The Pathogenic Mechanisms of and Novel Therapies for Lamin A/C-Related Dilated Cardiomyopathy Based on Patient-Specific Pluripotent Stem Cell Platforms and Animal Models
Abstract
1. Introduction
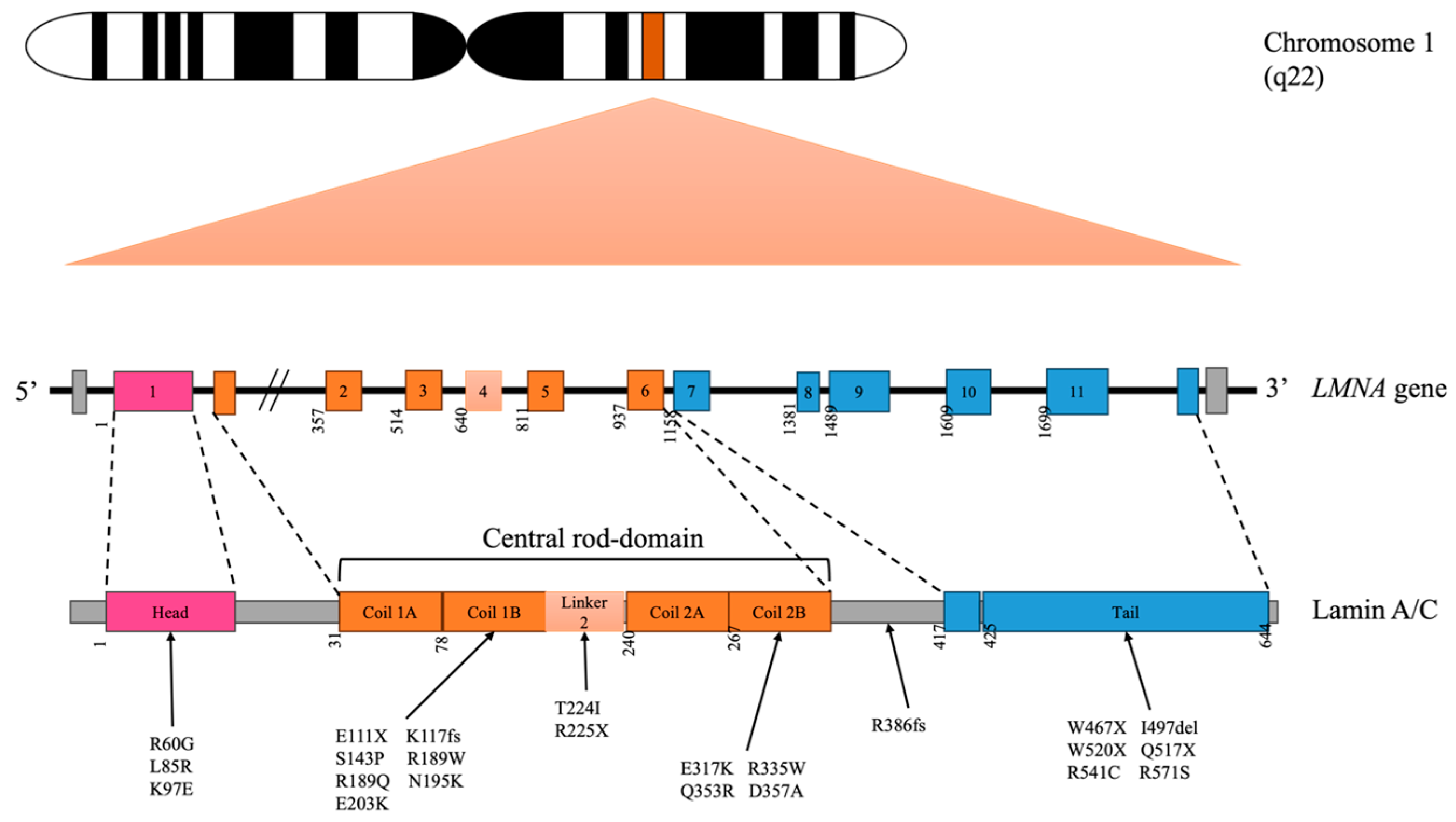
2. Lamin A/C Variants Related to DCM
3. Mechanisms of Lamin A/C-Related DCM
3.1. Mouse Models of LMNA A/C-Related DCM
3.2. Human-Induced Pluripotent Stem-Cell-Derived Cardiomyocyte Models
3.3. Potential Therapeutic Targets
3.3.1. Mitochondria Deficiency
3.3.2. Chromatin Modelling
3.3.3. MAPK-Related Pathway
3.3.4. TGF-β-Related Pathway
3.3.5. Abnormal Calcium Handling
4. Future Prospectives
5. Summary
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hasselberg, N.E.; Haland, T.F.; Saberniak, J.; Brekke, P.H.; Berge, K.E.; Leren, T.P.; Edvardsen, T.; Haugaa, K.H. Lamin A/C cardiomyopathy: Young onset, high penetrance, and frequent need for heart transplantation. Eur. Heart J. 2018, 39, 853–860. [Google Scholar] [CrossRef]
- Yeh, J.K.; Liu, W.H.; Wang, C.Y.; Lu, J.J.; Chen, C.H.; Wu-Chou, Y.H.; Chang, P.Y.; Chang, S.C.; Yang, C.H.; Tsai, M.L.; et al. Targeted Next Generation Sequencing for Genetic Mutations of Dilated Cardiomyopathy. Acta Cardiol. Sin. 2019, 35, 571–584. [Google Scholar]
- McNally, E.M.; Mestroni, L. Dilated Cardiomyopathy. Circ. Res. 2017, 121, 731–748. [Google Scholar] [CrossRef]
- Jacob, K.N.; Garg, A. Laminopathies: Multisystem dystrophy syndromes. Mol. Genet. Metab. 2006, 87, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Ciarambino, T.; Menna, G.; Sansone, G.; Giordano, M. Cardiomyopathies: An Overview. Int. J. Mol. Sci. 2021, 22, 7722. [Google Scholar] [CrossRef] [PubMed]
- Holaska, J.M.; Wilson, K.L.; Mansharamani, M. The nuclear envelope, lamins and nuclear assembly. Curr. Opin. Cell Biol. 2002, 14, 357–364. [Google Scholar] [CrossRef]
- Gaillard, M.-C.; Reddy, K.L. 14-The Nuclear Lamina and Genome Organization. In Nuclear Architecture and Dynamics; Lavelle, C., Victor, J.-M., Eds.; Academic Press: Boston, MA, USA, 2018; Volume 2, pp. 321–343. [Google Scholar]
- Shimoda, Y.; Murakoshi, N.; Mori, H.; Xu, D.; Tajiri, K.; Hemmi, Y.; Sato, I.; Noguchi, M.; Nakamura, Y.; Hayashi, Y.; et al. Generation of a human induced pluripotent stem cell line derived from a patient with dilated cardiomyopathy carrying LMNA nonsense mutation. Stem Cell Res. 2022, 62, 102793. [Google Scholar] [CrossRef] [PubMed]
- Goidescu, C.M. Dilated cardiomyopathy produced by lamin A/C gene mutations. Clujul Med. 2013, 86, 309–312. [Google Scholar] [PubMed]
- Reichart, D.; Magnussen, C.; Zeller, T.; Blankenberg, S. Dilated cardiomyopathy: From epidemiologic to genetic phenotypes. J. Intern. Med. 2019, 286, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Pereira, N.L. Genetics of Cardiomyopathy: Clinical and Mechanistic Implications for Heart Failure. Korean Circ. J. 2021, 51, 797–836. [Google Scholar] [CrossRef] [PubMed]
- Ferradini, V.; Cosma, J.; Romeo, F.; De Masi, C.; Murdocca, M.; Spitalieri, P.; Mannucci, S.; Parlapiano, G.; Di Lorenzo, F.; Martino, A.; et al. Clinical Features of LMNA-Related Cardiomyopathy in 18 Patients and Characterization of Two Novel Variants. J. Clin. Med. 2021, 10, 5075. [Google Scholar] [CrossRef]
- Wang, S.; Peng, D. Case series: LMNA-related dilated cardiomyopathy presents with reginal wall akinesis and transmural late gadolinium enhancement. ESC Heart Fail. 2020, 7, 3179–3183. [Google Scholar] [CrossRef] [PubMed]
- Stallmeyer, B.; Koopmann, M.; Schulze-Bahr, E. Identification of Novel Mutations in LMNA Associated with Familial Forms of Dilated Cardiomyopathy. Genet. Test. Mol. Biomark. 2012, 16, 543–549. [Google Scholar] [CrossRef]
