Ixeris polycephala Extract Alleviates Progression of Benign Prostatic Hyperplasia via Modification of Proliferation, Apoptosis, and Inflammation
Abstract
:1. Introduction
2. Results
2.1. Ultra-Performance Liquid Chromatography–Tandem Mass Spectrometry (Uplc-Ms/MS) Analyses of IP
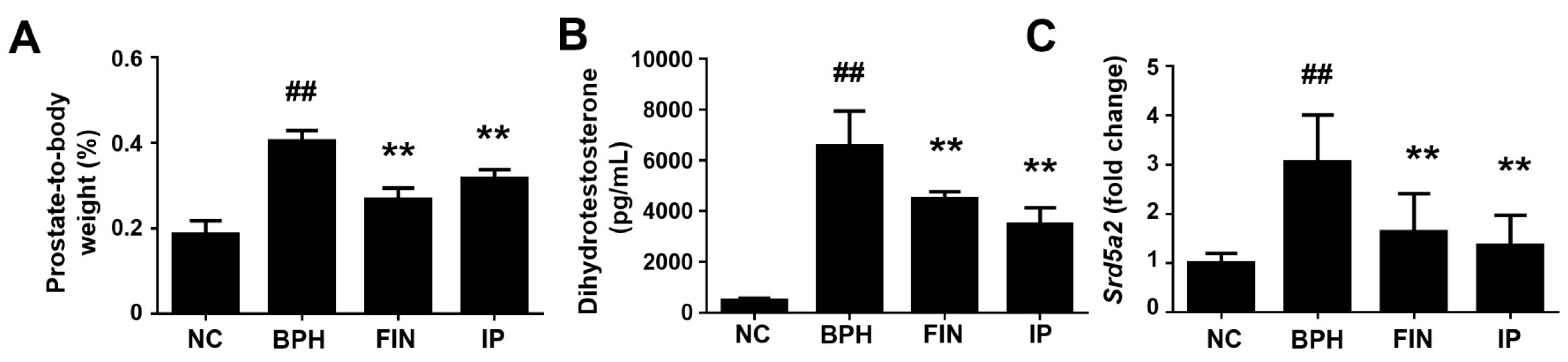
2.2. Prostatic Weight, Dht Level, and 5α-Reductase Level in a Rat Model of Bph
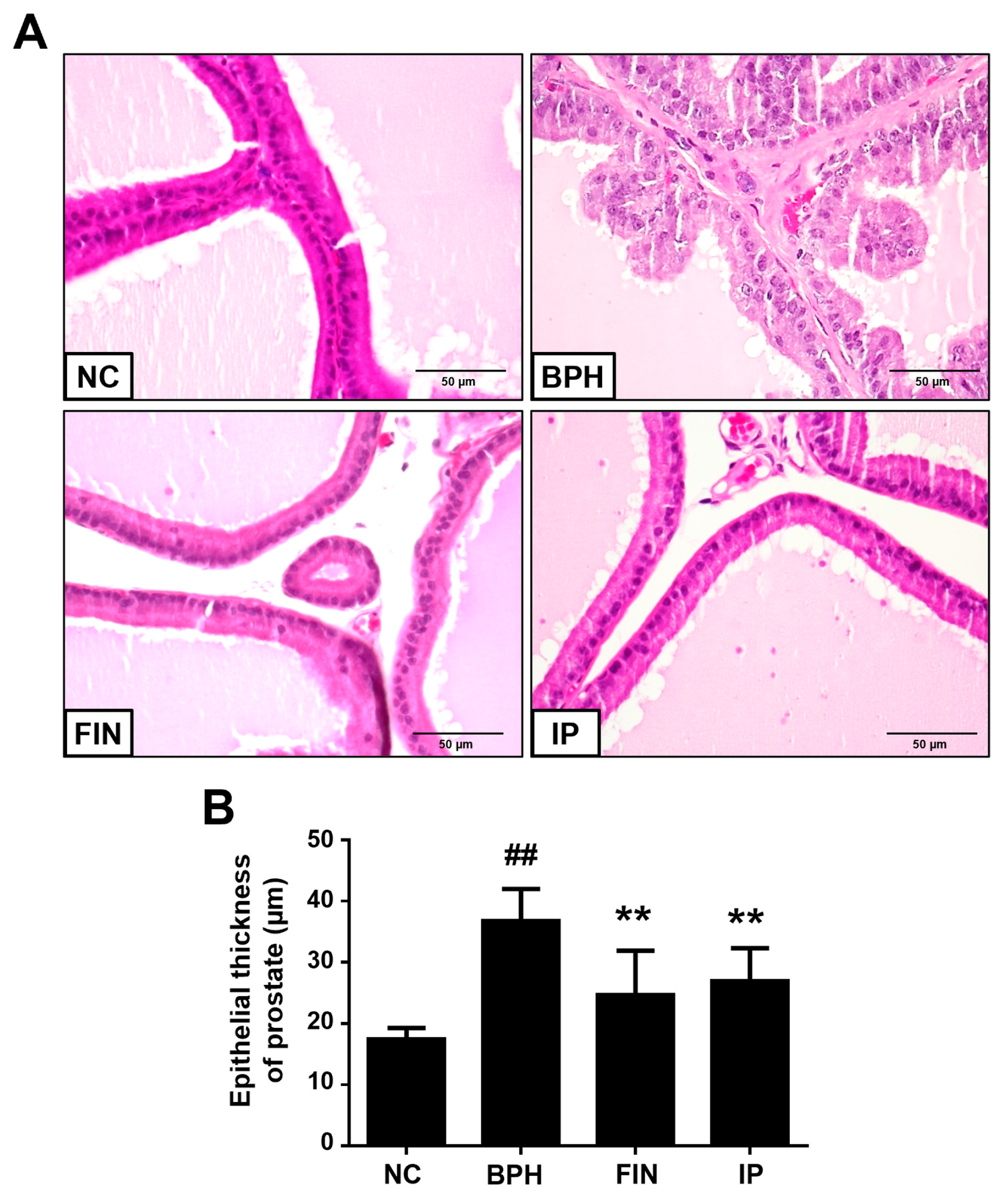
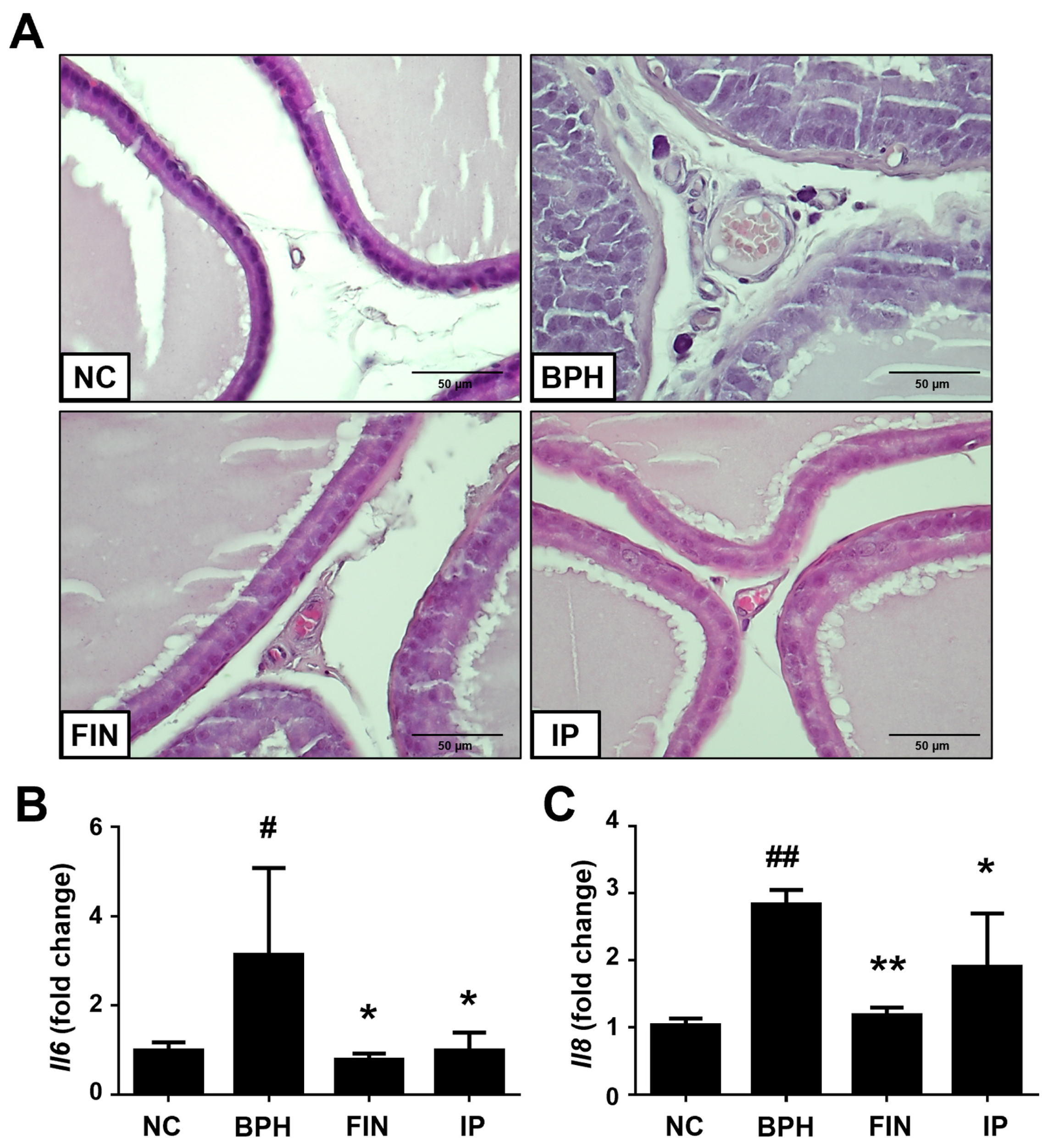
2.3. Histological Analysis of Prostatic Tissues from Bph Rats
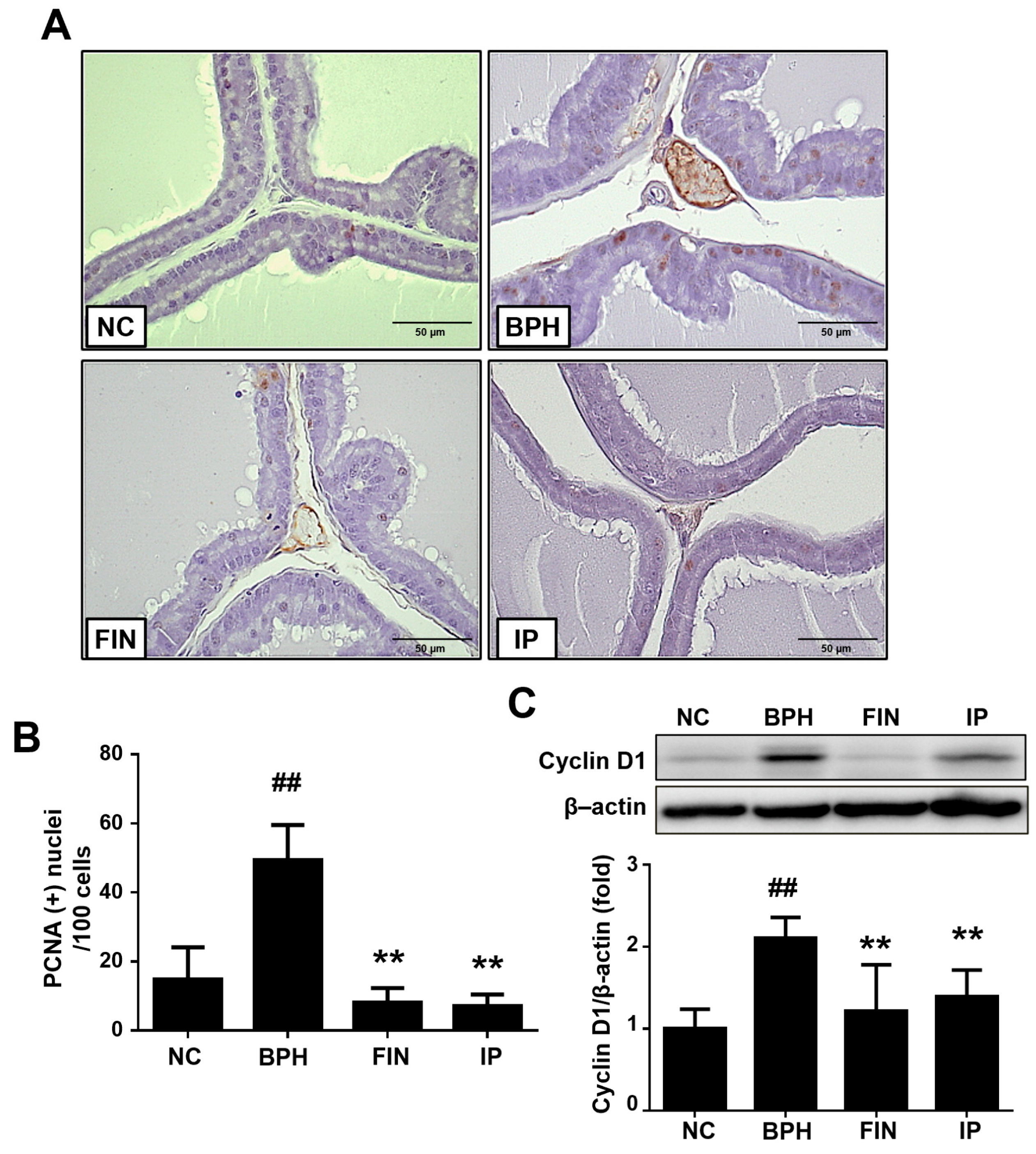
2.4. Prostatic Cell Proliferation in BPH Rats
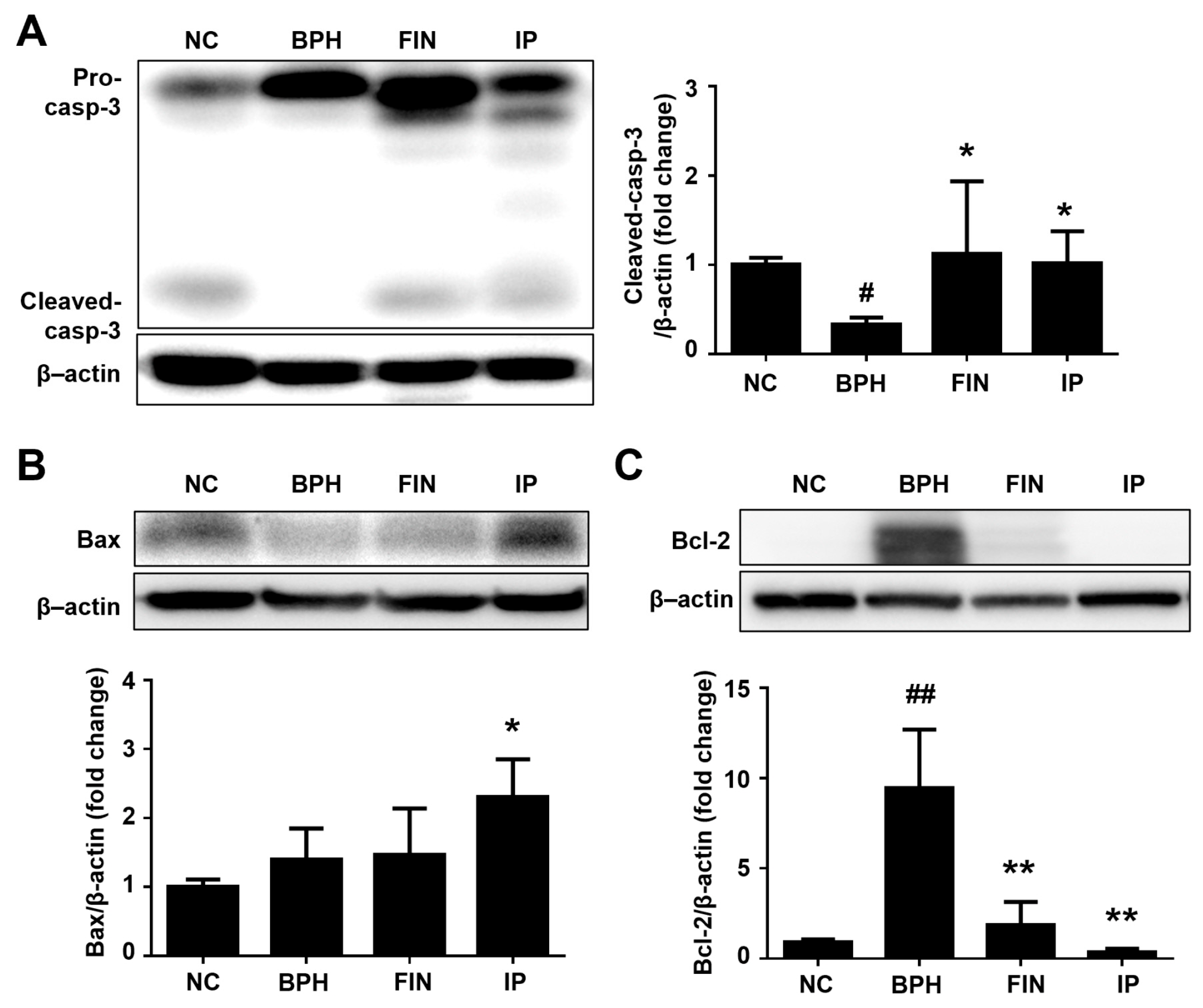
2.5. Prostatic Cell Apoptosis in BPH Rats
2.6. Inflammatory Cytokines in BPH Rats
2.7. Cyclooxygenase-2 (Cox-2) and Inducible Nitric Oxide Synthase (Inos) Expression in Bph Rats
3. Discussion
4. Materials and Methods
4.1. IP Extract
4.2. UPLC-MS/MS Analysis of IP
4.3. Animal Experiment
4.4. Enzyme Linked Immunosorbent Assay (ELISA) of DHT
4.5. Histological Examination
4.6. Immunohistochemical (IHC) Staining
4.7. Western Blotting
4.8. Quantitative Real-Time PCR (RT-qPCR)
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Madersbacher, S.; Sampson, N.; Culig, Z. Pathophysiology of Benign Prostatic Hyperplasia and Benign Prostatic Enlargement: A Mini-Review. Gerontology 2019, 65, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Ryl, A.; Rotter, I.; Miazgowski, T.; Slojewski, M.; Dolegowska, B.; Lubkowska, A.; Laszczynska, M. Metabolic syndrome and benign prostatic hyperplasia: Association or coincidence? Diabetol. Metab. Syndr. 2015, 7, 94. [Google Scholar] [CrossRef] [PubMed]
