Bee-Inspired Healing: Apitherapy in Veterinary Medicine for Maintenance and Improvement Animal Health and Well-Being
Abstract
1. Introduction
2. Honey
3. Propolis
4. Bee Venom (Apitoxin)
5. Pollen
6. Royal Jelly
7. Drone Larvae
8. Precautions and Final Remarks
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Weis, W.A.; Ripari, N.; Conte, F.L.; da Silva Honorio, M.; Sartori, A.A.; Matucci, R.H.; Sforcin, J.M. An overview about apitherapy and its clinical applications. Phytomedicine Plus 2022, 2, 100239. [Google Scholar] [CrossRef]
- Sawczuk, R.; Karpinska, J.; Miltyk, W. What do we need to know about drone brood homogenate and what is known. J. Ethnopharmacol. 2019, 245, 111581. [Google Scholar] [CrossRef] [PubMed]
- Vogt, N.A.; Vriezen, E.; Nwosu, A.; Sargeant, J.M. A scoping review of the evidence for the medicinal use of natural honey in animals. Front. Vet. Sci. 2021, 7, 618301. [Google Scholar] [CrossRef] [PubMed]
- Chatzimisios, K.; Tsioli, V.; Brellou, G.D.; Apostolopoulou, E.P.; Angelou, V.; Pratsinakis, E.D.; Cremers, N.A.J.; Papazoglou, L.G. Evaluation of the effectiveness of medical-grade honey and Hypericum perforatum ointment on second-intention healing of full-thickness skin wounds in cats. Animals 2023, 14, 36. [Google Scholar] [CrossRef] [PubMed]
- Bischofberger, A.S.; Dart, C.M.; Horadagoda, N.; Perkins, N.R.; Jeffcott, L.B.; Little, C.B.; Dart, A.J. Effect of Manuka honey gel on the transforming growth factor β1 and β3 concentrations, bacterial counts and histomorphology of contaminated full-thickness skin wounds in equine distal limbs. Aust. Vet. J. 2016, 94, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Lukanc, B.; Potokar, T.; Erjavec, V. Complete skin regeneration with medical honey after skin loss on the entire circumference of a leg in a cat. J. Tissue Viability 2020, 29, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Budak, Ö.; Çakıroğlu, H. Examination the effects of chestnut and Manuka honey for wound healing on mice experimental model. Med. Sci. Discov. 2022, 9, 170–174. [Google Scholar] [CrossRef]
- Blair, S.E.; Cokcetin, N.N.; Harry, E.J.; Carter, D.A. The unusual antibacterial activity of medical-grade Leptospermum honey: Antibacterial spectrum, resistance and transcriptome analysis. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Kwakman, P.H.; Zaat, S.A. Antibacterial components of honey. IUBMB Life 2012, 64, 48–55. [Google Scholar] [CrossRef]
- Majtan, J.; Bohova, J.; Prochazka, E.; Klaudiny, J. Methylglyoxal may affect hydrogen peroxide accumulation in manuka honey through the inhibition of glucose oxidase. J. Med. Food 2014, 17, 290–293. [Google Scholar] [CrossRef]
- Majtan, J.; Klaudiny, J.; Bohova, J.; Kohutova, L.; Dzurova, M.; Sediva, M.; Bartosova, M.; Majtan, V. Methylglyoxal-induced modifications of significant honeybee proteinous components in manuka honey: Possible therapeutic implications. Fitoterapia 2012, 83, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Unique Mānuka Factor, the Golden Standard in Mānuka Honey, the Rating System. Available online: https://www.umf.org.nz/unique-manuka-factor/ (accessed on 29 April 2024).
