A First Metabolite Analysis of Norfolk Island Pine Resin and Its Hepatoprotective Potential to Alleviate Methotrexate (MTX)-Induced Hepatic Injury
Abstract
:1. Introduction
2. Results
2.1. Phytochemical Analysis of AHR Extract
| No. | Identification | Rt (min) | [M] Exact Mass | Experimental Ion (m/z) | Formula | Ion | Ms/Ms | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1. | 13,14-dihydroagathic acid/ Labd-8(17)-en-15,19-dioic acid (Junicedric acid) | 9.55 | 336.2300 | 335.2226 | C20H32O4 | [M−H]− | 317.2110, 299.2001 | [31,32] |
| 2. | 10.63 | 336.2300 | 335.2228 | [M−H]− | 317.2110, 299.2001 | |||
| 3. | 12.54 | 336.2300 | 335.2205 | [M−H]− | 317.2110, 299.2001 | |||
| 4. | 15.57 | 336.2300 | 335.2202 | [M−H]− | 317.2110, 299.2001 | |||
| 5. | 16.37 | 336.2300 | 335.2207 | [M−H]− | 317.2110, 299.2001 | |||
| 6. | Agathic acid | 10.17 | 334.2144 | 333.2060 | C20H30O4 | [M−H]− | 315.1919, 289.1719, 273.1453 | [31] |
| 7. | 15-Formyloxylabd-8(17)-en-19-oic acid | 11.55 | 350.2457 | 349.2398 | C21H34O4 | [M−H]− | 315.1971, 301.1817 | [31,32] |
| 8. | 15.32 | 350.2457 | 373.2335 | [M+Na]+ | 323.1999, 301.2150 | |||
| 9. | trans-Communic acid | 11.64 | 302.2245 | 301.2151 | C20H30O2 | [M−H]− | 151.1190 | [31,33] |
| 10. | Dihydro-15-Acetoxy-8,9-epoxylabdane-19-oic acid/ Dihydro-15-Acetoxy-8,17-epoxylabdane-19-oic acid | 11.97 | 378.2406 | 377.2334 | C22H34O5 | [M−H]− | 316.9489, 301.1809 | [32,34] |
| 11. | 7-Oxodehydroabietic acid/6,7-Dehydroroyleanone | 11.88 | 314.1881 | 313.1749 | C20H26O3 | [M−H]− | 159.0800 | [35] |
| 12. | 11.57 | 314.1882 | 315.1955 | [M+H]+ | 297.1862, 269.1898 | |||
| 13. | Hardwickiic acid/Royleanone /20-Deoxocarnosol | 11.57 | 316.2038 | 315.1955 | C20H28O3 | [M−H]− | 297.1880, 271.2076 | [36] |
| 14. | 12.56 | 316.2038 | 315.1957 | [M−H]− | 297.1880, 271.2076 | |||
| 15. | 13.59 | 316.2038 | 315.19746 | [M−H]− | 297.1880, 271.2076 | |||
| 16. | 12.36 | 316.2038 | 317.2111 | [M+H]+ | 299.1913, 271.2040 | |||
| 17. | Hydroxy Copalic acid/ 19-Hydroxy-8,13E-labdadien-15-oic acid/ 15-Hydroxy-8,E-13-labdadien-19-oic acid/ (+)-Isocupressic acid | 11.61 | 320.2351 | 319.2256 | C20H32O3 | [M−H]− | 301.2172, 219.1386, 217.1199 | [37] |
| 18. | 12.65 | 320.2351 | 319.2253 | [M−H]− | 301.2172, 219.1386, 217.1199 | |||
| 19. | 13.91 | 320.2351 | 319.2253 | [M−H]− | 301.2172, 219.1386, 217.1199 | |||
| 20. | 15.59 | 320.2351 | 319.2257 | [M−H]− | 301.2172, 219.1386, 217.1199 | |||
| 21. | 3keto-copalic acid/ 12-Oxolabda-8(17),13E-dien-19 oic acid/ 7-Oxo-16-hydroxy-abiet-15(17)-en-19-al | 11.92 | 318.2194 | 317.2095 | C20H30O3 | [M−H]− | 299.2042, 273.1555 | [38] |
| 22. | 12.36 | 318.2194 | 317.2111 | [M−H]− | 299.2042, 273.1555 | |||
| 23. | 13.56 | 318.2194 | 317.2098 | [M−H]− | 299.2042, 273.1555 | |||
| 24. | 15.81 | 318.2194 | 317.2105 | [M−H]− | 299.2042, 273.1555 | |||
| 25. | 12.65 | 318.2194 | 319.2253 | [M+H]+ | 301.2151, 273.2099 | |||
| 26. | 8,13E-Labdadien-15,19-diol/Imbricatolal | 11.95 | 306.2558 | 305.2461 | C20H34O2 | [M−H]− | 287.2350, 257.2278 | [32] |
| 27. | 17.17 | 306.2558 | 305.2461 | [M−H]− | 287.2350, 257.2278 | |||
| 28. | 16.86 | 306.2558 | 305.2461 | [M−H]− | 287.2350, 257.2278 | |||
| 29. | 12-oxo-phytodienoic acid | 14.19 | 292.2038 | 291.1967 | C18H28O3 | [M−H]− | 249.0 | [39] |
| 30. | Acetoxy Copalic acid/Acetylisocupressic acid | 19.01 | 362.2457 | 361.2386 | C22H34O4 | [M−H]− | 301.2167, 219.1764, 217.8029, 189.6338 | [37] |
| 31. | 20.51 | 362.2457 | 361.2388 | [M−H]− | 301.2167, 219.1764, 217.8029, 189.6338 | |||
| 32. | 20.80 | 362.2457 | 361.2385 | [M−H]− | 301.2167, 219.1764, 217.8029, 189.6338 | |||
