Fatty Acid Amides Suppress Proliferation via Cannabinoid Receptors and Promote the Apoptosis of C6 Glioma Cells in Association with Akt Signaling Pathway Inhibition
Abstract
1. Introduction
2. Results
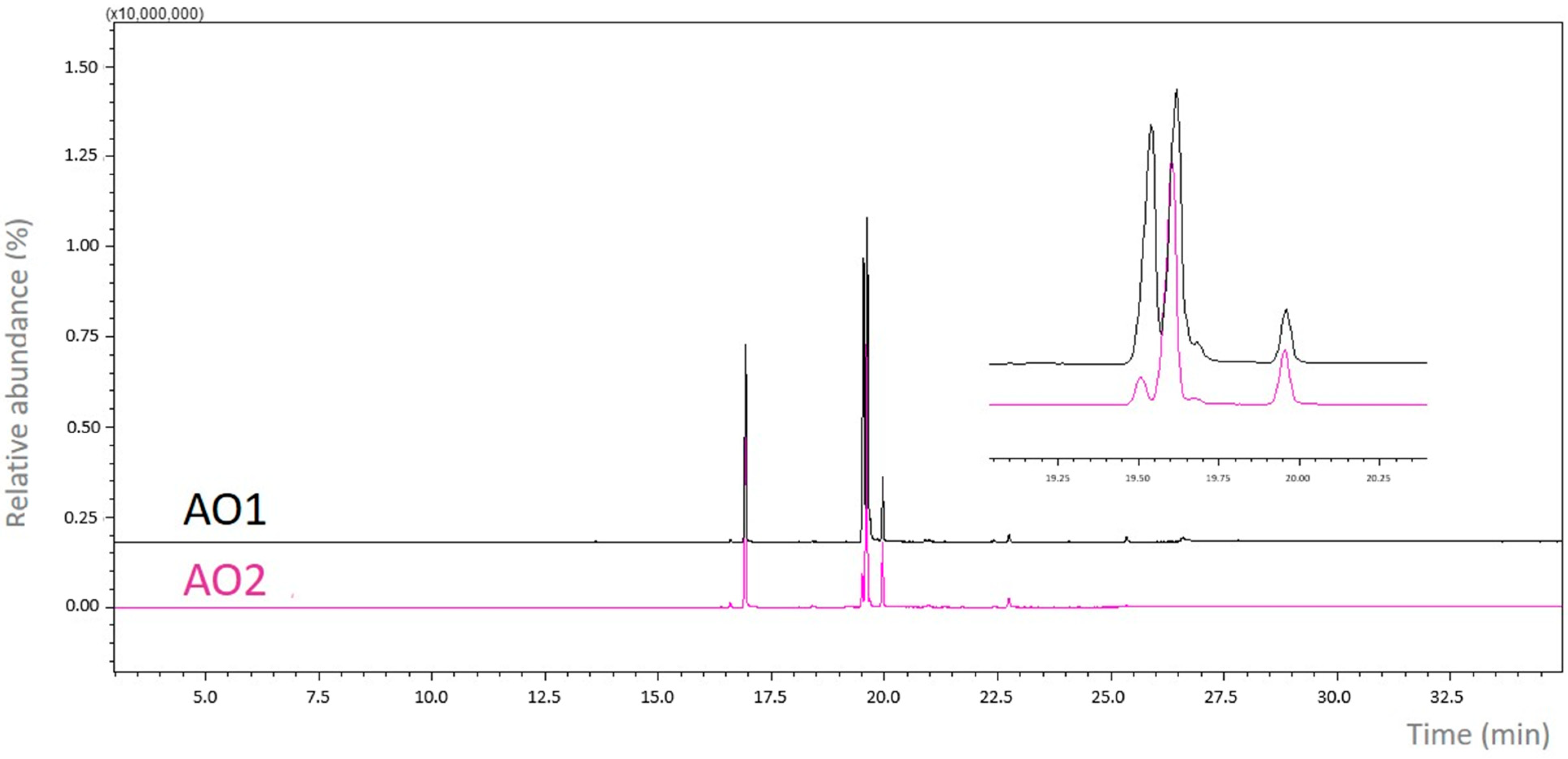
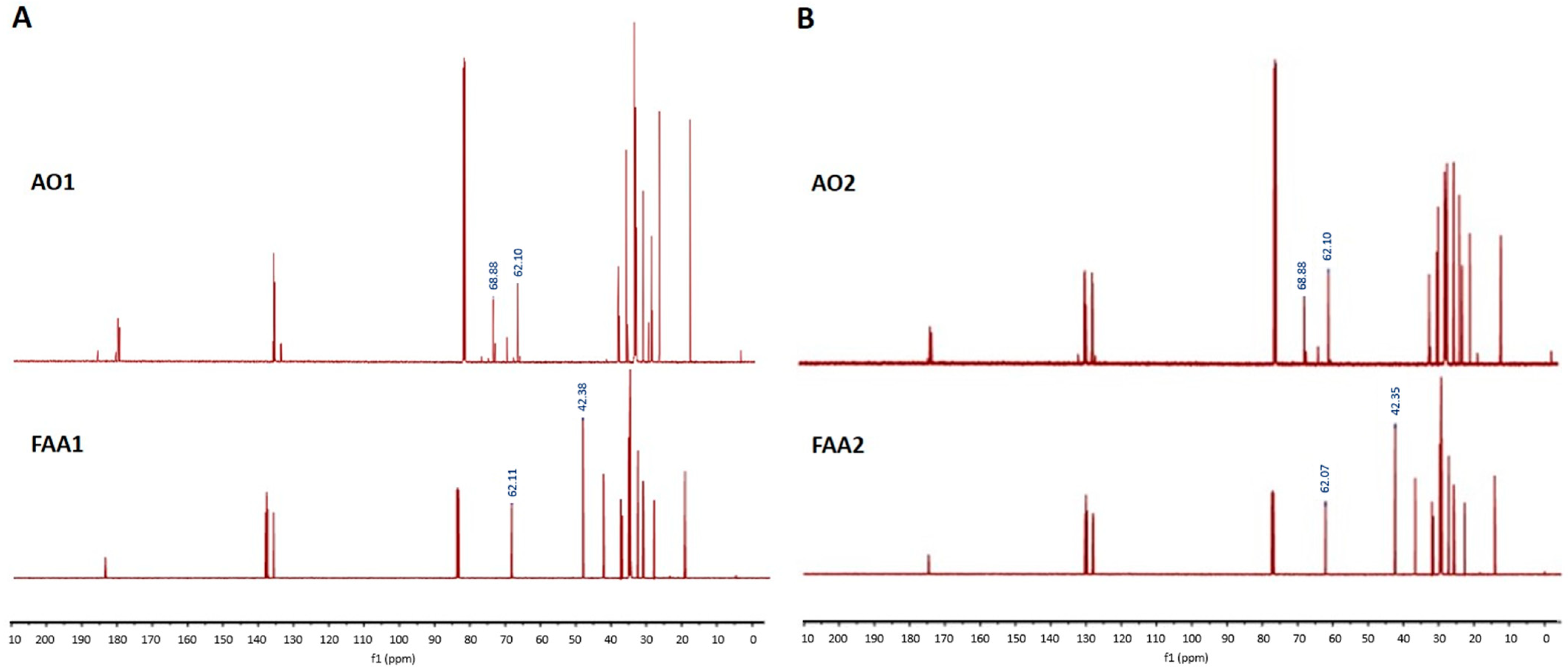
2.1. Spectroscopic Analysis
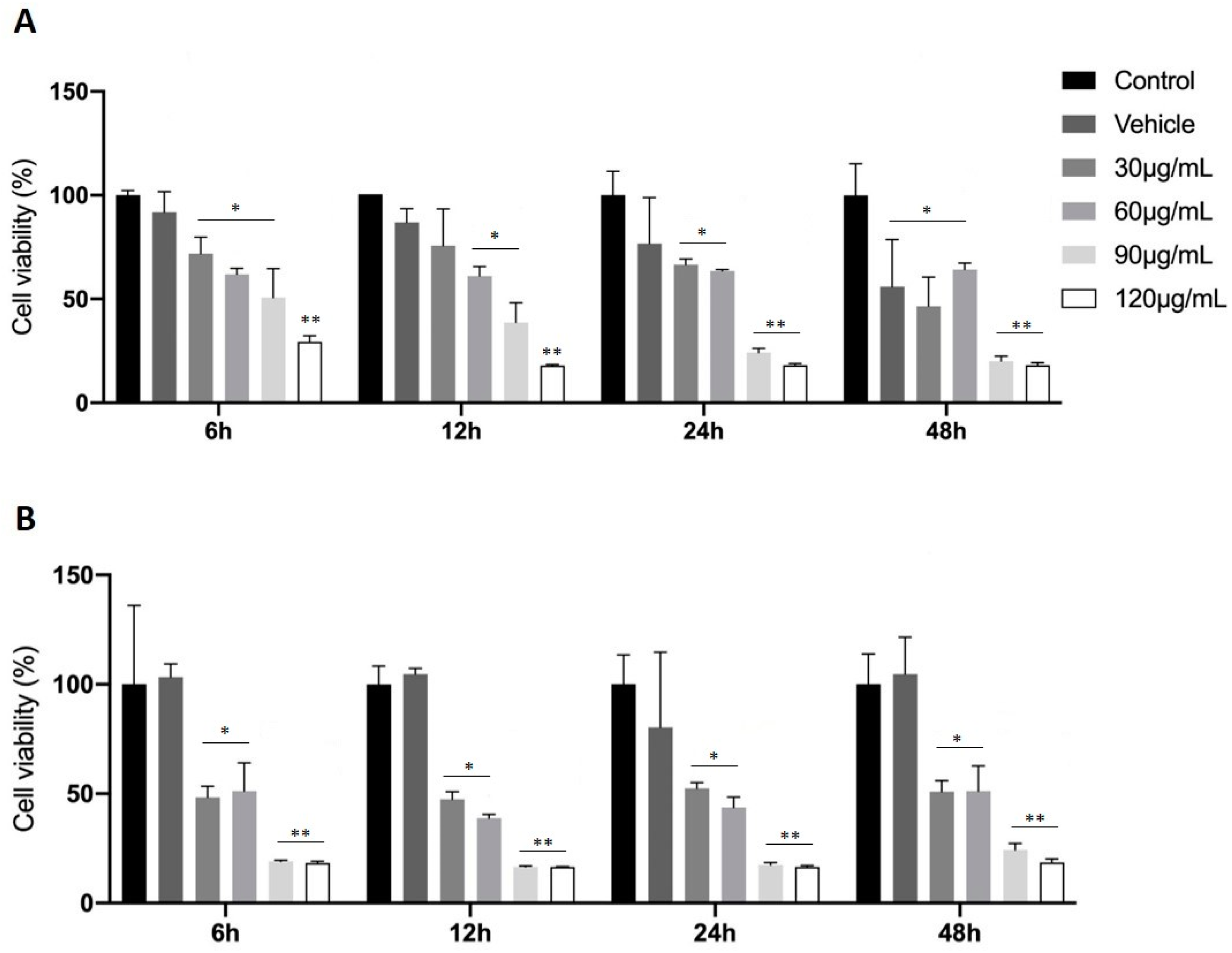
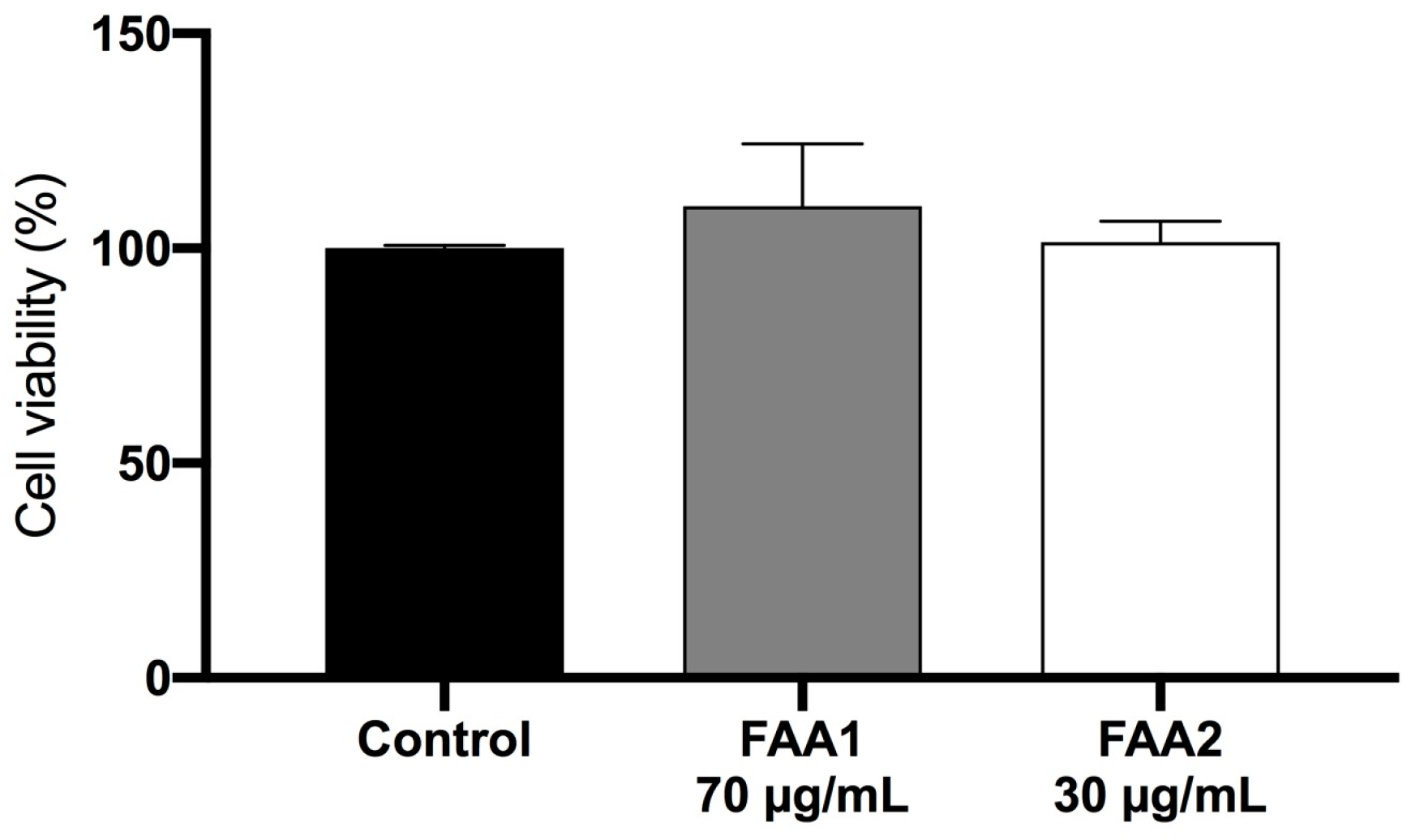
2.2. Treatment with FAAs Inhibits C6 Glioma Cell Growth
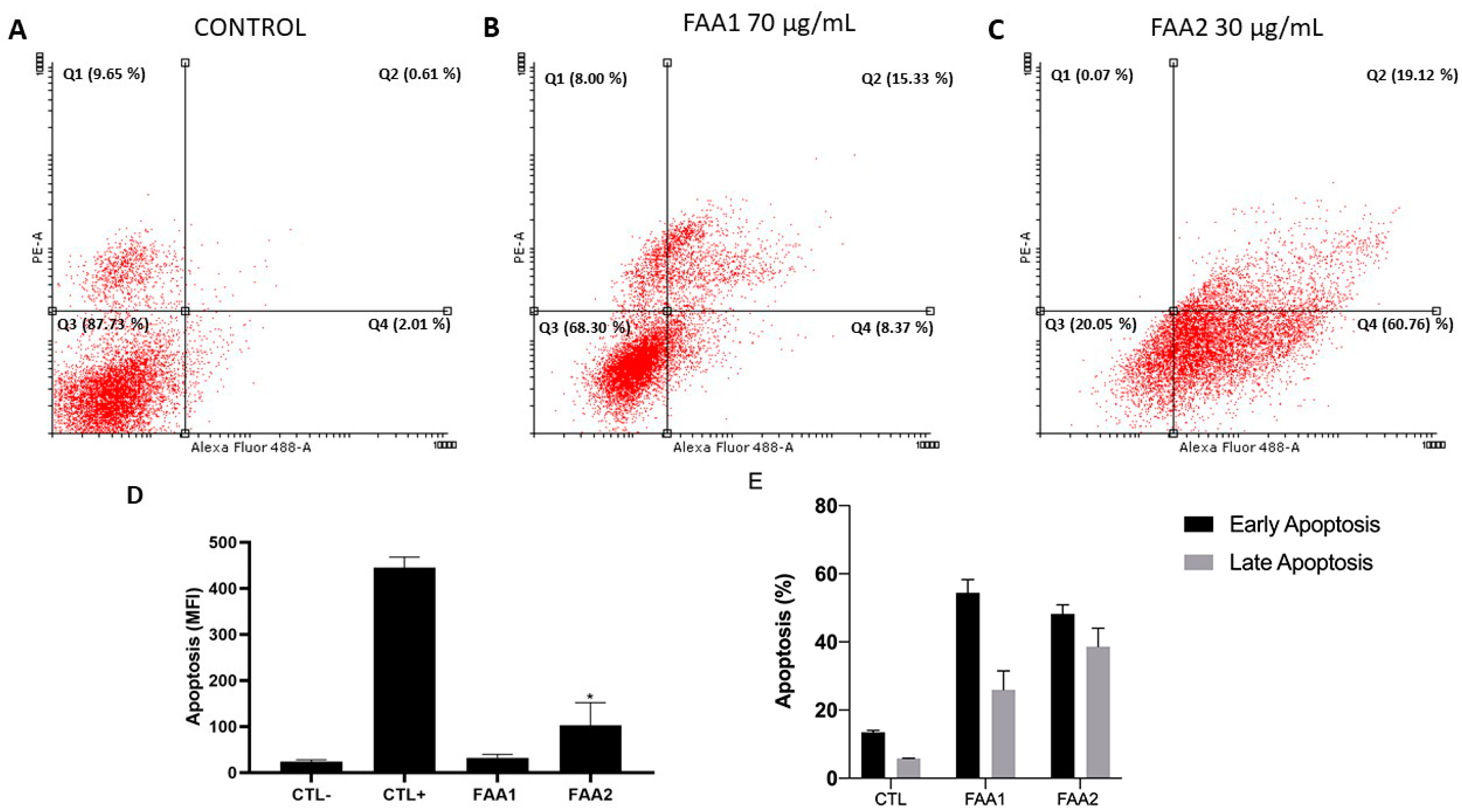
2.3. FAAs Induce Apoptosis in C6 Cells
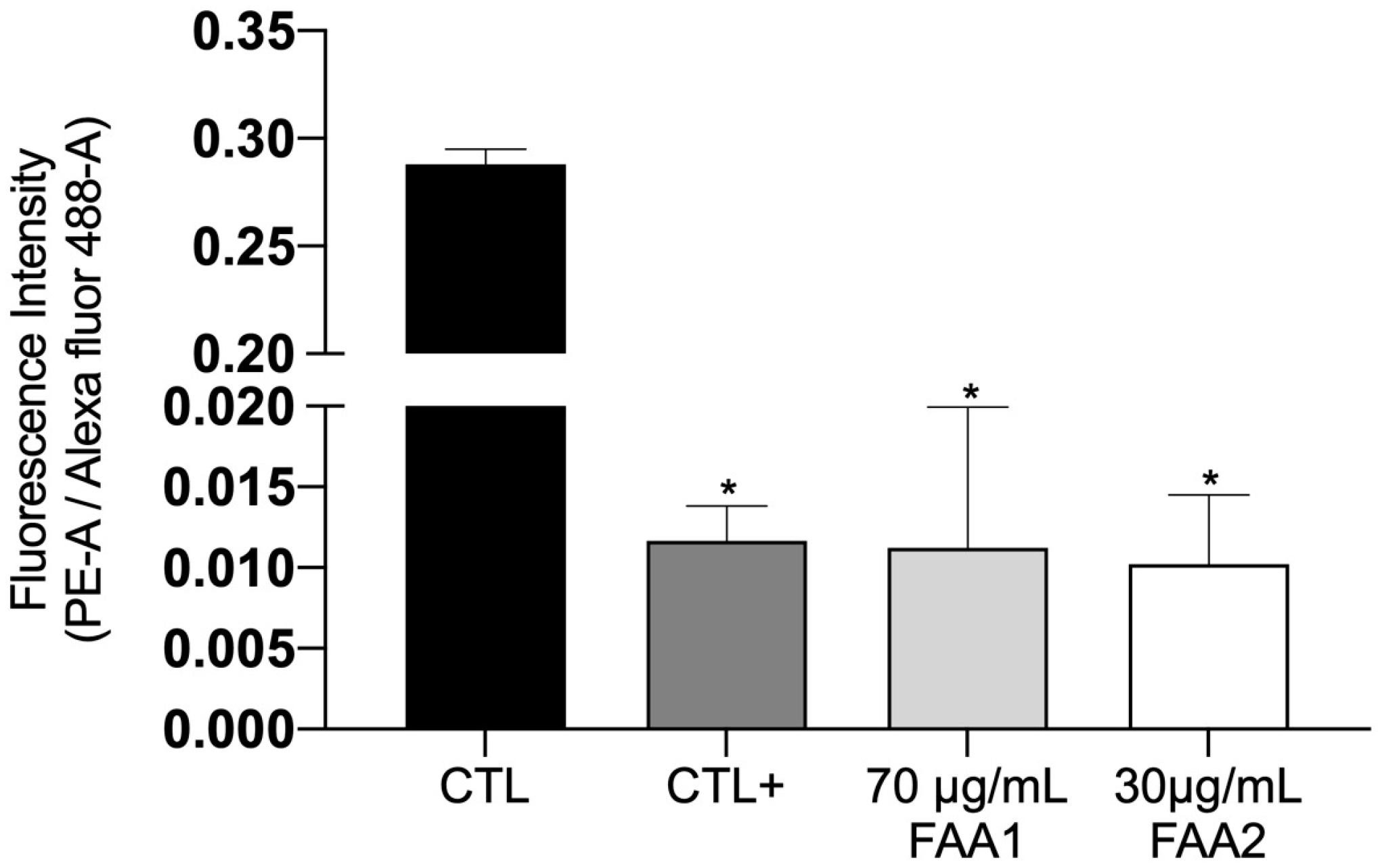
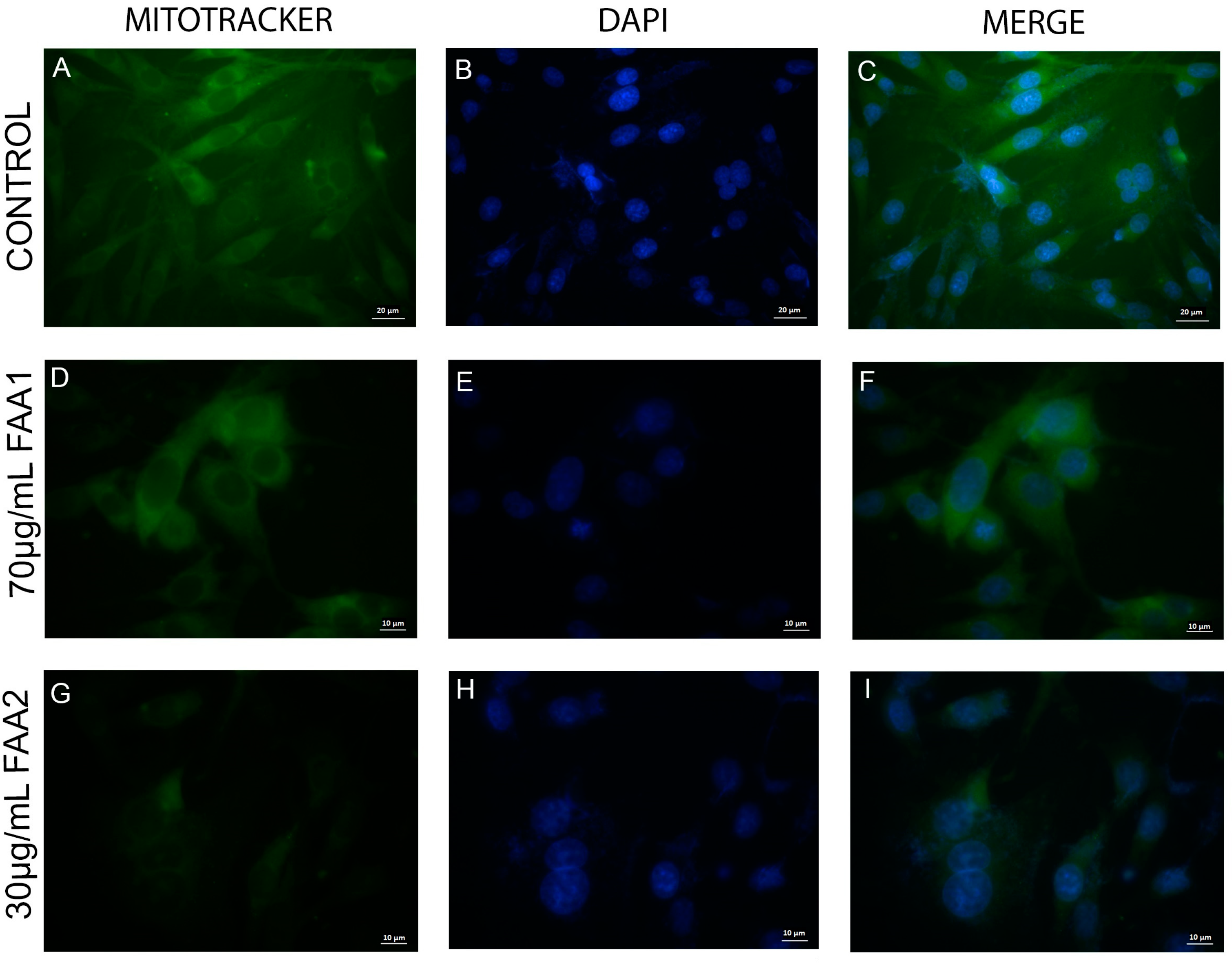
2.4. FAAs Trigger Mitochondrial Integrity (ΔΨm) Damage in Glioma Cells
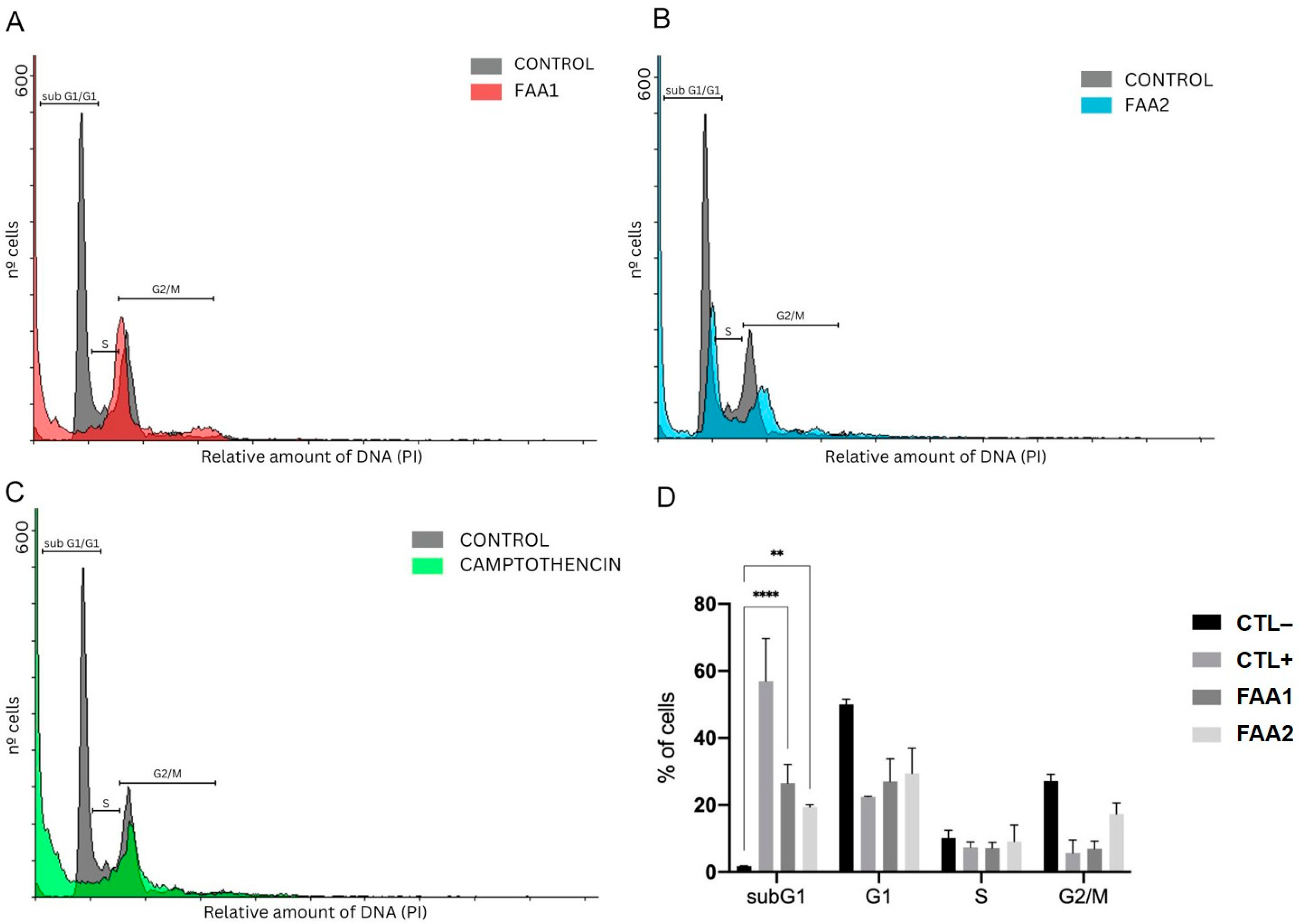
2.5. FAAs Cell Cycle Analysis
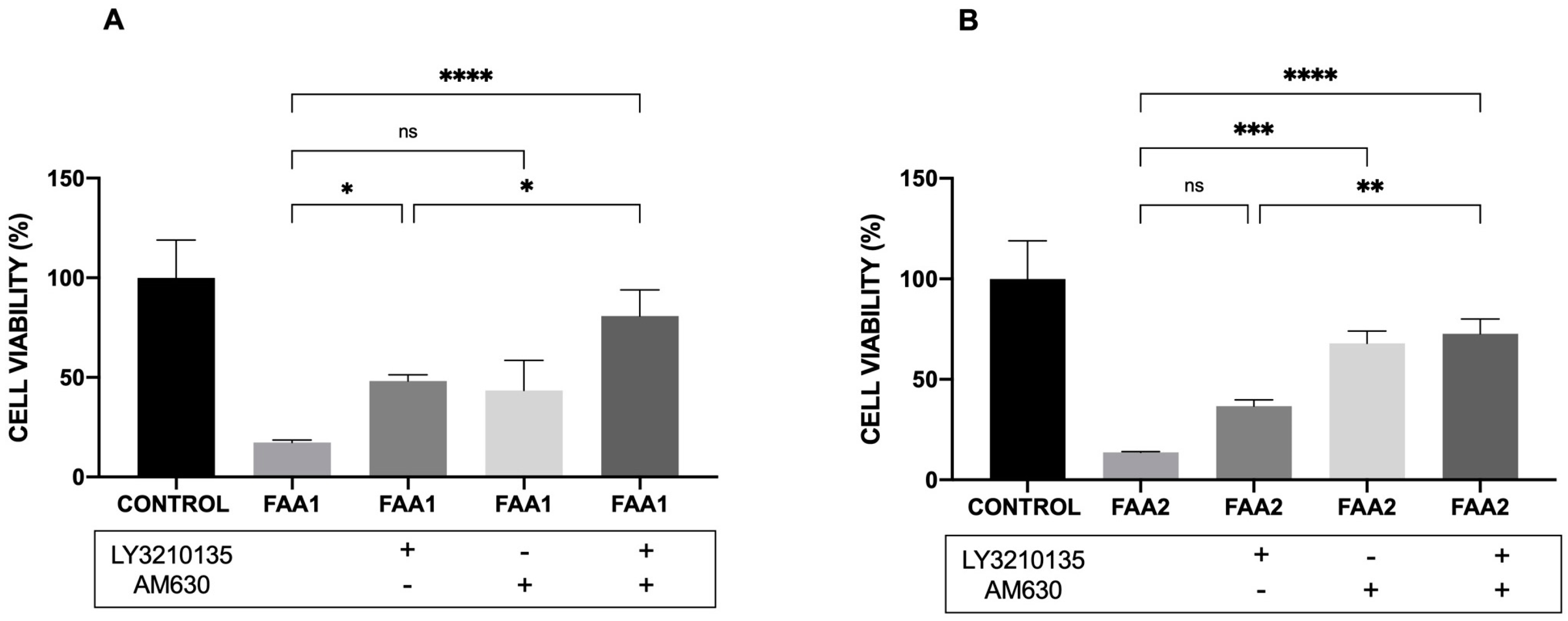
2.6. CB1 and CB2 Antagonist Analysis
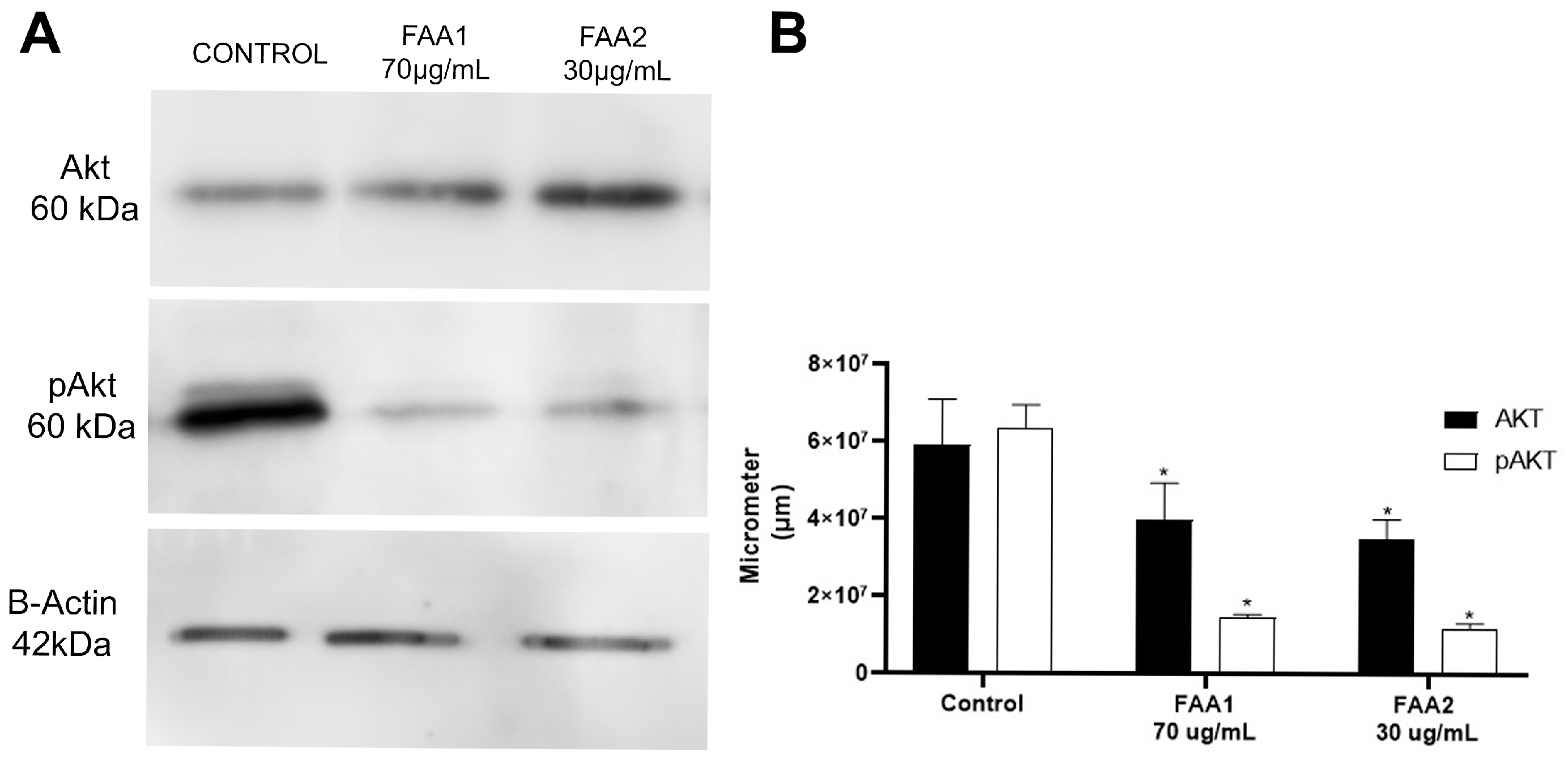
2.7. FAA1 and FAA2 Induce Apoptosis in Glioma Cells through Akt Signalling Pathway Suppression
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Transesterification of AO1 and AO2
4.3. Process for Obtaining Fatty Ethanolamides by Lipase from Candida Antarctica-B (CAL-B)
4.4. Analysis
4.4.1. GC-MS Analysis
4.4.2. Nuclear Magnetic Resonance (NMR) Analysis (1H and 13C)
4.5. Cell Viability Assay
4.6. Apoptosis Assay
4.7. Analysis of Mitochondrial Transmembrane Potential (ΔΨm)
4.8. Mitochondrial Labelling
4.9. Cell Cycle Analysis
4.10. Western Blot Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davis, M.E. Glioblastoma: Overview of Disease and Treatment. Clin. J. Oncol. Nurs. 2016, 20, S2–S8. [Google Scholar] [CrossRef] [PubMed]
- De Souza, R.M.; Shaweis, H.; Han, C.; Sivasubramiam, V.; Brazil, L.; Beaney, R.; Sadler, G.; Al-Sarraj, S.; Hampton, T.; Logan, J.; et al. Has the Survival of Patients with Glioblastoma Changed over the Years? Br. J. Cancer 2016, 114, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Silantyev, A.S.; Falzone, L.; Libra, M.; Gurina, O.I.; Kardashova, K.S.; Nikolouzakis, T.K.; Nosyrev, A.E.; Sutton, C.W.; Mitsias, P.D.; Tsatsakis, A. Current and Future Trends on Diagnosis and Prognosis of Glioblastoma: From Molecular Biology to Proteomics. Cells 2019, 8, 863. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Filho, A.; Piñeros, M.; Soerjomataram, I.; Deltour, I.; Bray, F. Cancers of the Brain and CNS: Global Patterns and Trends in Incidence. Neuro Oncol. 2017, 19, 270–280. [Google Scholar] [CrossRef]
