Iron-Based Metal-Organic Frameworks as Multiple Cascade Synergistic Therapeutic Effect Nano-Drug Delivery Systems for Effective Tumor Elimination
Abstract
:1. Introduction
2. Result and Discussion
2.1. Synthesis and Characterization of MDTM@P−Ag
2.2. Drug Release and Catalytic Ability of MDTM@P−Ag
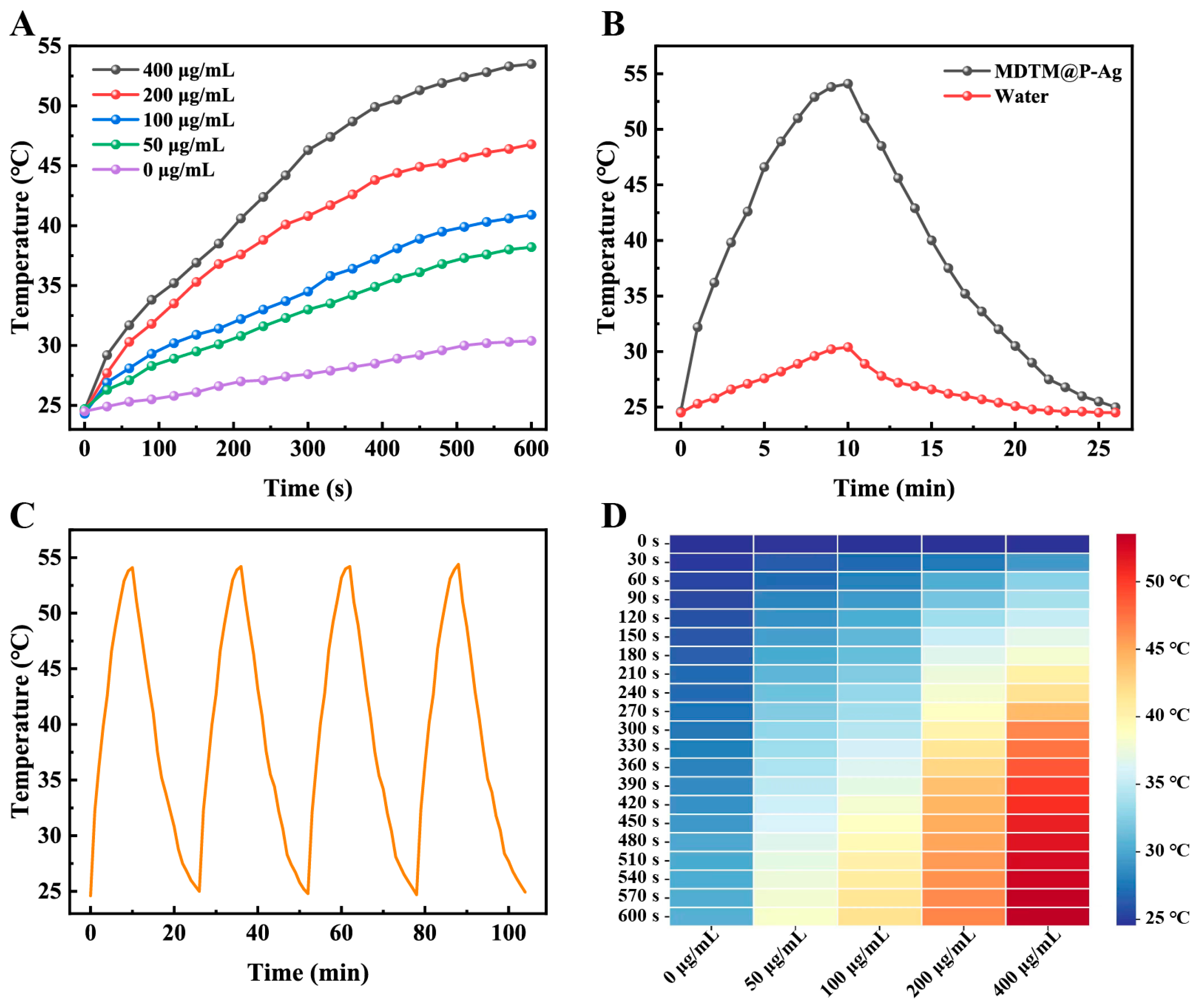
2.3. The Photothermal Performance of MDTM@P−Ag
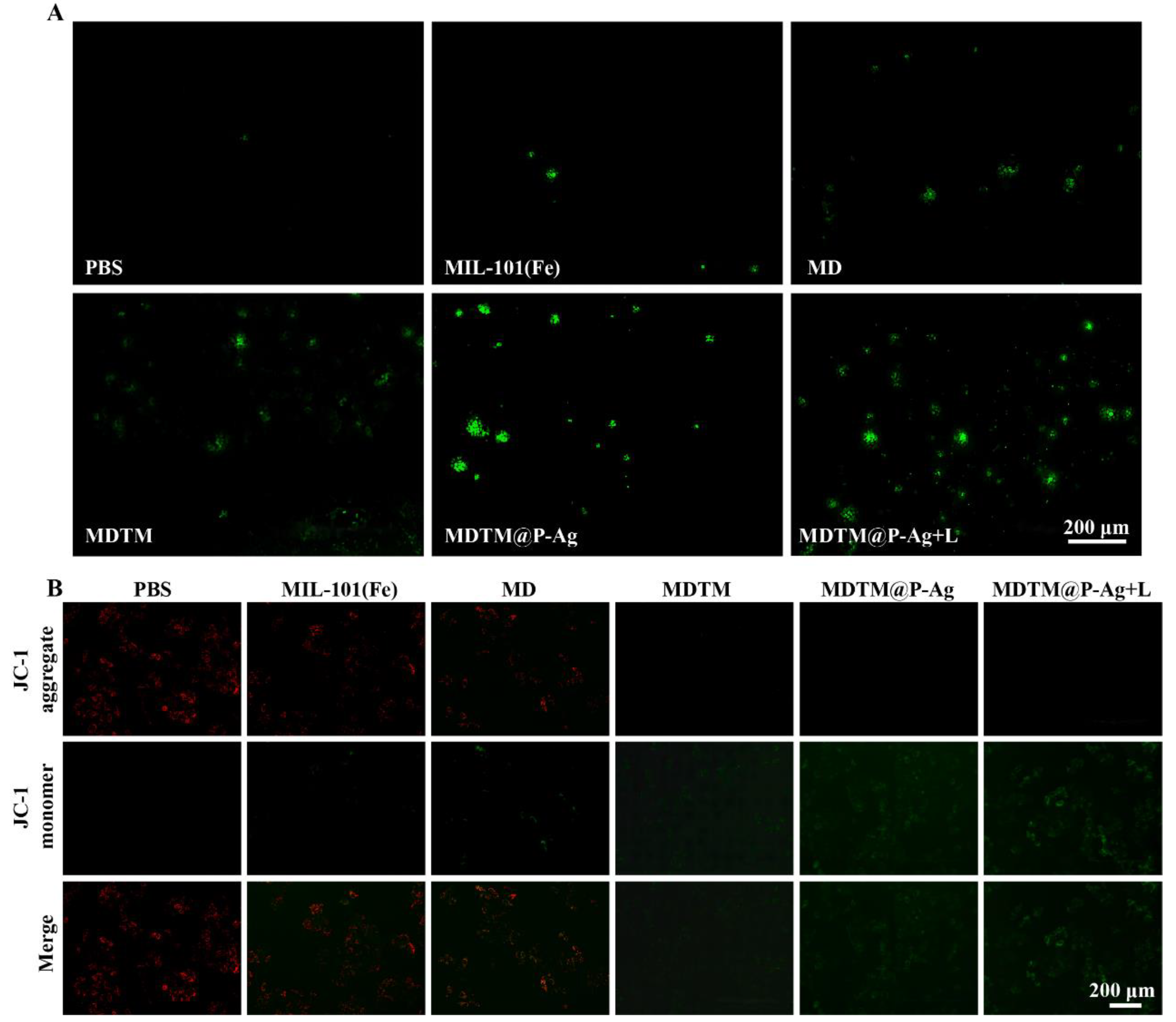
2.4. In Vitro Therapeutic Effects of MDTM@P−Ag
3. Conclusions
4. Experimental Sections
4.1. Synthesis of MIL−101(Fe)
4.2. Synthesis of MIL−101(Fe)−DOX
4.3. Synthesis of MIL−101(Fe)−DOX−TCPP
4.4. Synthesis of MIL−101(Fe)−DOX−TCPP−MnO2
4.5. Synthesis of Ag NPs
4.6. Synthesis of MIL−101(Fe)−DOX−TCPP−MnO2@PDA−Ag
4.7. Drug Loading and Drug Release Assays
4.8. The CDT Capacity Assays
4.9. The PDT Capacity Assays
4.10. The PTT Capacity Assays
4.11. Cell Culture
4.12. Cytotoxicity Test
4.13. Intracelluar GSH Generation
4.14. Intracelluar ROS Detection
4.15. JC-1 Assays
4.16. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kelley, R.K.; Ueno, M.; Yoo, C.; Finn, R.S.; Furuse, J.; Ren, Z.; Yau, T.; Klümpen, H.-J.; Chan, S.L.; Ozaka, M.; et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2023, 401, 1853–1865. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Pinyol, R.; Kelley, R.K.; El-Khoueiry, A.; Reeves, H.L.; Wang, X.W.; Gores, G.J.; Villanueva, A. Molecular pathogenesis and systemic therapies for hepatocellular carcinoma. Nat. Cancer 2022, 3, 386–401. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Meyer, T.; Sapisochin, G.; Salem, R.; Saborowski, A. Hepatocellular carcinoma. Lancet 2022, 400, 1345–1362. [Google Scholar] [CrossRef] [PubMed]
- Devarbhavi, H.; Asrani, S.K.; Arab, J.P.; Nartey, Y.A.; Pose, E.; Kamath, P.S. Global burden of liver disease: 2023 update. J. Hepatol. 2023, 79, 516–537. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Liu, C.; Qin, M.; Zhang, X.; Xi, T.; Yuan, S.; Hao, H.; Xiong, J. Metabolic dysregulation and emerging therapeutical targets for hepatocellular carcinoma. Acta Pharm. Sin. B 2022, 12, 558–580. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Li, X.; Ding, J. Characteristics of liver cancer stem cells and clinical correlations. Cancer Lett. 2016, 379, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Aloss, K.; Hamar, P. Recent Preclinical and Clinical Progress in Liposomal Doxorubicin. Pharmaceutics 2023, 15, 893. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Wang, X.; Zhou, Y.; Wang, X.; Yu, Y. Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor immunotherapy. Signal Transduct. Target. Ther. 2022, 7, 196. [Google Scholar] [CrossRef]
