Inula salicina L.: Insights into Its Polyphenolic Constituents and Biological Activity
Abstract
1. Introduction
2. Results and Discussion
2.1. Identification of Compounds in Inula salicina by UHPLC-MS/MS and NMR
2.2. Quantitative Determination of Total Phenolics, Total Flavonoids, Chlorogenic and Dicaffeoylquinic Acids
2.3. Antioxidant Potential of Inula salicina Extract
2.4. In Vitro Sun Protection Factor (SPF) of Inula salicina Extract
2.5. Biofilm Inhibition of Inula salicina Extract
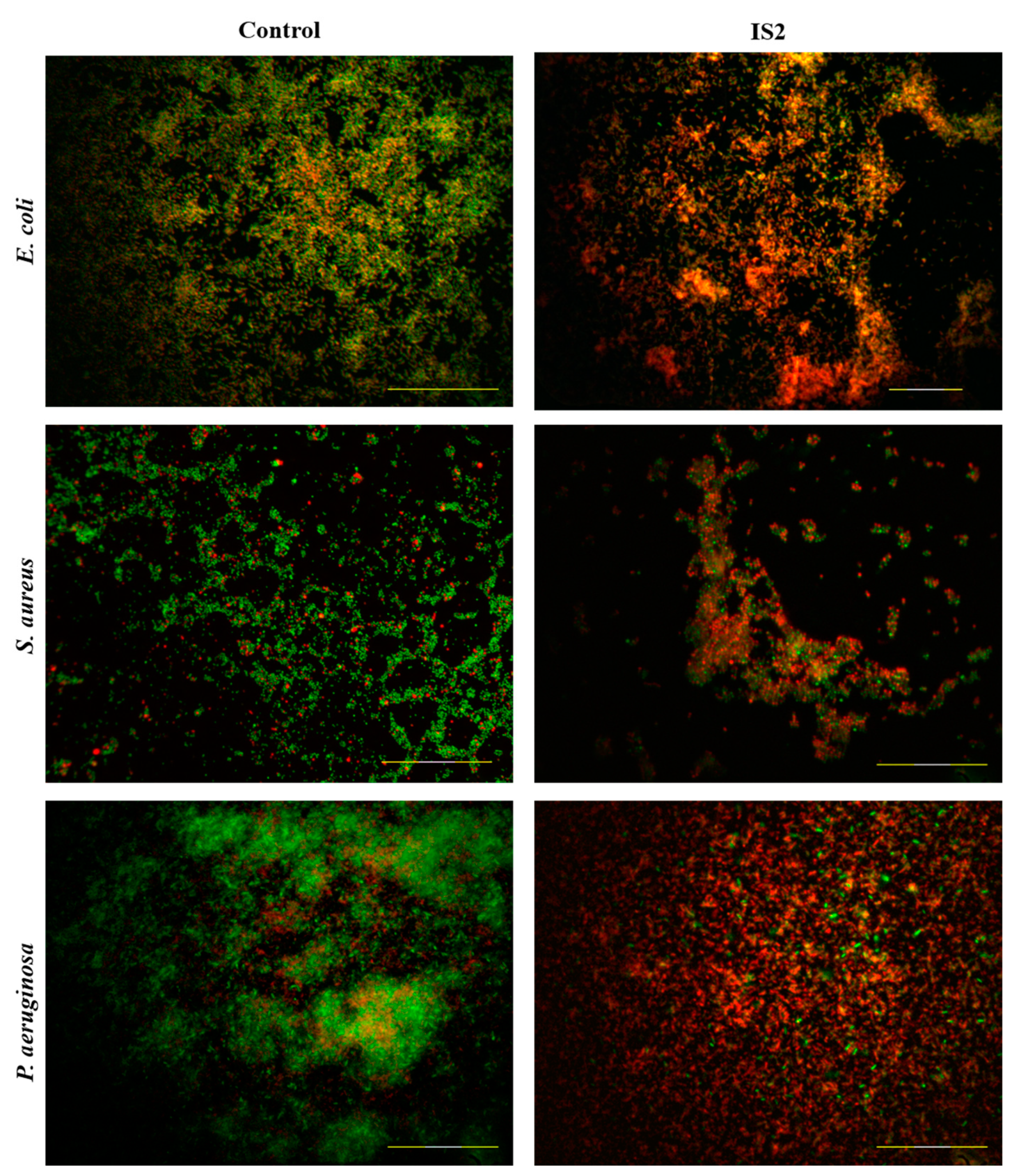
2.6. Live/Dead Biofilm Assays
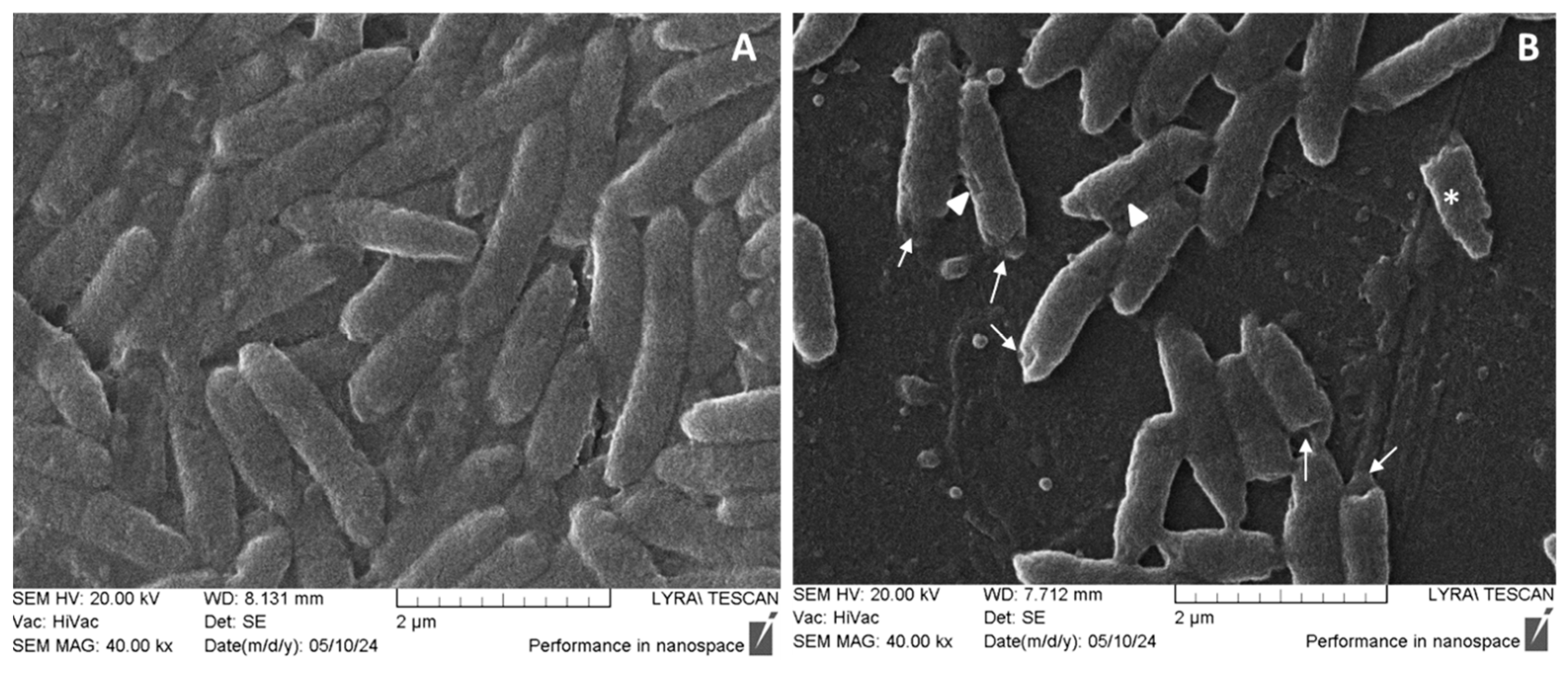
2.7. SEM on Pseudomonas aeruginosa Cell Morphology
3. Materials and Methods
3.1. Plant Material
3.2. Preparation of the Methanol Extract
3.3. Fractionation of the Methanol Extract and Isolation of Individual Compounds
3.4. NMR Analysis
3.5. UHPLC-HRMS Analysis
3.6. HPLC-DAD Quantification of Caffeoylquinic Acids
3.7. Evaluation of Total Phenolic (TPC) and Total Flavonoid (TFC) Contents
3.8. Assessment of Antioxidant Potential
3.9. SPF and UV-B Photoprotective Study
3.10. Biofilm Inhibition and Assessment of Biofilm Viability
3.10.1. Bacterial Strains and Growth Conditions
3.10.2. Biofilm Inhibition
3.10.3. Assessment of Biofilm Viability Using Live/Dead Staining
3.10.4. Scanning Electron Microscopy (SEM)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Szymanska, R.; Pospíšil, P.; Kruk, J. Plant-Derived Antioxidants in Disease Prevention 2018. Oxid. Med. Cell. Longev. 2018, 2018, 2068370. [Google Scholar] [CrossRef] [PubMed]
- Vara, D.; Pula, G. Reactive Oxygen Species: Physiological Roles in the Regulation of Vascular Cells. Curr. Mol. Med. 2014, 14, 1103–1125. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimzadeh, M.A.; Enayatifard, R.; Khalili, M.; Ghaffarloo, M.; Saeedi, M.; Charati, J.Y. Correlation between Sun Protection Factor and Antioxidant Activity, Phenol and Flavonoid Contents of Some Medicinal Plants. Iran. J. Pharm. Res. 2014, 13, 1041–1048. [Google Scholar] [PubMed]
- Wilson, B.D.; Moon, S.; Armstrong, F. Comprehensive Review of Ultraviolet Radiation and the Current Status on Sunscreens. J. Clin. Aesthet. Dermatol. 2012, 5, 18. [Google Scholar]
- Li, L.; Chong, L.; Huang, T.; Ma, Y.; Li, Y.; Ding, H. Natural Products and Extracts from Plants as Natural UV Filters for Sunscreens: A Review. Anim. Model. Exp. Med. 2023, 6, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M. Plant Extracts as Skin Care and Therapeutic Agents. Int. J. Mol. Sci. 2023, 24, 15444. [Google Scholar] [CrossRef]
- He, H.; Li, A.; Li, S.; Tang, J.; Li, L.; Xiong, L. Natural Components in Sunscreens: Topical Formulations with Sun Protection Factor (SPF). Biomed. Pharmacother. 2021, 134, 111161. [Google Scholar] [CrossRef] [PubMed]
