Fentanyl Overdose Causes Prolonged Cardiopulmonary Dysregulation in Male SKH1 Mice
Abstract
:1. Introduction
2. Results
3. Discussion
Limitations
4. Materials and Methods
4.1. Animals
4.2. Drug Treatment
4.3. Biomarker Assays
4.4. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mattson, C.L.; Tanz, L.J.; Quinn, K.; Kariisa, M.; Patel, P.; Davis, N.L. Trends and Geographic Patterns in Drug and Synthetic Opioid Overdose Deaths—United States, 2013–2019. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Zoorob, R.; Uptegrove, L.; Park, B.L. Case Report of Very-Low-Dose Fentanyl Causing Fentanyl-Induced Chest Wall Rigidity. Cureus 2023, 15, e43788. [Google Scholar] [CrossRef] [PubMed]
- Rzasa Lynn, R.; Galinkin, J.L. Naloxone Dosage for Opioid Reversal: Current Evidence and Clinical Implications. Ther. Adv. Drug Saf. 2018, 9, 63–88. [Google Scholar] [CrossRef]
- National EMS information System NEMSIS Database. Available online: https://nemsis.org/view-reports/public-reports/ (accessed on 1 April 2024).
- Chen, X.-Y.; Wang, L.; Ma, X.; Yang, F.; Wang, X.; Xu, P.; Xu, L.-L.; Di, B. Development of Fentanyl-Specific Monoclonal Antibody (MAb) to Antagonize the Pharmacological Effects of Fentanyl. Toxicol. Appl. Pharmacol. 2024, 486, 116918. [Google Scholar] [CrossRef]
- Furdui, A.; da Silveira Scarpellini, C.; Montandon, G. Fentanyl-Induced Respiratory Depression and Locomotor Hyperactivity Are Mediated by μ-Opioid Receptors Expressed in Somatostatin-Negative Neurons. eNeuro 2023, 10, ENEURO.0035-23.2023. [Google Scholar] [CrossRef]
- Du, K.; Shi, Q.; Zhou, X.; Zhang, L.; Su, H.; Zhang, C.; Wei, Z.; Liu, T.; Wang, L.; Wang, X.; et al. Melatonin Attenuates Fentanyl—Induced Behavioral Sensitization and Circadian Rhythm Disorders in Mice. Physiol. Behav. 2024, 279, 114523. [Google Scholar] [CrossRef] [PubMed]
- Newman, M.; Lynch, C.; Connery, H.; Goldsmith, W.; Nurkiewicz, T.; Raylman, R.; Boyd, J. Fentanyl Overdose: Temporal Effects and Prognostic Factors in SKH1 Mice. Basic Clin. Pharmacol. Toxicol. 2024, 134, 460–471. [Google Scholar] [CrossRef]
- Haouzi, P.; Tubbs, N. Effects of Fentanyl Overdose-Induced Muscle Rigidity and Dexmedetomidine on Respiratory Mechanics and Pulmonary Gas Exchange in Sedated Rats. J. Appl. Physiol. 2022, 132, 1407–1422. [Google Scholar] [CrossRef]
- Topacoglu, H.; Karcioglu, O.; Cimrin, A.H.; Arnold, J. Respiratory Arrest after Low-Dose Fentanyl. Ann. Saudi Med. 2005, 25, 508–510. [Google Scholar] [CrossRef]
- Ferdinand, P.; Roffe, C. Hypoxia after Stroke: A Review of Experimental and Clinical Evidence. Exp. Transl. Stroke Med. 2016, 8, 9. [Google Scholar] [CrossRef]
- Essentials of Pain Medicine and Regional Anesthesia; Elsevier: Philadelphia, PA, USA, 2005; ISBN 9780443066511.
