Research on the Mechanism and Material Basis of Corn (Zea mays L.) Waste Regulating Dyslipidemia
Abstract
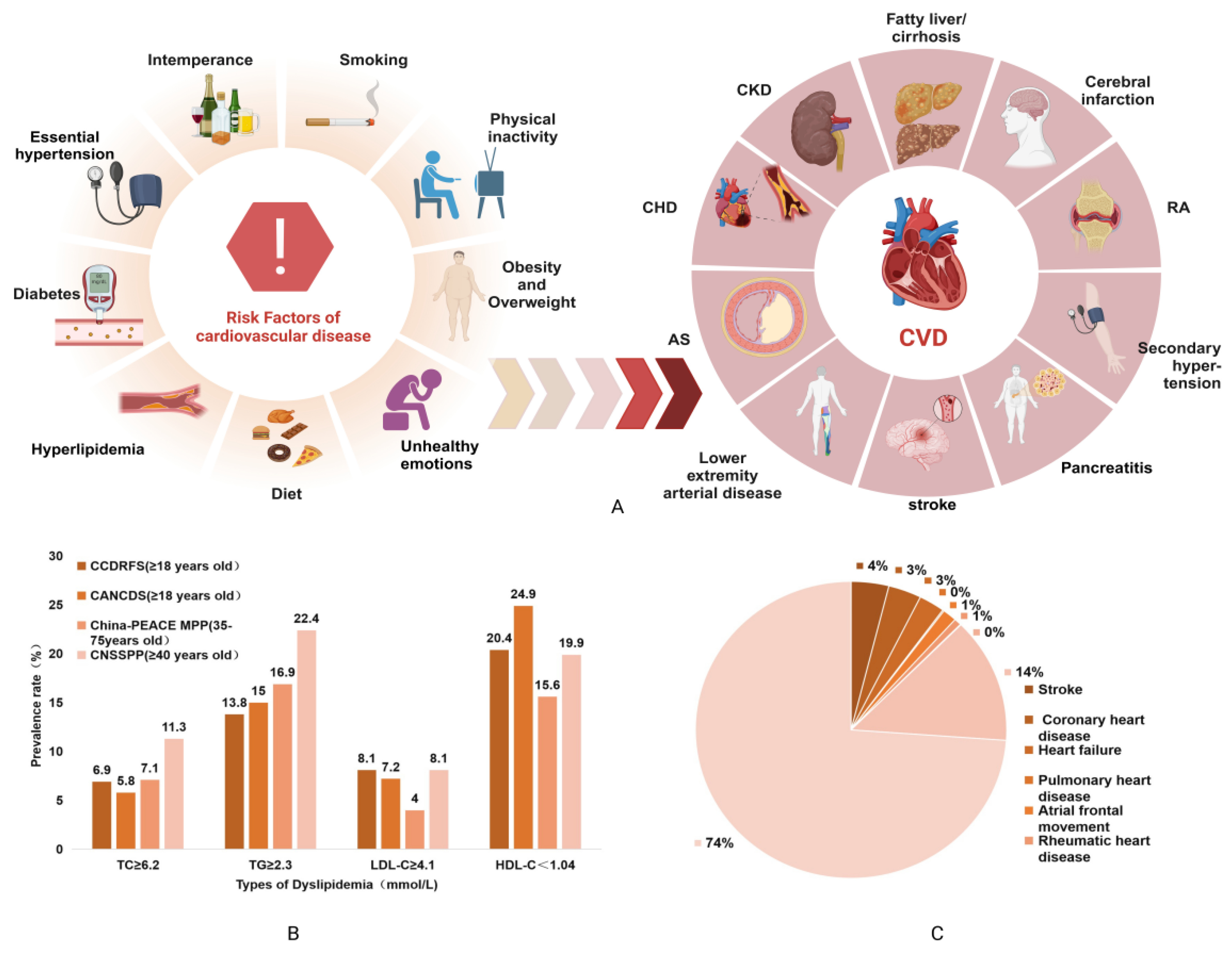
:1. Introduction
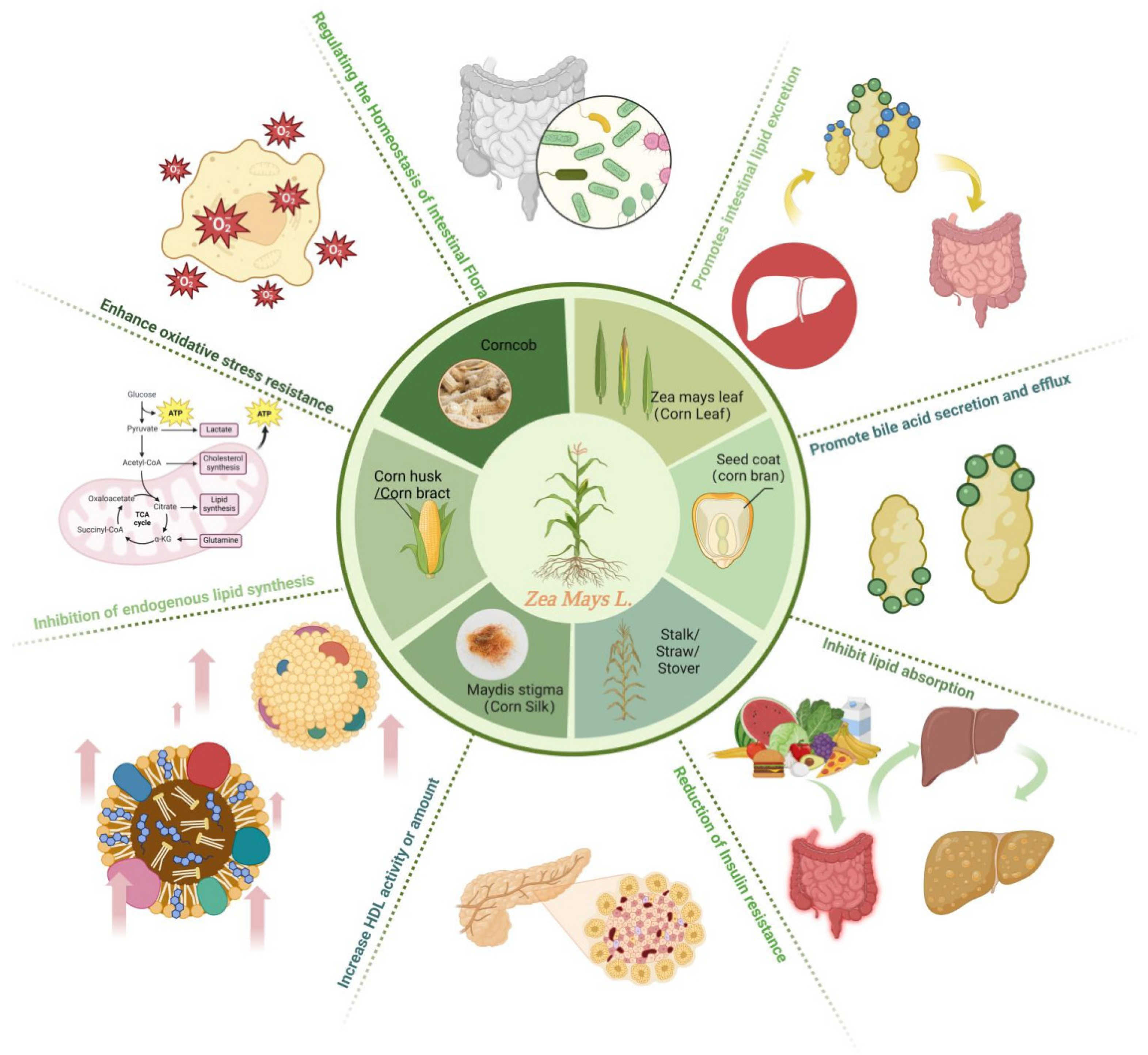
2. Corn Waste and Its Mechanism of Regulating Blood Lipids
2.1. Corn Bract
2.1.1. Chemical Composition
2.1.2. The Mechanism of Action
Increase HDL-C Activity and Quantity
Regulate Insulin Levels to Reduce Insulin Resistance and Promote Lipolysis of Adipose Tissue
Improve Antioxidant Capacity
Inhibit the Biosynthetic Pathway of TC and TG
2.2. Corn Stalks
2.2.1. Chemical Composition
2.2.2. The Mechanism of Action
2.3. Corn Silk
2.3.1. Chemical Composition
2.3.2. The Mechanism of Action
Promote the Breakdown of Cholesterol and Fatty Acids in the Body
Improve Oxidative Stress Capacity and Enhance Reverse Cholesterol Transport
Inhibit Cholesterol Synthesis Pathway
Promote the Secretion and Excretion of Bile Acids
2.4. Corn Bran
2.4.1. Chemical Composition
2.4.2. The Mechanism of Corn Bran in Lowering Blood Lipids
2.5. Others (Corn Leaves, Corn Cobs)
3. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CVD | cardiovascular disease | PPAR-γ | peroxisome proliferator-activated receptor-γ |
| AS | atherosclerosis | C/EBP-α | CCAAT/enhancer binding protein α |
| LDL | low-density lipoprotein | AMPK | adenosine 5′-monophosphate-activated protein kinase |
| TG | triglyceride | SREBP-1c | sterol regulatory element-binding transcription factor 1 |
| TC | cholesterol | CPT-1 | carnitine palmitoyltransferase-1 |
| HDL | high-density lipoprotein | IR | insulin resistance |
| VLDL | very low-density lipoprotein | CYP7A1 | cholesterol-7α-hydroxylase |
| RCT | cholesterol reverse transport | ACSP | acid corn silk polysaccharide |
