Use of Extracellular Monomeric Ubiquitin as a Therapeutic Option for Major Depressive Disorder
Abstract
1. Introduction
2. Neuroimmunoendocrine and Inflammation Alterations in MDD
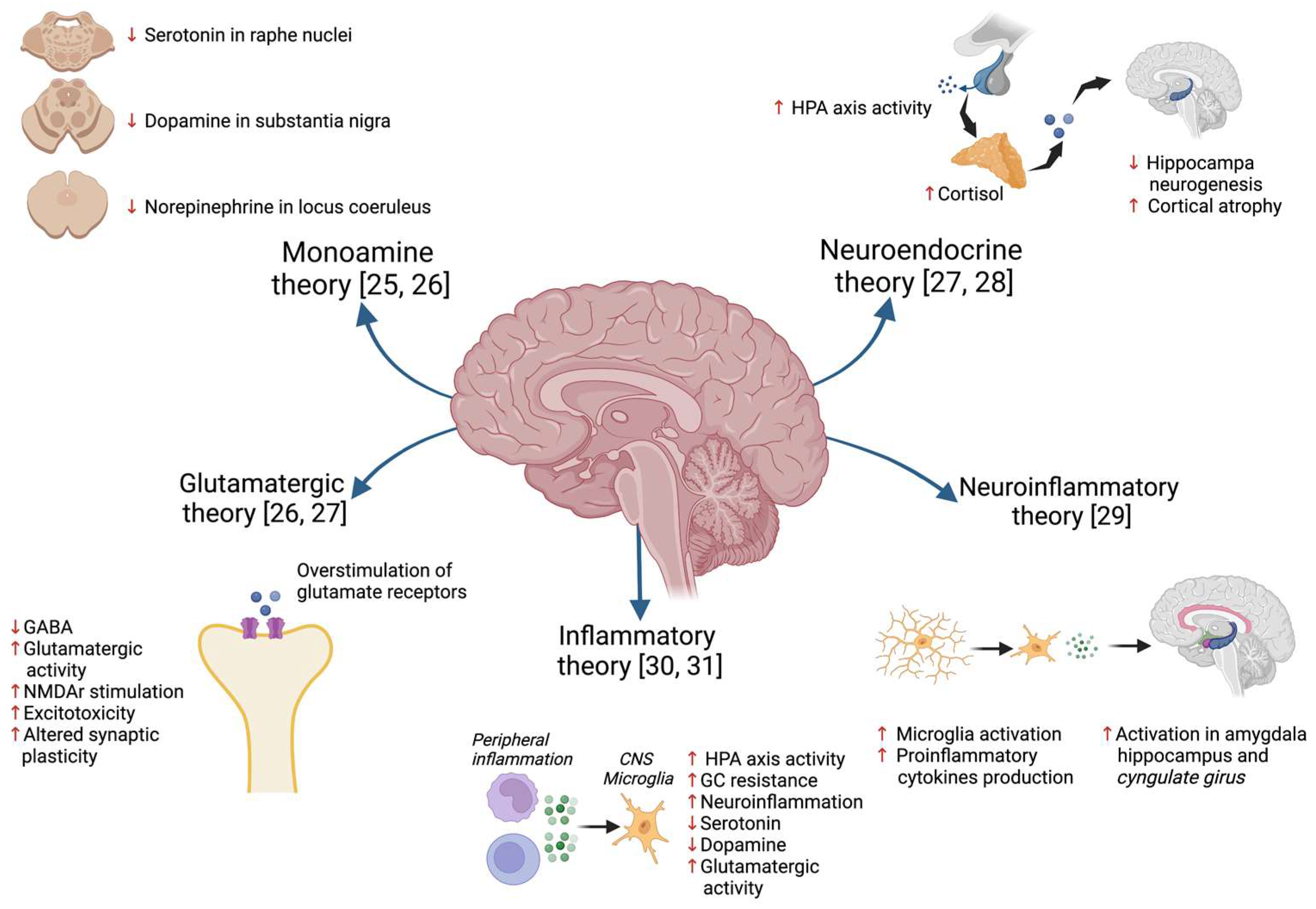
2.1. Hypothesis of MDD Pathophysiology
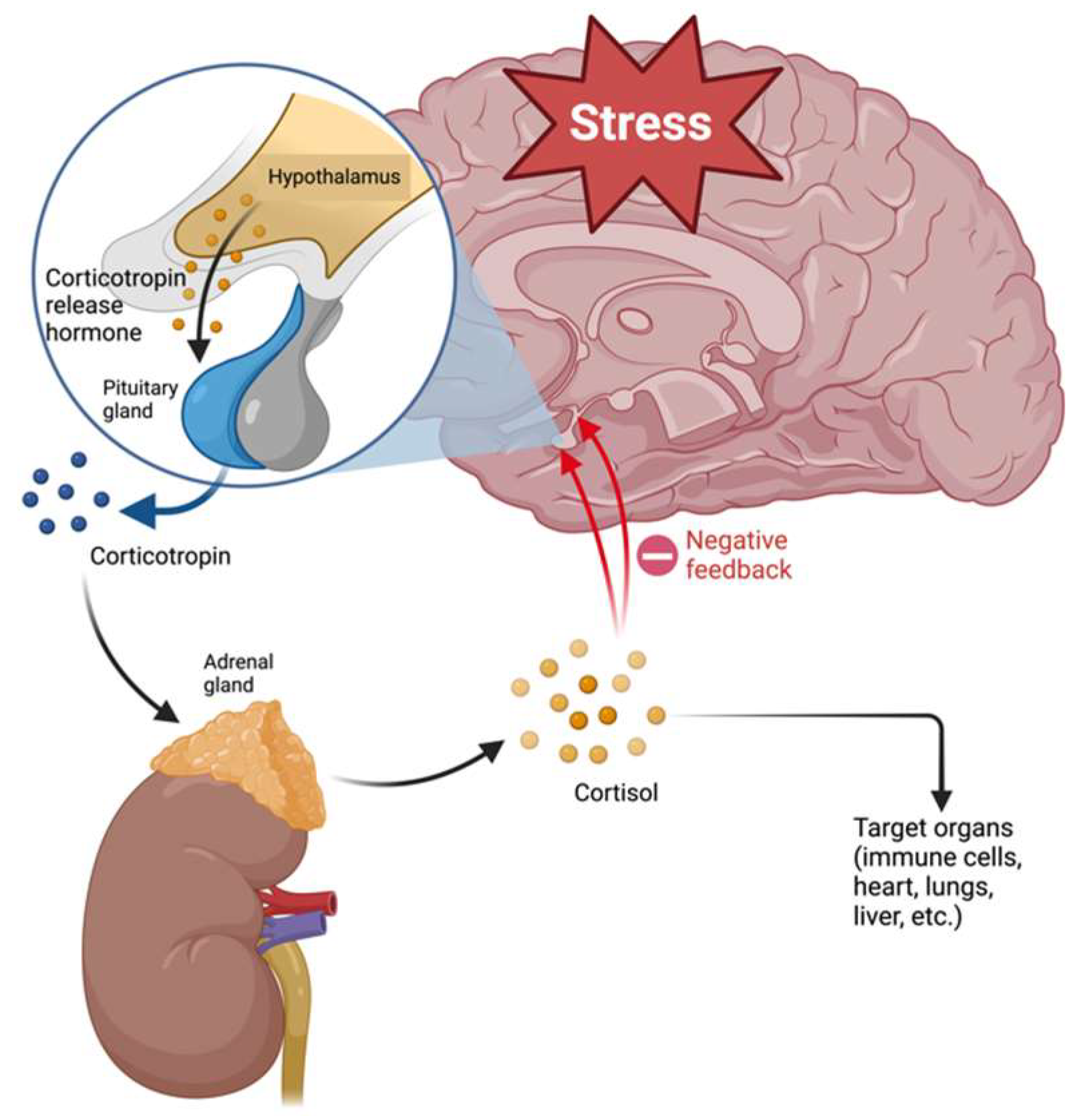
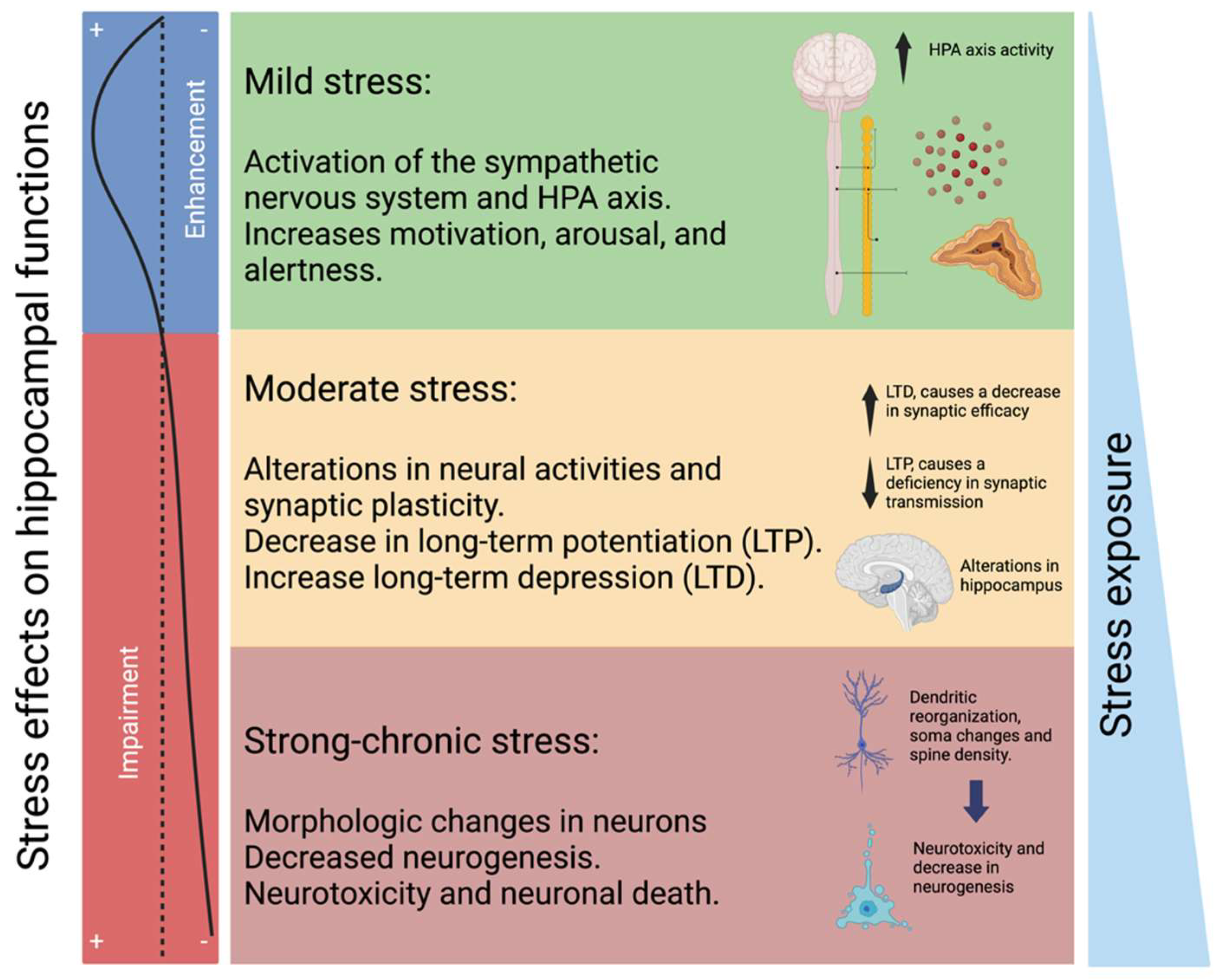
2.2. HPA Axis and Glucocorticoid Resistance
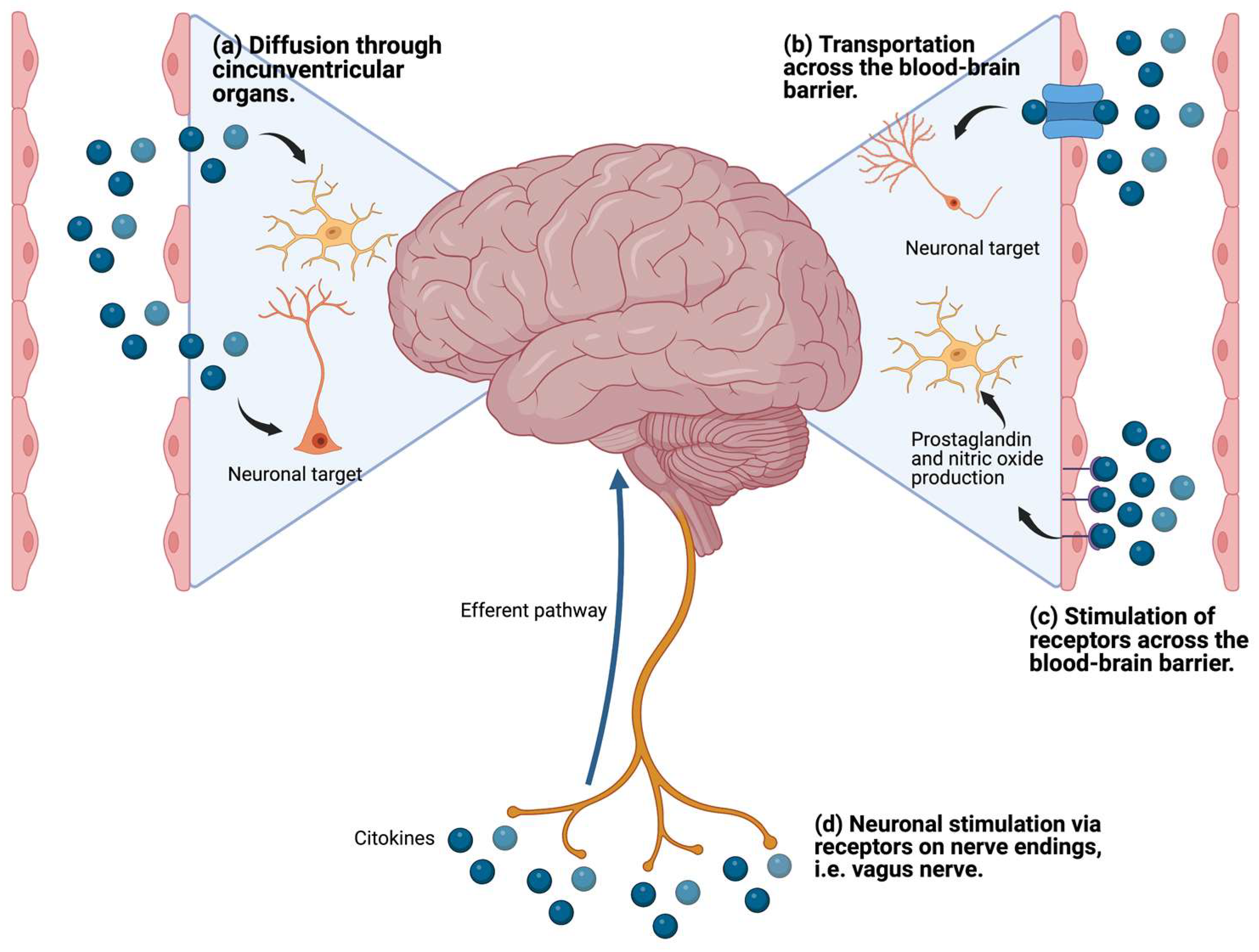
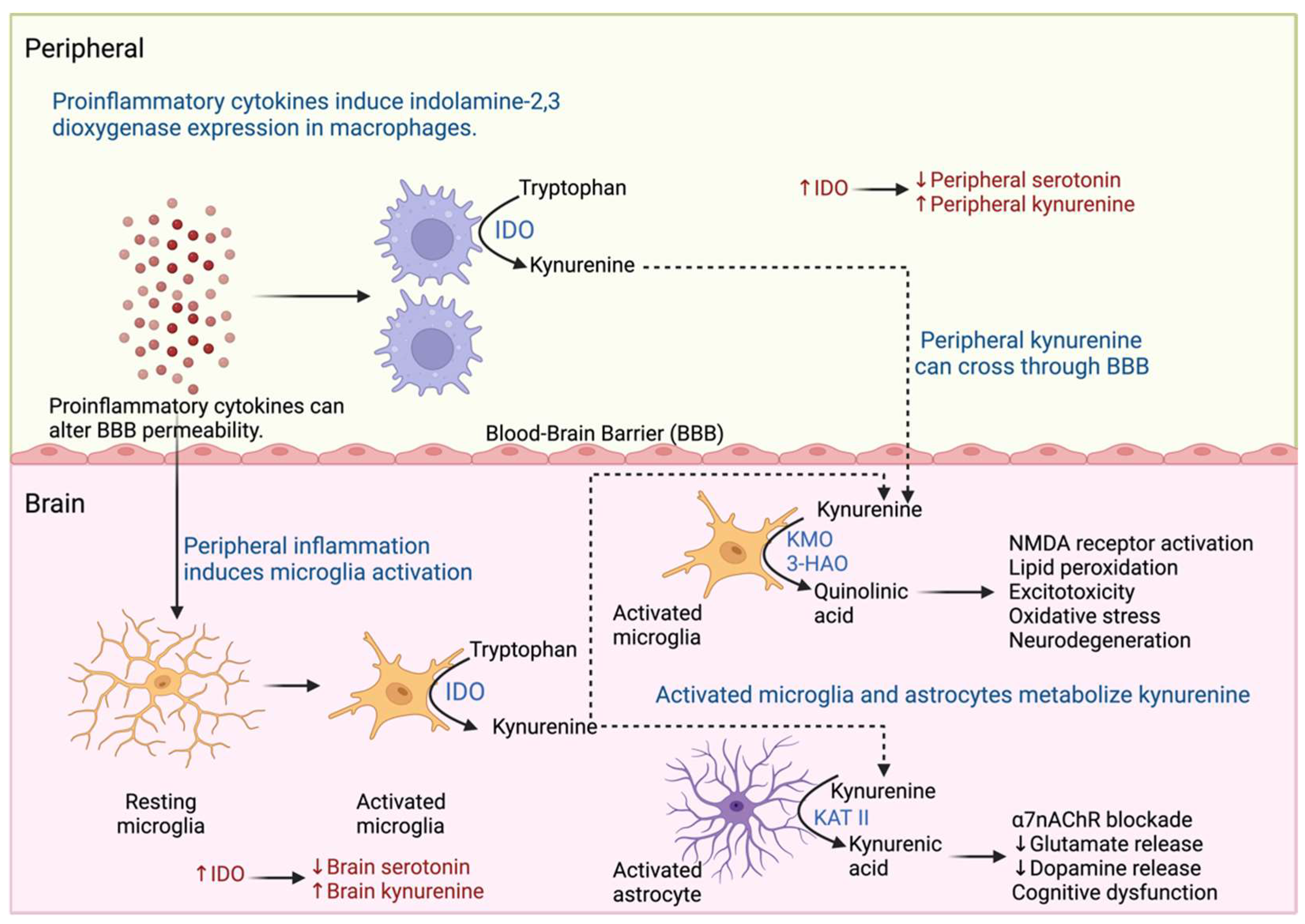
2.3. Proinflammatory Cytokines and Neurotransmitter Metabolism
3. Inflammation as a Therapeutic Target for MDD
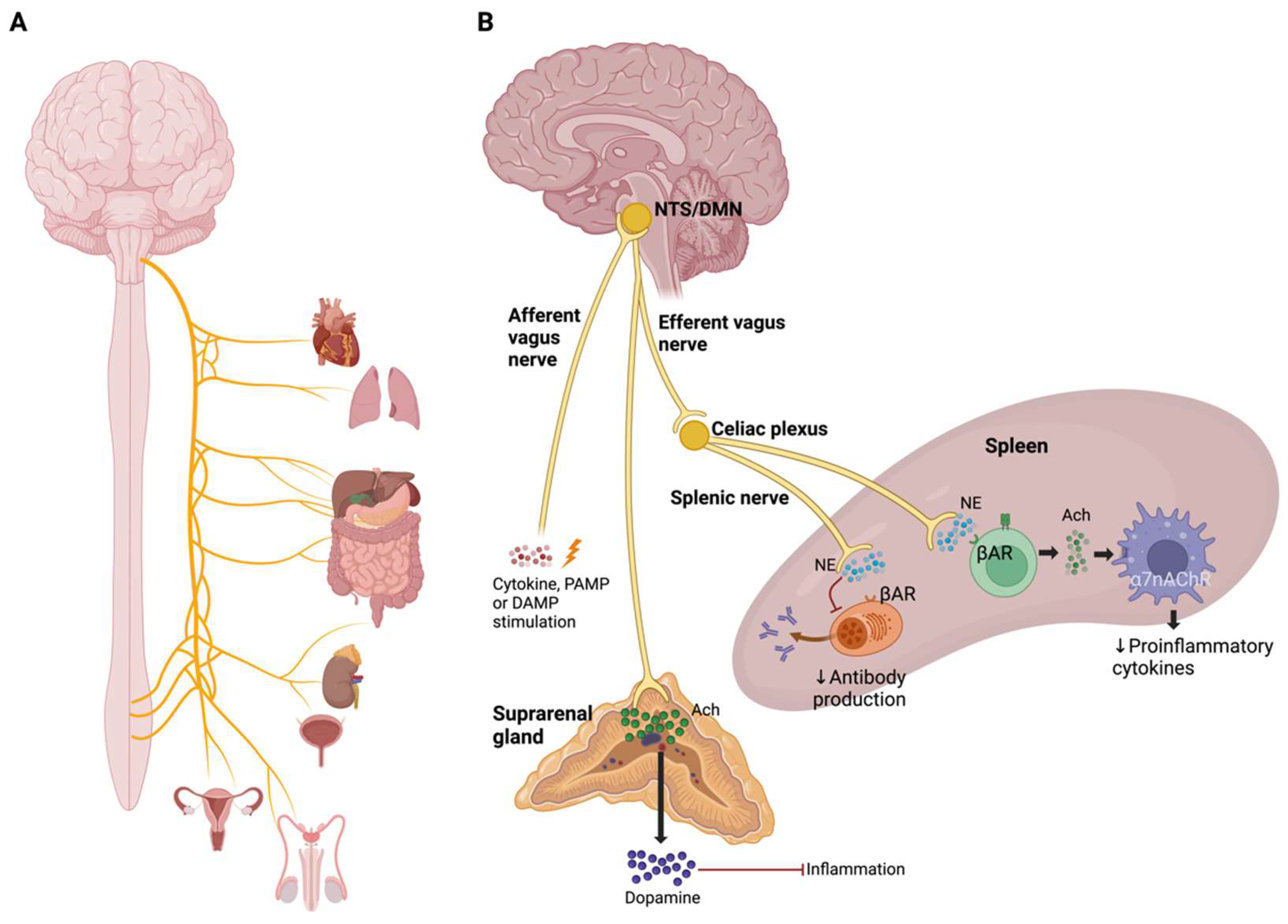
3.1. Cholinergic Anti-Inflammatory Pathway and Inflammatory Reflex
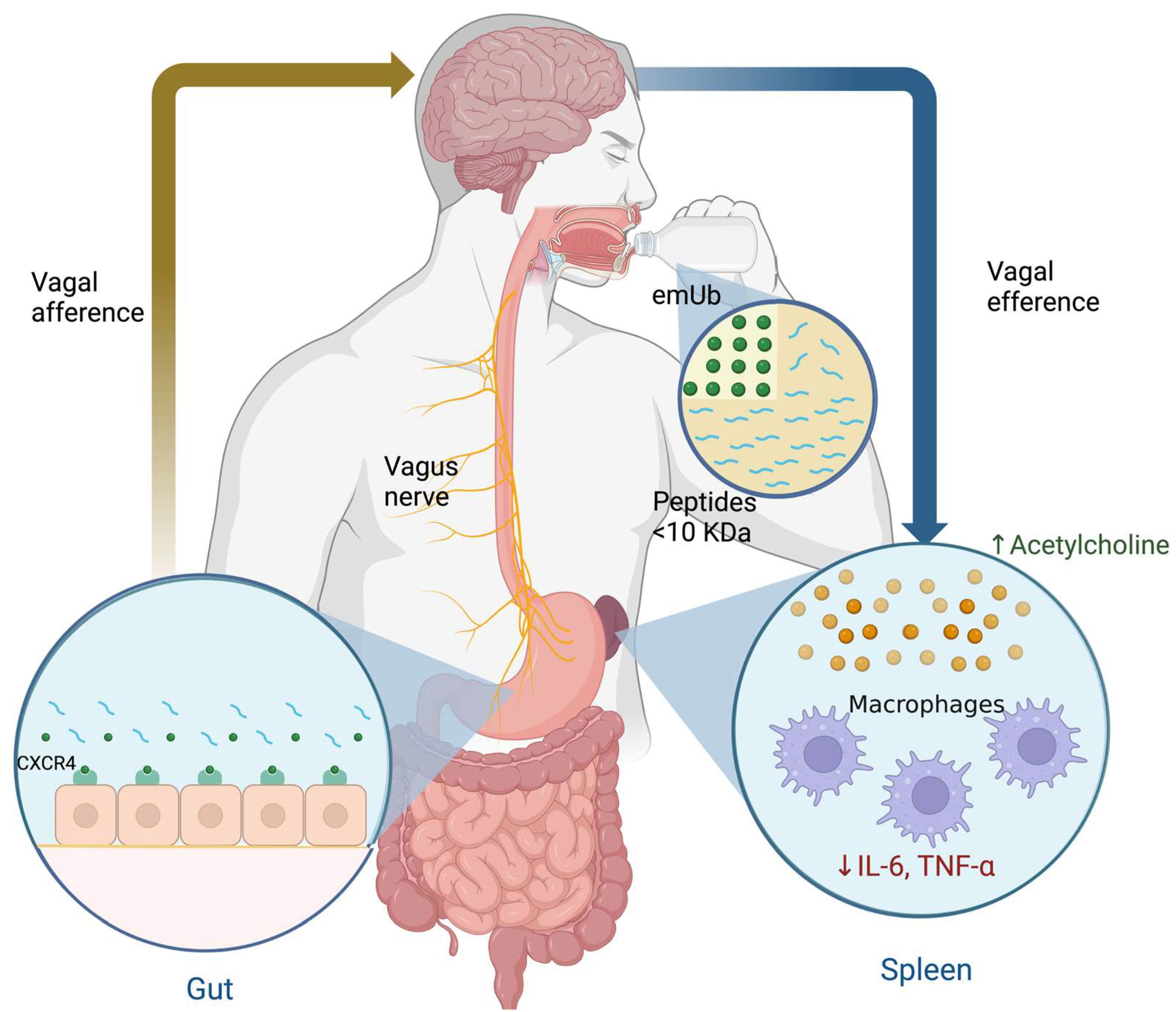
3.2. Extracellular Monomeric Ubiquitin (emUb)
4. Use of emUb as a Part of the Peptide Composition of the hDLE in MDD Treatment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marx, W.; Penninx, B.W.J.H.; Solmi, M.; Furukawa, T.A.; Firth, J.; Carvalho, A.F.; Berk, M. Major Depressive Disorder. Nat. Rev. Dis. Prim. 2023, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Otte, C.; Gold, S.M.; Penninx, B.W.; Pariante, C.M.; Etkin, A.; Fava, M.; Mohr, D.C.; Schatzberg, A.F. Major Depressive Disorder. Nat. Rev. Dis. Prim. 2016, 2, 16065. [Google Scholar] [CrossRef] [PubMed]
- Lopizzo, N.; Chiavetto, L.B.; Cattane, N.; Plazzotta, G.; Tarazi, F.I.; Pariante, C.M.; Riva, M.A.; Cattaneo, A. Gene–Environment Interaction in Major Depression: Focus on Experience-Dependent Biological Systems. Front. Psychiatry 2015, 6, 68. [Google Scholar] [CrossRef]
- Moussavi, S.; Chatterji, S.; Verdes, E.; Tandon, A.; Patel, V.; Ustun, B. Depression, Chronic Diseases, and Decrements in Health: Results from the World Health Surveys. Lancet 2007, 370, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Dhar, A.K.; Barton, D.A. Depression and the Link with Cardiovascular Disease. Front. Psychiatry 2016, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Al-Khatib, Y.; Akhtar, M.A.; Kanawati, M.A.; Mucheke, R.; Mahfouz, M.; Al-Nufoury, M. Depression and Metabolic Syndrome: A Narrative Review. Cureus 2022, 14, e22153. [Google Scholar] [CrossRef] [PubMed]
- Sanches, A.; Costa, R.; Marcondes, F.K.; Cunha, T.S. Relationship among Stress, Depression, Cardiovascular and Metabolic Changes and Physical Exercise. Fisioter. Mov. 2016, 29, 23–36. [Google Scholar] [CrossRef]
- Ferrari, A. Global, Regional, and National Burden of 12 Mental Disorders in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry 2022, 9, 137–150. [Google Scholar] [CrossRef]
- James, J.E. Mental Health. In The Health of Populations; Elsevier: Amsterdam, The Netherlands, 2016; pp. 429–464. ISBN 978-0-12-802812-4. [Google Scholar]
- Greenberg, P.E.; Fournier, A.A.; Sisitsky, T.; Simes, M.; Berman, R.; Koenigsberg, S.H.; Kessler, R.C. The Economic Burden of Adults with Major Depressive Disorder in the United States (2010 and 2018). Pharmacoeconomics 2021, 39, 653–665. [Google Scholar] [CrossRef]
- Bermudes, R.A. Rapid Response Therapies for Major Depression Could Dramatically Reduce Societal Cost. Psychiatr. News 2023, 58. [Google Scholar] [CrossRef]
- Karrouri, R.; Hammani, Z.; Otheman, Y.; Benjelloun, R. Major Depressive Disorder: Validated Treatments and Future Challenges. World J. Clin. Cases 2021, 9, 9350. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, R.S. Major Depressive Disorder; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780323581318. [Google Scholar]
- Sheffler, Z.M.; Patel, P.; Abdijadid, S. Antidepressants; StatPearls Publishing: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Sinyor, M.; Schaffer, A.; Levitt, A. The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) Trial: A Review. Can. J. Psychiatry 2010, 55, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Gaynes, B.N.; Rush, A.J.; Trivedi, M.H.; Wisniewski, S.R.; Spencer, D.; Fava, M. The STAR*D Study: Treating Depression in the Real World. Cleve. Clin. J. Med. 2008, 75, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, S.H. A Review of Antidepressant Therapy in Primary Care: Current Practices and Future Directions. Prim. Care Companion J. Clin. Psychiatry 2013, 15, 23071. [Google Scholar] [CrossRef] [PubMed]
- Almeida, S.S.; Zizzi, F.B.; Cattaneo, A.; Comandini, A.; Di Dato, G.; Lubrano, E.; Pellicano, C.; Spallone, V.; Tongiani, S.; Torta, R. Management and Treatment of Patients With Major Depressive Disorder and Chronic Diseases: A Multidisciplinary Approach. Front. Psychol. 2020, 11, 542444. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Khullar, S.; Singh, M.; Kaur, G.; Mastana, S. Diabetes to Cardiovascular Disease: Is Depression the Potential Missing Link? Med. Hypotheses 2015, 84, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Sartorius, N. Depression and Diabetes. Dialogues Clin. Neurosci. 2018, 20, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Tafet, G.E.; Nemeroff, C.B. The Links Between Stress and Depression: Psychoneuroendocrinological, Genetic, and Environmental Interactions. J. Neuropsychiatry Clin. Neurosci. 2016, 28, 77–88. [Google Scholar] [CrossRef]
- Boku, S.; Nakagawa, S.; Toda, H.; Hishimoto, A. Neural Basis of Major Depressive Disorder: Beyond Monoamine Hypothesis. Psychiatry Clin. Neurosci. 2018, 72, 3–12. [Google Scholar] [CrossRef]