- Bubier, J.A.; He, H.; Philip, V.M.; Roy, T.; Hernandez, C.M.; Bernat, R.; Donohue, K.D.; O’Hara, B.F.; Chesler, E.J. Genetic Variation Regulates Opioid-Induced Respiratory Depression in Mice. Sci. Rep. 2020, 10, 14970. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.D. Depression Effects on Long-Term Prescription Opioid Use, Abuse, and Addiction. Clin. J. Pain 2018, 34, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Allegri, N.; Mennuni, S.; Rulli, E.; Vanacore, N.; Corli, O.; Floriani, I.; De Simone, I.; Allegri, M.; Govoni, S.; Vecchi, T.; et al. Systematic Review and Meta-Analysis on Neuropsychological Effects of Long-Term Use of Opioids in Patients with Chronic Noncancer Pain. Pain. Pract. 2019, 19, 328–343. [Google Scholar] [CrossRef] [PubMed]
- Toska, E.; Mayrovitz, H.N. Opioid Impacts on Cardiovascular Health. Cureus 2023, 15, e46224. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Kersten, J.R.; Riess, M.L. Opioid-Induced Cardioprotection. Curr. Pharm. Des. 2014, 20, 5696–5705. [Google Scholar] [CrossRef] [PubMed]
- Radke, J.B.; Owen, K.P.; Sutter, M.E.; Ford, J.B.; Albertson, T.E. The Effects of Opioids on the Lung. Clin. Rev. Allergy Immunol. 2014, 46, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, T.; Sadikot, R.T. Opioid Effect on Lungs. Respirology 2013, 18, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, D.E.; Andrew, T.A. Common Findings and Predictive Measures of Opioid Overdoses. Acad. Forensic. Pathol. 2017, 7, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Yo, C.-H.; Hsu, W.-T.; Tsou, E.P.-Y.; Wang, Y.-C.; Ling, D.-A.; Lee, A.-F.; Liu, M.A.; Lee, C.-C. Use of Opioids and Outcomes of Pneumonia: Results from the US Nationwide Inpatient Sample. J. Acute Med. 2021, 11, 113–128. [Google Scholar] [CrossRef]
- Bataduwaarachchi, V.R.; Hansanie, S.; Rockwood, N.; D’Cruz, L.G. Immunomodulatory Properties of Morphine and the Hypothesised Role of Long-Term Opioid Use in the Immunopathogenesis of Tuberculosis. Front. Immunol. 2023, 14, 1265511. [Google Scholar] [CrossRef]
- Sun, Q.; Li, Z.; Wang, Z.; Wang, Q.; Qin, F.; Pan, H.; Lin, W.; Mu, X.; Wang, Y.; Jiang, Y.; et al. Immunosuppression by Opioids: Mechanisms of Action on Innate and Adaptive Immunity. Biochem. Pharmacol. 2023, 209, 115417. [Google Scholar] [CrossRef]
- Husain, S.; Liou, G.I.; Crosson, C.E. Opioid Receptor Activation: Suppression of Ischemia/Reperfusion-Induced Production of TNF-α in the Retina. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2577–2583. [Google Scholar] [CrossRef] [PubMed]
- Plein, L.M.; Rittner, H.L. Opioids and the Immune System—Friend or Foe. Br. J. Pharmacol. 2018, 175, 2717–2725. [Google Scholar] [CrossRef] [PubMed]
