Risk-Based Approach for Defining Retest Dates for Active Pharmaceutical Ingredients and Excipients
Abstract
:1. Introduction
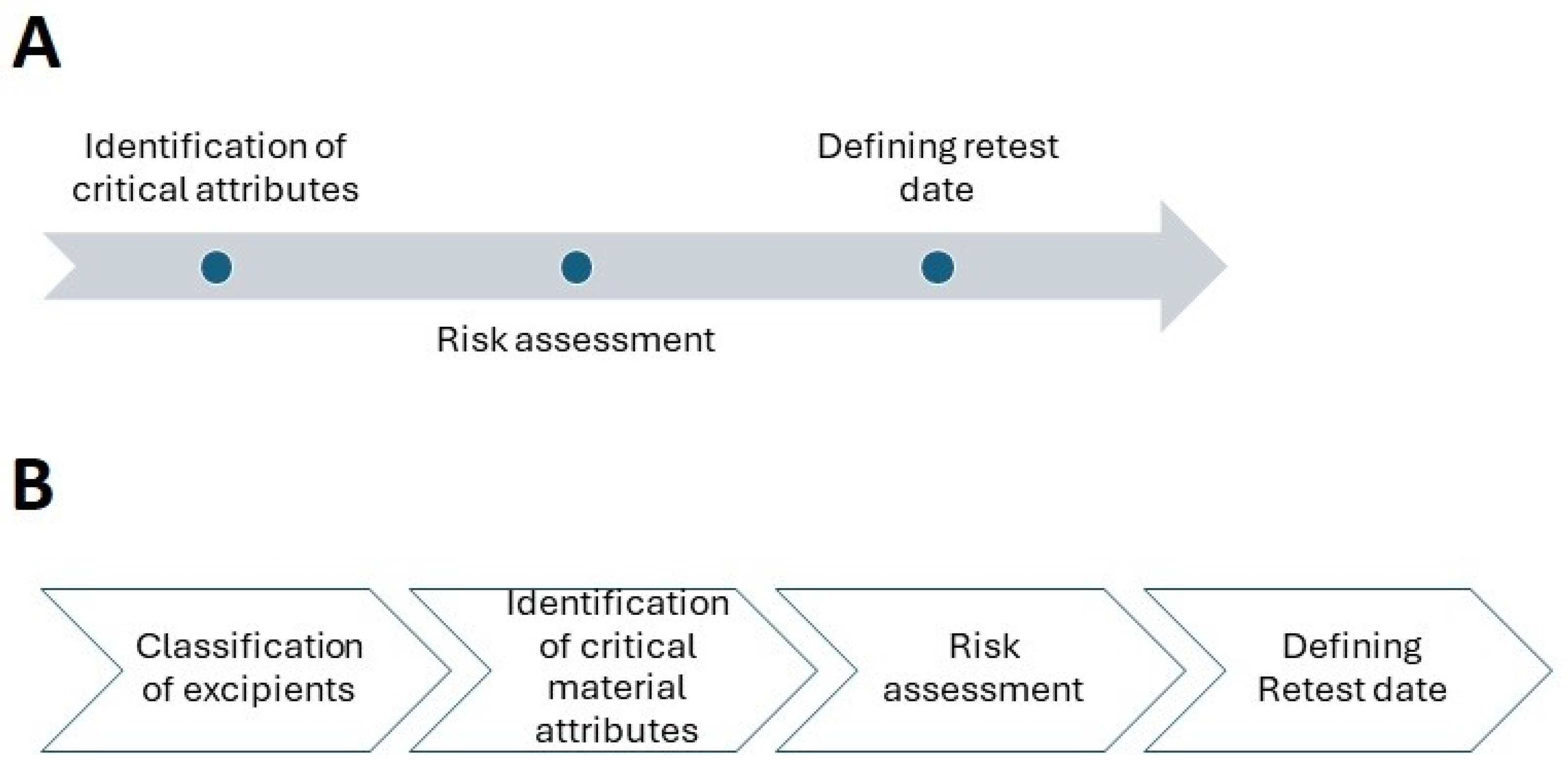
2. Establishing Retest Date for APIs
2.1. Identification of Critical Attributes
2.1.1. Particle Size and Shape
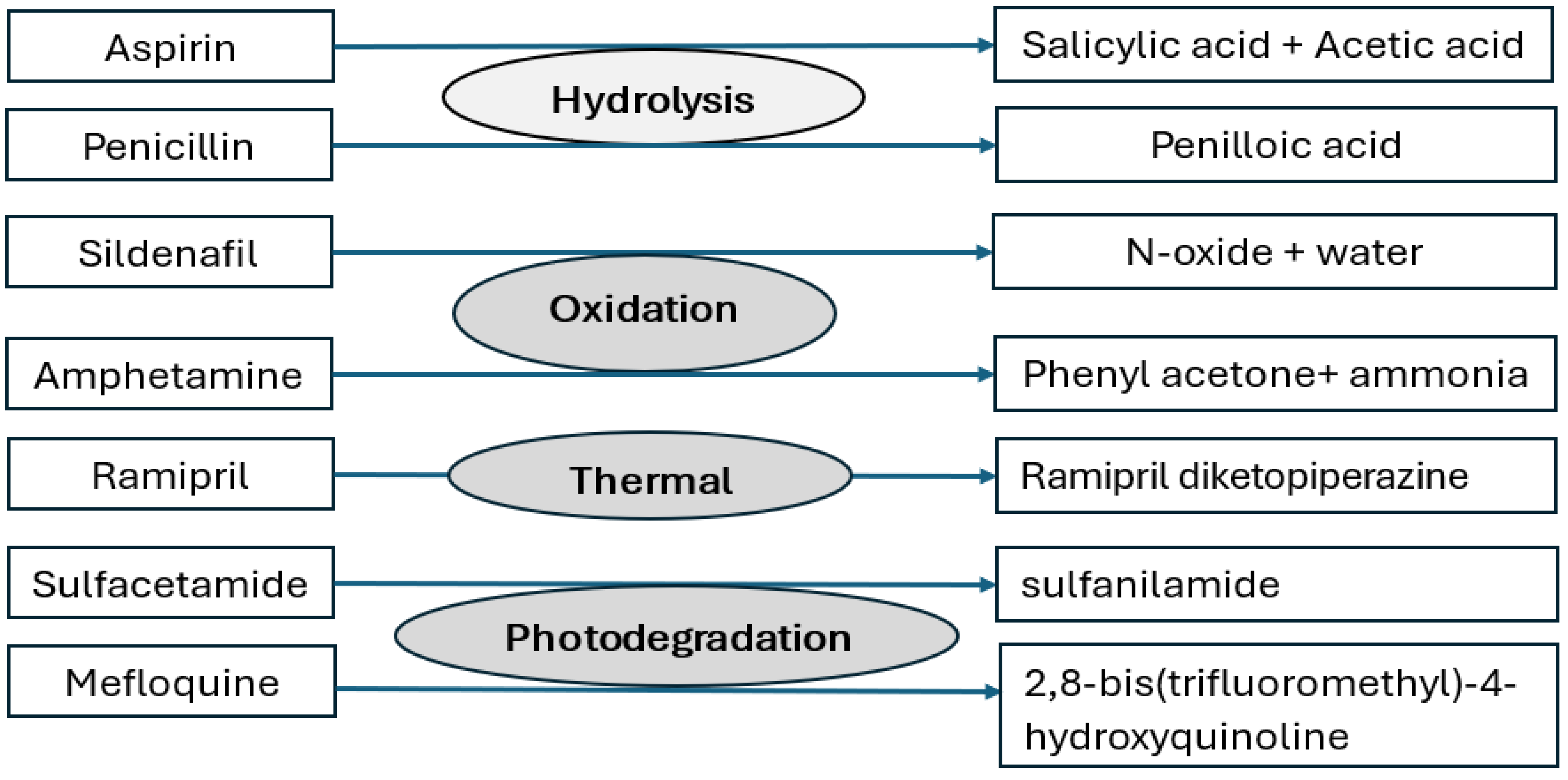
2.1.2. Assay, Impurity Profile, and Stability
2.1.3. Polymorphic Form and Solubility
2.1.4. Microbial Quality
2.1.5. Nitrosamines
2.2. Risk Assessment
2.3. Retest
3. Excipients
3.1. Classification of Excipients
3.1.1. Very Stable
3.1.2. Stable
3.1.3. Limited Stability
3.2. Identification of Critical Material Attributes and Risk Assessment
3.2.1. Polyplasdone XL-10 (Crospovidone): Example of Stable Excipient
3.2.2. Pearlitol® 200 SD (Mannitol): Example of Very Stable Excipient
3.2.3. Nitrosamine Risk Due to Excipients
3.3. Defining Retest Period
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- ICH Harmonised Tripartite Guideline. Stability Testing of New Drug Substances and Products, Q1A(R2). 2003. Available online: https://database.ich.org/sites/default/files/Q1A%28R2%29%20Guideline.pdf (accessed on 2 May 2024).