- Seki, K.; Yoshida, S.; Jaiswal, M. Molecular Mechanism of Noradrenaline during the Stress-Induced Major Depressive Disorder. Neural Regen. Res. 2018, 13, 1159–1169. [Google Scholar] [CrossRef]
- Bruno, A.; Dolcetti, E.; Rizzo, F.R.; Fresegna, D.; Musella, A.; Gentile, A.; De Vito, F.; Caioli, S.; Guadalupi, L.; Bullitta, S.; et al. Inflammation-Associated Synaptic Alterations as Shared Threads in Depression and Multiple Sclerosis. Front. Cell. Neurosci. 2020, 14, 169. [Google Scholar] [CrossRef] [PubMed]
- Cosci, F.; Chouinard, G. The Monoamine Hypothesis of Depression Revisited: Could It Mechanistically Novel Antidepressant Strategies? In Neurobiology of Depression; Elsevier: Amsterdam, The Netherlands, 2019; pp. 63–73. ISBN 9780128133330. [Google Scholar]
- Musazzi, L.; Treccani, G.; Popoli, M. Glutamate Hypothesis of Depression and Its Consequences for Antidepressant Treatments. Expert Rev. Neurother. 2012, 12, 1169–1172. [Google Scholar] [CrossRef] [PubMed]
- Onaolapo, A.Y.; Onaolapo, O.J. Glutamate and Depression: Reflecting a Deepening Knowledge of the Gut and Brain Effects of a Ubiquitous Molecule. World J. Psychiatry 2021, 11, 297. [Google Scholar] [CrossRef] [PubMed]
- Holsboer, F. Stress, Hypercortisolism and Corticosteroid Receptors in Depression: Implicatons for Therapy. J. Affect. Disord. 2001, 62, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Afridi, R.; Suk, K. Neuroinflammatory Basis of Depression: Learning From Experimental Models. Front. Cell. Neurosci. 2021, 15, 691067. [Google Scholar] [CrossRef] [PubMed]
- Alesci, S.; Martinez, P.E.; Kelkar, S.; Ilias, I.; Ronsaville, D.S.; Listwak, S.J.; Ayala, A.R.; Licinio, J.; Gold, H.K.; Kling, M.A.; et al. Major Depression Is Associated with Significant Diurnal Elevations in Plasma Interleukin-6 Levels, a Shift of Its Circadian Rhythm, and Loss of Physiological Complexity in Its Secretion: Clinical Implications. J. Clin. Endocrinol. Metab. 2005, 90, 2522–2530. [Google Scholar] [CrossRef] [PubMed]
- Gałecki, P.; Talarowska, M. Inflammatory Theory of Depression. Psychiatr. Pol. 2018, 52, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Chiu, W.C.; Su, Y.P.; Su, K.P.; Chen, P.C. Recurrence of Depressive Disorders after Interferon-Induced Depression. Transl. Psychiatry 2017, 7, e1026. [Google Scholar] [CrossRef]
- Amodio, P.; De Toni, E.N.; Cavalletto, L.; Mapelli, D.; Bernardinello, E.; Del Piccolo, F.; Bergamelli, C.; Costanzo, R.; Bergamaschi, F.; Poma, S.Z.; et al. Mood, Cognition and EEG Changes during Interferon Alpha (Alpha-IFN) Treatment for Chronic Hepatitis C. J. Affect. Disord. 2005, 84, 93–98. [Google Scholar] [CrossRef]
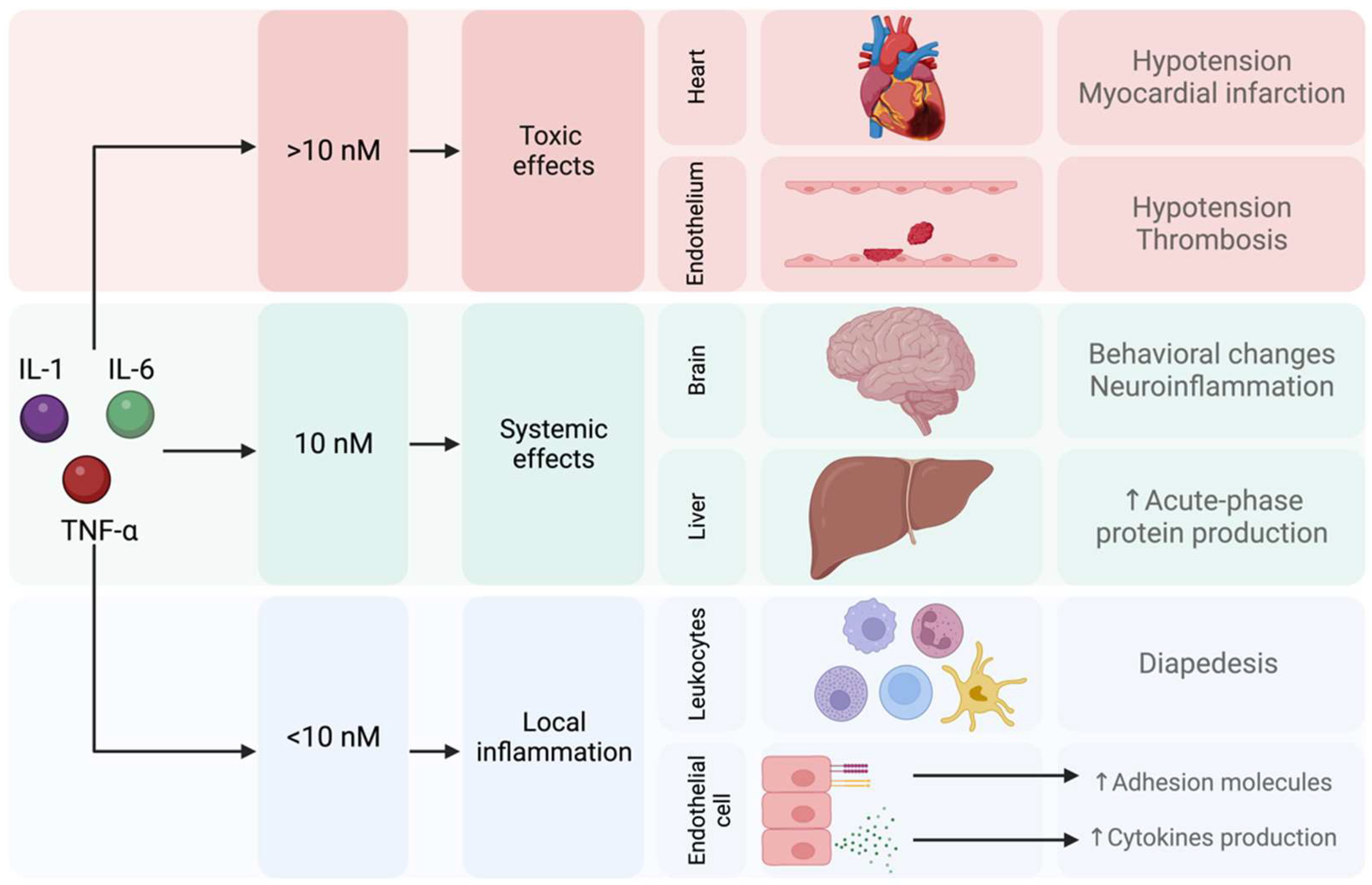
- Salvador, A.F.; de Lima, K.A.; Kipnis, J. Neuromodulation by the Immune System: A Focus on Cytokines. Nat. Rev. Immunol. 2021, 21, 526–541. [Google Scholar] [CrossRef]
- Brebner, K.; Hayley, S.; Zacharko, R.; Merali, Z.; Anisman, H. Synergistic Effects of Interleukin-1beta, Interleukin-6, and Tumor Necrosis Factor-Alpha: Central Monoamine, Corticosterone, and Behavioral Variations. Neuropsychopharmacology 2000, 22, 566–580. [Google Scholar] [CrossRef] [PubMed]
- Yabut, J.M.; Crane, J.D.; Green, A.E.; Keating, D.J.; Khan, W.I.; Steinberg, G.R. Emerging Roles for Serotonin in Regulating Metabolism: New Implications for an Ancient Molecule. Endocr. Rev. 2019, 40, 1092–1107. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From Inflammation to Sickness and Depression: When the Immune System Subjugates the Brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R. Cytokine, Sickness Behavior, and Depression. Immunol. Allergy Clin. N. Am. 2009, 29, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Raison, C.L.; Capuron, L.; Miller, A.H. Cytokines Sing the Blues: Inflammation and the Pathogenesis of Depression. Trends Immunol. 2006, 27, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.H.; Maletic, V.; Raison, C.L. Inflammation and Its Discontents: The Role of Cytokines in the Pathophysiology of Major Depression. Biol. Psychiatry 2009, 65, 732. [Google Scholar] [CrossRef] [PubMed]
- Derecki, N.C.; Cardani, A.N.; Yang, C.H.; Quinnies, K.M.; Crihfield, A.; Lynch, K.R.; Kipnis, J. Regulation of Learning and Memory by Meningeal Immunity: A Key Role for IL-4. J. Exp. Med. 2010, 207, 1067. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Brigas, H.C.; Temido-Ferreira, M.; Pousinha, P.A.; Regen, T.; Santa, C.; Coelho, J.E.; Marques-Morgado, I.; Valente, C.A.; Omenetti, S.; et al. Meningeal Γδ T Cell-Derived IL-17 Controls Synaptic Plasticity and Short-Term Memory. Sci. Immunol. 2019, 4, eaay5199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Rong, P.; Zhang, L.; He, H.; Zhou, T.; Fan, Y.; Mo, L.; Zhao, Q.; Han, Y.; Li, S.; et al. IL4-Driven Microglia Modulate Stress Resilience through BDNF-Dependent Neurogenesis. Sci. Adv. 2021, 7, 9888–9905. [Google Scholar] [CrossRef]
- Choi, G.B.; Yim, Y.S.; Wong, H.; Kim, S.; Kim, H.; Kim, S.V.; Hoeffer, C.A.; Littman, D.R.; Huh, J.R. The Maternal Interleukin-17a Pathway in Mice Promotes Autism-like Phenotypes in Offspring. Science 2016, 351, 933–939. [Google Scholar] [CrossRef]
- Filiano, A.J.; Xu, Y.; Tustison, N.J.; Marsh, R.L.; Baker, W.; Smirnov, I.; Overall, C.C.; Gadani, S.P.; Turner, S.D.; Weng, Z.; et al. Unexpected Role of Interferon-γ in Regulating Neuronal Connectivity and Social Behaviour. Nature 2016, 535, 425–429. [Google Scholar] [CrossRef]