- Kyprianou, N.; Tu, H.; Jacobs, S.C. Apoptotic versus proliferative activities in human benign prostatic hyperplasia. Hum. Pathol. 1996, 27, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Soler, R.; Andersson, K.E.; Chancellor, M.B.; Chapple, C.R.; de Groat, W.C.; Drake, M.J.; Gratzke, C.; Lee, R.; Cruz, F. Future direction in pharmacotherapy for non-neurogenic male lower urinary tract symptoms. Eur. Urol. 2013, 64, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Chughtai, B.; Lee, R.; Te, A.; Kaplan, S. Role of inflammation in benign prostatic hyperplasia. Rev. Urol. 2011, 13, 147–150. [Google Scholar] [PubMed]
- Yu, Z.J.; Yan, H.L.; Xu, F.H.; Chao, H.C.; Deng, L.H.; Xu, X.D.; Huang, J.B.; Zeng, T. Efficacy and Side Effects of Drugs Commonly Used for the Treatment of Lower Urinary Tract Symptoms Associated with Benign Prostatic Hyperplasia. Front. Pharmacol. 2020, 11, 658. [Google Scholar] [CrossRef]
- Hao, J.; Li, Y.; Jia, Y.; Wang, Z.; Rong, R.; Bao, J.; Zhao, M.; Fu, Z.; Ge, G. Comparative Analysis of Major Flavonoids among Parts of Lactuca indica during Different Growth Periods. Molecules 2021, 26, 7445. [Google Scholar] [CrossRef] [PubMed]
- Van Treuren, R.; van der Arend, A.J.M.; Schut, J.W. Distribution of downy mildew (Bremia lactucae Regel) resistances in a genebank collection of lettuce and its wild relatives. Plant Genet. Resour. 2013, 11, 15–25. [Google Scholar] [CrossRef]
- Luo, B.; Dong, L.M.; Xu, Q.L.; Zhang, Q.; Liu, W.B.; Wei, X.Y.; Zhang, X.; Tan, J.W. Characterization and immunological activity of polysaccharides from Ixeris polycephala. Int. J. Biol. Macromol. 2018, 113, 804–812. [Google Scholar] [CrossRef]
