.25 | 2.75 | 4.42 | 7.11 |
| Std. Deviation | 0.73 | 1.46 | 2.25 | 3.57 | 0.74 | 1.27 | 1.35 | 3.54 |
| Std. Error of Mean | 0.23 | 0.52 | 0.75 | 1.26 | 0.23 | 0.42 | 0.45 | 1.18 |
| (C) | ||||||||
| VEH/VEH/VEH | CBD/VEH/VEH | RIV/VEH/VEH | CBD/RIV/VEH | VEH/VEH/SCOP | CBD/VEH/SCOP | VEH/RIV/SCOP | CBD/RIV/SCOP | |
| Number of Mice | 9 | 9 | 8 | 8 | 10 | 8 | 8 | 8 |
| Mininmum | −0.47 | 3.26 | 1.41 | 3.08 | −0.66 | 0.69 | 1.52 | 3.00 |
| Maximum | 5.71 | 5.38 | 12.64 | 11.20 | 0.95 | 3.17 | 8.93 | 11.00 |
| Range | 6.19 | 2.12 | 11.22 | 8.12 | 1.61 | 2.48 | 7.41 | 8.00 |
| Mean | 2.37 | 3.68 | 4.79 | 7.26 | 0.49 | 2.30 | 6.09 | 7.85 |
| Std. Deviation | 2.18 | 0.69 | 4.05 | 3.58 | 0.45 | 0.94 | 2.72 | 2.80 |
| Std. Error of Mean | 0.73 | 0.23 | 1.43 | 1.27 | 0.14 | 0.33 | 0.96 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kruk-Slomka, M.; Slomka, T.; Biala, G. The Influence of an Acute Administration of Cannabidiol or Rivastigmine, Alone and in Combination, on Scopolamine-Provoked Memory Impairment in the Passive Avoidance Test in Mice. Pharmaceuticals 2024, 17, 809. https://doi.org/10.3390/ph17060809
Kruk-Slomka M, Slomka T, Biala G. The Influence of an Acute Administration of Cannabidiol or Rivastigmine, Alone and in Combination, on Scopolamine-Provoked Memory Impairment in the Passive Avoidance Test in Mice. Pharmaceuticals. 2024; 17(6):809. https://doi.org/10.3390/ph17060809
Chicago/Turabian StyleKruk-Slomka, Marta, Tomasz Slomka, and Grazyna Biala. 2024. "The Influence of an Acute Administration of Cannabidiol or Rivastigmine, Alone and in Combination, on Scopolamine-Provoked Memory Impairment in the Passive Avoidance Test in Mice" Pharmaceuticals 17, no. 6: 809. https://doi.org/10.3390/ph17060809
APA StyleKruk-Slomka, M., Slomka, T., & Biala, G. (2024). The Influence of an Acute Administration of Cannabidiol or Rivastigmine, Alone and in Combination, on Scopolamine-Provoked Memory Impairment in the Passive Avoidance Test in Mice. Pharmaceuticals, 17(6), 809. https://doi.org/10.3390/ph17060809