- Chen, S.; Cao, Q.; Wen, W.; Wang, H. Targeted therapy for hepatocellular carcinoma: Challenges and opportunities. Cancer Lett. 2019, 460, 1–9. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Y.W. Metal–Organic Frameworks for Biomedical Applications. Small 2020, 16, 1906846. [Google Scholar] [CrossRef]
- Hashemzadeh, A.; Drummen, G.P.C.; Avan, A.; Darroudi, M.; Khazaei, M.; Khajavian, R.; Rangrazi, A.; Mirzaei, M. When metal-organic framework mediated smart drug delivery meets gastrointestinal cancers. J. Mater. Chem. B 2021, 9, 3967–3982. [Google Scholar] [CrossRef]
- Chen, J.; Tan, X.; Huang, Y.; Xu, C.; Zeng, Z.; Shan, T.; Guan, Z.; Xu, X.; Huang, Z.; Zhao, C. Reactive oxygen species-activated self-amplifying prodrug nanoagent for tumor-specific Cu-chelate chemotherapy and cascaded photodynamic therapy. Biomaterials 2022, 284, 121513. [Google Scholar] [CrossRef]
- Ma, Y.; Su, Z.; Zhou, L.; He, L.; Hou, Z.; Zou, J.; Cai, Y.; Chang, D.; Xie, J.; Zhu, C.; et al. Biodegradable Metal-Organic-Framework-Gated Organosilica for Tumor-Microenvironment-Unlocked Glutathione-Depletion-Enhanced Synergistic Therapy. Adv. Mater. 2022, 34, 2107560. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Kuang, G.; Wang, H.; Zhao, Y.; Wei, J.; Shang, L. Multi-Bioinspired MOF Delivery Systems from Microfluidics for Tumor Multimodal Therapy. Adv. Sci. 2023, 10, 2303818. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; He, H.; Xiao, Z.; Xiao, H.; An, Y.; Zhong, H.; Lin, M.; Meng, X.; Han, S.; Shuai, X. GSH-Responsive Metal-Organic Framework for Intratumoral Release of NO and IDO Inhibitor to Enhance Antitumor Immunotherapy. Small 2022, 18, 2107732. [Google Scholar] [CrossRef]
- Karimi Alavijeh, R.; Akhbari, K. Cancer therapy by nano MIL-n series of metal-organic frameworks. Coord. Chem. Rev. 2024, 503, 215643. [Google Scholar] [CrossRef]
- Horcajada, P.; Serre, C.; Vallet-Regí, M.; Sebban, M.; Taulelle, F.; Férey, G. Metal-Organic Frameworks as Efficient Materials for Drug Delivery. Angew. Chem. Int. Ed. 2006, 118, 6120–6124. [Google Scholar] [CrossRef]