- Arbustini, E.; Pilotto, A.; Repetto, A.; Grasso, M.; Negri, A.; Diegoli, M.; Campana, C.; Scelsi, L.; Baldini, E.; Gavazzi, A.; et al. Autosomal dominant dilated cardiomyopathy with atrioventricular block: A lamin A/C defect-related disease. J. Am. Coll. Cardiol. 2002, 39, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Fatkin, D.M.D.; MacRae, C.M.D.; Sasaki, T.M.D.; Wolff, M.R.M.D.; Porcu, M.M.D.; Frenneaux, M.M.D.; Atherton, J.M.B.B.S.; Vidaillet, H.J.J.M.D.; Spudich, S.M.D.; De Girolami, U.M.D.; et al. Missense mutations in the rod domain of the lamin A/C gene as causes of dilated cardiomyopathy and conduction-system disease. N. Engl. J. Med. 1999, 341, 1715–1724. [Google Scholar] [CrossRef]
- Lazarte, J.; Hegele, R.A. Lamin A/C missense variants: From discovery to functional validation. NPJ Genom. Med. 2021, 6, 102. [Google Scholar] [CrossRef]
- Siu, C.W.; Lee, Y.K.; Ho, J.C.; Lai, W.H.; Chan, Y.C.; Ng, K.M.; Wong, L.Y.; Au, K.W.; Lau, Y.M.; Zhang, J.; et al. Modeling of lamin A/C mutation premature cardiac aging using patient-specific induced pluripotent stem cells. Aging 2012, 4, 803–822. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Zheng, W.; Wang, X.; Wang, S.; Song, L.; Zou, Y.; Yao, Y.; Hui, R. Mutation Glu82Lys in lamin A/C gene is associated with cardiomyopathy and conduction defect. Biochem. Biophys. Res. Commun. 2006, 344, 17–24. [Google Scholar] [CrossRef]
- Kärkkäinen, S.; Heliö, T.; Miettinen, R.; Tuomainen, P.; Peltola, P.; Rummukainen, J.; Ylitalo, K.; Kaartinen, M.; Kuusisto, J.; Toivonen, L.; et al. A novel mutation, Ser143Pro, in the lamin A/C gene is common in finnish patients with familial dilated cardiomyopathy. Eur. Heart J. 2004, 25, 885–893. [Google Scholar] [CrossRef]
- Botto, N.; Vittorini, S.; Colombo, M.G.; Biagini, A.; Paradossi, U.; Aquaro, G.; Andreassi, M.G. A novel LMNA mutation (R189W) in familial dilated cardiomyopathy: Evidence for a ‘hot spot’ region at exon 3: A case report. Cardiovasc. Ultrasound 2010, 8, 9. [Google Scholar] [CrossRef]
- Pan, H.; Richards, A.A.; Zhu, X.; Joglar, J.A.; Yin, H.L.; Garg, V. A novel mutation in LAMIN A/C is associated with isolated early-onset atrial fibrillation and progressive atrioventricular block followed by cardiomyopathy and sudden cardiac death. Heart Rhythm. 2009, 6, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Termglinchan, V.; Diecke, S.; Itzhaki, I.; Lam, C.K.; Garg, P.; Lau, E.; Greenhaw, M.; Seeger, T.; Wu, H.; et al. Activation of PDGF pathway links LMNA mutation to dilated cardiomyopathy. Nature 2019, 572, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Jakobs, P.M.; Hanson, E.L.; Crispell, K.A.; Toy, W.; Keegan, H.; Schilling, K.; Icenogle, T.B.; Litt, M.; Hershberger, R.E. Novel lamin A/C mutations in two families with dilated cardiomyopathy and conduction system disease. J. Card. Fail. 2001, 7, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Saga, A.; Karibe, A.; Otomo, J.; Iwabuchi, K.; Takahashi, T.; Kanno, H.; Kikuchi, J.; Keitoku, M.; Shinozaki, T.; Shimokawa, H. Lamin A/C Gene Mutations in Familial Cardiomyopathy with Advanced Atrioventricular Block and Arrhythmia. Tohoku J. Exp. Med. 2009, 218, 309–316. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zaragoza, M.V.; Hakim, S.A.; Hoang, V.; Elliott, A.M. Heart-hand syndrome IV: A second family with LMNA-related cardiomyopathy and brachydactyly. Clin. Genet. 2017, 91, 499–500. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Ko, T.; Ito, M.; Sassa, T.; Nomura, S.; Okuma, H.; Sato, M.; Imasaki, T.; Kikkawa, S.; Zhang, B.; et al. TEAD1 trapping by the Q353R-Lamin A/C causes dilated cardiomyopathy. Sci. Adv. 2023, 9, eade7047. [Google Scholar] [CrossRef] [PubMed]
