Neuroprotective Effects of Glycyrrhiza glabra Total Extract and Isolated Compounds
Abstract
:1. Introduction
2. Results
2.1. Chemical Characterization of the Isolated Compounds
2.2. Biological Study
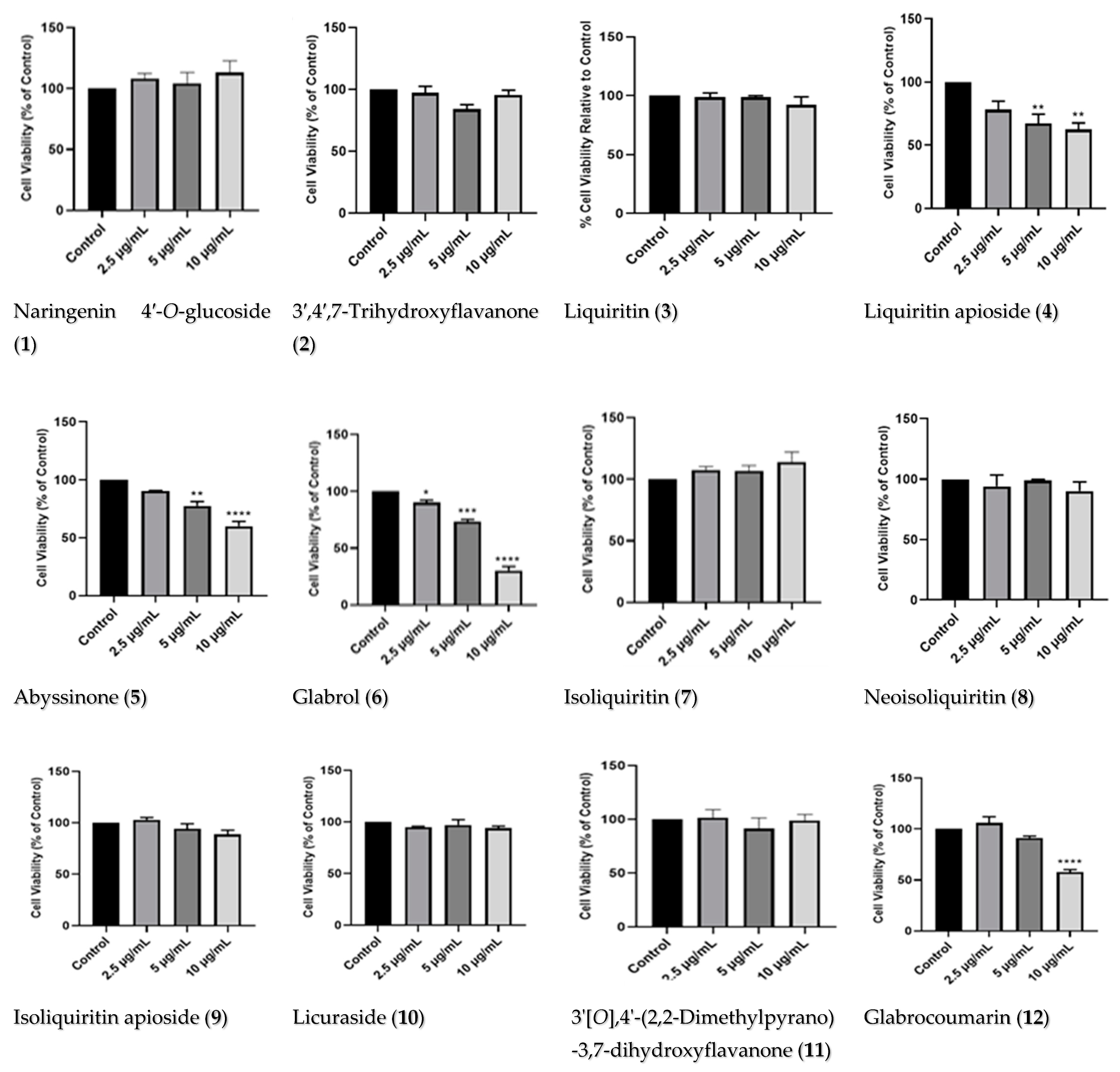
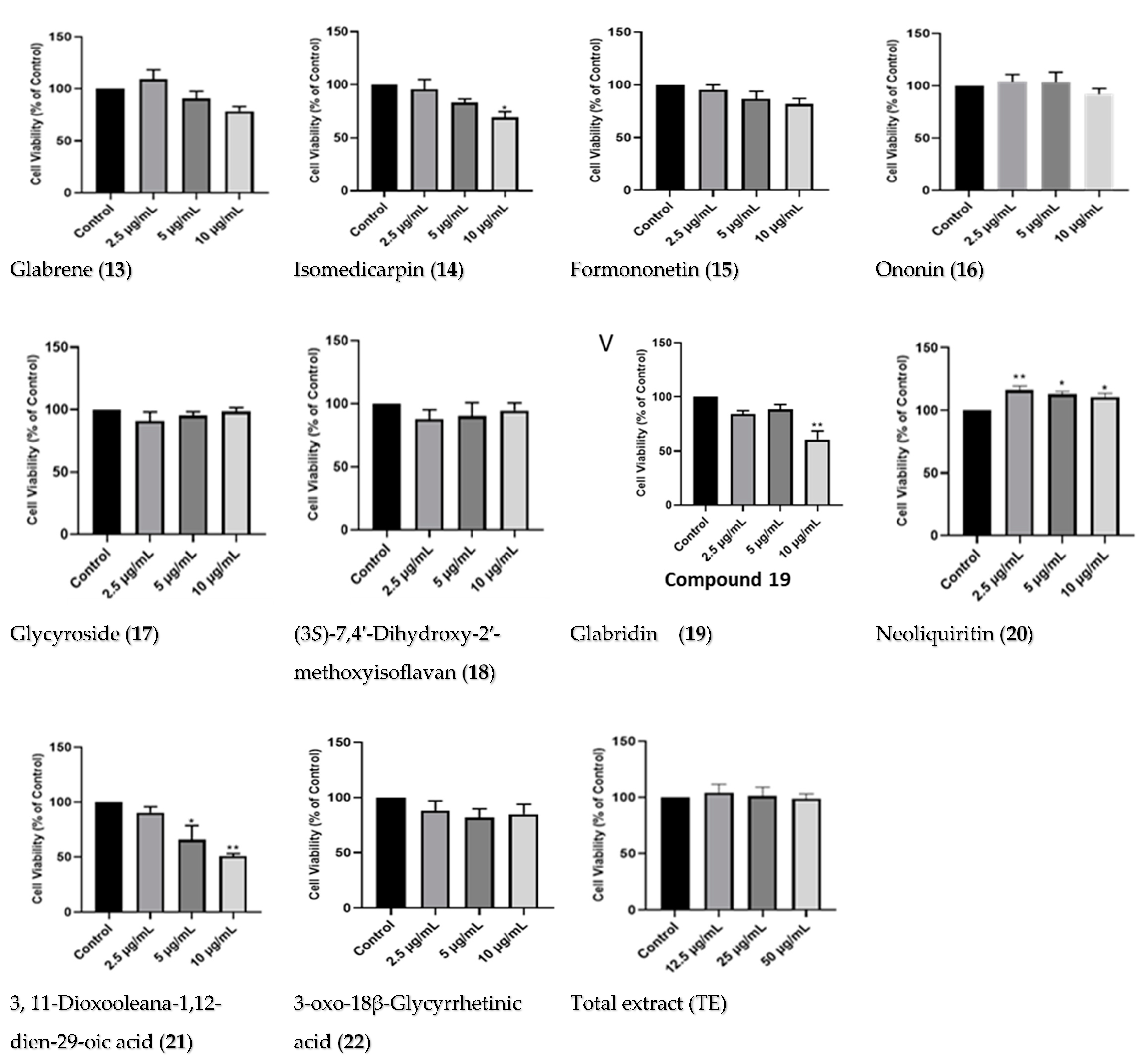
2.2.1. Dose–Response of Licorice TE and Compounds
2.2.2. Effects of Licorice TE and Compounds on MPP+-Induced Toxicity on SH-SY5Y Cells
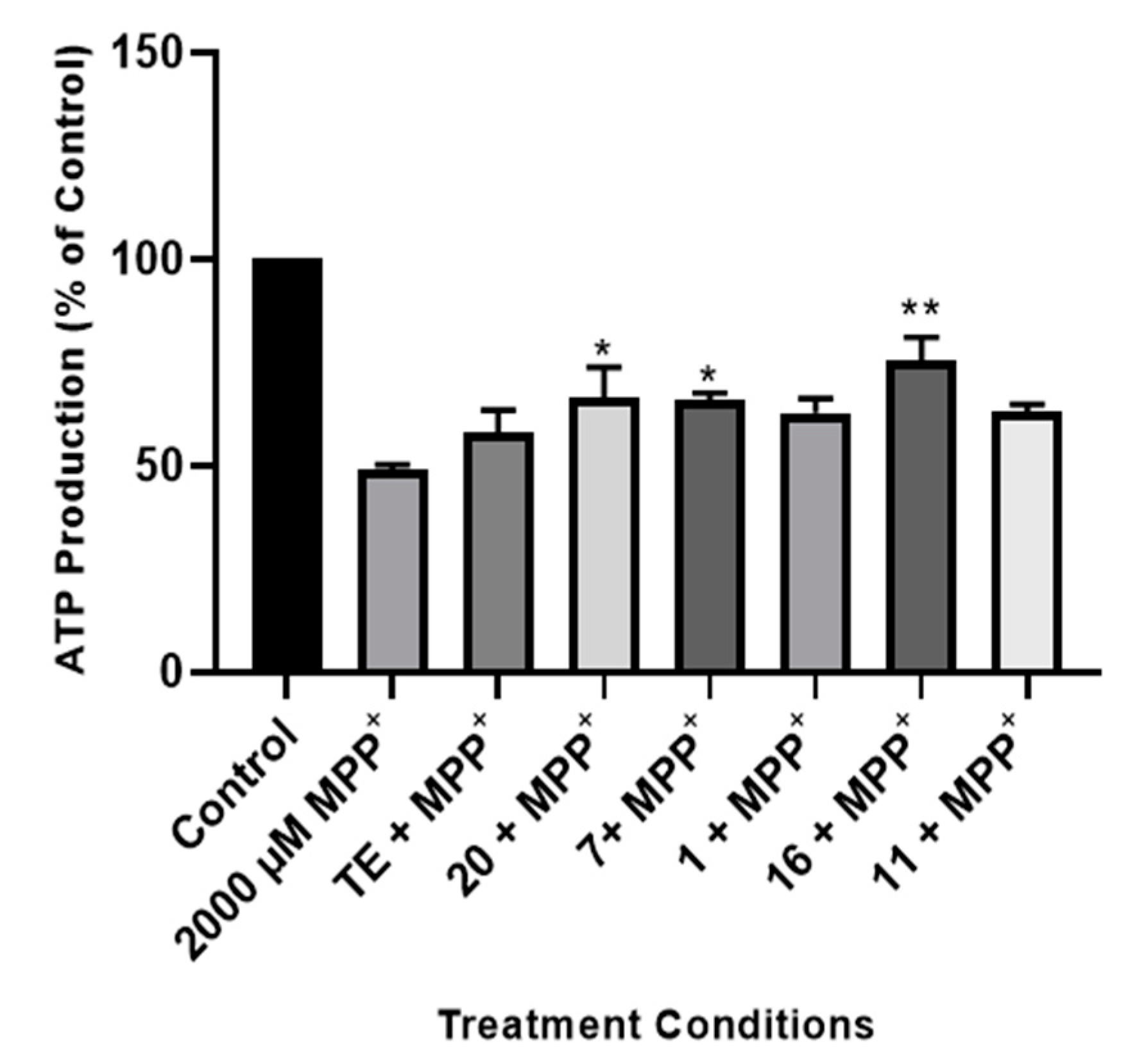
2.2.3. Effect of TE and Compounds on ATP Production in the Cells
2.2.4. Effect of TE and Compounds on Caspase 3/7 Activities in the Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals, Materials, and Reagents
4.2. Method
Extraction and Purification of Compounds
4.3. Compound 11
4.4. Cell Culture and Treatments
4.5. Treatments
4.6. Cell Viability Assays
4.7. Adenosine Triphosphate Assay
4.8. Caspase 3/7 Apoptosis Assay
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lamptey, R.N.L.; Chaulagain, B.; Trivedi, R.; Gothwal, A.; Layek, B.; Singh, J. A Review of the Common Neurodegenerative Disorders: Current Therapeutic Approaches and the Potential Role of Nanotherapeutics. Int. J. Mol. Sci. 2022, 23, 1851. [Google Scholar] [CrossRef] [PubMed]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet Neurol. 2015, 29, 896–912. [Google Scholar] [CrossRef] [PubMed]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef]
- Li, J.-L.; Lin, T.-Y.; Chen, P.-L.; Guo, T.-N.; Huang, S.-Y.; Chen, C.-H.; Lin, C.-H.; Chan, C.-C. Mitochondrial Function and Parkinson’s Disease: From the Perspective of the Electron Transport Chain. Front. Mol. Neurosci. 2021, 14, 797833. [Google Scholar] [CrossRef]
- Keane, P.; Kurzawa, M.; Blain, P.; Morris, C. Mitochondrial dysfunction in Parkinson’s disease. Park. Dis. 2011, 2011, 716871. [Google Scholar] [CrossRef]
- Nonnekes, J.; Post, B.; Tetrud, J.W.; Langston, J.W.; Bloem, B.R. MPTP-induced parkinsonism: An historical case series. Lancet Neurol. 2018, 17, 300–301. [Google Scholar]
- Reeve, A.; Simcox, E.; Turnbull, D. Ageing and Parkinson’s disease: Why is advancing age the biggest risk factor. Ageing Res. Rev. 2014, 14, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Thanvi, B.; Lo, N.; Robinson, T. Levodopa-induced dyskinesia in Parkinson’s disease: Clinical features, pathogenesis, prevention and treatment. Postgrad. Med. J. 2007, 83, 384–388. [Google Scholar] [CrossRef]
- Brahmachari, G. Discovery and Development of Neuroprotective Agents from Natural Products; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Abdolmaleki, A.; Akram, M.; Saeed, M.M.; Asadi, A.; Kajkolah, M. Herbal medicine as neuroprotective potential agent in human and animal models: A historical overview. J. Pharm. Care 2020, 8, 75–82. [Google Scholar] [CrossRef]
