Siraitia grosvenorii Extract Protects Lipopolysaccharide-Induced Intestinal Inflammation in Mice via Promoting M2 Macrophage Polarization
Abstract
:1. Introduction
2. Results
2.1. Chemical Component Analysis of SGE
2.2. Effect of SGE on Blood Indices of Mice
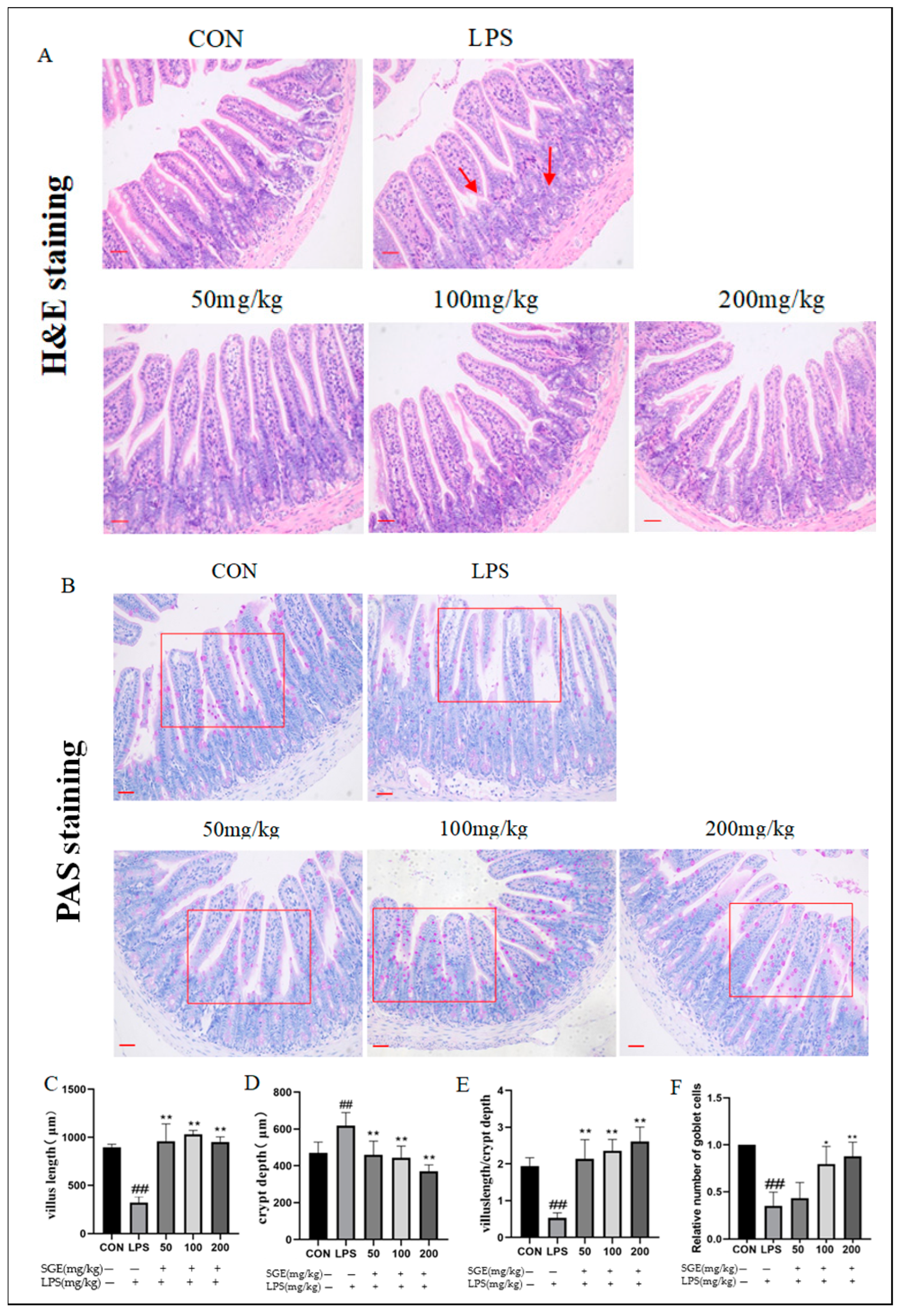
2.3. Effects of SGE on Ileum Histopathology in Mice with LPS-Induced Enteritis
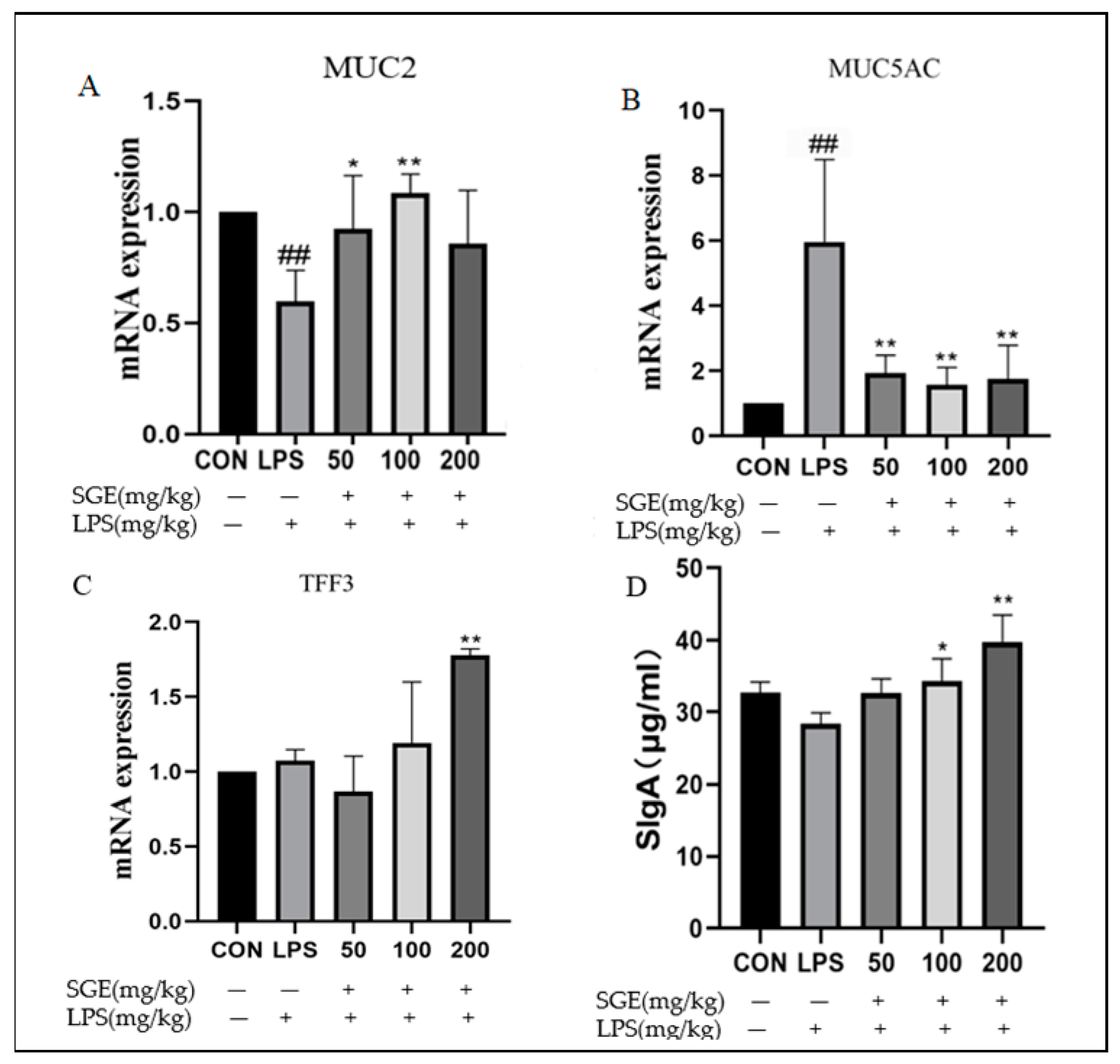
2.4. Protective Effect of SGE on Ileal Mucus Barrier of Mice
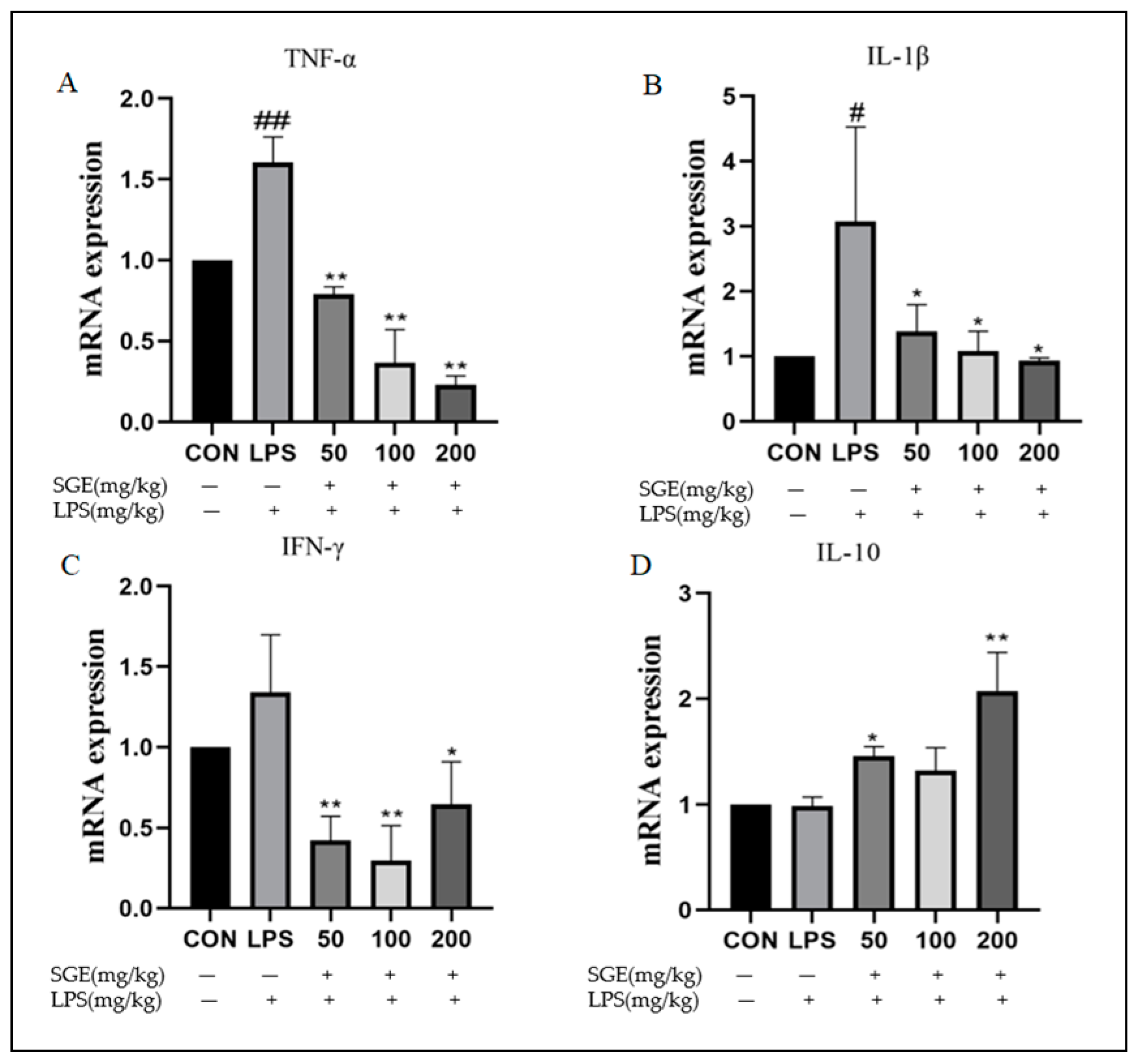
2.5. Effect of SGE on the Ileitis Factors in Mice
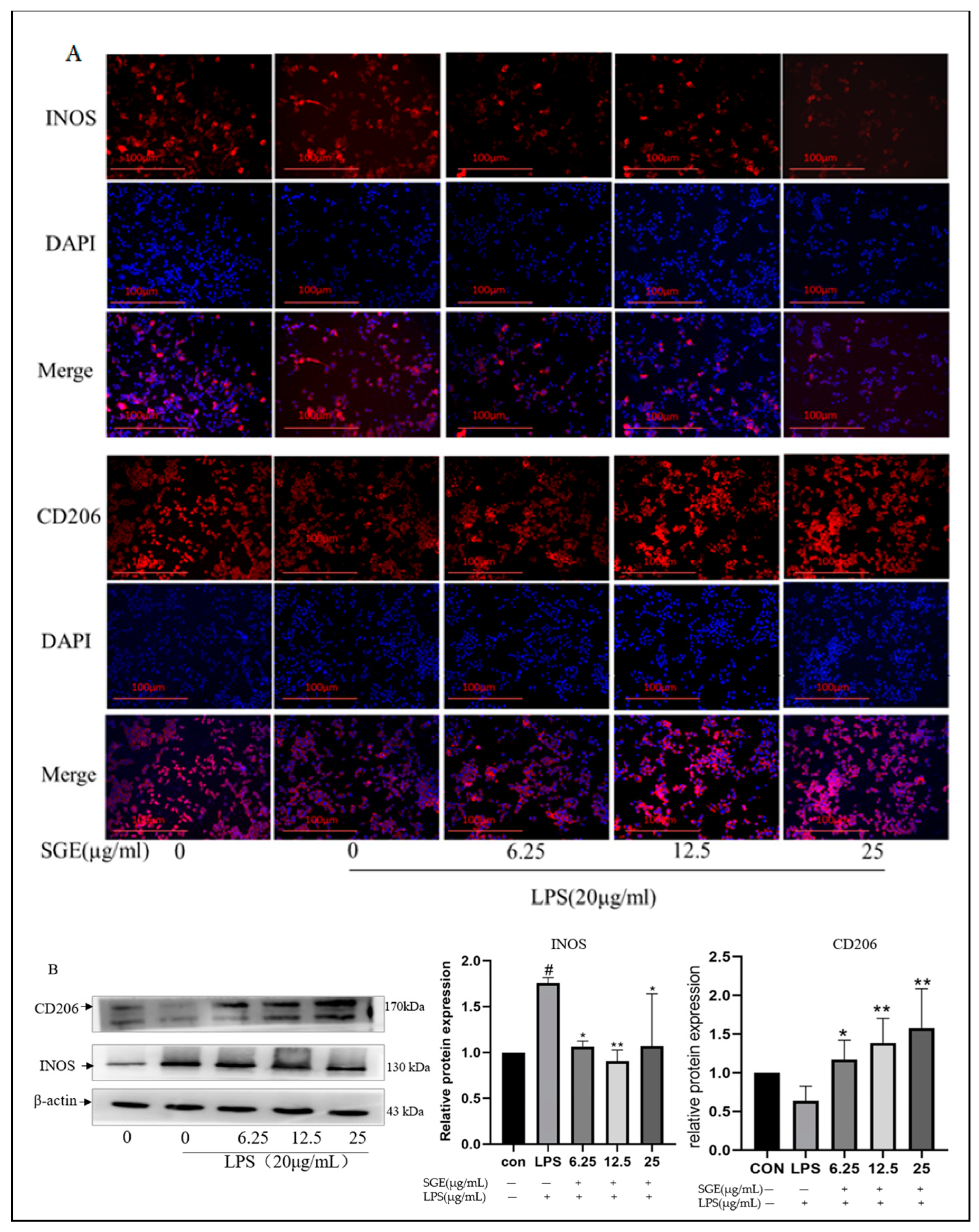
2.6. Effects of SGE on INOS and CD206 Protein in Ileum
2.7. Cytotoxicity of SGE and Its Application in Inflammatory Model
2.8. Effects of SGE on the Inflammatory Factors in RAW264.7 Cells
2.9. Effects of SGE on RAW264.7 Macrophage Polarization
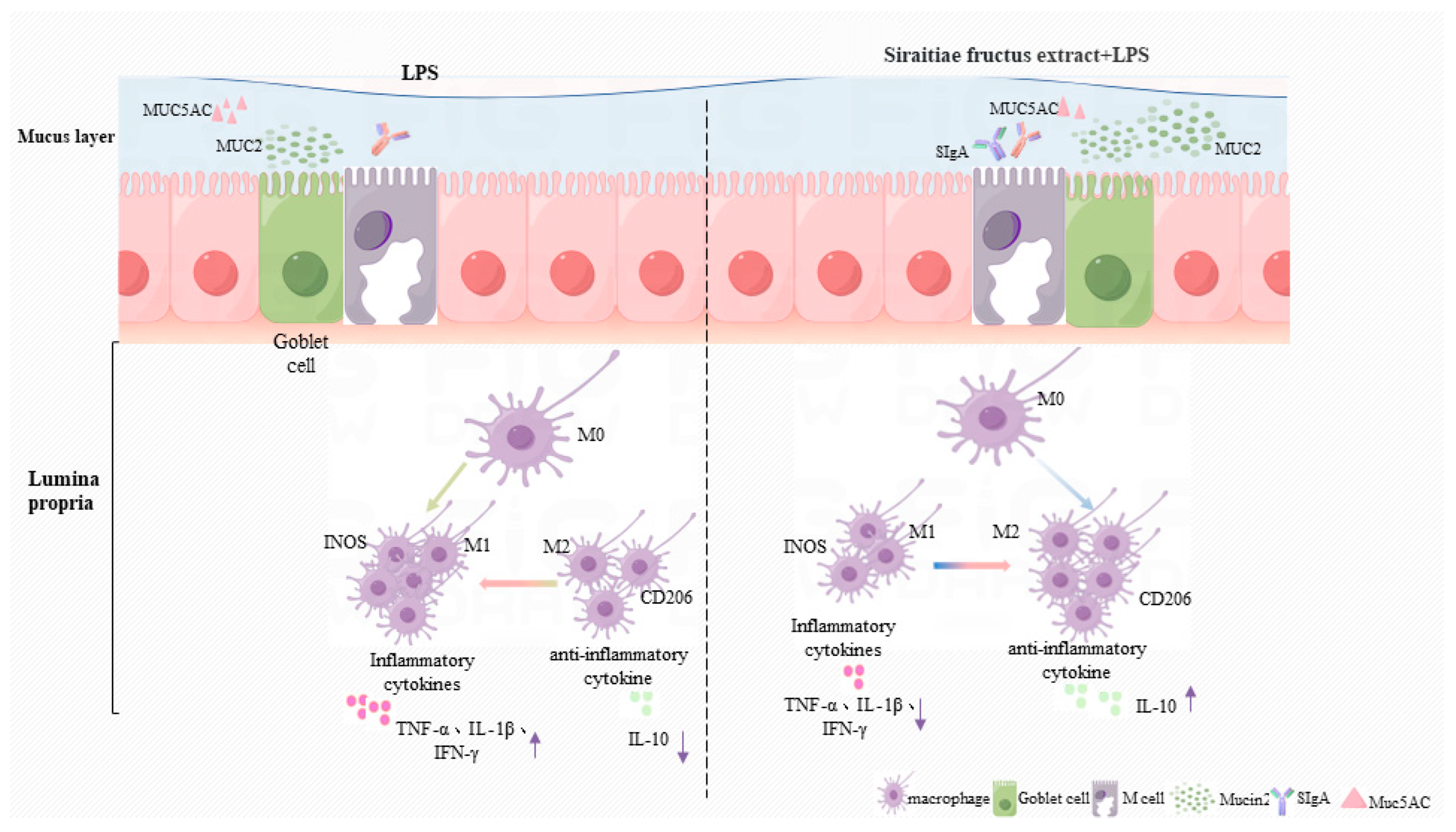
3. Discussion
4. Materials and Methods
4.1. Material
4.2. Preparation of SGE
4.3. Analysis of Chemical Constituents of SGE
4.4. Animal Experiment
4.5. Routine Analysis of Blood
4.6. ELISA
4.7. HE and PAS Staining
4.8. Cell Culture and Model Establishment
4.9. RTq-PCR
4.10. Western Blot
4.11. Immunofluorescence
4.12. Statistic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kang, L.; Pang, J.; Zhang, X.; Liu, Y.; Wu, Y.; Wang, J.; Han, D. L-arabinose Attenuates LPS-Induced Intestinal Inflammation and Injury through Reduced M1 Macrophage Polarization. J. Nutr. 2023, 153, 3327–3340. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Pelgrim, C.E.; Marzal, L.N.P.; Korver, S.; van Ark, I.; Leusink-Muis, T.; van Helvoort, A.; Keshavarzian, A.; Kraneveld, A.D.; Garssen, J. Changes in intestinal homeostasis and immunity in a cigarette smoke-and LPS-induced murine model for COPD: The lung-gut axis. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2022, 323, L266–L280. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Kaur, G.; Ali, S.A. IL-33’s role in the gut immune system: A comprehensive review of its crosstalk and regulation. Life Sci. 2023, 327, 121868. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Hu, Y.; Zhao, Y.; Cheng, M.; Qin, H.; Cheng, T.; Wang, Q.; Peng, X.; Zhang, X. Curcumin attenuates titanium particle-induced inflammation by regulating macrophage polarization in vitro and in vivo. Front. Immunol. 2017, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Na, Y.R.; Stakenborg, M.; Seok, S.H.; Matteoli, G. Macrophages in intestinal inflammation and resolution: A potential therapeutic target in IBD. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Hu, Z.; Ye, F.; Guo, T.; Luo, Y.; Zhou, W.; Qin, D.; Tang, Y.; Cao, F.; Luo, F. Mogroside V exerts anti-inflammatory effect via MAPK-NF-κB/AP-1 and AMPK-PI3K/Akt/mTOR pathways in ulcerative colitis. J. Funct. Food 2021, 87, 104807. [Google Scholar] [CrossRef]
- Wang, M.; Lou, H.; Zhang, N.; Zhang, J.; Cao, Y.; Li, J.; Pan, W. Research progress on chemical constituents and pharmacology of Siraitia grosvenorii. J. Guizhou Univ. Tradit. Chin. Med. 2021, 43, 80–84. [Google Scholar]
- Li, H.; Li, R.; Jiang, W.; Zhou, L. Research progress of pharmacological effects of Siraitia grosvenorii extract. J. Pharm. Pharmacol. 2022, 74, 953–960. [Google Scholar] [CrossRef]
- Liu, C.; Dai, L.; Liu, Y.; Dou, D.; Sun, Y.; Ma, L. Pharmacological activities of mogrosides. Future Sci. 2018, 10, 845–850. [Google Scholar] [CrossRef]
