Endometriosis: Molecular Pathophysiology and Recent Treatment Strategies—Comprehensive Literature Review
Abstract
1. Introduction
2. Aim
3. Methods
3.1. Article Selection Process and Selection Criteria
3.2. Inclusion Criteria
- Studies published in peer-reviewed journals;
- Articles in English;
- Research on the molecular mechanisms of endometriosis;
- Studies discussing current and emerging treatment strategies;
- Reviews, meta-analyses, clinical trials, and observational studies.
3.3. Exclusion Criteria
- Non-peer-reviewed articles;
- Studies not focused on endometriosis;
- Articles in languages other than English;
- Publications older than 20 years, unless they were seminal works.
3.4. Synthesis of Findings
4. Pathophysiology of Endometriosis
4.1. Inhibiting RAF/MEK/ERK Signal Pathway by Targeting RKIP Reduce Progression of Ectopic Lesions
4.2. Increased Levels of miRNAs Favor Survival Feature and Lead to Progesterone Resistance
4.3. Dysregulation of Immune System Concerning the Roles of Macrophages and Treg Cells
5. Surgical Treatment
5.1. Ablation vs. Excision
5.2. Peritoneal Endometriosis
5.3. Ovarian Endometriosis
5.4. Deep Endometriosis (DE)
5.5. Interruption of Pelvic Nerve Pathways: LUNA and PSN
6. Medical Approach
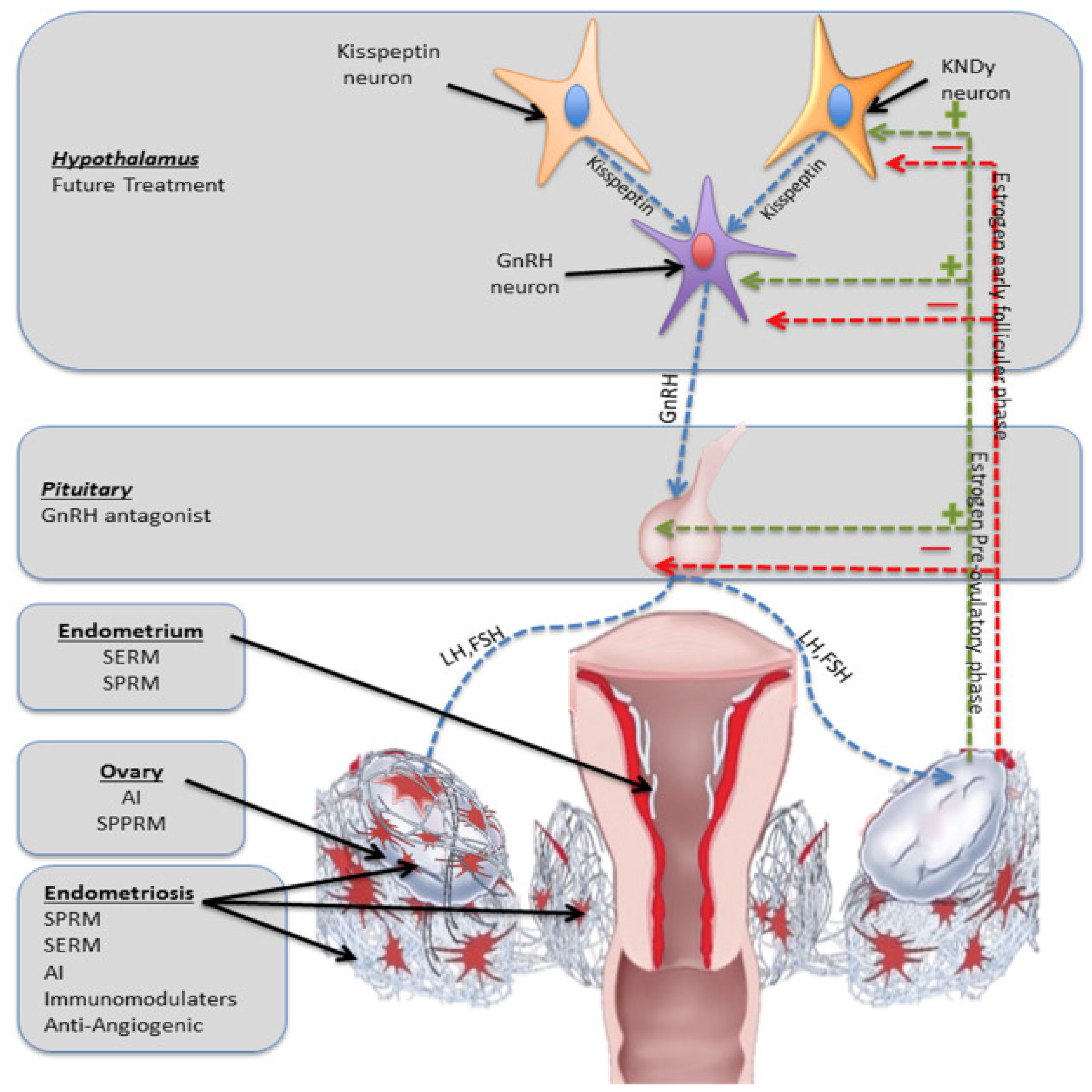
6.1. GnRH Antagonist and Agonist
- Progestogen—norethisterone octate;
- Combination of estrogen and progesterone;
- Selective estrogen receptor modulator (SERM);
- Bisphosphonates or testosterone [102].
