Prognostic Significance of Pan-Immune-Inflammation Value in Patients with HER2-Positive Metastatic Breast Cancer Treated with Trastuzumab Emtansine
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics
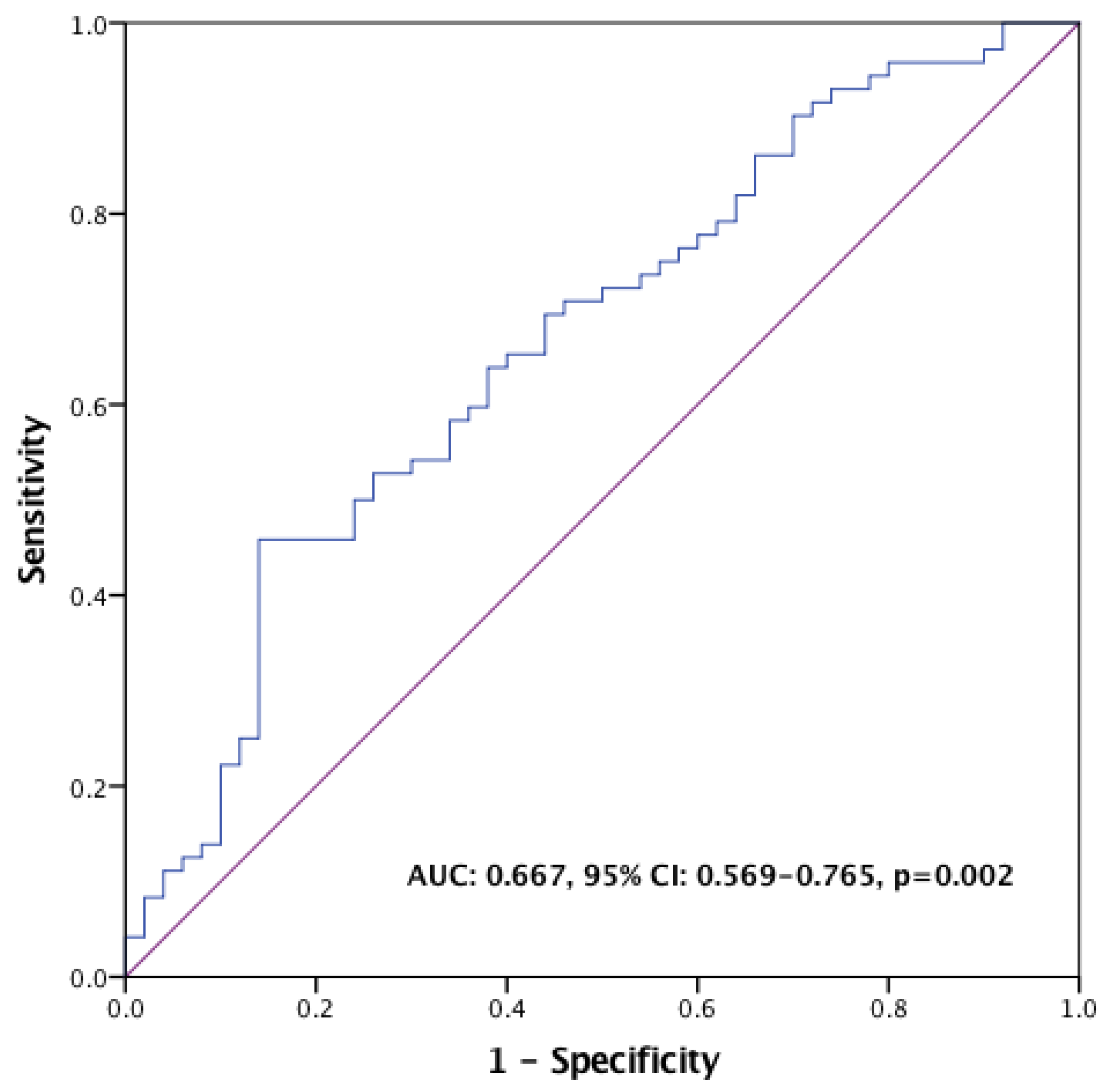
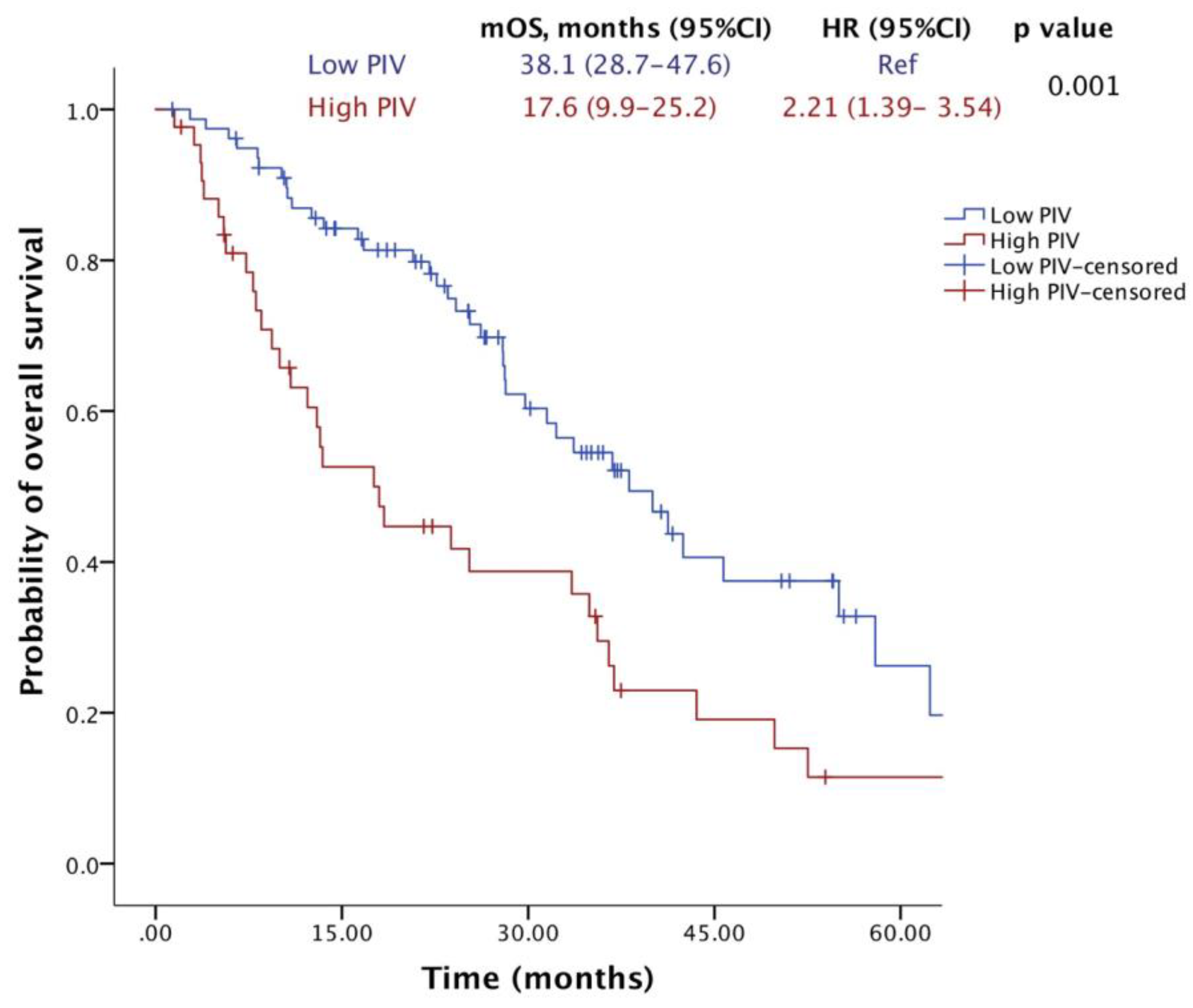
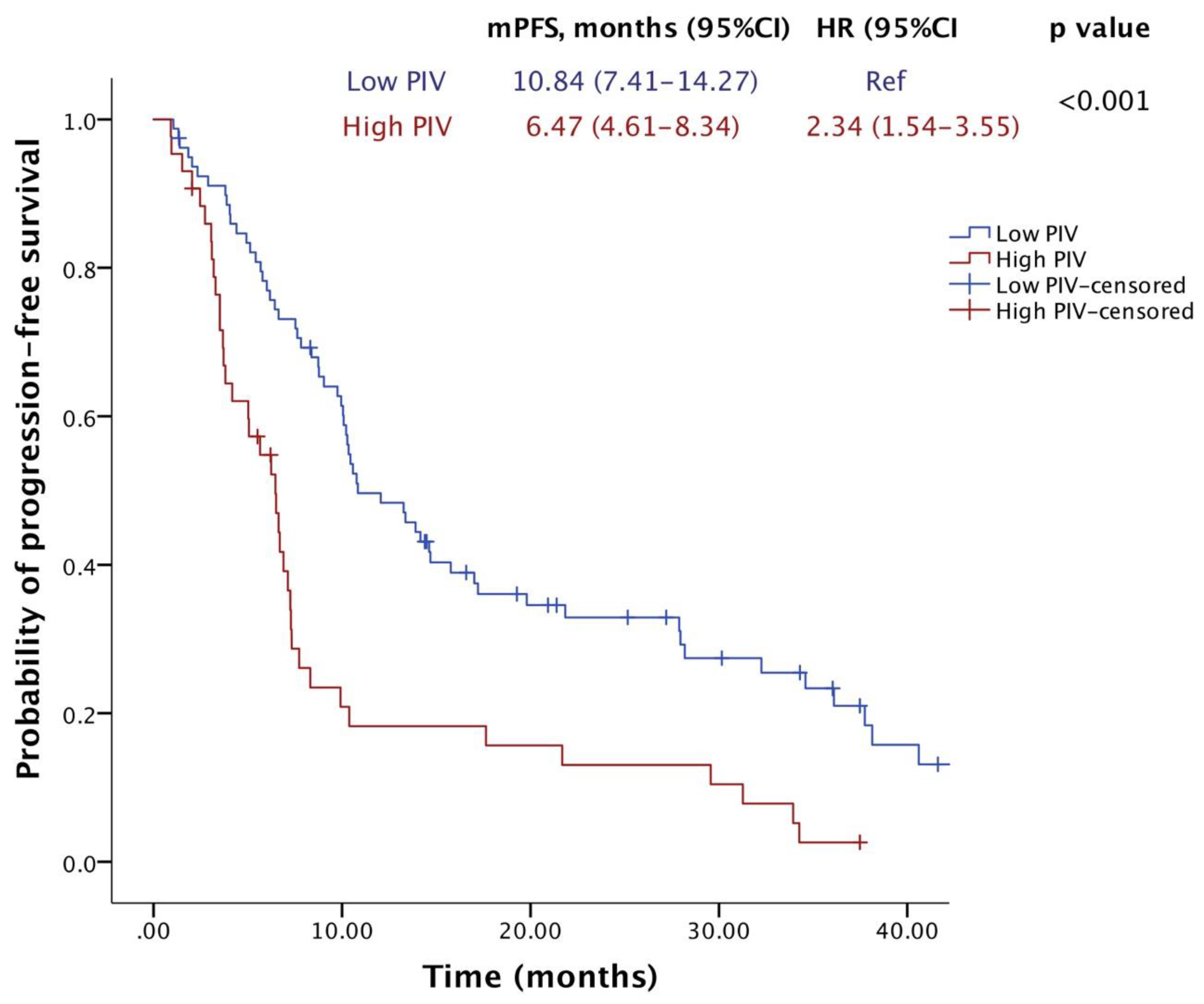
2.2. Survival Outcomes
2.3. Radiological Responses
3. Discussion
4. Materials and Methods
4.1. Patient Population
4.2. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cronin, K.A.; Harlan, L.C.; Dodd, K.W.; Abrams, J.S.; Ballard-Barbash, R. Population-based estimate of the prevalence of HER-2 positive breast cancer tumors for early stage patients in the US. Cancer Investig. 2010, 28, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Shastry, M.; Hamilton, E. Targeting HER2-positive breast cancer: Advances and future directions. Nat. Rev. Drug Discov. 2023, 22, 101–126. [Google Scholar] [CrossRef] [PubMed]
- Rinnerthaler, G.; Gampenrieder, S.P.; Greil, R. HER2 Directed Antibody-Drug-Conjugates beyond T-DM1 in Breast Cancer. Int. J. Mol. Sci. 2019, 20, 1115. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.-Y.; Diéras, V.; Guardino, E.; et al. Trastuzumab Emtansine for HER2-Positive Advanced Breast Cancer. N. Engl. J. Med. 2012, 367, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Krop, I.E.; Kim, S.B.; Martin, A.G.; LoRusso, P.M.; Ferrero, J.M.; Badovinac-Crnjevic, T.; Hoersch, S.; Smitt, M.; Wildiers, H. Trastuzumab emtansine versus treatment of physician’s choice in patients with previously treated HER2-positive metastatic breast cancer (TH3RESA): Final overall survival results from a randomised open-label phase 3 trial. Lancet Oncol. 2017, 18, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Kreuzaler, M.; Khan, T.; Thommen, D.S.; Martin, K.; Glatz, K.; Savic, S.; Harbeck, N.; Nitz, U.; Gluz, O. Trastuzumab emtansine (T-DM1) renders HER2+ breast cancer highly susceptible to CTLA-4/PD-1 blockade. Sci. Transl. Med. 2015, 7, ra188–ra315. [Google Scholar] [CrossRef] [PubMed]