- Shen, Y.; Lin, S.; Han, C.; Hou, X.; Long, Z.; Xu, K. Rapid identification and quantification of five major mogrosides in Siraitia grosvenorii (Luo-Han-Guo) by high performance liquid chromatography–triple quadrupole linear ion trap tandem mass spectrometry combined with microwave-assisted extraction. Microchem. J. 2014, 116, 142–150. [Google Scholar] [CrossRef]
- Sung, Y.; Yuk, H.; Yang, W.; Kim, S.; Kim, D. Siraitia grosvenorii Residual Extract Attenuates Atopic Dermatitis by Regulating Immune Dysfunction and Skin Barrier Abnormality. Nutrients 2020, 12, 3638. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, X.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. The role of MUC2 mucin in intestinal homeostasis and the impact of dietary components on MUC2 expression. Int. J. Biol. Macromol. 2020, 164, 884–891. [Google Scholar] [CrossRef]
- Hasnain, S.Z.; Evans, C.M.; Roy, M.; Gallagher, A.L.; Kindrachuk, K.N.; Barron, L.; Dickey, B.F.; Wilson, M.S.; Wynn, T.A.; Grencis, R.K. Muc5ac: A critical component mediating the rejection of enteric nematodes. J. Exp. Med. 2011, 208, 893–900. [Google Scholar] [CrossRef]
- Aamann, L.; Vestergaard, E.M.; Grønbæk, H. Trefoil factors in inflammatory bowel disease. World J. Gastroenterol. WJG 2014, 20, 3223. [Google Scholar] [CrossRef]
- Kumar, P.R.; Dinesh, S.R.; Rini, R. LCMS—A review and a recent update. J. Pharm. Pharm. Sci. 2016, 5, 377–391. [Google Scholar]
- Li, Y.; Zou, L.; Li, T.; Lai, D.; Wu, Y.; Qin, S. Mogroside V inhibits LPS-induced COX-2 expression/ROS production and overexpression of HO-1 by blocking phosphorylation of AKT1 in RAW264. 7 cells. Acta Biochim. Biophys. Sin. 2019, 51, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Jie, Z.; Qiaoling, Z.; Fenglai, L.; Jingru, S.; Hongwei, L.; Xiaohua, J. Study on Isolation, Purification and Immunological Activity of Polysaccharide from Siraitia grosvenorii Root. Chin. Med. herb. 2024, 55, 1100–1109. [Google Scholar]