6.2. Selective Progesterone Receptor Modulators (SPRMs)
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations
| ACOG | American college of obstetricians and gynecologists |
| AMH | Anti-Müllerian hormone |
| ASRM | American Society for Reproductive Medicine |
| CNGOF | Collège national des gynécologues et obstétriciens |
| COL7A1 | Collagen type VII alpha 1 chain |
| COX-2 | Cyclooxygenase-2 |
| CPP | Chronic pelvic pain |
| DE | Deep endometriosis |
| ESHRE | European Society of Human Reproduction and Embryology |
| FDA | Food and Drug Administration |
| FSH | Follicle-stimulating hormone |
| GnRH | Gonadotropin-releasing hormone |
| HEM15A cells | Endometriosis-derived immortalized eutopic endometrium stromal and epithelial cell lines |
| HPO | Hypothalamus–pituitary–ovarian axis |
| IDEA | International In-depth Endometriosis Analysis |
| IHEECs | Immortalized human endometrial epithelial cells |
| IVF | In vitro fertilization |
| LH | Luteinizing hormone |
| LUNA | Laparoscopic Uterine Nerve Ablation |
| MAPKs | Mitogen-activated protein kinases |
| MAPKs/ERKs | Mitogen-activated protein kinases (MAPKs), originally called extracellular signal-regulated kinases (ERKs) |
| miRNA | Micro-RNA |
| MMPs | Matrix metalloproteinases |
| MRI | Magnetic resonance imaging |
| NICE | National Institute for Health and Care Excellence |
| NF-kB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| PAECs | Progesterone receptor modulator-associated endometrial changes25 |
| PGE2 | Prostaglandin E2 |
| PI3K/AKT/mTOR pathway | An intracellular signaling pathway important in regulating the cell cycle |
| RKIP | RAF-kinase inhibitor protein |
| ROS | Reactive oxygen species |
| PR | Progesterone receptor |
| PSN | Presacral neurectomy |
| SERMs | Selective estrogen receptor modulators |
| SOGC | Society of Obstetricians and Gynaecologists of Canada |
| SPE | Superficial peritoneal endometriosis |
| SRC-1 | Steroid receptor coactivator-1 |
| SPRMs | Selective progesterone receptor modulators |
| RCTs | Randomized controlled trials |
| TIMPs | Tissue inhibitors of metalloproteinases |
| TFAP2C | Transcription factor family activator protein 2 |
| UPA | Ulipristal acetate |
| UPK1 B | Uroplakin-1b |
| WES | World Education Services |
References
- Zondervan, K.T.; Becker, C.M.; Missmer, S.A. Endometriosis. N. Engl. J. Med. 2020, 382, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.S.; Kotlyar, A.M.; Flores, V.A. Endometriosis is a chronic systemic disease: Clinical challenges and novel innovations. Lancet 2021, 397, 839–852. [Google Scholar] [CrossRef] [PubMed]
- Giudice, L.C. Clinical practice. Endometr. N. Engl. J. Med. 2010, 362, 2389–2398. [Google Scholar] [CrossRef] [PubMed]
- Shafrir, A.L.; Farland, L.V.; Shah, D.K.; Harris, H.R.; Kvaskoff, M.; Zondervan, K.; Missmer, S.A. Risk for and consequences of endometriosis: A critical epidemiologic review. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 51, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Simoens, S.; Dunselman, G.; Dirksen, C.; Hummelshoj, L.; Bokor, A.; Brandes, I.; Brodszky, V.; Canis, M.; Colombo, G.L.; DeLeire, T.; et al. The burden of endometriosis: Costs and quality of life of women with endometriosis and treated in referral centres. Hum. Reprod. 2012, 27, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Polak, G.; Banaszewska, B.; Filip, M.; Radwan, M.; Wdowiak, A. Environmental Factors and Endometriosis. Int. J. Environ. Res. Puvlic Health 2021, 18, 11025. [Google Scholar] [CrossRef] [PubMed]
- Klemmt, P.A.B.; Carver, J.G.; Koninckx, P.; McVeigh, E.J.; Mardon, H.J. Endometrial cells from women with endometriosis have increased adhesion and proliferative capacity in response to extracellular matrix components: Towards a mechanistic model for endometriosis progression. Hum. Reprod. 2007, 22, 3139–3147. [Google Scholar] [CrossRef] [PubMed]
- Alimi, Y.; Iwanaga, J.; Loukas, M.; Tubbs, R.S. The clinical anatomy of endometriosis: A review. Cureus J. Med. Sci. 2018, 10, e3361. [Google Scholar] [CrossRef]
- Sillem, M.; Prifti, S.; Neher, M.; Runnebaum, B. Extracellular matrix remodelling in the endometrium and its possible relevance to the pathogenesis of endometriosis. Hum. Reprod. Update 1998, 4, 730–735. [Google Scholar] [CrossRef]
- Reis, F.M.; Petraglia, F.; Taylor, R.N. Endometriosis: Hormone regulation and clinical consequences of chemotaxis and apoptosis. Hum. Reprod. Update 2013, 19, 406–418. [Google Scholar] [CrossRef]