| 33. | Abietic acid | 21.08 | 302.2245 | 301.2175 | C20H30O2 | [M−H]− | 283.2650, 265.1478, 227.1994 | [40] |
| 34. | 22.45 | 302.2245 | 301.2174 | [M−H]− | 283.2650, 265.1478, 227.1994 | |||
| 35. | 22.96 | 302.2245 | 301.2176 | [M−H]− | 283.2650, 265.1478, 227.1994 | |||
| 36. | 21.29 | 302.2245 | 303.2306 | [M+H]+ | 285.2190, 267.2028, 227.1756 | |||
| 37. | Copalic acid/Kolavenic acid/ent-4(18)-13E-Clerodadien-15-oic acid | 21.59 | 304.2402 | 303.2306 | C20H32O2 | [M−H]− | 259.2420, 219.1381 | [40] |
| 38. | 9β,13β-Epoxy-7-abietene | 22.76 | 288.2453 | 287.2519 | C20H32O | [M−H]− | 215.1700 | [41] |
| 39. | 22.76 | 288.2453 | 289.2517 | [M+H]+ | 215.1700 | |||
| 40. | Phyllocladanol | 23.12 | 290.2609 | 289.2152 | C20H34O | [M−H]− | 149.1295 | [42] |
| 41. | Myristyl Glucoside | 22.51 | 376.2824 | 375.2753 | C20H40O6 | [M−H]− | 301.2170, 255.2336 | [43] |
| 42. | Palmitic acid | 22.82 | 256.2402 | 255.2330 | C16H32O2 | [M−H]− | 255.2334 | [31] |
| 43. | Pinobanksin-3-O-phenylpropionate | 24.49 | 404.3137 | 403.3046 | C24H20O6 | [M−H]− | 271.2021, 253.0872 | [44] |
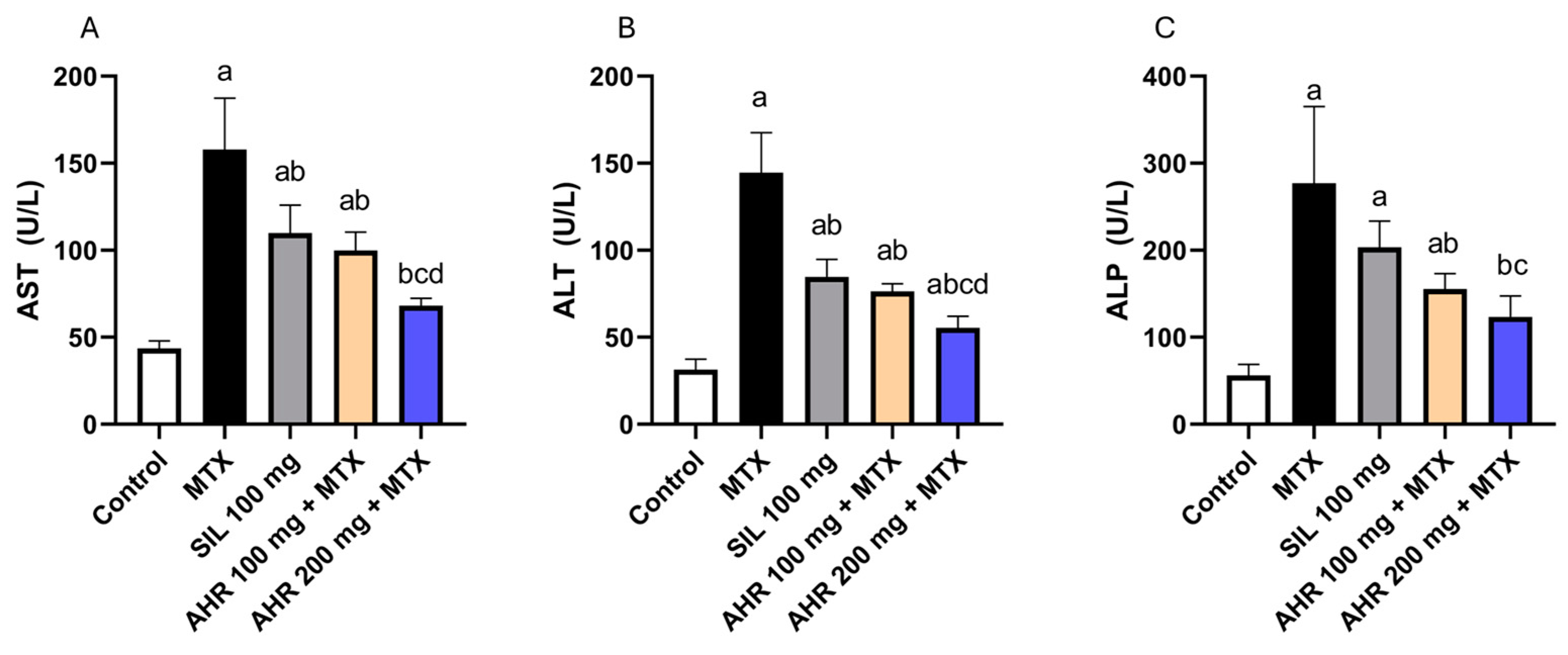
2.2. Effect of AHR on Hepatic Markers
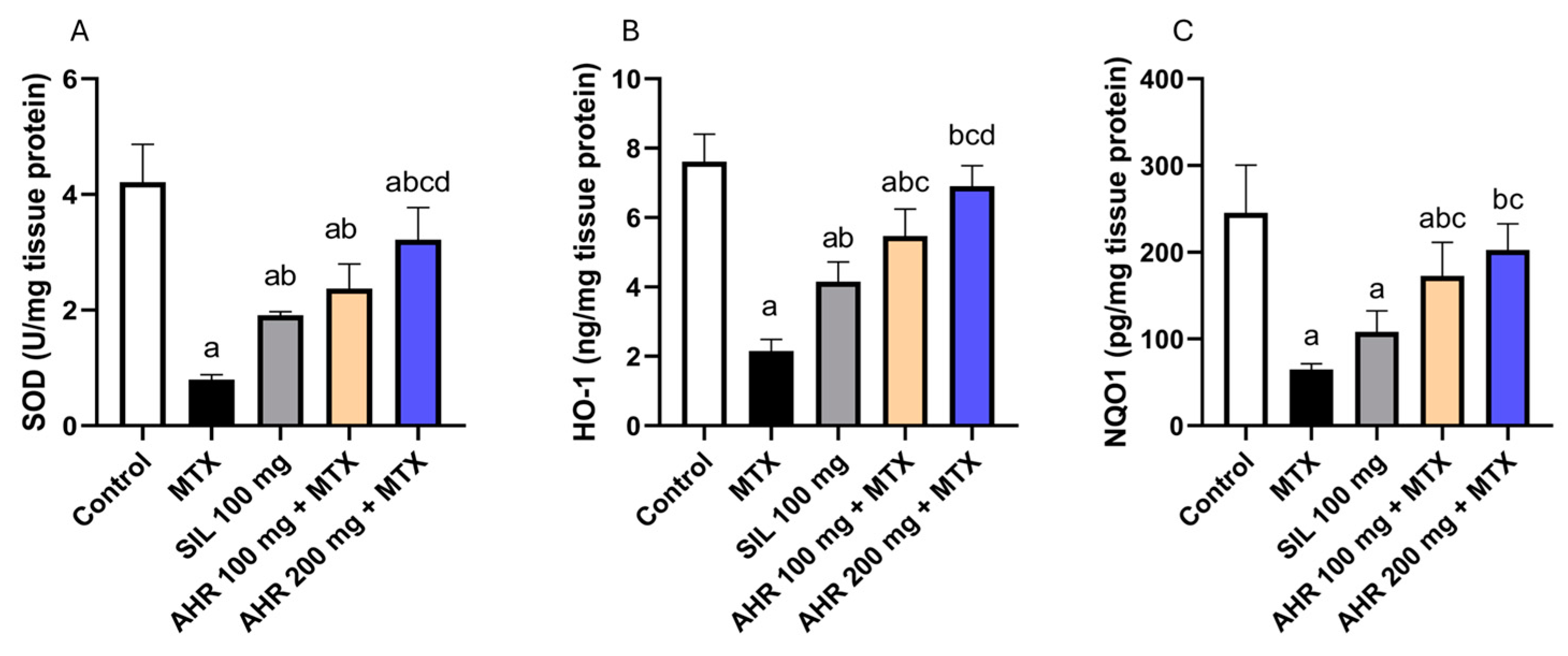
2.3. Effect of AHR on Antioxidant Parameters
2.4. Effect of AHR on NF-κB, IL-1β, IL-6 and TNF-α
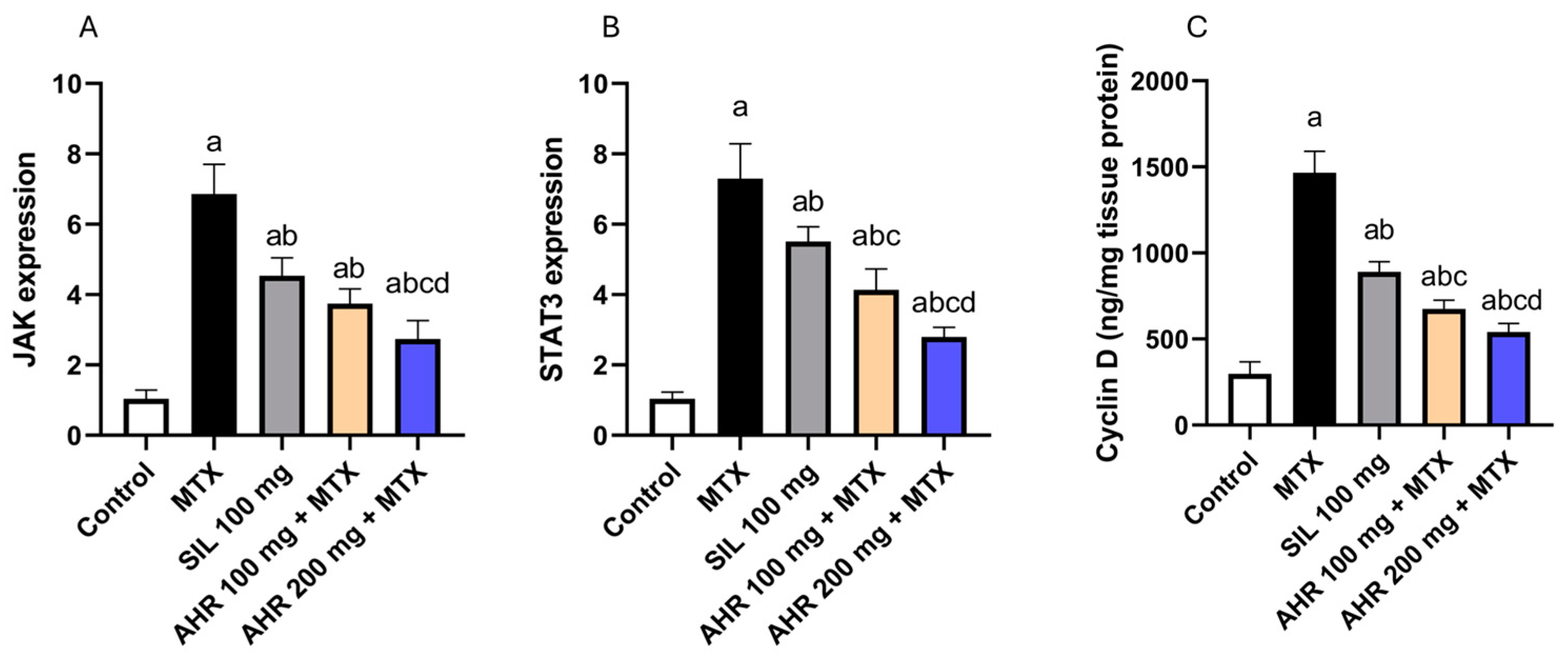
2.5. Effect of AHR on JAK, STAT3 and Cyclin D Signaling
2.6. Effect of AHR on p38 and BCL2
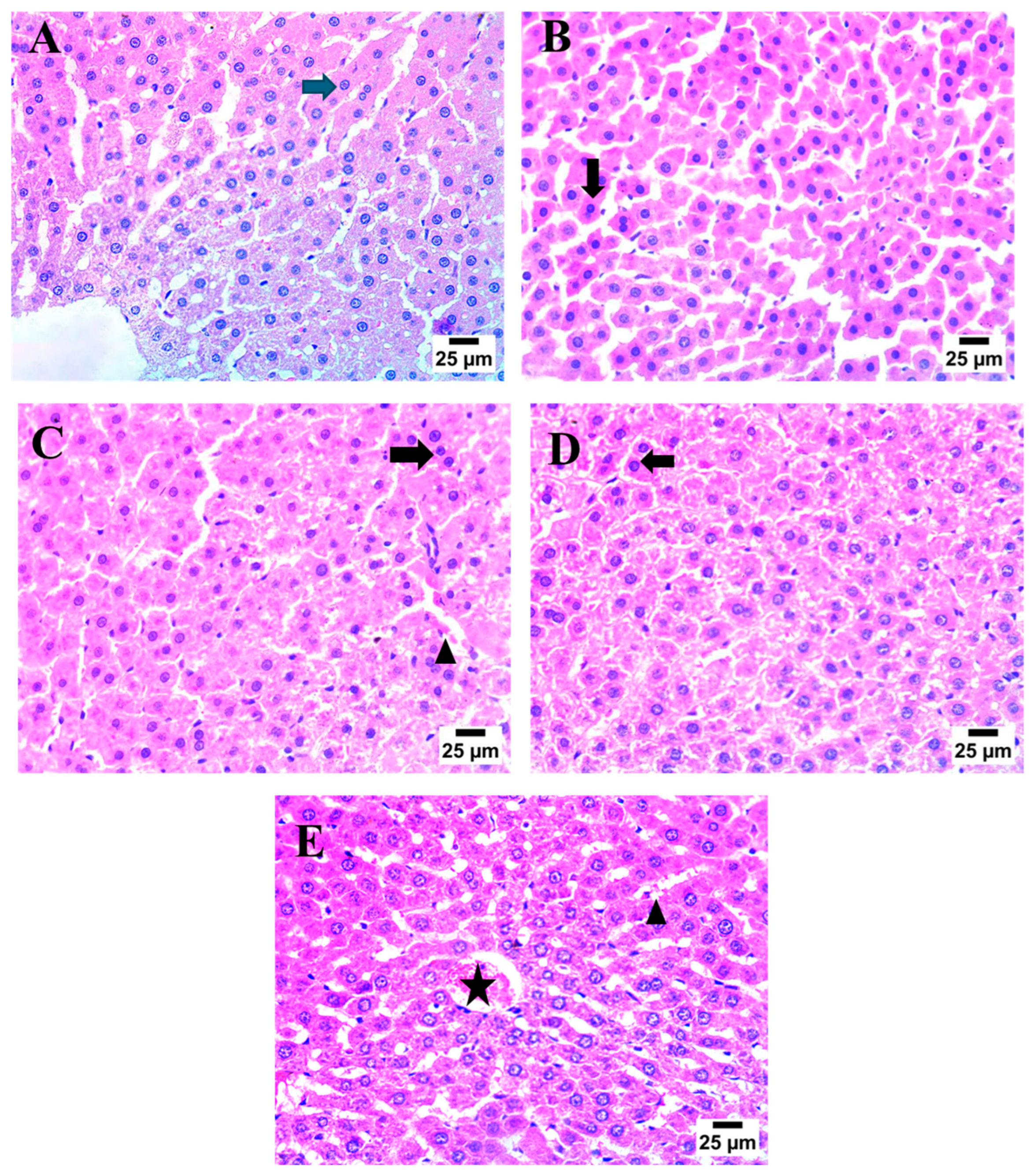
2.7. Effect of AHR on MTX-Induced Histopathological Alterations
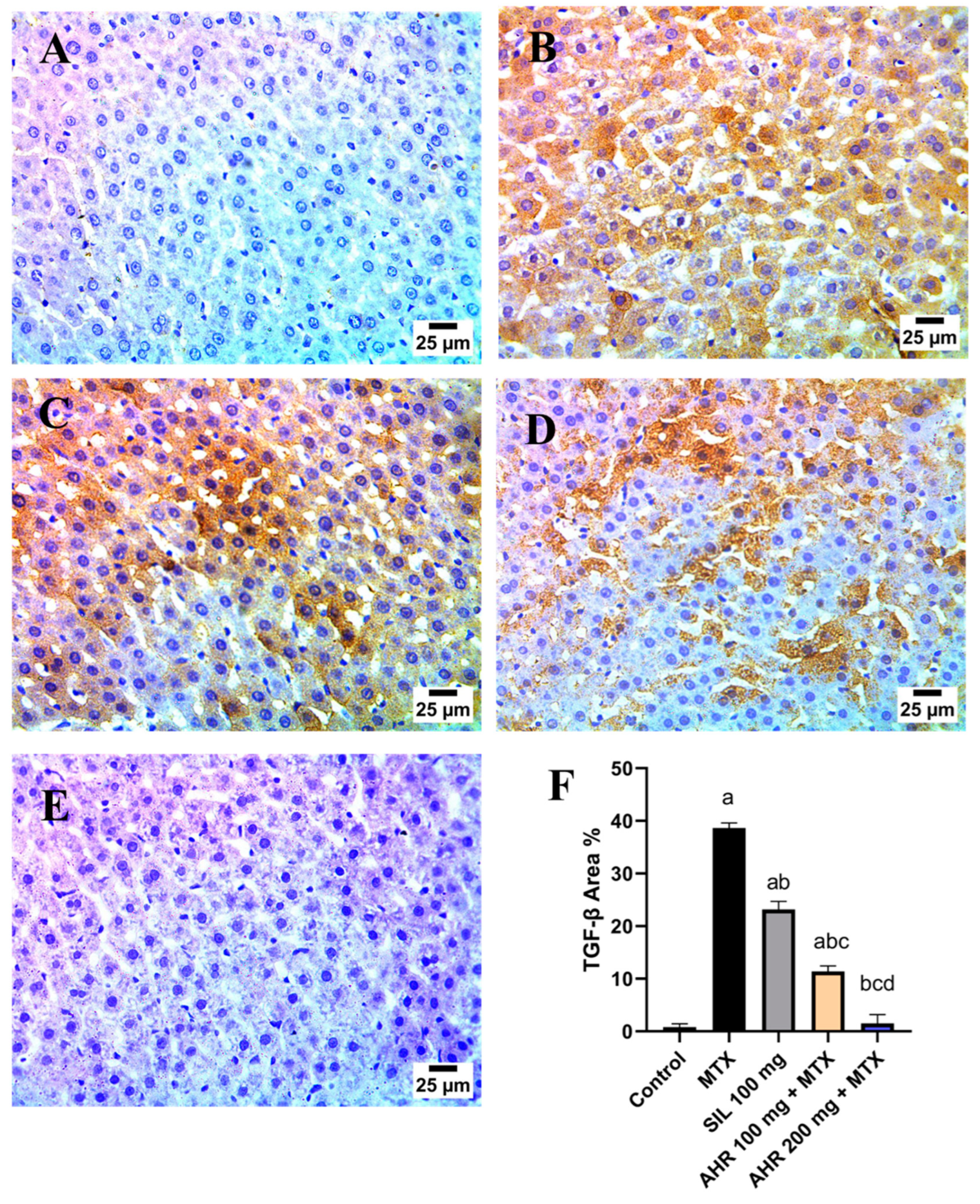
2.8. Effect of AHR on MTX-Induced Changes in TGF-β and VEGF Immunoreactivity
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Collection, Authentication, and Preparation of the Extract
4.3. LC-HRMS/MS Analysis
4.4. In Vivo Hepatoprotective Activity
4.4.1. Animals
4.4.2. Experimental Design
4.4.3. Evaluation of Hepatic Markers in Serum
4.4.4. Evaluation of Oxidative Stress Markers in Liver Tissue
4.4.5. Evaluation of NF-κB, IL-1β, IL-6, TNF-α and Cyclin D in Liver Tissue
4.4.6. Evaluation of JAK, STAT3, p38, and BCL2 in Liver Tissue
4.4.7. Histopathological Examination
4.4.8. Immunohistochemical Detection of TGF-β and VEGF
4.4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andrade, R.J.; Chalasani, N.; Björnsson, E.S.; Suzuki, A.; Kullak-Ublick, G.A.; Watkins, P.B.; Devarbhavi, H.; Merz, M.; Lucena, M.I.; Kaplowitz, N. Drug-induced liver injury. Nat. Rev. Dis. Primers 2019, 5, 58. [Google Scholar] [CrossRef]
- Leise, M.D.; Poterucha, J.J.; Talwalkar, J.A. Drug-Induced Liver Injury. Mayo Clin. Proc. 2014, 89, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Alfwuaires, M.A. Galangin mitigates oxidative stress, inflammation, and apoptosis in a rat model of methotrexate hepatotoxicity. Environ. Sci. Pollut. Res. 2021, 29, 20279–20288. [Google Scholar] [CrossRef] [PubMed]
- Wessels, J.A.M.; Huizinga, T.W.J.; Guchelaar, H.J. Recent insights in the pharmacological actions of methotrexate in the treatment of rheumatoid arthritis. Rheumatology 2007, 47, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Ezhilarasan, D. Hepatotoxic potentials of methotrexate: Understanding the possible toxicological molecular mechanisms. Toxicology 2021, 458, 152840. [Google Scholar] [CrossRef] [PubMed]
- Katturajan, R.; Vijayalakshmi, S.; Rasool, M.; Evan Prince, S. Molecular toxicity of methotrexate in rheumatoid arthritis treatment: A novel perspective and therapeutic implications. Toxicology 2021, 461, 152909. [Google Scholar] [CrossRef] [PubMed]
