Putative Pharmacological Depression and Anxiety-Related Targets of Calcitriol Explored by Network Pharmacology and Molecular Docking
Abstract
:1. Introduction
2. Results
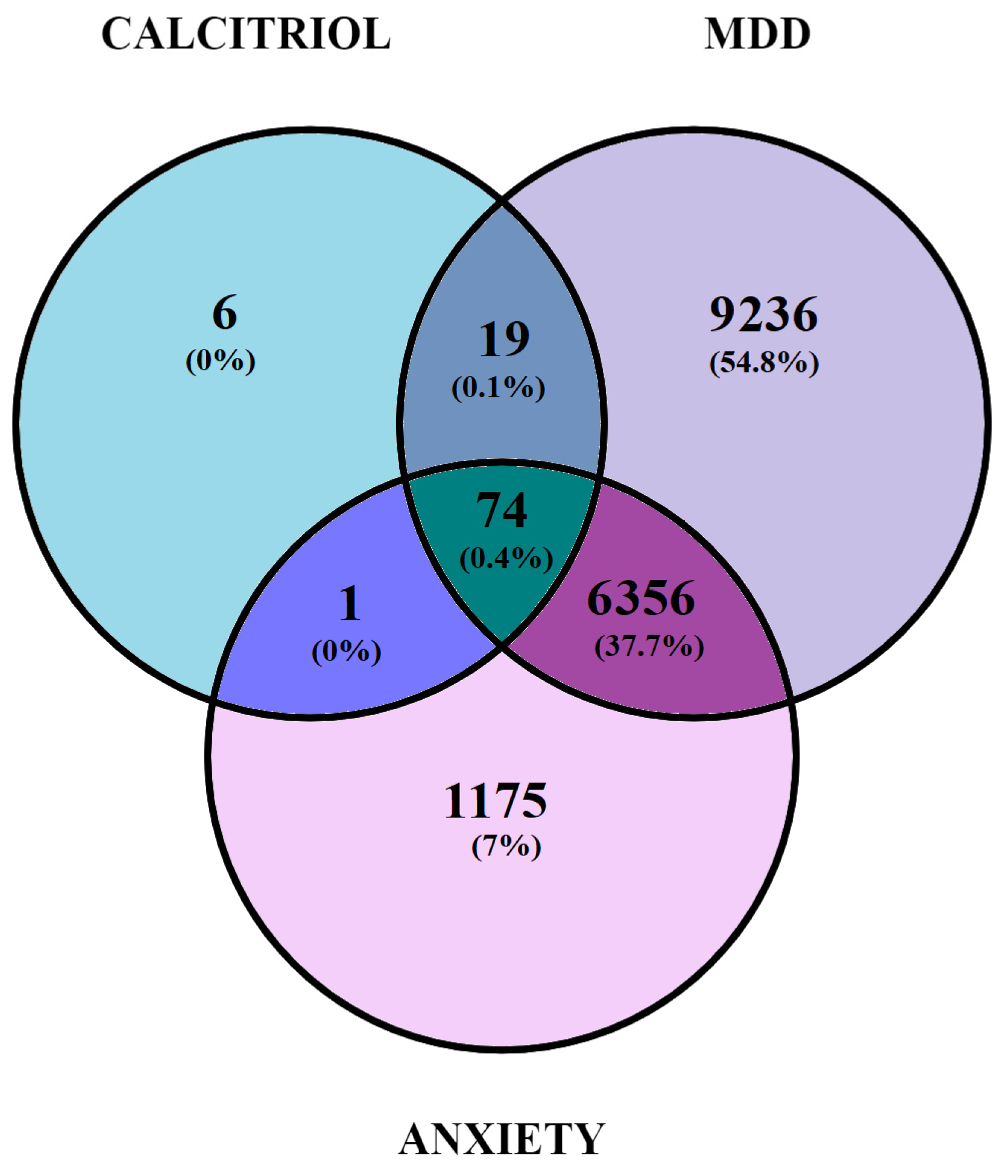
2.1. Identification and Gene Overlap between Potential Therapeutic Targets of Calcitriol and Genes Related to Depression and/or Anxiety
2.1.1. Elaboration of Pharmacological Networks of Calcitriol, Depression, and Anxiety-Overlapping Genes
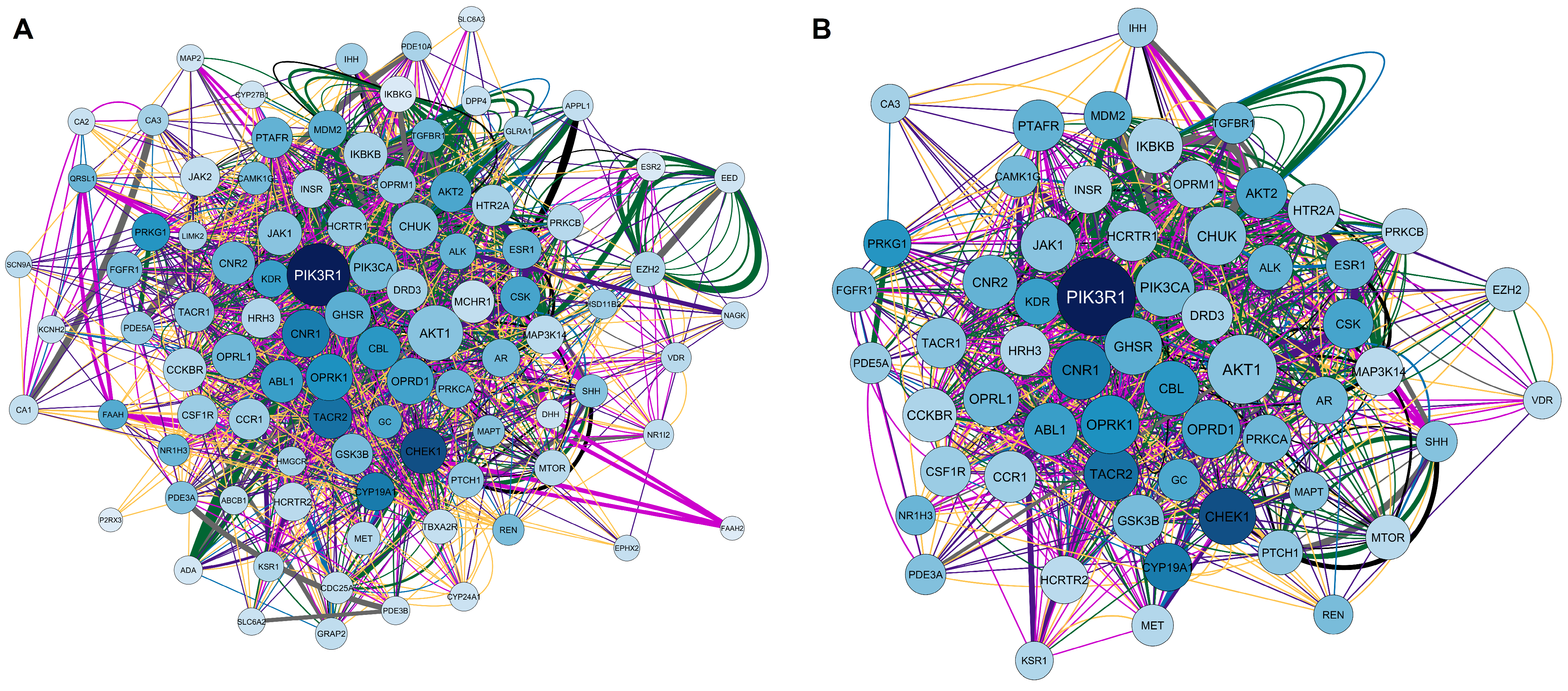
2.1.2. Elaboration of Pharmacological Networks of Genes Overlapping for Calcitriol and Depression
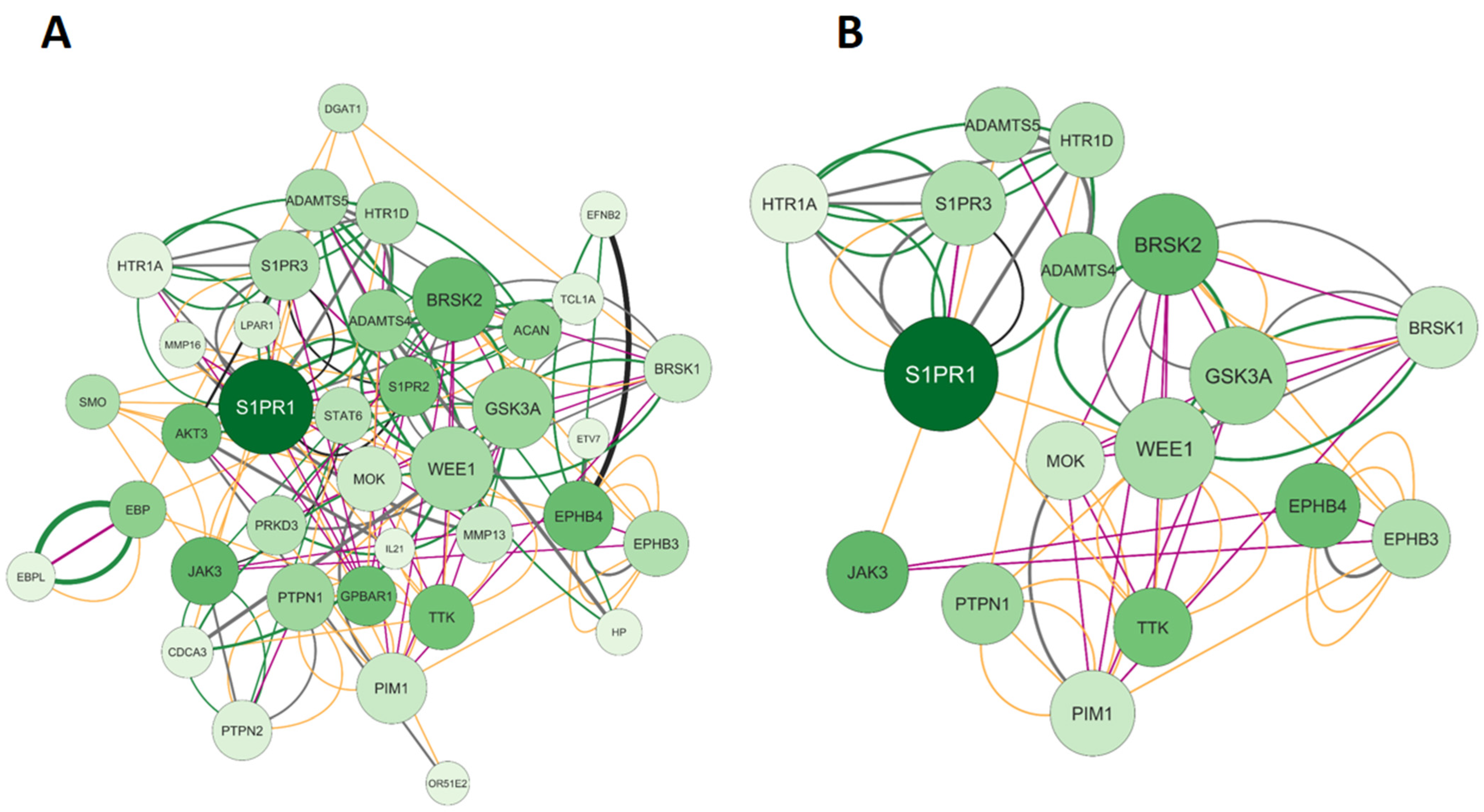
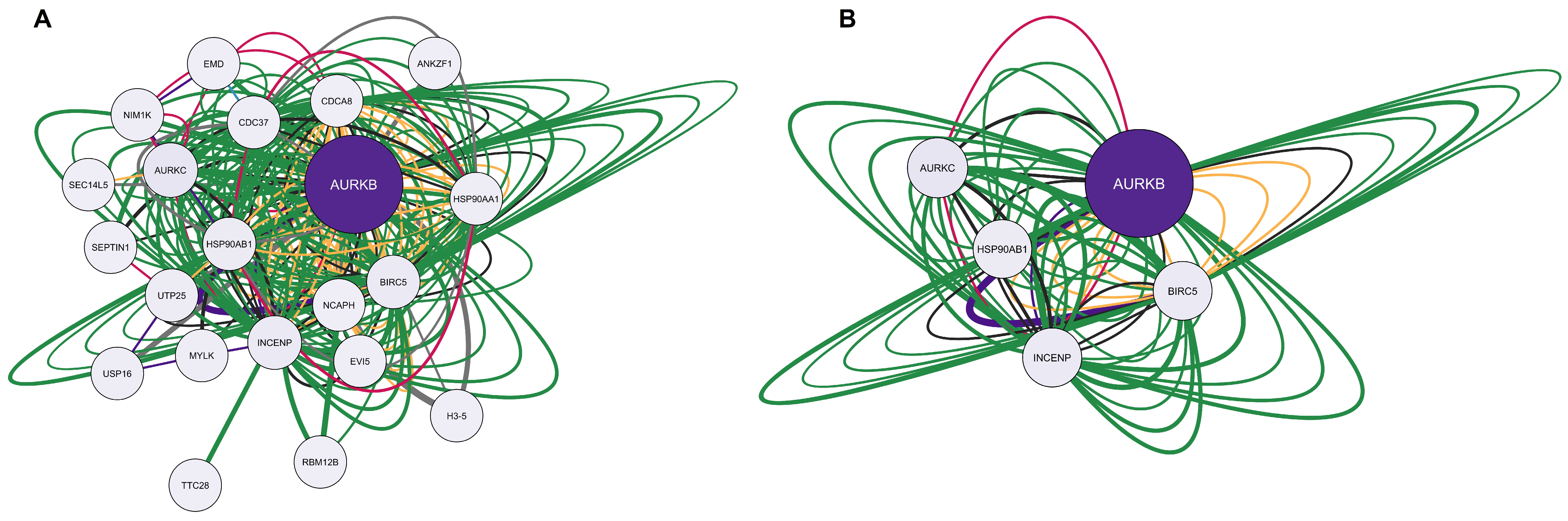
2.1.3. Elaboration of Pharmacological Networks of Genes Overlapping Calcitriol and Anxiety
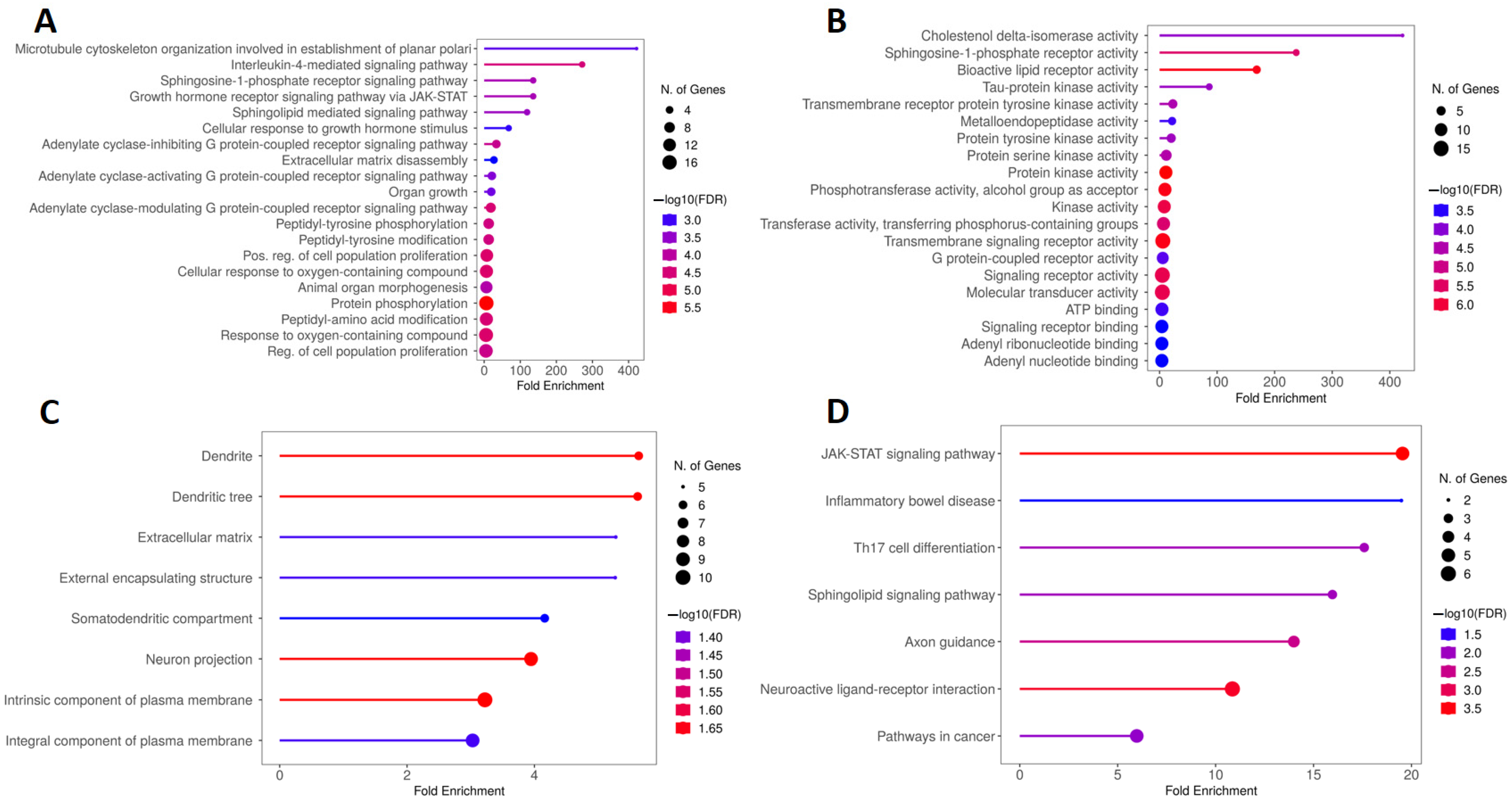
2.2. Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) Enrichment Analysis of Genes Overlapping Calcitriol, Depression and Anxiety
2.3. KEGG and GO Enrichment Analysis of Genes Overlapping Calcitriol and Depression
2.4. KEGG and GO Enrichment Analysis of Genes Overlapping Calcitriol and Anxiety
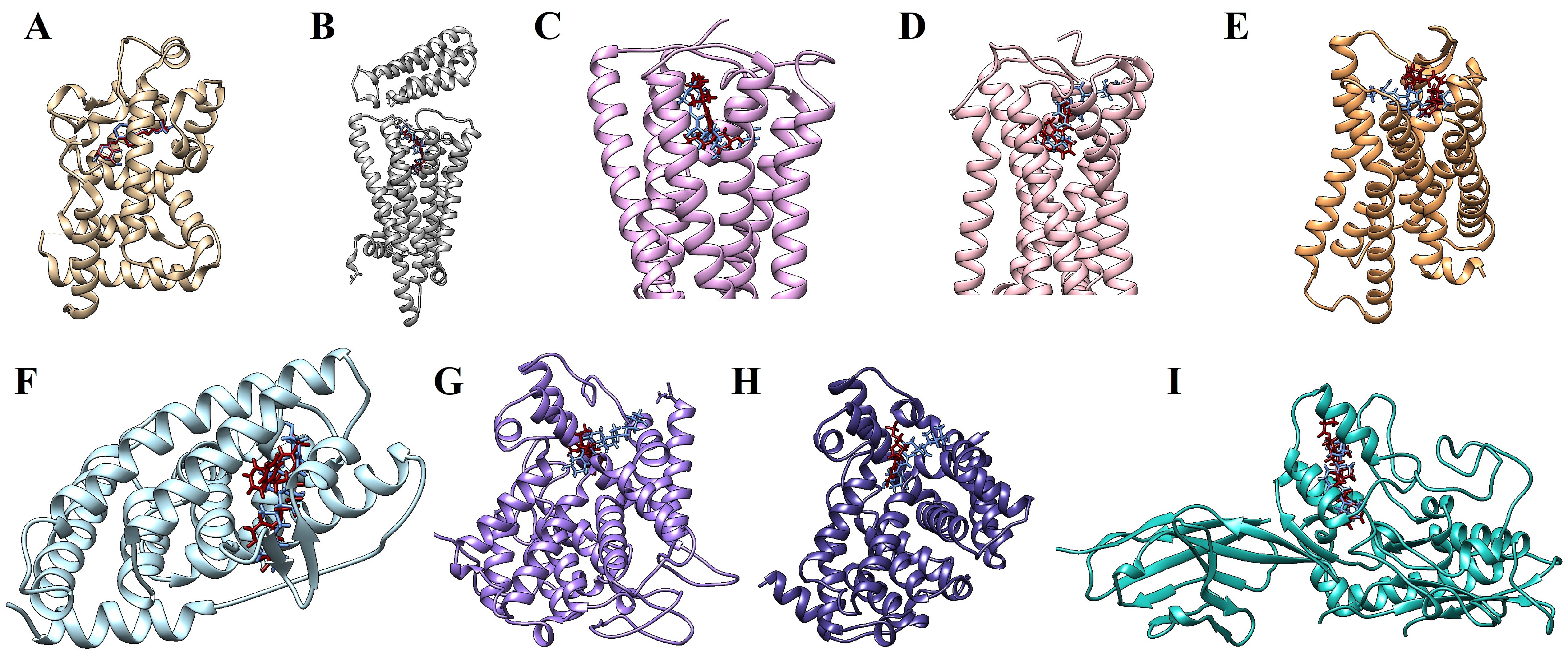
2.5. Molecular Docking Simulations
2.6. Bioinformatic Validation
3. Discussion
4. Materials and Methods
4.1. Prediction of Gene Pharmacological Potentials Modulated by Calcitriol in Depression and Anxiety
4.2. Gene Interaction Network Construction and Analysis
4.3. Network Enrichment Analysis
4.4. Molecular Docking Simulations
4.5. Bioinformatic Validation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Craske, M.G.; Stein, M.B. Anxiety. Lancet 2016, 388, 3048–3059. [Google Scholar] [CrossRef] [PubMed]
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- De Mello, A.J.; Moretti, M.; Rodrigues, A.L.S. SARS-CoV-2 Consequences for Mental Health: Neuroinflammatory Pathways Linking COVID-19 to Anxiety and Depression. World J. Psychiatry 2022, 12, 874–883. [Google Scholar] [CrossRef]
- Kaufman, J.; Charney, D. Comorbidity of Mood and Anxiety Disorders. Depress. Anxiety 2000, 12, 69–76. [Google Scholar] [CrossRef]
- Gorman, J.M. Comorbid Depression and Anxiety Spectrum Disorders. Depress. Anxiety 1996, 4, 160–168. [Google Scholar] [CrossRef]
- Otte, C.; Gold, S.M.; Penninx, B.W.; Pariante, C.M.; Etkin, A.; Fava, M.; Mohr, D.C.; Schatzberg, A.F. Major Depressive Disorder. Nat. Rev. Dis. Primers 2016, 2, 16065. [Google Scholar] [CrossRef]
- Casseb, G.A.S.; Kaster, M.P.; Rodrigues, A.L.S. Potential Role of Vitamin D for the Management of Depression and Anxiety. CNS Drugs 2019, 33, 619–637. [Google Scholar] [CrossRef] [PubMed]
- Alshahrani, F.; Aljohani, N. Vitamin D: Deficiency, Sufficiency and Toxicity. Nutrients 2013, 5, 3605–3616. [Google Scholar] [CrossRef]
- Mavar, M.; Sorić, T.; Bagarić, E.; Sarić, A.; Matek Sarić, M. The Power of Vitamin D: Is the Future in Precision Nutrition through Personalized Supplementation Plans? Nutrients 2024, 16, 1176. [Google Scholar] [CrossRef]
- Cui, X.; Eyles, D.W. Vitamin D and the Central Nervous System: Causative and Preventative Mechanisms in Brain Disorders. Nutrients 2022, 14, 4353. [Google Scholar] [CrossRef]
- Kouba, B.R.; Torrá, A.C.N.C.; Camargo, A.; Rodrigues, A.L.S. The Antidepressant-like Effect Elicited by Vitamin D3 Is Associated with BDNF/TrkB-Related Synaptic Protein Synthesis. Metab. Brain Dis. 2023, 38, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.; Dawson-Hughes, B. Seasonal Mood Changes in 250 Normal Women. Psychiatry Res. 1993, 49, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Parker, G.B.; Brotchie, H.; Graham, R.K. Vitamin D and Depression. J. Affect. Disord. 2017, 208, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Wirz-Justice, A. Seasonality in Affective Disorders. Gen. Comp. Endocrinol. 2018, 258, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Gooch, H.; Petty, A.; McGrath, J.J.; Eyles, D. Vitamin D and the Brain: Genomic and Non-Genomic Actions. Mol. Cell. Endocrinol. 2017, 453, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef]
- Fedotova, J.O. Vitamin D3 Treatment Differentially Affects Anxiety-like Behavior in the Old Ovariectomized Female Rats and Old Ovariectomized Female Rats Treated with Low Dose of 17β-Estradiol. BMC Med. Genet. 2019, 20, 49. [Google Scholar] [CrossRef] [PubMed]
- Koshkina, A.; Dudnichenko, T.; Baranenko, D.; Fedotova, J.; Drago, F. Effects of Vitamin D3 in Long-Term Ovariectomized Rats Subjected to Chronic Unpredictable Mild Stress: BDNF, NT-3, and NT-4 Implications. Nutrients 2019, 11, 1726. [Google Scholar] [CrossRef]
- Camargo, A.; Dalmagro, A.P.; Platt, N.; Rosado, A.F.; Neis, V.B.; Zeni, A.L.B.; Kaster, M.P.; Rodrigues, A.L.S. Cholecalciferol Abolishes Depressive-like Behavior and Hippocampal Glucocorticoid Receptor Impairment Induced by Chronic Corticosterone Administration in Mice. Pharmacol. Biochem. Behav. 2020, 196, 172971. [Google Scholar] [CrossRef]
- Kazemi, F.; Babri, S.; Keyhanmehr, P.; Farid-Habibi, M.; Rad, S.N.; Farajdokht, F. Maternal Vitamin D Supplementation and Treadmill Exercise Attenuated Vitamin D Deficiency-Induced Anxiety-and Depressive-like Behaviors in Adult Male Offspring Rats. Nutr. Neurosci. 2023, 26, 470–482. [Google Scholar] [CrossRef]
