Bioactivity Profiling of Daedaleopsis confragosa (Bolton) J. Schröt. 1888: Implications for Its Possible Application in Enhancing Women’s Reproductive Health
Abstract
1. Introduction
2. Results and Discussion
2.1. Extraction Yield
2.2. Determination of Total Phenolic Content and Total Flavonoid Content Alongside Antioxidant Activity
2.3. Cytotoxic Activity
2.4. Inhibition of AChE
2.5. Activity of PP1 Enzyme
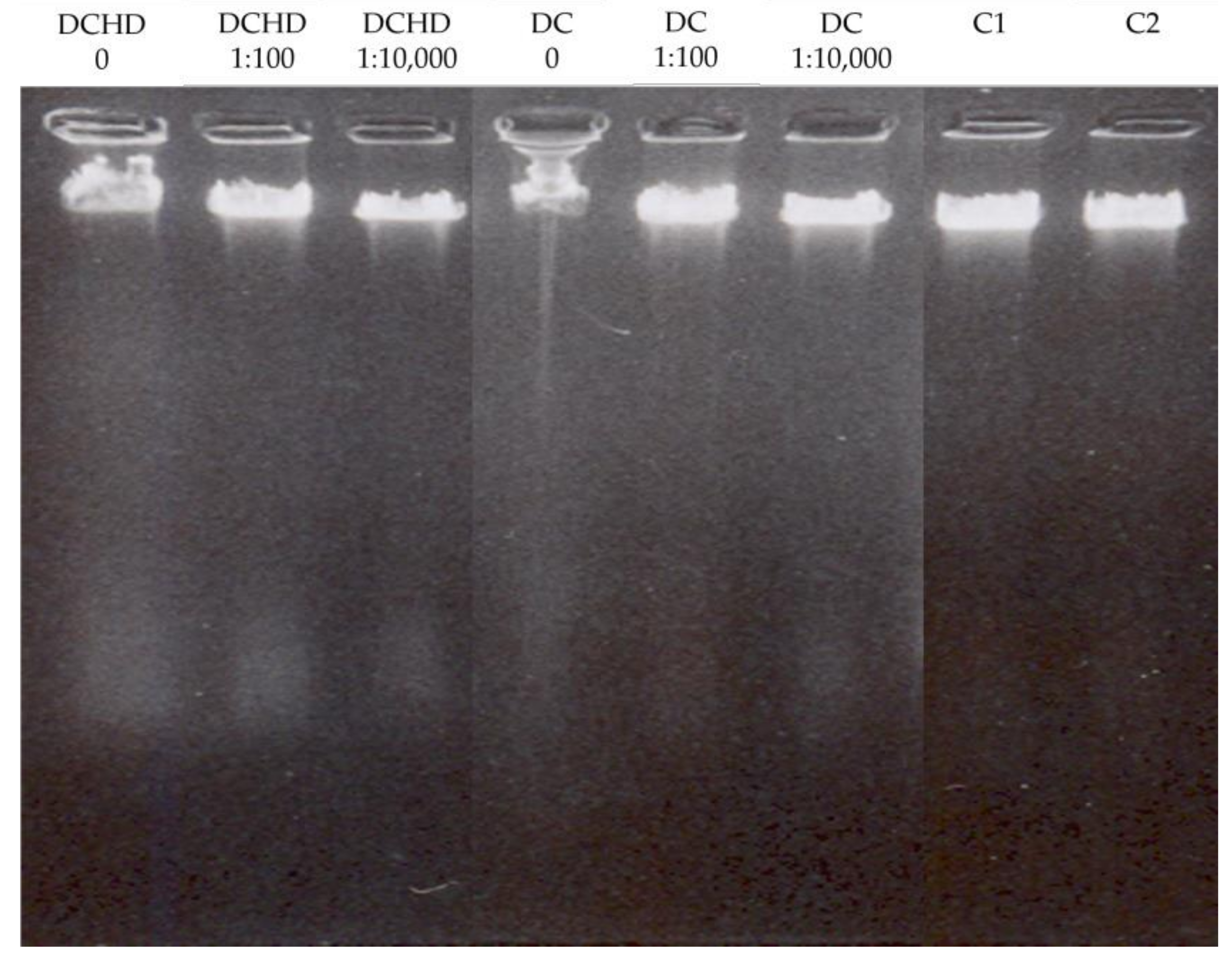
2.6. Extracts’ Effect on DNA Integrity
2.7. Hemolytic Activity
3. Materials and Methods
3.1. Fungal Material
3.2. Preparation of Fungal Extracts
3.3. Determination of TPC and TFC and Antioxidant Capacity
- h0—the height of the second peak of the DPPH radical ESR signal of the blank sample;
- hx—the height of the second peak of the ESR signal of the DPPH radical of the sample that contains extract.