- Gedik, M.E.; Saatci, O.; Oberholtzer, N.; Uner, M.; Akbulut-Caliskan, O.; Cetin, M.; Aras, M.; Ibis, K.; Caliskan, B.; Banoglu, E.; et al. Targeting TACC3 induces immunogenic cell death and enhances T-DM1 response in HER2-positive breast cancer. Cancer Res. 2024, 84, 1475–1490. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Zhang, L.P.; Xie, S.Y.; Huang, H.Y.; Chen, X.Y.; Jiang, T.C.; Guo, L.; Lin, H.X. Pan-Immune-Inflammation Value: A New Prognostic Index in Operative Breast Cancer. Front. Oncol. 2022, 12, 830138. [Google Scholar] [CrossRef] [PubMed]
- Ligorio, F.; Fucà, G.; Zattarin, E.; Lobefaro, R.; Zambelli, L.; Leporati, R.; Rea, C.; Mariani, G.; Bianchi, G.V.; Capri, G.; et al. The Pan-Immune-Inflammation-Value Predicts the Survival of Patients with Human Epidermal Growth Factor Receptor 2 (HER2)—Positive Advanced Breast Cancer Treated with First-Line Taxane-Trastuzumab-Pertuzumab. Cancers 2021, 13, 1964. [Google Scholar] [CrossRef]
- Su, Z.; Tang, J.; He, Y.; Zeng, W.H.; Yu, Q.; Cao, X.L.; Zou, G.R. Pan-immune-inflammation value as a novel prognostic biomarker in nasopharyngeal carcinoma. Oncol. Lett. 2024, 9, 252. [Google Scholar] [CrossRef] [PubMed]
- Şahin, A.B.; Cubukcu, E.; Ocak, B.; Deligonul, A.; Oyucu Orhan, S.; Tolunay, S.; Gokgoz, M.S.; Cetintas, S.; Yarbas, G.; Senol, K.; et al. Low pan-immune-inflammation-value predicts better chemotherapy response and survival in breast cancer patients treated with neoadjuvant chemotherapy. Sci. Rep. 2021, 19, 14662. [Google Scholar] [CrossRef] [PubMed]
- Efil, S.C.; Guner, G.; Guven, D.C.; Celikten, B.; Celebiyev, E.; Taban, H.; Akyol, A.; Isik, A.; Kilickap, S.; Yalcin, S.; et al. Prognostic and predictive value of tumor infiltrating lymphocytes in combination with systemic inflammatory markers in colon cancer. Clin. Res. Hepatol. Gastroenterol. 2023, 47, 102171. [Google Scholar] [CrossRef] [PubMed]