- Molina-Martínez, L.M.; González-Espinosa, C.; Cruz, S.L. Dissociation of Immunosuppressive and Nociceptive Effects of Fentanyl, but Not Morphine, after Repeated Administration in Mice: Fentanyl-Induced Sensitization to LPS. Brain Behav. Immun. 2014, 42, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Oh, W.S. Effect of Fentanyl on TNF-Alpha and IL-1beta Levels during Global Ischemia/Reperfusion in Rats. Int. J. Tissue React. 2002, 24, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Chu, H.; Jiang, Y.; Yuan, L. Morphine Enhances IL-1β Release through Toll-like Receptor 4-Mediated Endocytic Pathway in Microglia. Purinergic Signal. 2016, 12, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Alasmari, F.; Alasmari, M.S.; Assiri, M.A.; Alswayyed, M.; Rizwan Ahamad, S.; Alhumaydhi, A.I.; Arif, B.I.; Aljumayi, S.R.; AlAsmari, A.F.; Ali, N.; et al. Liver Metabolomics and Inflammatory Profiles in Mouse Model of Fentanyl Overdose Treated with Beta-Lactams. Metabolites 2023, 13, 965. [Google Scholar] [CrossRef] [PubMed]
- Mizher, H.; Zin, C.S.; Helal Uddin, A.B.; Mohamed, A.H.; Ling, T.H.; Izzat, M. Plasma Concentrations of Pro-Inflammatory Cytokine IL-6 and Antiinflammatory Cytokine IL-10 in Short- and Long-Term Opioid Users with Noncancer Pain. J. Pharm. Bioallied Sci. 2020, 12, S663–S666. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Liu, R.; Chen, C.; Ji, F.; Li, T. Opioid System Modulates the Immune Function: A Review. Transl. Perioper. Pain Med. 2016, 1, 5–13. [Google Scholar]
- Sutcliffe, K.J.; Corey, R.A.; Alhosan, N.; Cavallo, D.; Groom, S.; Santiago, M.; Bailey, C.; Charlton, S.J.; Sessions, R.B.; Henderson, G.; et al. Interaction with the Lipid Membrane Influences Fentanyl Pharmacology. Adv. Drug Alcohol Res. 2022, 2, 10280. [Google Scholar] [CrossRef]
- Torralva, R.; Eshleman, A.J.; Swanson, T.L.; Schmachtenberg, J.L.; Schutzer, W.E.; Bloom, S.H.; Wolfrum, K.M.; Reed, J.F.; Janowsky, A. Fentanyl but Not Morphine Interacts with Nonopioid Recombinant Human Neurotransmitter Receptors and Transporters. J. Pharmacol. Exp. Ther. 2020, 374, 376–391. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.B.; McCollum, D. Roberts and Hedges’ Clinical Procedures in Emergency Medicine and Acute Care, 7th ed.; Roberts, J.R., Custalow, C.B., Thomsen, T.W., Eds.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Elder, H.J.; Walentiny, D.M.; Beardsley, P.M. Theophylline Reverses Oxycodone’s but Not Fentanyl’s Respiratory Depression in Mice While Caffeine Is Ineffective against Both Opioids. Pharmacol. Biochem. Behav. 2023, 229, 173601. [Google Scholar] [CrossRef] [PubMed]
- Elder, H.J.; Walentiny, D.M.; Beardsley, P.M. Enantiomeric Contributions to Methamphetamine’s Bidirectional Effects on Basal and Fentanyl-Depressed Respiration in Mice. Pharmacol. Biochem. Behav. 2024, 238, 173735. [Google Scholar] [CrossRef] [PubMed]
- Rademeyer, K.M.; Nass, S.R.; Jones, A.M.; Ohene-Nyako, M.; Hauser, K.F.; McRae, M. Fentanyl Dysregulates Neuroinflammation and Disrupts Blood-Brain Barrier Integrity in HIV-1 Tat Transgenic Mice. J. Neurovirol. 2024, 30, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Torralva, R.; Janowsky, A. Noradrenergic Mechanisms in Fentanyl-Mediated Rapid Death Explain Failure of Naloxone in the Opioid Crisis. J. Pharmacol. Exp. Ther. 2019, 371, 453–475. [Google Scholar] [CrossRef] [PubMed]