- Han, S.J.; Jung, S.Y.; Wu, S.-P.; Hawkins, S.M.; Park, M.J.; Kyo, S.; Qin, J.; Lydon, J.P.; Tsai, S.Y.; Tsai, M.J.; et al. Estrogen receptor β modulates apoptosis complexes and the inflammasome to drive the pathogenesis of endometriosis. Cell 2015, 163, 960–974. [Google Scholar] [CrossRef] [PubMed]
- Donnez, J.; Smoes, P.; Gillerot, S.; Casanas-Roux, F.; Nisolle, M. Vascular endothelial growth factor (VEGF) in endometriosis. Hum. Reprod. 1998, 13, 1686–1690. [Google Scholar] [CrossRef] [PubMed]
- Symons, L.K.; Miller, J.E.; Kay, V.R.; Marks, R.M.; Liblik, K.; Koti, M.; Tayade, C. The immunopathophysiology of endometriosis. Trends Mol. Med. 2018, 24, 748–762. [Google Scholar] [CrossRef] [PubMed]
- Vinatier, D.; Cosson, M.; Dufour, P. Is endometriosis an endometrial disease? Eur. J. Obstet. Gynecol. Reprod. Biol. 2000, 91, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Borelli, V.; Martinelli, M.; Luppi, S.; Vita, F.; Romano, F.; Fanfani, F.; Trevisan, E.; Celsi, F.; Zabucchi, G.; Zanconati, F.; et al. Mast Cells in Peritoneal Fluid From Women with Endometriosis and Their Possible Role in Modulating Sperm Function. Front Physiol. 2020, 10, 1543. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Van Langendonckt, A.; Casanas-Roux, F.; Donnez, J. Oxidative stress and peritoneal endometriosis. Fertil. Steril. 2000, 77, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Donnez, J.; Binda, M.M.; Donnez, O.; Dolmans, M.M. Oxidative stress in the pelvic cavity and its role in the pathogenesis of endometriosis. Fertil. Steril. 2016, 106, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Nisolle, M.; Donnez, J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil. Steril. 1997, 68, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Nezhat, C.; Lindheim, S.R.; Backhus, L.; Vu, M.; Vang, N.; Nezhat, A.; Nezhat, C. Thoracic endometriosis syndrome: A review of diagnosis and management. JSLS 2019, 23, e2019. [Google Scholar] [CrossRef] [PubMed]
- Keckstein, J.; Saridogan, E. The Enzian classification: A comprehensive non-invasive and surgical description system for endometriosis. Acta Obstet. Gynecol. Scand. 2021, 100, 1165–1175. [Google Scholar] [CrossRef]
- Maignien, C.; Santulli, P.; Gayet, V.; Lafay-Pillet, M.C.; Korb, D.; Bourdon, M.; Marcellin, L.; de Ziegler, D.; Chapron, C. Prognostic factors for assisted reproductive technology in women with endometriosis-related infertility. Am. J. Obstet. Gynecol. 2017, 216, 280.e1–280.e9. [Google Scholar] [CrossRef]
- Maignien, C.; Santulli, P.; Marcellin, L.; Korb, D.; Bordonne, C.; Dousset, B.; Bourdon, M.; Chapron, C. Infertility in women with bowel endometriosis: First-line assisted reproductive technology results in satisfactory cumulative live-birth rates. Fertil. Steril. 2021, 115, 692–701. [Google Scholar] [CrossRef] [PubMed]
- The Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility: A committee opinion. Fertil. Steril. 2012, 98, 591–593. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nicholes, K.; Shih, I.M. The origin and pathogenesis of endometriosis. Annu. Rev. Pathol. 2020, 15, 71–95. [Google Scholar] [CrossRef] [PubMed]
- Laganà, A.S.; Garzon, S.; Götte, M.; Viganò, P.; Franchi, M.; Ghezzi, F.; Martin, D.C. The pathogenesis of endometriosis: Molecular and cell biology insights. Int. J. Mol. Sci. 2019, 20, 5615. [Google Scholar] [CrossRef]
- Romano, A.; Xanthoulea, S.; Giacomini, E.; Delvoux, B.; Alleva, E.; Vigano, P. Endometriotic cell culture contamination and authenticity: A source of bias in in vitro research? Hum. Reprod. 2020, 35, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Giuliano, C.J.; Palladino, A.; John, K.M.; Abramowicz, C.; Yuan, M.L.; Sausville, E.L.; Lukow, D.A.; Liu, L.; Chait, A.R.; et al. Off-target toxicity is a common mechanism of action of cancer drugs undergoing clinical trials. Sci. Transl. Med. 2019, 11, eaaw8412. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Chen, S.; Zhao, M.; Li, Z.; Zhu, L. Ginsenoside Rg3 attenuates endometriosis by inhibiting the viability of human ectopic endometrial stromal cells through the nuclear factor-kappaB signaling pathway. J. Gynecol. Obstet. Human. Reprod. 2020, 49, 101642. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.G.; Lenk, E.E.; Lebovic, D.I.; Shu, Y.; Yu, J.; Taylor, R.N. Pathogenesis of endometriosis: Interaction between endocrine and inflammatory pathways. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 50, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Lin, X.; Kapoor, A.; Gu, Y.; Xu, H.; Major, P.; Tang, D. Insights of RKIP-Derived suppression of prostate cancer. Cancers 2021, 13, 6388. [Google Scholar] [CrossRef]