- Bosch-Morell, F.; Villagrasa, V.; Ortega, T.; Acero, N.; Muñoz-Mingarro, D.; González-Rosende, M.E.; Castillo, E.; Sanahuja, M.A.; Soriano, P.; Martínez-Solís, I.P. Medicinal plants and natural products as neuroprotective agents in age-related macular degeneration. Neural Regen. Res. 2020, 15, 2207–2216. [Google Scholar]
- Öztürk, M.; Altay, V.; Hakeem, K.; Akçiçek, E. Liquorice: From Botany to Phytochemistry; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Hayashi, H.; Hosono, N.; Kondo, M.; Hiraoka, N.; Ikeshiro, Y.; Shibano, M.; Kusano, G.; Yamamoto, H.; Tanaka, T.; Inoue, K. Phylogenetic relationship of six Glycyrrhiza species based on rbcL sequences and chemical constituents. Biol. Pharm. Bull. 2000, 23, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Miwa, E.; Inoue, K. Phylogenetic relationship of Glycyrrhiza lepidota, American licorice, in genus Glycyrrhiza based on rbcL sequences and chemical constituents. Biol. Pharm. Bull. 2005, 28, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Avula, B.; Bae, J.-Y.; Chittiboyina, A.G.; Wang, Y.-H.; Wang, M.; Zhao, J.; Ali, Z.; Brinckmann, J.A.; Li, J.; Wu, C.; et al. Chemometric analysis and chemical characterization for the botanical identification of Glycyrrhiza species (G. glabra, G. uralensis, G. inflata, G. echinata and G. lepidota) using liquid chromatography-quadrupole time of flight mass spectrometry (LC-QTof). J. Food Compos. Anal. 2022, 112, 104679. [Google Scholar] [CrossRef]
- Akhtar, N.; Ihsan-ul-Haq; Mirza, B. Phytochemical analysis and comprehensive evaluation of antimicrobial and antioxidant properties of 61 medicinal plant species. Arab. J. Chem. 2018, 11, 1223–1235. [Google Scholar] [CrossRef]
- Sharma, V.; Katiyar, A.; Agrawal, R.C. Glycyrrhiza Glabra: Chemistry and Pharmacological Activity. In Sweeteners; Mérillon, J., Ramawat, K.G., Eds.; Springer: Cham, The Netherlands, 2018; pp. 87–100. [Google Scholar]
- Jiang, M.; Zhao, S.; Yang, S.; Lin, X.; He, X.; Wei, X.; Song, Q.; Li, R.; Fu, C.; Zhang, J.; et al. An “essential herbal medicine” Licorice: A review of phytochemicals and its effects in combination preparations. J. Ethnopharmacol. 2020, 249, 112439. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.S.; Ebrahimi, M.; Samsampour, D.; Abadía, J.; Khanahmadi, M.; Amirian, R.; Ghafoori, I.N.; Ghaderi-Zefrehei, M.; Gogorcena, Y. Association analysis and molecular tagging of phytochemicals in the endangered medicinal plant licorice (Glycyrrhiza glabra L.). Phytochemistry 2021, 183, 112629. [Google Scholar] [CrossRef]
- Chang, K.-H.; Chen, I.-C.; Lin, H.-Y.; Chen, H.-C.; Lin, C.-H.; Lin, T.-H.; Weng, Y.-T.; Chao, C.-Y.; Wu, Y.-R.; Lin, J.-Y.; et al. The aqueous extract of Glycyrrhiza inflata can upregulate unfolded protein response-mediated chaperones to reduce tau misfolding in cell models of Alzheimer’s disease. Drug Des. Dev. Ther. 2016, 10, 885–896. [Google Scholar]