| VEC | vascular endothelial cells | ACAT | acyl coenzyme a-cholesterol acyltransferase |
| VSMCs | vascular smooth muscle cells | COHO | corn husk oil |
| Fas | Fas protein | LTCH | lime-treated corn husks |
| ox-LDL | oxidized low density lipoprotein | ZMEAF | ethyl acetate extract of maize leaf |
| HMGCR | 3-hydroxy-3-methyl-glutaryl-CoA reductase | PPAR | peroxisome proliferator-activated receptor |
| CHM | corn husk powder | VCAM-1 | vascular cell adhesion molecule-1 |
| SOD | superoxide dismutase | HMG-CoA | 3-hydroxy-3-methyl glutaryl coenzyme A reductase |
| GSH-Px | glutathione peroxidase | Elovl6 | extension of long-chain fatty acid family member 6 |
| SCD-1 | stearoyl-coa desaturase-1 | ACC | acetyl Co-A carboxylase |
References
- Deng, J.G. Strategic Significance and Basic Ideas on Research into the Pharmaceutical Values for Crop Waste. Guangxi J. Tradit. Chin. Med. 2010, 33, 1–3. [Google Scholar] [CrossRef]
- Capanoglu, E.; Nemli, E.; Tomas-Barberan, F. Novel Approaches in the Valorization of Agricultural Wastes and Their Applications. J. Agric. Food Chem. 2022, 70, 6787–6804. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.J.; Wang, S.F. An Overview of Traditional Chinese Medicine Research on Dyslipidemia. Tradit. Chin. Med. Res. 2022, 35, 83–87. [Google Scholar] [CrossRef]
- Ma, L.Y.; Wang, Z.W.; Fan, J.; Hu, S.S. Interpretation of Report on Cardiovascular Health and Diseases in China 2022. Chin. Gen. Pract. 2023, 26, 3975–3994. [Google Scholar]
- Vekic, J.; Zeljkovic, A.; Stefanovic, A.; Jelic-Ivanovic, Z.; Spasojevic-Kalimanovska, V. Obesity and dyslipidemia. Metabolism 2019, 92, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Scientific Image and Illustration Software. BioRender. Available online: https://www.biorender.com/ (accessed on 3 June 2024).