- Coulthard, L.G.; Hawksworth, O.A.; Woodruff, T.M. Complement: The Emerging Architect of the Developing Brain. Trends Neurosci. 2018, 41, 373–384. [Google Scholar] [CrossRef]
- Westacott, L.J.; Humby, T.; Haan, N.; Brain, S.A.; Bush, E.L.; Toneva, M.; Baloc, A.I.; Moon, A.L.; Reddaway, J.; Owen, M.J.; et al. Complement C3 and C3aR Mediate Different Aspects of Emotional Behaviours; Relevance to Risk for Psychiatric Disorder. Brain Behav. Immun. 2022, 99, 70–82. [Google Scholar] [CrossRef]
- Allswede, D.M.; Zheutlin, A.B.; Chung, Y.; Anderson, K.; Hultman, C.M.; Ingvar, M.; Cannon, T.D. Complement Gene Expression Correlates with Superior Frontal Cortical Thickness in Humans. Neuropsychopharmacology 2018, 43, 525–533. [Google Scholar] [CrossRef]
- Sekar, A.; Bialas, A.R.; de Rivera, H.; Davis, A.; Hammond, T.R.; Kamitaki, N.; Tooley, K.; Presumey, J.; Baum, M.; Van Doren, V.; et al. Schizophrenia Risk from Complex Variation of Complement Component 4. Nature 2016, 530, 177–183. [Google Scholar] [CrossRef]
- Mikulska, J.; Juszczyk, G.; Gawrońska-Grzywacz, M.; Herbet, M. Hpa Axis in the Pathomechanism of Depression and Schizophrenia: New Therapeutic Strategies Based on Its Participation. Brain Sci. 2021, 11, 1298. [Google Scholar] [CrossRef]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr. Physiol. 2016, 6, 603. [Google Scholar] [CrossRef]
- Keller, J.; Gomez, R.; Williams, G.; Lembke, A.; Lazzeroni, L.; Murphy, G.M.; Schatzberg, A.F. HPA Axis in Major Depression: Cortisol, Clinical Symptomatology, and Genetic Variation Predict Cognition. Mol. Psychiatry 2017, 22, 527. [Google Scholar] [CrossRef]
- Gomez, R.G.; Fleming, S.H.; Keller, J.; Flores, B.; Kenna, H.; DeBattista, C.; Solvason, B.; Schatzberg, A.F. The Neuropsychological Profile of Psychotic Major Depression and Its Relation to Cortisol. Biol. Psychiatry 2006, 60, 472–478. [Google Scholar] [CrossRef]
- Lupien, S.J.; Gillin, C.J.; Hauger, R.L. Working Memory Is More Sensitive than Declarative Memory to the Acute Effects of Corticosteroids: A Dose-Response Study in Humans. Behav. Neurosci. 1999, 113, 420–430. [Google Scholar] [CrossRef]
- Rock, P.L.; Roiser, J.P.; Riedel, W.J.; Blackwell, A.D. Cognitive Impairment in Depression: A Systematic Review and Meta-Analysis. Psychol. Med. 2014, 44, 2029–2040. [Google Scholar] [CrossRef]
- Kim, E.J.; Kim, J.J. Neurocognitive Effects of Stress: A Metaparadigm Perspective. Mol. Psychiatry 2023, 28, 2750–2763. [Google Scholar] [CrossRef]
- Kim, E.J.; Pellman, B.; Kim, J.J. Stress Effects on the Hippocampus: A Critical Review. Learn. Mem. 2015, 22, 411. [Google Scholar] [CrossRef]
- Pariante, C.M. Why Are Depressed Patients Inflamed? A Reflection on 20 Years of Research on Depression, Glucocorticoid Resistance and Inflammation. Eur. Neuropsychopharmacol. 2017, 27, 554–559. [Google Scholar] [CrossRef]
- Silverman, M.N.; Sternberg, E.M. Glucocorticoid Regulation of Inflammation and Its Functional Correlates: From HPA Axis to Glucocorticoid Receptor Dysfunction. Ann. N. Y. Acad. Sci. 2012, 1261, 55–63. [Google Scholar] [CrossRef]
- Cohen, S.; Janicki-Deverts, D.; Doyle, W.J.; Miller, G.E.; Frank, E.; Rabin, B.S.; Turner, R.B. Chronic Stress, Glucocorticoid Receptor Resistance, Inflammation, and Disease Risk. Proc. Natl. Acad. Sci. USA 2012, 109, 5995–5999. [Google Scholar] [CrossRef]
- Zunszain, P.A.; Anacker, C.; Cattaneo, A.; Carvalho, L.A.; Pariante, C.M. Glucocorticoids, Cytokines and Brain Abnormalities in Depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 722–729. [Google Scholar] [CrossRef]
- Coutinho, A.E.; Chapman, K.E. The Anti-Inflammatory and Immunosuppressive Effects of Glucocorticoids, Recent Developments and Mechanistic Insights. Mol. Cell. Endocrinol. 2011, 335, 2. [Google Scholar] [CrossRef]
- Perrin, A.J.; Horowitz, M.A.; Roelofs, J.; Zunszain, P.A.; Pariante, C.M. Glucocorticoid Resistance: Is It a Requisite for Increased Cytokine Production in Depression? A Systematic Review and Meta-Analysis. Front. Psychiatry 2019, 10, 423. [Google Scholar] [CrossRef]
- Weber, M.D.; Godbout, J.P.; Sheridan, J.F. Repeated Social Defeat, Neuroinflammation, and Behavior: Monocytes Carry the Signal. Neuropsychopharmacology 2017, 42, 46–61. [Google Scholar] [CrossRef]
- Irwin, M.R.; Miller, A.H. Depressive Disorders and Immunity: 20 Years of Progress and Discovery. Brain Behav. Immun. 2007, 21, 374–383. [Google Scholar] [CrossRef]
- Rodriguez, J.M.; Monsalves-Alvarez, M.; Henriquez, S.; Llanos, M.N.; Troncoso, R. Glucocorticoid Resistance in Chronic Diseases. Steroids 2016, 115, 182–192. [Google Scholar] [CrossRef]
- Hernández, M.E.; Mendieta, D.; Martínez-Fong, D.; Loría, F.; Moreno, J.; Estrada, I.; Bojalil, R.; Pavón, L. Variations in Circulating Cytokine Levels during 52 Week Course of Treatment with SSRI for Major Depressive Disorder. Eur. Neuropsychopharmacol. 2008, 18, 917–924. [Google Scholar] [CrossRef]
- Himmerich, H.; Patsalos, O.; Lichtblau, N.; Ibrahim, M.A.A.; Dalton, B. Cytokine Research in Depression: Principles, Challenges, and Open Questions. Front. Psychiatry 2019, 10, 30. [Google Scholar] [CrossRef]
- Becher, B.; Spath, S.; Goverman, J. Cytokine Networks in Neuroinflammation. Nat. Rev. Immunol. 2016, 17, 49–59. [Google Scholar] [CrossRef]