- Lauridsen, C. Effects of dietary fatty acids on gut health and function of pigs pre-and post-weaning. J. Anim. Sci. 2020, 98, a86. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Yuan, M.; Guo, B.; Lin, J.; Yan, S.; Huang, H.; Chen, J.; Wang, S.; Ma, Y. Citric Acid Promotes Immune Function by Modulating the Intestinal Barrier. Int. J. Mol. Sci. 2024, 25, 1239. [Google Scholar] [CrossRef]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, C. The relationship between intestinal goblet cells and the immune response. Biosci. Rep. 2020, 40, R20201471. [Google Scholar] [CrossRef] [PubMed]
- McGuckin, M.A.; Lindén, S.K.; Sutton, P.; Florin, T.H. Mucin dynamics and enteric pathogens. Nat. Rev. Microbiol. 2011, 9, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Ryu, O.; Kiyoshi, T. Maintenance of intestinal homeostasis by mucosal barriers. Inflamm. Regen. 2018, 38, 5. [Google Scholar]
- Sun, J.; Shen, X.; Li, Y.; Guo, Z.; Zhu, W.; Zuo, L.; Zhao, J.; Gu, L.; Gong, J.; Li, J. Therapeutic Potential to Modify the Mucus Barrier in Inflammatory Bowel Disease. Nutrients 2016, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, F.; De Castro, C.; Silipo, A.; Molinaro, A. Lipopolysaccharide structures of Gram-negative populations in the gut microbiota and effects on host interactions. FEMS Microbiol. Rev. 2019, 43, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Bao, M.; Liang, M.; Sun, X.; Mohyuddin, S.G.; Chen, S.; Wen, J.; Yong, Y.; Ma, X.; Yu, Z.; Ju, X. Baicalin alleviates LPS-induced oxidative Stress via NF-κB and Nrf2–HO1 signaling Pathways in IPEC-J2 Cells. Front. Vet. Sci. 2022, 8, 808233. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhu, Y.; Wang, Y.; Li, Z.; Wang, Z.; Miao, Y.; Du, H.; Liu, D. The water-soluble subfraction from Artemisia argyi alleviates LPS-induced inflammatory responses via multiple pathways and targets in vitro and in vivo. J. Ethnopharmacol. 2024, 319, 117364. [Google Scholar] [CrossRef] [PubMed]
- Candelli, M.; Franza, L.; Pignataro, G.; Ojetti, V.; Covino, M.; Piccioni, A.; Gasbarrini, A.; Franceschi, F. Interaction between lipopolysaccharide and gut microbiota in inflammatory bowel diseases. Int. J. Mol. Sci. 2021, 22, 6242. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.; Liu, C.; Chen, Y.; Yu, M.; He, J.; Tan, B.; Fu, X.; He, J.; Xiao, D. Dietary Chito-oligosaccharide attenuates LPS-challenged intestinal inflammation via regulating mitochondrial apoptotic and MAPK signaling pathway. Int. Immunopharmacol. 2024, 126, 111153. [Google Scholar] [CrossRef]
- Boes, M.; Prodeus, A.P.; Schmidt, T.; Carroll, M.C.; Chen, J. A critical role of natural immunoglobulin M in immediate defense against systemic bacterial infection. J. Exp. Med. 1998, 188, 2381–2386. [Google Scholar] [CrossRef]
- Yates, J.L.; Racine, R.; McBride, K.M.; Winslow, G.M. T Cell–Dependent IgM Memory B Cells generated during bacterial infection are required for IgG responses to antigen challenge. J. Immunol. 2013, 191, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, N.R.; King, L.S.; D’Alessio, F.R. Diverse macrophage populations mediate acute lung inflammation and resolution. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2014, 306, L709–L725. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J. Macrophage polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ren, X.; Zhou, L.; Liu, K.; Deng, L.; Qing, Q.; Li, J.; Zhi, F.; Li, M. Tollip Orchestrates Macrophage Polarization to Alleviate Intestinal Mucosal Inflammation. J. Crohn’s Colitis 2022, 16, 1151–1167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Li, X.; Sheng, C.; Chen, X.; Chen, Y.; Zhu, D.; Gao, P. Macrophages activate iNOS signaling in adventitial fibroblasts and contribute to adventitia fibrosis. Nitric Oxide-Biol. Chem. 2016, 61, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Gensel, J.C.; Zhang, B. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res. 2015, 1619, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Helming, L.; Gordon, S. Alternative Activation of Macrophages: An Immunologic Functional Perspective. Annu. Rev. Immunol. 2009, 27, 451–483. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, D.; Yuk, H.J.; Kim, S.; Yang, W.; Park, G.D.; Kim, K.S.; Ham, W.J.; Sung, Y. Siraitia grosvenorii extract attenuates airway inflammation in a murine model of chronic obstructive pulmonary disease induced by cigarette smoke and lipopolysaccharide. Nutrients 2023, 15, 468. [Google Scholar] [CrossRef]
- Lu, R.; Hu, J.; Liu, X.; Yu, L.; Hu, J.; Jiang, H.; Liu, S.; Li, M.; He, J.; Yang, X. Mogroside-rich extract from Siraitia grosvenorii fruits protects against heat stress-induced intestinal damage by ameliorating oxidative stress and inflammation in mice. Food Funct. 2023, 14, 1238–1247. [Google Scholar] [CrossRef]