- Fabregat, I.; Moreno-Càceres, J.; Sánchez, A.; Dooley, S.; Dewidar, B.; Giannelli, G.; Ten Dijke, P.; Consortium, I.L. TGF-β signalling and liver disease. FEBS J. 2016, 283, 2219–2232. [Google Scholar] [CrossRef]
- Schmidt, S.; Messner, C.J.; Gaiser, C.; Hämmerli, C.; Suter-Dick, L. Methotrexate-Induced Liver Injury Is Associated with Oxidative Stress, Impaired Mitochondrial Respiration, and Endoplasmic Reticulum Stress In Vitro. Int. J. Mol. Sci. 2022, 23, 15116. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Ghosh, S.; Choudhury, S.; Adhikary, A.; Manna, K.; Dey, S.; Sa, G.; Das, T.; Chattopadhyay, S. Pomegranate reverses methotrexate-induced oxidative stress and apoptosis in hepatocytes by modulating Nrf2-NF-κB pathways. J. Nutr. Biochem. 2013, 24, 2040–2050. [Google Scholar] [CrossRef]
- Muriel, P. NF-κB in liver diseases: A target for drug therapy. J. Appl. Toxicol. 2009, 29, 91–100. [Google Scholar] [CrossRef]
- Choi, M.-J.; Zheng, H.-M.; Kim, J.M.; Lee, K.W.; Park, Y.H.; Lee, D.H. Protective effects of Centella asiatica leaf extract on dimethylnitrosamine-induced liver injury in rats. Mol. Med. Rep. 2016, 14, 4521–4528. [Google Scholar] [CrossRef] [PubMed]
- Miyazono, Y.; Gao, F.; Horie, T. Oxidative stress contributes to methotrexate-induced small intestinal toxicity in rats. Scand. J. Gastroenterol. 2004, 39, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Debacq-Chainiaux, F.; Boilan, E.; Le Moutier, J.D.; Weemaels, G.; Toussaint, O. p38MAPK in the Senescence of Human and Murine Fibroblasts. In Protein Metabolism and Homeostasis in Aging; Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 2010; pp. 126–137. [Google Scholar] [CrossRef]
- Kohandel, Z.; Farkhondeh, T.; Aschner, M.; Pourbagher-Shahri, A.M.; Samarghandian, S. Anti-inflammatory action of astaxanthin and its use in the treatment of various diseases. Biomed. Pharmacother. 2022, 145, 112179. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, X.; Wang, B.; Xu, X.; Wu, X.; Guo, M.; Wang, F. Effect of JAK2/STAT3 signaling pathway on liver injury associated with severe acute pancreatitis in rats. Exp. Ther. Med. 2018, 16, 2013–2021. [Google Scholar] [CrossRef]
- Dogra, A.; Gupta, D.; Bag, S.; Ahmed, I.; Bhatt, S.; Nehra, E.; Dhiman, S.; Kumar, A.; Singh, G.; Abdullah, S.T.; et al. Glabridin ameliorates methotrexate-induced liver injury via attenuation of oxidative stress, inflammation, and apoptosis. Life Sci. 2021, 278, 119583. [Google Scholar] [CrossRef] [PubMed]
- Kızıl, H.E.; Caglayan, C.; Darendelioğlu, E.; Ayna, A.; Gür, C.; Kandemir, F.M.; Küçükler, S. Hepatoprotective effect of morin against methotrexate-induced hepatotoxicity via targeting Nrf2/HO-1 and Bax/Bcl2/Caspase-3 signaling pathways. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Hassanein, E.H.M.; Shalkami, A.-G.S.; Khalaf, M.M.; Mohamed, W.R.; Hemeida, R.A.M. The impact of Keap1/Nrf2, P38MAPK/NF-κB and Bax/Bcl2/caspase-3 signaling pathways in the protective effects of berberine against methotrexate-induced nephrotoxicity. Biomed. Pharmacother. 2019, 109, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Zaulet, M.; Kevorkian, S.E.M.; Dinescu, S.; Cotoraci, C.; Suciu, M.; Herman, H.; Buburuzan, L.; Badulescu, L.; Ardelean, A.; Hermenean, A. Protective effects of silymarin against bisphenol A-induced hepatotoxicity in mouse liver. Exp. Ther. Med. 2017, 13, 821–828. [Google Scholar] [CrossRef]
- Surai, P.F. Silymarin as a Natural Antioxidant: An Overview of the Current Evidence and Perspectives. Antioxidants 2015, 4, 204–247. [Google Scholar] [CrossRef]
- Wadhwa, K.; Pahwa, R.; Kumar, M.; Kumar, S.; Sharma, P.C.; Singh, G.; Verma, R.; Mittal, V.; Singh, I.; Kaushik, D.; et al. Mechanistic Insights into the Pharmacological Significance of Silymarin. Molecules 2022, 27, 5327. [Google Scholar] [CrossRef]