- Yang, X.X.; Xu, X.; Wang, M.F.; Xu, H.Z.; Peng, X.C.; Han, N.; Yu, T.T.; Li, L.G.; Li, Q.R.; Chen, X.; et al. A nanoreactor boosts chemodynamic therapy and ferroptosis for synergistic cancer therapy using molecular amplifier dihydroartemisinin. J. Nanobiotechnol. 2022, 20, 230. [Google Scholar] [CrossRef]
- Zhou, Y.; Fan, S.; Feng, L.; Huang, X.; Chen, X. Manipulating Intratumoral Fenton Chemistry for Enhanced Chemodynamic and Chemodynamic-Synergized Multimodal Therapy. Adv. Mater. 2021, 33, 2104223. [Google Scholar] [CrossRef]
- Jia, C.; Guo, Y.; Wu, F.G. Chemodynamic Therapy via Fenton and Fenton-Like Nanomaterials: Strategies and Recent Advances. Small 2022, 18, e2103868. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Liu, Y.; He, M.; Bu, W. Chemodynamic Therapy: Tumour Microenvironment-Mediated Fenton and Fenton-like Reactions. Angew. Chem. Int. Ed. 2018, 58, 946–956. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhong, X.; Liu, Z.; Cheng, L. Recent progress of chemodynamic therapy-induced combination cancer therapy. Nano Today 2020, 35, 100946. [Google Scholar] [CrossRef]
- Cao, C.; Wang, X.; Yang, N.; Song, X.; Dong, X. Recent advances of cancer chemodynamic therapy based on Fenton/Fenton-like chemistry. Chem. Sci. 2022, 13, 863–889. [Google Scholar] [CrossRef]
- Li, S.L.; Jiang, P.; Jiang, F.L.; Liu, Y. Recent Advances in Nanomaterial-Based Nanoplatforms for Chemodynamic Cancer Therapy. Adv. Funct. Mater. 2021, 31, 2100243. [Google Scholar] [CrossRef]
- Ding, B.; Zheng, P.; Ma, P.; Lin, J. Manganese Oxide Nanomaterials: Synthesis, Properties, and Theranostic Applications. Adv. Mater. 2020, 32, e1905823. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Meng, H.; Tian, Y.; Yang, R.; Du, D.; Li, Z.; Qu, L.; Lin, Y. Recent advances in functionalized MnO2 nanosheets for biosensing and biomedicine applications. Nanoscale Horiz. 2019, 4, 321–338. [Google Scholar] [CrossRef]
- Yang, G.; Ji, J.; Liu, Z. Multifunctional MnO2 nanoparticles for tumor microenvironment modulation and cancer therapy. WIREs Nanomed. Nanobiotechnol. 2021, 13, e1720. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Ju, E.; Liu, Z.; Cao, F.; Chen, Z.; Ren, J.; Qu, X. Biomimetic nanoflowers by self-assembly of nanozymes to induce intracellular oxidative damage against hypoxic tumors. Nat. Commun. 2018, 9, 3334. [Google Scholar] [CrossRef]
