Long Non-Coding H19 in Lymphocytes: Prognostic Value in Acute Ischemic Stroke Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. The Collection of Clinical Data
2.3. RNA Extraction and Quality Detection
2.4. cDNA Synthesis and Real-Time Polymerase Chain Reaction (RT-PCR)
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Patients Grouped by 3-Month Functional Outcomes
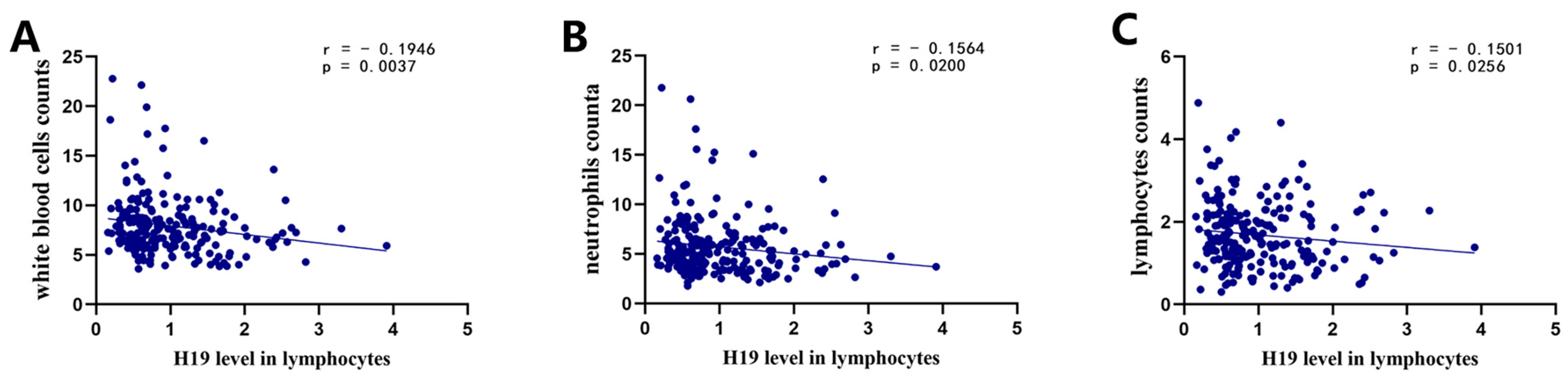
3.2. Relationship between the H19 Expression Level and Counts of Circulating Cells
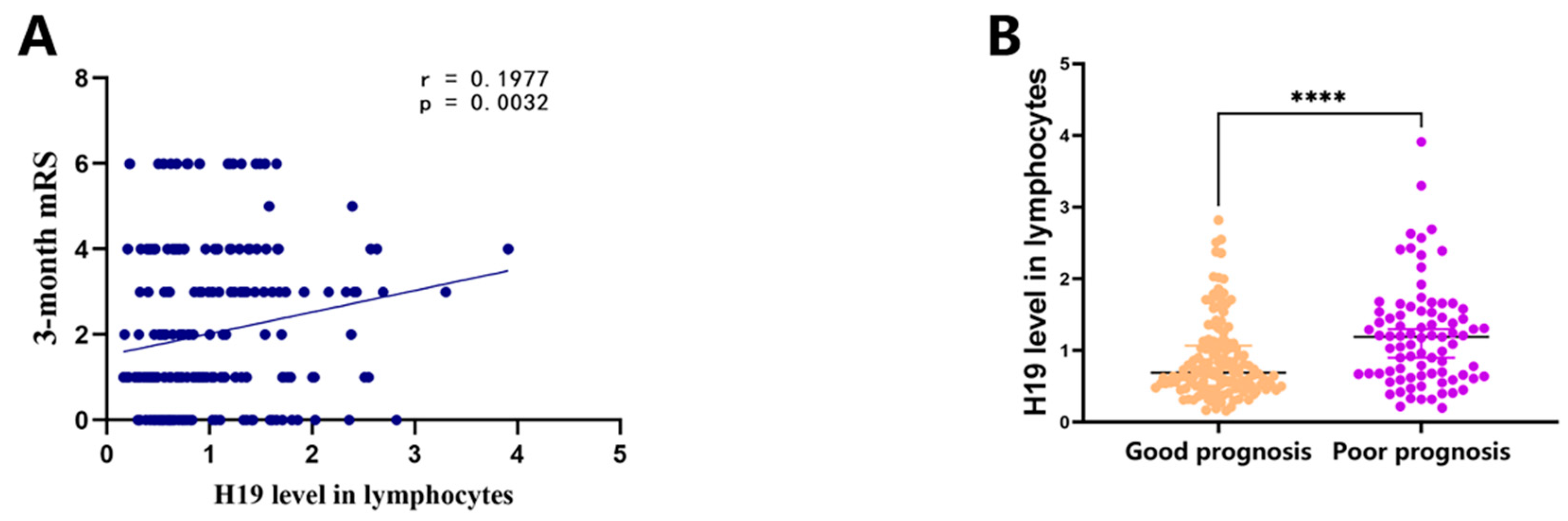
3.3. H19 Level in Lymphocytes Was Decreased in AIS Patients and Associated with Prognosis of AIS Patients
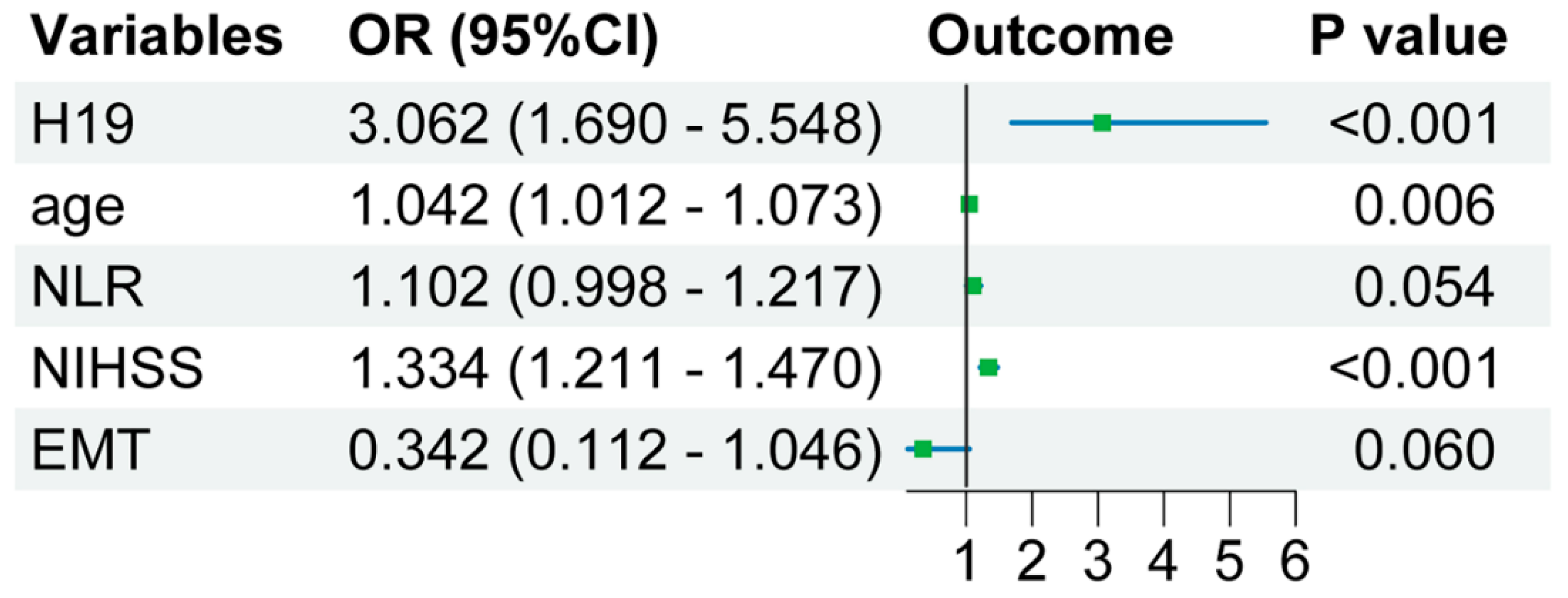
3.4. H19 Used as an Independent Predictor for Poor Prognosis in AIS
3.5. Incremental Predictive Value of lncRNA H19 in Lymphoctes for Poor Prognosis of AIS
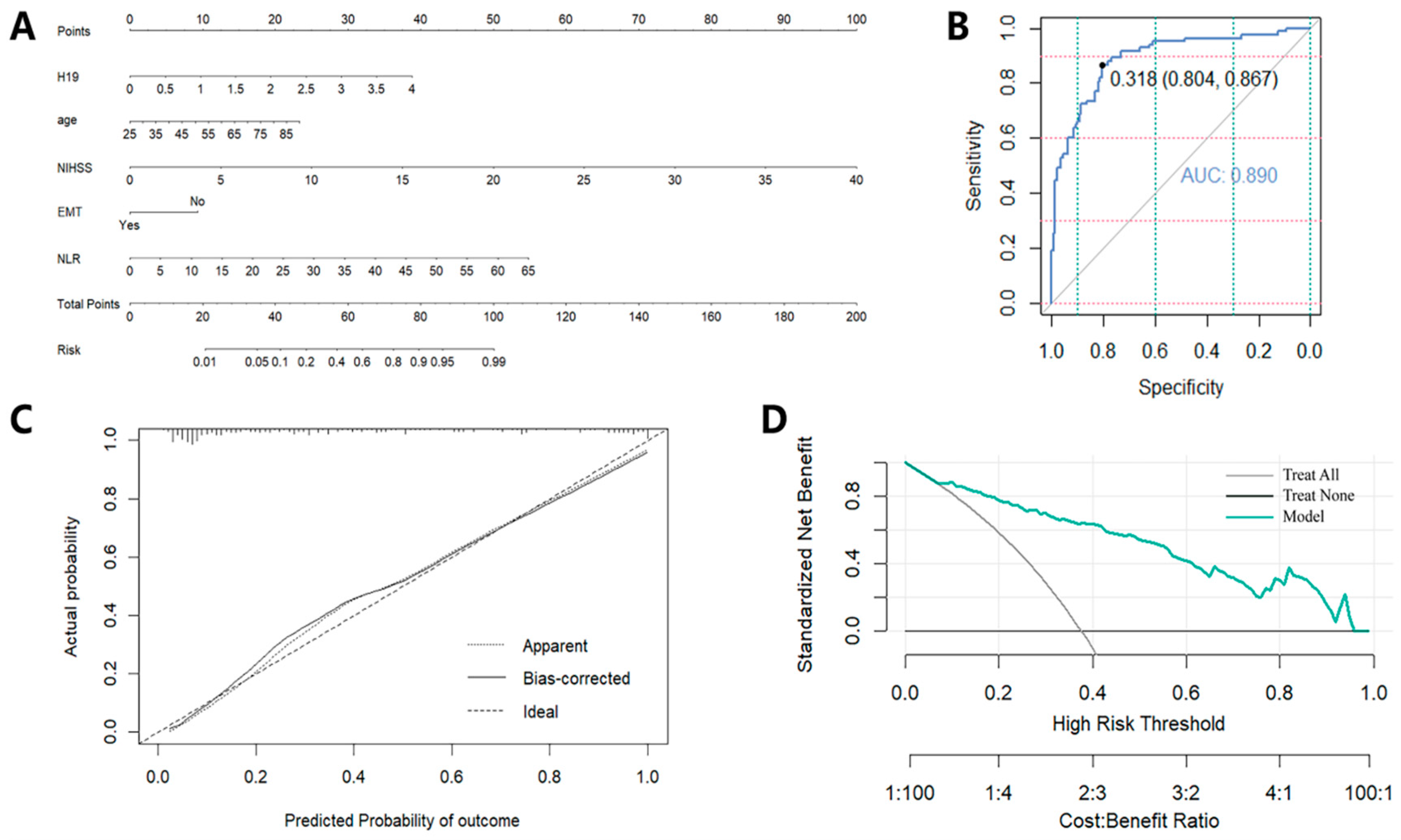
3.6. Creation and Evaluation of a Predictive Tool Based on the Independent Prognostic Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yao, G.Y.; Zhu, Q.; Xia, J.; Chen, F.J.; Huang, M.; Liu, J.; Zhou, T.T.; Wei, J.F.; Cui, G.Y.; Zheng, K.Y.; et al. Ischemic postconditioning confers cerebroprotection by stabilizing VDACs after brain ischemia. Cell Death Dis. 2018, 9, 1033. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Owolabi, M.O. Pragmatic solutions to reduce the global burden of stroke: A World Stroke Organization-Lancet Neurology Commission. Lancet Neurol. 2023, 22, 1160–1206. [Google Scholar] [CrossRef]
- Li, H.; Cai, H.; Lou, X.; Yu, M.; Li, Z. LOXL1-AS1 communicating with TIAR modulates vasculogenic mimicry in glioma via regulation of the miR-374b-5p/MMP14 axis. J. Cell. Mol. Med. 2022, 26, 475–490. [Google Scholar]
- Dharap, A.; Nakka, V.P.; Vemuganti, R. Effect of focal ischemia on long noncoding RNAs. Stroke 2012, 43, 2800–2802. [Google Scholar] [CrossRef] [PubMed]