- Sadia, S.; Sarkar, B.K.; Asma, J.; Ibne, S.M. Inhibitory Activity of Methanolic Extract of Araucaria heterophylla Leaves against Gram Negative Bacteria. Jagannath Univ. J. Life Earth Sci. 2019, 5, 216–2219. [Google Scholar]
- Elshamy, A.I.; Ammar, N.M.; Hassan, H.A.; Al-Rowaily, S.L.; Ragab, T.I.; El Gendy, A.E.-N.G.; Abd-ElGawad, A.M. Essential oil and its nanoemulsion of Araucaria heterophylla resin: Chemical characterization, anti-inflammatory, and antipyretic activities. Ind. Crops Prod. 2020, 148, 112272. [Google Scholar] [CrossRef]
- Abdel-Sattar, E.; Abdel Monem, A.R.; Ezzat, S.M.; El-Halawany, A.M.; Mouneir, S.M. Chemical and Biological Investigation of Araucaria heterophylla Salisb. Resin. Z. Naturforsch. C 2009, 64, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.S.; Choudhary, B.A.; Uzair, M.; Ijaz, A.S. Phytochemical and Ethno-Pharmacological Review of the Genus Araucaria—Review. Trop. J. Pharm. Res. 2013, 12, 651–659. [Google Scholar] [CrossRef]
- Frezza, C.; Venditti, A.; De Vita, D.; Toniolo, C.; Franceschin, M.; Ventrone, A.; Tomassini, L.; Foddai, S.; Guiso, M.; Nicoletti, M. Phytochemistry, chemotaxonomy, and biological activities of the Araucariaceae family—A review. Plants 2020, 9, 888. [Google Scholar] [CrossRef] [PubMed]
- Ali, D.E.; Bassam, S.M.; Elatrebi, S.; Habiba, E.S.; Allam, E.A.; Omar, E.M.; Ghareeb, D.A.; Abdulmalek, S.A.; Abdel-Sattar, E. HR LC-MS/MS metabolomic profiling of Yucca aloifolia fruit and the potential neuroprotective effect on rotenone-induced Parkinson’s disease in rats. PLoS ONE 2023, 18, e0282246. [Google Scholar] [CrossRef] [PubMed]
- Sameh, M.; Khalaf, H.M.; Anwar, A.M.; Osama, A.; Ahmed, E.A.; Mahgoub, S.; Ezzeldin, S.; Tanios, A.; Alfishawy, M.; Said, A.F.; et al. Integrated multiomics analysis to infer COVID-19 biological insights. Sci. Rep. 2023, 13, 1802. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, M.M.; Metwaly, A.M.; Mostafa, A.E.; Radwan, M.M.; Mehany, A.B.; Ahmed, E.; Enany, S.; Magdeldin, S.; Afifi, W.M.; ElSohly, M.A. Biological and Chemical Evaluation of Some African Plants Belonging to Kalanchoe Species: Antitrypanosomal, Cytotoxic, Antitopoisomerase I Activities and Chemical Profiling using Ultra-Performance Liquid Chromatography/Quadrupole-Time-of-Flight Mass Spectrometer. Pharmacogn. Mag. 2021, 17, 6–15. [Google Scholar]
- Kumar, A.; Singh, S.; Singh, M.K.; Gupta, A.; Tandon, S.; Verma, R.S. Chemistry, Biological Activities, and Uses of Araucaria Resin. In Gums, Resins and Latexes of Plant Origin: Chemistry, Biological Activities and Uses; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–20. [Google Scholar]
- Surek, M.; Fachi, M.M.; de Fátima Cobre, A.; de Oliveira, F.F.; Pontarolo, R.; Crisma, A.R.; de Souza, W.M.; Felipe, K.B. Chemical composition, cytotoxicity, and antibacterial activity of propolis from Africanized honeybees and three different Meliponini species. J. Ethnopharmacol. 2021, 269, 113662. [Google Scholar] [CrossRef]
- Schmeda-Hirschmanna, G.; Astudillo, L.; Sepúlveda, B.; Rodríguez, J.A.; Theoduloz, C.; Yáñez, T.; Palenzuela, J.A. Gastroprotective effect and cytotoxicity of natural and semisynthetic labdane diterpenes from Araucaria araucana resin. Z. Naturforschung C 2005, 60, 511–522. [Google Scholar] [CrossRef]