- Azar, I.; Alkassis, S.; Fukui, J.; Alsawah, F.; Fedak, K.; Al Hallak, M.N.; Sukari, A.; Nagasaka, M. Spotlight on Trastuzumab Deruxtecan (DS-8201,T-DXd) for HER2 Mutation Positive Non-Small Cell Lung Cancer. Lung Cancer (Auckl.) 2021, 12, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Cortés, J.; Kim, S.-B.; Chung, W.-P.; Im, S.-A.; Park, Y.H.; Hegg, R.; Kim, M.H.; Tseng, L.-M.; Petry, V.; Chung, C.-F.; et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. N. Engl. J. Med. 2022, 386, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Leyton, J.V. Improving Receptor-Mediated Intracellular Access and Accumulation of Antibody Therapeutics-The Tale of HER2. Antibodies 2020, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Lewis Phillips, G.D.; Verma, S.; Ro, J.; Huober, J.; Guardino, A.E.; Samant, M.K.; Olsen, S.; de Haas, S.L.; Pegram, M.D. Relationship between tumor biomarkers and efficacy in EMILIA, a phase III study of trastuzumab emtansine in HER2-positive metastatic breast cancer. Clin. Cancer Res. 2016, 22, 3755–3763. [Google Scholar] [CrossRef] [PubMed]
- Griguolo, G.; Brasó-Maristany, F.; Gonzalez-Farre, B.; Pascual, T.; Chic, N.; Saurí, T.; Kates, R.; Gluz, O.; Martínez, D.; Paré, L. ERBB2 mRNA expression and response to ado-trastuzumab emtansine (T-DM1) in HER2-positive breast cancer. Cancers 2020, 12, 1902. [Google Scholar] [CrossRef] [PubMed]
- Babar, Q.; Saeed, A.; Tabish, T.A.; Sarwar, M.; Thorat, N.D. Targeting the tumor microenvironment: Potential strategy for cancer therapeutics. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2023, 1869, 166746. [Google Scholar] [CrossRef] [PubMed]
- Luque, M.; Sanz-Álvarez, M.; Morales-Gallego, M.; Madoz-Gúrpide, J.; Zazo, S.; Domínguez, C.; Cazorla, A.; Izarzugaza, Y.; Arranz, J.L.; Cristóbal, I.; et al. Tumor-Infiltrating Lymphocytes and Immune Response in HER2-Positive Breast Cancer. Cancers 2022, 14, 6034. [Google Scholar] [CrossRef] [PubMed]