- Dykstra-Aiello, C.; Jickling, G.C.; Ander, B.P.; Shroff, N.; Zhan, X.; Liu, D.; Hull, H.; Orantia, M.; Stamova, B.S.; Sharp, F.R. Altered expression of long noncoding RNAs in blood after ischemic stroke and proximity to putative stroke risk loci. Stroke 2016, 47, 2896–2903. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Liu, P.; Fan, J.; Luo, Y. Long non-coding RNA H19: Physiological functions and involvements in central nervous system disorders. Neurochem. Int. 2021, 148, 105072. [Google Scholar] [CrossRef] [PubMed]
- Kraus, T.F.; Greiner, A.; Guibourt, V.; Kretzschmar, H.A. Long non-coding RNA normalisers in human brain tissue. J. Neural Transm. 2015, 122, 1045–1054. [Google Scholar] [CrossRef]
- Rezaei, M.; Mokhtari, M.J.; Bayat, M.; Safari, A.; Dianatpuor, M.; Tabrizi, R.; Asadabadi, T.; Borhani-Haghighi, A. Long non-coding RNA H19 expression and functional polymorphism rs217727 are linked to increased ischemic stroke risk. BMC Neurol. 2021, 21, 54. [Google Scholar] [CrossRef]
- Li, G.; Ma, X.; Zhao, H.; Fan, J.; Liu, T.; Luo, Y.; Guo, Y. Long non-coding RNA H19 promotes leukocyte inflammation in ischemic stroke by targeting the miR-29b/C1QTNF6 axis. CNS Neurosci. Ther. 2022, 28, 953–963. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, H.; Fan, Z.; Li, G.; Ma, Q.; Tao, Z.; Wang, R.; Feng, J.; Luo, Y. Long noncoding RNA h19 promotes neuroinflammation in ischemic stroke by driving histone deacetylase 1-Dependent m1 microglial polarization. Stroke 2017, 48, 2211–2221. [Google Scholar] [CrossRef]
- Gao, N.; Tang, H.; Gao, L.; Tu, G.L.; Luo, H.; Xia, Y. LncRNA h19 aggravates cerebral Ischemia/Reperfusion injury by functioning as a ceRNA for miR-19a-3p to target PTEN. Neuroscience 2020, 437, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Qiu, Y.; Lin, Y.; Medina, R.; Zhuang, S.; Rosenblum, J.S.; Cui, J.; Li, Z.; Zhang, X.; Guo, L. Blocking lncRNA H19-miR-19a-Id2 axis attenuates hypoxia/ischemia induced neuronal injury. Aging 2019, 11, 3585–3600. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, L.; Mao, Y.; Nan, G. Long Noncoding RNA-H19 Contributes to Atherosclerosis and Induces Ischemic Stroke via the Upregulation of Acid Phosphatase 5. Front. Neurol. 