- Lu, J.; Carter, D.A.; Turnbull, L.; Rosendale, D.; Hedderley, D.; Stephens, J.; Gannabathula, S.; Steinhorn, G.; Schlothauer, R.C.; Whitchurch, C.B.; et al. The effect of New Zealand kanuka, manuka and clover honeys on bacterial growth dynamics and cellular morphology varies according to the species. PLoS ONE 2013, 8, e55898. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Turnbull, L.; Burke, C.M.; Liu, M.; Carter, D.A.; Schlothauer, R.C.; Whitchurch, C.B.; Harry, E.J. Manuka-type honeys can eradicate biofilms produced by Staphylococcus aureus strains with different biofilm-forming abilities. PeerJ 2014, 2, e326. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Cokcetin, N.N.; Burke, C.M.; Turnbull, L.; Liu, M.; Carter, D.A.; Whitchurch, C.B.; Harry, E.J. Honey can inhibit and eliminate biofilms produced by Pseudomonas aeruginosa. Sci. Rep. 2019, 9, 18160. [Google Scholar] [CrossRef] [PubMed]
- Bouzo, D.; Cokcetin, N.N.; Li, L.; Ballerin, G.; Bottomley, A.L.; Lazenby, J.; Whitchurch, C.B.; Paulsen, I.T.; Hassan, K.A.; Harry, E.J. Characterizing the mechanism of action of an ancient antimicrobial, Manuka honey, against Pseudomonas aeruginosa using modern transcriptomics. MSystems 2020, 5, e00106-20. [Google Scholar] [CrossRef] [PubMed]
- Green, K.J.; Lawag, I.L.; Locher, C.; Hammer, K.A. Correlation of the antibacterial activity of commercial manuka and Leptospermum honeys from Australia and New Zealand with methylglyoxal content and other physicochemical characteristics. PLoS ONE 2022, 17, e0272376. [Google Scholar] [CrossRef] [PubMed]
- Girma, A.; Seo, W.; She, R.C. Antibacterial activity of varying UMF-graded Manuka honeys. PLoS ONE 2019, 14, e0224495. [Google Scholar] [CrossRef] [PubMed]
- Lukanc, B.; Potokar, T.; Erjavec, V. Observational study of the effect of L-mesitran® medical honey on wound healing in cats. Vet. Arhiv 2018, 88, 59–74. [Google Scholar] [CrossRef]
- Peteoaca, A.; Istrate, A.; Goanta, A.; Girdan, G.; Stefanescu, A.; Tanase, A. Therapeutic approach in managing degloving injuries of the front limbs in a dog—A case report. Sci. Work. Ser. C Vet. Med. 2019, 65, 70–78. [Google Scholar]
- Lukanc, B.; Šteh, T.; Erjavec, V. The effect of medical honey on second intention wound healing in dogs. Vet. Arh. 2023, 93, 569–580. [Google Scholar] [CrossRef]
- Yalcınkaya, E.; Basaran, M.M.; Tunckasık, M.E.; Yazici, G.N.; Elmas, Ç.; Kocaturk, S. Efficiency of Hypericum perforatum, povidone iodine, tincture benzoin and tretinoin on wound healing. Food Chem. Toxicol. 2022, 166, 113209. [Google Scholar] [CrossRef] [PubMed]
- Vatnikov, Y.; Shabunin, S.; Kulikov, E.; Karamyan, A.; Lenchenko, E.; Sachivkina, N.; Bobkova, N.; Bokov, D.; Zhilkina, V.; Tokar, A.; et al. Effectiveness of biologically active substances from Hypericum perforatum L. in the complex treatment of purulent wounds. Int. J. Pharm. Res. 2020, 12, 1108–1117. [Google Scholar]
- Sotirova, Y.; Kiselova-Kaneva, Y.; Vankova, D.; Tasinov, O.; Ivanova, D.; Popov, H.; Hristova, M.; Nikolova, K.; Andonova, V. Tissue regeneration and remodeling in rat models after application of Hypericum perforatum L. extract-loaded bigels. Gels 2024, 10, 341. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, M.H.; Khalil, J.; Qamar, S.; Qamar, Z.; Zahid, M.; Ansari, N.; Bakhshi, I.M. Comparative gastroprotective effects of natural honey, Nigella sativa and cimetidine against acetylsalicylic acid induced gastric ulcer in albino rats. JCPSP J. Coll. Physici. 2011, 21, 151–156. [Google Scholar]