3.4. Cytotoxic Activity
3.5. Enzyme Assays
3.5.1. Anti-Acetylcholinesterase Activity
3.5.2. Activity of Protein Phosphatase-1 Enzyme
3.6. Effect of Fungal Extracts on Viral DNA Integrity
3.7. Hemolytic Activity
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tiwari, P.; Bae, H. Endophytic fungi: Key insights, emerging prospects, and challenges in natural product drug discovery. Microorganisms 2022, 10, 360. [Google Scholar] [CrossRef] [PubMed]
- Karaman, M.; Matavulj, M.; Janjic, L. Antibacterial agents from lignicolous macrofungi. In Antimicrobial Agents; Bobbarala, V., Ed.; InTech Open: Rijeka, Croatia, 2012; Volume 18, pp. 361–386. [Google Scholar] [CrossRef][Green Version]
- Karaman, M.; Tesanovic, K.; Novakovic, A.; Jakovljevic, D.; Janjusevic, L.; Sibul, F.; Pejin, B. Coprinus comatus filtrate extract, a novel neuroprotective agent of natural origin. Nat. Prod. Res. 2020, 34, 2346–2350. [Google Scholar] [CrossRef] [PubMed]
- Mišković, J.; Karaman, M.; Rašeta, M.; Krsmanović, N.; Berežni, S.; Jakovljević, D.; Piattoni, F.; Zambonelli, A.; Gargano, M.L.; Venturella, G. Comparison of two Schizophyllum commune strains in production of acetylcholinesterase inhibitors and antioxidants from submerged cultivation. J. Fungi 2021, 7, 115. [Google Scholar] [CrossRef] [PubMed]
- Karaman, M.; Čapelja, E.; Rašeta, M.; Rakić, M. Diversity, chemistry, and environmental contamination of wild growing medicinal mushroom species as sources of biologically active substances (Antioxidants, Anti-Diabetics, and AChE Inhibitors). In Biology, Cultivation and Applications of Mushrooms; Arya, A., Rusevska, K., Eds.; Springer: Singapore, 2022; Volume 8, pp. 203–257. [Google Scholar] [CrossRef]
- Mišković, J.; Rašeta, M.; Čapelja, E.; Krsmanović, N.; Novaković, A.; Janjusevic, L.; Karaman, M. Mushroom species Stereum hirsutum as natural source of phenolics and fatty acids as antioxidants and acetylcholinesterase inhibitors. Chem. Biodivers. 2021, 18, e2100409. [Google Scholar] [CrossRef] [PubMed]
- Nagadesi, P.K.; Stephen, A. Mycochemicals and antidiabetic activity of lignocolous fungi—A critical review. Bionature 2022, 42, 13–30. [Google Scholar] [CrossRef]
- Rašeta, M.; Mišković, J.; Čapelja, E.; Zapora, E.; Petrović Fabijan, A.; Knežević, P.; Karaman, M. Do Ganoderma species represent novel sources of phenolic based antimicrobial agents? Molecules 2023, 28, 3264. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Zhou, L.; Zhang, C.; Xu, Q.; Sun, Y. Targeting protein phosphatases for the treatment of inflammation-related diseases: From signaling to therapy. Signal Transduct. Target. Ther. 2022, 7, 177. [Google Scholar] [CrossRef] [PubMed]
- Casamayor, A.; Ariño, J. Controlling Ser/Thr protein phosphatase PP1 activity and function through interaction with regulatory subunits. Adv. Protein Chem. Struct. Biol. 2020, 122, 231–288. [Google Scholar] [CrossRef] [PubMed]
- Karaman, M.; Janjušević, L.; Jakovljevic, D.; Sibul, F.; Pejin, B. Anti-hydroxyl radical activity, redox potential and anti-AChE activity of Amanita strobiliformis polysaccharide extract. Nat. Prod. Res. 2019, 33, 1522–1526. [Google Scholar] [CrossRef] [PubMed]