- Yang, K.; Wu, Z.; Zhang, H.; Zhang, N.; Wu, W.; Wang, Z.; Dai, Z.; Zhang, X.; Zhang, L.; Peng, Y.; et al. Glioma Targeted Therapy: Insight into Future of Molecular Approaches. Mol. Cancer 2022, 21, 39. [Google Scholar] [CrossRef] [PubMed]
- Shergalis, A.; Bankhead, A.; Luesakul, U.; Muangsin, N.; Neamati, N. Current Challenges and Opportunities in Treating Glioblastomas. Pharmacol. Rev. 2018, 70, 412–445. [Google Scholar] [CrossRef] [PubMed]
- Vengoji, R.; Macha, M.A.; Batra, S.K.; Shonka, N.A. Natural Products: A Hope for Glioblastoma Patients. Oncotarget 2018, 9, 22199–22224. [Google Scholar] [CrossRef] [PubMed]
- Laezza, C.; Pagano, C.; Navarra, G.; Pastorino, O.; Proto, M.C.; Fiore, D.; Piscopo, C.; Gazzerro, P.; Bifulco, M. The Endocannabinoid System: A Target for Cancer Treatment. Int. J. Mol. Sci. 2020, 21, 747. [Google Scholar] [CrossRef] [PubMed]
- Ellert-Miklaszewska, A.; Ciechomska, A.I.; Kaminska, B. Chapter e11—Cannabinoid signaling in glioma cells and therapeutic implications. In Handbook of Cannabis & Related Pathologies; Academic Press: Cambridge, MA, USA, 2017; pp. e111–e121. [Google Scholar]
- Lowe, H.; Toyang, N.; Steele, B.; Bryant, J.; Ngwa, W. The Endocannabinoid System: A Potential Target for the Treatment of Various Diseases. Int. J. Mol. Sci. 2021, 22, 9472. [Google Scholar] [CrossRef]
- Velasco, G.; Sánchez, C.; Guzmán, M. Endocannabinoids and Cancer. In Handbook of Experimental Pharmacology; Springer: Berlin, Germany, 2015; Volume 231, pp. 449–472. [Google Scholar]
- Chanda, D.; Neumann, D.; Glatz, J.F.C. The Endocannabinoid System: Overview of an Emerging Multi-Faceted Therapeutic Target. Prostaglandins Leukot. Essent. Fat. Acids 2019, 140, 51–56. [Google Scholar] [CrossRef]