- Almasaudi, S.B.; El-Shitany, N.A.; Abbas, A.T.; Abdel-Dayem, U.A.; Ali, S.S.; Al Jaouni, S.K.; Harakeh, S. Antioxidant, anti-inflammatory, and antiulcer potential of manuka honey against gastric ulcer in rats. Oxid. Med. Cell Longev. 2016, 2016, 3643824. [Google Scholar] [CrossRef] [PubMed]
- Almasaudi, S.B.; Abbas, A.T.; Al-Hindi, R.R.; El-Shitany, N.A.; Abdel-Dayem, U.A.; Ali, S.S.; Saleh, R.M.; Al Jaouni, S.K.; Kamal, M.A.; Harakeh, S.M. Manuka honey exerts antioxidant and anti-inflammatory activities that promote healing of acetic acid-induced gastric ulcer in rats. J. Evid. Based Complement. Altern. Med. 2017, 2017, 5413917. [Google Scholar] [CrossRef]
- Harakeh, S.; Saber, S.H.; Akefe, I.O.; Shaker, S.; Hussain, M.B.; Almasaudi, A.S.; Saleh, S.M.; Almasaudi, S. Saudi honey alleviates indomethacin-induced gastric ulcer via improving antioxidant and anti-inflammatory responses in male albino rats. Saudi. J. Biol. Sci. 2022, 29, 3040–3050. [Google Scholar] [CrossRef] [PubMed]
- Erejuwa, O.O.; Gurtu, S.; Sulaiman, S.A.; Wahab, M.S.; Sirajudeen, K.N.; Salleh, M.S. Hypoglycemic and antioxidant effects of honey supplementation in streptozotocin-induced diabetic rats. Int. J. Vitam. Nutr. Res. 2010, 80, 74. [Google Scholar] [PubMed]
- Erejuwa, O.O.; Sulaiman, S.A.; Wahab, M.S.; Sirajudeen, K.N.; Salleh, M.M.; Gurtu, S. Antioxidant protection of Malaysian tualang honey in pancreas of normal and streptozotocin-induced diabetic rats. Ann. Endocrinol. Paris 2010, 71, 291–296. [Google Scholar] [CrossRef]
- Erejuwa, O.O.; Nwobodo, N.N.; Akpan, J.L.; Okorie, U.A.; Ezeonu, C.T.; Ezeokpo, B.C.; Nwadike, K.I.; Erhiano, E.; Abdul Wahab, M.S.; Sulaiman, S.A. Nigerian honey ameliorates hyperglycemia and dyslipidemia in alloxan-induced diabetic rats. Nutrients 2016, 8, 95. [Google Scholar] [CrossRef]
- Arabmoazzen, S.; Sarkaki, A. Antidiabetic effect of honey feeding in noise induced hyperglycemic rat: Involvement of oxidative stress. Iran. J. Basic Med. Sci. 2015, 18, 745–751. [Google Scholar] [PubMed]
- Hemmati, M.; Karamian, M.; Malekaneh, M. Anti-atherogenic potential of natural honey: Anti-diabetic and antioxidant approaches. J. Pharm. Pharmacol. 2015, 3, 278–284. [Google Scholar] [CrossRef]
- Berretta, A.A.; de Castro, P.A.; Cavalheiro, A.H.; Fortes, V.S.; Bom, V.P.; Nascimento, A.P.; Marquele-Oliveira, F.; Pedrazzi, V.; Ramalho, L.N.; Goldman, G.H. Evaluation of mucoadhesive gels with propolis (EPP-AF) in preclinical treatment of candidiasis vulvovaginal infection. J. Evid. Based Complement. Altern. Med. 2013, 2013, 641480. [Google Scholar] [CrossRef] [PubMed]
- Bonfim, A.P.; Sakita, K.M.; Faria, D.R.; Arita, G.S.; Vendramini, F.A.; Capoci, I.R.; Braga, A.G.; Dos Santos, R.S.; Bruschi, M.L.; Becker, T.C.; et al. Preclinical approaches in vulvovaginal candidiasis treatment with mucoadhesive thermoresponsive systems containing propolis. PLoS ONE 2020, 15, e0243197. [Google Scholar] [CrossRef] [PubMed]
- Fiordalisi, S.A.; Honorato, L.A.; Loiko, M.R.; Avancini, C.A.; Veleirinho, M.B.; Machado Filho, L.C.; Kuhnen, S. The effects of Brazilian propolis on etiological agents of mastitis and the viability of bovine mammary gland explants. J. Dairy. Sci. 2016, 99, 2308–2318. [Google Scholar] [CrossRef]