- Yamanoue, T.; Brum, J.M.; Estafanous, F.G.; Khairallah, P.A.; Ferrario, C.M. Fentanyl Attenuates Porcine Coronary Arterial Contraction through M3-Muscarinic Antagonism. Anesth. Analg. 1993, 76, 382–390. [Google Scholar] [PubMed]
- Tao, R.; Karnik, M.; Ma, Z.; Auerbach, S.B. Effect of Fentanyl on 5-HT Efflux Involves Both Opioid and 5-HT1A Receptors. Br. J. Pharmacol. 2003, 139, 1498–1504. [Google Scholar] [CrossRef] [PubMed]
- Stevens, C.W.; Aravind, S.; Das, S.; Davis, R.L. Pharmacological Characterization of LPS and Opioid Interactions at the Toll-like Receptor 4. Br. J. Pharmacol. 2013, 168, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Jantsch, J.; Wiese, M.; Schödel, J.; Castiglione, K.; Gläsner, J.; Kolbe, S.; Mole, D.; Schleicher, U.; Eckardt, K.-U.; Hensel, M.; et al. Toll-like Receptor Activation and Hypoxia Use Distinct Signaling Pathways to Stabilize Hypoxia-Inducible Factor 1α (HIF1A) and Result in Differential HIF1A-Dependent Gene Expression. J. Leukoc. Biol. 2011, 90, 551–562. [Google Scholar] [CrossRef]
- Hirota, K.; Fukuda, R.; Takabuchi, S.; Kizaka-Kondoh, S.; Adachi, T.; Fukuda, K.; Semenza, G.L. Induction of Hypoxia-Inducible Factor 1 Activity by Muscarinic Acetylcholine Receptor Signaling. J. Biol. Chem. 2004, 279, 41521–41528. [Google Scholar] [CrossRef]
- Newman, M.; Connery, H.; Boyd, J. Opioids and Vitamin C: Known Interactions and Potential for Redox-Signaling Crosstalk. Antioxidants 2022, 11, 1267. [Google Scholar] [CrossRef] [PubMed]
- Laatifi, M.; Douzi, S.; Ezzine, H.; El Asry, C.; Naya, A.; Bouklouze, A.; Zaid, Y.; Naciri, M. Explanatory Predictive Model for COVID-19 Severity Risk Employing Machine Learning, Shapley Addition, and LIME. Sci. Rep. 2023, 13, 5481. [Google Scholar] [CrossRef] [PubMed]
- Franchi, S.; Moschetti, G.; Amodeo, G.; Sacerdote, P. Do All Opioid Drugs Share the Same Immunomodulatory Properties? A Review from Animal and Human Studies. Front. Immunol. 2019, 10, 2914. [Google Scholar] [CrossRef] [PubMed]
- Sacerdote, P.; Manfredi, B.; Mantegazza, P.; Panerai, A.E. Antinociceptive and Immunosuppressive Effects of Opiate Drugs: A Structure-Related Activity Study. Br. J. Pharmacol. 1997, 121, 834–840. [Google Scholar] [CrossRef]
- Marone, G.; Stellato, C.; Mastronardi, P.; Mazzarella, B. Mechanisms of Activation of Human Mast Cells and Basophils by General Anesthetic Drugs. Ann. Fr. Anesth. Reanim. 1993, 12, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Baldo, B.A.; Pham, N.H. Opioid Toxicity: Histamine, Hypersensitivity, and MRGPRX2. Arch. Toxicol. 2023, 97, 359–375. [Google Scholar] [CrossRef] [PubMed]