- WHO Expert Committee on Specifications for Pharmaceutical Preparations. Fifty-Second Report. Available online: https://iris.who.int/bitstream/handle/10665/272452/9789241210195-eng.pdf?sequence=1 (accessed on 2 May 2024).
- ICH Harmonised Tripartite Guideline. Evaluation for Stability Data, Q1E. 2003. Available online: https://database.ich.org/sites/default/files/Q1E_Guideline.pdf (accessed on 2 May 2024).
- Deng, T.; Garg, V.; Bradley, M.S.A. Electrostatic charging of fine powders and assessment of charge polarity using an inductive charge sensor. Nanomanufacturing 2023, 3, 281–292. [Google Scholar] [CrossRef]
- Peart, J. Powder electrostatics: Theory, techniques and applications. KONA Powder Part. J. 2001, 19, 34–45. [Google Scholar] [CrossRef]
- ICH Harmonised Tripartite Guideline. Good Manufacturing Practice Guide for Active Pharmaceutical Ingredients. Available online: https://database.ich.org/sites/default/files/Q7%20Guideline.pdf (accessed on 2 May 2024).
- ICH Harmonised Tripartite Guideline. Pharmaceutical Development Q8(R2). 2009. Available online: https://database.ich.org/sites/default/files/Q8_R2_Guideline.pdf (accessed on 2 May 2024).
- Cogdill, R.P.; Drennen, J.K. Risk-based quality by design (QbD): A Taguchi perspective on the assessment of product quality, and the quantitative linkage of drug product parameters and clinical performance. J. Pharm. Innov. 2008, 3, 23–29. [Google Scholar] [CrossRef]
- Somma, R. Development knowledge can increase manufacturingcapability and facilitate quality by design. J. Pharm. Innov. 2007, 2, 87–92. [Google Scholar] [CrossRef]
- Yu, L.X. Pharmaceutical quality by design: Product andprocess development, understanding, and control. Pharm. Res. 2008, 25, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Lionberger, R.A.; Lee, S.L.; Lee, L.; Raw, A.; Yu, L.X. Quality by design: Concepts for ANDAs. AAPS J. 2008, 10, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Shekunov, B.Y.; Chattopadhyay, P.; Tong, H.H.Y.; Chow, A.H.L. Particle size analysis in pharmaceutics: Principles, methods and applications. Pharm. Res. 2006, 24, 203–227. [Google Scholar] [CrossRef] [PubMed]
- Standish, N. Studies of size segregation in filling and emptying a hopper. Powder Technol. 1985, 45, 43–56. [Google Scholar] [CrossRef]
- Samadani, A.; Pradhan, A.; Kudrolli, A. Size segregation of granular matter in silo discharges. Phys. Rev. E 1999, 60, 7203–7209. [Google Scholar] [CrossRef]
- Charoo, N.A. Critical excipient attributes relevant to solid dosage formulation manufacturing. J. Pharm. Innov. 2020, 15, 163–181. [Google Scholar] [CrossRef]
- Hlinak, A.J.; Kuriyan, K.; Morris, K.R.; Reklaitis, G.W.; Basu, P.K. Understanding critical material properties for solid dosage form design. J. Pharm. Innov. 2006, 1, 12–17. [Google Scholar] [CrossRef]
- Abdullah, E.C.; Geldart, D. The use of bulk density measurements as flowability indicators. Powder Technol. 1999, 102, 151–165. [Google Scholar] [CrossRef]
- Fayed, M.E.; Otten, L. Handbook of Powder Science & Technology, 2nd ed.; Chapman & Hall: London, UK, 1997. [Google Scholar]
- Andrews, G.; Jones, D.; Zhai, H.; Diak, O.B.; Walker, G. Effects of grinding in pharmaceutical tablet production. In Pharmaceutical Manufacturing Handbook; Gad, S.C., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 1165–1190. [Google Scholar]