- Deepti Rawat, P.R. Comparative evaluation of antioxidant profiling and quantitative phytochemicals of Ixeris polycephala Cass. and Youngia japonica (L.) DC. (Asteraceae). Pharma Innov. J. 2020, 9, 05–09. [Google Scholar]
- Lu, B.; Smock, S.L.; Castleberry, T.A.; Owen, T.A. Molecular cloning and functional characterization of the canine androgen receptor. Mol. Cell. Biochem. 2001, 226, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Andriole, G.; Bruchovsky, N.; Chung, L.W.; Matsumoto, A.M.; Rittmaster, R.; Roehrborn, C.; Russell, D.; Tindall, D. Dihydrotestosterone and the prostate: The scientific rationale for 5alpha-reductase inhibitors in the treatment of benign prostatic hyperplasia. J. Urol. 2004, 172, 1399–1403. [Google Scholar] [CrossRef] [PubMed]
- Pawlicki, B.; Zielinski, H.; Dabrowski, M. Role of apoptosis and chronic prostatitis in the pathogenesis of benign prostatic hyperplasia. Pol. Merkur. Lek. 2004, 17, 307–310. [Google Scholar]
- Vickman, R.E.; Franco, O.E.; Moline, D.C.; Vander Griend, D.J.; Thumbikat, P.; Hayward, S.W. The role of the androgen receptor in prostate development and benign prostatic hyperplasia: A review. Asian J. Urol. 2020, 7, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, L.; So, A.; Fleshner, N.; Rendon, R.; Drachenberg, D.; Elhilali, M. The role of 5-alpha reductase inhibitors in prostate pathophysiology: Is there an additional advantage to inhibition of type 1 isoenzyme? Can. Urol. Assoc. J. 2009, 3, S109–S114. [Google Scholar] [CrossRef] [PubMed]