- Radice, M.; Manfredini, S.; Ziosi, P.; Dissette, V.; Buso, P.; Fallacara, A.; Vertuani, S. Herbal Extracts, Lichens and Biomolecules as Natural Photo-Protection Alternatives to Synthetic UV Filters. A Systematic Review. Fitoterapia 2016, 114, 144–162. [Google Scholar] [CrossRef]
- Damyanova, T.; Dimitrova, P.D.; Borisova, D.; Topouzova-Hristova, T.; Haladjova, E.; Paunova-Krasteva, T. An Overview of Biofilm-Associated Infections and the Role of Phytochemicals and Nanomaterials in Their Control and Prevention. Pharmaceutics 2024, 16, 162. [Google Scholar] [CrossRef]
- Dimitrova, P.D.; Damyanova, T.; Paunova-Krasteva, T. Chromobacterium Violaceum: A Model for Evaluating the Anti-Quorum Sensing Activities of Plant Substances. Sci. Pharm. 2023, 91, 33. [Google Scholar] [CrossRef]
- Seca, A.M.L.; Grigore, A.; Pinto, D.C.G.A.; Silva, A.M.S. The Genus Inula and Their Metabolites: From Ethnopharmacological to Medicinal Uses. J. Ethnopharmacol. 2014, 154, 286–310. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.M.; Zhang, M.L.; Shi, Q.W.; Kiyota, H. Chemical Constituents of Plants from the Genus Inula. Chem. Biodivers. 2006, 3, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Talebi, M.; Khoramjouy, M.; Feizi, A.; Ali, Z.; Khan, I.A.; Ayatollahi, N.A.; Ayatollahi, S.A.; Faizi, M. Novel Multi-Target Therapeutic Potential of the Genus Inula: Advances and Opportunities for Neuroprotection. Pharmacol. Res.-Mod. Chinese Med. 2023, 7, 100263. [Google Scholar] [CrossRef]
- Tavares, W.R.; Seca, A.M.L. Inula L. Secondary Metabolites against Oxidative Stress-Related Human Diseases. Antioxidants 2019, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.W.; Qin, J.J.; Cheng, X.R.; Shen, Y.H.; Shan, L.; Jin, H.Z.; Zhang, W.D. Inula Sesquiterpenoids: Structural Diversity, Cytotoxicity and Anti-Tumor Activity. Expert Opin. Investig. Drugs 2014, 23, 317–345. [Google Scholar] [CrossRef] [PubMed]
- Seca, A.M.L.; Pinto, D.C.G.A.; Silva, A.M.S. Metabolomic Profile of the Genus Inula. Chem. Biodivers. 2015, 12, 859–906. [Google Scholar] [CrossRef] [PubMed]
- Trendafilova, A.; Ivanova, V.; Rangelov, M.; Todorova, M.; Ozek, G.; Yur, S.; Ozek, T.; Aneva, I.; Veleva, R.; Moskova-Doumanova, V.; et al. Caffeoylquinic Acids, Cytotoxic, Antioxidant, Acetylcholinesterase and Tyrosinase Enzyme Inhibitory Activities of Six Inula Species from Bulgaria. Chem. Biodivers. 2020, 17, e2000051. [Google Scholar] [CrossRef] [PubMed]
- Inula Salicina in Flora of China @ Efloras.Org. Available online: http://www.efloras.org/florataxon.aspx?flora_id=2&taxon_id=200024067 (accessed on 20 May 2024).
- Inula Salicina|Euro+Med-Plantbase. Available online: https://europlusmed.org/cdm_dataportal/taxon/e4ecad7f-1304-4bf9-9736-3a87a4da505b (accessed on 20 May 2024).
- AGFonds—Irish Fleabane (Inula salicina L.). Available online: https://www.agfonds.lv/herbs/herbs-i-j/irish-fleabane-inula-salicina-l/ (accessed on 20 May 2024).