- Osorio, J.G.; Muzzio, F.J. Effects of powder flow properties on capsule filling weight uniformity. Drug Dev. Ind. Pharm. 2013, 39, 1464–1475. [Google Scholar] [CrossRef] [PubMed]
- Fichtner, F.; Rasmuson, A.; Alderborn, G. Particle size distribution and evolution in tablet structure during and after compaction. Int. J. Pharm. 2005, 292, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Kaerger, J.S.; Edge, S.; Price, R. Influence of particle size and shape on flowability and compactibility of binary mixtures of paracetamol and microcrystalline cellulose. Eur. J. Pharm. Sci. 2004, 22, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, K.; Miyata, S. Mechanism of density segregation of particles in filling vessels. Ind. Eng. Chem. Process Des. Dev. 1984, 23, 423–428. [Google Scholar] [CrossRef]
- Kuentz, M.; Leuenberger, H. A new theoretical approach to tabletstrength of a binary mixture consisting of a well and a poorly compactable substance. Eur. J. Pharm. Biopharm. 2000, 49, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Rácz, I. Mechanismen der Arzneimittelzersetzung—Arzneistoffstabilisierung [Mechanisms of drug decomposition-drug stabilization]. Pharmazie 1984, 39, 636–640. [Google Scholar]
- Bhangare, D.; Rajput, N.; Jadav, T.; Sengupta, P. Systematic strategies for degradation kinetic study of pharmaceuticals: An issue of utmost importance concerning current stability analysis practices. J. Anal. Sci. Technol. 2022, 13, 7. [Google Scholar] [CrossRef]
- Blessy, M.; Patel, R.D.; Prajapati, P.N.; Agrawal, Y.K. Development of forced degradation and stability indicating studies of drugs-A review. J. Pharm. Anal. 2014, 4, 159–165. [Google Scholar] [CrossRef]
- Baertschi, S.W.; Alsante, K.M.; Reed, R.A. Pharmaceutical Stress Testing: Predicting Drug Degradation; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Qiu, F.; Scrivens, G. Accelerated Predictive Stability (APS): Fundamentals and Pharmaceutical Industry Practices; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Huynh-Ba, K. Pharmaceutical Stability Testing to Support Global Markets; Springer Science & Business Media: Berlin, Germany, 2009. [Google Scholar]
- Gao, Y.; Glennon, B.; He, Y.; Donnellan, P. Dissolution kinetics of a BCS class II active pharmaceutical ingredient: Diffusion-based model validation and prediction. ACS Omega 2021, 6, 8056–8067. [Google Scholar] [CrossRef] [PubMed]
- Panda, R.; Lankalapalli, S. Bbioavailability and polymorphic stability challenges affecting drug product’s potential: A critical evaluation and pertinent solution. Asian J. Pharm. Cli. Res. 2023, 16, 9–23. [Google Scholar] [CrossRef]
- Censi, R.; Di Martino, P. Polymorph Impact on the Bioavailability and Stability of Poorly Soluble Drugs. Molecules 2015, 20, 18759–18776. [Google Scholar] [CrossRef]
- Charoo, N.A.; Cristofoletti, R.; Kim, S.K. Integrating biopharmaceutics risk assessment and in vivo absorption model in formulation development of BCS class I drug using the QbD approach. Drug Dev. Ind. Pharm. 2017, 43, 668–677. [Google Scholar] [CrossRef] [PubMed]
- ICH, Harmonised Tripartite Guideline Specifications: Test Procedures and Acceptance Criteria for New Drug Substances and New Drug Products: Chemical Substances, Q6A. 1999. Available online: https://database.ich.org/sites/default/files/Q6A%20Guideline.pdf (accessed on 2 May 2024).