- Wurzel, R.; Ray, P.; Major-Walker, K.; Shannon, J.; Rittmaster, R. The effect of dutasteride on intraprostatic dihydrotestosterone concentrations in men with benign prostatic hyperplasia. Prostate Cancer Prostatic Dis. 2007, 10, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Glover, M.; Soni, S.; Ren, Q.; Maclennan, G.T.; Fu, P.; Gupta, S. Influence of chronic inflammation on Bcl-2 and PCNA expression in prostate needle biopsy specimens. Oncol. Lett. 2017, 14, 3927–3934. [Google Scholar] [CrossRef] [PubMed]
- Schonenberger, F.; Deutzmann, A.; Ferrando-May, E.; Merhof, D. Discrimination of cell cycle phases in PCNA-immunolabeled cells. BMC Bioinform. 2015, 16, 180. [Google Scholar] [CrossRef]
- Sherr, C.J. D-type cyclins. Trends Biochem. Sci. 1995, 20, 187–190. [Google Scholar] [CrossRef]
- Yang, K.; Hitomi, M.; Stacey, D.W. Variations in cyclin D1 levels through the cell cycle determine the proliferative fate of a cell. Cell Div. 2006, 1, 32. [Google Scholar] [CrossRef]
- Barman, J.; Kumar, R.; Saha, G.; Tiwari, K.; Dubey, V.K. Apoptosis: Mediator Molecules, Interplay with Other Cell Death Processes and Therapeutic Potentials. Curr. Pharm. Biotechnol. 2018, 19, 644–663. [Google Scholar] [CrossRef] [PubMed]
- Robert, G.; Descazeaud, A.; Nicolaiew, N.; Terry, S.; Sirab, N.; Vacherot, F.; Maille, P.; Allory, Y.; de la Taille, A. Inflammation in benign prostatic hyperplasia: A 282 patients’ immunohistochemical analysis. Prostate 2009, 69, 1774–1780. [Google Scholar] [CrossRef] [PubMed]
- Kruslin, B.; Tomas, D.; Dzombeta, T.; Milkovic-Perisa, M.; Ulamec, M. Inflammation in Prostatic Hyperplasia and Carcinoma-Basic Scientific Approach. Front. Oncol. 