- Santana, H.F.; Barbosa, A.A.; Ferreira, S.O.; Mantovani, H.C. Bactericidal activity of ethanolic extracts of propolis against Staphylococcus aureus isolated from mastitic cows. World J. Microbiol. Biotechnol. 2012, 28, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Jin, X.L.; Shen, X.G.; Sun, L.P.; Wu, L.M.; Wei, J.Q.; Marcucci, M.C.; Hu, F.L.; Liu, J.X. Effects of Chinese propolis in protecting bovine mammary epithelial cells against mastitis pathogens-induced cell damage. Mediat. Inflamm. 2016, 2016, 8028291. [Google Scholar] [CrossRef]
- Shimizu, T.; Takeshita, Y.; Takamori, Y.; Kai, H.; Sawamura, R.; Yoshida, H.; Watanabe, W.; Tsutsumi, A.; Park, Y.K.; Yasukawa, K.; et al. Efficacy of Brazilian propolis against herpes simplex virus type 1 infection in mice and their modes of antiherpetic efficacies. J. Evid. Based Complement. Altern. Med. 2011, 2011, 976196. [Google Scholar] [CrossRef]
- Sartori, G.; Pesarico, A.P.; Pinton, S.; Dobrachinski, F.; Roman, S.S.; Pauletto, F.; Rodrigues, L.C.; Prigol, M. Protective effect of brown Brazilian propolis against acute vaginal lesions caused by herpes simplex virus type 2 in mice: Involvement of antioxidant and anti-inflammatory mechanisms. Cell Biochem. Funct. 2012, 30, 1–10. [Google Scholar] [CrossRef]
- Arismendi, N.; Vargas, M.; López, M.D.; Barría, Y.; Zapata, N. Promising antimicrobial activity against the honey bee parasite Nosema ceranae by methanolic extracts from Chilean native plants and propolis. J. Apicult. Res. 2018, 57, 522–535. [Google Scholar] [CrossRef]
- Burnham, A.J.; De Jong, E.; Jones, J.A.; Lehman, H.K. North American propolis extracts from upstate New York decrease Nosema ceranae (Microsporidia) spore levels in honey bees (Apis mellifera). Front. Microbiol. 2020, 11, 1719. [Google Scholar] [CrossRef] [PubMed]
- Naree, S.; Ellis, J.D.; Benbow, M.E.; Suwannapong, G. The use of propolis for preventing and treating Nosema ceranae infection in western honey bee (Apis mellifera Linnaeus, 1787) workers. J. Apicult. Res. 2021, 60, 686–696. [Google Scholar] [CrossRef]
- Nweze, N.E.; Okoro, H.O.; Al Robaian, M.; Omar, R.M.; Tor-Anyiin, T.A.; Watson, D.G.; Igoli, J.O. Effects of Nigerian red propolis in rats infected with. Comp. Clin. Pathol. 2017, 26, 1129–1133. [Google Scholar] [CrossRef]
- Afrouzan, H.; Zakeri, S.; Mehrizi, A.A.; Molasalehi, S.; Tahghighi, A.; Shokrgozar, M.A.; Es-Haghi, A.; Djadid, N.D. Anti-plasmodial assessment of four different Iranian propolis extracts. Arch. Iran. Med. 2017, 20, 270–281. [Google Scholar] [PubMed]
- AlGabbani, Q.; Mansour, L.; Elnakady, Y.A.; Al-Quraishy, S.; Alomar, S.; Al-Shaebi, E.M.; Abdel-Baki, A.A. In vivo assessment of the antimalarial and spleen-protective activities of the Saudi propolis methanolic extract. Parasitol. Res. 2017, 116, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.P.; Silva, T.M.; Mengarda, A.C.; Salvadori, M.C.; Teixeira, F.S.; Alencar, S.M.; Luz Filho, G.C.; Bueno-Silva, B.; de Moraes, J. Brazilian red propolis exhibits antiparasitic properties in vitro and reduces worm burden and egg production in an mouse model harboring either early or chronic Schistosoma mansoni infection. J. Ethnopharmacol. 2021, 264, 113387. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Alemzadeh, E.; Moshiri, A. Potential role of propolis in wound healing: Biological properties and therapeutic activities. Biomed. Pharmacother. 2018, 98, 469–483. [Google Scholar] [CrossRef]
- Berretta, A.A.; Nascimento, A.P.; Bueno, P.C.; Leite, M.M.; Marchetti, J.M. Propolis standardized extract (EPP-AF®), an innovative chemically and biologically reproducible pharmaceutical compound for treating wounds. Int. J. Biol. Sci. 2012, 8, 512–521. [Google Scholar] [CrossRef]