- Tardío, J.; Pardo-De-Santayana, M.; Morales, R. Ethnobotanical Review of Wild Edible Plants in Spain. Bot. J. Linn. Soc. 2006, 152, 27–71. [Google Scholar] [CrossRef]
- Yıldırım, A.; Şen, A.; Hacıoğlu, M.; Seher, A.; Tan, B.; Şenkardeş, İ.; Bitiş, L. In Vitro Investigation of Antimicrobial, Enzyme Inhibitory and Free Radical Scavenging Activities of Inula salicina L. Int. J. Agric. Environ. Food Sci. 2022, 6, 389–395. [Google Scholar] [CrossRef]
- Dimitrova, P.D.; Ivanova, V.; Trendafilova, A.; Paunova-Krasteva, T. Anti-Biofilm and Anti-Quorum-Sensing Activity of Inula Extracts: A Strategy for Modulating Chromobacterium Violaceum Virulence Factors. Pharmaceuticals 2024, 17, 573. [Google Scholar] [CrossRef]
- Sevindik, E.; Aydin, S.; Paksoy, M.Y.; Sokmen, B.B. Anti-Urease, Total Phenolic Content and Antioxidant Activities of Some Inula L. (Asteraceae) Taxa in Turkey. Genetika 2020, 52, 825–834. [Google Scholar] [CrossRef]
- Péter, A.; Dósa, G. Detection of Phenoloids in Some Hungarian Inula and Centaurea Species. Acta Bot. Hung. 2002, 44, 129–135. [Google Scholar] [CrossRef]
- Wollenweber, E.; Dörr, M.; Fritz, H.; Valant-Vetschera, K.M. Exudate Flavonoids in Several Asteroideae and Cichorioideae (Asteraceae). Zeitschrift fur Naturforsch. Sect. C-J. Biosci. 1997, 52, 137–143. [Google Scholar] [CrossRef]
- Bohlmann, F.; Mahanta, P.K.; Jakupovic, J.; Rastogi, R.C.; Natu, A.A. New Sesquiterpene Lactones from Inula Species. Phytochemistry 1978, 17, 1165–1172. [Google Scholar] [CrossRef]
- Trendafilova, A.; Ivanova, V.; Todorova, M.; Staleva, P.; Aneva, I. Terpenoids in Four Inula Species from Bulgaria. J. Serbian Chem. Soc. 2021, 86, 1229–1240. [Google Scholar] [CrossRef]
- Gevrenova, R.; Zengin, G.; Sinan, K.I.; Zheleva-Dimitrova, D.; Balabanova, V.; Kolmayer, M.; Voynikov, Y.; Joubert, O. An In-Depth Study of Metabolite Profile and Biological Potential of Tanacetum balsamita L. (Costmary). Plants 2023, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Wu, W.; Kirkpatrick, J.; Kuhnert, N. Profiling the Chlorogenic Acids and Other Caffeic Acid Derivatives of Herbal Chrysanthemum by LC-MSn. J. Agric. Food Chem. 2007, 55, 929–936. [Google Scholar] [CrossRef]
- Shahzad, M.N.; Ahmad, S.; Tousif, M.I.; Ahmad, I.; Rao, H.; Ahmad, B.; Basit, A. Profiling of Phytochemicals from Aerial Parts of Terminalia Neotaliala Using LC-ESI-MS2 and Determination of Antioxidant and Enzyme Inhibition Activities. PLoS ONE 2022, 17, e0266094. [Google Scholar] [CrossRef]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical Scheme for LC-MSn Identification of Chlorogenic Acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef]
- Clifford, M.N.; Zheng, W.; Kuhnert, N. Profiling the Chlorogenic Acids of Aster by HPLC–MSn. Phytochem. Anal. 2006, 17, 384–393. [Google Scholar] [CrossRef]
- Tolonen, A.; Joustamo, T.; Mattlla, S.; Kämäräinen, T.; Jalonen, J. Identification of Isomeric Dicaffeoylquinic Acids from Eleutheracoccus Senticosus Using HPLC-ESI/TOF/MS and H-NMR Methods. Phytochem. Anal. 2002, 13, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, R.; Halabi, E.A.; Karar, M.G.E.; Kuhnert, N. Identification and Characterisation of the Phenolics of Ilex Glabra L. Gray (Aquifoliaceae) Leaves by Liquid Chromatography Tandem Mass Spectrometry. Phytochemistry 2014, 106, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Marks, S.; Knight, S.; Kuhnert, N. Characterization by LC-MS(n) of Four New Classes of p-Coumaric Acid-Containing Diacyl Chlorogenic Acids in Green Coffee Beans. J. Agric. Food Chem. 2006, 54, 4095–4101. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, R.; Sovdat, T.; Vivan, F.; Kuhnert, N. Profiling and Characterization by LC-MSn of the Chlorogenic Acids and Hydroxycinnamoylshikimate Esters in Maté (Ilex paraguariensis). J. Agric. Food Chem. 2010, 58, 5471–5484. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Nilofar; Yildiztugay, E.; Bouyahya, A.; Cavusoglu, H.; Gevrenova, R.; Zheleva-Dimitrova, D. A Comparative Study on UHPLC-HRMS Profiles and Biological Activities of Inula Sarana Different Extracts and Its Beta-Cyclodextrin Complex: Effective Insights for Novel Applications. Antioxidants 2023, 12, 1842. [Google Scholar] [CrossRef] [PubMed]