- Jiao, Y.; Chen, H.D.; Han, H.; Chang, Y. Development and Utilization of Corn Processing by-Products: A Review. Foods 2022, 11, 3709. [Google Scholar] [CrossRef]
- Hou, J.M.; Zhen, Y.J.; Zhao, C.G.; Wu, M.F.; Liu, S.J.; Wang, J.S. The Impact of Corn Husks on Quail Blood Lipids. J. Hebei Tradit. Chin. Med. Pharmacol. 1997, 4, 4–5. [Google Scholar]
- Xiang, L.; Wenting, D.; Xu, Z.; Guodong, S.; Jianfeng, S.; Jinhai, H.; Weiming, W. Protective Effect of Corn Stigma on Liver Tissue of Hyperlipidemia Rats. Pharmacol. Clin. Chin. Mater. Medica 2021, 37, 84–90. [Google Scholar]
- Youhong, S.; Fei, W.U.; Yuyin, W.U.; An, D.; Jinzhi, F.; Renliang, W.U.; Zhiwei, W.; Xiang, C.; Liang, Z. Progress on the medicinal values of the maize silk. J. China Agric. Univ. 2020, 25, 12–23. [Google Scholar] [CrossRef]
- Yang, X.Q. Hypoglycemic Activity of Different Parts of Maize. Master’s Thesis, Changchun University of Chinese Medicine, Changchun, China, 2021. [Google Scholar]
- Zhang, K. Rats by Sweet Corncob Polysaccharide Pancreatic Protein Expression in Type 2 Diabetic. Master’s Thesis, Harbin University of Commerce, Harbin, China, 2020. [Google Scholar]
- Fu, C.; Jiang, Y.; Guo, J.; Su, Z. Natural Products with Anti-obesity Effects and Different Mechanisms of Action. J. Agric. Food Chem. 2016, 64, 9571–9585. [Google Scholar] [CrossRef]
- Liao, G.C.; Jhuang, J.H.; Yao, H.T. Artichoke leaf extract supplementation lowers hepatic oxidative stress and inflammation and increases multidrug resistance-associated protein 2 in mice fed a high-fat and high-cholesterol diet. Food Funct. 2021, 12, 7239–7249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kishi, H.; Kobayashi, S. Add-on therapy with traditional Chinese medicine: An efficacious approach for lipid metabolism disorders. Pharmacol. Res. 2018, 134, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Bartley, G.E.; Rimando, A.M.; Yokoyama, W. Hepatic gene expression related to lower plasma cholesterol in hamsters fed high-fat diets supplemented with blueberry peels and peel extract. J. Agric. Food Chem. 2010, 58, 3984–3991. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Guan, X.; Huang, K.; Zhang, Y.; Li, S.; Xia, J.A.; Shen, M. Flavonoids from Whole-Grain Oat Alleviated High-Fat Diet-Induced Hyperlipidemia via Regulating Bile Acid Metabolism and Gut Microbiota in Mice. J. Agric. Food Chem. 2021, 69, 7629–7640. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, Z.Y. Roles of Spicy Foods and Their Bioactive Compounds in Management of Hypercholesterolemia. J. Agric. Food Chem. 2018, 66, 8662–8671. [Google Scholar] [CrossRef] [PubMed]
- La Sala, L.; Prattichizzo, F.; Ceriello, A. The link between diabetes and atherosclerosis. Eur. J. Prev. Cardiol. 2019, 26, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ji, X.; Wu, H.; Li, X.; Zhang, H.; Tang, D. Mechanisms of traditional Chinese medicine in modulating gut microbiota metabolites-mediated lipid metabolism. J. Ethnopharmacol. 2021, 278, 114207. [Google Scholar] [CrossRef]
- Wang, Y. Studies on Flavonoids in the Bract of Zea mays L. Master’s Thesis, Jilin University, Changchun, China, 2010. [Google Scholar]
- Galeana-López, J.A.; Lizárraga-Velázquez, C.E.; Hernández, C.; Leyva-López, N.; Heredia, J.B. Corn Husk Phenolics Modulate Hepatic Antioxidant Response in Nile Tilapia (Oreochromis niloticus) Exposed to Hypoxia. Molecules 2021, 26, 6161. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Chen, J.; Zhu, L.; Guo, T.; Qin, D.; Hu, Z.; Han, S.; Zhou, Y.; Akan, O.D.; Wang, J.; et al. Oryzanol Attenuates High Fat and Cholesterol Diet-Induced Hyperlipidemia by Regulating the Gut Microbiome and Amino Acid Metabolism. J. Agric. Food Chem. 2022, 70, 6429–6443. [Google Scholar] [CrossRef]
- Ramjiganesh, T.; Roy, S.; Freake, H.C.; Fernandez, M.L.; McIntyre, J.C. Corn Fiber Oil Lowers Plasma Cholesterol by Altering Hepatic Cholesterol Metabolism and Up-Regulating LDL Receptors in Guinea Pigs. J. Nutr. 2002, 132, 335–340. [Google Scholar] [CrossRef]
- Jain, D.; Ebine, N.; Jia, X.; Kassis, A.; Marinangeli, C.; Fortin, M.; Beech, R.; Hicks, K.B.; Moreau, R.A.; Kubow, S.; et al. Corn fiber oil and sitostanol decrease cholesterol absorption independently of intestinal sterol transporters in hamsters. J. Nutr. Biochem. 2008, 19, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Kapcum, C.; Uriyapongson, S.; Uriyapongson, J. Phenolics, anthocyanins and antioxidant activities in waste products from different parts of purple waxy corn (Zea mays L.). Wārasān Songkhlā Nakharin 2021, 43, 398–405. [Google Scholar] [CrossRef]
- Jiayin, Y.; Mingli, K.; Xiaoting, W.; Lijun, S. Effect of Black Maize Anthocyanin on Blood Lipid Lowering in High-Fat Mice. Food Sci. Technol. 2020, 45, 196–200. [Google Scholar]
- iPlant. Available online: https://www.iplant.cn/ (accessed on 3 June 2024).