- Abu-Seida, A.M. Effect of propolis on experimental cutaneous wound healing in dogs. Vet. Med. Int. 2015, 2015, 672643. [Google Scholar] [CrossRef]
- Olczyk, P.; Komosinska-Vassev, K.; Wisowski, G.; Mencner, L.; Stojko, J.; Kozma, E.M. Propolis modulates fibronectin expression in the matrix of thermal injury. BioMed Res. Int. 2014, 2014, 748101. [Google Scholar] [CrossRef]
- Staniczek, J.; Jastrzębska-Stojko, Ż.; Stojko, R. Biological activity of propolis ointment with the addition of 1% nanosilver in the treatment of experimentally-evoked burn wounds. Polymers 2021, 13, 2312. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Guo, Z.; Shen, Z.; Wang, J.; Hu, Y.; Wang, D. The immune enhancement of propolis adjuvant on inactivated porcine parvovirus vaccine in guinea pig. Cell Immunol. 2011, 270, 13–18. [Google Scholar] [CrossRef]
- Ferreira, L.N.; Fonseca, F.P.; Caetano de Castro, C.; Sica Siedler, B.; Silveira Munhoz, L.; D’Avila Vargas, G.; Fischer, G.; Oliveira Hubner, S. Effect of the etanolic extract from green propolis on production of antibodies after immunization against Canine Parvovirus (CPV) and Canine Coronavirus (CCoV). Braz. J. Vet. Res. Anim. Sci. 2012, 49, 116–122. [Google Scholar] [CrossRef]
- Liu, S.; Wang, D.; Cao, Y.; Lu, T.; Liu, H.; Li, S. Effects of propolis on the immune enhancement of the formalin-inactivated Aeromonas salmonicida vaccine. Aquac. Res. 2020, 51, 4759–4770. [Google Scholar] [CrossRef]
- de Mendonça, M.A.A.; Ribeiro, A.R.S.; de Lima, A.K.; Bezerra, G.B.; Pinheiro, M.S.; de Albuquerque-Júnior, R.L.C.; Gomes, M.Z.; Padilha, F.F.; Thomazzi, S.M.; Novellino, E.; et al. Red propolis and its dyslipidemic regulator formononetin: Evaluation of antioxidant activity and gastroprotective effects in rat model of gastric ulcer. Nutrients 2020, 12, 2951. [Google Scholar] [CrossRef] [PubMed]
- Babińska, I.; Kleczek, K.; Makowski, W.; Szarek, J. Effect of feed supplementation with propolis on liver and kidney morphology in broiler chickens. Pak. Vet. J. 2013, 33, 1–4. [Google Scholar]
- Salehi, A.; Hosseini, S.M.; Kazemi, S. Antioxidant and anticarcinogenic potentials of propolis for dimethylhydrazine-induced colorectal cancer in Wistar rats. BioMed Res. Int. 2022, 2022, 8497562. [Google Scholar] [CrossRef]
- Rizk, S.M.; Zaki, H.F.; Mina, M.A. Propolis attenuates doxorubicin-induced testicular toxicity in rats. Food Chem. Toxicol. 2014, 67, 176–186. [Google Scholar] [CrossRef]
- Sameni, H.R.; Yosefi, S.; Alipour, M.; Pakdel, A.; Torabizadeh, N.; Semnani, V.; Bandegi, A.R. Co-administration of 5FU and propolis on AOM/DSS induced colorectal cancer in BALB-c mice. Life Sci. 2021, 276, 119390. [Google Scholar] [CrossRef]
- Cavalcante, D.R.R.; de Oliveira, P.S.; Góis, S.M.; Soares, A.F.; Cardoso, J.C.; Padilha, F.F.; de Albuquerque Júnior, R.L.C. Effect of green propolis on oral epithelial dysplasia in rats. Rev. Bras. Otorrinolaringol. 2011, 77, 278–284. [Google Scholar] [CrossRef]
- Arslan, S.; Silici, S.; Percin, D.; Koç, A.N.; Er, Ö. Antimicrobial activity of poplar propolis on mutans streptococci and caries development in rats. Turk. J. Biol. 2012, 36, 65–73. [Google Scholar] [CrossRef]