- Sinosaki, N.B.M.; Tonin, A.P.P.; Ribeiro, M.A.S.; Poliseli, C.B.; Roberto, S.B.; da Silveira, R.; Visentainer, J.V.; Santos, O.O.; Meurer, E.C. Structural Study of Phenolic Acids by Triple Quadrupole Mass Spectrometry with Electrospray Ionization in Negative Mode and H/D Isotopic Exchange. J. Braz. Chem. Soc. 2020, 31, 402–408. [Google Scholar] [CrossRef]
- Brahmi-Chendouh, N.; Piccolella, S.; Crescente, G.; Pacifico, F.; Boulekbache, L.; Hamri-Zeghichi, S.; Akkal, S.; Madani, K.; Pacifico, S. A Nutraceutical Extract from Inula Viscosa Leaves: UHPLC-HR-MS/MS Based Polyphenol Profile, and Antioxidant and Cytotoxic Activities. J. Food Drug Anal. 2019, 27, 692–702. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, Y.; Li, S.; He, X.; Gu, R. Comparative Bioactivity Evaluation and Metabolic Profiling of Different Parts of Duhaldea Nervosa Based on GC-MS and LC-MS. Front. Nutr. 2023, 10, 1301715. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Xie, J.; Shi, S.; Luo, L.; Li, K.; Xiong, P.; Cai, W. Diagnostic Fragment-Ion-Based for Rapid Identification of Chlorogenic Acids Derivatives in Inula Cappa Using UHPLC-Q-Exactive Orbitrap Mass Spectrometry. J. Anal. Methods Chem. 2021, 2021, 6393246. [Google Scholar] [CrossRef] [PubMed]
- Stojakowska, A.; Malarz, J.; Kiss, A.K. Hydroxycinnamates from Elecampane (Inula helenium L.) Callus Culture. Acta Physiol. Plant. 2016, 38, 1–5. [Google Scholar] [CrossRef]
- Rechek, H.; Haouat, A.; Hamaidia, K.; Pinto, D.C.G.A.; Boudiar, T.; Válega, M.S.G.A.; Pereira, D.M.; Pereira, R.B.; Silva, A.M.S. Inula viscosa (L.) Aiton Ethanolic Extract Inhibits the Growth of Human AGS and A549 Cancer Cell Lines. Chem. Biodivers. 2023, 20, e202200890. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, V.; Todorova, M.; Rangelov, M.; Aneva, I.; Trendafilova, A. Phenolic Content and Antioxidant Capacity of Inula Britannica from Different Habitats in Bulgaria. Bulg. Chem. Commun. 2020, 52, 168–173. [Google Scholar]
- Fraisse, D.; Felgines, C.; Texier, O.; Lamaison, J.-L. Caffeoyl Derivatives: Major Antioxidant Compounds of Some Wild Herbs of the Asteraceae Family. Food Nutr. Sci. 2011, 2, 181–192. [Google Scholar] [CrossRef]
- Wianowska, D.; Gil, M. Recent Advances in Extraction and Analysis Procedures of Natural Chlorogenic Acids. Phytochem. Rev. 2018, 18, 273–302. [Google Scholar] [CrossRef]
- Liu, W.; Li, J.; Zhang, X.; Zu, Y.; Yang, Y.; Liu, W.; Xu, Z.; Gao, H.; Sun, X.; Jiang, X.; et al. Current Advances in Naturally Occurring Caffeoylquinic Acids: Structure, Bioactivity, and Synthesis. J. Agric. Food Chem. 2020, 68, 10489–10516. [Google Scholar] [CrossRef]
- Kłeczek, N.; Michalak, B.; Malarz, J.; Kiss, A.K.; Stojakowska, A. Carpesium Divaricatum Sieb. & Zucc. Revisited: Newly Identified Constituents from Aerial Parts of the Plant and Their Possible Contribution to the Biological Activity of the Plant. Molecules 2019, 24, 1614. [Google Scholar] [CrossRef]
- Malarz, J.; Michalska, K.; Galanty, A.; Kiss, A.K.; Stojakowska, A. Constituents of Pulicaria Inuloides and Cytotoxic Activities of Two Methoxylated Flavonols. Molecules 2023, 28, 480. [Google Scholar] [CrossRef] [PubMed]
- Kłeczek, N.; Malarz, J.; Gierlikowska, B.; Kiss, A.K.; Stojakowska, A. Constituents of Xerolekia speciosissima (L.) Anderb. (Inuleae), and Anti-Inflammatory Activity of 7,10-Diisobutyryloxy-8,9-Epoxythymyl Isobutyrate. Molecules 2020, 25, 4913. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, V.; Trendafilova, A.; Todorova, M.; Danova, K.; Dimitrov, D. Phytochemical Profile of Inula Britannica from Bulgaria. Nat. Prod. Commun. 2017, 12, 153–154. [Google Scholar] [CrossRef]
- Wollenweber, E.; Christ, M.; Dunstan, R.H.; Roitman, J.N.; Stevens, J.F. Exudate Flavonoids in Some Gnaphalieae and Inuleae (Asteraceae). Z. Naturforsch. C. 2005, 60, 671–678. [Google Scholar] [CrossRef]
- Trendafilova, A.; Todorova, M.; Ivanova, V.; Aneva, I. Phenolic Constituents and Antioxidant Capacity of Inula Oculus-Christi from Bulgaria. Bulg. Chem. Commun. 2017, 49, 176–180. [Google Scholar]