- Bauer, M.E. Accelerated Immunosenescence in Rheumatoid Arthritis: Impact on Clinical Progression. Immun. Ageing 2020, 17, 6. [Google Scholar] [CrossRef]
- Roohi, E.; Jaafari, N.; Hashemian, F. On Inflammatory Hypothesis of Depression: What Is the Role of IL-6 in the Middle of the Chaos? J. Neuroinflamm. 2021, 18, 45. [Google Scholar] [CrossRef]
- Corrigan, M.; O’Rourke, A.M.; Moran, B.; Fletcher, J.M.; Harkin, A. Inflammation in the Pathogenesis of Depression: A Disorder of Neuroimmune Origin. Neuronal Signal. 2023, 7, 20220054. [Google Scholar] [CrossRef]
- Herselman, M.F.; Bailey, S.; Bobrovskaya, L. The Effects of Stress and Diet on the “Brain–Gut” and “Gut–Brain” Pathways in Animal Models of Stress and Depression. Int. J. Mol. Sci. 2022, 23, 2013. [Google Scholar] [CrossRef]
- Miller, A.H. Mechanisms of Cytokine-Induced Behavioral Changes: Psychoneuroimmunology at the Translational Interface. Brain Behav. Immun. 2009, 23, 149–158. [Google Scholar] [CrossRef]
- Huang, Y.S.; Ogbechi, J.; Clanchy, F.I.; Williams, R.O.; Stone, T.W. IDO and Kynurenine Metabolites in Peripheral and CNS Disorders. Front. Immunol. 2020, 11, 493984. [Google Scholar] [CrossRef] [PubMed]
- Haroon, E.; Raison, C.L.; Miller, A.H. Psychoneuroimmunology Meets Neuropsychopharmacology: Translational Implications of the Impact of Inflammation on Behavior. Neuropsychopharmacology 2012, 37, 137–162. [Google Scholar] [CrossRef] [PubMed]
- Mithaiwala, M.N.; Santana-Coelho, D.; Porter, G.A.; O’connor, J.C. Neuroinflammation and the Kynurenine Pathway in CNS Disease: Molecular Mechanisms and Therapeutic Implications. Cells 2021, 10, 1548. [Google Scholar] [CrossRef] [PubMed]
- Becerril-Villanueva, E.; Olvera-Alvarez, M.I.; Alvarez-Herrera, S.; Maldonado-García, J.L.; López-Torres, A.; Ramírez-Marroquín, O.A.; González-Ruiz, O.; Nogueira-Fernández, J.M.; Mendoza-Contreras, J.M.; Sánchez-García, H.O.; et al. Screening of SERT and P11 MRNA Levels in Airline Pilots: A Translational Approach. Front. Psychiatry 2022, 13, 859768. [Google Scholar] [CrossRef] [PubMed]
- Vancassel, S.; Capuron, L.; Castanon, N. Brain Kynurenine and BH4 Pathways: Relevance to the Pathophysiology and Treatment of Inflammation-Driven Depressive Symptoms. Front. Neurosci. 2018, 12, 499. [Google Scholar] [CrossRef] [PubMed]
- Hassamal, S. Chronic Stress, Neuroinflammation, and Depression: An Overview of Pathophysiological Mechanisms and Emerging Anti-Inflammatories. Front. Psychiatry 2023, 14, 1130989. [Google Scholar] [CrossRef] [PubMed]
- Beurel, E.; Toups, M.; Nemeroff, C.B. The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron 2020, 107, 234–256. [Google Scholar] [CrossRef] [PubMed]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctôt, K.L. A Meta-Analysis of Cytokines in Major Depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef]
- Howren, M.B.; Lamkin, D.M.; Suls, J. Associations of Depression with C-Reactive Protein, IL-1, and IL-6: A Meta-Analysis. Psychosom. Med. 2009, 71, 171–186. [Google Scholar] [CrossRef]
- Liu, Y.; Ho, R.C.M.; Mak, A. Interleukin (IL)-6, Tumour Necrosis Factor Alpha (TNF-α) and Soluble Interleukin-2 Receptors (SIL-2R) Are Elevated in Patients with Major Depressive Disorder: A Meta-Analysis and Meta-Regression. J. Affect. Disord. 2012, 139, 230–239. [Google Scholar] [CrossRef]
- Köhler, C.A.; Freitas, T.H.; Maes, M.; de Andrade, N.Q.; Liu, C.S.; Fernandes, B.S.; Stubbs, B.; Solmi, M.; Veronese, N.; Herrmann, N.; et al. Peripheral Cytokine and Chemokine Alterations in Depression: A Meta-Analysis of 82 Studies. Acta Psychiatr. Scand. 2017, 135, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Osimo, E.F.; Pillinger, T.; Rodriguez, I.M.; Khandaker, G.M.; Pariante, C.M.; Howes, O.D. Inflammatory Markers in Depression: A Meta-Analysis of Mean Differences and Variability in 5166 Patients and 5083 Controls. Brain Behav. Immun. 2020, 87, 901. [Google Scholar] [CrossRef]
- Min, X.; Wang, G.; Cui, Y.; Meng, P.; Hu, X.; Liu, S.; Wang, Y. Association between Inflammatory Cytokines and Symptoms of Major Depressive Disorder in Adults. Front. Immunol. 2023, 14, 1110775. [Google Scholar] [CrossRef] [PubMed]
- Harsanyi, S.; Kupcova, I.; Danisovic, L.; Klein, M. Selected Biomarkers of Depression: What Are the Effects of Cytokines and Inflammation? Int. J. Mol. Sci. 2023, 24, 578. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Halbreich, U.; Han, C.; Leonard, B.E.; Luo, H. Imbalance between Pro- and Anti-Inflammatory Cytokines, and between Th1 and Th2 Cytokines in Depressed Patients: The Effect of Electroacupuncture or Fluoxetine Treatment. Pharmacopsychiatry 2009, 42, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Myint, A.M.; Leonard, B.E.; Steinbusch, H.W.M.; Kim, Y.K. Th1, Th2, and Th3 Cytokine Alterations in Major Depression. J. Affect. Disord. 2005, 88, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yu, K. Th1, Th2, and Th17 Cells and Their Corresponding Cytokines Are Associated with Anxiety, Depression, and Cognitive Impairment in Elderly Gastric Cancer Patients. Front. Surg. 2022, 9, 996680. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.; Fahimi, A. Immune and Neuroprotective Effects of Physical Activity on the Brain in Depression. Front. Neurosci. 2018, 12, 498. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Tu, H.; Chen, T. The Microbiota–Gut–Brain Axis in Depression: The Potential Pathophysiological Mechanisms and Microbiota Combined Antidepression Effect. Nutrients 2022, 14, 2081. [Google Scholar] [CrossRef]