- Bueno-Silva, B.; Koo, H.; Falsetta, M.L.; Alencar, S.M.; Ikegaki, M.; Rosalen, P.L. Effect of neovestitol–vestitol containing Brazilian red propolis on accumulation of biofilm in vitro and development of dental caries in vivo. Biofouling 2013, 29, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.R.; Alves, A.V.; dos Santos, E.P.; Padilha, F.F.; Gomes, M.Z.; Rabelo, A.S.; Cardoso, J.C.; Massarioli, A.P.; de Alencar, S.M.; de Albuquerque-Junior, R.L. Inhibition of DMBA-induced oral squamous cells carcinoma growth by Brazilian red propolis in rodent model. Basic. Clin. Physiol. Pharmacol. 2015, 117, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Darweesh, F.A.; Zaher, A.R.; Zaghlool, A.E.; Helal, M.E.; El-Sabaa, H.M. Honey bee propolis as a storage medium on teeth replantation in dogs (histological, histochemical and radiographic study). Mansoura J. Dent. 2014, 1, 1–6. [Google Scholar]
- Lozina, L.A.; Peichoto, M.E.; Boehringer, S.I.; Koscinczuk, P.; Granero, G.E.; Acosta, O.C. Efficacy of Argentine propolis formulation for topical treatment of canine otitis externa. Arq. Bras. Med. Vet. Zootec. 2010, 62, 1359–1366. [Google Scholar] [CrossRef]
- Martin, L.F.; Rocha, E.M.; Garcia, S.B.; Paula, J.S. Topical Brazilian propolis improves corneal wound healing and inflammation in rats following alkali burns. BMC Complement. Altern. Med. 2013, 13, 337. [Google Scholar] [CrossRef] [PubMed]
- Oršolić, N.; Sirovina, D.; Končić, M.Z.; Lacković, G.; Gregorović, G. Effect of Croatian propolis on diabetic nephropathy and liver toxicity in mice. BMC Complement. Altern. Med. 2012, 12, 117. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, T.; Farooqui, A.A. Molecular mechanism underlying the therapeutic activities of propolis: A critical review. Curr. Nutr. Food Sci. 2010, 6, 186–199. [Google Scholar] [CrossRef]
- Araujo, M.A.; Libério, S.A.; Guerra, R.N.; Ribeiro, M.N.; Nascimento, F.R. Mechanisms of action underlying the anti-inflammatory and immunomodulatory effects of propolis: A brief review. Rev. Bras. Farmacogn. 2012, 22, 208–219. [Google Scholar] [CrossRef]
- Oršolić, N.; Jazvinšćak Jembrek, M. Molecular and cellular mechanisms of propolis and its polyphenolic compounds against cancer. Int. J. Mol. Sci. 2022, 23, 10479. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Ye, Y.; Wang, X.R.; Lin, L.T.; Xiao, L.Y.; Zhou, P.; Shi, G.X.; Liu, C.Z. Bee venom therapy: Potential mechanisms and therapeutic applications. Toxicon 2018, 148, 64–73. [Google Scholar] [CrossRef]
- Kocyigit, A.; Guler, E.M.; Kaleli, S.; Kocyigit, A.; Guler, E.M.; Kaleli, S. Anti-inflammatory and antioxidative properties of honey bee venom on Freund’s Complete Adjuvant-induced arthritis model in rats. Toxicon 2019, 161, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.Y.; Hsieh, C.L. Clinical applications of bee venom acupoint injection. Toxins 2020, 12, 618. [Google Scholar] [CrossRef] [PubMed]
- Oršolić, N. Bee venom in cancer therapy. Cancer Metastasis Rev. 2012, 31, 173–194. [Google Scholar] [CrossRef] [PubMed]
- Aufschnaiter, A.; Kohler, V.; Khalifa, S.; Abd El-Wahed, A.; Du, M.; El-Seedi, H.; Büttner, S. Apitoxin and its components against cancer, neurodegeneration and rheumatoid arthritis: Limitations and possibilities. Toxins 2020, 12, 66. [Google Scholar] [CrossRef] [PubMed]
- Tsai, L.C.; Lin, Y.W.; Hsieh, C.L. Effects of bee venom injections at acupoints on neurologic dysfunction induced by thoracolumbar intervertebral disc disorders in canines: A randomized, controlled prospective study. BioMed Res. Int. 2015, 2015, 363801. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Fischer, D.; Noelker, C.; Vulinović, F.; Grünewald, A.; Chevarin, C.; Klein, C.; Oertel, W.H.; Hirsch, E.C.; Michel, P.P.; Hartmann, A. Bee venom and its component apamin as neuroprotective agents in a Parkinson disease mouse model. PLoS ONE 2013, 8, e61700. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi-Rad, M.; Ghasemi, N.; Aliomrani, M. Evaluation of apamin effects on myelination process in C57BL/6 mice model of multiple sclerosis. Res. Pharm. Sci. 2019, 14, 424. [Google Scholar] [PubMed]