- Costas-Insua, C.; Guzmán, M. Endocannabinoid signaling in glioma. Glia 2023, 71, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Han, L.; Zhang, X.; Li, L.; Jiang, C.; Qiu, Y.; Huang, R.; Xie, B.; Lin, Z.; Ren, J.; et al. Alteration of endocannabinoid system in human gliomas. J. Neurosci. 2012, 120, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Bifulco, M.; Laezza, C.; Pisanti, S.; Gazzerro, P. Cannabinoids and Cancer: Pros and Cons of an Antitumour Strategy. Br. J. Pharmacol. 2006, 148, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Fraguas-Sánchez, A.I.; Martín-Sabroso, C.; Torres-Suárez, A.I. Insights into the Effects of the Endocannabinoid System in Cancer: A Review. Br. J. Pharmacol. 2018, 175, 2566–2580. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, R.; Ramer, R.; Hinz, B. Targeting the Endocannabinoid System as a Potential Anticancer Approach. Drug Metab. Rev. 2018, 50, 26–53. [Google Scholar] [CrossRef] [PubMed]
- Tomko, A.; O’Leary, L.; Trask, H.; Achenbach, J.C.; Hall, S.R.; Goralski, K.B.; Ellis, L.D.; Dupré, D.J. Antitumor Activity of Abnormal Cannabidiol and Its Analog O-1602 in Taxol-Resistant Preclinical Models of Breast Cancer. Front. Pharmacol. 2019, 10, 1124. [Google Scholar] [CrossRef] [PubMed]
- Filipiuc, L.E.; Ababei, D.C.; Alexa-Stratulat, T.; Pricope, C.V.; Bild, V.; Stefanescu, R.; Stanciu, G.D.; Tamba, B.I. Major Phytocannabinoids and Their Related Compounds: Should We Only Search for Drugs That Act on Cannabinoid Receptors? Pharmaceutics 2021, 13, 1823. [Google Scholar] [CrossRef] [PubMed]
- De Petrocellis, L.; Melck, D.; Bisogno, T.; Di Marzo, V. Endocannabinoids and fatty acid amides in cancer, inflammation and related disorders. Chem. Phys. Lipids 2000, 108, 191–209. [Google Scholar] [CrossRef]
- Massi, P.; Valenti, M.; Solinas, M.; Parolaro, D. Molecular Mechanisms Involved in the Antitumor Activity of Cannabinoids on Gliomas: Role for Oxidative Stress. Cancers 2010, 2, 1013–1026. [Google Scholar] [CrossRef]
- Pereira da Silva, V.; de Carvalho Brito, L.; Mesquita Marques, A.; da Cunha Camillo, F.; Raquel Figueiredo, M. Bioactive limonoids from Carapa guianensis seeds oil and the sustainable use of its by-products. Curr. Res. Toxicol. 2023, 4, 100104. [Google Scholar] [CrossRef]