- Mu-Xin, Z.; Yin-Yan, L.; Wei, S.; Xiao-Hong, Y.; Guang-Shu, W. Isolation and Identification of Novel Flavonoids from the Bract of Zea mays L. Chem. J. Chin. Univ. 2011, 32, 2554–2557. [Google Scholar]
- Zeng, L.; Luo, L.; Xue, Q.; He, Q.; Chen, X.; Meng, J.; Wang, S.; Liang, S. LC-MS based plasma metabolomics study of the intervention effect of different polar parts of Hawthorn on hyperlipidemia rats. J. Sep. Sci. 2021, 44, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Imm, J.Y. Antiobesity Effect of Tricin, a Methylated Cereal Flavone, in High-Fat-Diet-Induced Obese Mice. J. Agric. Food Chem. 2018, 66, 9989–9994. [Google Scholar] [CrossRef]
- Wei-Wei, Y.; Upp, A.; Wei-Qun, W. Regulatory Effect of Berberine Hydrochloride on AMPK Signal Pathway and Renal Protective Effect in Streptozotocin-Induced Diabetic Nephropathy Rats. Guid. J. Tradit. Chin. Med. Pharmacol. 2020, 26, 1–5. [Google Scholar]
- Sun, L.; He, Z.D.; Yang, R.M.; Gao, N.N.; Xu, L.J.; Jin, W. Hypolipidemic activity of total phenylpropanoid glycosides from Ligustrum robustum (Roxb.) Blume and its mechanisms on AMPK pathway. Chin. Pharmacol. Bull. 2017, 33, 1073–1079. [Google Scholar]
- Naidan, Z.; Zhaohang, Z.; Ying, W.; Wei, S.; Weiqiao, P. Effect of Corn Ferulic Acid on Lipid Metabolism and Liver Injury in Hyperlipidemia Rats. Sci. Technol. Food Ind. 2023, 44, 8–14. [Google Scholar] [CrossRef]
- Yang, X.Q.; Zhi, H.; Zhang, H.; Sun, J.M. Research Progress on Chemical Constituents, Pharmacological Activity and Utilization Status of Different Parts of Corn. Jilin J. Chin. Med. 2019, 39, 837–840. [Google Scholar]
- Shin, K.; Kim, Y.; Lee, K.; Choi, K. Effect of In Vitro Antioxidant Properties and Extract of Corn Husk on Serum Lipids in Mice. J. East. Asian Soc. Diet. Life 2015, 25, 261. [Google Scholar] [CrossRef]
- Roh, K.B.; Kim, H.; Shin, S.; Kim, Y.S.; Lee, J.A.; Kim, M.O.; Jung, E.; Lee, J.; Park, D. Anti-inflammatory effects of Zea mays L. husk extracts. BMC Complement. Altern. Med. 2016, 16, 298. [Google Scholar] [CrossRef] [PubMed]
- Okokon, J.E.; Antia, B.S.; Mohanakrishnan, D.; Sahal, D. Antimalarial and antiplasmodial activity of husk extract and fractions of Zea mays. Pharm. Biol. 2017, 55, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- Martin R Bennett, S.S.G.K. Vascular Smooth Muscle Cells in Atherosclerosis. Circ. Res. 2016, 118, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Barter, P.J.; Nicholls, S.; Rye, K.; Anantharamaiah, G.M.; Navab, M.; Fogelman, A.M. Antiinflammatory Properties of HDL. Circ. Res. 2004, 95, 764–872. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Y.J.; Hou, J.M.; Wu, Z.Q.; Liu, S.J.; Wu, M.F.; Wang, L.; Wang, J.S. The Effects of Corn Bract Decotion on Endothelin and Prostacyclin and Morphology of Atherosclerosis in Rabbits. Chin. J. Arterioscler. 2003, 11, 207–210. [Google Scholar]
- Chen, B.X.; Zhu, S.S.; Cai, M.X. The Effect of Compound Hawthorn Fruit Tea on the Levels of Thromboxane and Prostaglandin in Rat Plasma. Chin. J. Gerontol. 1997, 2, 45–47. [Google Scholar]
- Zheng, G.; An-rong, J.; Xiao-Gang, P.; Man, Y. Typha angustifolia extract reduces diet-induced hyperlipidemia in rats. Chin. Herb. Med. 2019, 11, 98–102. [Google Scholar]
- Zhen, Y.J.; Hou, J.M.; Jiang, X.J.; Ding, T.; Zhang, D.H.; Cao, H. Effect of Corn Bract on the Level of NO, ET and Apoptosis of Endothelial Cells. Liaoning J. Tradit. Chin. Med. 2009, 36, 307–308. [Google Scholar]
- Zhen, Y.J.; Hou, J.M.; Zhao, C.G.; Wu, M.F.; Liu, S.J.; Wang, J.S.; Wang, L.H. Experimental Study on the Effects of Corn Husk on Hyperlipidemia and Atherosclerosis in Domestic Rabbits. J. Hebei Tradit. Chin. Med. Pharmacol. 1998, 4, 4–5. [Google Scholar]
- Siqi, Y.; Jian, W.; Lan, T.; Lingyao, Q.I.; Xu, C.; Lin, C. Research progress in apoptosis of vascular smooth muscle cells in atherosclerosis. J. Cent. South Univ. 2021, 46, 872–876. [Google Scholar] [CrossRef]
- Jiang, X.J.; Zhen, Y.J.; Ding, T.; Ding, X.M.; Hou, J.B.; Zhang, X.J.; Wu, M.F.; Zhang, F.M.; Liu, Z.M. The reaction of proliferation and apoptosis of endothelial cells and smooth muscle cells of hyperlipidemia rat to the role of corn bract decoction. Chin. J. Gerontol. 2008, 28, 1579–1580. [Google Scholar] [CrossRef]
- Jiang, X.J.; Wang, X.H.; Li, Y.; Ding, X.M.; Zhen, Y.J. Corn Husk’s Regulation of Apoptosis in Hyperlipidemic Rat VSMCs and its Effects on Fas and Caspase-3. Lishizhen Med. Mater. Medica Res. 2012, 23, 1147–1148. [Google Scholar]
- Ding, X.M.; Li, G.L.; Jiang, X.J.; Wang, H.; Li, Y.; Wang, Z.J.; Zhen, Y.J. Study on the Medicinal Effects of Corn Husk on Apoptosis of Vascular Endothelium and Smooth Muscle and p53 Expression in Rats. Lishizhen Med. Mater. Medica Res. 2010, 21, 2878–2879. [Google Scholar]