- Blunk, J.A.; Schmelz, M.; Zeck, S.; Skov, P.; Likar, R.; Koppert, W. Opioid-Induced Mast Cell Activation and Vascular Responses Is Not Mediated by Mu-Opioid Receptors: An in Vivo Microdialysis Study in Human Skin. Anesth. Analg. 2004, 98, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Henthorn, T.K.; Liu, Y.; Mahapatro, M.; Ng, K.Y. Active Transport of Fentanyl by the Blood-Brain Barrier. J. Pharmacol. Exp. Ther. 1999, 289, 1084–1089. [Google Scholar] [PubMed]
- Huo, X.-C.; Yang, H.-W.; Huang, L.-H.; Zhang, F.; Pan, Z.-Y.; Liao, Q. Organic Anion Transporting Polypeptide 1A2 Mediates Fentanyl Uptake in Cultured Cells. Mol. Med. Rep. 2020, 21, 485–492. [Google Scholar] [CrossRef]
- Froese, L.; Dian, J.; Batson, C.; Gomez, A.; Unger, B.; Zeiler, F.A. Cerebrovascular Response to Propofol, Fentanyl, and Midazolam in Moderate/Severe Traumatic Brain Injury: A Scoping Systematic Review of the Human and Animal Literature. Neurotrauma Rep. 2020, 1, 100–112. [Google Scholar] [CrossRef]
- Chi, O.Z.; Hunter, C.; Liu, X.; Chokshi, S.K.; Weiss, H.R. Effects of Fentanyl Pretreatment on Regional Cerebral Blood Flow in Focal Cerebral Ischemia in Rats. Pharmacology 2010, 85, 153–157. [Google Scholar] [CrossRef] [PubMed]
- de Nadal, M.; Munar, F.; Poca, M.A.; Sahuquillo, J.; Garnacho, A.; Rosselló, J. Cerebral Hemodynamic Effects of Morphine and Fentanyl in Patients with Severe Head Injury: Absence of Correlation to Cerebral Autoregulation. Anesthesiology 2000, 92, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The Pro- and Anti-Inflammatory Properties of the Cytokine Interleukin-6. Biochim. Biophys. Acta 2011, 1813, 878–888. [Google Scholar] [CrossRef]
- Bentley, J.B.; Conahan, T.J.; Cork, R.C. Fentanyl Sequestration in Lungs during Cardiopulmonary Bypass. Clin. Pharmacol. Ther. 1983, 34, 703–706. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.S. Opioid Metabolism. Mayo Clin. Proc. 2009, 84, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Akorn Pharmaceuticals Fentanyl Citrate Injection, USP Product Insert. Reference ID: 3336008. 2012. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/016619s034lbl.pdf (accessed on 10 March 2024).
- Kharasch, E.D. Opioid Half-Lives and Hemlines: The Long and Short of Fashion. Anesthesiology 2015, 122, 969–970. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.S.; Lee, H.J.; Nishida, M.; Lee, M.-S.; Tamura, R.; Yamashita, S.; Matsuzawa, Y.; Lee, I.-K.; Koh, G.Y. Betacellulin and Amphiregulin Induce Upregulation of Cyclin D1 and DNA Synthesis Activity through Differential Signaling Pathways in Vascular Smooth Muscle Cells. Circ. Res. 2003, 93, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Mifune, M.; Ohtsu, H.; Suzuki, H.; Frank, G.D.; Inagami, T.; Utsunomiya, H.; Dempsey, P.J.; Eguchi, S. Signal Transduction of Betacellulin in Growth and Migration of Vascular Smooth Muscle Cells. Am. J. Physiol. Cell Physiol. 2004, 287, C807–C813. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.R.; Dahlhoff, M.; Herbach, N.; Renner-Mueller, I.; Dalke, C.; Puk, O.; Graw, J.; Wanke, R.; Wolf, E. Betacellulin Overexpression in Transgenic Mice Causes Disproportionate Growth, Pulmonary Hemorrhage Syndrome, and Complex Eye Pathology. Endocrinology 2005, 146, 5237–5246. [Google Scholar] [CrossRef]