- Gulati, V.; Singh, M.; Gulati, P. Role of mushrooms in gestational diabetes mellitus. AIMS Med. Sci. 2019, 6, 49–66. [Google Scholar] [CrossRef]
- Sun, L.; Niu, Z. A mushroom diet reduced the risk of pregnancy-induced hypertension and macrosomia: A randomized clinical trial. Food Nutr. Res. 2020, 64, 4451. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, J.; Vaz, J.A.; Ricardo, S. The potential of mushroom extracts to improve chemotherapy efficacy in cancer cells: A systematic review. Cells 2024, 13, 510. [Google Scholar] [CrossRef] [PubMed]
- Gariboldi, M.B.; Marras, E.; Ferrario, N.; Vivona, V.; Prini, P.; Vignati, F.; Perletti, G. Anti-cancer potential of edible/medicinal mushrooms in breast cancer. Int. J. Mol. Sci. 2023, 24, 10120. [Google Scholar] [CrossRef] [PubMed]
- Rai, N.; Gupta, P.; Verma, A.; Tiwari, R.K.; Madhukar, P.; Kamble, S.C.; Kumar, A.; Kumar, R.; Singh, S.K.; Gautam, V. Ethyl acetate extract of Colletotrichum gloeosporioides promotes cytotoxicity and apoptosis in human breast cancer cells. ACS Omega 2023, 8, 3768–3784. [Google Scholar] [CrossRef] [PubMed]
- Clusan, L.; Ferrière, F.; Flouriot, G.; Pakdel, F. A basic review on estrogen receptor signaling pathways in breast cancer. Int. J. Mol. Sci. 2023, 24, 6834. [Google Scholar] [CrossRef]
- Hong, S.A.; Kim, K.; Nam, S.J.; Kong, G.; Kim, M. A case–control study on the dietary intake of mushrooms and breast cancer risk among Korean women. Int. J. Cancer 2008, 122, 919–923. [Google Scholar] [CrossRef]
- Shin, A.; Kim, J.; Lim, S.Y.; Kim, G.H.; Sung, M.; Lee, E.; Ro, J. Dietary mushroom intake and the risk of breast cancer based on hormone receptor status. Nutr. Cancer 2010, 62, 476–483. [Google Scholar] [CrossRef]
- Panda, S.K.; Sahoo, G.; Swain, S.S.; Luyten, W. Anticancer activities of mushrooms: A neglected source for drug discovery. Pharmaceuticals 2022, 15, 176. [Google Scholar] [CrossRef] [PubMed]
- Ismaya, W.T.; Tjandrawinata, R.R.; Rachmawati, H. Lectins from the edible mushroom Agaricus bisporus and their therapeutic potentials. Molecules 2020, 25, 2368. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Montemayor, M.M.; Ling, T.; Suárez-Arroyo, I.J.; Ortiz-Soto, G.; Santiago-Negrón, C.L.; Lacourt-Ventura, M.Y.; Valentín-Acevedo, A.; Lang, W.H.; Rivas, F. Identification of biologically active Ganoderma lucidum compounds and synthesis of improved derivatives that confer anti-cancer activities in vitro. Front. Pharmacol. 2019, 10, 115. [Google Scholar] [CrossRef]
- Ćilerdžić, J.; Galić, M.; Ivanović, Ž.; Brčeski, I.; Vukojević, J.; Stajić, M. Stimulation of wood degradation by Daedaleopsis confragosa and D. tricolor. Appl. Biochem. Biotechnol. 2019, 187, 1371–1383. [Google Scholar] [CrossRef] [PubMed]
- Karadžić, D.; Radulović, Z.; Jovanović, D.; Milenković, I. A contribution to the knowledge of fungi Daedaleopsis confragosa (Bolton) Schröt the cause of white rot of hardwood. Glas. Sumar. Fak. 2023, 128, 31–46. [Google Scholar] [CrossRef]