- Grootaert, M.O.J.; Moulis, M.; Roth, L.; Martinet, W.; Vindis, C.; Bennett, M.R.; De Meyer, G.R.Y. Vascular smooth muscle cell death, autophagy and senescence in atherosclerosis. Cardiovasc. Res. 2018, 114, 622–634. [Google Scholar] [CrossRef]
- Obermayer, G.; Afonyushkin, T.; Binder, C.J. Oxidized low-density lipoprotein in inflammation-driven thrombosis. J. Thromb. Haemost. 2018, 16, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Grootaert, M.; Uryga, A.; Finigan, A.; Figg, N.; Bennett, M. Role of SIRTUIN 6 in vascular smooth muscle cells in atherosclerosis. Atherosclerosis 2020, 315, e18. [Google Scholar] [CrossRef]
- Shan, L.; Yun, L.; Gui-bo, S.; Xiao-Bo, S. Traditional Chinese medicines treating macrophage: A particular strategy for atherosclerosis. Chin. Herb. Med. 2019, 11, 3–9. [Google Scholar]
- Hou, J.M.; Zhen, Y.J.; Wang, L.; Wu, Z.Q.; Wu, M.F.; Liu, S.J.; Wang, J.S. Corn bract reduces the leukocyte apoptosis and CD44 expression in rabbits with atherosclerosis. Chin. J. Gerontol. 2004, 11, 1036–1037. [Google Scholar]
- Cuff, C.A.; Kothapalli, D.; Azonobi, I.; Chun, S.; Zhang, Y.; Belkin, R.; Yeh, C.; Secreto, A.; Assoian, R.K.; Rader, D.J.; et al. The adhesion receptor CD44 promotes atherosclerosis by mediating inflammatory cell recruitment and vascular cell activation. J. Clin. Investig. 2001, 108, 1031–1040. [Google Scholar] [CrossRef]
- Paublini, H.; Lopez, G.A.; Busquets-Cortes, C.; Tomas-Gil, P.; Riutord-Sbert, P.; Ramirez-Manent, J.I. Relationship between Atherogenic Dyslipidaemia and Lipid Triad and Scales That Assess Insulin Resistance. Nutrients 2023, 15, 2105. [Google Scholar] [CrossRef]
- Riaz, M.; Nawaz, S.; Ilyas, I.; Rehman, M.; Qadir, R.; Mehmood, T.; Afzal, M.; Abdul, R.N.; Ali, A. Evaluation of antidiabetic, antioxidant, and cytotoxic potential of maize (Zea mays L.) husk leaf extracts. Cell Mol. Biol. 2021, 67, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.S.; Li, T.D.; Zeng, Z.H. Mechanisms underlying direct actions of hyperlipidemia on myocardium: An updated review. Lipids Health Dis. 2020, 19, 23. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Bungau, S.; Kumar, K.; Zengin, G.; Khan, F.; Kumar, A.; Kaur, R.; Venkatachalam, T.; Tit, D.M.; Vesa, C.M.; et al. Pleotropic Effects of Polyphenols in Cardiovascular System. Biomed. Pharmacother. 2020, 130, 110714. [Google Scholar] [CrossRef] [PubMed]
- Erlao, Z.; Minghua, W.; Junling, Z.; Jie, Y. Optimization of Microwave Assisted Extraction Technology of Corn Bracts Polyphenol by Response Surface Methodology and Its Antioxidant Activity. Mol. Plant Breed. 2018, 16, 5789–5795. [Google Scholar] [CrossRef]
- Xiao-xu, K.; Yan-yang, X.U.; Zhuo-yang, J.; Yan-ling, S. Optimization of extraction process for polyphenol from corn bracts by response surface methodology. Food Sci. Techol. 2015, 40, 229–235. [Google Scholar]
- Ping, Z.; Hong-Lei, T.; Kai-Xiong, L.I. Extraction of corn-bract flavonoid and related antioxidative activity. Chin. J. Eco-Agric. 2007, 15, 108–112. [Google Scholar]
- Lei, Z.; Wei, Z.; Man, J. Study on Micro-assisted Extracting and Antioxidant Activity of Flavonoids from the Corn-bract. Food Res. Dev. 2015, 36, 12–15. [Google Scholar] [CrossRef]
- Schmelz, E.A.; Kaplan, F.; Huffaker, A.; Dafoe, N.J.; Vaughan, M.M.; Ni, X.; Rocca, J.R.; Alborn, H.T.; Teal, P.E. Identity, regulation, and activity of inducible diterpenoid phytoalexins in maize. Proc. Natl. Acad. Sci. USA 2011, 108, 5455–5460. [Google Scholar] [CrossRef]
- Tao, Y.; Zhongxiang, Z.; Mengyuan, L. Analysis of the chemical constituents of flavonoids in corn stigama by HPLC-Q-TOF-MS. China Pharm. 2019, 22, 1776–1780. [Google Scholar]
- Mian, Z.; Chuan-Shui, L.; Tian-Peng, Y.; Hai-Xian, F.; Le, C.; Zhong-Tao, D. Chemical constituents of the style of Zea mays L. from Yunnan. Chem. Res. Appl. 2013, 25, 846–850. [Google Scholar] [CrossRef]
- Christensen, S.A.; Huffaker, A.; Sims, J.; Hunter, C.T.; Block, A.; Vaughan, M.M.; Willett, D.; Romero, M.; Mylroie, J.E.; Williams, W.P.; et al. Fungal and herbivore elicitation of the novel maize sesquiterpenoid, zealexin A4, is attenuated by elevated CO2. Planta 2018, 247, 863–873. [Google Scholar] [CrossRef]
- Xia, Y.; Cong-Yong, S.; Jiang-Nan, Y.U.; Xi-Ming, X.U. Structure characterization and hypolipidemic activity of acid corn silk polysaccharide. J. Jiangsu Univ. 2018, 28, 333–338. [Google Scholar] [CrossRef]
- Yumei Wang, M.G.J.M. Phytochemical study: Fragmentation patterns of flavonoid-C-glycosides in the enriched flavonoids from corn silk using high-efficiency ultra-high performance liquid chromatography combined with quadrupole time-of-flight mass spectrometry. Sep. Sci. Plus 2023, 7, 2300156. [Google Scholar] [CrossRef]