- Lee, D.; Kim, S.K.; Lee, J.R.; Jee, B.C. Management of endometriosis-related infertility: Considerations and treatment options. Clin. Exp. Reprod. Med. 2020, 23, 1–11. [Google Scholar] [CrossRef]
- Tang, X.-L.; Zhang, F.-L.; Jiang, X.-J.; Yang, X.-J. Telocytes enhanced the proliferation, adhesion and motility of endometrial stromal cells as mediated by the ERK pathway in vitro. Am. J. Transl. Res. 2019, 11, 572–585. [Google Scholar] [PubMed]
- Dong, P.; Ling, L.; Hu, L. Systematic review and meta-analysis of traditional Chinese medicine compound in treating infertility caused by endometriosis. Ann. Palliat. Med. 2021, 10, 12631–12642. [Google Scholar] [CrossRef] [PubMed]
- Koninckx, P.R.; Fernandes, R.; Ussia, A.; Schindler, L.; Wattiez, A.; Al-Suwaidi, S.; Amro, B.; Al-Maamari, B.; Hakim, Z.; Tahlak, M. Pathogenesis based diagnosis and treatment of endometriosis. Front. Endocrinol. 2021, 12, 745548. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Hang, Y.; Zhang, T.; Tan, L.; Li, S.; Jin, Y. USP10 promotes proliferation and migration and inhibits apoptosis of endometrial stromal cells in endometriosis through activating the raf-1/MEK/ERK pathway. Am. J. Physiol. Cell Physiol. 2018, 315, C863–C872. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, J.J.; Wang, X.F.; Wang, Q. Clinical and prognostic significance of raf kinase inhibitory protein expression in gastrointestinal stromal tumors. World J. Gastroenterol. 2018, 24, 2508–2517. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Meenakshisundaram, S.; Manickam, M.; Sankaranarayanan, M. A medicinal chemistry perspective of drug repositioning: Recent advances and challenges in drug discovery. Eur. J. Med. Chem. 2020, 195, 112275. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.-F.; Chiu, T.-L.; Huang, S.-Y.; Hsieh, T.-F.; Chang, S.-F.; Ruan, J.-W.; Chen, S.-P.; Pang, C.-Y.; Chiu, S.-C. Anti-cancer effects of Radix angelica sinensis (Danggui) and N-butylidenephthalide on gastric cancer: Implications for REDD1 activation and mTOR inhibition. Cell Physiol. Biochem. 2018, 48, 2231–2246. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.M.; Sureda, A.; Xiao, J.; Dehpour, A.R.; Shirooie, S.; Silva, A.S.; Baldi, A.; Khan, H.; Daglia, M. Anti-inflammatory effects of melatonin: A mechanistic review. Crit. Rev. Food Sci. Nutr. 2019, 59 (Suppl. S1), S4–S16. [Google Scholar] [CrossRef]
- Gou, Y.; Li, X.; Li, P.; Zhang, H.; Xu, T.; Wang, H.; Wang, B.; Ma, X.; Jiang, X.; Zhang, Z. Estrogen receptor β upregulates CCL2 via NF-κB signaling in endometriotic stromal cells and recruits macrophages to promote the pathogenesis of endometriosis. Hum. Reprod. 2019, 34, 646–658. [Google Scholar] [CrossRef]
- Carafa, V.; Altucci, L.; Nebbioso, A. Dual tumor suppressor and tumor promoter action of sirtuins in determining malignant phenotype. Front. Pharmacol. 2019, 10, 38. [Google Scholar] [CrossRef]
- Zhang, T.; de Carolis, C.; Man, G.C.W.; Wang, C.C. The link between immunity, autoimmunity and endometriosis: A literature update. Autoimmun. Rev. 2018, 17, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Sadłocha, M.; Toczek, J.; Czuba, Z.; Stojko, R. Effects of Macrophage Inflammatory Protein 1 Alpha and Beta Chemokine Concentrations in the Progression of Endometriosis. Clin. Exp. Obstet. Gynecol. 2022, 49, 153. [Google Scholar] [CrossRef]
- Viganò, P.; Ottolina, J.; Bartiromo, L.; Bonavina, G.; Schimberni, M.; Villanacci, R.; Candiani, M. Cellular components contributing to fibrosis in endometriosis: A literature review. J. Minim. Invasive Gynecol. 2020, 27, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, G.; Bao, Y.; Wu, Y.; You, Q. Evaluation and Application of Tools for the Identification of Known MicroRNAs in Plants. Appl. Plant Sci. 2021, 9, e11414. [Google Scholar] [CrossRef]
- Nguyen, J.-M.; Jézéquel, P.; Gillois, P.; Silva, L.; Ben Azzouz, F.; Lambert-Lacroix, S.; Juin, P.; Campone, M.; Gaultier, A.; Moreau-Gaudry, A.; et al. Random Forest of Perfect Trees: Concept, Performance, Applications, and Perspectives. Bioinformatic 2021, 37, 2165–2174. [Google Scholar] [CrossRef] [PubMed]
- Lecointre, L.; Bund, V.; Sangnier, E.; Ouldamer, L.; Bendifallah, S.; Koskas, M.; Bolze, P.-A.; Collinet, P.; Canlorbe, G.; Touboul, C.; et al. Status of Surgical Management of Borderline Ovarian Tumors in France: Are Recommendations Being Followed? Multicentric French Study by the FRANCOGYN Group. Ann. Surg. Oncol. 2021, 28, 7616–7623. [Google Scholar] [CrossRef]