- Yan, H.; Chang, H. Antioxidant and antitumor activities of selenium-and zinc-enriched oyster mushroom in mice. Biol. Trace Elem. Res. 2012, 150, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Knežević, A.; Stajić, M.; Živković, L.; Milovanović, I.; Spremo-Potparević, B.; Vukojević, J. Antifungal, antioxidative, and genoprotective properties of extracts from the blushing bracket mushroom, Daedaleopsis confragosa (Agaricomycetes). Int. J. Med. Mushrooms 2017, 19, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Na, M.W.; Lee, E.; Kang, D.M.; Jeong, S.Y.; Ryoo, R.; Kim, C.Y.; Ahn, M.J.; Kang, K.B.; Kim, K.H. Identification of antibacterial sterols from Korean wild mushroom Daedaleopsis confragosa via bioactivity-and LC-MS/MS profile-guided fractionation. Molecules 2022, 27, 1865. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, E.; Graham, A.L.; Úbeda, F.; Wild, G. On maternity and the stronger immune response in women. Nat. Commun. 2022, 13, 4858. [Google Scholar] [CrossRef] [PubMed]
- Bogavac, M.A.; Ćelić, D.D.; Perić, T.M. A prospective study of mid-trimester MCP-1 levels as a predictor of preterm delivery. Medicines 2023, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Ilic, D.; Ilic, A.; Stojsic, S.; Stojsic-Milosavljevic, A.; Papovic, J.; Grkovic, D.; Rankov, O.; Milovancev, A.; Velicki, L. Effect of dipping pattern of gestational hypertension on maternal symptoms and physical findings, birth weight and preterm delivery. Acta Clin. Croat. 2021, 60, 641–650. [Google Scholar] [PubMed]
- Geng, P.; Siu, K.; Wang, Z.; Wu, J. Antifatigue functions and mechanisms of edible and medicinal mushrooms. BioMed. Res. Int. 2017, 2017, 9648496. [Google Scholar] [CrossRef] [PubMed]
- Rašeta, M.; Popović, M.; Beara, I.; Šibul, F.; Zengin, G.; Krstić, S.; Karaman, M. Anti-inflammatory, antioxidant and enzyme inhibition activities in correlation with mycochemical profile of selected indigenous Ganoderma spp. from Balkan region (Serbia). Chem. Biodivers. 2020, 18, e2000828. [Google Scholar] [CrossRef] [PubMed]
- Bogavac, M.A.; Jakovljević, A.; Stajić, Z.; Nikolić, A.; Milosević-Tosić, M.; Dejanović, J.; Lozanov-Crvenkovic, Z. Preeclampsia and level of oxidative stress in the first trimester of pregnancy. Vojnosanit. Pregl. 2017, 74, 633–638. [Google Scholar] [CrossRef]
- Stajić, D.; Ilić, D.; Vuković, J.; Baturan, B.; Ilić, A.; Milovančev, A. The effect of continuous positive airway pressure treatment on hypertensive disorder in pregnant women with obstructive sleep apnea. Sleep Breath. 2021, 26, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Bogavac, M.A.; Jakovljević, A.; Nikolić, A.; Milošević Tošić, M.; Perić, T.; Belopavlović, Z. Biomarkers of oxidative stress in pregnant women with recurrent miscarriages. J. Lab. Med. 2019, 43, 101–114. [Google Scholar] [CrossRef]
- Rašeta, M.; Popović, M.; Knežević, P.; Šibul, F.; Kaišarević, S.; Karaman, M. Bioactive phenolic compounds of two medicinal mushroom species Trametes versicolor and Stereum subtomentosum as antioxidant and antiproliferative agents. Chem. Biodivers. 2020, 17, e2000683. [Google Scholar] [CrossRef] [PubMed]