- Chamberlin, S.R.; Blucher, A.; Wu, G.; Shinto, L.; Choonoo, G.; Kulesz-Martin, M.; McWeeney, S. Natural Product Target Network Reveals Potential for Cancer Combination Therapies. Front. Pharmacol. 2019, 10, 557. [Google Scholar] [CrossRef]
- Porfírio-Dias, C.L.; Melo, K.M.; Bastos, C.E.M.C.; Ferreira, T.A.A.; Azevedo, L.F.C.; Salgado, H.L.; Santos, A.S.; Rissino, J.D.; Nagamachi, C.Y.; Pieczarka, J.C. Andiroba Oil (Carapa guianensis Aubl) Shows Cytotoxicity but No Mutagenicity in the ACPP02 Gastric Cancer Cell Line. J. Appl. Toxicol. 2020, 40, 1060–1066. [Google Scholar] [CrossRef] [PubMed]
- Melo, K.M.; Oliveira, L.F.S.; da Rocha, R.M.; Ferreira, M.A.P.; Fascineli, M.L.; Milhomem-Paixão, S.S.R.; Grisolia, C.K.; Santos, A.S.; Salgado, H.L.C.; Muehlmann, L.A.; et al. Andiroba Oil and Nanoemulsion (Carapa guianensis Aublet) Reduce Lesion Severity Caused by the Antineoplastic Agent Doxorubicin in Mice. Biomed. Pharmacother. 2021, 138, 111505. [Google Scholar] [CrossRef] [PubMed]
- Gertsch, J. Immunomodulatory Lipids in Plants: Plant Fatty Acid Amides and the Human Endocannabinoid System. Planta Med. 2008, 74, 638–650. [Google Scholar] [CrossRef] [PubMed]
- Cabral, E.C.; Da Cruz, G.F.; Simas, R.C.; Sanvido, G.B.; Gonçalves, L.V.; Leal, R.V.P.; Da Silva, R.C.F.; Da Silva, J.C.T.; Barata, L.E.S.; Da Cunha, V.S.; et al. Typification and Quality Control of the Andiroba (Carapa guianensis) Oil via Mass Spectrometry Fingerprinting. Anal. Methods 2013, 5, 1385–1391. [Google Scholar] [CrossRef]
- Milhomem-Paixão, S.S.R.; Fascineli, M.L.; Roll, M.M.; Longo, J.P.F.; Azevedo, R.B.; Pieczarka, J.C.; Salgado, H.L.C.; Santos, A.; Grisolia, C.K. The Lipidome, Genotoxicity, Hematotoxicity and Antioxidant Properties of Andiroba Oil from the Brazilian Amazon. Genet. Mol. Biol. 2016, 39, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.R.; Rodrigues, K.E.; Hamoy, M.; Sarquis, I.R.; Hamoy, A.O.; Crespo-Lopez, M.E.; Ferreira, I.M.; Macchi, B.M.; do Nascimento, J.L.M. Fatty Acid Amides Synthesized from Andiroba Oil (Carapa guianensis Aublet.) Exhibit Anticonvulsant Action with Modulation on GABA-A Receptor in Mice: A Putative Therapeutic Option. Pharmaceuticals 2020, 13, 43. [Google Scholar] [CrossRef]