- Guo, Y.-X.; He, L.-Y.; Zhang, M.; Wang, F.; Liu, F.; Peng, W.-X. 1,25-Dihydroxyvitamin D3 Regulates Expression of LRP1 and RAGE in Vitro and in Vivo, Enhancing Aβ1–40 Brain-to-Blood Efflux and Peripheral Uptake Transport. Neuroscience 2016, 322, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Carmeliet, G.; Verlinden, L.; Van Etten, E.; Verstuyf, A.; Luderer, H.F.; Lieben, L.; Mathieu, C.; Demay, M. Vitamin D and Human Health: Lessons from Vitamin D Receptor Null Mice. Endocr. Rev. 2008, 29, 726–776. [Google Scholar] [CrossRef] [PubMed]
- Lasoń, W.; Jantas, D.; Leśkiewicz, M.; Regulska, M.; Basta-Kaim, A. The Vitamin D Receptor as a Potential Target for the Treatment of Age-Related Neurodegenerative Diseases Such as Alzheimer’s and Parkinson’s Diseases: A Narrative Review. Cells 2023, 12, 660. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Zhang, L.-H.; Cai, H.-L.; Li, H.-D.; Liu, Y.-P.; Tang, M.-M.; Dang, R.-L.; Zhu, W.-Y.; Xue, Y.; He, X. Neurochemical Effects of Chronic Administration of Calcitriol in Rats. Nutrients 2014, 6, 6048–6059. [Google Scholar] [CrossRef] [PubMed]
- Sabir, M.S.; Haussler, M.R.; Mallick, S.; Kaneko, I.; Lucas, D.A.; Haussler, C.A.; Whitfield, G.K.; Jurutka, P.W. Optimal Vitamin D Spurs Serotonin: 1,25-Dihydroxyvitamin D Represses Serotonin Reuptake Transport (SERT) and Degradation (MAO-A) Gene Expression in Cultured Rat Serotonergic Neuronal Cell Lines. Genes Nutr. 2018, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Naveilhan, P.; Neveu, I.; Wion, D.; Brachet, P. 1,25-Dihydroxyvitamin D3, an Inducer of Glial Cell Line-Derived Neurotrophic Factor. NeuroReport 1996, 7, 2171–2175. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, H.A.; Rasouli, J.; Ciric, B.; Rostami, A.; Zhang, G.-X. 1,25-Dihydroxyvitamin D3 Enhances Neural Stem Cell Proliferation and Oligodendrocyte Differentiation. Exp. Mol. Pathol. 2015, 98, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Niu, Z.; Xue, Y.; Gao, J.; Zhang, M.; Li, M.; Peng, Y.; Zhang, S.; Li, W.; Zhang, Q.; et al. Chronic Vitamin D3 Supplementation Alleviates Cognition Impairment via Inhibition of Oxidative Stress Regulated by PI3K/AKT/Nrf2 in APP/PS1 Transgenic Mice. Neurosci. Lett. 2022, 783, 136725. [Google Scholar] [CrossRef]
- Calvello, R.; Cianciulli, A.; Nicolardi, G.; De Nuccio, F.; Giannotti, L.; Salvatore, R.; Porro, C.; Trotta, T.; Panaro, M.A.; Lofrumento, D.D. Vitamin D Treatment Attenuates Neuroinflammation and Dopaminergic Neurodegeneration in an Animal Model of Parkinson’s Disease, Shifting M1 to M2 Microglia Responses. J. Neuroimmune Pharmacol. 2017, 12, 327–339. [Google Scholar] [CrossRef]
- Usategui-Martín, R.; De Luis-Román, D.-A.; Fernández-Gómez, J.M.; Ruiz-Mambrilla, M.; Pérez-Castrillón, J.-L. Vitamin D Receptor (VDR) Gene Polymorphisms Modify the Response to Vitamin D Supplementation: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 360. [Google Scholar] [CrossRef]
- Sun, D.; Song, M.; Zeng, C.; Chen, H.; Zhang, J.; Liu, F.; Luo, S.; Liao, Q.; Xiao, Y.; Xu, W.; et al. Associations of Vitamin D-Related Single Nucleotide Polymorphisms with Post-Stroke Depression among Ischemic Stroke Population. Front. Psychiatry 2023, 14, 1148047. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Sabião, T.; Alves De Menezes-Júnior, L.A.; Batista, A.P.; Silva De Moura, S.; Meireles, A.L.; Carvalho De Menezes, M.; Lins Machado-Coelho, G.L.; Cardoso Carraro, J.C. Interaction between Fokl Polymorphism and Vitamin D Deficiency in the Symptoms of Mental Disorders in Adults: A Population-Based Study. Sci. Rep. 2024, 14, 6925. [Google Scholar] [CrossRef]
- Kalueff, A.; Lou, Y.; Laaksi, I.; Tuohimaa, P. Increased Grooming Behavior in Mice Lacking Vitamin D Receptors. Physiol. Behav. 2004, 82, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Zhang, W.-Y.; Li, H.-D.; Cai, H.-L.; Liu, Y.-P.; Chen, L.-Y. Stress and Vitamin D: Altered Vitamin D Metabolism in Both the Hippocampus and Myocardium of Chronic Unpredictable Mild Stress Exposed Rats. Psychoneuroendocrinology 2013, 38, 2091–2098. [Google Scholar] [CrossRef]
- He, Y.; Wu, Z.; Lan, T.; Wang, Y.; Tian, Y.; Chen, X.; Li, Y.; Bai, M.; Liu, J.; Gong, X.; et al. The 25(OH)D/VDR Signaling May Play a Role in Major Depression. Biochem. Biophys. Res. Commun. 2020, 523, 405–410. [Google Scholar] [CrossRef]
- Iida, T.; Yoshikawa, T.; Kárpáti, A.; Matsuzawa, T.; Kitano, H.; Mogi, A.; Harada, R.; Naganuma, F.; Nakamura, T.; Yanai, K. JNJ10181457, a Histamine H3 Receptor Inverse Agonist, Regulates in Vivo Microglial Functions and Improves Depression-like Behaviours in Mice. Biochem. Biophys. Res. Commun. 2017, 488, 534–540. [Google Scholar] [CrossRef]
- Alhusaini, M.; Eissa, N.; Saad, A.K.; Beiram, R.; Sadek, B. Revisiting Preclinical Observations of Several Histamine H3 Receptor Antagonists/Inverse Agonists in Cognitive Impairment, Anxiety, Depression, and Sleep–Wake Cycle Disorder. Front. Pharmacol. 2022, 13, 861094. [Google Scholar] [CrossRef]
- Gao, Z.; Hurst, W.J.; Czechtizky, W.; Hall, D.; Moindrot, N.; Nagorny, R.; Pichat, P.; Stefany, D.; Hendrix, J.A.; George, P.G. Identification and Profiling of 3,5-Dimethyl-Isoxazole-4-Carboxylic Acid [2-Methyl-4-((2S,3′S)-2-Methyl-[1,3′]Bipyrrolidinyl-1′-Yl)Phenyl] Amide as Histamine H3 Receptor Antagonist for the Treatment of Depression. Bioorg. Med. Chem. Lett. 2013, 23, 6269–6273. [Google Scholar] [CrossRef]