2017, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Mechergui, Y.B.; Ben Jemaa, A.; Mezigh, C.; Fraile, B.; Ben Rais, N.; Paniagua, R.; Royuela, M.; Oueslati, R. The Profile of Prostate Epithelial Cytokines and its Impact on Sera Prostate Specific Antigen Levels. Inflammation 2009, 32, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.R.; Li, Q.J.; Han, P.; Li, X.; Zeng, H.; Zhu, Y.C.; Wei, Q. Evaluation of Interleukin-8 in Expressed Prostatic Secretion as a Reliable Biomarker of Inflammation in Benign Prostatic Hyperplasia. Urology 2009, 74, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Baltaci, S.; Orhan, D.; Gogus, C.; Turkolmez, K.; Tulunay, O.; Gogus, O. Inducible nitric oxide synthase expression in benign prostatic hyperplasia, low- and high-grade prostatic intraepithelial neoplasia and prostatic carcinoma. BJU Int. 2001, 88, 100–103. [Google Scholar] [CrossRef]
- Lee, L.M.; Pan, C.C.; Cheng, C.J.; Chi, C.W.; Liu, T.Y. Expression of cyclooxygenase-2 in prostate adenocarcinoma and benign prostatic hyperplasia. Anticancer. Res. 2001, 21, 1291–1294. [Google Scholar]
- Wu, S.; Huang, D.; Su, X.; Yan, H.; Ma, A.; Li, L.; Wu, J.; Sun, Z. The prostaglandin synthases, COX-2 and L-PGDS, mediate prostate hyperplasia induced by low-dose bisphenol A. Sci. Rep. 2020, 10, 13108. [Google Scholar] [CrossRef] [PubMed]
- Bouyahya, A.; Taha, D.; Benali, T.; Zengin, G.; El Omari, N.; El Hachlafi, N.; Khalid, A.; Abdalla, A.N.; Ardianto, C.; Tan, C.S.; et al. Natural sources, biological effects, and pharmacological properties of cynaroside. Biomed. Pharmacother. 2023, 161, 114337. [Google Scholar] [CrossRef]
- Bektic, J.; Guggenberger, R.; Spengler, B.; Christoffel, V.; Pelzer, A.; Berger, A.P.; Ramoner, R.; Bartsch, G.; Klocker, H. The flavonoid apigenin inhibits the proliferation of prostatic strornal cells via the MAPK-pathway and cell-cycle arrest in G1/S. Maturitas 2006, 55, S37–S46. [Google Scholar] [CrossRef]