- Hookana, E.; Junttila, M.J.; Särkioja, T.; Sormunen, R.; Niemelä, M.; Raatikainen, M.J.P.; Uusimaa, P.; Lizotte, E.; Peuhkurinen, K.; Brugada, R.; et al. Cardiac Arrest and Left Ventricular Fibrosis in a Finnish Family with the Lamin A/C Mutation. J. Cardiovasc. Electrophysiol. 2008, 19, 743–747. [Google Scholar] [CrossRef]
- Zaragoza, M.V.; Fung, L.; Jensen, E.; Oh, F.; Cung, K.; McCarthy, L.A.; Tran, C.K.; Hoang, V.; Hakim, S.A.; Grosberg, A. Exome Sequencing Identifies a Novel LMNA Splice-Site Mutation and Multigenic Heterozygosity of Potential Modifiers in a Family with Sick Sinus Syndrome, Dilated Cardiomyopathy, and Sudden Cardiac Death. PLoS ONE 2016, 11, e0155421. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Hou, Y.; Jia, X.; Lan, Y.; Wu, X.; Wu, J.; Jie, W.; Liu, H.; Huang, S.; Wan, Z.; et al. Characterization of cardiac involvement in patients with LMNA splice-site mutation-related dilated cardiomyopathy and sudden cardiac death. Front. Genet. 2023, 14, 1291411. [Google Scholar] [CrossRef]
- Saj, M.; Jankowska, A.; Lewandowski, M.; Szwed, H.; Szperl, M.; Płoski, R.; Bilińska, Z.T. Dilated cardiomyopathy with profound segmental wall motion abnormalities and ventricular arrhythmia caused by the R541C mutation in the LMNA gene. Int. J. Cardiol. 2010, 144, e51–e53. [Google Scholar] [CrossRef]
- Forissier, J.-F.; Bonne, G.; Bouchier, C.; Duboscq-Bidot, L.; Richard, P.; Wisnewski, C.; Briault, S.; Moraine, C.; Dubourg, O.; Schwartz, K.; et al. Apical left ventricular aneurysm without atrio-ventricular block due to a lamin A/C gene mutation. Eur. J. Heart Fail. 2003, 5, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, M.; Morimoto, S. Experimental models of inherited cardiomyopathy and its therapeutics. World J. Cardiol. 2014, 6, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Jung, G.; Bernstein, D. hiPSC Modeling of Inherited Cardiomyopathies. Curr. Treat. Options Cardiovasc. Med. 2014, 16. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, V.; Leimena, C.; McMahon, A.C.; Tan, J.C.; Chandar, S.; Jogia, D.; Kesteven, S.H.; Michalicek, J.; Otway, R.; Verheyen, F.; et al. Defects in nuclear structure and function promote dilated cardiomyopathy in lamin A/C-deficient mice. J. Clin. Investig. 2004, 113, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Frock, R.L.; Chen, S.C.; Da, D.F.; Frett, E.; Lau, C.; Brown, C.; Pak, D.N.; Wang, Y.; Muchir, A.; Worman, H.J.; et al. Cardiomyocyte-specific expression of lamin a improves cardiac function in Lmna-/- mice. PLoS ONE 2012, 7, e42918. [Google Scholar] [CrossRef]
- Lu, D.; Lian, H.; Zhang, X.; Shao, H.; Huang, L.; Qin, C.; Zhang, L. LMNA E82K mutation activates FAS and mitochondrial pathways of apoptosis in heart tissue specific transgenic mice. PLoS ONE 2010, 5, e15167. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.; Virtanen, L.; Prajapati, C.; Kiamehr, M.; Gullmets, J.; West, G.; Kreutzer, J.; Pekkanen-Mattila, M.; Heliö, T.; Kallio, P.; et al. Modeling of LMNA-Related Dilated Cardiomyopathy Using Human Induced Pluripotent Stem Cells. Cells 2019, 8, 594. [Google Scholar] [CrossRef] [PubMed]
- Arimura, T.; Helbling-Leclerc, A.; Massart, C.; Varnous, S.; Niel, F.; Lacène, E.; Fromes, Y.; Toussaint, M.; Mura, A.-M.; Keller, D.I.; et al. Mouse model carrying H222P- Lmna mutation develops muscular dystrophy and dilated cardiomyopathy similar to human striated muscle laminopathies. Hum. Mol. Genet. 2004, 14, 155–169. [Google Scholar] [CrossRef]