- Fougere, L.; Zubrzycki, S.; Elfakir, C.; Destandau, E. Characterization of Corn Silk Extract Using HPLC/HRMS/MS Analyses and Bioinformatic Data Processing. Plants 2023, 12, 721. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Jing, R.Q.; Hu, T.H. The Effect of Total Flavonoids from Corn Silk on Blood Lipids, Blood Sugar Levels, and Antioxidant Action in Diabetic Dyslipidemic Rats. Pharmacol. Clin. Chin. Mater. Medica 2011, 27, 85–86. [Google Scholar]
- Tian, M.; Bai, Y.; Tian, H.; Zhao, X. The Chemical Composition and Health-Promoting Benefits of Vegetable Oils-A Review. Molecules 2023, 28, 6393. [Google Scholar] [CrossRef]
- Bunzel, M.; Ralph, J.; Bruning, P.; Steinhart, H. Structural identification of dehydrotriferulic and dehydrotetraferulic acids isolated from insoluble maize bran fiber. J. Agric. Food Chem. 2006, 54, 6409–6418. [Google Scholar] [CrossRef]
- Ou, J.Y.; Huang, J.Q.; Song, Y.; Yao, S.W.; Peng, X.C.; Wang, M.F.; Ou, S.Y. Feruloylated Oligosaccharides from Maize Bran Modulated the Gut Microbiota in Rats. Plant Food Hum. Nutr. 2016, 71, 123–128. [Google Scholar] [CrossRef]
- Choi, M.K.K.I. The Inhibitory Effect of Hydroxycinnamic Acid Derivatives from Corn (Zea may L.) Bran on Melanogenesis. J. Soc. Cosmet. Sci. Korea 2009, 35, 143–149. [Google Scholar]
- Farcas, A.C.; Socaci, S.A.; Nemes, S.A.; Pop, O.L.; Coldea, T.E.; Fogarasi, M.; Biris-Dorhoi, E.S. An Update Regarding the Bioactive Compound of Cereal By-Products: Health Benefits and Potential Applications. Nutrients 2022, 14, 3470. [Google Scholar] [CrossRef]
- Lin, W.T.; Hong, H.R. Effect of Dietary Fiber in Carrot Residue on B lood Lipid Regulation. Acta Nutr. Sin. 2008, 30, 530–531. [Google Scholar] [CrossRef]
- Hong, H.R.; Lin, W.T. The Fermentation of Dietary Fiber and Its Related Physiological Functions. Food Nutr. China 2007, 1, 54–56. [Google Scholar] [CrossRef]
- Jin-ting, W. The Advance of Dietary Fiber and Its Functions of Physiological Health Care. Prog. Mod. Biomed. 2007, 7, 1414–1416. [Google Scholar] [CrossRef]
- Jung, Y.J.; Park, J.H.; Cho, J.G.; Seo, K.H.; Lee, D.S.; Kim, Y.C.; Kang, H.C.; Song, M.C.; Baek, N.I. Lignan and flavonoids from the stems of Zea mays and their anti-inflammatory and neuroprotective activities. Arch. Pharm. Res. 2015, 38, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Huffaker, A.; Kaplan, F.; Vaughan, M.M.; Dafoe, N.J.; Ni, X.; Rocca, J.R.; Alborn, H.T.; Teal, P.E.; Schmelz, E.A. Novel acidic sesquiterpenoids constitute a dominant class of pathogen-induced phytoalexins in maize. Plant Physiol. 2011, 156, 2082–2097. [Google Scholar] [CrossRef] [PubMed]
- Kollner, T.G.; Held, M.; Lenk, C.; Hiltpold, I.; Turlings, T.C.; Gershenzon, J.; Degenhardt, J. A maize (E)-beta-caryophyllene synthase implicated in indirect defense responses against herbivores is not expressed in most American maize varieties. Plant Cell 2008, 20, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.Y.; Du, J.; Liu, Z.; Wu, Y.K.; Li, N.; Zhu, F. Research Progress on Dietary Fiber of Corn Husk and Hyperlipidemia. Farm Prod. Process. 2022, 6, 69–71+76. [Google Scholar] [CrossRef]
- Sim, J.Y.; Kim, M.; Kim, M.J.; Chun, W.; Kwon, Y. Acetylcholinesterase inhibitors from the Stem of Zea mays. Nat. Prod. Sci. 2014, 20, 13–16. [Google Scholar]
- Hasanudin, K.; Hashim, P.; Mustafa, S. Corn silk (Stigma maydis) in healthcare: A phytochemical and pharmacological review. Molecules 2012, 17, 9697–9715. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Liu, C.; Wang, X.; Chen, B.; Yao, L.; Qiao, Y.; Zheng, H. Recent developments in stigma maydis polysaccharides: Isolation, structural characteristics, biological activities and industrial application. Int. J. Biol. Macromol. 2020, 150, 246–252. [Google Scholar] [CrossRef]
- Wang, Y.; Mao, J.; Zhang, M.; Liu, L.; Zhu, Y.; Gu, M.; Zhang, J.; Bu, H.; Sun, Y.; Sun, J.; et al. An Umbrella Insight into the Phytochemistry Features and Biological Activities of Corn Silk: A Narrative Review. Molecules 2024, 29, 891. [Google Scholar] [CrossRef]
- Zongkai, L.; Jinling, Z.; Lina, G.; Yukun, M.; Linlin, W.; Jicheng, L. Research Progress on Chemical Constituents of Stigma maydis. Chem. Ind. Times 2022, 36, 23–31. [Google Scholar] [CrossRef]
- Tian, J. Studies on Liposoluble Constituents of Corn Silk. Master’s Thesis, Tianjin University, Tianjin, China, 2014. [Google Scholar]
- Zhenyan, L.; Wenqin, Y.; Hong, C.; Lei, Q.I.; Hong, G.; Yu, C.; Jicheng, L. Analysis of amino acids composition and evaluation of the nutritional value and favor in aqueous extract of maize silk. China Food Addit. 2022, 33, 109–114. [Google Scholar] [CrossRef]