- Chen, C.-M.; Weng, Y.-T.; Chen, W.-L.; Lin, T.-H.; Chao, C.-Y.; Lin, C.-H.; Chen, I.-C.; Lee, L.-C.; Lin, H.-Y.; Wu, Y.-R.; et al. Aqueous extract of Glycyrrhiza inflata inhibits aggregation by upregulating PPARGC1A and NFE2L2–ARE pathways in cell models of spinocerebellar ataxia 3. Free Radic. Biol. Med. 2014, 71, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.-H.; Chen, X.; Hua, H.-P.; Liang, L.; Liu, L.-J. The oral pretreatment of glycyrrhizin prevents surgery-induced cognitive impairment in aged mice by reducing neuroinflammation and Alzheimer’s-related pathology via HMGB1 inhibition. J. Mol. Neurosci. 2017, 63, 385–395. [Google Scholar] [CrossRef]
- Ravanfar, P.; Namazi, G.; Atigh, M.; Zafarmand, S.; Hamedi, A.; Salehi, A.; Izadi, S.; Borhani-Haghighi, A. Efficacy of whole extract of licorice in neurological improvement of patients after acute ischemic stroke. J. Herb. Med. 2016, 6, 12–17. [Google Scholar] [CrossRef]
- Hasan, M.K.; Ara, I.; Mondal, M.S.A.; Kabir, Y. Phytochemistry, pharmacological activity, and potential health benefits of Glycyrrhiza glabra. Heliyon 2021, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Zhang, M.; Xue, H.; Yu, R.; Bao, Y.-O.; Kuang, Y.; Chai, Y.; Ma, W.; Wang, J.; Shi, X.; et al. Schaftoside inhibits 3CLpro and PLpro of SARS-CoV-2 virus and regulates immune response and inflammation of host cells for the treatment of COVID-19. Acta Pharm. Sin. B 2022, 12, 4154–4164. [Google Scholar] [CrossRef] [PubMed]
- Xiang, C.; Qiao, X.; Ye, M.; Guo, D. Classification and distribution analysis of components in Glycyrrhiza using licorice compounds database. Acta Pharm. Sin. 2012, 47, 1023–1030. [Google Scholar]
- Omoruyi, S.I.; Ibrakaw, A.S.; Ekpo, O.E.; Boatwright, J.S.; Cupido, C.N.; Hussein, A.A. Neuroprotective activities of crossyne flava bulbs and amaryllidaceae alkaloids: Implications for parkinson’s disease. Molecules 2021, 26, 3990. [Google Scholar] [CrossRef] [PubMed]
- Egunlusi, A.O.; Malan, S.F.; Omoruyi, S.I.; Ekpo, O.E.; Palchykov, V.A.; Joubert, J. Open and rearranged norbornane derived polycyclic cage molecules as potential neuroprotective agents through attenuation of MPP+-and calcium overload-induced excitotoxicity in neuroblastoma SH-SY5Y cells. Eur. J. Med. Chem. 2020, 204, 112617. [Google Scholar] [CrossRef] [PubMed]
- Oyama, K.-i.; Kondo, T. Total synthesis of apigenin 7, 4’-di-O-β-glucopyranoside, a component of blue flower pigment of Salvia patens, and seven chiral analogues. Tetrahedron 2004, 60, 2025–2034. [Google Scholar] [CrossRef]