- Chatzifrangkeskou, M.; Yadin, D.; Marais, T.; Chardonnet, S.; Cohen-Tannoudji, M.; Mougenot, N.; Schmitt, A.; Crasto, S.; Di Pasquale, E.; Macquart, C.; et al. Cofilin-1 phosphorylation catalyzed by ERK1/2 alters cardiac actin dynamics in dilated cardiomyopathy caused by lamin A/C gene mutation. Hum. Mol. Genet. 2018, 27, 3060–3078. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Lau, Y.M.; Cai, Z.J.; Lai, W.H.; Wong, L.Y.; Tse, H.F.; Ng, K.M.; Siu, C.W. Modeling Treatment Response for Lamin A/C Related Dilated Cardiomyopathy in Human Induced Pluripotent Stem Cells. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef]
- Lu, J.; Lee, Y.-K.; Ran, X.; Lai, W.-H.; Li, R.A.; Keung, W.; Tse, K.; Tse, H.-F.; Yao, X. An abnormal TRPV4-related cytosolic Ca2+ rise in response to uniaxial stretch in induced pluripotent stem cells-derived cardiomyocytes from dilated cardiomyopathy patients. Biochim. Et. Biophys. Acta. Mol. Basis Dis. 2017, 1863, 2964–2972. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.-J.; Lee, Y.-K.; Lau, Y.-M.; Ho, J.C.-Y.; Lai, W.-H.; Wong, N.L.-Y.; Huang, D.; Hai, J.-J.; Ng, K.-M.; Tse, H.-F.; et al. Expression of Lmna-R225X nonsense mutation results in dilated cardiomyopathy and conduction disorders (DCM-CD) in mice: Impact of exercise training. Int. J. Cardiol. 2020, 298, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Sun, J.; Chen, Z.; Liu, L.; Sun, Y.; Lin, J.; Hu, X.; Zhao, M.; Ma, Y.; Lu, D.; et al. The LMNA p.R541C mutation causes dilated cardiomyopathy in human and mice. Int. J. Cardiol. 2022, 363, 149–158. [Google Scholar] [CrossRef]
- Walker, S.G.; Langland, C.J.; Viles, J.; Hecker, L.A.; Wallrath, L.L. Drosophila Models Reveal Properties of Mutant Lamins That Give Rise to Distinct Diseases. Cells 2023, 12, 1142. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Jia, H.; Wang, F.; Mo, H.; Kang, Y.; Zhang, N.; Zhao, L.; Xu, L.; Yang, Z.; Yang, Q.; et al. Primate Model Carrying LMNA Mutation Develops Dilated Cardiomyopathy. JACC Basic. Transl. Sci. 2024, 9, 380–395. [Google Scholar] [CrossRef] [PubMed]
- Mounkes, L.C.; Kozlov, S.V.; Rottman, J.N.; Stewart, C.L. Expression of an LMNA-N195K variant of A-type lamins results in cardiac conduction defects and death in mice. Hum. Mol. Genet. 2005, 14, 2167–2180. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, T.; Escalante-Alcalde, D.; Bhatt, H.; Anver, M.; Bhat, N.; Nagashima, K.; Stewart, C.L.; Burke, B. Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J. Cell Biol. 1999, 147, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Jahn, D.; Schramm, S.; Schnölzer, M.; Heilmann, C.J.; de Koster, C.G.; Schütz, W.; Benavente, R.; Alsheimer, M. A truncated lamin A in the Lmna -/- mouse line: Implications for the understanding of laminopathies. Nucleus 2012, 3, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Wolf, C.M.; Wang, L.; Alcalai, R.; Pizard, A.; Burgon, P.G.; Ahmad, F.; Sherwood, M.; Branco, D.M.; Wakimoto, H.; Fishman, G.I.; et al. Lamin A/C haploinsufficiency causes dilated cardiomyopathy and apoptosis-triggered cardiac conduction system disease. J. Mol. Cell Cardiol. 2008, 44, 293–303. [Google Scholar] [CrossRef]
- Markandeya, Y.S.; Tsubouchi, T.; Hacker, T.A.; Wolff, M.R.; Belardinelli, L.; Balijepalli, R.C. Inhibition of late sodium current attenuates ionic arrhythmia mechanism in ventricular myocytes expressing LaminA-N195K mutation. Heart Rhythm. 2016, 13, 2228–2236. [Google Scholar] [CrossRef]
- Cardoso-Moreira, M.; Sarropoulos, I.; Velten, B.; Mort, M.; Cooper, D.N.; Huber, W.; Kaessmann, H. Developmental Gene Expression Differences between Humans and Mammalian Models. Cell Rep. 2020, 33, 108308. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Tellez, N.; Greenway, S.C. Cellular models for human cardiomyopathy: What is the best option? World J. Cardiol. 2019, 11, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Pourrier, M.; Fedida, D. The Emergence of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes (hiPSC-CMs) as a Platform to Model Arrhythmogenic Diseases. Int. J. Mol. Sci. 2020, 21, 657. [Google Scholar] [CrossRef] [PubMed]