- Geoffron, S.; Lier, A.; de Kermadec, E.; Sermondade, N.; Varinot, J.; Thomassin-Naggara, I.; Bendifallah, S.; Daraï, E.; Chabbert-Buffet, N.; Kolanska, K. Fertility Preservation in Women with Malignant and Borderline Ovarian Tumors: Experience of the French ESGO-Certified Center and Pregnancy-Associated Cancer Network (CALG). Gynecol. Oncol. 2021, 161, 817–824. [Google Scholar] [CrossRef]
- Kern, F.; Krammes, L.; Danz, K.; Diener, C.; Kehl, T.; Küchler, O.; Fehlmann, T.; Kahraman, M.; Rheinheimer, S.; Aparicio- Puerta, E.; et al. Validation of Human MicroRNA Target Pathways Enables Evaluation of Target Prediction Tools. Nucleic Acids Res. 2021, 49, 127–144. [Google Scholar] [CrossRef]
- Monnaka, V.U.; Hernandes, C.; Heller, D.; Podgaec, S. Overview of MiRNAs for the Non-Invasive Diagnosis of Endometriosis: Evidence, Challenges and Strategies. A Systematic Review. Einstein Sao Paulo Braz. 2021, 19, eRW5704. [Google Scholar] [CrossRef]
- El-Mogy, M.; Lam, B.; Haj-Ahmad, T.A.; McGowan, S.; Yu, D.; Nosal, L.; Rghei, N.; Roberts, P.; Haj-Ahmad, Y. Diversity and Signature of Small RNA in Different Bodily Fluids Using next Generation Sequencing. BMC Genom. 2018, 19, 408. [Google Scholar] [CrossRef]
- Moustafa, S.; Burn, M.; Mamillapalli, R.; Nematian, S.; Flores, V.; Taylor, H.S. Accurate Diagnosis of Endometriosis Using Serum MicroRNAs. Am. J. Obstet. Gynecol. 2020, 223, 557.e1–557.e11. [Google Scholar] [CrossRef] [PubMed]
- Maged, A.M.; Deeb, W.S.; El Amir, A.; Zaki, S.S.; El Sawah, H.; Al Mohamady, M.; Metwally, A.A.; Katta, M.A. Diagnostic Accuracy of Serum MiR-122 and MiR-199a in Women with Endometriosis. Int. J. Gynaecol. Obstet. Off. Organ. Int. Fed. Gynaecol. Obstet. 2018, 141, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson Teague, E.M.; Van der Hoek, K.H.; Van der Hoek, M.B.; Perry, N.; Wagaarachchi, P.; Robertson, S.A.; Print, C.G.; Hull, L.M. MicroRNA-regulated pathways associated with endometriosis. Mol. Endocrinol. 2009, 23, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Filigheddu, N.; Gregnanin, I.; Porporato, P.E.; Surico, D.; Perego, B.; Galli, L.; Patrignani, C.; Graziani, A.; Surico, N. Differential expression of microRNAs between eutopic and ectopic endometrium in ovarian endometriosis. J. Biomed. Biotechnol. 2010, 2010, 369549. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, S.M.; Creighton, C.J.; Hay, D.Y.; Zariff, A.; Anderson, M.L.; Gunaratne, P.H.; Matzuk, M.M. Functional microRNA involved in endometriosis. Mol. Endocrinol. 2011, 25, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Braza-Boils, A.; Mari-Alexandre, J.; Gilabert, J.; Sanchez-Izquierdo, D.; Espana, F.; Estelles, A.; ilabert-Estelles, J. MicroRNA expression profile in endometriosis: Its relation to angiogenesis and fibrinolytic factors. Hum. Reprod. 2014, 29, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-Y.; Hsieh, T.-H.; Tsai, C.-F.; Tsai, H.-P.; Chen, H.-S.; Chang, Y.; Chuang, H.Y.; Lee, J.N.; Hsu, Y.L.; Tsai, E.M. MiRNA-199a-5p regulates VEGFA in endometrial mesenchymal stem cells and contributes to the pathogenesis of endometriosis. J. Pathol. 2014, 232, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Wan, X.; La, X.; Gong, X.; Cai, X. miR-29c is downregulated in the ectopic endometrium and exerts its effects on endometrial cell proliferation, apoptosis and invasion by targeting c-Jun. Int. J. Mol. Med. 2015, 35, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lu, J.; Sun, M.; Mi, S.; Zhang, H.; Luo, R.T.; Chen, P.; Wang, Y.; Yan, M.; Qian, Z.; et al. Distinct microRNA expression profiles in acute myeloid leukemia with common translocations. Proc. Nat. Acad. Sci. USA 2008, 105, 15535–15540. [Google Scholar] [CrossRef]
- Tavazoie, S.F.; Alarcón, C.; Oskarsson, T.; Padua, D.; Wang, Q.; Bos, P.D.; Gerald, W.L.; Massagué, J. Endogenous human microRNAs that suppress breast cancer metastasis. Nature 2008, 451, 147–152. [Google Scholar] [CrossRef]
- Joshi, N.R.; Miyadahira, E.H.; Afshar, Y.; Jeong, J.W.; Young, S.L.; Lessey, B.A.; Serafini, P.C.; Fazleabas, A.T. Progesterone resistance in endometriosis is modulated by the altered expression of microRNA-29c and FKBP4. J. Clin. Endocrinol. Metab. 2017, 102, 141–149. [Google Scholar] [CrossRef]
- Riccio, L.D.G.C.; Santulli, P.; Marcellin, L.; Abrăo, M.S.; Batteux, F.; Chapron, C. Immunology of Endometriosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 50, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Santoso, B.; Sa’adi, A.; Dwiningsih, S.R.; Tunjungseto, A.; Widyanugraha, M.Y.A.; Mufid, A.F.; Rahmawati, K.Y.; Ahsan, F. Soluble Immune Checkpoints CTLA-4, HLA-G, PD-1, and PD-L1 Are Associated with Endometriosis-Related Infertility. Am. J. Reprod. Immunol. 2020, 84, e13296. [Google Scholar] [CrossRef] [PubMed]