- Ivanova, V.; Todorova, M.; Nedialkov, P.; Trendafilova, A. A New Flavonol Acylglucoside from Inula Aschersoniana Janka Var. Aschersoniana. Comptes Rendus L’Academie Bulg. des Sci. 2021, 74, 514–520. [Google Scholar] [CrossRef]
- Krasteva, I.; Bratkov, V.; Bucar, F.; Kunert, O.; Kollroser, M.; Kondeva-Burdina, M.; Ionkova, I. Flavoalkaloids and Flavonoids from Astragalus Monspessulanus. J. Nat. Prod. 2015, 78, 2565–2571. [Google Scholar] [CrossRef]
- Ilkei, V.; Hazai, L.; Antus, S.; Bölcskei, H. Flavonoid Alkaloids: Isolation, Bioactivity, and Synthesis. Stud. Nat. Prod. Chem. 2018, 56, 247–285. [Google Scholar] [CrossRef]
- Khadem, S.; Marles, R.J. Chromone and Flavonoid Alkaloids: Occurrence and Bioactivity. Molecules 2011, 17, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380–3410. [Google Scholar] [CrossRef] [PubMed]
- Özcan, F.Ş.; Özcan, N.; Dikmen Meral, H.; Çetin, Ö.; Çelik, M.; Trendafilova, A. Extraction of Sesquiterpene Lactones from Inula Helenium Roots by High-Pressure Homogenization and Effects on Antimicrobial, Antioxidant, and Antiglycation Activities. Food Bioprocess Technol. 2024, 1–12. [Google Scholar] [CrossRef]
- Ceylan, R.; Zengin, G.; Mahomoodally, M.F.; Sinan, K.I.; Ak, G.; Jugreet, S.; Cakır, O.; Ouelbani, R.; Paksoy, M.Y.; Yılmaz, M.A. Enzyme Inhibition and Antioxidant Functionality of Eleven Inula Species Based on Chemical Components and Chemometric Insights. Biochem. Syst. Ecol. 2021, 95, 104225. [Google Scholar] [CrossRef]
- Mansur, J.S.; Breder, M.N.; Mnasur, M.C.; Azulay, R.D. Determinacao Do Fator de Protecao Solar Por Espectrofotometria. An. Bras. Dermatol 1986, 61, 121–124. [Google Scholar]
- Kurz, H.; Karygianni, L.; Argyropoulou, A.; Hellwig, E.; Skaltsounis, A.L.; Wittmer, A.; Vach, K.; Al-Ahmad, A. Antimicrobial Effects of Inula Viscosa Extract on the In Situ Initial Oral Biofilm. Nutrients 2021, 13, 4029. [Google Scholar] [CrossRef]
- Asraoui, F.; El Mansouri, F.; Cacciola, F.; Brigui, J.; Louajri, A.; Simonetti, G. Biofilm Inhibition of Inula viscosa (L.) Aiton and Globularia alypum L. Extracts Against Candida Infectious Pathogens and In Vivo Action on Galleria Mellonella Model. Adv. Biol. 2023, 7, 2300081. [Google Scholar] [CrossRef]
- Wojnicz, D.; Kucharska, A.Z.; Sokół-Łętowska, A.; Kicia, M.; Tichaczek-Goska, D. Medicinal Plants Extracts Affect Virulence Factors Expression and Biofilm Formation by the Uropathogenic Escherichia Coli. Urol. Res. 2012, 40, 683–697. [Google Scholar] [CrossRef]
- Paunova-Krasteva, T.; Hemdan, B.A.; Dimitrova, P.D.; Damyanova, T.; El-Feky, A.M.; Elbatanony, M.M.; Stoitsova, S.; El-Liethy, M.A.; El-Taweel, G.E.; El Nahrawy, A.M. Hybrid Chitosan/CaO-Based Nanocomposites Doped with Plant Extracts from Azadirachta Indica and Melia Azedarach: Evaluation of Antibacterial and Antibiofilm Activities. Bionanoscience 2023, 13, 88–102. [Google Scholar] [CrossRef]
- Chen, K.; Peng, C.; Chi, F.; Yu, C.; Yang, Q.; Li, Z. Antibacterial and Antibiofilm Activities of Chlorogenic Acid Against Yersinia Enterocolitica. Front. Microbiol. 2022, 13, 885092. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, D.; Sun, Z.; Liu, F.; Du, L.; Wang, D. The Combination of Ultrasound and Chlorogenic Acid to Inactivate Staphylococcus Aureus under Planktonic, Biofilm, and Food Systems. Ultrason. Sonochem. 2021, 80, 105801. [Google Scholar] [CrossRef]
- Wang, L.; Pan, X.; Jiang, L.; Chu, Y.; Gao, S.; Jiang, X.; Zhang, Y.; Chen, Y.; Luo, S.; Peng, C. The Biological Activity Mechanism of Chlorogenic Acid and Its Applications in Food Industry: A Review. Front. Nutr. 2022, 9, 943911. [Google Scholar] [CrossRef] [PubMed]
- Hemeg, H.A.; Moussa, I.M.; Ibrahim, S.; Dawoud, T.M.; Alhaji, J.H.; Mubarak, A.S.; Kabli, S.A.; Alsubki, R.A.; Tawfik, A.M.; Marouf, S.A. Antimicrobial Effect of Different Herbal Plant Extracts against Different Microbial Population. Saudi J. Biol. Sci. 2020, 27, 3221. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Zhu, S.; Ma, C.; Wang, Z. Antibacterial Activity and Mechanism of Action of Chlorogenic Acid. J. Food Sci. 2011, 76, M398–M403. [Google Scholar] [CrossRef]