- Fonseca, F.N.; Ferreira, A.J.; Sartorelli, P.; Lopes, N.P.; Floh, E.I.; Handro, W.; Kato, M.J. Phenylpropanoid derivatives and biflavones at different stages of differentiation and development of Araucaria angustifolia. Phytochemistry 2000, 55, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Yamamoto, M.; Yanaka, T.; Ito, Y.; Nohara, T. Ten new labdane-type diterpenes from the fruit of Vitex rotundifolia. Chem. Pharm. Bull. 2001, 49, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Areche, C.; Schmeda-Hirschmann, G.; Theoduloz, C.; Rodríguez, J.A. Gastroprotective effect and cytotoxicity of abietane diterpenes from the Chilean Lamiaceae Sphacele chamaedryoides (Balbis) Briq. J. Pharm. Pharmacol. 2009, 61, 1689–1697. [Google Scholar] [CrossRef] [PubMed]
- Baglyas, M.; Ott, P.G.; Schwarczinger, I.; Nagy, J.K.; Darcsi, A.; Bakonyi, J.; Móricz, Á.M. Antimicrobial Diterpenes from Rough Goldenrod (Solidago rugosa Mill.). Molecules 2023, 28, 3790. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, G.P.; Crevelin, E.J.; Dias, H.J.; Ambrósio, S.R.; Bastos, J.K.; Heleno, V.C.; Vessecchi, R.; Crotti, A.E. Electrospray ionization tandem mass spectrometry of labdane-type acid diterpenes. J. Mass Spectrom. 2018, 53, 1086–1096. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, C.J.d.; Violante, I.M.P.; Garcez, W.S.; Pott, A.; Garcez, F.R. Biologically active abietane and ent-kaurane diterpenoids and other constituents from Erythroxylum suberosum. Phytochem. Lett. 2012, 5, 401–406. [Google Scholar] [CrossRef]
- Taki, N.; Sasaki-Sekimoto, Y.; Obayashi, T.; Kikuta, A.; Kobayashi, K.; Ainai, T.; Yagi, K.; Sakurai, N.; Suzuki, H.; Masuda, T.; et al. 12-oxo-phytodienoic acid triggers expression of a distinct set of genes and plays a role in wound-induced gene expression in Arabidopsis. Plant Physiol. 2005, 139, 1268–1283. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.J.M.; Crevelin, E.J.; Carneiro, L.J.; Rogez, H.; Veneziani, R.C.S.; Ambrósio, S.R.; Moraes, L.A.B.; Bastos, J.K. Development of a validated ultra-high-performance liquid chromatography tandem mass spectrometry method for determination of acid diterpenes in Copaifera oleoresins. J. Chromatogr. A 2017, 1515, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Gazim, Z.C.; Rodrigues, F.; Amorin, A.C.L.; de Rezende, C.M.; Soković, M.; Tešević, V.; Vučković, I.; Krstić, G.; Cortez, L.E.R.; Colauto, N.B. New natural diterpene-type abietane from Tetradenia riparia essential oil with cytotoxic and antioxidant activities. Molecules 2014, 19, 514–524. [Google Scholar] [CrossRef]
- Brophy, J.J.; Goldsack, R.J.; Wu, M.Z.; Fookes, C.J.; Forster, P.I. The steam volatile oil of Wollemia nobilis and its comparison with other members of the Araucariaceae (Agathis and Araucaria). Biochem. Syst. Ecol. 2000, 28, 563–578. [Google Scholar] [CrossRef]
- Pérusse, D.; Guégan, J.; Rolland, H.; Guilbot, J.; Benvegnu, T. Efficient solvent-free cationization of alkylpolyglycoside based surfactant compositions using natural glycine betaine. Green. Chem. 2016, 18, 1664–1673. [Google Scholar] [CrossRef]
- Xu, X.; Pu, R.; Li, Y.; Wu, Z.; Li, C.; Miao, X.; Yang, W. Chemical Compositions of Propolis from China and the United States and their Antimicrobial Activities Against Penicillium notatum. Molecules 2019, 24, 3576. [Google Scholar] [CrossRef] [PubMed]
- Acquaviva, R.; Malfa, G.A.; Loizzo, M.R.; Xiao, J.; Bianchi, S.; Tundis, R. Advances on Natural Abietane, Labdane and Clerodane Diterpenes as Anti-Cancer Agents: Sources and Mechanisms of Action. Molecules 2022, 27, 4791. [Google Scholar] [CrossRef] [PubMed]
- Barrero, A.F.; Herrador, M.M.; Arteaga, P.; Arteaga, J.F.; Arteaga, A.F. Communic acids: Occurrence, properties and use as chirons for the synthesis of bioactive compounds. Molecules 2012, 17, 1448–1467. [Google Scholar] [CrossRef] [PubMed]