- Chang, L.; Wei, Y.; Hashimoto, K. Brain–Gut–Microbiota Axis in Depression: A Historical Overview and Future Directions. Brain Res. Bull. 2022, 182, 44–56. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, J.; Chen, Y. Regulation of Neurotransmitters by the Gut Microbiota and Effects on Cognition in Neurological Disorders. Nutrients 2021, 13, 2099. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between Microbiota and Immunity in Health and Disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Medina-Rodriguez, E.M.; Watson, J.; Reyes, J.; Trivedi, M.; Beurel, E. Th17 Cells Sense Microbiome to Promote Depressive-like Behaviors. Microbiome 2023, 11, 92. [Google Scholar] [CrossRef] [PubMed]
- Medina-Rodriguez, E.M.; Madorma, D.; O’Connor, G.; Mason, B.L.; Han, D.; Deo, S.K.; Oppenheimer, M.; Nemeroff, C.B.; Trivedi, M.H.; Daunert, S.; et al. Identification of a Signalling Mechanism by Which the Microbiome Regulates Th17 Cell-Mediated Depressive-like Behaviors in Mice. Am. J. Psychiatry 2020, 177, 974. [Google Scholar] [CrossRef] [PubMed]
- Rusch, J.A.; Layden, B.T.; Dugas, L.R. Signalling Cognition: The Gut Microbiota and Hypothalamic-Pituitary-Adrenal Axis. Front. Endocrinol. 2023, 14, 1130689. [Google Scholar] [CrossRef] [PubMed]
- Farzi, A.; Fröhlich, E.E.; Holzer, P. Gut Microbiota and the Neuroendocrine System. Neurotherapeutics 2018, 15, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, H.; Chen, X.; Zhang, Y.; Zhang, H.; Xie, P. Gut Microbiota and Its Metabolites in Depression: From Pathogenesis to Treatment. eBioMedicine 2023, 90, 104527. [Google Scholar] [CrossRef] [PubMed]
- Gujral, S.; Aizenstein, H.; Reynolds, C.F.; Butters, M.A.; Erickson, K.I. Exercise Effects on Depression: Possible Neural Mechanisms. Gen. Hosp. Psychiatry 2017, 49, 2. [Google Scholar] [CrossRef]
- Zhao, J.L.; Jiang, W.T.; Wang, X.; Cai, Z.D.; Liu, Z.H.; Liu, G.R. Exercise, Brain Plasticity, and Depression. CNS Neurosci. Ther. 2020, 26, 885. [Google Scholar] [CrossRef] [PubMed]
- Kohler, O.; Krogh, J.; Mors, O.; Eriksen Benros, M. Inflammation in Depression and the Potential for Anti-Inflammatory Treatment. Curr. Neuropharmacol. 2016, 14, 732–742. [Google Scholar] [CrossRef]
- Miller, A.H.; Raison, C.L. The Role of Inflammation in Depression: From Evolutionary Imperative to Modern Treatment Target. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef]
- Alvarez-Herrera, S.; Pavon, L. Interacciones Neuroendocrinoinmunológicas. In Inmunología Molecular, Celular y Traslacional; Pavón, L., Ed.; Wolters Kluwer: Mexico City, Mexico, 2021; pp. 227–242. ISBN 9788417949181. [Google Scholar]
- Fioranelli, M.; Roccia, M.G.; Flavin, D.; Cota, L. Regulation of Inflammatory Reaction in Health and Disease. Int. J. Mol. Sci. 2021, 22, 5277. [Google Scholar] [CrossRef]
- Balkan, B.; Pogun, S. Nicotinic Cholinergic System in the Hypothalamus Modulates the Activity of the Hypothalamic Neuropeptides during the Stress Resp. Curr. Neuropharmacol. 2017, 15, 371–387. [Google Scholar] [CrossRef]
- Pavlov, V.A.; Tracey, K.J. Neural Regulation of Immunity: Molecular Mechanisms and Clinical Translation. Nat. Neurosci. 2017, 20, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, P.S.; Rosas-Ballina, M.; Levine, Y.A.; Tracey, K.J. Rethinking Inflammation: Neural Circuits in the Regulation of Immunity. Immunol. Rev. 2012, 248, 188–204. [Google Scholar] [CrossRef]
- Pavlov, V.A.; Tracey, K.J. The Vagus Nerve and the Inflammatory Reflex-Linking Immunity and Metabolism. Nat. Rev. Endocrinol. 2012, 8, 743–754. [Google Scholar] [CrossRef]
- Pavlov, V.A.; Wang, H.; Czura, C.J.; Friedman, S.G.; Tracey, K.J. The Cholinergic Anti-Inflammatory Pathway: A Missing Link in Neuroimmunomodulation. Mol. Med. 2003, 9, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Vida, G.; Peña, G.; Deitch, E.A.; Ulloa, L. A7-Cholinergic Receptor Mediates Vagal Induction of Splenic Norepinephrine. J. Immunol. 2011, 186, 4340–4346. [Google Scholar] [CrossRef]
- Zhou, J.; Yan, J.; Liang, H.; Jiang, J. Epinephrine Enhances the Response of Macrophages under LPS Stimulation. Biomed. Res. Int. 2014, 2014, 254686. [Google Scholar] [CrossRef]
- Staedtke, V.; Bai, R.Y.; Kim, K.; Darvas, M.; Davila, M.L.; Riggins, G.J.; Rothman, P.B.; Papadopoulos, N.; Kinzler, K.W.; Vogelstein, B.; et al. Disruption of a Self-Amplifying Catecholamine Loop Reduces Cytokine Release Syndrome. Nature 2018, 564, 273–277. [Google Scholar] [CrossRef]
- Macias, A.E.; Guaní-Guerra, E. Transfer Factor: Myths and Facts. Arch. Med. Res. 2020, 51, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Islas-Weinstein, L.; Maldonado-García, J.L. Immunomodulatory Supplements; Elsevier Inc.: Amsterdam, The Netherlands, 2021; ISBN 9780128187319. [Google Scholar]
- Vallejo-Castillo, L.; Favari, L.; Vázquez-Leyva, S.; Mellado-Sánchez, G.; Macías-Palacios, Z.; López-Juárez, L.E.; Valencia-Flores, L.; Medina-Rivero, E.; Chacón-Salinas, R.; Pavón, L.; et al. Sequencing Analysis and Identification of the Primary Peptide Component of the Dialyzable Leukocyte Extract “Transferon Oral”: The Starting Point to Understand Its Mechanism of Action. Front. Pharmacol. 2020, 11, 569039. [Google Scholar] [CrossRef] [PubMed]