- Izumi, G.; Koga, K.; Takamura, M.; Makabe, T.; Satake, E.; Takeuchi, A.; Taguchi, A.; Urata, Y.; Fujii, T.; Osuga, Y. Involvement of Immune Cells in the Pathogenesis of Endometriosis. J. Obstet. Gynaecol. Res. 2018, 44, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Bacci, M.; Capobianco, A.; Monno, A.; Cottone, L.; Puppo, F.D.; Camisa, B.; Mariani, M.; Brignole, C.; Ponzoni, M.; Ferrari, S.; et al. Macrophages Are Alternativel Activated in Patients with Endometriosis and Required for Growth and Vascularization of Lesions in a Mouse Model of Disease. Am. J. Pathol. 2009, 175, 547–556. [Google Scholar] [CrossRef]
- Lukács, L.; Kovács, A.R.; Pál, L.; Szűcs, S.; Kövér, Á.; Lampé, R. Phagocyte Function of Peripheral keutrophil Granulocytes and Monocytes in Endometriosis before and after Surgery. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 101796. [Google Scholar] [CrossRef]
- Berbic, M.; Schulke, L.; Markham, R.; Tokushige, K.; Russell, P.; Fraser, I.S. Macrophage Expression in Endometrium of Women with and without Endometriosis. Hum. Reprod. 2009, 24, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Greaves, E.; Temp, J.; Esnal-Zufiurre, A.; Mechsner, S.; Horne, A.W.; Saunders, P.T.K. Estradiol Is a Critical Mediator of Macrophage-kerve Cross Talk in Peritoneal Endometriosis. Am. J. Pathol. 2015, 185, 2286–2297. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Yamaguchi, T.; Komura, T.; Ono, M. Regulatory T cells and immune tolerance. Cell 2008, 133, 775–787. [Google Scholar] [CrossRef]
- Davis, A.C.; Goldberg, J.M. Extrapelvic Endometriosis. Semin. Reprod. Med. 2017, 35, 098–101. [Google Scholar]
- Mechsner, S.; Weichbrodt, M.; Riedlinger, W.F.J.; Bartley, J.; Kaufmann, A.M.; Schneider, A.; Köhler, C. Estrogen and Progestogen Receptor Positive Endometriotic Lesions and Disseminated Cells in Pelvic Sentinel Lymph kodes of Patients with Deep Infiltrating Rectovaginal Endometriosis: A Pilot Study. Hum. Reprod. 2008, 23, 2202–2209. [Google Scholar] [CrossRef] [PubMed]
- Olkowska-Truchanowicz, J.; Bocian, K.; Maksym, R.B.; Białoszewska, A.; Włodarczyk, D.; Baranowski, W.; Ząbek, J.; Korczak-Kowalska, G.; Malejczyk, J. CD4+ CD25+ FOXP3+ Regulatory T Cells inPeripheral Blood and Peritoneal Fluid of Patients with Endometriosis. Hum. Reprod. 2013, 28, 119–124. [Google Scholar] [CrossRef]
- Parazzini, F.; Esposito, G.; Tozzi, L.; Koli, S.; Bianchi, S. Epidemiology of Endometriosis and Its Comorbidities. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 209, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Montagna, P.; Capellino, S.; Villaggio, B.; Remorgida, V.; Ragni, k.; Cutolo, M.; Ferrero, S. PeritonealFluid Macrophages in Endometriosis: Correlation between the Expression of Estrogen Receptors and Inflammation. Fertil. Steril. 2008, 90, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Maksym, R.B.; Hoffmann-Młodzianowska, M.; Skibińska, M.; Rabijewski, M.; Mackiewicz, A.; Kieda, C. Immunology and Immunotherapy of Endometriosis. J. Clin. Med. 2021, 10, 5879. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.eshre.eu/Guideline/Endometriosis (accessed on 2 February 2022).
- Pundir, J.; Omanwa, K.; Kovoor, E.; Pundir, V.; Lancaster, G.; Barton-Smith, P. Laparoscopic excision versus ablation for endometriosis-associated pain: An updated systematic review and meta-analysis. J. Minim. Invasive Gynecol. 2017, 24, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Bafort, C.; Beebeejaun, Y.; Tomassetti, C.; Bosteels, J.; Duffy, J.M. Laparoscopic surgery for endometriosis. Cochrane Database Syst. Rev. 2020, 10, CD011031. [Google Scholar]
- Liu, Y.; Li, M.; Wei, C.; Tang, L.; Sheng, Y.; Liu, Y.; Li, D.; Ding, D.; Qiu, J.; Zhu, X. TSP1-CD47-SIRPαSignaling Facilitates the Development of Endometriosis by Mediating the Survival of Ectopic Endometrium. Am. J. Reprod. Immunol. 2020, 83, e13236. [Google Scholar] [CrossRef] [PubMed]
- Horne, A.W.; Daniels, J.; Hummelshoj, L.; Cox, E.; Cooper, K.G. Surgical removal of superficial peritoneal endometriosis for managing women with chronic pelvic pain: Time for a rethink? BJOG 2019, 126, 1414. [Google Scholar] [CrossRef]
- Carmona, F.; Martínez-Zamora, M.A.; Rabanal, A.; Martínez-Román, S.; Balasch, J. Ovarian cystectomy versus laser vaporization in the treatment of ovarian endometriomas: A randomized clinical trial with a five-year follow-up. Fertil. Steril. 2011, 96, 251–254. [Google Scholar] [CrossRef]