- Aminimoghadamfarouj, N.; Nematollahi, A. Propolis Diterpenes as a Remarkable Bio-Source for Drug Discovery Development: A Review. Int. J. Mol. Sci. 2017, 18, 1290. [Google Scholar] [CrossRef] [PubMed]
- Fronza, M.; Murillo, R.; Ślusarczyk, S.; Adams, M.; Hamburger, M.; Heinzmann, B.; Laufer, S.; Merfort, I. In vitro cytotoxic activity of abietane diterpenes from Peltodon longipes as well as Salvia miltiorrhiza and Salvia sahendica. Bioorg. Med. Chem. 2011, 19, 4876–4881. [Google Scholar] [CrossRef] [PubMed]
- Shimira, F. Tetradenia riparia, an ethnobotanical plant with diverse applications, from antimicrobial to anti-proliferative activity against cancerous cell lines: A systematic review. J. Herb. Med. 2022, 32, 100537. [Google Scholar] [CrossRef]
- Babarinde, S.A.; Kemabonta, K.A.; Olatunde, O.Z.; Ojutiku, E.O.; Adeniyi, A.K. Composition and toxicity of rough lemon (Citrus jambhiri Lush.) rind essential oil against red flour beetle. Acta Ecol. Sin. 2021, 41, 325–331. [Google Scholar] [CrossRef]
- Medeiros, V.; Duran, F.J.; Lang, K. Copalic Acid: Occurrence, Chemistry, and Biological Activities. Rev. Bras. Farmacogn. 2021, 31, 375–386. [Google Scholar] [CrossRef]
- Tsao, N.-W.; Lin, Y.-C.; Tseng, Y.-H.; Chien, S.-C.; Wang, S.-Y. Composition analysis of exudates produced by conifers grown in Taiwan and their antifungal activity. J. Wood Sci. 2022, 68, 46. [Google Scholar] [CrossRef]
- Caputo, R.; Mangoni, L.; Monaco, P.; Previtera, L. New labdane diterpenes from Araucaria cooki. Phytochemistry 1974, 13, 471–474. [Google Scholar] [CrossRef]
- Santos, A.O.; Izumi, E.; Ueda-Nakamura, T.; Dias-Filho, B.P.; Veiga-Júnior, V.F.; Nakamura, C.V. Antileishmanial activity of diterpene acids in copaiba oil. Mem. Do Inst. Oswaldo Cruz 2013, 108, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Vardi, N.; Parlakpinar, H.; Cetin, A.; Erdogan, A.; Cetin Ozturk, I. Protective Effect of β-Carotene on Methotrexate–Induced Oxidative Liver Damage. Toxicol. Pathol. 2010, 38, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.K.; Bansal, M.; Soni, G.; Bhatnagar, D. Protective role of Vitamin E pre-treatment on N-nitrosodiethylamine induced oxidative stress in rat liver. Chem.-Biol. Interact. 2005, 156, 101–111. [Google Scholar] [CrossRef]
- Ali, N.; Rashid, S.; Nafees, S.; Hasan, S.K.; Shahid, A.; Majed, F.; Sultana, S. Protective effect of Chlorogenic acid against methotrexate induced oxidative stress, inflammation and apoptosis in rat liver: An experimental approach. Chem.-Biol. Interact. 2017, 272, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Hussein, O.E.; Hozayen, W.G.; Bin-Jumah, M.; Abd El-Twab, S.M. Ferulic acid prevents oxidative stress, inflammation, and liver injury via upregulation of Nrf2/HO-1 signaling in methotrexate-induced rats. Environ. Sci. Pollut. Res. 2019, 27, 7910–7921. [Google Scholar] [CrossRef]
- Ishii, T.; Itoh, K.; Takahashi, S.; Sato, H.; Yanagawa, T.; Katoh, Y.; Bannai, S.; Yamamoto, M. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J. Biol. Chem. 2000, 275, 16023–16029. [Google Scholar] [CrossRef]
- Mohamed, W.R.; Kotb, A.S.; Abd El-Raouf, O.M.; Mohammad Fikry, E. Apigenin alleviated acetaminophen-induced hepatotoxicity in low protein-fed rats: Targeting oxidative stress, STAT3, and apoptosis signals. J. Biochem. Mol. Toxicol. 2020, 34, e22472. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.-H.; Lee, G.-P.; Jeong, W.-I.; Do, S.-H.; Yang, H.-J.; Yuan, D.-W.; Park, H.-Y.; Kim, K.-J.; Jeong, K.-S. Alterations of mast cells and TGF-beta1 on the silymarin treatment for CCl(4)-induced hepatic fibrosis. World J. Gastroenterol. 2005, 11, 1141–1148. [Google Scholar] [CrossRef]