- Galve-Roperh, I.; Sánchez, C.; Cortés, M.L.; Del Pulgar, T.G.; Izquierdo, M.; Guzmán, M. Anti-Tumoral Action of Cannabinoids: Involvement of Sustained Ceramide Accumulation and Extracellular Signal-Regulated Kinase Activation. Nat. Med. 2000, 6, 313–319. [Google Scholar] [CrossRef]
- Dos Santos, D.S.; Piovesan, L.A.; D’Oca, C.R.M.; Hack, C.R.L.; Treptow, T.G.M.; Rodrigues, M.O.; Vendramini-Costa, D.B.; Ruiz, A.L.T.G.; De Carvalho, J.E.; D’Oca, M.G.M. Antiproliferative Activity of Synthetic Fatty Acid Amides from Renewable Resources. Bioorg. Med. Chem. 2015, 23, 340–347. [Google Scholar] [CrossRef]
- Moreno, E.; Cavic, M.; Krivokuca, A.; Casadó, V.; Canela, E. The Endocannabinoid System as a Target in Cancer Diseases: Are We There Yet? Front. Pharmacol. 2019, 10, 339. [Google Scholar] [CrossRef]
- Ezzili, C.; Otrubova, K.; Boger, D.L. Fatty Acid Amide Signaling Molecules. Bioorg. Med. Chem. Lett. 2010, 20, 5959–5968. [Google Scholar] [CrossRef] [PubMed]
- Maccarrone, M.; Pauselli, R.; Di Rienzo, M.; Finazzi-Agrò, A. Binding, Degradation and Apoptotic Activity of Stearoylethanolamide in Rat C6 Glioma Cells. Biochem. J. 2002, 366, 137–144. [Google Scholar] [CrossRef] [PubMed]
- da Silva, V.P.; Oliveira, R.R.; Figueiredo, M.R. Isolation of Limonoids from Seeds of Carapa guianensis Aublet (Meliaceae) by High-Speed Countercurrent Chromatography. Phytochem. Anal. 2009, 20, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Fowler, C.J.; Jonsson, K.O.; Andersson, A.; Juntunen, J.; Järvinen, T.; Vandevoorde, S.; Lambert, D.M.; Jerman, J.C.; Smart, D. Inhibition of C6 Glioma Cell Proliferation by Anandamide, 1-Arachidonoylglycerol, and by a Water Soluble Phosphate Ester of Anandamide: Variability in Response and Involvement of Arachidonic Acid. Biochem. Pharmacol. 2003, 66, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Torres-Román, A.L.; García-Hernández, V.M.; Rangel-López, E.; Ruiz-García, E.; Meneses-García, A.; Santamaría, A.; Aschner, M.; Prospero-García, O.; Ortega-Gómez, A. Oleamide Induces Cell Death in Glioblastoma RG2 Cells by a Cannabinoid Receptor–Independent Mechanism. Neurotox. Res. 2020, 38, 941–956. [Google Scholar] [CrossRef] [PubMed]