- Chen, K.; Hu, Z.-m.; Song, W.; Wang, Z.-l.; He, J.-b.; Shi, X.-m.; Cui, Q.-h.; Qiao, X.; Ye, M. Diversity of O-glycosyltransferases contributes to the biosynthesis of flavonoid and triterpenoid glycosides in Glycyrrhiza uralensis. Am. Chem. Soc. Synth. Biol. 2019, 8, 1858–1866. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Ding, H.-Y.; Wang, T.-Y.; Cai, C.-Z.; Chang, T.-S. Application of Biotransformation-Guided Purification in Chinese Medicine: An Example to Produce Butin from Licorice. Catalysts 2022, 12, 1–10. [Google Scholar] [CrossRef]
- Tian, G.; Zhang, U.; Zhang, T.; Yang, F.; Ito, Y. Separation of flavonoids from the seeds of Vernonia anthelmintica Willd by high-speed counter-current chromatography. J. Chromatogr. A 2004, 1049, 219–222. [Google Scholar] [CrossRef]
- Ji, S.; Li, Z.; Song, W.; Wang, Y.; Liang, W.; Li, K.; Tang, S.; Wang, Q.; Qiao, X.; Zhou, D.; et al. Bioactive Constituents of Glycyrrhiza uralensis (Licorice): Discovery of the Effective Components of a Traditional Herbal Medicine. J. Nat. Prod. 2016, 79, 281–292. [Google Scholar] [CrossRef]
- Pastorino, G.; Cornara, L.; Soares, S.; Rodrigues, F.; Oliveira, M.B.P. Liquorice (Glycyrrhiza glabra): A phytochemical and pharmacological review. Phytother. Res. 2018, 32, 2323–2339. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Wu, Q.; Yoo, K.-H.; Yong, H.-I.; Cho, S.-M.; Chung, I.-S.; Baek, N.-I. Cytotoxic effect of flavonoids from the roots of Glycyrrhiza uralensis on human cancer cell lines. J. Appl. Biol. Chem. 2011, 54, 67–70. [Google Scholar] [CrossRef]
- Asada, Y.; Li, W.; Yoshikawa, T. Biosynthesis of the dimethylallyl moiety of glabrol in Glycyrrhiza glabra hairy root cultures via a non-mevalonate pathway. Phytochemistry 2000, 55, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Fukai, T.; Akiyama, T. Chemistry of phenolic compounds of licorice (Glycyrrhiza species) and their estrogenic and cytotoxic activities. Pure Appl. Chem. 2002, 74, 1199–1206. [Google Scholar] [CrossRef]
- Lee, J.E.; Lee, J.Y.; Kim, J.; Lee, K.; Choi, S.U.; Ryu, S.Y. Two minor chalcone acetylglycosides from the roots extract of Glycyrrhiza uralensis. Arch. Pharmacal Res. 2015, 38, 1299–1303. [Google Scholar] [CrossRef]
- Wang, D.; Liang, J.; Zhang, J.; Wang, Y.; Chai, X. Natural chalcones in Chinese materia medica: Licorice. Evid.-Based Complement. Altern. Med. 2020, 2020, 3821248. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Kaur, S.; Kumar, N.; Singh, B.; Kumar, S. Evaluation of antigenotoxic activity of isoliquiritin apioside from Glycyrrhiza glabra L. Toxicol. Vitr. 2009, 23, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Li, H.; Wang, X.; Lee, F.S.C.; Cui, S. Isolation and Identification of Flavonoids in Licorice and a Study of Their Inhibitory Effects on Tyrosinase. J. Agric. Food Chem. 2005, 53, 7408–7414. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Simmler, C.; Chen, L.; Nikolic, D.; Chen, S.-N.; Pauli, G.F.; van Breemen, R.B. Cytochrome P450 inhibition by three licorice species and fourteen licorice constituents. Eur. J. Pharm. Sci. 2017, 109, 182–190. [Google Scholar] [CrossRef]