- Miao, M.; Xiang, L. Pharmacological Action and Potential Targets of Chlorogenic Acid. Adv. Pharmacol. 2020, 87, 71–88. [Google Scholar] [CrossRef]
- D’Abrosca, B.; Buommino, E.; D’Angelo, G.; Coretti, L.; Scognamiglio, M.; Severino, V.; Pacifico, S.; Donnarumma, G.; Fiorentino, A. Spectroscopic Identification and Anti-Biofilm Properties of Polar Metabolites from the Medicinal Plant Helichrysum Italicum against Pseudomonas Aeruginosa. Bioorg. Med. Chem. 2013, 21, 7038–7046. [Google Scholar] [CrossRef]
- Garayev, E.; Di Giorgio, C.; Herbette, G.; Mabrouki, F.; Chiffolleau, P.; Roux, D.; Sallanon, H.; Ollivier, E.; Elias, R.; Baghdikian, B. Bioassay-Guided Isolation and UHPLC-DAD-ESI-MS/MS Quantification of Potential Anti-Inflammatory Phenolic Compounds from Flowers of Inula Montana L. J. Ethnopharmacol. 2018, 226, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Stefanakis, M.K.; Tsiftsoglou, O.S.; Mašković, P.Z.; Lazari, D.; Katerinopoulos, H.E. Chemical Constituents and Anticancer Activities of the Extracts from Phlomis × commixta Rech. f. (P. Cretica × P. Lanata). Int. J. Mol. Sci. 2024, 25, 816. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Sun, Y.; Wang, L.; Wang, J.; Wu, B.; Yan, T.; Jia, Y. An Investigation of the Anti-Depressive Properties of Phenylpropanoids and Flavonoids in Hemerocallis Citrina Baroni. Molecules 2022, 27, 5809. [Google Scholar] [CrossRef] [PubMed]
- Hajiaghaee, R.; Monsef-Esfahani, H.R.; Khorramizadeh, M.R.; Saadat, F.; Shahverdi, A.R.; Attar, F. Inhibitory Effect of Aerial Parts of Scrophularia Striata on Matrix Metalloproteinases Expression. Phyther. Res. 2007, 21, 1127–1129. [Google Scholar] [CrossRef] [PubMed]
- Krzyzaniak, L.M.; Antonelli-Ushirobira, T.M.; Panizzon, G.; Sereia, A.L.; De Souza, J.R.P.; Zequi, J.A.C.; Novello, C.R.; Lopes, G.C.; De Medeiros, D.C.; Silva, D.B.; et al. Larvicidal Activity against Aedes Aegypti and Chemical Characterization of the Inflorescences of Tagetes Patula. Evid.-Based Complement. Altern. Med. 2017, 2017, 9602368. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.B.; Hwang, S.H.; Suh, H.W.; Lim, S.S. Phytochemical Analysis of Agrimonia Pilosa Ledeb, Its Antioxidant Activity and Aldose Reductase Inhibitory Potential. Int. J. Mol. Sci. 2017, 18, 379. [Google Scholar] [CrossRef] [PubMed]
- Bojilov, D.; Manolov, S.; Ahmed, S.; Dagnon, S.; Ivanov, I.; Marc, G.; Oniga, S.; Oniga, O.; Nedialkov, P.; Mollova, S. HPLC Analysis and In Vitro and In Silico Evaluation of the Biological Activity of Polyphenolic Components Separated with Solvents of Various Polarities from Helichrysum Italicum. Molecules 2023, 28, 6198. [Google Scholar] [CrossRef] [PubMed]
- Yoo, K.M.; Lee, C.H.; Lee, H.; Moon, B.K.; Lee, C.Y. Relative Antioxidant and Cytoprotective Activities of Common Herbs. Food Chem. 2008, 106, 929–936. [Google Scholar] [CrossRef]
- Jia, Z.; Tang, M.; Wu, J. The Determination of Flavonoid Contents in Mulberry and Their Scavenging Effects on Superoxide Radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC Assays for Estimating Antioxidant Activity from Guava Fruit Extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Trendafilova, A.; Ivanova, V.; Trusheva, B.; Kamenova-Nacheva, M.; Tabakov, S.; Simova, S. Chemical Composition and Antioxidant Capacity of the Fruits of European Plum Cultivar “Čačanska Lepotica” Influenced by Different Rootstocks. Foods 2022, 11, 2844. [Google Scholar] [CrossRef] [PubMed]
- De Soyza, A.; Hall, A.J.; Mahenthiralingam, E.; Drevinek, P.; Kaca, W.; Drulis-Kawa, Z.; Stoitsova, S.R.; Toth, V.; Coenye, T.; Zlosnik, J.E.A.; et al. Developing an International Pseudomonas Aeruginosa Reference Panel. Microbiologyopen 2013, 2, 1010–1023. [Google Scholar] [CrossRef] [PubMed]
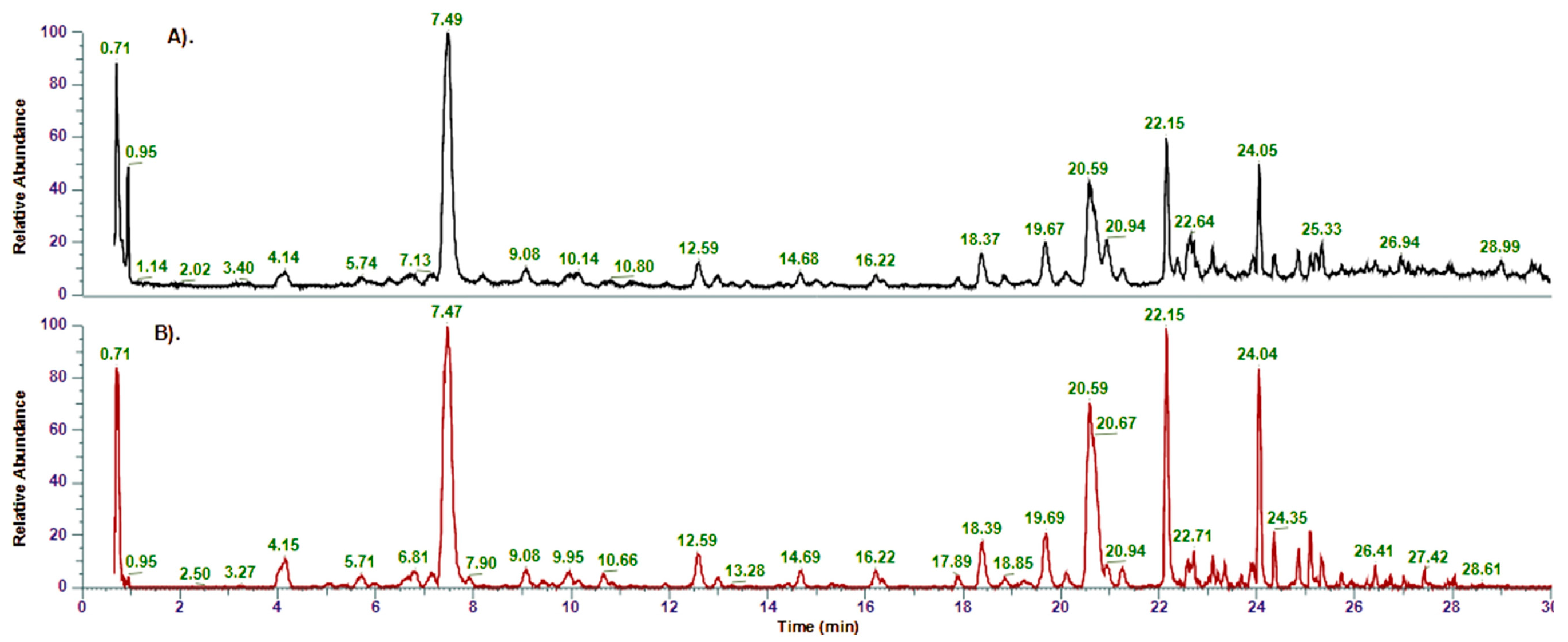
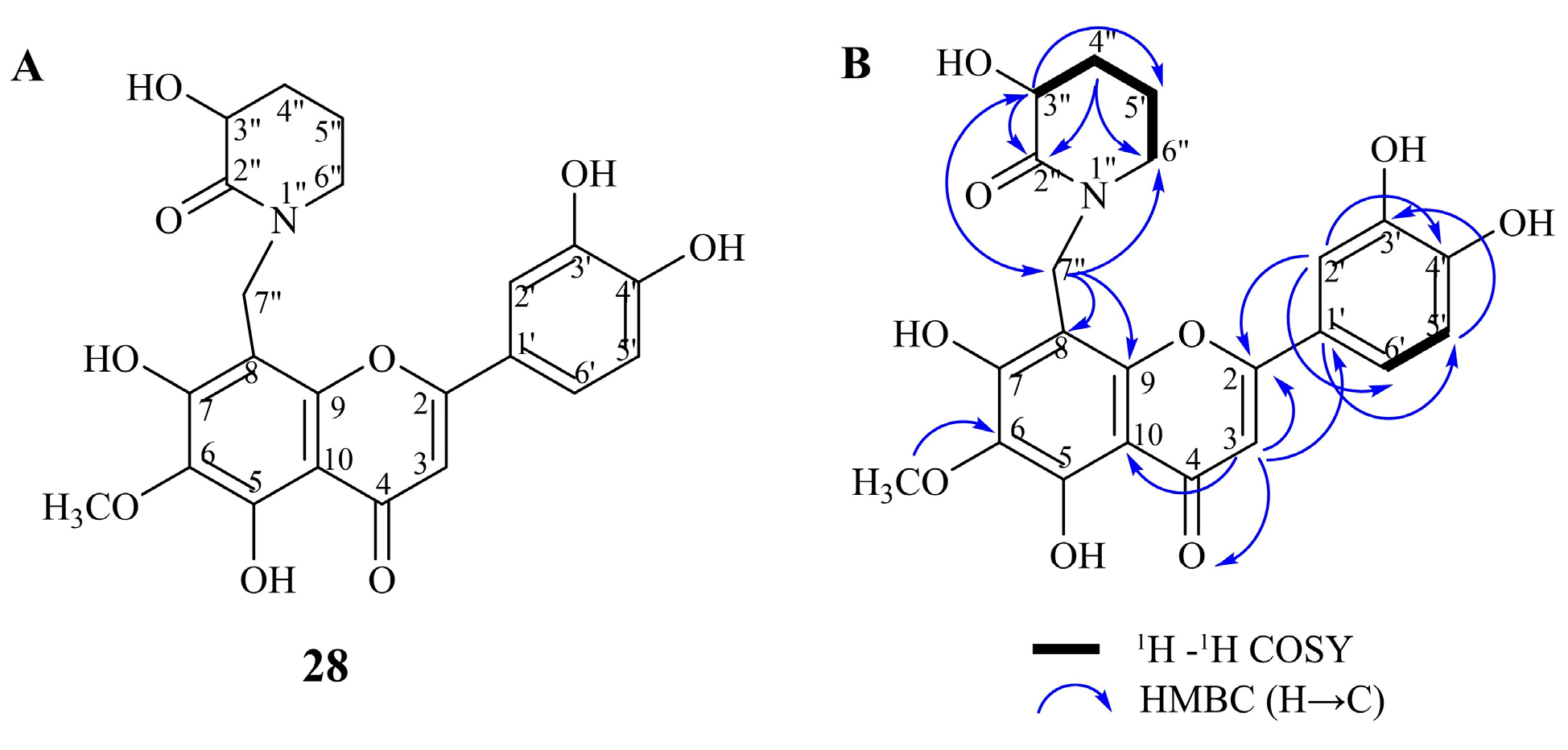
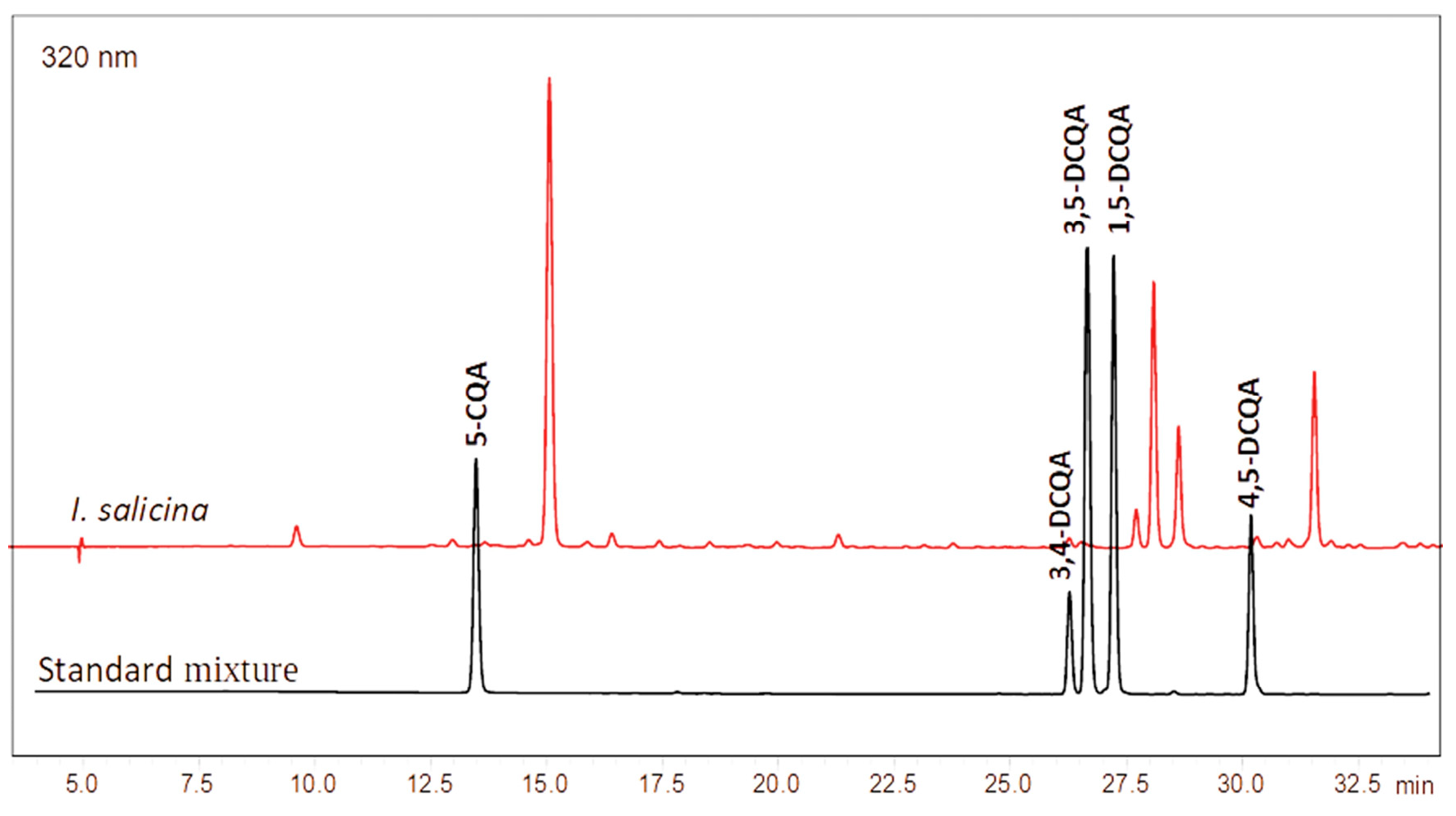
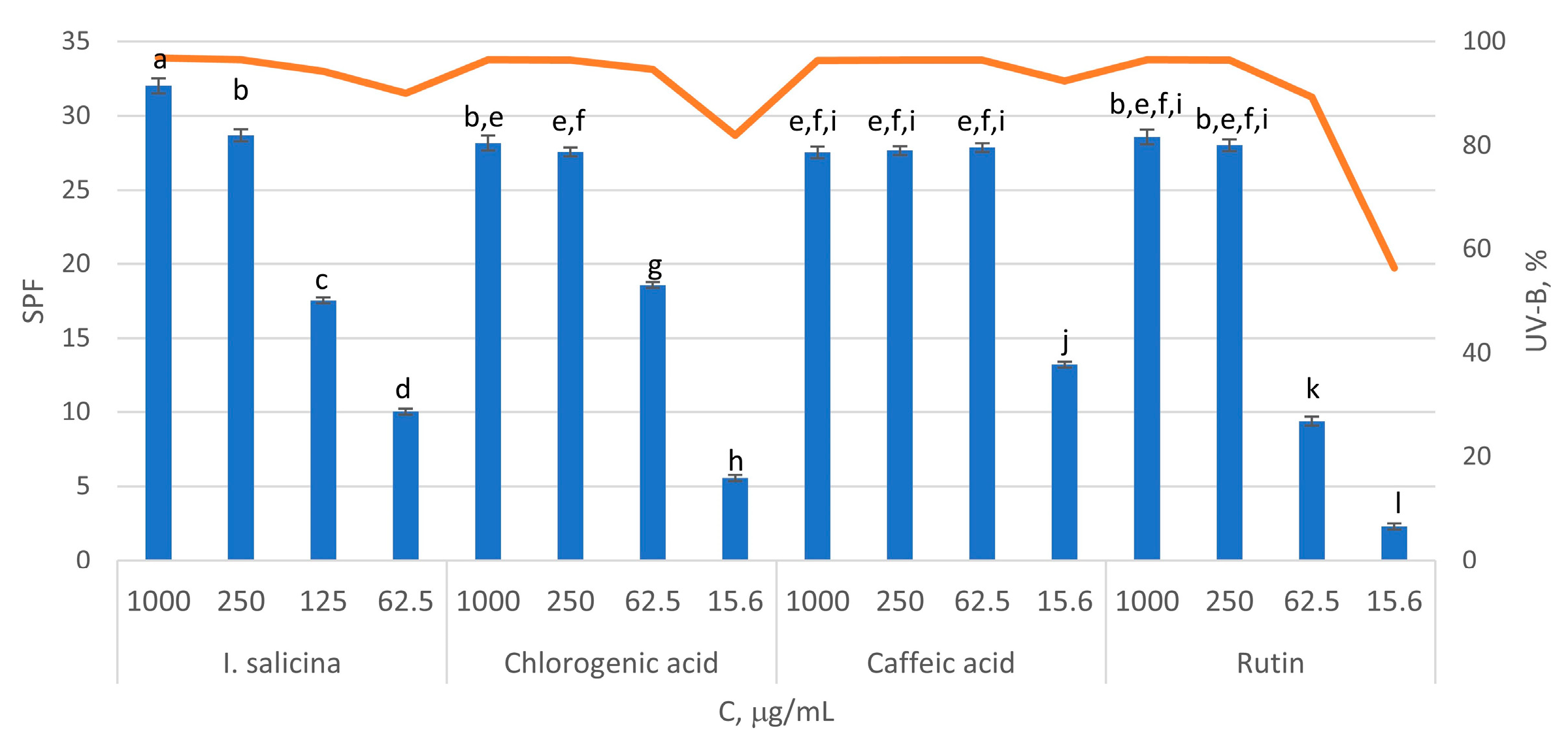
| No | Rt (min) | Compound Name | Formula | [M-H]−, m/z | Δ, ppm | MS/MS Fragments | Identification * |
|---|---|---|---|---|---|---|---|