- Ellert-Miklaszewska, A.; Ciechomska, I.A.; Kaminska, B. Synthetic Cannabinoids Induce Autophagy and Mitochondrial Apoptotic Pathways in Human Glioblastoma Cells Independently of Deficiency in TP53 or Pten Tumor Suppressors. Cancers 2021, 13, 419. [Google Scholar] [CrossRef] [PubMed]
- Ciaglia, E.; Torelli, G.; Pisanti, S.; Picardi, P.; D’Alessandro, A.; Laezza, C.; Malfitano, A.M.; Fiore, D.; Pagano Zottola, A.C.; Proto, M.C.; et al. Cannabinoid Receptor CB1 Regulates STAT3 Activity and Its Expression Dictates the Responsiveness to SR141716 Treatment in Human Glioma Patients’ Cells. Oncotarget 2015, 6, 15464–15481. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.; Bashi, S.; Zali, A. The Expression Level of Cannabinoid Receptors Type 1 and 2 in the Different Types of Astrocytomas. Mol. Biol. Rep. 2020, 47, 5461–5467. [Google Scholar] [CrossRef] [PubMed]
- Nabissi, M.; Morelli, M.B.; Santoni, M.; Santoni, G. Triggering of the TRPV2 Channel by Cannabidiol Sensitizes Glioblastoma Cells to Cytotoxic Chemotherapeutic Agents. Carcinogenesis 2013, 34, 48–57. [Google Scholar] [CrossRef]
- Contassot, E.; Wilmotte, R.; Tenan, M.; Belkouch, M.C.; Schnüriger, V.; de Tribolet, N.; Burkhardt, K.; Dietrich, P.Y. Arachidonylethanolamide induces apoptosis of human glioma cells through vanilloid receptor-1. J. Neuropathol. Exp. Neurol. 2004, 63, 956–963. [Google Scholar] [CrossRef]
- Wu, H.Y.; Huang, C.H.; Lin, Y.H.; Wang, C.C.; Jan, T.R. Cannabidiol Induced Apoptosis in Human Monocytes through Mitochondrial Permeability Transition Pore-Mediated ROS Production. Free Radic. Biol. Med. 2018, 124, 311–318. [Google Scholar] [CrossRef]
- Ellert-Miklaszewska, A.; Kaminska, B.; Konarska, L. Cannabinoids Down-Regulate PI3K/Akt and Erk Signalling Pathways and Activate Proapoptotic Function of Bad Protein. Cell Signal 2005, 17, 25–37. [Google Scholar] [CrossRef]