2019, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cao, B.; Zhao, H.; Gao, Y.; Luo, Y.; Chen, Y.; Feng, J. Long noncoding RNA H19 prevents neurogenesis in ischemic stroke through p53/Notch1 pathway. Brain Res. Bull. 2019, 150, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cao, B.; Gao, Y.; Chen, Y.H.; Feng, J. Exosome-transported lncRNA H19 regulates insulin-like growth factor-1 via the H19/let-7a/insulin-like growth factor-1 receptor axis in ischemic stroke. Neural Regen. Res. 2023, 18, 1316–1320. [Google Scholar] [PubMed]
- Lapikova-Bryhinska, T.; Ministrini, S.; Puspitasari, Y.M.; Kraler, S.; Mohamed, S.A.; Costantino, S.; Paneni, F.; Khetsuriani, M.; Bengs, S.; Liberale, L.; et al. Long non-coding RNAs H19 and NKILA are associated with the risk of death and lacunar stroke in the elderly population. Eur. J. Intern. Med. 2024, 123, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yuan, Y.; Cho, J.H.; McClarty, S.; Baxter, D.; Galas, D.J. Comparing the MicroRNA spectrum between serum and plasma. PLoS ONE 2012, 7, e41561. [Google Scholar] [CrossRef] [PubMed]
- Weitbrecht, L.; Berchtold, D.; Zhang, T.; Jagdmann, S.; Dames, C.; Winek, K.; Meisel, C.; Meisel, A. CD4(+) T cells promote delayed B cell responses in the ischemic brain after experimental stroke. Brain Behav. Immun. 2021, 91, 601–614. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, L.; Li, Y.; Zhang, Q.; Fang, Q.; Tang, X. Association of the Neutrophil-to-Lymphocyte ratio with 90-Day functional outcomes in patients with acute ischemic stroke. Brain Sci. 2024, 14, 250. [Google Scholar] [CrossRef]
- Goyal, N.; Tsivgoulis, G.; Chang, J.J.; Malhotra, K.; Pandhi, A.; Ishfaq, M.F.; Alsbrook, D.; Arthur, A.S.; Elijovich, L.; Alexandrov, A.V. Admission Neutrophil-to-Lymphocyte ratio as a prognostic biomarker of outcomes in large vessel occlusion strokes. Stroke 2018, 49, 1985–1987. [Google Scholar] [CrossRef]