- Sadek, B.S.; Bahi, A.; Schwed, J.S.; Walter, M.; Stark, H. Anxiolytic and Antidepressant-like Activities of the Novel and Potent Non-Imidazole Histamine H3 Receptor Antagonist ST-1283. Drug Des. Dev. Ther. 2014, 8, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Soliani, A.; Kubota, S.M.; Corrêa, M.F.; Cerutti, S.M.; Fernandes, J.P.S. Differential Contribution of H3R Antagonism by LINS01 Compounds on Memory, Anxiety-like Behaviour and Spontaneous Locomotor Activity in Healthy Rats. Behav. Brain Res. 2020, 377, 112230. [Google Scholar] [CrossRef]
- Venkatachalam, K.; Zhong, S.; Dubiel, M.; Satała, G.; Sadek, B.; Stark, H. The Novel Pimavanserin Derivative ST-2300 with Histamine H3 Receptor Affinity Shows Reduced 5-HT2A Binding, but Maintains Antidepressant- and Anxiolytic-like Properties in Mice. Biomolecules 2022, 12, 683. [Google Scholar] [CrossRef] [PubMed]
- Rizk, A.; Curley, J.; Robertson, J.; Raber, J. Anxiety and Cognition in Histamine H3 Receptor-/- Mice. Eur. J. Neurosci. 2004, 19, 1992–1996. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, A.; Yoshikawa, T.; Miura, Y.; Nakamura, T.; Naganuma, F.; Shibuya, K.; Iida, T.; Harada, R.; Okamura, N.; Watanabe, T.; et al. Mechanism of the Histamine H3 Receptor-Mediated Increase in Exploratory Locomotor Activity and Anxiety-like Behaviours in Mice. Neuropharmacology 2014, 81, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.S.; Schiweck, C.; Arteaga-Henríquez, G.; Poletti, S.; Haarman, B.C.M.; Dik, W.A.; Schwarz, M.; Vrieze, E.; Mikova, O.; Joergens, S.; et al. Monocyte Mitochondrial Dysfunction, Inflammaging, and Inflammatory Pyroptosis in Major Depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 111, 110391. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhou, C.; Li, J.; Chen, Z.; Shi, H.; Yang, W.; Qin, Y.; Lü, L.; Zhao, L.; Fang, L.; et al. Quantitative Proteomic Study of the Plasma Reveals Acute Phase Response and LXR/RXR and FXR/RXR Activation in the Chronic Unpredictable Mild Stress Mouse Model of Depression. Mol. Med. Rep. 2018, 17, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Schulman, I.G. Liver X Receptors Link Lipid Metabolism and Inflammation. FEBS Lett. 2017, 591, 2978–2991. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Kim, T.-K.; Qayyum, S.; Song, Y.; Janjetovic, Z.; Oak, A.S.W.; Slominski, R.M.; Raman, C.; Stefan, J.; Mier-Aguilar, C.A.; et al. Vitamin D and Lumisterol Derivatives Can Act on Liver X Receptors (LXRs). Sci. Rep. 2021, 11, 8002. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Deng, B.; Jia, J.; Hou, W.; Hu, S.; Deng, J.; Lin, W.; Hou, L.; Sang, H. Liver X Receptor β in the Hippocampus: A Potential Novel Target for the Treatment of Major Depressive Disorder? Neuropharmacology 2018, 135, 514–528. [Google Scholar] [CrossRef]
- Zhu, P.; Tang, J.; Liang, X.; Luo, Y.; Wang, J.; Li, Y.; Xiao, K.; Li, J.; Deng, Y.; Jiang, L.; et al. Activation of Liver X Receptors Protects Oligodendrocytes in CA3 of Stress-Induced Mice. Front. Pharmacol. 2022, 13, 936045. [Google Scholar] [CrossRef]
- Deng, C.; Liu, Q.; Zhao, H.; Qian, L.; Lei, W.; Yang, W.; Liang, Z.; Tian, Y.; Zhang, S.; Wang, C.; et al. Activation of NR1H3 Attenuates the Severity of Septic Myocardial Injury by Inhibiting NLRP3 Inflammasome. Bioeng. Transl. Med. 2023, 8, e10517. [Google Scholar] [CrossRef]
- Ibrahim, K.S.; Craft, J.A.; Biswas, L.; Spencer, J.; Shu, X. Etifoxine Reverses Weight Gain and Alters the Colonic Bacterial Community in a Mouse Model of Obesity. Biochem. Pharmacol. 2020, 180, 114151. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Parker, L.A. The Endocannabinoid System and the Brain. Annu. Rev. Psychol. 2013, 64, 21–47. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Li, Y.; Tian, T.; Quan, W.; Wang, L.; Shao, Q.; Fu, L.-Q.; Zhang, X.-H.; Wang, X.-Y.; Zhang, H.; et al. Role of the Endocannabinoid System in the Formation and Development of Depression. Pharmazie 2017, 72, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Urits, I.; Gress, K.; Charipova, K.; Li, N.; Berger, A.A.; Cornett, E.M.; Hasoon, J.; Kassem, H.; Kaye, A.D.; Viswanath, O. Cannabis Use and Its Association with Psychological Disorders. Psychopharmacol. Bull. 2020, 50, 56–67. [Google Scholar] [PubMed]
- Peng, J.; Fan, M.; An, C.; Ni, F.; Huang, W.; Luo, J. A Narrative Review of Molecular Mechanism and Therapeutic Effect of Cannabidiol (CBD). Basic Clin. Pharmacol. Toxicol. 2022, 130, 439–456. [Google Scholar] [CrossRef] [PubMed]
- Galve-Roperh, I.; Aguado, T.; Palazuelos, J.; Guzmán, M. The Endocannabinoid System and Neurogenesis in Health and Disease. Neuroscientist 2007, 13, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Olivas-Aguirre, M.; Gutiérrez-Iñiguez, C.; Pottosin, I.; Dobrovinskaya, O. Molecular Targets for Cannabinoids in Natural Killer Cells: Do They Modulate the Antitumor Activity? Receptors 2024, 3, 122–144. [Google Scholar] [CrossRef]
- Ilyasov, A.A.; Milligan, C.E.; Pharr, E.P.; Howlett, A.C. The Endocannabinoid System and Oligodendrocytes in Health and Disease. Front. Neurosci. 2018, 12, 733. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Thomas, B.F.; Zhang, Y. Overcoming the Psychiatric Side Effects of the Cannabinoid CB1 Receptor Antagonists: Current Approaches for Therapeutics Development. Curr. Top. Med. Chem. 2019, 19, 1418–1435. [Google Scholar] [CrossRef]
- Howlett, A.C. The CB1 Cannabinoid Receptor in the Brain. Neurobiol. Dis. 1998, 5, 405–416. [Google Scholar] [CrossRef]