- Zhan, M.; Wang, F.; Liu, Y.; Zhou, J.; Zhao, W.; Lu, L.; Li, J.; He, X. Dual-Cascade Activatable Nanopotentiators Reshaping Adenosine Metabolism for Sono-Chemodynamic-Immunotherapy of Deep Tumors. Adv. Sci. 2023, 10, 2207200. [Google Scholar] [CrossRef]
- Xu, D.; Duan, Q.; Yu, H.; Dong, W. Photodynamic therapy based on porphyrin-based metal–organic frameworks. J. Mater. Chem. B 2023, 11, 5976–5989. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Li, X.; Chen, X.; Zhou, J.; Li, Y.; Peng, J.; Lin, Z.; Liu, G.; Zeng, X.; Li, C.; et al. Fe/MOF based platform for NIR laser induced efficient PDT/PTT of cancer. Front. Bioeng. Biotechnol. 2023, 11, 1156079. [Google Scholar] [CrossRef] [PubMed]
- Larue, L.; Myrzakhmetov, B.; Ben-Mihoub, A.; Moussaron, A.; Thomas, N.; Arnoux, P.; Baros, F.; Frochot, C. Frochot, Fighting Hypoxia to Improve PDT. Pharmaceuticals 2019, 12, 163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, M.; Mu, Y.; Li, J.; Foda, M.F.; Zhang, W.; Han, K.; Han, H. Reasonably retard O2 consumption through a photoactivity conversion nanocomposite for oxygenated photodynamic therapy. Biomaterials 2019, 218, 119312. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Cheng, H.; Jiang, C.; Qiu, X.; Wang, K.; Huan, W.; Yuan, A.; Wu, J.; Hu, Y. Perfluorocarbon nanoparticles enhance reactive oxygen levels and tumour growth inhibition in photodynamic therapy. Nat. Commun. 2015, 6, 8785. [Google Scholar] [CrossRef] [PubMed]
- Han, H.S.; Choi, K.Y. Advances in Nanomaterial-Mediated Photothermal Cancer Therapies: Toward Clinical Applications. Biomedicines 2021, 9, 305. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yong, T.; Wei, Z.; Bie, N.; Zhang, X.; Zhan, G.; Li, J.; Qin, J.; Yu, J.; Zhang, B.; et al. Reversing insufficient photothermal therapy-induced tumor relapse and metastasis by regulating cancer-associated fibroblasts. Nat. Commun. 2022, 13, 2101625. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Zhang, X.; Zhang, S.; Wei, G.; Su, Z. Biomedical and bioactive engineered nanomaterials for targeted tumor photothermal therapy: A review. Mater. Sci. Eng. C 2019, 104, 109891. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hou, M.; Sun, W.; Wu, Q.; Xu, J.; Xiong, L.; Chai, Y.; Liu, Y.; Yu, M.; Wang, H.; et al. Sequential PDT and PTT Using Dual-Modal Single-Walled Carbon Nanohorns Synergistically Promote Systemic Immune Responses against Tumor Metastasis and Relapse. Adv. Sci. 2020, 7, 2001088. [Google Scholar] [CrossRef]