- Li, W.; Hou, M.; Ding, Z.; Liu, X.; Shao, Y.; Li, X. Prognostic value of Neutrophil-to-Lymphocyte ratio in stroke: A systematic review and Meta-Analysis. Front. Neurol. 2021, 12, 686983. [Google Scholar] [CrossRef] [PubMed]
- Brott, T.; Adams, H.P., Jr.; Olinger, C.P.; Marler, J.R.; Barsan, W.G.; Biller, J.; Spilker, J.; Holleran, R.; Eberle, R.; Hertzberg, V. Measurements of acute cerebral infarction: A clinical examination scale. Stroke 1989, 20, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Bonita, R.; Beaglehole, R. Recovery of motor function after stroke. Stroke 1988, 19, 1497–1500. [Google Scholar] [CrossRef] [PubMed]
- Adams, H.P., Jr.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E., 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Wu, E.R.; Chou, Y.E.; Liu, Y.F. Association of lncRNA h19 gene polymorphisms with the occurrence of hepatocellular carcinoma. Genes 2019, 10, 506. [Google Scholar] [CrossRef]
- Dey, A. Structural modifications and novel Protein-Binding sites in Pre-miR-675-Explaining its regulatory mechanism in carcinogenesis. Noncoding RNA 2023, 9, 45. [Google Scholar] [CrossRef]
- Allen, M.; Pearn, K.; Ford, G.A.; White, P.; Rudd, A.G.; McMeekin, P.; Stein, K.; James, M. National implementation of reperfusion for acute ischaemic stroke in England: How should services be configured? A modelling study. Eur. Stroke J. 2022, 7, 28–40. [Google Scholar] [CrossRef]
- Wang, J.; Cao, B.; Han, D.; Sun, M.; Feng, J. Long non-coding RNA h19 induces cerebral ischemia reperfusion injury via activation of autophagy. Aging Dis. 2017, 8, 71–84. [Google Scholar] [CrossRef]
- Li, H.; Tang, C.; Wang, D. LncRNA H19 promotes inflammatory response induced by cerebral ischemia-reperfusion injury through regulating the miR-138-5p-p65 axis. Biochem. Cell Biol. 2020, 98, 525–536. [Google Scholar] [CrossRef]