- Li, X. Study on the effect of reducing blood lipid and improving liver injury of corn silk. Master’s Thesis, Heilongjiang Academy of Traditional Chinese Medicine, Harbin, China, 2021. [Google Scholar]
- Ross, S.; Gerstein, H.; Paré, G. The Genetic Link Between Diabetes and Atherosclerosis. Can. J. Cardiol. 2018, 34, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Zeyue, Z.; Ying, G.; Chen, Z. Effects of corn silk polysaccharides with different molecular weight on hypolipidemic and its mechanism. China Food Addit. 2023, 34, 241–248. [Google Scholar] [CrossRef]
- Miura, T. The lipid-lowering effect of corn whiskers. Int. J. Tradit. Chin. Med. 2006, 28, 98. [Google Scholar]
- Sun, Q.; Wang, H.Y.; Zhang, T.L.; Li, L.G.; Kong, Y.; Gao, J.; Yao, J.Q. Effect of Corn Extract on Blood Glucose, Blood Lipid and Hemorheology in Diabetic Mice Models. Acta Chin. Med. Pharmacol. 2016, 44, 25–28. [Google Scholar]
- Ding, L. The Effect of Stigma Maydis and Its Polysaccharideson Blood Glucose and Blood Lipids. Master’s Thesis, Heilongjiang University of Chinese Medicine, Harbin, China, 2016. [Google Scholar]
- Deng, W.; Yang, X.; Zhu, Y.; Yu, J.; Xu, X. Structural characterization and hypolipidemic activities of purified stigma maydis polysaccharides. Food Sci. Nutr. 2019, 7, 2674–2683. [Google Scholar] [CrossRef]
- Saulnier, L.; Vigouroux, J.; Thibault, J.F. Isolation and partial characterization of feruloylated oligosaccharides from maize bran. Carbohyd Res. 1995, 272, 241–253. [Google Scholar] [CrossRef]
- Allerdings, E.; Ralph, J.; Steinhart, H.; Bunzel, M. Isolation and structural identification of complex feruloylated heteroxylan side-chains from maize bran. Phytochemistry 2006, 67, 1276–1286. [Google Scholar] [CrossRef] [PubMed]
- Funk, C.; Ralph, J.; Steinhart, H.; Bunzel, M. Isolation and structural characterisation of 8-O-4/8-O-4- and 8-8/8-O-4-coupled dehydrotriferulic acids from maize bran. Phytochemistry 2005, 66, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Rice brans, rice bran oils, and rice hulls: Composition, food and industrial uses, and bioactivities in humans, animals, and cells. J. Agric. Food Chem. 2013, 61, 10626–10641. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Han, Y.; McClements, D.J.; Xu, D.; Chen, S. Production, Characterization, Delivery, and Cholesterol-Lowering Mechanism of Phytosterols: A Review. J. Agric. Food Chem. 2022, 70, 2483–2494. [Google Scholar] [CrossRef] [PubMed]
- Ntanios, F.Y.; Jones, P.J.H. Dietary sitostanol reciprocally influences cholesterol absorption and biosynthesis in hamsters and rabbits. Atherosclerosis 1999, 143, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Ramjiganesh, T.; Roy, S.; Nicolosi, R.J.; Young, T.L.; McIntyre, J.C.; Fernandez, M.L. Corn husk oil lowers plasma LDL cholesterol concentrations by decreasing cholesterol absorption and altering hepatic cholesterol metabolism in guinea pigs. J. Nutr. Biochem. 2000, 11, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Quintanar, R.L.; Hernandez, L.; Conde, K.; Vergara-Jimenez, M.; Fernandez, M.L. Lime-treated corn husks lower plasma LDL cholesterol in guinea pigs by altering hepatic cholesterol metabolism. J. Nutr. Biochem. 1997, 8, 479–486. [Google Scholar] [CrossRef]
- Wang, D.; Hu, D.; Yang, L.J. The Health Benefits of Dietary Fiber. Food Nutr. China 2006, 6, 48–49. [Google Scholar]
- Zhong, L.Y.; Lin, W.T. Overview of Research on the Role and Mechanism of Dietary Fiber in Lipid Reduction. Strait J. Prev. Med. 2008, 14, 26–28. [Google Scholar] [CrossRef]
- Guangdong Province Food and Drug Administration. Standards for Traditional Chinese Medicine Materials in Guangdong Province Volume 3; Guangdong Science and Technology Publishing House: Guangzhou, China, 2018; p. 512. [Google Scholar]
- Yongqiang, M.A.; Kai, Z.; Xin, W.; Xuechun, L.U.; Congyu, L. Hypoglycemic Effect of Sweet Corn Cob Polysaccharide on Diabetic Rats. Food Sci. 2020, 41, 169–173. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, X.; Ma, Y.Q.; Chen, J.J.; Xiao, E.L. Effect of Sweet Corn Core Polysaccharide on Starch and Digestive Enzyme. Food Ind. 2019, 40, 199–202. [Google Scholar]
- Ma, Y.Q.; Wang, X.; Gao, S. Hypoglycemic Activity of Polysaccharides from Sweet Corncob on Streptozotocin-Induced Diabetic Rats. J. Food Sci. 2017, 82, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Soppert, J.; Lehrke, M.; Marx, N.; Jankowski, J.; Noels, H. Lipoproteins and lipids in cardiovascular disease: From mechanistic insights to therapeutic targeting. Adv. Drug Deliv. Rev. 2020, 159, 4–33. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M.; Gao, Z.D.; Li, Q.; Tian, Z.F.; Guo, Z.L. Effects of Apple Polyphenol on Blood Lipid, Blood Glucose Level and PPARy Gene Expression of Diabetic Mice. J. Anhui Agric. Sci. 2015, 43, 1–3. [Google Scholar]