- de Haas, S.L.; Slamon, D.J.; Martin, M.; Press, M.F.; Lewis, G.D.; Lambertini, C.; Prat, A.; Lopez-Valverde, V.; Boulet, T.; Hurvitz, S.A. Tumor biomarkers and efficacy in patients treated with trastuzumab emtansine + pertuzumab versus standard of care in HER2-positive early breast cancer: An open-label, phase III study (KRISTINE). Breast Cancer Res. 2023, 25, 2. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Kim, E.Y.; Yun, J.S.; Park, Y.L.; Do, S.-I.; Chae, S.W.; Park, C.H. The prognostic and predictive value of tumor-infiltrating lymphocytes and hematologic parameters in patients with breast cancer. BMC Cancer 2018, 18, 938. [Google Scholar] [CrossRef] [PubMed]
- Ulas, A.; Avci, N.; Kos, T.; Cubukcu, E.; Olmez, O.F.; Bulut, N.; Degirmenci, M. Are neutrophil/lymphocyte ratio and platelet/lymphocyte ratio associated with prognosis in patients with HER2-positive early breast cancer receiving adjuvant trastuzumab? Lung Cancer 2015, 18, 20. [Google Scholar]
- Imamura, M.; Morimoto, T.; Egawa, C.; Fukui, R.; Bun, A.; Ozawa, H.; Miyagawa, Y.; Fujimoto, Y.; Higuchi, T.; Miyoshi, Y. Significance of baseline neutrophil-to-lymphocyte ratio for progression-free survival of patients with HER2-positive breast cancer treated with trastuzumab emtansine. Sci. Rep. 2019, 9, 1811. [Google Scholar] [CrossRef] [PubMed]
- Guven, D.C.; Yildirim, H.C.; Bilgin, E.; Aktepe, O.H.; Taban, H.; Sahin, T.K.; Cakir, I.Y.; Akin, S.; Dizdar, O.; Aksoy, S.; et al. PILE: A candidate prognostic score in cancer patients treated with immunotherapy. Clin. Transl. Oncol. 2021, 23, 1630–1636. [Google Scholar] [CrossRef] [PubMed]