- Han, Y.; Ma, J.; Wang, J.; Wang, L. Silencing of H19 inhibits the adipogenesis and inflammation response in ox-LDL-treated Raw264.7 cells by up-regulating miR-130b. Mol. Immunol. 2018, 93, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Pan, W.; Wang, X.; Wei, M.; He, A.; Zhao, A.; Chopp, M.; Zhang, Z.G.; Liu, X.S. Long noncoding RNA mediates stroke-induced neurogenesis. Stem Cells 2020, 38, 973–985. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Hu, S.; Li, Y.; Sun, Y.; Xiong, X.; Hu, X.; Chen, J.; Qiu, S. Interleukins and ischemic stroke. Front. Immunol. 2022, 13, 828447. [Google Scholar] [CrossRef]
- Arumugam, T.V.; Granger, D.N.; Mattson, M.P. Stroke and t-cells. Neuromolecular Med. 2005, 7, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Alemseged, F.; Rocco, A.; Arba, F.; Schwabova, J.P.; Wu, T.; Cavicchia, L.; Ng, F.; Ng, J.L.; Zhao, H.; Williams, C.; et al. Posterior national institutes of health stroke scale improves prognostic accuracy in posterior circulation stroke. Stroke 2022, 53, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Rahim, A.H.; Fulton, R.L.; Sucharew, H.; Kleindorfer, D.; Khatri, P.; Broderick, J.P.; Lees, K.R.; VISTA collaborators Alexandrov, A.; Bath, P.M.; Bluhmki, E. National institutes of health stroke scale item profiles as predictor of patient outcome: External validation on independent trial data. Stroke 2015, 46, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Kelly-Hayes, M.; Beiser, A.; Kase, C.S.; Scaramucci, A.; D’Agostino, R.B.; Wolf, P.A. The influence of gender and age on disability following ischemic stroke: The Framingham study. J. Stroke Cerebrovasc. Dis. 2003, 12, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Tárraga, C.; Giralt-Steinhauer, E.; Mola-Caminal, M.; Ois, A.; Rodríguez-Campello, A.; Cuadrado-Godia, E.; Fernández-Cadenas, I.; Cullell, N.; Roquer, J.; Jiménez-Conde, J. Biological Age is a predictor of mortality in Ischemic Stroke. Sci. Rep. 2018, 8, 4148. [Google Scholar] [CrossRef]
- Goyal, M.; Menon, B.K.; Van Zwam, W.H.; Dippel, D.W.; Mitchell, P.J.; Demchuk, A.M.; Dávalos, A.; Majoie, C.B.; van Der Lugt, A.; De Miquel, M.A.; et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet 2016, 387, 1723–1731. [Google Scholar] [CrossRef]