- Wang, M. Effect of Geniposide on NF-κB/IAPin LPS-induced Intestinal Damage in Mice. Master’s Thesis, Heilongjiang Bayi Agricultural University, Daqing, China, 2020. [Google Scholar]
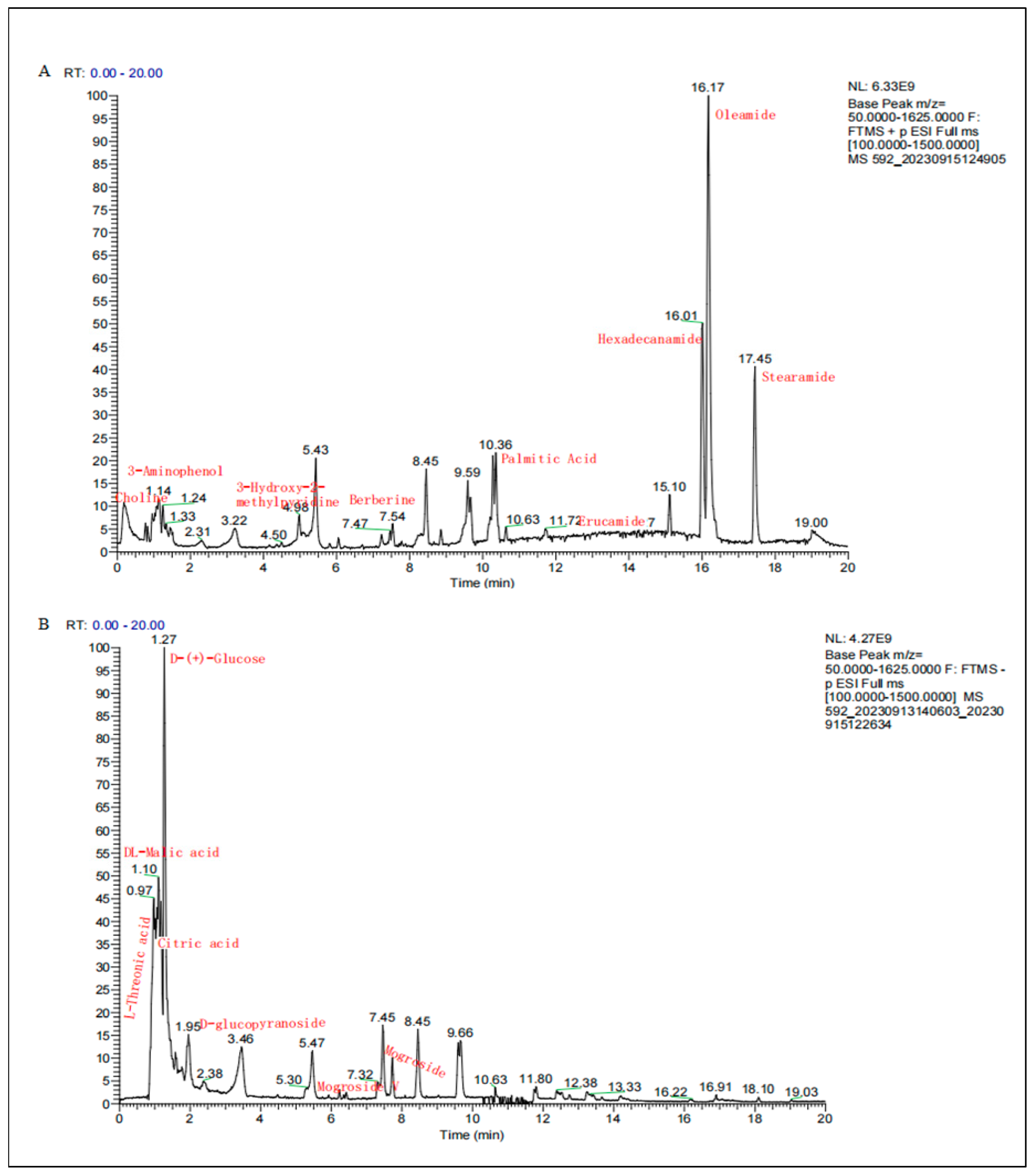




| Mode | Chemical Compound | Molecular Formula | Molecular Weight | Retention (min) | Peak Area | Peak Area Percentage % |
|---|---|---|---|---|---|---|
| P | Oleamide | C18H35NO | 281.27138 | 16.168 | 4.90 × 1010 | 19.20 |
| P | Hexadecanamide | C16H33NO | 255.25575 | 16.002 | 2.11 × 1010 | 8.28 |
| P | Stearamide | C18H37NO | 283.28706 | 17.444 | 1.69 × 1010 | 6.61 |
| P | N-Methylanthranilic acid | C8H9NO2 | 151.06336 | 5.082 | 1.03 × 1010 | 4.05 |
| P | Erucamide | C22H43NO | 337.33409 | 13.409 | 9.96 × 109 | 3.90 |
| P | Palmitic acid | C16H32O2 | 273.26619 | 10.283 | 6.70 × 109 | 2.62 |
| P | 3-Aminophenol | C6H7NO | 109.0528 | 1.149 | 4.36 × 109 | 1.71 |
| P | Choline | C5H13NO | 103.09977 | 0.99 | 4.02 × 109 | 1.57 |
| P | Trigonelline HCl | C7H7NO2 | 137.04791 | 1.033 | 3.63 × 109 | 1.42 |
| P | 3-Hydroxy-2-methylpyridine | C6H7NO | 109.05282 | 4.957 | 2.97 × 109 | 1.16 |
| N | DL-Malic acid | C4H6O5 | 134.02132 | 1.074 | 4.33 × 1010 | 16.93 |
| N | Citric acid | C6H8O7 | 192.02679 | 1.166 | 1.46 × 1010 | 5.72 |
| N | D-(+)-Glucose | C6H12O6 | 226.06866 | 1.245 | 9.63 × 109 | 3.77 |
| N | L-Threonic acid | C4H8O5 | 136.03703 | 0.888 | 4.34 × 109 | 1.70 |
| N | Gluconic acid | C6H12O7 | 196.05797 | 1.012 | 2.92 × 109 | 1.14 |
| N | Mogroside | C60H102O29 | 1286.64704 | 6.4–8.2 | 4.66 × 108 | 1.27 |
| Time (min) | A (0.1% FA/Water) | B (0.1% FA/ACN) |
|---|---|---|
| 0 | 90 | 10 |
| 10 | 0 | 100 |
| 15 | 0 | 100 |
| 17.1 | 90 | 10 |
| 20 | 90 | 10 |
| Gene | Primer Sequence (5′ to 3′) | Login Number | Prodsize |