- Dong, S.; Dong, Y.; Zhao, Z.; Liu, J.; Liu, S.; Feng, L.; He, F.; Gai, S.; Xie, Y.; Yang, P. “Electron Transport Chain Interference” Strategy of Amplified Mild-Photothermal Therapy and Defect-Engineered Multi-Enzymatic Activities for Synergistic Tumor-Personalized Suppression. J. Am. Chem. Soc. 2023, 145, 9488–9507. [Google Scholar] [CrossRef]
- Peng, J.; Xiao, Y.; Li, W.; Yang, Q.; Tan, L.; Jia, Y.; Qu, Y.; Qian, Z. Photosensitizer Micelles Together with IDO Inhibitor Enhance Cancer Photothermal Therapy and Immunotherapy. Adv. Sci. 2018, 5, 1700891. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Xu, D.; Ge, M.; Luo, J.; Chen, L.; Chen, Z.; You, Y.; Zhu, Y.-X.; Lin, H.; Shi, J. Blocking glutathione regeneration: Inorganic NADPH oxidase nanozyme catalyst potentiates tumoral ferroptosis. Nano Today 2022, 46, 101574. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Niu, H.; Wang, Y.; Wu, A.; Shu, C.; Zhu, Y.; Bian, Y.; Lin, K. Metal-Organic Framework-Based Nanoagents for Effective Tumor Therapy by Dual Dynamics-Amplified Oxidative Stress. ACS Appl. Mater. Interfaces 2021, 13, 45201–45213. [Google Scholar] [CrossRef] [PubMed]
- Uematsu, T.; Miyamoto, Y.; Ogasawara, Y.; Suzuki, K.; Yamaguchi, K.; Mizuno, N. Molybdenum-doped α−MnO2 as an efficient reusable heterogeneous catalyst for aerobic sulfide oxygenation. Catal. Sci. Technol. 2016, 6, 222–233. [Google Scholar] [CrossRef]
- Liu, J.; Li, L.; Zhang, B.; Xu, Z.P. MnO2-shelled Doxorubicin/Curcumin nanoformulation for enhanced colorectal cancer chemo-immunotherapy. J. Colloid Interface Sci. 2022, 617, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, Z.; Xu, M.; Yang, S.; Xie, Y.; Zhang, B.; Fan, J. A Curcumin-Based MOF Embedded Ag NPs and Modified with Polydopamine for Dual-Drug Delivery and Dual Biological Window Responsive Synergetic Cancer Therapy. Part. Part. Syst. Charact. 2023, 40, 2300047. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, Z.; He, W.; Ren, J.; Wong, C.-Y. One-pot synthesis of a self-reinforcing cascade bioreactor for combined photodynamic/chemodynamic/starvation therapy. J. Colloid Interface Sci. 2021, 599, 543–555. [Google Scholar] [CrossRef]