- Fucà, G.; Guarini, V.; Antoniotti, C.; Morano, F.; Moretto, R.; Corallo, S.; Marmorino, F.; Lonardi, S.; Rimassa, L.; Sartore-Bianchi, A. The Pan-Immune-Inflammation Value is a new prognostic biomarker in metastatic colorectal cancer: Results from a pooled-analysis of the Valentino and TRIBE first-line trials. Br. J. Cancer 2020, 123, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Huang, W.; Wu, Y.; Luo, Y.; Wu, B.; Cheng, J.; Chen, J.; Liu, D.; Li, C. Prognostic role of pretreatment blood lymphocyte count in patients with solid tumors: A systematic review and meta-analysis. Cancer Cell Int. 2020, 20, 15. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Saxena, S.; Singh, R.K. Neutrophils in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1224, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Que, H.; Fu, Q.; Lan, T.; Tian, X.; Wei, X. Tumor-associated neutrophils and neutrophil-targeted cancer therapies. Biochim. Biophys. Acta (BBA) Rev. Cancer 2022, 1877, 188762. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Yu, Y.; Wang, X.; Zhang, T. Tumor-Associated Macrophages in Tumor Immunity. Front. Immunol. 2020, 11, 583084. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Baselga, J.; Kim, S.-B.; Ro, J.; Semiglazov, V.; Campone, M.; Ciruelos, E.; Ferrero, J.-M.; Schneeweiss, A.; Heeson, S. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N. Engl. J. Med. 2015, 372, 724–734. [Google Scholar] [CrossRef]
- Guven, D.C.; Sahin, T.K.; Erul, E.; Kilickap, S.; Gambichler, T.; Aksoy, S. The Association between the Pan-Immune-Inflammation Value and Cancer Prognosis: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 2675. [Google Scholar] [CrossRef] [PubMed]
- Ruopp, M.D.; Perkins, N.J.; Whitcomb, B.W.; Schisterman, E.F. Youden Index and optimal cut-point estimated from observations affected by a lower limit of detection. Biom. J. 2008, 50, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1. 1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | n, (%) |
|---|---|
| Age at T-DM1 treatment, median (IQR) | 51 (43–61) |
| Hormone receptor status, n (%) | |
| ER+ or PR+ | 67 (54.9) |
| ER− and PR− | 55 (45.1) |
| HER2 status, n (%) | |
| IHC3+ | 93 (76.2) |
| IHC2+ and ISH+ | 29 (23.8) |