- Montoro, P.; Maldini, M.; Russo, M.; Postorino, S.; Piacente, S.; Pizza, C. Metabolic profiling of roots of liquorice (Glycyrrhiza glabra) from different geographical areas by ESI/MS/MS and determination of major metabolites by LC-ESI/MS and LC-ESI/MS/MS. J. Pharm. Biomed. Anal. 2011, 54, 535–544. [Google Scholar] [CrossRef]
- Kinoshit, T.; Tamur, Y.; Mizutani, K. The Isolation and Structure Elucidation of Minor Isoflavonoids from Licorice of Glycyrrhiza glabra Origin. Chem. Pharm. Bull. 2005, 53, 847–849. [Google Scholar] [CrossRef]
- Fukai, T.; Sheng, C.-B.; Horikoshi, T.; Nomura, T. Isoprenylated flavonoids from underground parts of Glycyrrhiza glabra. Phytochemistry 1996, 43, 1119–1124. [Google Scholar] [CrossRef]
- Adesanya, S.; O’Neill, M.J.; Roberts, M.F. Structure-related fungitoxicity of isoflavonoids. Physiol. Mol. Plant Pathol. 1986, 29, 95–103. [Google Scholar] [CrossRef]
- Piccinelli, A.L.; Fernandez, M.C.; Cuesta-Rubio, O.; Hernández, I.M.; Simone, F.D.; Rastrelli, L. Isoflavonoids Isolated from Cuban Propolis. J. Agric. Food Chem. 2005, 53, 9010–9016. [Google Scholar] [CrossRef]
- Liaoa, W.C.; Linb, Y.-H.; Changc, T.-M.; Huang, W.-Y. Identification of two licorice species, Glycyrrhiza uralensis and Glycyrrhiza glabra, based on separation and identification of their bioactive components. Food Chem. 2012, 132, 2188–2193. [Google Scholar] [CrossRef]
- Chintharlapalli, S.; Papineni, S.; Jutooru, I.; McAlees, A.; Safe, S. Structure-dependent activity of glycyrrhetinic acid derivatives as peroxisome proliferator–activated receptor γ agonists in colon cancer cells. Mol. Cancer Ther. 2007, 6, 1588–1598. [Google Scholar] [CrossRef] [PubMed]
- Baltina, L.A.; Budaev, A.S.; Mikhailova, L.R.; Baltina, J.L.A.; Spirikhin, L.V.; Makara, N.S.; Zarudii, F.S. New stereoisomeric glycyrrhetic acid derivatives. Chem. Nat. Compd. 2014, 50, 1042–1046. [Google Scholar] [CrossRef]
- Kuroda, M.; Mimakia, Y.; Honda, S.; Tanaka, H.; Yokota, S.; Mae, T. Phenolics from Glycyrrhiza glabra roots and their PPAR-c ligand-binding activity. Bioorganic Med. Chem. 2010, 18, 962–970. [Google Scholar] [CrossRef]
- Hoglinger, G.U.; Carrard, G.; Michel, P.P.; Medja, F.; Lombès, A.; Ruberg, M.; Friguet, B.; Hirsch, E.C. Dysfunction of mitochondrial complex I and the proteasome:interactions between two biochemical deficits in a cellular modelof Parkinson’s disease. J. Neurochem. 2003, 86, 1297–1307. [Google Scholar] [CrossRef]