- Brittain, H.G.; Grant, D.J.R.; Myrdal, P.B. Effects of Polymorphism and Solid-State Solvation on Solubility and Dissolution Rate. In Polymorphism in Pharmaceutical Solids, 2nd ed.; Brittain, H.G., Ed.; CRC Press: Boca Raton, FL, USA, 2009; pp. 436–480. [Google Scholar]
- Zhou, Y.; Wang, J.; Xiao, Y.; Wang, T.; Huang, X. The effects of polymorphism on physicochemical properties and pharmacodynamics of solid drugs. Curr. Pharm. Des. 2018, 24, 2375–2382. [Google Scholar] [CrossRef] [PubMed]
- Avicel® for Solid Dosage Forms. Available online: http://www.fmcbiopolymer.com/Pharmaceutical/Products/Avicelforsoliddoseforms.aspx (accessed on 2 May 2024).
- Rumondor, A.C.; Taylor, L.S. Effect of polymer hygroscopicity on the phase behavior of amorphous solid dispersions in the presence of moisture. Mol. Pharm. 2010, 7, 477–490. [Google Scholar] [CrossRef]
- Talaczynska, A.; Dzitko, J.; Cielecka-Piontek, J. Benefits and limitations of polymorphic and amorphous forms of active pharmaceutical ingredients. Curr. Pharm. Des. 2016, 22, 4975–4980. [Google Scholar] [CrossRef]
- Valenti, S.; Barrio, M.; Negrier, P.; Romanini, M.; Macovez, R.; Tamarit, J.L. Comparative physical study of three pharmaceutically active benzodiazepine derivatives: Crystalline versus amorphous state and crystallization tendency. Mol. Pharm. 2021, 18, 1819–1832. [Google Scholar] [CrossRef]
- Červinka, C.; Fulem, M. Structure and glass transition temperature of amorphous dispersions of model pharmaceuticals with nucleobases from molecular dynamics. Pharmaceutics 2021, 13, 1253. [Google Scholar] [CrossRef]
- Yoshinari, T.; Forbes, R.T.; York, P.; Kawashima, Y. Moisture induced polymorphic transition of mannitol and its morphological transformation. Int. J. Pharm. 2002, 247, 69–77. [Google Scholar] [CrossRef]
- Sutton, S.; Jimenez, L. A Review of reported recalls involving microbiological control 2004-2011 with emphasis on FDA considerations of ‘objectionable organisms. Am. Pharm. Rev. 2012, 15, 42–57. [Google Scholar]
- Dao, H.; Lakhani, P.; Police, A.; Kallakunta, V.; Ajjarapu, S.S.; Wu, K.W.; Ponkshe, P.; Repka, M.A.; Narasimha Murthy, S. Microbial stability of pharmaceutical and cosmetic products. AAPS Pharmscitech 2018, 19, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, M.; Kubicka, M.M.; Kamińska, D.; Sawicka, P.; Długaszewska, J. Microbiological quality of non-sterile pharmaceutical products. Saudi Pharm. J. 2015, 23, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Bermúdez, A.; Rodríguez-de Lecea, J.; Soto-Esteras, T.; Vázquez-Estévez, C.; Chena-Cañete, C. Tipos de contaminantes microbianos de materias primas farmacéuticas [Types of microbial contaminants in pharmaceutical raw materials]. Rev. Latinoam. De Microbiol. 1991, 33, 153–157. [Google Scholar]
- Cundell, T. Mold monitoring and control in pharmaceutical manufacturing areas. Am. Pharm. Rev. 2016, 19. Available online: https://www.americanpharmaceuticalreview.com/Featured-Articles/190686-Mold-Monitoring-and-Control-in-Pharmaceutical-Manufacturing-Areas/ (accessed on 23 June 2024).