| Disease extent at diagnosis, n (%) | |
| Early | 66 (54.1) |
| Metastatic | 56 (45.9) |
| ECOG performance status, n (%) | |
| 0 | 55 (45.1) |
| 1 | 61 (50.0) |
| 2 | 6 (4.9) |
| Distribution of distant metastatic sites at initiation of T-DM1, n (%) | |
| Bone | 41 (33.6) |
| Liver | 20 (16.4) |
| Brain | 33 (27.0) |
| Lung | 30 (24.6) |
| Number of sites of distant metastasis at T-DM1 initiation, n (%) | |
| 1 | 80 (65.6) |
| 2 | 32 (26.2) |
| 3+ | 10 (8.2) |
| Prior line of therapy | |
| Anti-HER2 therapy | |
| Trastuzumab | 121 (99.2) |
| Pertuzumab | 45 (36.9) |
| Lapatinib | 26 (21.3) |
| Chemotherapy | |
| Anthracycline-based | 68 (55.7) |
| Taxane-based | 106 (86.9) |
| T-DM1 treatment line in metastatic setting, n (%) | |
| Second | 53 (43.5) |
| Third | 36 (29.5) |
| Fourth or more | 33 (27) |
| Characteristics | Low-PIV Group (n = 79) | High-PIV Group (n = 43) | p Value |
|---|---|---|---|
| Age group, n (%) | 0.186 | ||
| <60 years | 57 (72.2) | 26 (60.5) | |
| ≥60 years | 22 (27.8) | 17 (39.5) | |
| Hormone receptor status, n (%) | 0.319 | ||
| ER+ or PR+ | 46 (58.2) | 21 (48.8) | |
| ER− and PR− | 33 (41.8) | 22 (51.2) | |
| HER2 status, n (%) | 0.152 | ||
| IHC3+ | 57 (72.2) | 36 (83.7) | |
| IHC2+ and ISH+ | 22 (27.8) | 7 (16.3) | |
| Disease extent at diagnosis, n (%) | 0.779 | ||
| Early | 42 (53.2) | 24 (55.8) | |
| Metastatic | 37 (46.8) | 19 (44.2) | |
| ECOG performance status, n (%) | 0.851 | ||
| 0 | 35 (44.3) | 20 (46.5) | |
| 1–2 | 44 (55.7) | 23 (53.5) | |
| Distribution of distant metastatic sites at initiation of T-DM1, n (%) | |||
| Bone | 25 (31.6) | 16 (37.2) | 0.534 |
| Liver | 15 (19.0) | 5 (11.6) | 0.443 |
| Brain | 22 (27.8) | 11 (25.6) | 0.788 |
| Lung | 18 (22.8) | 12 (27.9) | 0.530 |
| Number of sites of distant metastasis at T-DM1 initiation, n (%) | 0.127 | ||
| <2 | 55 (69.6) | 24 (55.8) | |
| 2 or more | 24 (30.4) | 19 (44.2) | |
| Prior line of therapy | |||