- Leo, L.M.; Abood, M.E. CB1 Cannabinoid Receptor Signaling and Biased Signaling. Molecules 2021, 26, 5413. [Google Scholar] [CrossRef] [PubMed]
- Colangeli, R.; Teskey, G.C.; Di Giovanni, G. Endocannabinoid-Serotonin Systems Interaction in Health and Disease. Prog. Brain Res. 2021, 259, 83–134. [Google Scholar] [CrossRef] [PubMed]
- Haller, J.; Varga, B.; Ledent, C.; Freund, T.F. CB1 Cannabinoid Receptors Mediate Anxiolytic Effects: Convergent Genetic and Pharmacological Evidence with CB1-Specific Agents. Behav. Pharmacol. 2004, 15, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Naderi, N.; Haghparast, A.; Saber-Tehrani, A.; Rezaii, N.; Alizadeh, A.-M.; Khani, A.; Motamedi, F. Interaction between Cannabinoid Compounds and Diazepam on Anxiety-like Behaviour of Mice. Pharmacol. Biochem. Behav. 2008, 89, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Gobira, P.H.; LaMar, J.; Marques, J.; Sartim, A.; Silveira, K.; Santos, L.; Wegener, G.; Guimaraes, F.S.; Mackie, K.; Lu, H.-C.; et al. CB1 Receptor Silencing Attenuates Ketamine-Induced Hyperlocomotion without Compromising Its Antidepressant-Like Effects. Cannabis Cannabinoid Res. 2023, 8, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-J.; Zheng, D.; Li, K.-X.; Yang, J.-M.; Pan, H.-Q.; Yu, X.-D.; Fu, J.-Y.; Zhu, Y.; Sun, Q.-X.; Tang, M.-Y.; et al. Cannabinoid CB1 Receptors in the Amygdalar Cholecystokinin Glutamatergic Afferents to Nucleus Accumbens Modulate Depressive-like Behavior. Nat. Med. 2019, 25, 337–349. [Google Scholar] [CrossRef]
- Campos, A.C.; Ortega, Z.; Palazuelos, J.; Fogaça, M.V.; Aguiar, D.C.; Díaz-Alonso, J.; Ortega-Gutiérrez, S.; Vázquez-Villa, H.; Moreira, F.A.; Guzmán, M.; et al. The Anxiolytic Effect of Cannabidiol on Chronically Stressed Mice Depends on Hippocampal Neurogenesis: Involvement of the Endocannabinoid System. Int. J. Neuropsychopharmacol. 2013, 16, 1407–1419. [Google Scholar] [CrossRef] [PubMed]
- Melas, P.A.; Scherma, M.; Fratta, W.; Cifani, C.; Fadda, P. Cannabidiol as a Potential Treatment for Anxiety and Mood Disorders: Molecular Targets and Epigenetic Insights from Preclinical Research. Int. J. Mol. Sci. 2021, 22, 1863. [Google Scholar] [CrossRef] [PubMed]
- Traynor, K. Panel Advises against Rimonabant Approval. Am. J. Health Syst. Pharm. 2007, 64, 1460–1461. [Google Scholar] [CrossRef]
- Ward, S.J.; Raffa, R.B. Rimonabant Redux and Strategies to Improve the Future Outlook of CB1 Receptor Neutral-Antagonist/Inverse-Agonist Therapies. Obesity 2011, 19, 1325–1334. [Google Scholar] [CrossRef]
- Duffy, S.S.; Hayes, J.P.; Fiore, N.T.; Moalem-Taylor, G. The Cannabinoid System and Microglia in Health and Disease. Neuropharmacology 2021, 190, 108555. [Google Scholar] [CrossRef] [PubMed]
- Vieira, G.; Cavalli, J.; Gonçalves, E.C.D.; Braga, S.F.P.; Ferreira, R.S.; Santos, A.R.S.; Cola, M.; Raposo, N.R.B.; Capasso, R.; Dutra, R.C. Antidepressant-Like Effect of Terpineol in an Inflammatory Model of Depression: Involvement of the Cannabinoid System and D2 Dopamine Receptor. Biomolecules 2020, 10, 792. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.A.; Aguiar, R.P.; Scarante, F.F.; Fusse, E.J.; de Oliveira, R.M.W.; Guimaraes, F.S.; Campos, A.C. Spontaneous Activity of CB2 Receptors Attenuates Stress-Induced Behavioral and Neuroplastic Deficits in Male Mice. Front. Pharmacol. 2021, 12, 805758. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, H.; Liu, D.; Li, X.; He, L.; Pan, J.; Shen, Q.; Peng, Y. CB2R Activation Ameliorates Late Adolescent Chronic Alcohol Exposure-Induced Anxiety-like Behaviors during Withdrawal by Preventing Morphological Changes and Suppressing NLRP3 Inflammasome Activation in Prefrontal Cortex Microglia in Mice. Brain Behav. Immun. 2023, 110, 60–79. [Google Scholar] [CrossRef] [PubMed]
- Guida, F.; Boccella, S.; Belardo, C.; Iannotta, M.; Piscitelli, F.; De Filippis, F.; Paino, S.; Ricciardi, F.; Siniscalco, D.; Marabese, I.; et al. Altered Gut Microbiota and Endocannabinoid System Tone in Vitamin D Deficiency-Mediated Chronic Pain. Brain Behav. Immun. 2020, 85, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Skoda, A.M.; Simovic, D.; Karin, V.; Kardum, V.; Vranic, S.; Serman, L. The Role of the Hedgehog Signaling Pathway in Cancer: A Comprehensive Review. Bosn. J. Basic Med. Sci. 2018, 18, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wang, Y.; Qiu, F.; Hou, G.; Liu, J.; Yang, H.; Wu, M.; Dong, X.; Guo, D.; Zhong, Z.; et al. Ablated Sonic Hedgehog Signaling in the Dentate Gyrus of the Dorsal and Ventral Hippocampus Impairs Hippocampal-Dependent Memory Tasks and Emotion in a Rat Model of Depression. Mol. Neurobiol. 2023. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Li, S.; Li, H.; Yang, C.; Lin, J. The Role of Shh Signalling Pathway in Central Nervous System Development and Related Diseases. Cell Biochem. Funct. 2021, 39, 180–189. [Google Scholar] [CrossRef]
- Meng, P.; Zhang, X.; Li, D.; Yang, H.; Lin, X.; Zhao, H.; Li, P.; Wang, Y.; Wang, X.; Ge, J. Leonurine Regulates Hippocampal Nerve Regeneration in Rats with Chronic and Unpredictable Mild Stress by Activating SHH/GLI Signaling Pathway and Restoring Gut Microbiota and Microbial Metabolic Homeostasis. Neural Plast. 2023, 2023, 1455634. [Google Scholar] [CrossRef]