- Semwal, R.B.; Semwal, D.K.; Combrinck, S.; Trill, J.; Gibbons, S.; Viljoen, A. Acacetin-A simple flavone exhibiting diverse pharmacological activities. Phytochem. Lett. 2019, 32, 56–65. [Google Scholar] [CrossRef]
- Ntalouka, F.; Tsirivakou, A. Luteolin: A promising natural agent in management of pain in chronic conditions. Front. Pain. Res. 2023, 4, 1114428. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Jin, L.; Huang, X.; Deng, H.; Shen, Q.K.; Quan, Z.S.; Zhang, C.; Guo, H.Y. Arctigenin: Pharmacology, total synthesis, and progress in structure modification. J. Enzym. Inhib. Med. Chem. 2022, 37, 2452–2477. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, H.; Zhou, X.; Wu, X.; Hu, E.; Jiang, Z. Inhibition effects of chlorogenic acid on benign prostatic hyperplasia in mice. Eur. J. Pharmacol. 2017, 809, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Park, J.; Kim, H.L.; Youn, D.H.; Kang, J.; Lim, S.; Jeong, M.Y.; Sethi, G.; Park, S.J.; Ahn, K.S.; et al. Vanillic acid attenuates testosterone-induced benign prostatic hyperplasia in rats and inhibits proliferation of prostatic epithelial cells. Oncotarget 2017, 8, 87194–87208. [Google Scholar] [CrossRef]
- Baek, E.B.; Hwang, Y.H.; Park, S.; Hong, E.J.; Won, Y.S.; Kwun, H.J. Eriochloa villosa Alleviates Progression of Benign Prostatic Hyperplasia in vitro and in vivo. Res. Rep. Urol. 2022, 14, 313–326. [Google Scholar] [CrossRef]
- Ub Wijerathne, C.; Park, H.S.; Jeong, H.Y.; Song, J.W.; Moon, O.S.; Seo, Y.W.; Won, Y.S.; Son, H.Y.; Lim, J.H.; Yeon, S.H.; et al. Quisqualis indica Improves Benign Prostatic Hyperplasia by Regulating Prostate Cell Proliferation and Apoptosis. Biol. Pharm. Bull. 2017, 40, 2125–2133. [Google Scholar] [CrossRef]