- Hedegger, K.; Algül, H.; Lesina, M.; Blutke, A.; Schmid, R.M.; Schneider, M.R.; Dahlhoff, M. Unraveling ERBB Network Dynamics upon Betacellulin Signaling in Pancreatic Ductal Adenocarcinoma in Mice. Mol. Oncol. 2020, 14, 1653–1669. [Google Scholar] [CrossRef]
- Jackson, L.F. Defective Valvulogenesis in HB-EGF and TACE-Null Mice Is Associated with Aberrant BMP Signaling. EMBO J. 2003, 22, 2704–2716. [Google Scholar] [CrossRef]
- Nakanishi, K.; Tsutsui, H.; Yoshimoto, T. Importance of IL-18-Induced Super Th1 Cells for the Development of Allergic Inflammation. Allergol. Int. 2010, 59, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Dima, E.; Koltsida, O.; Katsaounou, P.; Vakali, S.; Koutsoukou, A.; Koulouris, N.G.; Rovina, N. Implication of Interleukin (IL)-18 in the Pathogenesis of Chronic Obstructive Pulmonary Disease (COPD). Cytokine 2015, 74, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Seys, L.J.M.; Verhamme, F.M.; Schinwald, A.; Hammad, H.; Cunoosamy, D.M.; Bantsimba-Malanda, C.; Sabirsh, A.; McCall, E.; Flavell, L.; Herbst, R.; et al. Role of B Cell-Activating Factor in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2015, 192, 706–718. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.C.L.; Lam, C.W.K.; Tam, L.-S.; Wong, C.K. IL33: Roles in Allergic Inflammation and Therapeutic Perspectives. Front. Immunol. 2019, 10, 364. [Google Scholar] [CrossRef] [PubMed]
- Zharichenko, N.; Njoku, D.B. The Role of Pro-Inflammatory and Regulatory Signaling by IL-33 in the Brain and Liver: A Focused Systematic Review of Mouse and Human Data and Risk of Bias Assessment of the Literature. Int. J. Mol. Sci. 2020, 21, 3933. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, E.O.; Shimpo, M.; De Keulenaer, G.W.; MacGillivray, C.; Tominaga, S.; Solomon, S.D.; Rouleau, J.-L.; Lee, R.T. Expression and Regulation of ST2, an Interleukin-1 Receptor Family Member, in Cardiomyocytes and Myocardial Infarction. Circulation 2002, 106, 2961–2966. [Google Scholar] [CrossRef]
- Wei, P.; Liu, L.; Wang, X.; Zong, B.; Liu, X.; Zhang, M.; Fu, Q.; Wang, L.; Cao, B. Expression of Soluble ST2 in Patients with Essential Hypertension and Its Relationship with Left Ventricular Hypertrophy. ESC Heart Fail. 2023, 10, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Bayés-Genis, A.; González, A.; Lupón, J. ST2 in Heart Failure. Circ. Heart Fail. 2018, 11, e005582. [Google Scholar] [CrossRef]
- Veeraveedu, P.T.; Sanada, S.; Okuda, K.; Fu, H.Y.; Matsuzaki, T.; Araki, R.; Yamato, M.; Yasuda, K.; Sakata, Y.; Yoshimoto, T.; et al. Ablation of IL-33 Gene Exacerbate Myocardial Remodeling in Mice with Heart Failure Induced by Mechanical Stress. Biochem. Pharmacol. 2017, 138, 73–80. [Google Scholar] [CrossRef]