- Vidović, S.; Zeković, Z.; Mujić, I.; Lepojević, Ž.; Radojković, M.; Živković, J. The antioxidant properties of polypore mushroom Daedaleopsis confragosa. Cent. Eur. J. Biol. 2011, 6, 575–582. [Google Scholar] [CrossRef]
- Chandrawanshi, N.K.; Tandia, D.K.; Jadhav, S.K. Determination of antioxidant and antidiabetic activities of polar solvent extracts of Daedaleopsis confragosa (Bolton) J. Schröt. Res. J. Pharm. Technol. 2018, 11, 5623–5630. [Google Scholar] [CrossRef]
- Glumac, M.; Pejin, B.; Karaman, M.; Mojović, M.; Matavulj, M. Lignicolous fungi hydrodistilled extracts may represent a promising source of natural phenolics. Nat. Prod. Res. 2017, 31, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Sulkowska-Ziaja, K.; Muszynska, B.; Motyl, P.; Pasko, P.; Ekiert, H. Phenolic compounds and antioxidant activity in some species of polyporoid mushrooms from Poland. Int. J. Med. Mushrooms 2012, 14, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Karaman, M.; Stahl, M.; Vulić, J.; Vesić, M.; Čanadanović-Brunet, J. Wild-growing lignicolous mushroom species as sources of novel agents with antioxidative and antibacterial potentials. Int. J. Food Sci. Nutr. 2014, 65, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Manjusha, P.G.S. Molecular identification and isolation of antimicrobial, phytochemical, antioxidant properties of different solvent extracts from wild mushrooms. Saudi J. Pathol. Microbiol. 2020, 5, 98–107. [Google Scholar] [CrossRef]
- Hossen, S.M.; Hossain, M.S.; Akbar, S.; Tahmida, U.; Mawa, J.; Emon, N.U. Wild mushrooms showed analgesic and cytotoxic properties along with phytoconstituent’s binding affinity to COX-1, COX-2 and cytochrome P450 2C9. Heliyon 2021, 7, e07997. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Ahn, N.S.; Yang, X.; Lee, Y.S.; Kang, K.S. Ganoderma lucidum extract induces cell cycle arrest and apoptosis in MCF-7 human breast cancer cell. Int. J. Cancer 2002, 102, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Rašeta, M.; Karaman, M.; Jakšić, M.; Šibul, F.; Kebert, M.; Novaković, A.; Popović, M. Mineral composition, antioxidant and cytotoxic biopotentials of wild-growing Ganoderma species (Serbia): G. lucidum (Curtis) P. Karst vs. G. applanatum (Pers.). Pat. Int. J. Food Sci. Technol. 2016, 51, 2583–2590. [Google Scholar] [CrossRef]
- Tomasi, S.; Lohezic-Le Devehat, F.; Sauleau, P.; Bezivin, C.; Boustie, J. Cytotoxic activity of methanol extracts from Basidiomycete mushrooms on murine cancer cell lines. Pharmazie 2004, 59, 290–293. [Google Scholar] [PubMed]
- Karaman, M.; Vesic, M.; Stahl, M.; Novakovic, M.; Janjic, L.; Matavuly, M. Bioactive properties of wild-growing mushroom species Ganorderma applanatum (Pers.) Pat. from Fruska Gora Forest (Serbia). In Recent Progress in Medicinal Plants, RPMP Ethnomedicine and Therapeutic Validation; Govil, J.N., Ed.; Studium Press: New Delhi, India, 2012; Volume 32, pp. 361–377. [Google Scholar]
- James, C.D.; Morgan, I.M.; Bristol, M.L. The Relationship between estrogen-related signaling and human papillomavirus positive cancers. Pathogens 2020, 9, 403. [Google Scholar] [CrossRef] [PubMed]