- Balasubramanian, K.; Padma, P.R. Anticancer Activity of Zea mays Leaf Extracts on Oxidative Stress-induced Hep2 Cells. J. Acupunct. Meridian 2013, 6, 149–158. [Google Scholar] [CrossRef]
- Adeyi, A.; Gbadamosi, I.; Olanrewaju, O.D.; Badejo, J.A. IDF21-0319 Zea mays leaves partitions ameliorated diabetic nephropathy via NRF2 antioxidant signaling pathways in Wistar rats. Diabetes Res. Clin. Pract. 2022, 186, 109646. [Google Scholar] [CrossRef]


| Plant Part | Class of Compound | Name of Compound | Chemical Formula | Molecular Weight | Structural Formula | Reference |
|---|---|---|---|---|---|---|
| corn bracts | Flavonoid | Tricin | C17H14O7 | 330.29 | Compound (1) in Figure 3 | [21] |
| corn bracts | Flavonoids | Tricin-5-O-β-D-glucoside | C23H24O12 | 492.4 | Compound (2) in Figure 3 | [29] |
| corn bracts | Flavonoids | Tricin-7-O-β-D-glucoside | C23H24O12 | 492.4 | Compound (3) in Figure 3 | [29] |
| corn bracts | Flavonoids | Tricin-7-O-[β-D-apifuranosyl(1→2)]-β-D-glucopyranoside | C28H32O16 | —— | Compound (4) in Figure 3 | [29] |
| corn bracts, corn bran | phenolic acids | Ferulic acid | C10H10O4 | 194.18 | Compound (5) in Figure 3 | [22,30] |
| corn bracts | phenolic acids | p-coumaric acid | C9H8O3 | 164.16 | Compound (6) in Figure 3 | [22] |
| corn bracts | phenolic acids | p-hydroxybenzoic acid | C7H6O3 | 138.12 | Compound (7) in Figure 3 | [22] |
| corn bracts | phenolic acids | Chlorogenic acid | C16H18O9 | 354.31 | Compound (8) in Figure 3 | [22] |
| corn stalks | Flavonoids | trans-4′-methoxy-4-nitrochalcone | C16H13NO4 | 283.28 | Compound (9) in Figure 3 | [31] |
| corn stalks | Flavonoids | diethyl2-acetamido-6-(1-cyano-2-ethoxy-2-oxoethyl)-1,3-azulenedicarboxylate | C23H24N2O7 | 410 | Compound (10) in Figure 3 | [11] |
| corn stalks | Flavonoids | 4′-methyl-epigallocatechin-3′-glucuronide | C22H24O13 | 496 | Compound (11) in Figure 3 | [11] |
| corn stalks | Flavonoids | 4′,8-dimethoxy-epigallocatechin-3′-glucUronide | C23H26O14 | 526 | Compound (12) in Figure 3 | [11] |
| corn stalks | Phytosterols | sitosterol | C29H50O | 414.7 | —— | [25] |
| corn stalks | Anthocyanin | anthocyanin | —— | —— | —— | [27,32] |
| corn cob | Polysaccharides | SCP-80-1 | Ara:Man:Glu:Gal= 0.369:0.824:10.759:0.333 | 18.350 kDa | —— | [33,34] |
| corn silk | Polysaccharides | CSP-3 | Man:Rha:Glu:Gal:Ara:Xyl:Gala= 1.53:0.00:0.43:1.06:0.46:0.35:1.46 | 5.9 ± 0.06 kDa | —— | [35] |
| Name | Possible Active Components | Hypolipidemic Effects | Possible Mechanisms | References |
|---|---|---|---|---|
| corn bract | corn bract water extract | TC↓, TG↓, LDL-C↓, VLDL-C↓, HDL-C↑ |
| [41,44,45,47,48,49,54] |
| corn bract | total flavonoids, total phenols | alpha-amylase↓, CAT↑, DPPH↑ |
| [22,59] |
| corn bract | tricin | TG↓, ALT↓, AST↓ |
| [60,61] |
| cornstalk | flavonoids | alpha-amylase↓ |
| [11,31] |
| corn silk | corn silk water extract | TC↓, TG↓, LDL↓, HDL↑, SOD↑, MDA↓, GSH-PX↑, ALT↓, AST↓ |
| [64,65,66] |
| corn silk | corn silk polysaccharide | TC↓, TG↓, LDL-C↓, HDL-C↑ |
| [35,67,68] |
| corn silk | corn silk acidic polysaccharide | TC↓, TG↓, Kch↓, LDL-C↓ |
| [69] |
| corn silk | flavonoids | TC↓, TG↓, LDL-C↓, HDL-C↑, SOD↑, MDA↓ |
| [70] |
| corn bran | dietary fiber | TC↓, TG↓ |
| [71,72] |
| corn bran/corn fiber oil | phytosterols | TC↓, TG↓, LDL-C↓ |
| [73,74,75] |
| corn bran | lime-treated corn brans | LDL-C, VLDL-C↓ |
| [76] |
| corn cob | polysaccharide (SCP-80-1) | alpha-amylase↑, TC↓, TG↓, LDL-C↓, HDL-C↑ |
| [77] |
| corn leaves | ethyl acetate extract | - |
| [78,79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Cao, L.; Tang, J.; Deng, J.; Hao, E.; Bai, G.; Tang, P.L.; Yang, J.; Li, H.; Yao, L.; et al. Research on the Mechanism and Material Basis of Corn (Zea mays L.) Waste Regulating Dyslipidemia. Pharmaceuticals 2024, 17, 868. https://doi.org/10.3390/ph17070868
Wang X, Cao L, Tang J, Deng J, Hao E, Bai G, Tang PL, Yang J, Li H, Yao L, et al. Research on the Mechanism and Material Basis of Corn (Zea mays L.) Waste Regulating Dyslipidemia. Pharmaceuticals. 2024; 17(7):868. https://doi.org/10.3390/ph17070868
Chicago/Turabian StyleWang, Xiaodong, Lewei Cao, Jiajun Tang, Jiagang Deng, Erwei Hao, Gang Bai, Pei Ling Tang, Jieyi Yang, Huaying Li, Lihao Yao, and et al. 2024. "Research on the Mechanism and Material Basis of Corn (Zea mays L.) Waste Regulating Dyslipidemia" Pharmaceuticals 17, no. 7: 868. https://doi.org/10.3390/ph17070868
APA StyleWang, X., Cao, L., Tang, J., Deng, J., Hao, E., Bai, G., Tang, P. L., Yang, J., Li, H., Yao, L., He, C., & Hou, X. (2024). Research on the Mechanism and Material Basis of Corn (Zea mays L.) Waste Regulating Dyslipidemia. Pharmaceuticals, 17(7), 868. https://doi.org/10.3390/ph17070868