- Dooley, S.; ten Dijke, P. TGF-β in progression of liver disease. Cell Tissue Res. 2012, 347, 245–256. [Google Scholar] [CrossRef]
- Salminen, A.; Huuskonen, J.; Ojala, J.; Kauppinen, A.; Kaarniranta, K.; Suuronen, T. Activation of innate immunity system during aging: NF-kB signaling is the molecular culprit of inflamm-aging. Ageing Res. Rev. 2008, 7, 83–105. [Google Scholar] [CrossRef] [PubMed]
- Tak, P.P.; Firestein, G.S. NF-kappaB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Alkharfy, K.M.; Bin Jardan, Y.A.; Shahid, M.; Ansari, M.A.; Alqahtani, S.; Jan, B.L.; Al-Jenoobi, F.I.; Raish, M. Sinapic acid mitigates methotrexate-induced hepatic injuries in rats through modulation of Nrf-2/HO-1 signaling. Environ. Toxicol. 2021, 36, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Ali, D.E.; Abd El-Aziz, M.M.; Ibrahim, S.S.A.; Sheta, E.; Abdel-Sattar, E. Gastroprotective and anti-Helicobacter pylori potentials of essential oils from the oleoresins of Araucaria bidwillii and Araucaria heterophylla. Inflammopharmacology 2023, 31, 465–483. [Google Scholar] [CrossRef] [PubMed]
- Bode, J.G.; Albrecht, U.; Häussinger, D.; Heinrich, P.C.; Schaper, F. Hepatic acute phase proteins—Regulation by IL-6- and IL-1-type cytokines involving STAT3 and its crosstalk with NF-κB-dependent signaling. Eur. J. Cell Biol. 2012, 91, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Kagan, P.; Sultan, M.; Tachlytski, I.; Safran, M.; Ben-Ari, Z. Both MAPK and STAT3 signal transduction pathways are necessary for IL-6-dependent hepatic stellate cells activation. PLoS ONE 2017, 12, e0176173. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Qi, Y.-F.; Yu, Y.-R. STAT3: A key regulator in liver fibrosis. Ann. Hepatol. 2021, 21, 100224. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.F.; Elimam, D.M.; El-Senduny, F.F.; Alossaimi, M.A.; Alamri, M.; Abdel Bar, F.M. Design, biological evaluation, and molecular modelling insights of cupressic acid derivatives as promising anti-inflammatory agents. J. Enzym. Inhib. Med. Chem. 2023, 38, 2187327. [Google Scholar] [CrossRef] [PubMed]
- Darmadi, D.; Habriel Ruslie, R.; Pakpahan, C. Vascular Endothelial Growth Factor (VEGF) in Liver Disease. In Tumor Angiogenesis and Modulators; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Manie, M.F.; Fawzy, H.M.; El-Sayed, E.-S.M. Hydroxytyrosol Alleviates Methotrexate-Induced Pulmonary Fibrosis in Rats: Involvement of TGF-β1, Tissue Factor, and VEGF. Biol. Pharm. Bull. 2024, 47, 303–310. [Google Scholar] [CrossRef]
- Han, D.; Shinohara, M.; Ybanez, M.D.; Saberi, B.; Kaplowitz, N. Signal Transduction Pathways Involved in Drug-Induced Liver Injury. In Adverse Drug Reactions; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 267–310. [Google Scholar] [CrossRef]
- Abbaszadeh, H.; Keikhaei, B.; Mottaghi, S. A review of molecular mechanisms involved in anticancer and antiangiogenic effects of natural polyphenolic compounds. Phytother. Res. 2019, 33, 2002–2014. [Google Scholar] [CrossRef]
- Dumitraș, D.-A.; Andrei, S. Recent Advances in the Antiproliferative and Proapoptotic Activity of Various Plant Extracts and Constituents against Murine Malignant Melanoma. Molecules 2022, 27, 2585. [Google Scholar] [CrossRef] [PubMed]
- Leslie, K.; Lang, C.; Devgan, G.; Azare, J.; Berishaj, M.; Gerald, W.; Kim, Y.B.; Paz, K.; Darnell, J.E.; Albanese, C.; et al. Cyclin D1 Is Transcriptionally Regulated by and Required for Transformation by Activated Signal Transducer and Activator of Transcription 3. Cancer Res. 2006, 66, 2544–2552. [Google Scholar] [CrossRef] [PubMed]