- Campostrini, G.; Kosmidis, G.; Ward-van Oostwaard, D.; Davis, R.P.; Yiangou, L.; Ottaviani, D.; Veerman, C.C.; Mei, H.; Orlova, V.V.; Wilde, A.A.M.; et al. Maturation of hiPSC-derived cardiomyocytes promotes adult alternative splicing of SCN5A and reveals changes in sodium current associated with cardiac arrhythmia. Cardiovasc. Res. 2022, 119, 167–182. [Google Scholar] [CrossRef]
- Sharma, A.; Wu, J.C.; Wu, S.M. Induced pluripotent stem cell-derived cardiomyocytes for cardiovascular disease modeling and drug screening. Stem Cell Res. Ther. 2013, 4, 150. [Google Scholar] [CrossRef]
- Mura, M.; Lee, Y.K.; Pisano, F.; Ginevrino, M.; Boni, M.; Calabrò, F.; Crotti, L.; Valente, E.M.; Schwartz, P.J.; Tse, H.F.; et al. Generation of the human induced pluripotent stem cell (hiPSC) line PSMi004-A from a carrier of the KCNQ1-R594Q mutation. Stem Cell Res. 2019, 37, 101431. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Yazawa, M.; Liu, J.; Han, L.; Sanchez-Freire, V.; Abilez, O.J.; Navarrete, E.G.; Hu, S.; Wang, L.; Lee, A.; et al. Patient-specific induced pluripotent stem cells as a model for familial dilated cardiomyopathy. Sci. Transl. Med. 2012, 4, 130ra147. [Google Scholar] [CrossRef]
- Shah, P.P.; Lv, W.; Rhoades, J.H.; Poleshko, A.; Abbey, D.; Caporizzo, M.A.; Linares-Saldana, R.; Heffler, J.G.; Sayed, N.; Thomas, D.; et al. Pathogenic LMNA variants disrupt cardiac lamina-chromatin interactions and de-repress alternative fate genes. Cell Stem Cell 2021, 28, 938–954.e939. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hua, Y.; Miyagawa, S.; Zhang, J.; Li, L.; Liu, L.; Sawa, Y. hiPSC-Derived Cardiac Tissue for Disease Modeling and Drug Discovery. Int. J. Mol. Sci. 2020, 21, 8893. [Google Scholar] [CrossRef]
- Wang, P.H.; Fang, Y.H.; Liu, Y.W.; Yeh, M.L. Merits of hiPSC-Derived Cardiomyocytes for In Vitro Research and Testing Drug Toxicity. Biomedicines 2022, 10, 2764. [Google Scholar] [CrossRef]
- Feng, W.; Schriever, H.; Jiang, S.; Bais, A.; Wu, H.; Kostka, D.; Li, G. Computational profiling of hiPSC-derived heart organoids reveals chamber defects associated with NKX2-5 deficiency. Commun. Biol. 2022, 5, 399. [Google Scholar] [CrossRef]
- Marini, V.; Marino, F.; Aliberti, F.; Giarratana, N.; Pozzo, E.; Duelen, R.; Cortés Calabuig, Á.; La Rovere, R.; Vervliet, T.; Torella, D.; et al. Long-term culture of patient-derived cardiac organoids recapitulated Duchenne muscular dystrophy cardiomyopathy and disease progression. Front. Cell Dev. Biol. 2022, 10, 878311. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, P.; Jackson, C.B.; Ozhathil, L.C.; Agarkova, I.; Galindo, C.L.; Sawyer, D.B.; Suter, T.M.; Zuppinger, C. 3D Co-culture of hiPSC-Derived Cardiomyocytes With Cardiac Fibroblasts Improves Tissue-Like Features of Cardiac Spheroids. Front. Mol. Biosci. 2020, 7, 14. [Google Scholar] [CrossRef]
- Helle, E.; Ampuja, M.; Dainis, A.; Antola, L.; Temmes, E.; Tolvanen, E.; Mervaala, E.; Kivelä, R. HiPS-Endothelial Cells Acquire Cardiac Endothelial Phenotype in Co-culture With hiPS-Cardiomyocytes. Front. Cell Dev. Biol. 2021, 9, 715093. [Google Scholar] [CrossRef] [PubMed]
- Ramaccini, D.; Montoya-Uribe, V.; Aan, F.J.; Modesti, L.; Potes, Y.; Wieckowski, M.R.; Krga, I.; Glibetić, M.; Pinton, P.; Giorgi, C.; et al. Mitochondrial Function and Dysfunction in Dilated Cardiomyopathy. Front. Cell Dev. Biol. 2020, 8, 624216. [Google Scholar] [CrossRef]