- Klein, K.; Stolk, P.; De Bruin, M.L.; Leufkens, H.G.M.; Crommelin, D.J.A.; De Vlieger, J.S.B. The EU Regulatory Landscape of Non-Biological Complex Drugs (NBCDs) Follow-on Products: Observations and Recommendations. Eur. J. Pharm. Sci. 2019, 133, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Medina-Rivero, E.; Merchand-Reyes, G.; Pavón, L.; Vázquez-Leyva, S.; Pérez-Sánchez, G.; Salinas-Jazmín, N.; Estrada-Parra, S.; Velasco-Velázquez, M.; Pérez-Tapia, S.M. Batch-to-Batch Reproducibility of TransferonTM. J. Pharm. Biomed. Anal. 2014, 88, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Medina-Rivero, E.; Vallejo-Castillo, L.; Vázquez-Leyva, S.; Pérez-Sánchez, G.; Favari, L.; Velasco-Velázquez, M.; Estrada-Parra, S.; Pavón, L.; Pérez-Tapia, S.M. Physicochemical Characteristics of TransferonTM Batches. Biomed. Res. Int. 2016, 2016, 7935181. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Leyva, S.; Vallejo-Castillo, L.; López-Morales, C.A.; Herbert-Pucheta, J.E.; Zepeda-Vallejo, L.G.; Velasco-Velázquez, M.; Pavón, L.; Pérez-Tapia, S.M.; Medina-Rivero, E. Identity Profiling of Complex Mixtures of Peptide Products by Structural and Mass Mobility Orthogonal Analysis. Anal. Chem. 2019, 91, 14392–14400. [Google Scholar] [CrossRef] [PubMed]
- Herbert-Pucheta, J.E.; López-Morales, C.A.; Medina-Rivero, E.; Estrada-Parra, S.; Pérez-Tapia, S.M.; Zepeda-Vallejo, L.G. Consistency of a Dialyzable Leucocyte Extract Manufactured at GMP Facilities by Nuclear Magnetic Resonance Spectroscopy. J. Pharm. Biomed. Anal. 2021, 196, 113940. [Google Scholar] [CrossRef]
- Pickart, C.M.; Eddins, M.J. Ubiquitin: Structures, Functions, Mechanisms. Biochim. Biophys. Acta-Mol. Cell Res. 2004, 1695, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Chen, Z.J. The Novel Functions of Ubiquitination in Signaling. Curr. Opin. Cell Biol. 2004, 16, 119–126. [Google Scholar] [CrossRef]
- Mendoza-Salazar, I.; Fragozo, A.; González-Martínez, A.P.; Trejo-Martínez, I.; Arreola, R.; Pavón, L.; Almagro, J.C.; Vallejo-Castillo, L.; Aguilar-Alonso, F.A.; Pérez-Tapia, S.M. Almost 50 Years of Monomeric Extracellular Ubiquitin (EUb). Pharmaceuticals 2024, 17, 185. [Google Scholar] [CrossRef]
- Schmidt, M.F.; Gan, Z.Y.; Komander, D.; Dewson, G. Ubiquitin Signalling in Neurodegeneration: Mechanisms and Therapeutic Opportunities. Cell Death Differ. 2021, 28, 570–590. [Google Scholar] [CrossRef] [PubMed]
- Swatek, K.N.; Komander, D. Ubiquitin Modifications. Cell Res. 2016, 26, 399–422. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.M.; Laporte, H.M.; Albee, L.J.; Baker, T.A.; Bach, H.H.; Vana, P.G.; Evans, A.E.; Gamelli, R.L.; Majetschak, M. Ubiquitin Urine Levels in Burn Patients. J. Burn Care Res. 2017, 38, e133–e143. [Google Scholar] [CrossRef] [PubMed]
- Majetschak, M.; Krehmeier, U.; Bardenheuer, M.; Denz, C.; Quintel, M.; Voggenreiter, G.; Obertacke, U. Extracellular Ubiquitin Inhibits the TNF-Alpha Response to Endotoxin in Peripheral Blood Mononuclear Cells and Regulates Endotoxin Hyporesponsiveness in Critical Illness. Blood 2003, 101, 1882–1890. [Google Scholar] [CrossRef] [PubMed]
- Majetschak, M. Extracellular Ubiquitin: Immune Modulator and Endogenous Opponent of Damage-Associated Molecular Pattern Molecules. J. Leukoc. Biol. 2011, 89, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Majetschak, M.; Cohn, S.M.; Nelson, J.A.; Burton, E.H.; Obertacke, U.; Proctor, K.G. Effects of Exogenous Ubiquitin in Lethal Endotoxemia. Surgery 2004, 135, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Baker, T.A.; Romero, J.; Bach, H.H.; Strom, J.A.; Gamelli, R.L.; Majetschak, M. Effects of Exogenous Ubiquitin in a Polytrauma Model with Blunt Chest Trauma. Crit. Care Med. 2012, 40, 2376–2384. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Parra, S.; Nagaya, A.; Serrano, E.; Rodriguez, O.; Santamaria, V.; Ondarza, R.; Chavez, R.; Correa, B.; Monges, A.; Cabezas, R.; et al. Comparative Study of Transfer Factor and Acyclovir in the Treatment of Herpes Zoster. Int. J. Immunopharmacol. 1998, 20, 521–535. [Google Scholar] [CrossRef] [PubMed]
- Salinas-Jazmin, N.; Estrada-Parra, S.; Becerril-Garcia, M.A.; Limon-Flores, A.Y.; Vazquez-Leyva, S.; Medina-Rivero, E.; Pavon, L.; Velasco-Velazquez, M.A.; Perez-Tapia, S.M. Herpes Murine Model as a Biological Assay to Test Dialyzable Leukocyte Extracts Activity. J. Immunol. Res. 2015, 2015, 146305. [Google Scholar] [CrossRef]
- Muñoz, A.I.; Vallejo-Castillo, L.; Fragozo, A.; Vázquez-Leyva, S.; Pavón, L.; Pérez-Sánchez, G.; Soria-Castro, R.; Mellado-Sánchez, G.; Cobos-Marin, L.; Pérez-Tapia, S.M. Increased Survival in Puppies Affected by Canine Parvovirus Type II Using an Immunomodulator as a Therapeutic Aid. Sci. Rep. 2021, 11, 19864. [Google Scholar] [CrossRef]
- Muñoz, A.I.; Maldonado-García, J.L.; Fragozo, A.; Vallejo-Castillo, L.; Lucas-Gonzalez, A.; Trejo-Martínez, I.; Pavón, L.; Pérez-Sánchez, G.; Cobos-Marin, L.; Pérez-Tapia, S.M. Altered Neutrophil-to-Lymphocyte Ratio in Sepsis Secondary to Canine Parvoviral Enteritis Treated with and without an Immunomodulator in Puppies. Front. Vet. Sci. 2022, 9, 995443. [Google Scholar] [CrossRef] [PubMed]