- Wu, Q.; Niu, M.; Chen, X.; Tan, L.; Fu, C.; Ren, X.; Ren, J.; Li, L.; Xu, K.; Zhong, H.; et al. Biocompatible and biodegradable zeolitic imidazolate framework/polydopamine nanocarriers for dual stimulus triggered tumor thermo-chemotherapy. Biomaterials 2018, 162, 132–143. [Google Scholar] [CrossRef]
- Liebscher, J. Chemistry of Polydopamine-Scope, Variation, and Limitation. Eur. J. Org. Chem. 2019, 2019, 4976–4994. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, W.; Mao, D.; Teh, C.; Wang, B.; Liu, B. Metal–Organic Framework Assisted and Tumor Microenvironment Modulated Synergistic Image-Guided Photo-Chemo Therapy. Adv. Funct. Mater. 2020, 30, 2002431. [Google Scholar] [CrossRef]
- Yang, P.; Tao, J.; Chen, F.; Chen, Y.; He, J.; Shen, K.; Zhao, P.; Li, Y. Multienzyme-Mimic Ultrafine Alloyed Nanoparticles in Metal Organic Frameworks for Enhanced Chemodynamic Therapy. Small 2021, 17, 2005865. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Li, Y.; Zhang, Z.; Ren, J.; Zhang, L.; Xu, Z.; Kang, Y.; Xue, P. Silk fibroin-capped metal-organic framework for tumor-specific redox dyshomeostasis treatment synergized by deoxygenation-driven chemotherapy. Acta Biomater. 2022, 138, 545–560. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Lin, B.; Chen, C.; Wang, H.; Lin, X.; Liu, J.; Ren, Q.; Tao, J.; Zhao, P.; Xu, Y. Synergistic Reinforcing of Immunogenic Cell Death and Transforming Tumor-Associated Macrophages Via a Multifunctional Cascade Bioreactor for Optimizing Cancer Immunotherapy. Adv. Mater. 2022, 34, e2207593. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Zhang, W.; Zhou, X.; Fu, J.; He, C. Tumor cell membrane-camouflaged responsive nanoparticles enable MRI-guided immuno-chemodynamic therapy of orthotopic osteosarcoma. Bioact. Mater. 2022, 17, 221–233. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, Z.; Wu, S.; Zhu, C.; Meng, X.; Li, C.; Cheng, S.; Tao, W.; Wang, F. Glutathione-Sensitive Nanoglue Platform with Effective Nucleic Acids Gluing onto Liposomes for Photo-Gene Therapy. ACS Appl. Mater. Interfaces 2022, 14, 25126–25134. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Liu, H.; Xue, Y.; Lin, J.; Fu, Y.; Xia, Z.; Pan, D.; Zhang, J.; Qiao, K.; Zhang, Z.; et al. Reversing cold tumors to hot: An immunoadjuvant-functionalized metal-organic framework for multimodal imaging-guided synergistic photo-immunotherapy. Bioact Mater 2021, 6, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lin, S.; Zhao, D.; Guan, L.; Hu, Y.; Wang, Y.; Lin, K.; Zhu, Y. Palladium Nanocrystals-Engineered Metal–Organic Frameworks for Enhanced Tumor Inhibition by Synergistic Hydrogen/Photodynamic Therapy. Adv. Funct. Mater. 2020, 31, 2006853. [Google Scholar] [CrossRef]