- Rho, J.; Seo, C.S.; Park, H.S.; Wijerathne, C.U.; Jeong, H.Y.; Moon, O.S.; Seo, Y.W.; Son, H.Y.; Won, Y.S.; Kwun, H.J. Ulmus macrocarpa Hance improves benign prostatic hyperplasia by regulating prostatic cell apoptosis. J. Ethnopharmacol. 2019, 233, 115–122. [Google Scholar] [CrossRef]
- Park, H.S.; Wijerathne, C.U.B.; Jeong, H.Y.; Seo, C.S.; Ha, H.; Kwun, H.J. Gastroprotective effects of Hwanglyeonhaedok-tang against Helicobacter pylori-induced gastric cell injury. J. Ethnopharmacol. 2018, 216, 239–250. [Google Scholar] [CrossRef]


| No. | Identification | Formula | Rt (min) | Adduct | Calculated (m/z) | Measured (m/z) | Error (ppm) | MS/MS Fragments (m/z) | Measured Area (×108) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | p-Hydroxy-cinnamic acid | C9H8O3 | 6.58 | [M+H]+ | 165.0546 | 165.0548 | 1.333 | 147, 84 | 0.10 |
| 2 | Vanillin | C8H8O3 | 6.67 | [M+H]+ | 153.0546 | 153.0547 | 0.740 | 153, 125, 133, 93, 72 | 0.08 |
| 3 | Cynaroside | C21H20O11 | 7.05 | [M+H]+ | 449.1078 | 449.1078 | −0.048 | 287 | 28.65 |
| 4 | Luteolin | C15H10O6 | 9.55 | [M+H]+ | 287.0550 | 287.0549 | −0.379 | 133 | 2.06 |
| 5 | Apigenin | C15H10O5 | 10.83 | [M+H]+ | 271.0601 | 271.0600 | −0.378 | 159 | 7.40 |
| 6 | Arctigenin | C21H24O6 | 12.67 | [M+H]+ | 373.1646 | 373.1646 | 0.057 | 305, 237, 177, 137 | 1.48 |
| 7 | Acacetin | C16H12O5 | 14.20 | [M+H]+ | 285.0758 | 285.0756 | −0.554 | 130 | 4.07 |
| 8 | Chlorogenic Acid | C16H18O9 | 5.25 | [M−H]− | 353.0878 | 353.0870 | −2.278 | 191 | 1.84 |
| 9 | Isoquercitrin | C21H20O12 | 6.93 | [M−H]− | 463.0882 | 463.0871 | −2.382 | 301 | 0.14 |