- Kyriakis, J.M.; Avruch, J. Mammalian Mitogen-Activated Protein Kinase Signal Transduction Pathways Activated by Stress and Inflammation. Physiol. Rev. 2001, 81, 807–869. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.M.; Aldhahrani, A.; Alkhedaide, A.; Nassan, M.A.; Althobaiti, F.; Mohamed, W.A. The ameliorative impacts of Moringa oleifera leaf extract against oxidative stress and methotrexate-induced hepato-renal dysfunction. Biomed. Pharmacother. 2020, 128, 110259. [Google Scholar] [CrossRef] [PubMed]
- Shalkami, A.-G.S.; Hassanein, E.H.M.; Sayed, A.M.; Mohamed, W.R.; Khalaf, M.M.; Hemeida, R.A.M. Hepatoprotective effects of phytochemicals berberine and umbelliferone against methotrexate-induced hepatic intoxication: Experimental studies and in silico evidence. Environ. Sci. Pollut. Res. 2021, 28, 67593–67607. [Google Scholar] [CrossRef] [PubMed]
- Wang, K. Molecular mechanisms of hepatic apoptosis. Cell Death Dis. 2014, 5, e996. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Patra, J.K.; Bonesi, M.; Das, S.; Nath, R.; Das Talukdar, A.; Das, G.; Loizzo, M.R. Anti-Cancer Agent: The Labdane Diterpenoid-Andrographolide. Plants 2023, 12, 1969. [Google Scholar] [CrossRef]
- Chen, J.-J.; Ting, C.-W.; Wu, Y.-C.; Hwang, T.-L.; Cheng, M.-J.; Sung, P.-J.; Wang, T.-C.; Chen, J.-F. New Labdane-Type Diterpenoids and Anti-Inflammatory Constituents from Hedychium coronarium. IJMS 2013, 14, 13063–13077. [Google Scholar] [CrossRef] [PubMed]
- Mehrzadi, S.; Fatemi, I.; Esmaeilizadeh, M.; Ghaznavi, H.; Kalantar, H.; Goudarzi, M. Hepatoprotective effect of berberine against methotrexate induced liver toxicity in rats. Biomed. Pharmacother. 2018, 97, 233–239. [Google Scholar] [CrossRef]
- Sokar, S.S.; El-Sayad, M.E.-S.; Ghoneim, M.E.-S.; Shebl, A.M. Combination of Sitagliptin and Silymarin ameliorates liver fibrosis induced by carbon tetrachloride in rats. Biomed. Pharmacother. 2017, 89, 98–107. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques; Elsevier Health Sciences: Amsterdam, The Netherlands, 2008. [Google Scholar]





| mRNA Species | Accession Number | Sequence (5′→3′) |
|---|---|---|
| JAK | ON706994 | F: TTTGGATCCCTGGATACATACCTGA R: TGGCACACACATTCCCATGA |
| STAT3 | XM_054316993 | F: CCCCGTACCTGAAGACCAAGT R: CCGTTATTTCCAAACTGCATCA |
| P38 | NM_001109891 | F: TCATAGGCATCCGAGACATCC R: CGTCTCCATGAGGTCCTGAAC |
| BCL2 | XM_047437733 | F: ATCGCTCTGTGGATGACTGAGTAC R: AGAGACAGCCAGGAGAAATCAAAC |
| GAPDH | NM_002046.7 | F: GTCTCCTCTGACTTCAACAGCG R: ACCACCCTGTTGCTGTAGCCAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sweilam, S.H.; Ali, D.E.; Atwa, A.M.; Elgindy, A.M.; Mustafa, A.M.; Esmail, M.M.; Alkabbani, M.A.; Senna, M.M.; El-Shiekh, R.A. A First Metabolite Analysis of Norfolk Island Pine Resin and Its Hepatoprotective Potential to Alleviate Methotrexate (MTX)-Induced Hepatic Injury. Pharmaceuticals 2024, 17, 970. https://doi.org/10.3390/ph17070970
Sweilam SH, Ali DE, Atwa AM, Elgindy AM, Mustafa AM, Esmail MM, Alkabbani MA, Senna MM, El-Shiekh RA. A First Metabolite Analysis of Norfolk Island Pine Resin and Its Hepatoprotective Potential to Alleviate Methotrexate (MTX)-Induced Hepatic Injury. Pharmaceuticals. 2024; 17(7):970. https://doi.org/10.3390/ph17070970
Chicago/Turabian StyleSweilam, Sherouk Hussein, Dalia E. Ali, Ahmed M. Atwa, Ali M. Elgindy, Aya M. Mustafa, Manar M. Esmail, Mahmoud Abdelrahman Alkabbani, Mohamed Magdy Senna, and Riham A. El-Shiekh. 2024. "A First Metabolite Analysis of Norfolk Island Pine Resin and Its Hepatoprotective Potential to Alleviate Methotrexate (MTX)-Induced Hepatic Injury" Pharmaceuticals 17, no. 7: 970. https://doi.org/10.3390/ph17070970