- Donica, C.L.; Ramirez, V.I.; Awwad, H.O.; Zaveri, N.T.; Toll, L.; Standifer, K.M. Orphanin FQ/Nociceptin Activates Nuclear Factor Kappa B. J. Neuroimmune Pharmacol. 2011, 6, 617–625. [Google Scholar] [CrossRef]
- Ubaldi, M.; Cannella, N.; Borruto, A.M.; Petrella, M.; Micioni Di Bonaventura, M.V.; Soverchia, L.; Stopponi, S.; Weiss, F.; Cifani, C.; Ciccocioppo, R. Role of Nociceptin/Orphanin FQ-NOP Receptor System in the Regulation of Stress-Related Disorders. Int. J. Mol. Sci. 2021, 22, 12956. [Google Scholar] [CrossRef]
- Donica, C.L.; Awwad, H.O.; Thakker, D.R.; Standifer, K.M. Cellular Mechanisms of Nociceptin/Orphanin FQ (N/OFQ) Peptide (NOP) Receptor Regulation and Heterologous Regulation by N/OFQ. Mol. Pharmacol. 2013, 83, 907–918. [Google Scholar] [CrossRef]
- Toll, L.; Bruchas, M.R.; Calo’, G.; Cox, B.M.; Zaveri, N.T. Nociceptin/Orphanin FQ Receptor Structure, Signaling, Ligands, Functions, and Interactions with Opioid Systems. Pharmacol. Rev. 2016, 68, 419–457. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, D.; Yan, Y.; Li, Q.; Xing, W.; Liu, Y.; Chen, Y.; Wang, D.; Yuan, Y.; Xie, J.; et al. The Nociceptin Receptor Promotes Autophagy through NF-kB Signaling and Is Transcriptionally Regulated by E2F1 in HCC. Cell Death Discov. 2022, 8, 165. [Google Scholar] [CrossRef] [PubMed]
- Gavioli, E.C.; De Medeiros, I.U.; Monteiro, M.C.; Calo, G.; Romão, P.R.T. Nociceptin/Orphanin FQ-NOP Receptor System in Inflammatory and Immune-Mediated Diseases. In Vitamins & Hormones; Elsevier: Amsterdam, The Netherlands, 2015; Volume 97, pp. 241–266. ISBN 978-0-12-802443-0. [Google Scholar]
- Fu, X.; Zhu, Z.-H.; Wang, Y.-Q.; Wu, G.-C. Regulation of Proinflammatory Cytokines Gene Expression by Nociceptin/Orphanin FQ in the Spinal Cord and the Cultured Astrocytes. Neuroscience 2007, 144, 275–285. [Google Scholar] [CrossRef]
- Jenck, F.; Moreau, J.-L.; Martin, J.R.; Kilpatrick, G.J.; Reinscheid, R.K.; Monsma, F.J.; Nothacker, H.-P.; Civelli, O. Orphanin FQ Acts as an Anxiolytic to Attenuate Behavioral Responses to Stress. Proc. Natl. Acad. Sci. USA 1997, 94, 14854–14858. [Google Scholar] [CrossRef] [PubMed]
- Asth, L.; Correia, N.; Lobão-Soares, B.; De Lima, T.C.M.; Guerrini, R.; Calo’, G.; Soares-Rachetti, V.P.; Gavioli, E.C. Nociceptin/Orphanin FQ Induces Simultaneously Anxiolytic and Amnesic Effects in the Mouse Elevated T-Maze Task. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2015, 388, 33–41. [Google Scholar] [CrossRef]
- Redrobe, J.; Calo’, G.; Regoli, D.; Quirion, R. Nociceptin Receptor Antagonists Display Antidepressant-like Properties in the Mouse Forced Swimming Test. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2002, 365, 164–167. [Google Scholar] [CrossRef]
- Holanda, V.A.D.; Medeiros, I.U.; Asth, L.; Guerrini, R.; Calo’, G.; Gavioli, E.C. Antidepressant Activity of Nociceptin/Orphanin FQ Receptor Antagonists in the Mouse Learned Helplessness. Psychopharmacology 2016, 233, 2525–2532. [Google Scholar] [CrossRef] [PubMed]
- Gavioli, E.C.; Marzola, G.; Guerrini, R.; Bertorelli, R.; Zucchini, S.; De Lima, T.C.M.; Rae, G.A.; Salvadori, S.; Regoli, D.; Calo, G. Blockade of Nociceptin/Orphanin FQ–NOP Receptor Signalling Produces Antidepressant-like Effects: Pharmacological and Genetic Evidences from the Mouse Forced Swimming Test. Eur. J. Neurosci. 2003, 17, 1987–1990. [Google Scholar] [CrossRef]
- Holanda, V.A.D.; Santos, W.B.; Asth, L.; Guerrini, R.; Calo’, G.; Ruzza, C.; Gavioli, E.C. NOP Agonists Prevent the Antidepressant-like Effects of Nortriptyline and Fluoxetine but Not R-Ketamine. Psychopharmacology 2018, 235, 3093–3102. [Google Scholar] [CrossRef] [PubMed]
- Vitale, G.; Filaferro, M.; Micioni Di Bonaventura, M.V.; Ruggieri, V.; Cifani, C.; Guerrini, R.; Simonato, M.; Zucchini, S. Effects of [Nphe 1, Arg 14, Lys 15 ] N/OFQ-NH 2 (UFP-101), a Potent NOP Receptor Antagonist, on Molecular, Cellular and Behavioural Alterations Associated with Chronic Mild Stress. J. Psychopharmacol. 2017, 31, 691–703. [Google Scholar] [CrossRef]
- Medeiros, I.U.; Ruzza, C.; Asth, L.; Guerrini, R.; Romão, P.R.T.; Gavioli, E.C.; Calo, G. Blockade of Nociceptin/Orphanin FQ Receptor Signaling Reverses LPS-Induced Depressive-like Behavior in Mice. Peptides 2015, 72, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Gavioli, E.C.; Rizzi, A.; Marzola, G.; Zucchini, S.; Regoli, D.; Calo’, G. Altered Anxiety-Related Behavior in Nociceptin/Orphanin FQ Receptor Gene Knockout Mice. Peptides 2007, 28, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, M.F.; Faucz, F.R.; Bimpaki, E.; Horvath, A.; Levy, I.; De Alexandre, R.B.; Ahmad, F.; Manganiello, V.; Stratakis, C.A. Clinical and Molecular Genetics of the Phosphodiesterases (PDEs). Endocr. Rev. 2014, 35, 195–233. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-Z.; Zhang, Y.; Zhang, H.-T.; Li, Y.-F. Phosphodiesterase: An Interface Connecting Cognitive Deficits to Neuropsychiatric and Neurodegenerative Diseases. Curr. Pharm. Des. 2014, 21, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Delhaye, S.; Bardoni, B. Role of Phosphodiesterases in the Pathophysiology of Neurodevelopmental Disorders. Mol. Psychiatry 2021, 26, 4570–4582. [Google Scholar] [CrossRef] [PubMed]