- Consoli, A.; Gory, B. Long-term results of mechanical thrombectomy for large ischaemic stroke. Lancet 2024, 403, 700–701. [Google Scholar] [CrossRef]
| Baseline Characteristics | Total | Good Prognosis | Poor Prognosis | p Value |
|---|---|---|---|---|
| (N = 221) | (N = 138) | (N = 83) | ||
| Age, x ± s or median (IQR) | 65.01 ± 13.17 | 62.50 [54.75–71.00] | 70.00 [60.00–82.00] | <0.001 * |
| Male, n (%) | 164 (74.21) | 107 (77.54) | 57 (68.67) | 0.145 |
| Onset-to-treatment time, h, median (IQR) | 2.90 [1.45–5.05] | 2.80 [1.40–4.70] | 3.00 [1.70–6.30] | 0.387 |
| Systolic blood pressure, mm Hg, median (IQR) | 150.00 [138.00–165.00] | 150.00 [136.00–166.50] | 148.50 [140.00–164.00] | 0.703 |
| Diastolic blood pressure, mm Hg, median (IQR) | 83.00 [77.00–91.00] | 83.00 [77.00–92.00] | 86.00 [75.00–90.00] | 0.536 |
| Admission NIHSS scores, median (IQR) | 6.00 [3.00–11.00] | 4.00 [2.00–7.00] | 12.00 [7.00–17.00] | <0.001 * |
| Treatment | ||||
| Intravenous thrombolysis, n (%) | 93 (42.08) | 64 (46.38) | 29 (34.94) | 0.095 |
| EMT, n (%) | 34 (15.38) | 15 (10.87) | 19 (22.89) | 0.016 * |
| Prior risk factors | ||||
| Hypertension, n (%) | 150 (67.87) | 89 (64.49) | 61 (73.49) | 0.165 |
| Diabetes mellitus, n (%) | 75 (33.94) | 41 (29.71) | 34 (40.96) | 0.087 |
| Coronary heart disease, n (%) | 48 (21.72) | 21 (15.22) | 27 (32.53) | 0.003 * |
| Atrial fibrillation, n (%) | 39 (17.73) | 16 (11.59) | 23 (27.71) | 0.002 * |
| Stroke etiology | 0.149 | |||
| Large artery atherosclerosis, n (%) | 117 (52.94) | 77 (55.80) | 40 (48.19) | |
| Small vessel occlusion, n (%) | 55 (24.89) | 37 (26.81) | 18 (47.37) | |
| Cardioembolic, n (%) | 10 (4.52) | 4 (2.90) | 6 (15.79) | |
| Other determined and undetermined, n (%) | 39 (17.65) | 20 (14.49) | 19 (50.00) | |
| Clinical parameters | ||||
| White blood cells, ×109/L, median (IQR) | 7.38 [6.03–8.85] | 7.21 [5.98–8.62] | 7.87 [6.27–9.41] | 0.104 |