- Omoruyi, S.I.; Akinfenwa, A.O.; Ekpo, O.E.; Hussein, A.A. Aspalathin and linearthin from Aspalathus linearis (Rooibos) protect SH-SY5Y cells from MPP+-induced neuronal toxicity. South Afr. J. Bot. 2023, 157, 53–63. [Google Scholar] [CrossRef]
- Kouli, A.; Torsney, K.M.; Kuan, W.-L. Parkinson’s disease: Etiology, neuropathology, and pathogenesis. Exon Publ. 2018, 3–26. [Google Scholar] [CrossRef]
- Pang, S.Y.-Y.; Ho, P.W.-L.; Liu, H.-F.; Leung, C.-T.; Li, L.; Chang, E.E.S.; Ramsden, D.B.; Ho, S.-L. The interplay of aging, genetics and environmental factors in the pathogenesis of Parkinson’s disease. Transl. Neurodegener. 2019, 8, 23. [Google Scholar] [CrossRef]
- Massano, J.; Bhatia, K. Clinical approach to Parkinson’s disease: Features, diagnosis, and principles of management. Cold Spring Harb. Perspect. Med. 2012, 2, a008870. [Google Scholar] [CrossRef] [PubMed]
- HOhno; Araho, D.; Uesawa, Y.; Kagaya, H.; Ishihara, M.; Sakagami, H.; Yamamoto, M. Evaluation of Cytotoxiciy and Tumor-specificity of Licorice Flavonoids Based on Chemical Structure. Anticancer Res. 2013, 33, 3061–3068. [Google Scholar]
- Goel, B.; Sharma, A.; Tripathi, N.; Bhardwaj, N.; Sahu, B.; Kaur, G.; Singh, B.; Jain, S.K. In-vitro antitumor activity of compounds from Glycyrrhiza glabra against C6 glioma cancer cells: Identification of natural lead for further evaluation. Nat. Prod. Res. 2021, 35, 5489–5492. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Kim, Y.J.; Lee, M.S.; Han, E.S.; Lee, S.J. 18β-Glycyrrhetinic acid induces apoptotic cell death in SiHa cells and exhibits a synergistic effect against antibiotic anti-cancer drug toxicity. Life Sci. 2008, 83, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, A.; Kalinowska-Lis, U. 18β-Glycyrrhetinic acid: Its core biological properties and dermatological applications. Int. J. Cosmet. Sci. 2019, 41, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Tay, K.-C.; Tan, L.T.-H.; Chan, C.K.; Hong, S.L.; Chan, K.-G.; Yap, W.H.; Pusparajah, P.; Lee, L.-H.; Goh, B.-H. Formononetin: A Review of Its Anticancer Potentials and Mechanisms. Front. Pharmacol. 2019, 10, 820. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.-S.; Kim, T.-H.; Park, J.-H.; Lim, H.; Cho, I.-A.; You, J.-S.; Lee, G.-J.; Seo, Y.-S.; Kim, D.K.; Kim, C.S.; et al. Formononetin induces apoptotic cell death through the suppression of mitogen activated protein kinase and nuclear factor κB phosphorylation in FaDu human head and neck squamous cell carcinoma cells. Oncol. Rep. 2020, 43, 700–710. [Google Scholar] [CrossRef]
- Jiang, D.; Rasul, A.; Batool, R.; Sarfraz, I.; Hussain, G.; Tahir, M.M.; Qin, Z.S.T.; Ali, M.; Li, J.; Li, X. Potential Anticancer Properties and Mechanisms of Action of Formononetin. BioMed Res. Int. 2019, 2019, 854315. [Google Scholar] [CrossRef]