- Meng, X.; Lu, Z.; Lv, Q.; Jiang, Y.; Zhang, L.; Wang, Z. Tumor metabolism destruction via metformin-based glycolysis inhibition and glucose oxidase-mediated glucose deprivation for enhanced cancer therapy. Acta Biomater 2022, 145, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, C.; Ying, Y.; Lv, S.; Wang, Y.; Zhang, X.; Cai, Z.; Gu, W.; Li, Z.; Jiang, G.; et al. Smart PdH@MnO2 Yolk–Shell Nanostructures for Spatiotemporally Synchronous Targeted Hydrogen Delivery and Oxygen-Elevated Phototherapy of Melanoma. ACS Nano 2022, 16, 5597–5614. [Google Scholar] [CrossRef]
- Xi, D.; Xiao, M.; Cao, J.; Zhao, L.; Xu, N.; Long, S.; Fan, J.; Shao, K.; Sun, W.; Yan, X.; et al. NIR Light-Driving Barrier-Free Group Rotation in Nanoparticles with an 88.3% Photothermal Conversion Efficiency for Photothermal Therapy. Adv. Mater. 2020, 32, 1907855. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, H.; An, G.; Yang, X.; Huang, L.; Wang, N.; Zhu, Y. Iron-Based Metal-Organic Frameworks as Multiple Cascade Synergistic Therapeutic Effect Nano-Drug Delivery Systems for Effective Tumor Elimination. Pharmaceuticals 2024, 17, 812. https://doi.org/10.3390/ph17060812
Zheng H, An G, Yang X, Huang L, Wang N, Zhu Y. Iron-Based Metal-Organic Frameworks as Multiple Cascade Synergistic Therapeutic Effect Nano-Drug Delivery Systems for Effective Tumor Elimination. Pharmaceuticals. 2024; 17(6):812. https://doi.org/10.3390/ph17060812
Chicago/Turabian StyleZheng, Heming, Guanghui An, Xiaohui Yang, Lei Huang, Nannan Wang, and Yanqiu Zhu. 2024. "Iron-Based Metal-Organic Frameworks as Multiple Cascade Synergistic Therapeutic Effect Nano-Drug Delivery Systems for Effective Tumor Elimination" Pharmaceuticals 17, no. 6: 812. https://doi.org/10.3390/ph17060812
APA StyleZheng, H., An, G., Yang, X., Huang, L., Wang, N., & Zhu, Y. (2024). Iron-Based Metal-Organic Frameworks as Multiple Cascade Synergistic Therapeutic Effect Nano-Drug Delivery Systems for Effective Tumor Elimination. Pharmaceuticals, 17(6), 812. https://doi.org/10.3390/ph17060812