| Neutrophils, ×109/L, median (IQR) | 5.01 [3.84–6.52] | 4.72 [3.70–5.93] | 5.95 [4.04–7.56] | 0.006 * |
| Lymphocytes, ×109/L, median (IQR) | 1.54 [1.12–2.16] | 1.73 [1.25–2.22] | 1.31 [0.91–1.90] | <0.001 * |
| NLR, median (IQR) | 2.88 [2.03–5.61] | 2.61 [1.77–4.33] | 4.14 [2.34–7.43] | <0.001 * |
| Platelet counts, ×1000/mm3, median (IQR) | 208.00 [169.00–244.50] | 214.50 [171.00–250.75] | 200.00 [163.00–236.00] | 0.125 |
| TG, mmol/L, median (IQR) | 1.45 [0.96–2.53] | 1.59 [1.03–2.70] | 1.30 [0.84–2.01] | 0.042 * |
| TC, mmol/L, median (IQR) | 4.53 [3.72–5.41] | 4.58 [3.85–5.48] | 4.43 [3.60–5.10] | 0.196 |
| HDL, mmol/L, median (IQR) | 1.18 (1.00–1.40] | 1.20 [0.99–1.43] | 1.17 [1.01–1.37] | 0.524 |
| LDL, mmol/L, x ± s or median (IQR) | 2.69 [2.01–3.42] | 2.78 ± 0.95 | 2.64 ± 0.93 | 0.282 |
| Biomarkers | ||||
| H19 level in lymphocytes, median (IQR) | 0.78 [0.55–1.33] | 0.69 [0.51–1.07] | 1.19 [0.66–1.54] | <0.001 * |
| Univariate Analysis | ||
|---|---|---|
| Parameter | OR (95% CI) | p |
| Age, y | 1.045 [1.021–1.070] | <0.001 * |
| Male | 1.547 [0.853–2.904] | 0.146 |
| Onset-to-treatment time, h | 1.032 [0.964–1.105] | 0.360 |
| Systolic blood pressure, mm Hg | 0.999 [0.988–1.010] | 0.829 |
| Diastolic blood pressure, mm Hg | 0.997 [0.979–1.015] | 0.766 |
| Admission NIHSS scores | 1.293 [1.203–1.389] | <0.001 * |
| Intravenous thrombolysis (%) | 0.621 [0.354–1.089] | 0.096 |
| EMT (%) | 0.411 [0.196–0.862] | 0.019 * |
| Hypertension | 1.527 [0.838–2.780] | 0.167 |
| Diabetes mellitus | 1.642 [0.929–2.902] | 0.088 |
| Coronary heart disease | 2.686 [1.398–5.162] | 0.003 * |
| Atrial fibrillation | 2.923 [1.439–5.939] | 0.003 * |
| White blood cells, ×109/L | 1.064 [0.972–1.164] | 0.176 |
| Neutrophils, ×109/L | 1.119 [1.018–1.231] | 0.020 * |
| Lymphocytes, ×109/L | 0.487 [0.324–0.732] | <0.001 * |
| NLR | 1.176 [1.082–1.278] | <0.001 * |
| Platelet counts, ×1000/mm3 | 0.996 [0.991–1.001] | 0.116 |
| TG, mmol/L | 1.049 [0.977–1.125] | 0.187 |