- Gross, C.; Ramirez, D.A.; McGrath, S.; Gustafson, D.L. Cannabidiol Induces Apoptosis and Perturbs Mitochondrial Function in Human and Canine Glioma Cells. Front. Pharmacol. 2021, 12, 725136. [Google Scholar] [CrossRef]
- Hinz, B.; Ramer, R. Anti-Tumour Actions of Cannabinoids. Br. J. Pharmacol. 2019, 176, 1384–1394. [Google Scholar] [CrossRef]
- Maués, L.A.L.; Alves, G.M.; Couto, N.M.G.; da Silva, B.J.M.; Arruda, M.S.P.; Macchi, B.M.; Sena, C.B.C.; Prado, A.F.; Crespo-Lopez, M.E.; Silva, E.O.; et al. Flavonoids from the Amazon Plant Brosimum Acutifolium Induce C6 Glioma Cell Line Apoptosis by Disrupting Mitochondrial Membrane Potential and Reducing AKT Phosphorylation. Biomed. Pharmacother. 2019, 113, 108728. [Google Scholar] [CrossRef]
- Vrechi, T.A.M.; Leão, A.H.F.F.; Morais, I.B.M.; Abílio, V.C.; Zuardi, A.W.; Hallak, J.E.C.; Crippa, J.A.; Bincoletto, C.; Ureshino, R.P.; Smaili, S.S.; et al. Cannabidiol Induces Autophagy via ERK1/2 Activation in Neural Cells. Sci. Rep. 2021, 11, 5434. [Google Scholar] [CrossRef]
- Shinkai, V.M.T.; Sampaio, I.M.O.; Santos, E.G.; Galué-Parra, A.J.; Ferreira, D.P.; Baliza, D.D.M.S.; Ramos, N.F.; Pimenta, R.S.; Burbano, R.M.R.; Sena, C.B.C.; et al. In Vitro Cytotoxic Effects and Mechanisms of Action of Eleutherine Isolated from Eleutherine plicata Bulb in Rat Glioma C6 Cells. Molecules 2022, 27, 8850. [Google Scholar] [CrossRef]
- Malfitano, A.M.; Ciaglia, E.; Gangemi, G.; Gazzerro, P.; Laezza, C.; Bifulco, M. Update on the endocannabinoid system as an anticancer target. Expert Opin. Ther. Targets 2011, 15, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Gousias, K.; Theocharous, T.; Simon, M. Mechanisms of Cell Cycle Arrest and Apoptosis in Glioblastoma. Biomedicines 2022, 10, 564. [Google Scholar] [CrossRef]
- Dumitru, C.A.; Sandalcioglu, E.; Karsak, M. Cannabinoids in Glioblastoma Therapy: New Applications for Old Drugs. Front. Mol. Neurosci. 2018, 11, 159. [Google Scholar] [CrossRef]