|---|---|---|---|
| β-actin | F: ACTGCCGCATCCTCTTCCTC | NC_000071.7 | 80 |
| R: AACCGCTCGTTGCCAATAGTG | |||
| TNF-α | F: CGCTCTTCTGTCTACTGAACTTCGG R: GTGGTTTGTGAGTGTGAGGGTCTG | NC_000083.7 | 113 |
| IL-1β | F: TCGCAGCAGCACATCAACAAG R:TCCACGGGAAAGACACAGGTAG | NC_000068.8 | 94 |
| IFN-γ | F: CTGGAGGAACTGGCAAAAGGATGG R: GACGCTTATGTTGTTGCTGATGGC | NC_000076.7 | 121 |
| IL-10 | F:GGACAACATACTGCTAACCGACTCC R: AGCCGCATCCTGAGGGTCTTC | NC_000067.7 | 186 |
| MUC2 | F:GCTGACGAGTGGTTGGTGAATG R:GATGAGGTGGCAGACAGGAGAC | NC_000073.7 | 135 |
| MUC5AC | F:TCACTCTACCACTCCCTGCTTCTG R:CACCTGACAATCCTGGCTACACATC | NC_000073.7 | 127 |
| TFF3 | F:AATGCTGTTGGTGGTCCTGGTTG R:GGGCACATTTGGGATACTGGAGTC | NC_000083.7 | 181 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Guo, M.; Zhao, L.; Zhang, J.; He, J.; Xu, A.; Yu, Z.; Ma, X.; Yong, Y.; Li, Y.; et al. Siraitia grosvenorii Extract Protects Lipopolysaccharide-Induced Intestinal Inflammation in Mice via Promoting M2 Macrophage Polarization. Pharmaceuticals 2024, 17, 1023. https://doi.org/10.3390/ph17081023
Wu H, Guo M, Zhao L, Zhang J, He J, Xu A, Yu Z, Ma X, Yong Y, Li Y, et al. Siraitia grosvenorii Extract Protects Lipopolysaccharide-Induced Intestinal Inflammation in Mice via Promoting M2 Macrophage Polarization. Pharmaceuticals. 2024; 17(8):1023. https://doi.org/10.3390/ph17081023
Chicago/Turabian StyleWu, Huining, Mengru Guo, Linlu Zhao, Jin Zhang, Jieyi He, Anning Xu, Zhichao Yu, Xingbin Ma, Yanhong Yong, Youquan Li, and et al. 2024. "Siraitia grosvenorii Extract Protects Lipopolysaccharide-Induced Intestinal Inflammation in Mice via Promoting M2 Macrophage Polarization" Pharmaceuticals 17, no. 8: 1023. https://doi.org/10.3390/ph17081023