- Matthews, A.N.; Friend, D.S.; Zimmermann, N.; Sarafi, M.N.; Luster, A.D.; Pearlman, E.; Wert, S.E.; Rothenberg, M.E. Eotaxin Is Required for the Baseline Level of Tissue Eosinophils. Proc. Natl. Acad. Sci. USA 1998, 95, 6273–6278. [Google Scholar] [CrossRef] [PubMed]
- Diny, N.L.; Hou, X.; Barin, J.G.; Chen, G.; Talor, M.V.; Schaub, J.; Russell, S.D.; Klingel, K.; Rose, N.R.; Čiháková, D. Macrophages and Cardiac Fibroblasts Are the Main Producers of Eotaxins and Regulate Eosinophil Trafficking to the Heart. Eur. J. Immunol. 2016, 46, 2749–2760. [Google Scholar] [CrossRef] [PubMed]
- Jamaluddin, M.S.; Wang, X.; Wang, H.; Rafael, C.; Yao, Q.; Chen, C. Eotaxin Increases Monolayer Permeability of Human Coronary Artery Endothelial Cells. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 2146–2152. [Google Scholar] [CrossRef] [PubMed]
- Rothenberg, M.E.; MacLean, J.A.; Pearlman, E.; Luster, A.D.; Leder, P. Targeted Disruption of the Chemokine Eotaxin Partially Reduces Antigen-Induced Tissue Eosinophilia. J. Exp. Med. 1997, 185, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Zini, G. Abnormalities in Leukocyte Morphology and Number. In Blood and Bone Marrow Pathology; Elsevier: Amsterdam, The Netherlands, 2011; pp. 247–261. [Google Scholar]
- Alkhalil, M.; Kearney, A.; Hegarty, M.; Stewart, C.; Devlin, P.; Owens, C.G.; Spence, M.S. Eosinopenia as an Adverse Marker of Clinical Outcomes in Patients Presenting with Acute Myocardial Infarction. Am. J. Med. 2019, 132, e827–e834. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.-M.; Qin, W.-Q.; Wang, P.-J.; Wen, Z.-M. Eosinopenia Is a Predictive Factor for the Severity of Acute Ischemic Stroke. Neural Regen. Res. 2019, 14, 1772–1779. [Google Scholar] [CrossRef]
- Mancuso, G.; Berdondini, R.M.; Passarini, B. Eosinophilic Pustular Eruption Associated with Transdermal Fentanyl. J. Eur. Acad. Dermatol. Venereol. 2001, 15, 70–72. [Google Scholar] [CrossRef] [PubMed]
- Giannoglou, G.D.; Chatzizisis, Y.S.; Zamboulis, C.; Parcharidis, G.E.; Mikhailidis, D.P.; Louridas, G.E. Elevated Heart Rate and Atherosclerosis: An Overview of the Pathogenetic Mechanisms. Int. J. Cardiol. 2008, 126, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Crooks, G.M.; Hao, Q.L.; Petersen, D.; Barsky, L.W.; Bockstoce, D. IL-3 Increases Production of B Lymphoid Progenitors from Human CD34+CD38- Cells. J. Immunol. 2000, 165, 2382–2389. [Google Scholar] [CrossRef]
- Chen, D.; Tang, T.-X.; Deng, H.; Yang, X.-P.; Tang, Z.-H. Interleukin-7 Biology and Its Effects on Immune Cells: Mediator of Generation, Differentiation, Survival, and Homeostasis. Front. Immunol. 2021, 12, 747324. [Google Scholar] [CrossRef]