- Russell, M. Microbial Quality Assurance in Cosmetics, Toiletries and Non-Sterile Pharmaceuticals; Taylor & Francis: London, UK, 1996; pp. 31–47. [Google Scholar]
- Grant, W.D. Life at low water activity. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2004, 359, 1249–1267. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, Y.; Salazar, J.K.; Yang, J.; Tortorello, M.L.; Zhang, W. Increased water activity reduces the thermal resistance of salmonella enterica in peanut butter. Appl. Environ. Microbiol. 2013, 79, 4763–4767. [Google Scholar] [CrossRef] [PubMed]
- United States Pharmacopoeia. Microbiological Examination of Non-Sterile Products: Tests for Specified Microorganisms, 31st ed.; Twin brook, Parkway Rockvalie; United States Pharmacopoeial Convention: Frederick, MD, USA, 2012; Volume 1, pp. 56–65. [Google Scholar]
- Denyer, S.P.; Baird, R.M. Microbial contamination, spoilage and hazard. In Guide to Microbiological Control in Pharmaceuticals and Medical Devices, 2nd ed.; Taylor & Francis: London, UK, 2007; pp. 24–44. [Google Scholar]
- Tuesuwan, B.; Vongsutilers, V. Nitrosamine contamination in pharmaceuticals: Threat, impact, and control. J. Pharm. Sci. 2021, 110, 3118–3128. [Google Scholar] [CrossRef] [PubMed]
- FDA-U.S. Food & Drug Administration. FDA Requests Removal of All Ranitidine Products (Zantac) from the Market. 2020. Available online: https://www.fda.gov/news-events/press-announcements/fda-requests-removalall-ranitidine-products-zantac-market (accessed on 10 December 2021).
- EMA-European Medicines Agency. EMA to Review Ranitidine Medicines following Detection of NDMA. Press Release. 2019. Available online: https://www.ema.europa.eu/en/news/ema-review-ranitidine-medicines-following-detection-ndma. (accessed on 1 December 2020).
- Charoo, N.A.; Dharani, S.; Khan, M.A.; Rahman, Z. Nitroso impurities in drug products: An overview of risk assessment, regulatory milieu, and control strategy. AAPS PharmSciTech 2023, 24, 60. [Google Scholar] [CrossRef]
- Ashworth, I.W.; Dirat, O.; Teasdale, A.; Whiting, M. Potential for the formation of N-nitrosamines during the manufacture of active pharmaceutical ingredients: An assessment of the risk posed by trace nitrite in water. Org. Process Res. Dev. 2020, 24, 1629–1646. [Google Scholar] [CrossRef]
- ICH, Harmonised Guideline. Quality Risk Management Q9(R1). 2023. Available online: https://database.ich.org/sites/default/files/ICH_Q9%28R1%29_Guideline_Step4_2023_0126_0.pdf (accessed on 2 May 2024).
- Stamatis, D.H. Failure Mode and Effect Analysis, Fmea from Theory to Execution, 2nd ed.; ASQ Quality Press: Milwaukee, WI, USA, 2003. [Google Scholar]
- Charoo, N.A.; Ali, A.A. Quality risk management in pharmaceutical development. Drug Dev. Ind. Pharm. 2013, 39, 947–960. [Google Scholar] [CrossRef] [PubMed]
- Githens, G.D.; Peterson, R.J. Using risk management in the front end of projects. In Proceedings of the Project Management Institute 32nd Annual Seminars & Symposium, Nashville, TN, USA, 1–10 November 2001; p. 4. [Google Scholar]
- Florence, A.T.; Attwood, D. Drug Stability. In Physicochemical Principles of Pharmacy; Palgrave: London, UK, 1998. [Google Scholar]
- Charoo, N.; Cristofoletti, R.; Graham, A.; Lartey, P.; Abrahamsson, B.; Groot, D.W.; Kopp, S.; Langguth, P.; Polli, J.; Shah, V.P.; et al. Biowaiver monograph for immediate-release solid oral dosage forms: Fluconazole. J. Pharm. Sci. 2014, 103, 3843–3858. [Google Scholar] [CrossRef] [PubMed]
- Alkhamis, K.A.; Obaidat, A.A.; Nuseirat, A.F. Solid-state characterization of fluconazole. Pharm. Dev. Technol. 2002, 7, 491–503. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, M.S.; Kim, J.S.; Cho, W.; Park, J.; Cha, K.H.; Kang, Y.S.; Hwang, S.J. Solid-state carbon NMR characterization and investigation of intrinsic dissolution behavior of fluconazole polymorphs, anhydrate forms I and II. Chem. Pharm. Bull. 2010, 58, 1243–1247. [Google Scholar] [CrossRef] [PubMed]
- Public Assessment Report Scientific Discussion: Losartan kalium Pharmaclan 50 mg and 100 mg Film-Coated Tablets (Losartan Potassium). NL/H/5449/001-002/DC, 2023. Available online: https://www.geneesmiddeleninformatiebank.nl/pars/h128848.pdf (accessed on 2 May 2024).