| Anti-HER2 therapy | |||
| Trastuzumab | 79 (100) | 42 (97.7) | 0.352 |
| Pertuzumab | 24 (30.4) | 21 (48.8) | 0.044 |
| Lapatinib | 15 (19.0) | 11 (25.6) | 0.488 |
| Chemotherapy | |||
| Anthracycline-based | 45 (66.2) | 23 (53.5) | 0.712 |
| Taxane-based | 69 (87.3) | 37 (86.0) | 0.840 |
| T-DM1 treatment line in metastatic setting, n (%) | 0.903 | ||
| <3 | 34 (43) | 19 (44.2) | |
| 3 or more | 45 (57) | 24 (55.8) |
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | p | HR | 95% CI | p |
| Age (≥60 vs. <60) | 1.403 | 0.868–2.270 | 0.167 | |||
| ECOG status (>0 vs. 0) | 1.996 | 1.235–3.226 | 0.005 | 2.726 | 1.628–4.564 | <0.001 |
| Hormone receptor, positive (yes vs. no) | 1.228 | 0.767–1.966 | 0.393 | |||
| HER2 status (IHC 2+/ISH + vs. IHC 3+) | 1.226 | 0.692–2.173 | 0.485 | |||
| CNS metastasis (yes vs. no) | 1.377 | 0.832–2.278 | 0.214 | |||
| Liver metastasis (yes vs. no) | 1.404 | 0.782–2.524 | 0.256 | |||
| Bone metastasis (yes vs. no) | 1.270 | 0.775–2.079 | 0.343 | |||
| Number of metastatic sites (≥2 vs. <2) | 1.545 | 0.957–2.493 | 0.075 | 1.603 | 1.026–2.685 | 0.046 |
| Treatment line of T-DM1(≥2 vs. <2) | 1.804 | 1.113–2.926 | 0.017 | 1.325 | 0.775–2.265 | 0.303 |
| Prior pertuzumab use | 1.714 | 1.022–2.876 | 0.047 | 1.358 | 0.780–2.363 | 0.279 |
| NLR (high vs. low) | 1.748 | 1.080–2.827 | 0.023 | 1.590 | 0.939–2.693 | 0.084 |
| PLR (high vs. low) | 1.554 | 0.966–2.499 | 0.069 | 1.239 | 0.745–2.059 | 0.408 |
| PIV value (high vs. low) | 2.216 | 1.388–3.539 | 0.001 | 2.332 | 1.408–3.861 | 0.001 |
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | p Value | HR | 95% CI | p Value |
| Age (≥60 vs. <60) | 0.978 | 0.641–1.491 | 0.916 | |||
| ECOG status (>0 vs. 0) | 1.290 | 0.867–1.919 | 0.209 | |||
| Hormone receptor, positive (yes vs. no) | 1.352 | 0.908–2.014 | 0.138 | |||
| HER2 status (IHC 2+/ISH + vs. IHC 3+) | 0.905 | 0.570–1.436 | 0.671 | |||
| CNS metastasis (yes vs. no) | 0.992 | 0.646–1.524 | 0.972 | |||