- Tešanović, K.; Pejin, B.; Šibul, F.; Matavulj, M.; Rašeta, M.; Janjušević, L.; Karaman, M. A comparative overview of antioxidative properties and phenolic profiles of different fungal origins: Fruiting bodies and submerged cultures of Coprinus comatus and Coprinellus truncorum. J. Food Sci. Technol. 2017, 54, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Canivenc-Lavier, M.-C.; Bennetau-Pelissero, C. Phytoestrogens and health effects. Nutrients 2023, 15, 317. [Google Scholar] [CrossRef] [PubMed]
- Aissani, F.; Grara, N.; Bensouici, C.; Bousbia, A.; Ayed, H.; Idris, M.H.M.; Teh, L.K. Algerian Sonchus oleraceus L.: A comparison of different extraction solvent on phytochemical composition, antioxidant properties and anti-cholinesterase activity. Adv. Tradit. Med. 2022, 22, 1–12. [Google Scholar] [CrossRef]
- Fagbemi, K.O.; Aina, D.A.; Olajuyigbe, O.O. Soxhlet extraction versus hydrodistillation using the clevenger apparatus: A comparative study on the extraction of a volatile compound from Tamarindus indica Seeds. Sci. World J. 2021, 2, 5961586. [Google Scholar] [CrossRef]
- Bhambri, A.; Srivastava, M.; Mahale, V.G.; Mahale, S.; Karn, S.K. Mushrooms as potential sources of active metabolites and medicines. Front. Microbiol. 2022, 13, 837266. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Li, H.; Liu, Z.; Feng, G.; Shi, C.; Wu, Y. Unveiling the therapeutic potentials of mushroom bioactive compounds in Alzheimer’s disease. Foods 2023, 12, 2972. [Google Scholar] [CrossRef] [PubMed]
- Chapla, V.M.; Honório, A.E.; Gubiani, J.R.; Vilela, A.F.; Young, M.C.; Cardoso, C.L.; Pavan, F.R.; Cicarelli, R.M.; Ferreira, P.M.; Bolzani, V.D.; et al. Acetylcholinesterase inhibition and antifungal activity of cyclohexanoids from the endophytic fungus Saccharicola sp. Phytochem. Lett. 2020, 39, 116–123. [Google Scholar] [CrossRef]
- Zhao, H. What do we learn from enzyme behaviors in organic solvents? –Structural functionalization of ionic liquids for enzyme activation and stabilization. Biotechnol. Adv. 2020, 45, 107638. [Google Scholar] [CrossRef] [PubMed]
- Villa-Moruzzi, E.; Puntoni, F.; Marin, O. Activation of protein phosphatase-1 isoforms and glycogen synthase kinase-3 beta in muscle from mdx mice. Int. J. Biochem. Cell. Biol. 1996, 28, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Rathee, S.; Rathee, D.; Rathee, D.; Kumar, V.; Rathee, P. Mushrooms as therapeutic agents. Rev. Bras. Farmacogn./Braz. J. Pharmacogn. 2012, 22, 459–474. [Google Scholar] [CrossRef]
- Liu, X.; Luo, D.; Guan, J.; Chen, J.; Xu, X. Mushroom polysaccharides with potential in anti-diabetes: Biological mechanisms, extraction, and future perspectives: A review. Front Nutr. 2022, 9, 1087826. [Google Scholar] [CrossRef] [PubMed]
- Andonova, T.; Muhovski, Y.; Vrancheva, R.; Slavov, I.; Apostolova, E.; Naimov, S.; Pavlov, A.; Dimitrova-Dyulgerova, I. Antioxidant and DNA-protective potentials, main phenolic compounds, and microscopic features of Koelreuteria paniculata aerial parts. Antioxidants 2022, 11, 1154. [Google Scholar] [CrossRef] [PubMed]