- Saini, V.; Marchese, A.; Majetschak, M. CXC Chemokine Receptor 4 Is a Cell Surface Receptor for Extracellular Ubiquitin. J. Biol. Chem. 2010, 285, 15566–15576. [Google Scholar] [CrossRef]
- Saini, V.; Staren, D.M.; Ziarek, J.J.; Nashaat, Z.N.; Campbell, E.M.; Volkman, B.F.; Marchese, A.; Majetschak, M. The CXC Chemokine Receptor 4 Ligands Ubiquitin and Stromal Cell-Derived Factor-1α Function through Distinct Receptor Interactions. J. Biol. Chem. 2011, 286, 33466–33477. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Davis, J.D.; Staren, D.M.; Volkman, B.F.; Majetschak, M. CXC Chemokine Receptor 4 Signaling upon Co-Activation with Stromal Cell-Derived Factor-1α and Ubiquitin. Cytokine 2014, 65, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Xie, G.; Xiao, H.; Ding, F.; Bao, W.; Zhang, M. CXCR4 Knockdown Prevents Inflammatory Cytokine Expression in Macrophages by Suppressing Activation of MAPK and NF-ΚB Signaling Pathways. Cell Biosci. 2019, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Guyon, A. CXCL12 Chemokine and Its Receptors as Major Players in the Interactions between Immune and Nervous Systems. Front. Cell. Neurosci. 2014, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Martínez, I.E.; Rodríguez, M.C.; Cerbón, M.; Ramos-Martínez, J.C.; Ramos-Martínez, E.G. Role of the Cholinergic Anti-Inflammatory Reflex in Central Nervous System Diseases. Int. J. Mol. Sci. 2021, 22, 13427. [Google Scholar] [CrossRef] [PubMed]
- Abarca-Castro, E.A.; Talavera-Peña, A.K.; Reyes-Lagos, J.J.; Becerril-Villanueva, E.; Pérez-Sanchez, G.; de la Peña, F.R.; Maldonado-García, J.L.; Pavón, L. Modulation of Vagal Activity May Help Reduce Neurodevelopmental Damage in the Offspring of Mothers with Pre-Eclampsia. Front. Immunol. 2023, 14, 1280334. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Sun, S.C. Ubiquitin Signaling in Immune Responses. Cell Res. 2016, 26, 457–483. [Google Scholar] [CrossRef]
- Liu, H.; Wilson, K.R.; Schriek, P.; Macri, C.; Blum, A.B.; Francis, L.; Heinlein, M.; Nataraja, C.; Harris, J.; Jones, S.A.; et al. Ubiquitination of MHC Class II Is Required for Development of Regulatory but Not Conventional CD4+ T Cells. J. Immunol. 2020, 205, 1207–1216. [Google Scholar] [CrossRef]
- Schriek, P.; Liu, H.; Ching, A.C.; Huang, P.; Gupta, N.; Wilson, K.R.; Tsai, M.H.; Yan, Y.; Macri, C.F.; Dagley, L.F.; et al. Physiological Substrates and Ontogeny-Specific Expression of the Ubiquitin Ligases MARCH1 and MARCH8. Curr. Res. Immunol. 2021, 2, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Uribe, A.P.; Valencia-Martínez, H.; Carballo-Uicab, G.; Vallejo-Castillo, L.; Medina-Rivero, E.; Chacón-Salinas, R.; Pavón, L.; Velasco-Velázquez, M.A.; Mellado-Sánchez, G.; Estrada-Parra, S.; et al. CD80 Expression Correlates with IL-6 Production in THP-1-like Macrophages Costimulated with LPS and Dialyzable Leukocyte Extract (Transferon®). J. Immunol. Res. 2019, 2019, 2198508. [Google Scholar] [CrossRef] [PubMed]
- Pavón, L.; Sandoval-López, G.; Eugenia Hernández, M.; Loría, F.; Estrada, I.; Pérez, M.; Moreno, J.; Ávila, U.; Leff, P.; Antón, B.; et al. Th2 Cytokine Response in Major Depressive Disorder Patients before Treatment. J. Neuroimmunol. 2006, 172, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, M.E.; Mendieta, D.; Pérez-Tapia, M.; Bojalil, R.; Estrada-Garcia, I.; Estrada-Parra, S.; Pavón, L. Effect of Selective Serotonin Reuptake Inhibitors and Immunomodulator on Cytokines Levels: An Alternative Therapy for Patients with Major Depressive Disorder. Clin. Dev. Immunol. 2013, 2013, 267871. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-García, J.L.; Pérez-Sánchez, G.; Becerril Villanueva, E.; Alvarez-Herrera, S.; Pavón, L.; Gutiérrez-Ospina, G.; López-Santiago, R.; Maldonado-Tapia, J.O.; Pérez-Tapia, S.M.; Moreno-Lafont, M.C. Behavioral and Neurochemical Shifts at the Hippocampus and Frontal Cortex Are Associated to Peripheral Inflammation in Balb/c Mice Infected with Brucella Abortus 2308. Microorganisms 2021, 9, 1937. [Google Scholar] [CrossRef]
- Maldonado-García, J.L.; Pérez-Sánchez, G.; Becerril-Villanueva, E.; Alvarez-Herrera, S.; Pavón, L.; Sánchez-Torres, L.; Gutiérrez-Ospina, G.; Girón-Pérez, M.I.; Damian-Morales, G.; Maldonado-Tapia, J.O.; et al. Imipramine Administration in Brucella Abortus 2308-Infected Mice Restores Hippocampal Serotonin Levels, Muscle Strength, and Mood, and Decreases Spleen CFU Count. Pharmaceuticals 2023, 16, 1525. [Google Scholar] [CrossRef]
| Category | Mechanism of Action | Examples |
|---|---|---|
| Selective serotonin reuptake inhibitors (SSRIs) | Inhibit serotonin reuptake, thus increasing serotonin activity. | Citalopram Escitalopram Paroxetine Sertraline Fluoxetine Fluvoxamine |
| Serotonin–norepinephrine reuptake inhibitors (SNRIs) | Block serotonin and norepinephrine reuptake in the synaptic button, increasing postsynaptic receptors’ stimulation | Venlafaxine Desvenlafaxine Duloxetine Milnacipran Levomilnacipran |
| Atypical antidepressants | This group is characterized by different mechanisms of action, with the following examples: Bupropion inhibits dopamine and norepinephrine reuptake. Mirtazapine blocks α-2 adrenergic receptors on the cell bodies and nerve terminals and increases the release of norepinephrine into the synapse. Mirtazapine works by blocking alpha-2 adrenergic receptors on the cell bodies and nerve terminals, promoting the release of norepinephrine into the synapse, and in addition antagonizes 5-HT receptors | Bupropion Mirtazapine Agomelatine |