- Hsu, Y.-C.; Hsieh, W.-C.; Chen, S.-H.; Li, Y.-Z.; Liao, H.-F.; Lin, M.-Y.; Sheu, S.-M. 18β-glycyrrhetinic Acid Modulated Autophagy is Cytotoxic to Breast Cancer Cells. Int. J. Med. Sci. 2023, 20, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Ren, D.; Fan, P.; Shen, T.; Lou, H. Protective effects of naringenin-7-O-glucoside on doxorubicin-induced apoptosis in H9C2 cells. Eur. J. Pharmacol. 2008, 581, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Sugumar, M.; Sevanan, M.; Sekar, S. Neuroprotective effect of naringenin against MPTP-induced oxidative stress. Int. J. Neurosci. 2019, 129, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.M.; Elnagar, M.R.; Abdelrazik, E.; Mahdi, M.R.; Hamza, E.; Elattar, E.M.; ElNashar, E.M.; Alghamdi, M.A.; Al-Qahtani, Z.; Al-Khater, K.M.; et al. Neuroprotective effect of naringin against cerebellar changes in Alzheimer’s disease through modulation of autophagy, oxidative stress and tau expression: An experimental study. Front. Neuroanat. 2022, 16, 1012422. [Google Scholar] [CrossRef] [PubMed]
- Mansour, L.; Elshopakey, G.; Abdelhamid, F.; Albukhari, T.; Almehmadi, S.; Refaat, B.; El-Boshy, M.; Risha, E. Hepatoprotective and Neuroprotective Effects of Naringenin against Lead-Induced Oxidative Stress, Inflammation, and Apoptosis in Rats. Biomedicines 2023, 11, 1080. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Song, W.; Tong, Y.; Zhang, X.; Zhao, J.; Gao, X.; Yong, J.; Wang, H. Isoliquiritin ameliorates depression by suppressing NLRP3-mediated pyroptosis via miRNA-27a/SYK/NF-κB axis. J. Neuroinflammation 2021, 18, 1. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, R.; Seong, S.H.; Park, S.E.; Paudel, P.; Jung, H.A.; Choi, J.S. Isoliquiritigenin, a potent human monoamine oxidase inhibitor, modulates dopamine D1, D3, and vasopressin V1A receptors. Sci. Rep. 2021, 11, 23528. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Chen, Y.; Luo, Z.-H.; Shen, X.-L.; Hu, Y.-J. 7-methoxyflavanone alleviates neuroinflammation in lipopolysaccharide-stimulated microglial cells by inhibiting TLR4/MyD88/MAPK signalling and activating the Nrf2/NQO-1 pathway. J. Pharm. Pharmacol. 2020, 72, 385–395. [Google Scholar] [CrossRef]
- Pöltl, D.; Schildknecht, S.; Karreman, C.; Leist, M. Uncoupling of ATP-depletion and cell death in human dopaminergic neurons. NeuroToxicology 2012, 33, 769–779. [Google Scholar] [CrossRef]
- Babu, V.; Khurana, N. A review on mitochondrial dysfunction and oxidative stress due to complex-i in Parkinson disease. Res. J. Pharmacol. Pharmacodyn. 2021, 13, 167–170. [Google Scholar] [CrossRef]