- IPEC. Excipient Stability Guide. 2022. Available online: https://www.ipec-europe.org/guidelines.html (accessed on 2 May 2024).
- Polyplasdone™ Crospovidone Superdisintegrants. Product Overview. Available online: http://www.ashland.com/Ashland/Static/Documents/ASI/PC_11319_Polyplasdone_Overview.pdf (accessed on 2 May 2024).
- Wu, Y.; Levons, J.; Narang, A.S.; Raghavan, K.; Rao, V.M. Reactive impurities in excipients: Profiling, identification and mitigation of drug-excipient incompatibility. AAPS PharmSciTech 2011, 12, 1248–1263. [Google Scholar] [CrossRef]
- Crospovidone. In Handbook of Pharmaceutical Excipients, 6th ed.; Rowe, R.C.; Sheskey, P.J.; Quinn, M.E. (Eds.) Pharmaceutical Press: London, UK; American Pharmaceutical Association: Washington, DC, USA, 2009; pp. 208–210. [Google Scholar]
- Boetzel, R.; Schlingemann, J.; Hickert, S.; Korn, C.; Kocks, G.; Luck, B.; Blom, G.; Harrison, M.; François, M.; Allain, L.; et al. A nitrite excipient database: A useful tool to support N-nitrosamine risk assessments for drug products. J. Pharm. Sci. 2022, 112, 1615–1624. [Google Scholar] [CrossRef]
- Mannitol. In Handbook of Pharmaceutical Excipients, 6th ed.; Rowe, R.C.; Sheskey, P.J.; Quinn, M.E. (Eds.) Pharmaceutical Press: London, UK; American Pharmaceutical Association: Washington, DC, USA, 2009; pp. 424–428. [Google Scholar]
- Pearlitol® 200 SD. The Ultimate Mannitol for DC Tablets. Available online: http://www.roquette-pharma.com/brochures/17/visio.html (accessed on 2 May 2024).
- Nasr, N.E.H.; Metwaly, M.G.; Ahmed, E.O.; Fares, A.R.; ElMeshad, A.N. Investigating the root cause of N-nitrosodimethylamine formation in metformin pharmaceutical products. Expert Opin. Drug Saf. 2021, 20, 855–862. [Google Scholar] [CrossRef]
- FDA-U.S. Food & Drug Administration. Control of Nitrosamine Impurities in Human Drugs Guidance for Industry. 2021. Available online: https://www.fda.gov/media/141720/download (accessed on 10 January 2022).