- Nguyêñ-Trân, V.T.B.; Kubalak, S.W.; Minamisawa, S.; Fiset, C.; Wollert, K.C.; Brown, A.B.; Ruiz-Lozano, P.; Barrere-Lemaire, S.; Kondo, R.; Norman, L.W.; et al. A Novel Genetic Pathway for Sudden Cardiac Death via Defects in the Transition between Ventricular and Conduction System Cell Lineages. Cell 2000, 102, 671–682. [Google Scholar] [CrossRef]
- Cheedipudi, S.M.; Matkovich, S.J.; Coarfa, C.; Hu, X.; Robertson, M.J.; Sweet, M.; Taylor, M.; Mestroni, L.; Cleveland, J.; Willerson, J.T.; et al. Genomic Reorganization of Lamin-Associated Domains in Cardiac Myocytes Is Associated With Differential Gene Expression and DNA Methylation in Human Dilated Cardiomyopathy. Circ. Res. 2019, 124, 1198–1213. [Google Scholar] [CrossRef]
- Aguado-Alvaro, L.P.; Garitano, N.; Pelacho, B. Fibroblast Diversity and Epigenetic Regulation in Cardiac Fibrosis. Int. J. Mol. Sci. 2024, 25, 6004. [Google Scholar] [CrossRef]
- Shao, J.; Liu, J.; Zuo, S. Roles of Epigenetics in Cardiac Fibroblast Activation and Fibrosis. Cells 2022, 11, 2347. [Google Scholar] [CrossRef]
- Liu, R.; Lee, J.; Kim, B.S.; Wang, Q.; Buxton, S.K.; Balasubramanyam, N.; Kim, J.J.; Dong, J.; Zhang, A.; Li, S.; et al. Tead1 is required for maintaining adult cardiomyocyte function, and its loss results in lethal dilated cardiomyopathy. JCI Insight 2017, 2, 93343. [Google Scholar] [CrossRef]
- Guo, Y.J.; Pan, W.W.; Liu, S.B.; Shen, Z.F.; Xu, Y.; Hu, L.L. ERK/MAPK signalling pathway and tumorigenesis (Review). Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef]
- Wu, W.; Muchir, A.; Shan, J.; Bonne, G.; Worman, H.J. Mitogen-Activated Protein Kinase Inhibitors Improve Heart Function and Prevent Fibrosis in Cardiomyopathy Caused by Mutation in Lamin A/C Gene. Circulation 2011, 123, 53–61. [Google Scholar] [CrossRef]
- West, G.; Turunen, M.; Aalto, A.; Virtanen, L.; Li, S.P.; Heliö, T.; Meinander, A.; Taimen, P. A heterozygous p.S143P mutation in LMNA associates with proteasome dysfunction and enhanced autophagy-mediated degradation of mutant lamins A and C. Front. Cell Dev. Biol. 2022, 10, 932983. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.R. Apoptosis in the cardiovascular system. Heart 2002, 87, 480–487. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Cardiac fibrosis. Cardiovasc. Res. 2021, 117, 1450–1488. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Li, Z.; Kong, C.W.; Tang, N.L.; Huang, Y.; Li, R.A.; Yao, X. Uniaxial cyclic stretch stimulates TRPV4 to induce realignment of human embryonic stem cell-derived cardiomyocytes. J. Mol. Cell Cardiol. 2015, 87, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Sejersted, O.M. Calcium controls cardiac function--by all means! J. Physiol. 2011, 589 Pt 12, 2919–2920. [Google Scholar] [CrossRef]
- Zima, A.V.; Bovo, E.; Mazurek, S.R.; Rochira, J.A.; Li, W.; Terentyev, D. Ca handling during excitation-contraction coupling in heart failure. Pflügers Arch. Eur. J. Physiol. 2014, 466, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Chaigne, S.; Barbeau, S.; Ducret, T.; Guinamard, R.; Benoist, D. Pathophysiological Roles of the TRPV4 Channel in the Heart. Cells 2023, 12, 1654. [Google Scholar] [CrossRef]
- Miller, M.; Koch, S.E.; Veteto, A.; Domeier, T.; Rubinstein, J. Role of Known Transient Receptor Potential Vanilloid Channels in Modulating Cardiac Mechanobiology. Front. Physiol. 2021, 12, 734113. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, 1063. [Google Scholar] [CrossRef]
- Cowan, J.R.; Van Spaendonck-Zwarts, K.Y.; Hershberger, R.E. Dilated Cardiomyopathy; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 77–97. [Google Scholar]