- Sanders, O.; Rajagopal, L. Phosphodiesterase Inhibitors for Alzheimer’s Disease: A Systematic Review of Clinical Trials and Epidemiology with a Mechanistic Rationale. J. Alzheimer’s Dis. Rep. 2020, 4, 185–215. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Zhang, S.; Wu, L.; Kumar, G.; Liao, Y.; Gk, P.; Fan, H. Inhibition of Phosphodiesterase: A Novel Therapeutic Target for the Treatment of Mild Cognitive Impairment and Alzheimer’s Disease. Front. Aging Neurosci. 2022, 14, 1019187. [Google Scholar] [CrossRef]
- Sadeghi, M.A.; Nassireslami, E.; Yousefi Zoshk, M.; Hosseini, Y.; Abbasian, K.; Chamanara, M. Phosphodiesterase Inhibitors in Psychiatric Disorders. Psychopharmacology 2023, 240, 1201–1219. [Google Scholar] [CrossRef]
- Tomaz, V.S.; Cordeiro, R.C.; Costa, A.M.N.; De Lucena, D.F.; Nobre Júnior, H.V.; De Sousa, F.C.F.; Vasconcelos, S.M.M.; Vale, M.L.; Quevedo, J.; Macêdo, D. Antidepressant-like Effect of Nitric Oxide Synthase Inhibitors and Sildenafil against Lipopolysaccharide-Induced Depressive-like Behavior in Mice. Neuroscience 2014, 268, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Hafez, M.; Kazaz, S. The Impact of Phosphodiesterase-5 Inhibitor (Sildenafil Citrate) on Some Hippocampal Neurotransmitters, Oxidative Stress Status, Minerals, and Anxiety-like Behavior in Rats. J. Adv. Vet. Anim. Res. 2020, 7, 281. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.R.; Kim, H.N.; Hong, K.W.; Shin, H.K.; Choi, B.T. Anti-Depressant Effects of Phosphodiesterase 3 Inhibitor Cilostazol in Chronic Mild Stress-Treated Mice after Ischemic Stroke. Psychopharmacology 2016, 233, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.A.; Hemmati, S.; Yousefi-Manesh, H.; Foroutani, L.; Nassireslami, E.; Yousefi Zoshk, M.; Hosseini, Y.; Abbasian, K.; Dehpour, A.R.; Chamanara, M. Cilostazol Pretreatment Prevents PTSD-Related Anxiety Behavior through Reduction of Hippocampal Neuroinflammation. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Tai, S.-Y.; Chen, C.-H.; Chien, C.-Y.; Yang, Y.-H. Cilostazol as an Add-on Therapy for Patients with Alzheimer’s Disease in Taiwan: A Case Control Study. BMC Neurol. 2017, 17, 40. [Google Scholar] [CrossRef] [PubMed]
- Chapman, T.M.; Goa, K.L. Cilostazol: A Review of Its Use in Intermittent Claudication. Am. J. Cardiovasc. Drugs 2003, 3, 117–138. [Google Scholar] [CrossRef] [PubMed]
- Prickaerts, J.; Heckman, P.R.A.; Blokland, A. Investigational Phosphodiesterase Inhibitors in Phase I and Phase II Clinical Trials for Alzheimer’s Disease. Expert Opin. Investig. Drugs 2017, 26, 1033–1048. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem in 2021: New Data Content and Improved Web Interfaces. Nucleic Acids Res. 2021, 49, D1388–D1395. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated Data and New Features for Efficient Prediction of Protein Targets of Small Molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef]
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Stein, T.I.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr. Protoc. Bioinform. 2016, 54, 1–30. [Google Scholar] [CrossRef]
- Franz, M.; Rodriguez, H.; Lopes, C.; Zuberi, K.; Montojo, J.; Bader, G.D.; Morris, Q. GeneMANIA Update 2018. Nucleic Acids Res. 2018, 46, W60–W64. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Peng, W.; Wu, F. Computational Approaches to Predicting Essential Proteins: A Survey. Proteom. Clin. Appl. 2013, 7, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Altê, G.A.; Rodrigues, A.L.S. Exploring the Molecular Targets for the Antidepressant and Antisuicidal Effects of Ketamine Enantiomers by Using Network Pharmacology and Molecular Docking. Pharmaceuticals 2023, 16, 1013. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.X.; Jung, D.; Yao, R. ShinyGO: A Graphical Gene-Set Enrichment Tool for Animals and Plants. Bioinformatics 2020, 36, 2628–2629. [Google Scholar] [CrossRef] [PubMed]
- Guedes, I.A.; Costa, L.S.C.; Dos Santos, K.B.; Karl, A.L.M.; Rocha, G.K.; Teixeira, I.M.; Galheigo, M.M.; Medeiros, V.; Krempser, E.; Custódio, F.L.; et al. Drug Design and Repurposing with DockThor-VS Web Server Focusing on SARS-CoV-2 Therapeutic Targets and Their Non-Synonym Variants. Sci. Rep. 2021, 11, 5543. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure Visualization for Researchers, Educators, and Developers. Protein Sci. 2021, 30, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING V11: Protein–Protein Association Networks with Increased Coverage, Supporting Functional Discovery in Genome-Wide Experimental Datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Piñero, J.; Ramírez-Anguita, J.M.; Saüch-Pitarch, J.; Ronzano, F.; Centeno, E.; Sanz, F.; Furlong, L.I. The DisGeNET Knowledge Platform for Disease Genomics: 2019 Update. Nucleic Acids Res. 2020, 48, D845–D855. [Google Scholar] [CrossRef]





| Gene Symbol | Codified Proteins |
|---|---|
| ABCB1 | ATP Binding Cassette Subfamily B Member 1 |
| ABL1 | ABL Proto-Oncogene 1, Non-Receptor Tyrosine Kinase |
| AKT1 | AKT Serine/Threonine Kinase 1 |
| AKT2 | AKT Serine/Threonine Kinase 2 |
| ALK | ALK Receptor Tyrosine Kinase |
| AR | Androgen Receptor |
| CA1 | Carbonic Anhydrase 1 |
| CA2 | Carbonic Anhydrase 2 |
| CCKBR | Cholecystokinin B Receptor |
| CCR1 | C-C Motif Chemokine Receptor 1 |
| CDC25A | Cell Division Cycle 25A |
| CHEK1 | Checkpoint Kinase 1 |
| CHUK | Component Of Inhibitor Of Nuclear Factor Kappa B Kinase Complex |
| CNR1 | Cannabinoid Receptor 1 |
| CNR2 | Cannabinoid Receptor 2 |