| 1 | 0.75 | Quinic acid | C7H11O6 | 191.0554 | 1.64 | 191, 173, 163, 145, 129, 115, 101 | MS |
| 2 | 3.27 | Protocatechuic acid | C7H5O4 | 153.0183 | −3.49 | 153, 109 | MS |
| 3 | 4.14 | Neochlorogenic acid (3-O-caffeoylquinic acid) | C16H17O9 | 353.0878 | −0.10 | 353, 191, 179, 135 | MS, St |
| 4 | 5.75 | O-Caffeoyl hexose | C15H17O9 | 341.0879 | 0.16 | 341, 179, 135 | MS |
| 5 | 6.07 | 3-O-p-Coumaroylquinic acid | C16H17O8 | 337.0929 | −0.08 | 337, 191, 163 | MS |
| 6 | 6.85 | O-Caffeoyl hexose isomer | C15H17O9 | 341.0877 | −0.29 | 341, 281, 251, 221, 179, 161, 135 | MS |
| 7 | 6.97 | Ferulic acid | C10H9O4 | 193.0499 | −3.26 | 193, 178, 149, 134 | MS |
| 8 | 7.47 | Chlorogenic acid (5-O-caffeoylquinic acid) | C16H17O9 | 353.0881 | 0.76 | 353, 191, 179, 135 | MS, St, NMR |
| 9 | 7.89 | Caffeic acid | C9H7O4 | 179.0342 | −1.08 | 179, 135 | MS, St |
| 10 | 9.41 | Chlorogenic acid isomer | C16H17O9 | 353.0877 | −0.45 | 353, 191, 179, 161 | MS |
| 11 | 9.95 | Chlorogenic acid isomer | C16H17O9 | 353.0876 | −0.62 | 353, 191, 179, 161, 135 | MS |
| 12 | 10.15 | 4-O-p-Coumaroylquinic acid | C16H17O8 | 337.0934 | 1.55 | 337, 191, 173, 163 | MS |
| 13 | 10.65 | 5-O-p-Coumaroylquinic acid | C16H17O8 | 337.0932 | 1.01 | 337, 191, 173, 163 | MS |
| 14 | 11.76 | p-Coumaric acid | C9H7O3 | 163.0388 | −4.58 | 163, 135, 119 | MS |
| 15 | 12.44 | p-Coumaroylquinic acid isomer | C16H17O8 | 337.0933 | 1.28 | 337, 191, 163 | MS |
| 16 | 12.55 | 5-O-Feruloylquinic acid | C17H19O9 | 367.1033 | −0.58 | 367, 191, 173 | MS |
| 17 | 17.81 | Rutin (quercetin 3-O-rutinoside) | C27H29O16 | 609.1465 | 0.41 | 609, 301 | MS, St, NMR |
| 18 | 18.39 | Isoquercitrin (quercetin 3-O-glucoside) | C21H19O12 | 463.0882 | 0.06 | 463, 301, 300, | MS, St, NMR |
| 19 | 18.55 | Luteolin 7-O-glucoside | C21H19O11 | 447.0933 | 0.09 | 447, 285, 284 | MS, St, NMR |
| 20 | 18.87 | Patulitrin (patuletin 7-O-glucoside) | C22H21O13 | 493.0992 | 0.91 | 493, 331, 330, 316 | MS, NMR |
| 21 | 19.35 | Caffeoyl-(salicyl)-hexoside | C22H21O11 | 461.1089 | −0.04 | 461, 323, 221, 179, 161, 137 | MS |
| 22 | 19.63 | Nepitrin (nepetin 7-O-glucoside) | C22H21O12 | 477.1036 | −0.51 | 477, 315 | MS, NMR |
| 23 | 19.66 | 3,4-Di-O-caffeoylquinic acid | C25H23O12 | 515.1191 | −0.81 | 515, 353, 335, 191, 179, 173 | MS, St |
| 24 | 20.12 | Isorhamnetin hexoside | C22H21O12 | 477.1038 | −0.12 | 477, 315, 299 | MS |
| 25 | 20.56 | 3,5-Di-O-caffeoylquinic acid | C25H23O12 | 515.1191 | −0.81 | 515, 353, 191, 179 | MS, St, NMR |
| 26 | 20.67 | 1,5-Di-O-caffeoylquinic acid | C25H23O12 | 515.1191 | −0.81 | 515, 353, 191, 179 | MS, St, NMR |
| 27 | 20.87 | Tricaffeoylhexaric acid | C33H27O17 | 695.1261 | 1.84 | 695, 533, 371, 209, 191 | MS |
| 28 | 20.94 | N-(8-methylnepetin)-3-hydroxypiperidin-2-one | C22H20O9N | 442.1150 | 1.37 | 442, 327, 312, 284, 256 | MS, NMR |
| 29 | 21.28 | Kaempferol 3-O-glucoside (astragallin) | C21H19O11 | 447.0937 | 0.97 | 447, 285, 284, 255 | MS, NMR |
| 30 | 22.16 | 4,5-Di-O-caffeoylquinic acid | C25H23O12 | 515.1190 | −1.05 | 515, 353, 191, 179, 173 | MS, St |
| 31 | 22.19 | 3-O-p-Coumaroyl-4-O-caffeoylquinic acid | C25H23O11 | 499.1250 | 0.77 | 499, 353, 337, 335, 319, 173, 163 | MS |
| 32 | 22.20 | Tricaffeoylhexaric acid isomer | C33H27O17 | 695.1261 | 1.84 | 695, 533, 371, 209, 191 | MS |
| 33 | 22.36 | 3-O-Caffeoyl-4-O-p-coumaroylquinic acid | C25H23O11 | 499.1249 | 0.53 | 499, 353, 337, 335, 319, 173, 163 | MS |
| 34 | 22.39 | Tricaffeoylhexaric acid isomer | C33H27O17 | 695.1261 | 1.84 | 695, 533, 371, 209, 191 | MS |
| 35 | 22.53 | Tricaffeoylhexaric acid isomer | C33H27O17 | 695.1261 | 1.84 | 695, 533, 371, 209, 191 | MS |
| 36 | 22.57 | 3-O-p-Coumaroyl-5-O-caffeoylquinic acid | C25H23O11 | 499.1252 | 1.26 | 499, 353, 337, 191, 163 | MS |
| 37 | 22.67 | 3-O-Caffeoyl-5-O-p-coumaroylquinic acid | C25H23O11 | 499.1251 | 1.08 | 499, 353, 337, 191, 179, 163 | MS |
| 38 | 22.79 | Caffeoylferuloyl quinic acid | C26H25O12 | 529.1353 | 0.20 | 529, 367, 161 | MS |
| 39 | 23.03 | Caffeoylferuloyl quinic acid isomer | C26H25O12 | 529.1357 | 1.01 | 529, 367, 353, 191 | MS |