| Drug | Polymorph Used in Formulation | Polymorphic Transformation |
|---|---|---|
| Ritonavir | Form I | Conversion of Form I to Form II led to failure in meeting dissolution specification. The two forms differ in solubility significantly. |
| Rifaximin | Rifaximin-α | Conversion to amorphous rifaximin, may lead to systemic absorption of this otherwise locally acting GIT (gastro-intestinal tract) drug resulting in serious safety issues. |
| Celiprolol hydrochloride | Form I | Form I converts to form II on exposure to high humidity (>80% RH) over a period of one month. Therefore, it is essential to control humidity condition during its storage and processing. |
| Carbamazepine | Form III | Form III is unstable and absorbs high percentage of water on storage and converts to carbamazepine dihydrate resulting in drop in dissolution. Form I is relatively stable. The dissolution rate rank orders as III > form I > dihydrate. |
| Risk Category | Retest Period | |
|---|---|---|
| Non-Significant | Significant | |
| Low | 6 months | 3 months |
| Medium | 6 months | 2 months |
| High | 3 months | 1 month |
| Parameters | USP * | Ph.Eur. * | JP * | Polyplasdone™ XL-10 | ||||
|---|---|---|---|---|---|---|---|---|
| Stability | Impact on Product CQAs | Occurrence Probability (Change in Material Attributes) | Detection @ | Tests to Be Performed at Retest | ||||
| Definition (description/appearance) | + | + | + | Stable | High Can impact CQAs of product such as disintegration time, dissolution and impurity profile. | Low to moderate | High Control strategy for finished product | + |
| Assay (Nitrogen) | - | |||||||
| Identification | + | + | + | - | ||||
| Peroxides | + | + | + | + | ||||
| Water soluble substances | + | + | + | - | ||||
| Impurity A | + | - | + | + | ||||
| Loss on drying | + | + | + | + | ||||
| Residue on ignition/sulfated ash | + | + | + | - | ||||
| Assay | + | + | + | + | ||||
| Storage | + | + | + | - | ||||
| Microbial enumeration test | - | - | - | - | ||||
| Particle size | In-house test | + | ||||||
| Parameters | USP * | Ph.Eur. * | JP * | Pearlitol ® | ||||
|---|---|---|---|---|---|---|---|---|
| Stability | Impact on Product CQAs | Occurrence Probability (Change in Material Attributes) | Detection@ | Tests to Be Performed at Retest | ||||
| Definition (description/appearance) | + | + | + | Very stable | High Can impact CQAs of product such as uniformity of dosage units, dissolution and influence compressibility. | Low | High Control strategy for finished product | + |
| Identification | + | + | + | - | ||||
| Assay | + | + | + | + | ||||
| Related substances (impurities) | + | + | + | + | ||||
| Reducing sugars | + | + | + | + | ||||
| Nickel | + | - | + | - | ||||
| Melting range or temperature | + | + | + | - | ||||
| Appearance of solution | + | + | + | - | ||||
| Loss on drying | + | + | + | + | ||||
| Conductivity | + | + | + | - | ||||
| Microbial enumeration test | + | + | - | + | ||||
| Bacterial endotoxin test | + | + | - | + | ||||
| Labeling | + | + | - | - | ||||
| Particle size | In-house test | + | ||||||
| Retest Period | ||
|---|---|---|
| First Retest Date | Second Retest Date | |
| Within the boundaries of the retest date assigned by the excipient manufacturer | Within the boundaries of the retest date assigned by the excipient manufacturer | Beyond the retest date designated by the excipient manufacturer |
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charoo, N.A.; Akanji, O.; Rahman, Z.; Khan, A.A.; Badshah, A. Risk-Based Approach for Defining Retest Dates for Active Pharmaceutical Ingredients and Excipients. Pharmaceuticals 2024, 17, 903. https://doi.org/10.3390/ph17070903
Charoo NA, Akanji O, Rahman Z, Khan AA, Badshah A. Risk-Based Approach for Defining Retest Dates for Active Pharmaceutical Ingredients and Excipients. Pharmaceuticals. 2024; 17(7):903. https://doi.org/10.3390/ph17070903
Chicago/Turabian StyleCharoo, Naseem A., Omotayo Akanji, Ziyaur Rahman, Aqeel A. Khan, and Aqal Badshah. 2024. "Risk-Based Approach for Defining Retest Dates for Active Pharmaceutical Ingredients and Excipients" Pharmaceuticals 17, no. 7: 903. https://doi.org/10.3390/ph17070903
APA StyleCharoo, N. A., Akanji, O., Rahman, Z., Khan, A. A., & Badshah, A. (2024). Risk-Based Approach for Defining Retest Dates for Active Pharmaceutical Ingredients and Excipients. Pharmaceuticals, 17(7), 903. https://doi.org/10.3390/ph17070903