| CSF1R | Colony Stimulating Factor 1 Receptor |
| CSK | C-Terminal Src Kinase |
| CYP19A1 | Cytochrome P450 Family 19 Subfamily A Member 1 |
| CYP24A1 | Cytochrome P450 Family 24 Subfamily A Member 1 |
| CYP27B1 | Cytochrome P450 Family 27 Subfamily B Member 1 |
| DPP4 | Dipeptidyl Peptidase 4 |
| DRD3 | Dopamine Receptor D3 |
| EPHX2 | Epoxide Hydrolase 2 |
| ESR1 | Estrogen Receptor 1 |
| ESR2 | Estrogen Receptor 2 |
| EZH2 | Enhancer Of Zeste 2 Polycomb Repressive Complex 2 Subunit |
| FAAH | Fatty Acid Amide Hydrolase |
| FGFR1 | Fibroblast Growth Factor Receptor 1 |
| GC | GC Vitamin D Binding Protein |
| GHSR | Growth Hormone Secretagogue Receptor |
| GLRA1 | Glycine Receptor Alpha 1 |
| GSK3B | Glycogen Synthase Kinase 3 Beta |
| HCRTR1 | Hypocretin Receptor 1 |
| HCRTR2 | Hypocretin Receptor 2 |
| HMGCR | 3-Hydroxy-3-Methylglutaryl-CoA Reductase |
| HRH3 | Histamine Receptor H3 |
| HSD11B2 | Hydroxysteroid 11-Beta Dehydrogenase 2 |
| HTR2A | 5-Hydroxytryptamine Receptor 2A |
| IKBKB | Inhibitor Of Nuclear Factor Kappa B Kinase Subunit Beta |
| INSR | Insulin Receptor |
| JAK1 | Janus Kinase 1 |
| JAK2 | Janus Kinase 2 |
| KCNH2 | Potassium Voltage-Gated Channel Subfamily H Member 2 |
| KDR | Kinase Insert Domain Receptor |
| LIMK2 | LIM Domain Kinase 2 |
| MAP2 | Microtubule Associated Protein 2 |
| MAP3K14 | Mitogen-Activated Protein Kinase Kinase Kinase 14 |
| MCHR1 | Melanin Concentrating Hormone Receptor 1 |
| MDM2 | MDM2 Proto-Oncogene |
| MET | MET Proto-Oncogene, Receptor Tyrosine Kinase |
| MTOR | Mechanistic Target Of Rapamycin Kinase |
| NR1H3 | Nuclear Receptor Subfamily 1 Group H Member 3 |
| NR1I2 | Nuclear Receptor Subfamily 1 Group I Member 2 |
| OPRD1 | Opioid Receptor Delta 1 |
| OPRK1 | Opioid Receptor Kappa 1 |
| OPRM1 | Opioid Receptor Mu 1 |
| P2RX3 | Purinergic Receptor P2X 3 |
| PDE10A | Phosphodiesterase 10A |
| PDE3A | Phosphodiesterase 3A |
| PDE3B | Phosphodiesterase 3B |
| PIK3CA | Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit Alpha |
| PRKCB | Protein Kinase C Beta |
| PRKG1 | Protein Kinase CGMP-Dependent 1 |
| PTAFR | Platelet Activating Factor Receptor |
| REN | Renin |
| SCN9A | Sodium Voltage-Gated Channel Alpha Subunit 9 |
| SHH | Sonic Hedgehog Signaling Molecule |
| SLC6A2 | Solute Carrier Family 6 Member 2 |
| SLC6A3 | Solute Carrier Family 6 Member 3 |
| TACR1 | Tachykinin Receptor 1 |
| TACR2 | Tachykinin Receptor 2 |
| TBXA2R | Thromboxane A2 Receptor |
| TGFBR1 | Transforming Growth Factor Beta Receptor 1 |
| VDR | Vitamin D Receptor |
| Gene Symbol | Codified Proteins |
|---|---|
| ABL1 | ABL proto-oncogene 1, non-receptor tyrosine kinase |
| AKT1 | AKT serine/threonine kinase 1 |
| AKT2 | AKT serine/threonine kinase 2 |
| ALK | ALK receptor tyrosine kinase |
| AR | Androgen receptor |
| CA3 | Carbonic anhydrase 3 |
| CAMK1G | Calcium/calmodulin-dependent protein kinase IG |
| CBL | Cbl proto-oncogene |
| CCKBR | Cholecystokinin B receptor |
| CCR1 | C-C motif chemokine receptor 1 |
| CHEK1 | Checkpoint kinase 1 |
| CHUK | Component of inhibitor of nuclear factor kappa B kinase complex |
| CNR1 | Cannabinoid receptor 1 |
| CNR2 | Cannabinoid receptor 2 |
| CSF1R | Colony stimulating factor 1 receptor |
| CSK | C-terminal Src kinase |
| CYP19A1 | Cytochrome P450 family 19 subfamily A member 1 |
| DRD3 | Dopamine receptor D3 |
| ESR1 | Estrogen receptor |
| EZH2 | Enhancer of zeste 2 polycomb repressive complex 2 subunit |
| FGFR1 | Fibroblast growth factor receptor 1 |
| GC | GC vitamin D binding protein |
| GHSR | Growth hormone secretagogue receptor |
| GSK3B | Glycogen synthase kinase 3 beta |
| HCRTR1 | Hypocretin receptor 1 |
| HCRTR2 | Hypocretin receptor 2 |
| HRH3 | Histamine receptor H3 |
| HTR2A | 5-hydroxytryptamine receptor 2A |
| IHH | Indian hedgehog signaling molecule |
| IKBKB | Inhibitor of nuclear factor kappa B kinase subunit beta |
| INSR | Insulin receptor |
| JAK1 | Janus kinase 1 |
| KDR | Kinase insert domain receptor |
| KSR1 | Kinase suppressor of ras 1 |
| MAP3K14 | Mitogen-activated protein kinase kinase kinase 14 |
| MAPT | Microtubule associated protein tau |
| MDM2 | MDM2 proto-oncogene |
| MET | MET proto-oncogene, receptor tyrosine kinase |
| MTOR | Mechanistic target of rapamycin kinase |
| NR1H3 | Nuclear receptor subfamily 1 group H member 3 |
| OPRD1 | Opioid receptor delta 1 |
| OPRK1 | Opioid receptor kappa 1 |
| OPRL1 | Opioid related nociceptin receptor 1 |
| OPRM1 | Opioid receptor mu 1 |
| PDE3A | Phosphodiesterase 3A |
| PDE5A | Phosphodiesterase 5A |
| PIK3CA | Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha |
| PIK3R1 | Phosphoinositide-3-kinase regulatory subunit 1 |
| PRKCA | Protein kinase C alpha |
| PRKCB | Protein kinase C beta |
| PRKG1 | Protein kinase cGMP-dependent 1 |
| PTAFR | Platelet-activating factor receptor |
| PTCH1 | Patched 1 |
| REN | Renin |
| SHH | Sonic hedgehog signaling molecule |
| TACR1 | Tachykinin receptor 1 |
| TACR2 | Tachykinin receptor 2 |
| TGFBR1 | Transforming growth factor beta receptor 1 |
| VDR | Vitamin D receptor |
| Gene Symbol | PDB ID | Ligand ID (Binding Site) | Positive Control (Ligand ID) Docking Score (kcal/mol) | Calcitriol Docking Score (kcal/mol) |
|---|---|---|---|---|
| VDR | 1DB1 | VDX | −12.116 | −12.116 |
| HRH3 | 7F61 | 1IB | −10.509 | −12.077 |