- Dorszewska, J.D.P.; Kowalska, M.; Prendecki, M.; Piekut, T.; Kozłowska, J.; Kozubski, W. Oxidative stress factors in Parkinson’s disease. Neural Regen. Res. 2021, 16, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Błaszczyk, J.W. Energy metabolism decline in the aging brain—Pathogenesis of neurodegenerative disorders. Metabolites 2020, 10, 450. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Delgado, A.; Ortiz, G.G.; Delgado-Lara, D.L.; González-Usigli, H.A.; González-Ortiz, L.J.; Cid-Hernández, M.; Cruz-Serrano, J.A.; Pacheco-Moisés, F.P. Effect of Melatonin Administration on Mitochondrial Activity and Oxidative Stress Markers in Patients with Parkinson’s Disease. Oxidative Med. Cell. Longev. 2021, 2021, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-z.; Li, X.; Gong, W.-x.; Tian, J.-s.; Gao, X.-x.; Gao, L.; Zhang, X.; Du, G.-h.; Qin, X.-m. Protective effect of isoliquiritin against corticosterone-induced neurotoxicity in PC12 cells. Food Funct. 2017, 8, 1235–1244. [Google Scholar] [CrossRef]
- Abramov, A.; Angelova, P.R. Mitochondrial dysfunction and energy deprivation in the mechanism of neurodegeneration. Turk. J. Biochem. 2019, 44, 723–729. [Google Scholar] [CrossRef]




| Position | Compound 11 | 3,4′,7-Trihydroxy-3′-prenylflavanone [51] | ||
|---|---|---|---|---|
| δC | δH, multi, J (Hz) | δC | δH, multi, J (Hz) | |
| 2 | 83.7 | 4.92 (d, 11.8) | 84.6 | 5.03 (d, 11.9) |
| 3 | 72.9 | 4.50 (d, 11.8) | 73.4 | 4.59 (d, 11.9) |
| 4 | 192.7 | - | 192.7 | - |
| 5 | 128.6 | 7.72 (d, 8.6) | 129.3 | 7.74 (d, 8.6) |
| 6 | 111.5 | 6.51 (dd, 8.6, 2.0) | 111.2 | 6.64 (dd, 8.6, 2.2) |
| 7 | 162.7 | - | 165.4 | - |
| 8 | 102.5 | 6.30 (d, 2.0) | 103.1 | 6.41 (d, 2.2) |
| 9 | 163.2 | - | 164.1 | - |
| 10 | 111.9 | - | 112.5 | - |
| 1′ | 129.6 | - | 128.9 | - |
| 2′ | 126.2 | 7.23 (br s) | 130.0 | 7.35 (d, 2.0) |
| 3′ | 120.7 s | - | 128.1 | - |
| 4′ | 152.6 | - | 155.8 | - |
| 5′ | 115.5 | 6.76 (d, 7.9) | 115.0 | 6.90 (d, 8.2) |
| 6′ | 129.1 d | 7.25 (br d, 7.9) | 127.1 | 7.27 (dd, 8.2, 2.2) |
| 1″ | 121.6 | 6.43 (d, 9.7) | 28.7 | 3.38 (2H, d, 7.3) |
| 2″ | 131.3 | 5.58 (d, 9.7) | 123.1 | 5.39 (m) |
| 3″ | 76.3 | - | 132.0 | - |
| 4″ | 27.7 | 1.37 (s) | 25.4 | 1.72 (br s) |
| 5″ | 27.7 | 1.37 (s) | 17.4 | 1.74 (br s) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eltahir, A.O.E.; Omoruyi, S.I.; Augustine, T.N.; Luckay, R.C.; Hussein, A.A. Neuroprotective Effects of Glycyrrhiza glabra Total Extract and Isolated Compounds. Pharmaceuticals 2024, 17, 852. https://doi.org/10.3390/ph17070852
Eltahir AOE, Omoruyi SI, Augustine TN, Luckay RC, Hussein AA. Neuroprotective Effects of Glycyrrhiza glabra Total Extract and Isolated Compounds. Pharmaceuticals. 2024; 17(7):852. https://doi.org/10.3390/ph17070852
Chicago/Turabian StyleEltahir, Ali O. E., Sylvester I. Omoruyi, Tanya N. Augustine, Robert C. Luckay, and Ahmed A. Hussein. 2024. "Neuroprotective Effects of Glycyrrhiza glabra Total Extract and Isolated Compounds" Pharmaceuticals 17, no. 7: 852. https://doi.org/10.3390/ph17070852