| 10 | Syringaresinol glucoside | C28H36O13 | 7.78 | [M−H]− | 579.2083 | 579.2069 | −2.419 | 417, 402, 181, 166 | 0.10 |
| Group | Treatment | Absolute Prostate Weight (g) | Body Weight (g) |
|---|---|---|---|
| NC | Corn oil/PBS | 0.81 ± 0.13 | 436.67 ± 7.74 |
| BPH | Testosterone/PBS | 1.56 ± 0.04 ## | 387.33 ± 26.21 |
| FIN | Testosterone/Finasteride | 1.07 ± 0.12 ** | 404.00 ± 60.67 |
| IP | Testosterone/IP | 1.35 ± 0.11 * | 428.80 ± 24.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, E.-B.; Hwang, Y.-H.; Hong, E.-J.; Won, Y.-S.; Kwun, H.-J. Ixeris polycephala Extract Alleviates Progression of Benign Prostatic Hyperplasia via Modification of Proliferation, Apoptosis, and Inflammation. Pharmaceuticals 2024, 17, 1032. https://doi.org/10.3390/ph17081032
Baek E-B, Hwang Y-H, Hong E-J, Won Y-S, Kwun H-J. Ixeris polycephala Extract Alleviates Progression of Benign Prostatic Hyperplasia via Modification of Proliferation, Apoptosis, and Inflammation. Pharmaceuticals. 2024; 17(8):1032. https://doi.org/10.3390/ph17081032
Chicago/Turabian StyleBaek, Eun-Bok, Youn-Hwan Hwang, Eun-Ju Hong, Young-Suk Won, and Hyo-Jung Kwun. 2024. "Ixeris polycephala Extract Alleviates Progression of Benign Prostatic Hyperplasia via Modification of Proliferation, Apoptosis, and Inflammation" Pharmaceuticals 17, no. 8: 1032. https://doi.org/10.3390/ph17081032
APA StyleBaek, E.-B., Hwang, Y.-H., Hong, E.-J., Won, Y.-S., & Kwun, H.-J. (2024). Ixeris polycephala Extract Alleviates Progression of Benign Prostatic Hyperplasia via Modification of Proliferation, Apoptosis, and Inflammation. Pharmaceuticals, 17(8), 1032. https://doi.org/10.3390/ph17081032