- Tan, C.Y.; Chan, P.S.; Tan, H.; Tan, S.W.; Lee, C.J.M.; Wang, J.W.; Ye, S.; Werner, H.; Loh, Y.J.; Lee, Y.L.; et al. Systematic in vivo candidate evaluation uncovers therapeutic targets for LMNA dilated cardiomyopathy and risk of Lamin A toxicity. J. Transl. Med. 2023, 21, 690. [Google Scholar] [CrossRef]
- Wilschanski, M.; Miller, L.L.; Shoseyov, D.; Blau, H.; Rivlin, J.; Aviram, M.; Cohen, M.; Armoni, S.; Yaakov, Y.; Pugatch, T.; et al. Chronic ataluren (PTC124) treatment of nonsense mutation cystic fibrosis. Eur. Respir. J. 2011, 38, 59–69. [Google Scholar] [CrossRef]
- Wang, J.-H.; Gessler, D.J.; Zhan, W.; Gallagher, T.L.; Gao, G. Adeno-associated virus as a delivery vector for gene therapy of human diseases. Signal Transduct. Target. Ther. 2024, 9, 78. [Google Scholar] [CrossRef]
- Jung, P.; Seibertz, F.; Fakuade, F.E.; Ignatyeva, N.; Sampathkumar, S.; Ritter, M.; Li, H.; Mason, F.E.; Ebert, A.; Voigt, N. Increased cytosolic calcium buffering contributes to a cellular arrhythmogenic substrate in iPSC-cardiomyocytes from patients with dilated cardiomyopathy. Basic. Res. Cardiol. 2022, 117, 5. [Google Scholar] [CrossRef]
- Eisner, D.A.; Caldwell, J.L.; Kistamás, K.; Trafford, A.W. Calcium and Excitation-Contraction Coupling in the Heart. Circ. Res. 2017, 121, 181–195. [Google Scholar] [CrossRef]
- Rüegg, J.C. Cardiac contractility: How calcium activates the myofilaments. Naturwissenschaften 1998, 85, 575–582. [Google Scholar] [CrossRef]
- Falcón, D.; Galeano-Otero, I.; Calderón-Sánchez, E.; Del Toro, R.; Martín-Bórnez, M.; Rosado, J.A.; Hmadcha, A.; Smani, T. TRP Channels: Current Perspectives in the Adverse Cardiac Remodeling. Front. Physiol. 2019, 10, 159. [Google Scholar] [CrossRef]

| Domain | Variant | Codon | Type of Variant | Onset | CDS | EF (%) | |
|---|---|---|---|---|---|---|---|
| Missense | Nonsense | ||||||
| N-terminal head | R60G | 188G>C | ✓ | Early [16] | AVB, bradycardia | N/A | |
| E82K | 244G>A [19] | ✓ | Early [19] | AVB | N/A | ||
| L85R | 254G>T | ✓ | Early [16] | AF | N/A | ||
| K97E | N/A | ✓ | Early [15] | AVB | Severe | ||
| Coil 1B | E111X | N/A | ✓ | Late [15] | AVB | Severe | |
| K117fs | 348-349insG | ✓ | Late [23] | AF, AVB | Normal | ||
| N120Lfs*5 | 357-2A>G | ✓ | Late [29] | N/A | Normal | ||
| S143P | 427T>C | ✓ | Late [20] | AF, AVB, bradycardia | Severe | ||
| K171K | 513+1G>A | ✓ | Late [30] | AF, AVB | N/A | ||
| R189W | 565C>T | ✓ | Late [12,21] | AF | Severe | ||
| R190W | N/A | ✓ | Late [15] | AVB | Severe | ||
| N195K | 585G>C | ✓ | Late [16] | AF | N/A | ||
| E203K | 707G>A | ✓ | Late [24] | AF, AVB | N/A | ||
| Linker2 | T224I | N/A | ✓ | Early [12] | AF | Severe | |
| R225X | 675C>T | ✓ | Early [24] Late [12] | AF, AVB, bradycardia | Moderate | ||
| Coil 2B | E317K | 949G>A | ✓ | Late [12,15] | AF, AVB, bradycardia | Moderate | |
| R335W | 1003C>T | ✓ | Early [14] | AF | Moderate | ||
| Q353R | 1058A>G | ✓ | N/A [27] | N/A | N/A | ||
| D357A | 1070A>C | ✓ | Early [14] | AF, AVB | Moderate | ||
| C-terminal tail | R386SfsX21 | 1157+1G>T | ✓ | Early [14] | N/A | Severe | |
| W467X | N/A | ✓ | Early [12] | AF, AVB | moderate | ||
| I497-E536del | 1489-1G>T | ✓ | Late [14] | AF | Normal | ||
| Q517X | 1549C>T | ✓ | Late [14] | AF, AVB | Normal | ||
| W520X | 1560G>A | ✓ | Late [14] | N/A | N/A | ||
| R541C | 1621C>T | ✓ | Early [13,28] | N/A | Moderate | ||
| R541H | 1621G>A | ✓ | Early [13] | N/A | Severe | ||
| R541G | 1621C>G | ✓ | Early [13] | N/A | Moderate | ||
| R571S | 1711A>C | ✓ | Late [16] | AVB | N/A | ||
| LMNA Variant | Models | Phenotypes | Mechanisms | Treatment |
|---|---|---|---|---|