- KaJailzopoulos, D.R.; Mitsoupoulou, A.; Ilipoulou, S.M. Association between and gynecological cancer a critical review of literarures. Arch. Gynecol. Obst. 2020, 301, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Sepulveda, J.; Romeral, C.; Niño, G.; Pérez-Benavente, A. The Effect of Laparoscopic Surgery on Anti Miillerian Hormone: A Systematic Review of the Literature and MetaAnalysis. JBRA Assist. Reprod. 2022, 26, 88104. [Google Scholar]
- Pascoal, E.; Wessels, J.M.; Aas-Eng, M.K.; Abrao, M.S.; Condous, G.; Jurkovic, D.; Espada, M.; Exacoustos, C.; Ferrero, S.; Guerriero, S.; et al. Strengths and limitations of diagnostic tools for endometriosis and relevance in diagnostic test accuracy research. Ultrasound Obstet. Gynecol. 2022, 3, 309327. [Google Scholar] [CrossRef] [PubMed]
- Johan, M.Z.; Ingman, W.V.; Robertson, S.A.; Hull, M.L. Macrophages Infiltrating Endometriosislike Lesions Exhibit Progressive Phenotype Changes in a Heterologous Mouse Model. J. Reprod. Immunol. 2019, 132, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Duffy, J.M.; Arambage, K.; Correa, F.J.; Olive, D.; Farquhar, C.; Garry, R.; Barlow, D.H.; Jacobson, T.Z. Laparoscopic surgery for endometriosis. Cochrane Database Syst. Rev. 2014, 4, CD011031. [Google Scholar]
- Koninckx, P.R.; Ussia, A.; Adamyan, L.; Wattiez, A.; Donnez, J. Deep endometriosis: Definition, diagnosis, and treatment. Fertil. Steril. 2012, 98, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.sciencedirect.com/science/article/abs/pii/S1521693420301759 (accessed on 2 February 2022).
- Dunselman, G.A.J.; Vermeulen, N.; Becker, C.; Calhaz-Jorge, C.; D’Hooghe, T.; De Bie, B.; Heikinheimo, O.; Horne, A.W.; Kiesel, L.; Nap, A.; et al. ESHRE guideline: Management of women with endometriosis. Hum. Reprod. 2014, 29, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Fedele, L.; Aimi, G.; Pietropaolo, G.; Consonni, D.; Crosignani, P.G. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: A analysis of over 1000 patients. Hum. Reprod. 2007, 22, 266271. [Google Scholar] [CrossRef] [PubMed]
- Chapron, C.; Fauconnier, A.; Vieira, M.; Barakat, H.; Dousset, B.; Pansini, V.; Vacher-Lavenu, M.C.; Dubuisson, J.B. Anatomical distribution of deeply infiltrating endometriosis: Surgical implications and proposition for a classification. Hum. Reprod. 2003, 18, 157–161. [Google Scholar] [CrossRef]
- Bazot, M.; Gasner, A.; Ballester, M.; Darai, E. Value of thinsection oblique axial T2weighted magnetic resonance images to assess uterosacral ligament endometriosis. Hum. Reprod. 2011, 26, 346–353. [Google Scholar] [CrossRef]
- Redwine, D.B. Intestinal Endometriosis. Surgical Management of Endometriosis; Informa Healthcare: New York, NY, USA, 2004. [Google Scholar]
- Available online: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD011031.pub3/full (accessed on 2 February 2022).
- Roman, H.; Bubenheim, M.; Huet, E.; Bridoux, V.; Zacharopoulou, C.; Collinet, P.; Daraï, E.; Tuech, J.-J. Baseline severe constipation negatively impacts functional outcomes of surgery for deep endometriosis infiltrating the rectum: Results of the ENDORE randomized trial. J. Gynecol. Obstet. Hum. Reprod. 2019, 48, 6259. [Google Scholar] [CrossRef] [PubMed]
- Duffy, J.; Hirsch, M.; Vercoe, M.; Abbott, J.; Barker, C.; Collura, B.; Drake, R.; Evers, J.; Hickey, M.; Horne, A.; et al. A core outcome set for future endometriosis research: An international consensus development study. BJOG 2020, 127, 96774. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Somigliana, E.; Vigano, P.; Abbiati, A.; Barbara, G.; Crosignani, P.G. Surgery for endometriosis associated infertility: A pragmatic approach. Hum. Reprod. 2009, 24, 25469. [Google Scholar] [CrossRef] [PubMed]
- Audebert, A.; Petousis, S.; Margioula-Siarkou, C.; Ravanos, K.; Prapas, N.; Prapas, Y. Anatomic distribution of endometriosis: A reappraisal based on series of 1101 patients. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 230, 36. [Google Scholar] [CrossRef]
- Tang, H.; Wu, R.; Li, X.; Zhou, Y.; Liu, Z.; Wang, C.; Chen, Y.; Zhang, F. Curative effect of 1.88 mg and 3.75 mg gonadotrophinreleasing hormone agonist on stage IIIIV endometriosis: Randomized contro IIed study. J. Obstet. Gynaecol. Res. 2017, 43, 5501554. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Hu, M.; Hong, L.; Hong, S.; Ding, W.; Min, J.; Fang, G.; Guo, W. Clinical eficacy of addback therapy in treatment of endometriosis: A metaanalysis. Arch. Gynecol. Obstet. 2014, 290, 513523. [Google Scholar] [CrossRef]