- Di Marzo, V.; Piscitelli, F. The Endocannabinoid System and its Modulation by Phytocannabinoids. Neurotherapeutics 2015, 12, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Khoo, N.K.H.; Hebbar, S.; Zhao, W.; Moore, S.A.; Domann, F.E.; Robbins, M.E. Differential Activation of Catalase Expression and Activity by PPAR Agonists: Implications for Astrocyte Protection in Anti-Glioma Therapy. Redox. Biol. 2013, 1, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Chinigò, G.; Castel, H.; Chever, O.; Gkika, D. TRP Channels in Brain Tumors. Front. Cell Dev. Biol. 2021, 9, 617801. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, N.M.d.; Lopes, I.C.S.; Galué-Parra, A.J.; Ferreira, I.M.; Sena, C.B.C.d.; Silva, E.O.d.; Macchi, B.d.M.; Oliveira, F.R.d.; do Nascimento, J.L.M. Fatty Acid Amides Suppress Proliferation via Cannabinoid Receptors and Promote the Apoptosis of C6 Glioma Cells in Association with Akt Signaling Pathway Inhibition. Pharmaceuticals 2024, 17, 873. https://doi.org/10.3390/ph17070873
Silva NMd, Lopes ICS, Galué-Parra AJ, Ferreira IM, Sena CBCd, Silva EOd, Macchi BdM, Oliveira FRd, do Nascimento JLM. Fatty Acid Amides Suppress Proliferation via Cannabinoid Receptors and Promote the Apoptosis of C6 Glioma Cells in Association with Akt Signaling Pathway Inhibition. Pharmaceuticals. 2024; 17(7):873. https://doi.org/10.3390/ph17070873
Chicago/Turabian StyleSilva, Nágila Monteiro da, Izabella Carla Silva Lopes, Adan Jesus Galué-Parra, Irlon Maciel Ferreira, Chubert Bernardo Castro de Sena, Edilene Oliveira da Silva, Barbarella de Matos Macchi, Fábio Rodrigues de Oliveira, and José Luiz Martins do Nascimento. 2024. "Fatty Acid Amides Suppress Proliferation via Cannabinoid Receptors and Promote the Apoptosis of C6 Glioma Cells in Association with Akt Signaling Pathway Inhibition" Pharmaceuticals 17, no. 7: 873. https://doi.org/10.3390/ph17070873
APA StyleSilva, N. M. d., Lopes, I. C. S., Galué-Parra, A. J., Ferreira, I. M., Sena, C. B. C. d., Silva, E. O. d., Macchi, B. d. M., Oliveira, F. R. d., & do Nascimento, J. L. M. (2024). Fatty Acid Amides Suppress Proliferation via Cannabinoid Receptors and Promote the Apoptosis of C6 Glioma Cells in Association with Akt Signaling Pathway Inhibition. Pharmaceuticals, 17(7), 873. https://doi.org/10.3390/ph17070873