| TC, mmol/L | 1.025 [0.933–1.127] | 0.605 |
| HDL, mmol/L | 0.914 [0.398–2.101] | 0.833 |
| LDL, mmol/L | 0.850 [0.633–1.142] | 0.281 |
| H19 level in lymphocytes | 2.411 (1.510–3.849) | <0.001 * |
| Clinical Model | Clinical Model + H19 | |
|---|---|---|
| Logistic regression | ||
| R2 (Cox and snell) | 0.366 | 0.409 |
| Age, y | OR = 1.040 [1.011–1.069], p = 0.006 * | OR = 1.042 [1.012–1.073], p = 0.006 * |
| Admission NIHSS scores | OR = 1.330 [1.212–1.459], p < 0.001 * | OR = 1.334 [1.211–1.470], p < 0.001 * |
| EMT | OR = 3.339 [1.123–9.930], p = 0.030 * | OR = 0.342 [0.112–1.046], p = 0.060 |
| NLR | OR = 1.096 [0.998–1.203], p = 0.055 | OR = 1.102 [0.998–1.217], p = 0.054 |
| H19 level in lymphocytes | - | OR = 3.062 [1.69–5.548], p < 0.001 |
| AUC | ||
| 0.870 | 0.890 | |
| IDI index, % | ||
| Total IDI | - | 5.03 [1.76–8.30] |
| p value | - | 0.0026 * |
| NRI index, % | ||
| Continuous NRI | - | 68.33 [42.86–93.79] |
| p value | - | <0.0001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, L.; Xie, Z.; Han, Z.; Fan, J.; Wang, R.; Tao, Z.; Ma, Q.; Luo, Y. Long Non-Coding H19 in Lymphocytes: Prognostic Value in Acute Ischemic Stroke Patients. Pharmaceuticals 2024, 17, 1008. https://doi.org/10.3390/ph17081008
Zhong L, Xie Z, Han Z, Fan J, Wang R, Tao Z, Ma Q, Luo Y. Long Non-Coding H19 in Lymphocytes: Prognostic Value in Acute Ischemic Stroke Patients. Pharmaceuticals. 2024; 17(8):1008. https://doi.org/10.3390/ph17081008
Chicago/Turabian StyleZhong, Liyuan, Zixian Xie, Ziping Han, Junfen Fan, Rongliang Wang, Zhen Tao, Qingfeng Ma, and Yumin Luo. 2024. "Long Non-Coding H19 in Lymphocytes: Prognostic Value in Acute Ischemic Stroke Patients" Pharmaceuticals 17, no. 8: 1008. https://doi.org/10.3390/ph17081008
APA StyleZhong, L., Xie, Z., Han, Z., Fan, J., Wang, R., Tao, Z., Ma, Q., & Luo, Y. (2024). Long Non-Coding H19 in Lymphocytes: Prognostic Value in Acute Ischemic Stroke Patients. Pharmaceuticals, 17(8), 1008. https://doi.org/10.3390/ph17081008