- Lobiuc, A.; Pavăl, N.-E.; Mangalagiu, I.I.; Gheorghiță, R.; Teliban, G.-C.; Amăriucăi-Mantu, D.; Stoleru, V. Future antimicrobials: Natural and functionalized phenolics. Molecules 2023, 28, 1114. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Espinoza, M.-C.; Villeneuve, P. Phenolic acids enzymatic lipophilization. J. Agric. Food Chem. 2005, 53, 2779–2787. [Google Scholar] [CrossRef] [PubMed]
- Taofiq, O.; Calhelha, R.C.; Heleno, S.; Barros, L.; Martins, A.; Santos-Buelga, C.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. The contribution of phenolic acids to the anti-inflammatory activity of mushrooms: Screening in phenolic extracts, individual parent molecules and synthesized glucuronated and methylated derivatives. Food Res. Int. 2015, 76, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Fakoya, S.; Adegbehingbe, K.T.; Ogundiimu, A.A. Biopharmaceutical assessment of active components of Deadaleopsis confragosa and Ganoderma lucidum. Open J. Med. Microbiol. 2013, 3, 135–138. [Google Scholar] [CrossRef][Green Version]
- Sikder, L.; Khan, M.R.; Smrity, S.Z.; Islam, M.T.; Khan, S.A. Phytochemical and pharmacological investigation of the ethanol extract of Byttneria pilosa Roxb. Clin. Phytosci. 2022, 8, 1. [Google Scholar] [CrossRef]
- Karaman, M.; Kaišarević, S.; Somborski, J.; Kebert, M.; Matavulj, M. Biological activities of the lignicolous fungus Meripilus giganteus (Pers.: Pers.) Karst. Arch. Biol. Sci. 2009, 61, 853–861. [Google Scholar] [CrossRef]
- Francis, G.; Kerem, Z.; Makkar, H.P.; Becker, K. The biological action of saponins in animal systems: A review. Br. J. Nutr. 2002, 88, 587–605. [Google Scholar] [CrossRef] [PubMed]
- Rafique, S.; Murtaza, M.A.; Hafiz, I.; Ameer, K.; Qayyum, M.M.; Yaqub, S.; Mohamed Ahmed, I.A. Investigation of the antimicrobial, antioxidant, hemolytic, and thrombolytic activities of Camellia sinensis, Thymus vulgaris, and Zanthoxylum armatum ethanolic and methanolic extracts. Food Sci. Nutr. 2023, 11, 6303–6311. [Google Scholar] [CrossRef] [PubMed]
- Pino, A.; Bartolo, E.; Caggia, C.; Cianci, A.; Randazzo, C.L. Detection of vaginal lactobacilli as probiotic candidates. Sci. Rep. 2019, 9, 3355. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela–Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Chang, C.; Yang, M.; When, H.; Chern, J. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar] [CrossRef]
- Espin, C.J.; Soler-Rivas, G.; Wichers, J.H. Characterization of the total free radical scavenger capacity of vegetable oils and oil fractions using 2,2-diphenyl-1-picrylhydrazyl radical. J. Agric. Food Chem. 2000, 48, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid and concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- An, J.S.; Carmichael, W.W. Use of a colorimetric protein phosphatase inhibition assay and enzyme linked immunosorbent assay for the study of microcystins and nodularins. Toxicon 1994, 32, 1495–1507. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, P.; Obreht, D.; Curcin, S.; Petrusic, M.; Aleksic, V.; Kostanjsek, R.; Petrovic, O. Phages of Pseudomonas aeruginosa: Response to environmental factors and in vitro ability to inhibit bacterial growth and biofilm formation. J. Appl. Microbiol. 2011, 111, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Russel, D.W. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2001. [Google Scholar]