- Urbantat, R.M.; Blank, A.; Kremenetskaia, I.; Vajkoczy, P.; Acker, G.; Brandenburg, S. The CXCL2/IL8/CXCR2 Pathway Is Relevant for Brain Tumor Malignancy and Endothelial Cell Function. Int. J. Mol. Sci. 2021, 22, 2634. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hou, W.; Zuo, J.; Huang, Z.; Ding, X.; Bu, X. CXCL2 Acts as a Prognostic Biomarker and Associated with Immune Infiltrates in Stomach Adenocarcinoma. Medicine 2022, 101, e31096. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Lan, T.; Wei, Y.; Wei, X. CCL5/CCR5 Axis in Human Diseases and Related Treatments. Genes Dis. 2022, 9, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Peng, N.; Tang, Y.; Yu, N.; Wang, C.; Cai, X.; Zhang, L.; Hu, D.; Ciccia, F.; Lu, L. Roles of IL-25 in Type 2 Inflammation and Autoimmune Pathogenesis. Front. Immunol. 2021, 12, 691559. [Google Scholar] [CrossRef] [PubMed]
- Korbecki, J.; Gąssowska-Dobrowolska, M.; Wójcik, J.; Szatkowska, I.; Barczak, K.; Chlubek, M.; Baranowska-Bosiacka, I. The Importance of CXCL1 in Physiology and Noncancerous Diseases of Bone, Bone Marrow, Muscle and the Nervous System. Int. J. Mol. Sci. 2022, 23, 4205. [Google Scholar] [CrossRef] [PubMed]
- Sá, V.C.; Silva, T.A.; Reis, C.M.S.; Cunha, F.Q.; Figueiredo, F.; Bocca, A.L. The Pattern of Immune Cell Infiltration in Chromoblastomycosis: Involvement of Macrophage Inflammatory Protein-1 Alpha/CCL3 and Fungi Persistence. Rev. Inst. Med. Trop. S. Paulo 2007, 49, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Sutlović, D.; Definis-Gojanović, M. Suicide by Fentanyl. Arh. Hig. Rada Toksikol. 2007, 58, 317–321. [Google Scholar] [CrossRef]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Blighe, K.; Lun, A. PCAtools: PCAtools: Everything Principal Components Analysis. R Package Version 2.16.0. Available online: https://github.com/kevinblighe/PCAtools (accessed on 1 March 2024).
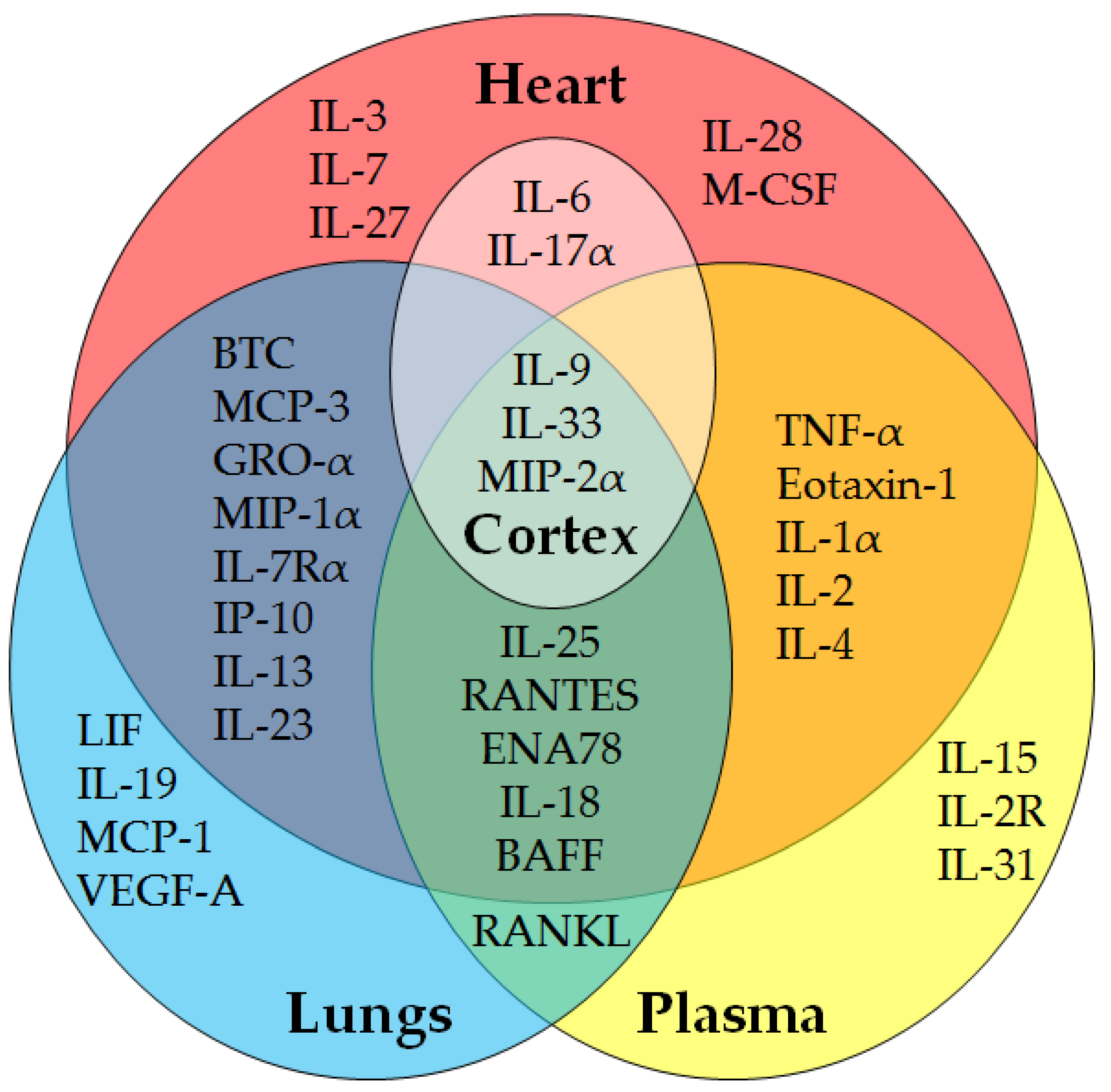
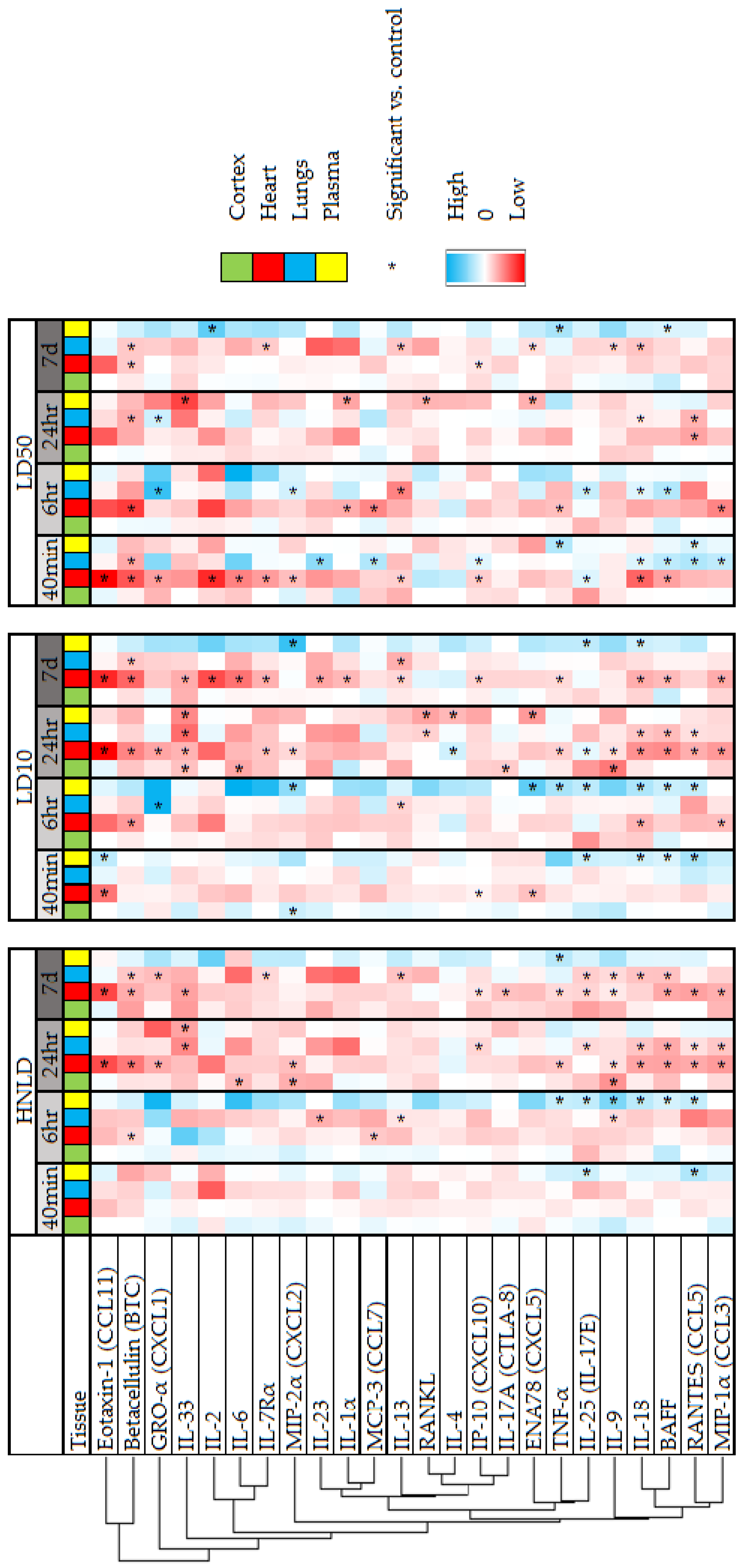
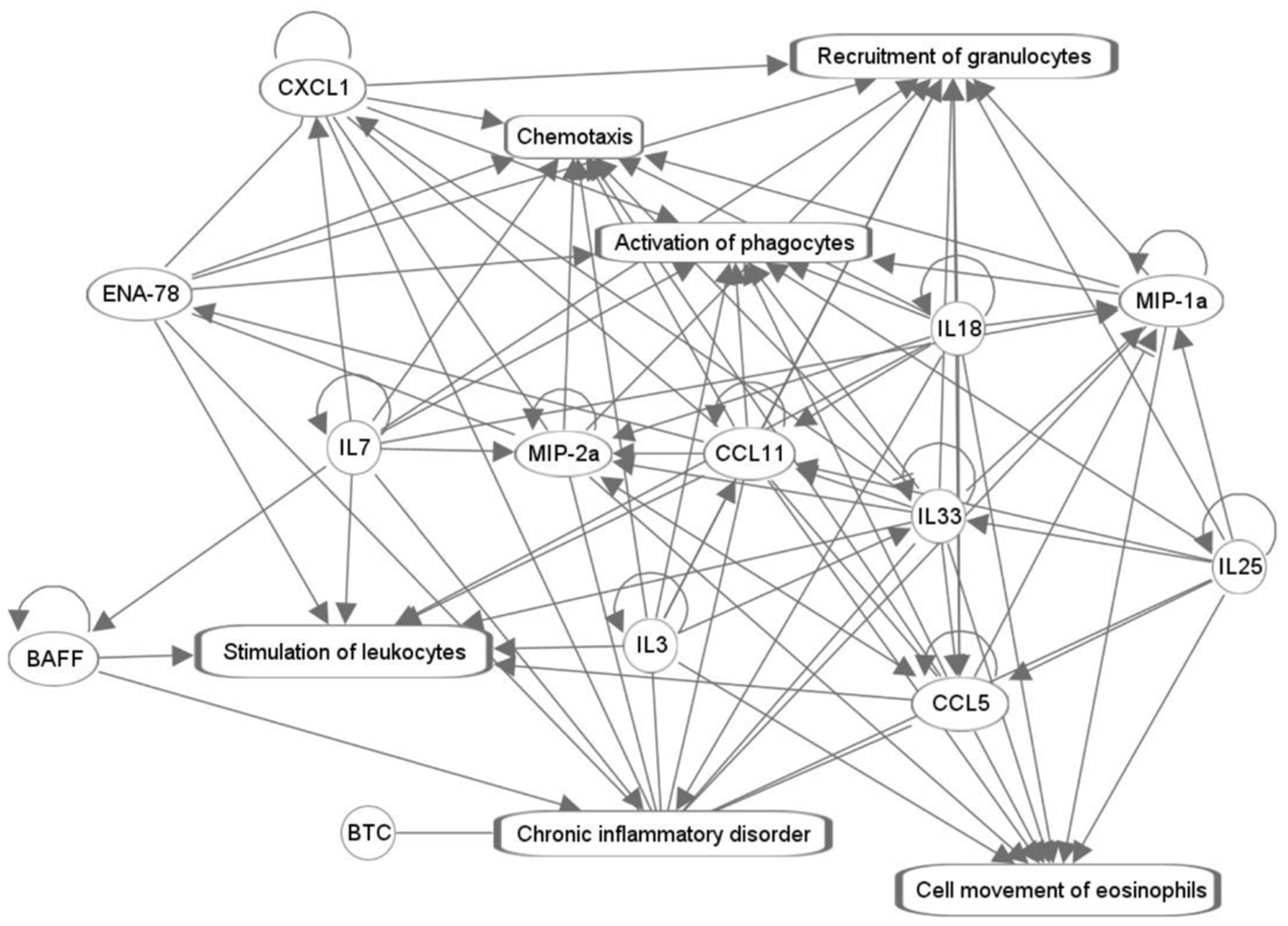
| Analyte | PC1 Loading | Analyte | PC2 Loading | |
|---|---|---|---|---|
| Eotaxin-1 | −0.5097 | BTC | −0.2070 | |
| BTC | −0.3312 | IL-7 | −0.2053 | |
| IL-7 | −0.3155 | MIP2α | 0.2133 | |
| IL-3 | −0.3146 | ENA78 | 0.2542 | |
| IL-18 | −0.2799 | IL-25 | 0.2668 | |
| BAFF | −0.2647 | BAFF | 0.2696 | |
| MIP-1α | −0.2055 | RANTES | 0.2858 | |
| GRO-α | −0.2053 | IL-33 | 0.4629 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Newman, M.; Connery, H.; Kannan, S.; Gautam, A.; Hammamieh, R.; Chakraborty, N.; Boyd, J. Fentanyl Overdose Causes Prolonged Cardiopulmonary Dysregulation in Male SKH1 Mice. Pharmaceuticals 2024, 17, 941. https://doi.org/10.3390/ph17070941
Newman M, Connery H, Kannan S, Gautam A, Hammamieh R, Chakraborty N, Boyd J. Fentanyl Overdose Causes Prolonged Cardiopulmonary Dysregulation in Male SKH1 Mice. Pharmaceuticals. 2024; 17(7):941. https://doi.org/10.3390/ph17070941
Chicago/Turabian StyleNewman, Mackenzie, Heather Connery, Swapna Kannan, Aarti Gautam, Rasha Hammamieh, Nabarun Chakraborty, and Jonathan Boyd. 2024. "Fentanyl Overdose Causes Prolonged Cardiopulmonary Dysregulation in Male SKH1 Mice" Pharmaceuticals 17, no. 7: 941. https://doi.org/10.3390/ph17070941