| Liver metastasis (yes vs. no) | 1.381 | 0.835–2.283 | 0.209 | |||
| Bone metastasis (yes vs. no) | 1.450 | 0.948–2.217 | 0.086 | 1.120 | 0.673–1.866 | 0.662 |
| Number of metastatic sites (≥2 vs. <2) | 1.831 | 1.212–2.767 | 0.004 | 1.435 | 0.952–2.163 | 0.085 |
| Treatment line of T-DM1(≥2 vs. <2) | 1.716 | 1.139–2.585 | 0.010 | 1.735 | 1.141–2.637 | 0.010 |
| Prior pertuzumab use | 1.475 | 0.971–2.240 | 0.068 | 1.159 | 0.748–1.196 | 0.509 |
| NLR (high vs. low) | 1.300 | 0.873–1.935 | 0.197 | |||
| PLR (high vs. low) | 1.207 | 0.811–1.797 | 0.353 | |||
| PIV value (high vs. low) | 2.341 | 1.545–3.548 | <0.001 | 2.423 | 1.585–3.702 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahin, T.K.; Akyildiz, A.; Dogan, O.T.; Kavgaci, G.; Guven, D.C.; Aksoy, S. Prognostic Significance of Pan-Immune-Inflammation Value in Patients with HER2-Positive Metastatic Breast Cancer Treated with Trastuzumab Emtansine. Pharmaceuticals 2024, 17, 824. https://doi.org/10.3390/ph17070824
Sahin TK, Akyildiz A, Dogan OT, Kavgaci G, Guven DC, Aksoy S. Prognostic Significance of Pan-Immune-Inflammation Value in Patients with HER2-Positive Metastatic Breast Cancer Treated with Trastuzumab Emtansine. Pharmaceuticals. 2024; 17(7):824. https://doi.org/10.3390/ph17070824
Chicago/Turabian StyleSahin, Taha Koray, Arif Akyildiz, Osman Talha Dogan, Gozde Kavgaci, Deniz Can Guven, and Sercan Aksoy. 2024. "Prognostic Significance of Pan-Immune-Inflammation Value in Patients with HER2-Positive Metastatic Breast Cancer Treated with Trastuzumab Emtansine" Pharmaceuticals 17, no. 7: 824. https://doi.org/10.3390/ph17070824
APA StyleSahin, T. K., Akyildiz, A., Dogan, O. T., Kavgaci, G., Guven, D. C., & Aksoy, S. (2024). Prognostic Significance of Pan-Immune-Inflammation Value in Patients with HER2-Positive Metastatic Breast Cancer Treated with Trastuzumab Emtansine. Pharmaceuticals, 17(7), 824. https://doi.org/10.3390/ph17070824