- Sæbø, I.P.; Bjørås, M.; Franzyk, H.; Helgesen, E.; Booth, J.A. Optimization of the hemolysis assay for the assessment of cytotoxicity. Int. J. Mol. Sci. 2023, 24, 2914. [Google Scholar] [CrossRef] [PubMed]

| Assay | D. confragosa | ||
|---|---|---|---|
| EtOH–DC | HD–DCHD | ||
| Total Content | |||
| TPC (mg GAE/g d.w.) | 25.30 ± 1.05 | 12.12 ± 0.89 | |
| TFC (mg QE/g d.w.) | 2.84 ± 0.85 | 1.01 ± 0.13 | |
| Antioxidant Activity | |||
| DPPH (IC50 (μg/mL)) | 8.53 ± 0.39 | 10.13 ± 0.76 | |
| ESR DPPH (IC50 (μg/mL)) | 0.981 | 1.565 | |
| FRAP (mg AAE/g d.w.) | 29.74 ± 0.81 | 9.24 ± 0.74 | |
| Enzyme Modulation | Galantamine * | ||
| Anti-AChE (IC50 (μg/mL)) | 3.59 ± 0.78 | 3.11 ± 0.45 | 0.08 ± 0.002 |
| Cytotoxic Activity | Ellagic Acid ** | ||
| MTT (IC50 (μg/mL)) 24 h | 88.23 ± 1.43 | 12.15 ± 0.99 | 35.82 ± 1.52 |
| MTT (IC50 (μg/mL)) 72 h | 81.45 ± 1.32 | 27.76 ± 0.89 | 40.07 ± 0.97 |
| Parameters | D. confragosa | Control | |
|---|---|---|---|
| EtOH–DC | HD–DCHD | 80% EtOH | |
| Basic concentration (mg/mL) | 125 | 125 | - |
| Final concentration (mg/mL) | 3.0 | 3.0 | - |
| AE PP1 (%) | 339.39 ± 1.16 | 217.42 ± 1.09 | 46.96 ± 0.65 |
| AF | 3.39 | 2.17 | 0.47 |
| Parameters | D. confragosa | Control | |
|---|---|---|---|
| EtOH–DC | HD–DCHD | 80% EtOH | |
| Tested concentration (mg/mL) | 125 | 125 | - |
| t50 (min) | <50 | >20 | 0 |
| Intensity of hemolysis | +, −, colored | ++++ | - |
| Hemagglutination (HGA) | - | - | - |
| Tested concentration (mg/mL) | n.a. | 150 | - |
| t50 (min) | n.a. | 6.06 | n.a. |
| 1/t50 (min−1) | n.a. | 0.17 | n.a. |
| HC50 (mg/mL) | n.a. | 81.0 | n.a. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilić, D.; Karaman, M.; Bogavac, M.; Mišković, J.; Rašeta, M. Bioactivity Profiling of Daedaleopsis confragosa (Bolton) J. Schröt. 1888: Implications for Its Possible Application in Enhancing Women’s Reproductive Health. Pharmaceuticals 2024, 17, 600. https://doi.org/10.3390/ph17050600
Ilić D, Karaman M, Bogavac M, Mišković J, Rašeta M. Bioactivity Profiling of Daedaleopsis confragosa (Bolton) J. Schröt. 1888: Implications for Its Possible Application in Enhancing Women’s Reproductive Health. Pharmaceuticals. 2024; 17(5):600. https://doi.org/10.3390/ph17050600
Chicago/Turabian StyleIlić, Djordje, Maja Karaman, Mirjana Bogavac, Jovana Mišković, and Milena Rašeta. 2024. "Bioactivity Profiling of Daedaleopsis confragosa (Bolton) J. Schröt. 1888: Implications for Its Possible Application in Enhancing Women’s Reproductive Health" Pharmaceuticals 17, no. 5: 600. https://doi.org/10.3390/ph17050600
APA StyleIlić, D., Karaman, M., Bogavac, M., Mišković, J., & Rašeta, M. (2024). Bioactivity Profiling of Daedaleopsis confragosa (Bolton) J. Schröt. 1888: Implications for Its Possible Application in Enhancing Women’s Reproductive Health. Pharmaceuticals, 17(5), 600. https://doi.org/10.3390/ph17050600