| NR1H3 | 3IPQ | 965 | −13.750 | −11.927 |
| CNR1 | 7WV9 | 9GF | −11.133 | −11.810 |
| PTCH1 | 6RTW | Y01 | −11.908 | −11.570 |
| CNR2 | 6PT0 | WI5 | −11.502 | −11.333 |
| HTR2A | 6A93 | 8NU | −11.220 | −10.639 |
| CYP19A1 | 3ST9 | ASD | −9.048 | −10.024 |
| HCRTR2 | 4S0V | SUV | −10.610 | −9.920 |
| TACR2 | 7XWO | α-helix | N.A. | −9.903 |
| MTOR | 4DRH | RAP | −14.977 | −9.871 |
| GHSR | 7NA8 | 1KD | −10.476 | −9.803 |
| CCKBR | 7XOW | gastrine | N.A. | −9.674 |
| ESR1 | 1A52 | EST | −9.860 | −9.622 |
| TACR1 | 6E59 | L76 | −10.985 | −9.607 |
| HCRTR1 | 4ZJ8 | SUV | −10.690 | −9.450 |
| PRKCA | 3IW4 | LW4 | −10.166 | −9.324 |
| OPRL1 | 5DHG | DGV | −8.291 | −9.275 |
| ABL1 | 1OPL | P16 | −10.757 | −9.245 |
| PDE5A | 1T9S | 5GP | −6.430 | −9.230 |
| TGFBR1 | 1PY5 | PY1 | −9.168 | −9.230 |
| REN | 1BIL | 0IU | −11.031 | −9.219 |
| PRKCB | 2I0E | PDS | −9.299 | −9.160 |
| CHUK | 5EBZ | 5TL | −8.347 | −9.137 |
| OPRD1 | 6PT2 | KGCHM07 (peptide) | N.A. | −9.128 |
| CCR1 | 7VL9 | CLR | −8.753 | −9.090 |
| DRD3 | 8IRT | R5F | −10.321 | −9.054 |
| KDR | 1Y6A | AAZ | −9.908 | −8.914 |
| INSR | 1GAG | 112 | −7.707 | −8.901 |
| CSK | 1BYG | STU | −9.931 | −8.810 |
| CAMK1G | 2JAM | J60 | −9.240 | −8.754 |
| PDE3A | 7KWE | X5M | −7.975 | −8.740 |
| OPRM1 | 8EF5 | 7V7 | −9.767 | −8.639 |
| CNR2 | 6PT0 | CLR | −8.361 | −8.625 |
| GSK3B | 6B8J | 65C | −9.243 | −8.612 |
| PIK3CA | 3ZIM | KKR | −10.432 | −8.518 |
| OPRK1 | 4DJH | JDC | −11.363 | −8.457 |
| EZH2 | 5HYN | SAH | −8.392 | −8.394 |
| FGFR1 | 3DPK | 8C5 | −9.543 | −8.309 |
| AKT2 | 1GZK | α-helix | N.A. | −8.157 |
| CNR1 | 7WV9 | 7IC | −8.213 | −8.038 |
| GC | 1J78 | VDY | −8.621 | −8.034 |
| PTAFR | P25105 * | α-helix | N.A. | −8.020 |
| MDM2 | 1RV1 | IMZ | −9.773 | −7.993 |
| CSF1R | 3BEA | IXH | −10.084 | −7.959 |
| KSR1 | 7JUY | ANP | −6.572 | −7.950 |
| MAPT | 7NRS | center of protein | N.A. | −7.891 |
| ALK | 2XP2 | VGH | −8.907 | −7.871 |
| IHH | 3K7I | α-helix | N.A. | −7.851 |
| AR | 1T5Z | DHT | −10.094 | −7.746 |
| IKBKB | 4KIK | KSA | −10.662 | −7.730 |
| JAK1 | 4E4L | 0NH | −8.583 | −7.342 |
| AKT1 | 2UVM | GVF | −7.453 | −7.268 |
| PIK3R | 1H9O | PTR | −6.556 | −7.267 |
| SHH | 6PJV | GOL | N.A. | −7.187 |
| MAP3K14 | 4DN5 | AGS | −6.658 | −7.130 |
| CHEK1 | 1ZLT | HYM | −7.915 | −7.127 |
| CA3 | 3UYQ | α-helix | N.A. | −6.963 |
| Gene Symbol | PDB ID | Ligand ID (Binding Site) | Positive Control (Ligand ID) Docking Score (kcal/mol) | Calcitriol Docking Score (kcal/mol) |
|---|---|---|---|---|
| SMO | 4QIM | ANTAXV(A8T) | −11.921 | −10.995 |
| HTR1D | 7E32 | SRO | −7.735 | −10.563 |
| EBP | 6OHU | CTX | −11.329 | −10.509 |
| GPBAR1 | 7CFM | P395 (FWX) | −9.368 | −10.253 |
| S1PR2 | 7T6B | S1P | −8.881 | −9.907 |
| S1PR1 | 7E04 | BAF312 (J8C) | −10.775 | −9.701 |
| ADAMTS5 | 3B8Z | 294 | −10.223 | −8.761 |
| GSK3A | 7SXG | BIO8546(D1E) | −8.959 | −8.426 |
| ACAN | 4MD4 | α-helix | N.A. | −8.421 |
| ADAMTS4 | 2RJP | 886 | −10.875 | −8.416 |
| BRSK2 | Q8IWQ3 * | α-helix | N.A. | −8.348 |
| S1PR3 | 7EW2 | EFTY720(J89) | −9.144 | −8.273 |
| STAT6 | 4Y5W | α-helix | N.A. | −8.244 |
| HTR1D | 7E32 | CLR | −8.750 | −8.153 |
| EPHB4 | 2VWU | 7 × 1 | −10.345 | −8.062 |
| JAK3 | 3ZC6 | VFC | −9.065 | −7.872 |
| TTK | 2X9E | NMS-P715 (SVE) | −10.407 | −7.823 |
| EPHB3 | 5L6O | 6P6 | −8.676 | −7.622 |
| WEE1 | 3BI6 | PD352396 (396) | −10.779 | −7.510 |
| AKT3 | 2 × 18 | EPE | −6.106 | −7.379 |
| PRKD3 | 2D9Z | α-helix | N.A. | −7.125 |
| PTPN1 | 1BZC | TPI | −7.353 | −6.496 |
| Gene Symbol | PDB ID | Ligand ID (Binding Site) | Positive Control (Ligand ID) Docking Score (kcal/mol) | Calcitriol Docking Score (kcal/mol) |
|---|---|---|---|---|
| AURKB | 4AF3 | VX6 | −10.203 | −10.268 |
| HSP90B | 3NMQ | EC44/7PP | −9.434 | −10.195 |
| AURKC | 6GR9 | VX6 | −7.638 | −9.892 |
| INCENP | 6GR8 | α-helix | N.A. | −7.920 |
| BIRC5 | 2QFA | MES | −6.717 | −6.374 |
| Gene/ Protein Symbol | Degree | Betweenness Centrality | Closeness Centrality | Clustering Coefficient |
|---|---|---|---|---|
| CNR1/CB1 | 27 | 0.0019 | 0.3379 | 0.2821 |
| CNR2/CB2 | 7 | 0.0017 | 0.2923 | 0.4286 |
| PDE3A | 8 | 9.82 × 108 | 0.2651 | 0.7857 |
| PDE5A | 9 | 1.15 × 1010 | 0.2652 | 0.8056 |
| PTCH1 | 3 | 0.0000 | 0.2173 | 1.0000 |
| SMO | 4 | 0.0049 | 0.2775 | 0.5000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kouba, B.R.; Altê, G.A.; Rodrigues, A.L.S. Putative Pharmacological Depression and Anxiety-Related Targets of Calcitriol Explored by Network Pharmacology and Molecular Docking. Pharmaceuticals 2024, 17, 893. https://doi.org/10.3390/ph17070893
Kouba BR, Altê GA, Rodrigues ALS. Putative Pharmacological Depression and Anxiety-Related Targets of Calcitriol Explored by Network Pharmacology and Molecular Docking. Pharmaceuticals. 2024; 17(7):893. https://doi.org/10.3390/ph17070893
Chicago/Turabian StyleKouba, Bruna R., Glorister A. Altê, and Ana Lúcia S. Rodrigues. 2024. "Putative Pharmacological Depression and Anxiety-Related Targets of Calcitriol Explored by Network Pharmacology and Molecular Docking" Pharmaceuticals 17, no. 7: 893. https://doi.org/10.3390/ph17070893
APA StyleKouba, B. R., Altê, G. A., & Rodrigues, A. L. S. (2024). Putative Pharmacological Depression and Anxiety-Related Targets of Calcitriol Explored by Network Pharmacology and Molecular Docking. Pharmaceuticals, 17(7), 893. https://doi.org/10.3390/ph17070893