| Serotonin modulators | This group has different mechanisms of action on the serotonergic system. Trazodone acts upon postsynaptic serotonin 5-HT2A and 5-HT2C receptors and weakly inhibits presynaptic serotonin reuptake. In addition, it has additional postsynaptic alpha-adrenergic receptors and histamine receptors blocking activity. In addition, it blocks α-adrenergic receptors and histamine receptors in the postsynaptic button. Nefazodone antagonizes postsynaptic serotonin 5-HT2A receptors and inhibits presynaptic serotonin and norepinephrine reuptake. Vortioxetine acts as a 5-HT1A receptor agonist and a 5-HT3 and 5-HT7 receptor antagonist. | Nefazodone Trazodone Vilazodone Vortioxetine |
| Tricyclic antidepressants (TCAs) | Inhibit the reuptake of norepinephrine and serotonin at the presynaptic neuronal membrane. | Amitriptyline Clomipramine Doxepin Imipramine Trimipramine Desipramine Nortriptyline Protriptyline Maprotiline Amoxapine |
| Monoamine oxidase inhibitors (MAOIs) | Inhibit the monoamine oxidase enzyme responsible for catabolizing serotonin, norepinephrine, and dopamine. | Selegiline Moclobemide Tranylcypromine Isocarboxazid Phenelzine |
| Cytokine | Effect |
|---|---|
| IFN-α | Fatigue, depression, thought disorders, psychosis and suicidal ideation, stress, anxiety, decreased substance P, myalgia, psychomotor retardation, anorexia, social isolation, irritability, and cognitive disorders (lack of concentration, memory impairment, and bradypsychia) |
| IFN-β | Fatigue, depression, and bradypsychia |
| IFN-γ | Modulates social behavior by regulating the connection of social interaction brain areas |
| TNF-α | Anorexia, fatigue, stress, upregulation of substance P expression, rapid eye movements during sleep, and increased release of excitatory neurotransmitters; noradrenaline and adrenaline stimulate its release |
| IL-1β | Somnolence, confusion, hallucinations, hyperalgesia, fatigue, fever, sleepiness, myalgia, and substance P antinociception (increased GABA and decreased NMDA); noradrenaline and adrenaline stimulate its release |
| IL-2 | Confusion, delusions, depression, psychosis, myalgias, and cognitive dysfunction |
| IL-4 | Regulates higher mental functions such as memory and learning |
| IL-6 | Stress, fatigue, hyperalgesia, depression, and activation of the sympathetic nervous system; noradrenaline, adrenaline, and substance P stimulate their release |
| IL-8 | Mediates sympathetic pain; substance P stimulates its production |
| IL-10 | Blocks pain |
| IL-17A | Modulates anxiety through meningeal γδ T cells |
| Variables | Effect |
|---|---|
| Cortisol | ↑ |
| T helper cells | ns |
| T cytotoxic cells | ↑ |
| NK cells | ↑ |
| B cells | ↓ |
| IL-1β | ↓ |
| TNF-α | ↑ |
| IL-6 | ns |
| IL-2 | ↓ |
| IFN-γ | ↓ |
| IL-4 | ↑ |
| IL-13 | ↑ |
| Variables | Comparison of Patients with MDD vs. Healthy Volunteers | ||
|---|---|---|---|
| W0 vs. HV | W20 vs. HV | W52 vs. HV | |
| Cortisol | ↑ | ns | ↑ |
| IL-1β | ↓ | ↑ | ↑ |
| IL-2 | ↓ | ns | ↓ |
| IFN-γ | ↓ | ns | ↑ |
| IL-10 | ↑ | ↓ | ↓ |
| Variables | Comparison of Patients with MDD vs. Healthy Volunteers (HV) | |||||
|---|---|---|---|---|---|---|
| W0 | W20 | W52 | ||||
| SSRI + hDLE vs. HV | SSRI + hDLE vs. SSRI | SSRI + hDLE vs. HV | SSRI + hDLE vs. SSRI | SSRI + hDLE vs. HV | SSRI + hDLE vs. SSRI | |
| Cortisol | ↑ | ns | ns | ↓ | ns | ↓ |
| IL-1β | ↓ | ns | ↑ | ns | ns | ↓ |
| IL-2 | ↓ | ns | ns | ns | ns | ↑ |
| IFN-γ | ↓ | ns | ns | ↑ | ns | ↑ |
| IL-10 | ↑ | ns | ns | ↑ | ns | ↑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maldonado-García, J.L.; García-Mena, L.H.; Mendieta-Cabrera, D.; Pérez-Sánchez, G.; Becerril-Villanueva, E.; Alvarez-Herrera, S.; Homberg, T.; Vallejo-Castillo, L.; Pérez-Tapia, S.M.; Moreno-Lafont, M.C.; et al. Use of Extracellular Monomeric Ubiquitin as a Therapeutic Option for Major Depressive Disorder. Pharmaceuticals 2024, 17, 841. https://doi.org/10.3390/ph17070841
Maldonado-García JL, García-Mena LH, Mendieta-Cabrera D, Pérez-Sánchez G, Becerril-Villanueva E, Alvarez-Herrera S, Homberg T, Vallejo-Castillo L, Pérez-Tapia SM, Moreno-Lafont MC, et al. Use of Extracellular Monomeric Ubiquitin as a Therapeutic Option for Major Depressive Disorder. Pharmaceuticals. 2024; 17(7):841. https://doi.org/10.3390/ph17070841
Chicago/Turabian StyleMaldonado-García, José Luis, Lissette Haydee García-Mena, Danelia Mendieta-Cabrera, Gilberto Pérez-Sánchez, Enrique Becerril-Villanueva, Samantha Alvarez-Herrera, Toni Homberg, Luis Vallejo-Castillo, Sonia Mayra Pérez-Tapia, Martha C. Moreno-Lafont, and et al. 2024. "Use of Extracellular Monomeric Ubiquitin as a Therapeutic Option for Major Depressive Disorder" Pharmaceuticals 17, no. 7: 841. https://doi.org/10.3390/ph17070841
APA StyleMaldonado-García, J. L., García-Mena, L. H., Mendieta-Cabrera, D., Pérez-Sánchez, G., Becerril-Villanueva, E., Alvarez-Herrera, S., Homberg, T., Vallejo-Castillo, L., Pérez-Tapia, S. M., Moreno-Lafont, M. C., Ortuño-Sahagún, D., & Pavón, L. (2024). Use of Extracellular Monomeric Ubiquitin as a Therapeutic Option for Major Depressive Disorder. Pharmaceuticals, 17(7), 841. https://doi.org/10.3390/ph17070841