| Null | Mice [35,36] | -Nuclear deformation -Cardiac conduction defects -Cardiac contractility dysfunction -Irregular desmin | Altered nuclear–desmin interaction Altered pERK1/2 ↓ Cx43 | FLAG-tagged transgenic human lamin A |
| p.E82K | Mice [37] | -Nuclear deformation -Abnormal sarcomeres -Mitochondria defects | FAS/mitochondrial-related apoptosis pathway | N/A |
| p.K117fs | iPSC-CMs [23] | -Arrythmias -Abnormal Ca2+ handling -Fragile lamina -Altered heterochromatin distribution | Altered PDGF pathway ↑ CAMK2D ↑ RYR2 ↑ PDGRB | PDGRB inhibitors |
| p.S143P | iPSC-CMs [38] | -Fragile lamina -Cellular stress -Abnormal Ca2+ handling -Dysrhythmias | Altered pERK1/2 ↑ peIF2α ↑ hsp90, hsp70, hsp 60 ↑ γH2AX | N/A |
| p.H222P | Mice [39,40] | -Conduction defects -Altered heterochromatin distribution -Disrupted sarcomere organisation | Altered pERK1/2 pathway ↑ pERK1/2 ↑ p-cofilin-1 ↑ TGF-β ↑ pSmad 2/3 | ERK inhibitor JNK inhibitor |
| p.R225X | iPSC-CMs [18,41,42] | -Abnormal Ca2+ handling -Nuclear deformation -Cell apoptosis | Altered ERK1/2 & pMEK1 | TRPV4 inhibitor PTC124 MEK1/2 inhibitor |
| Mice [43] | -Fibrosis in AV node -Cardiac dysfunction | ↑ Itgb3, Itgb2, Fn1, Col2a ↓ Kcnj2, Kcnj3 | Swimming exercise | |
| p.Q353R | iPSC-CMs [27] | -Deformed nuclei -Reduced sarcomere density | ↓ TEAD1 | Activator of YES-associated (YAP)-TEAD activity (TT-10) |
| Mice [27] | -Poor sarcomere formation -Nuclear deformation | |||
| p.R541C | Mice [44] | -Mitochondria defects -Altered heterochromatin distribution | N/A | N/A |
| Variant | Description | Phenotype Onset | Other Diseases | |
|---|---|---|---|---|
| Knockout mice | ||||
| Null | No Lamin A/C | +/− | at 10 weeks | N/A |
| −/− | Onset DCM at 4–6 weeks; died by 6–8 weeks | |||
| Knock-in mice | ||||
| N195K | Missense variant | +/− | No Phenotype | EDMD |
| −/− | Late onset | |||
| H222P | Missense variant | +/− | No Phenotype | EDMD |
| −/− | Onset at 2 months in males | |||
| Later onset in females | ||||
| R541C | Missense variant | +/− | N/A | EDMD |
| −/− | Onset at 6 months | |||
| Transgenic mice | ||||
| E82K | Missense variant | Not indicated | Onset at 2 months | N/A |
| R225X | Nonsense variant | +/− | Onset at 6–8 months | N/A |
| −/− | Lethal in neonates, died by 12 days | |||
| Q353R | Missense variant | +/− | Perinatally lethal | N/A |
| −/− | Cannot be born | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.-Y.; Lee, Y.-K.; Lau, Y.-M.; Au, K.-W.; Tse, Y.-L.; Ng, K.-M.; Wong, C.-K.; Tse, H.-F. The Pathogenic Mechanisms of and Novel Therapies for Lamin A/C-Related Dilated Cardiomyopathy Based on Patient-Specific Pluripotent Stem Cell Platforms and Animal Models. Pharmaceuticals 2024, 17, 1030. https://doi.org/10.3390/ph17081030
Wu X-Y, Lee Y-K, Lau Y-M, Au K-W, Tse Y-L, Ng K-M, Wong C-K, Tse H-F. The Pathogenic Mechanisms of and Novel Therapies for Lamin A/C-Related Dilated Cardiomyopathy Based on Patient-Specific Pluripotent Stem Cell Platforms and Animal Models. Pharmaceuticals. 2024; 17(8):1030. https://doi.org/10.3390/ph17081030
Chicago/Turabian StyleWu, Xin-Yi, Yee-Ki Lee, Yee-Man Lau, Ka-Wing Au, Yiu-Lam Tse, Kwong-Man Ng, Chun-Ka Wong, and Hung-Fat Tse. 2024. "The Pathogenic Mechanisms of and Novel Therapies for Lamin A/C-Related Dilated Cardiomyopathy Based on Patient-Specific Pluripotent Stem Cell Platforms and Animal Models" Pharmaceuticals 17, no. 8: 1030. https://doi.org/10.3390/ph17081030
APA StyleWu, X.-Y., Lee, Y.-K., Lau, Y.-M., Au, K.-W., Tse, Y.-L., Ng, K.-M., Wong, C.-K., & Tse, H.-F. (2024). The Pathogenic Mechanisms of and Novel Therapies for Lamin A/C-Related Dilated Cardiomyopathy Based on Patient-Specific Pluripotent Stem Cell Platforms and Animal Models. Pharmaceuticals, 17(8), 1030. https://doi.org/10.3390/ph17081030