- Taylor, H.S.; Giudice, L.C.; Lessey, B.A.; Abrao, M.S.; Kotarski, J.; Archer, D.F.; Diamond, M.P.; Surrey, E.; Johnson, N.P.; Watts, N.B.; et al. Treatment of Endometriosis-Associated Pain with Elagolix, an Oral GnRH Antagonist. N. Engl. I Med. 2017, 377, 284. [Google Scholar] [CrossRef] [PubMed]
- As-Sanie, S.; Becker, C.M.; Johnson, N. Efficacy and safety of relugolix combination therapy in women with endometriosis associated pain: Phase 3 randomized, doubleblind, placebocontrolled study [spirit 2]. Fertil. Steril. 2020, 114, e77. [Google Scholar] [CrossRef]
- Giudice, L.C.; As-Sanie, S.; Ferreira, J.C.A.; Becker, C.M.; Abrao, M.S.; Lessey, B.A.; Brown, E.; Dynowski, K.; Wilk, K.; Li, Y.; et al. Once daily oral relugolix combination therapy versus placebo in patients with endometriosisassociated pain: Two replicate phase randomised, doubleblind, studies (SPIRIT I and II). Lancet 2022, 10343, 2267–2279, Erratum in Lancet 2022, 10353, 660. [Google Scholar] [CrossRef]
- Leyland, N.; Estes, S.J.; Lessey, B.A.; Advincula, A.P.; Taylor, H.S. A Clinician’s Guide to the Treatment of Endometriosis with Elagolix. J. Womens Health 2021, 30, 569–578. [Google Scholar] [CrossRef]
- Agarwal, S.K.; Foster, W.G. Reduction in Endometrioza Size with Three Months of Aromatase Inhibition and Progestin Add Back. BioMed Res. Int. 2015, 2015, 878517. [Google Scholar] [CrossRef] [PubMed]
- Lusher, S.J.; Raaijmakers, H.C.A.; VuPham, D.; Dechering, K.; Lam, T.W.; Brown, A.R.; Hamilton, N.M.; Nimz, O.; Bosch, R.; McGuire, R.; et al. Structural Basis for Agonism and Antagonism for a Set of Chemically Related Progesterone Receptor Modulators. J. Biol. Chem. 2011, 286, 35079–35086. [Google Scholar] [CrossRef]
- Flores, V.A.; Vanhie, A.; Dang, T.; Taylor, H.S. Progesterone receptor status predicts response to progestin therapy in endome-triosis. J. Clin. Endocrinol. Metab. 2018, 103, 4561–4568. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, L.H.R.; Murray, A.A.; Matthews, R.; Shaw, G.; Williams, A.R.W.; Saunders, P.T.K.; Critchley, H.O.D. Selective Progesterone Receptor Modulator (SPRM) Ulipristal Acetate (UPA) and Its Effects on the Human Endometrium. Hum. Reprod. 2017, 32, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Schultze-Mosgau, M.-H.; Höchel, J.; Prien, O.; Zimmermann, T.; Brooks, A.; Bush, J.; Rottmann, A. Characterization of the Pharmacokinetics of Vilaprisan: Bioavailability, Excretion, Biotransformation, and Drug–Drug Interaction Potential. Clin. Pharmacokinet. 2018, 57, 1001–1015. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Man, G.C.; Hung, S.W.; Zhang, T.; Fung, L.W.; Cheung, C.W.; Chung, J.P.; Li, T.C.; Wang, C.C. Therapeutic effects of green tea on endometriosis. Crit. Rev. Food Sci. Nutr. 2021, 63, 3222–3235. [Google Scholar] [CrossRef]
- D’Alterio, M.K.; Saponara, S.; Agus, M.; Laganà, A.S.; Koventa, M.; Loi, E.S.; Feki, A.; Angioni, S. Medical and surgical interventions to improve the quality of life for endometriosis patients: A systematic review. Gynecol. Surg. 2021, 18, 13. [Google Scholar] [CrossRef]
- Brichant, G.; Laraki, I.; Henry, L.; Munaut, C.; Kisolle, M. New Therapeutics in Endometriosis: A Review of Hormonal, non-Hormonal, and non-Coding RkA Treatments. Int. J. Mol. Sci. 2021, 22, 10498. [Google Scholar] [CrossRef]
- Bedaiwy, M.A.; Alfaraj, S.; Yong, P.; Casper, R. New developments in the medical treatment of endometriosis. Fertil. Steril. 2017, 107, 555–565. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadłocha, M.; Toczek, J.; Major, K.; Staniczek, J.; Stojko, R. Endometriosis: Molecular Pathophysiology and Recent Treatment Strategies—Comprehensive Literature Review. Pharmaceuticals 2024, 17, 827. https://doi.org/10.3390/ph17070827
Sadłocha M, Toczek J, Major K, Staniczek J, Stojko R. Endometriosis: Molecular Pathophysiology and Recent Treatment Strategies—Comprehensive Literature Review. Pharmaceuticals. 2024; 17(7):827. https://doi.org/10.3390/ph17070827
Chicago/Turabian StyleSadłocha, Marcin, Jakub Toczek, Katarzyna Major, Jakub Staniczek, and Rafał Stojko. 2024. "Endometriosis: Molecular Pathophysiology and Recent Treatment Strategies—Comprehensive Literature Review" Pharmaceuticals 17, no. 7: 827. https://doi.org/10.3390/ph17070827
APA StyleSadłocha, M., Toczek, J., Major, K., Staniczek, J., & Stojko, R. (2024). Endometriosis: Molecular Pathophysiology and Recent Treatment Strategies—Comprehensive Literature Review. Pharmaceuticals, 17(7), 827. https://doi.org/10.3390/ph17070827