| 40 | 23.09 | 4-O-p-Coumaroyl-5-O-caffeoylquinic acid | C25H23O11 | 499.1250 | 0.89 | 499, 337, 191, 173, 163 | MS |
| 41 | 23.20 | 4-O-Caffeoyl-5-O-p-coumaroylquinic acid | C25H23O11 | 499.1250 | 0.83 | 499, 353, 337, 191, 179, 173 | MS |
| 42 | 23.34 | Caffeoylferuloyl quinic acid isomer | C26H25O12 | 529.1352 | 0.08 | 529, 367, 179, 161 | MS |
| 43 | 23.52 | 3,5-di-O-p-Coumaroylquinic acid | C25H23O10 | 483.1298 | 0.19 | 483, 337, 319, 191, 163 | MS |
| 44 | 23.66 | Tetracaffeoylhexaric acid | C42H33O20 | 857.1575 | 1.11 | 857, 695, 533,371, 209, 191 | MS |
| 45 | 23.69 | Caffeoylferuloyl quinic acid isomer | C26H25O12 | 529.1353 | 0.20 | 529, 367, 179, 161 | MS |
| 46 | 23.76 | 2-Methylbutanoyl/isovaleryl dicaffeoylhexaric acid | C29H29O15 | 617.1505 | −1.1 | 617, 455, 293, 191, 179 | MS |
| 47 | 23.81 | Quercetin | C15H9O7 | 301.0353 | −0.22 | 301, 179, 151 | MS, St |
| 48 | 23.85 | Luteolin | C15H9O6 | 285.0404 | −0.20 | 285 | MS, St, NMR |
| 49 | 23.89 | 4,5-di-O-p-Coumaroylquinic acid | C25H23O10 | 483.1298 | 0.19 | 483, 337, 191, 173, 163 | MS |
| 50 | 23.91 | 3,4,5-Tricaffeoylquinic acid | C34H29O15 | 677.1516 | 1.34 | 677, 515, 353, 335, 191, 179, 173, 161 | MS |
| 51 | 23.95 | Patuletin (6-methoxyquercetin) | C16H11O8 | 331.0459 | −0.22 | 331, 316, 287, 271 | MS, NMR |
| 52 | 24.18 | Nepetin (6-methoxyluteolin) | C16H11O7 | 315.0511 | 0.29 | 315, 301, 300 | MS, NMR |
| 53 | 24.32 | Isobutanoyl tricaffeoylhexaric acid | C37H33O18 | 765.1679 | 1.59 | 765, 603, 441, 279, 261, 191 | MS |
| 54 | 24.85 | 2-Methylbutanoyl/isovaleryl tricaffeoylhexaric acid isomer | C38H35O18 | 779.1830 | 0.80 | 779, 617, 455, 293, 275, 191 | MS |
| 55 | 25.12 | Chrysoeriol | C16H11O6 | 299.0563 | 0.75 | 299, 284 | MS |
| 56 | 25.31 | Jaceosidin | C17H13O7 | 329.0667 | −0.08 | 329, 314, 299 | MS |
| 57 | 25.49 | Quercetagetin trimethyl ether | C18H15O8 | 359.0773 | 0.25 | 359, 344, 329, 301 | MS |
| 58 | 25.99 | Apigenin | C15H9O5 | 269.0457 | 0.63 | 269 | MS, St, NMR |
| № | δH | δC * | № | δH | δC * |
|---|---|---|---|---|---|
| 2 | 164.5 | 3′ | 145.8 | ||
| 3 | 6.59 (s) | 101.9 | 4′ | 150.3 | |
| 4 | 182.5 | 5′ | 6.94 (d, 8.4) | 115.6 | |
| 5 | 168.5 | 6′ | 7.45 (dd, 2.2, 8.4) | 119.0 | |
| 6 | 132.5 | OMe | 3.89 (s) | 59.6 | |
| 7 | 160.5 | 2” | 172.4 | ||
| 8 | 97.3 | 3” | 4.07 (dd, 5.5, 9.3) | 69.3 | |
| 9 | 152.5 | 4″ | 2.18 (m)/2.48 (m) | 28.8 | |
| 10 | 103.1 | 5″ | 2.09 (m)/1.95 (m) | 23.0 | |
| 1′ | 122.1 | 6″ | 3.57 (m)/3.36 (m) | 53.4 | |
| 2′ | 7.62 (d, 2.2) | 113.5 | 7″ | 4.58 (d, 13.3)/4.72 (d, 13.3) | 47.4 |
| TPC a | TFC b | 5-CQA c | 3,4-DCQA c | 3,5-DCQA c | 1,5-DCQA c | 4,5-DCQA c | DPPH d | ABTS d | FRAP e | |
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 215.57 | 87.44 | 103.39 | 5.38± | 30.62 | 18.58 | 23.33 | 0.741 | 0.711 | 5.77 |
| SD | 2.52 | 0.52 | 1.30 | 0.26 | 0.79 | 0.69 | 0.50 | 0.006 | 0.007 | 0.08 |
| Time (min) | Solvent A * (%) | Solvent B * (%) |
|---|---|---|
| 0→1 | 95 | 5 |
| 1→20 | 95→82 | 5→18 |
| 20→24 | 82→60 | 18→40 |
| 24→27 | 60→30 | 40→70 |
| 27→29 | 30→5 | 70→95 |
| 29→31 | 5 | 95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanova, V.; Nedialkov, P.; Dimitrova, P.; Paunova-Krasteva, T.; Trendafilova, A. Inula salicina L.: Insights into Its Polyphenolic Constituents and Biological Activity. Pharmaceuticals 2024, 17, 844. https://doi.org/10.3390/ph17070844
Ivanova V, Nedialkov P, Dimitrova P, Paunova-Krasteva T, Trendafilova A. Inula salicina L.: Insights into Its Polyphenolic Constituents and Biological Activity. Pharmaceuticals. 2024; 17(7):844. https://doi.org/10.3390/ph17070844
Chicago/Turabian StyleIvanova, Viktoria, Paraskev Nedialkov, Petya Dimitrova, Tsvetelina Paunova-Krasteva, and Antoaneta Trendafilova. 2024. "Inula salicina L.: Insights into Its Polyphenolic Constituents and Biological Activity" Pharmaceuticals 17, no. 7: 844. https://doi.org/10.3390/ph17070844
APA StyleIvanova, V., Nedialkov, P., Dimitrova, P., Paunova-Krasteva, T., & Trendafilova, A. (2024). Inula salicina L.: Insights into Its Polyphenolic Constituents and Biological Activity. Pharmaceuticals, 17(7), 844. https://doi.org/10.3390/ph17070844