- Nguyen, C.D.; Lee, G. Neuroprotective activity of melittin—The main component of bee venom—Against oxidative stress induced by Aβ25–35 in in vitro and in vivo models. Antioxidants 2021, 10, 1654. [Google Scholar] [CrossRef]
- Khalil, W.K.; Assaf, N.; ElShebiney, S.A.; Salem, N.A. Neuroprotective effects of bee venom acupuncture therapy against rotenone-induced oxidative stress and apoptosis. Neurochem. Int. 2015, 80, 79–86. [Google Scholar] [CrossRef]
- Choi, J.; Jeon, C.; Lee, J.H.; Jang, J.U.; Quan, F.S.; Lee, K.; Kim, W.; Kim, S.K. Suppressive effects of bee venom acupuncture on paclitaxel-induced neuropathic pain in rats: Mediation by spinal α2-adrenergic receptor. Toxins 2017, 9, 351. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chung, G.; Kim, S.K. The involvement of central noradrenergic pathway in the analgesic effect of bee venom acupuncture on vincristine-induced peripheral neuropathy in rats. Toxins 2020, 12, 775. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.J.; Park, H.M. Therapeutic trial of bee venom acupuncture for idiopathic facial paralysis in a dog. J. Vet. Clin. 2013, 30, 107–110. [Google Scholar]
- El-Hanoun, A.; El-Komy, A.; El-Sabrout, K.; Abdella, M. Effect of bee venom on reproductive performance and immune response of male rabbits. Physiol. Behav. 2020, 223, 112987. [Google Scholar] [CrossRef] [PubMed]
- Elkomy, A.; El-Hanoun, A.; Abdella, M.; El-Sabrout, K. Improving the reproductive, immunity and health status of rabbit does using honey bee venom. J. Anim. Physiol. Anim. Nutr. 2021, 105, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, W.A.; Abd-Elhakim, Y.M.; Ismail, S.A. Involvement of the anti-inflammatory, anti-apoptotic, and anti-secretory activity of bee venom in its therapeutic effects on acetylsalicylic acid-induced gastric ulceration in rats. Toxicology 2019, 419, 11–23. [Google Scholar] [CrossRef]
- Han, S.M.; Lee, K.G.; Yeo, J.H.; Oh, B.Y.; Kim, B.S.; Lee, W.; Baek, H.J.; Kim, S.T.; Hwang, S.J.; Pak, S.C. Effects of honeybee venom supplementation in drinking water on growth performance of broiler chickens. Poult. Sci. 2010, 89, 2396–2400. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Han, S.M.; Keum, M.C.; Lee, S.; An, B.K.; Lee, S.R.; Lee, K.W. Evaluation of bee venom as a novel feed additive in fast-growing broilers. Brit. Poult. Sci. 2018, 59, 435–442. [Google Scholar] [CrossRef]
- Han, S.M.; Lee, K.G.; Yeo, J.H.; Hwang, S.J.; Jang, C.H.; Chenoweth, P.J.; Pak, S.C. Effects of bee venom treatment on growth performance of young pigs. Am. J. Chin. Med. 2009, 37, 253–260. [Google Scholar] [CrossRef]
- Shin, J.C.; Kim, S.H.; Park, H.J.; Seo, K.W.; Song, K.H. Effect of aromatherapy and apipuncture on Malassezia-related otitis externa in dogs. J. Vet. Clin. 2012, 29, 470–473. [Google Scholar]
- Jung, B.G.; Lee, J.A.; Park, S.B.; Hyun, P.M.; Park, J.K.; Suh, G.H.; Lee, B.J. Immunoprophylactic effects of administering honeybee (Apis melifera) venom spray against Salmonella gallinarum in broiler chicks. J. Vet. Med. Sci. 2013, 75, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.B.; Lee, B.H.; Nikapitiya, C.; Kim, J.H.; Kim, T.H.; Lee, H.C.; Kim, C.G.; Lee, J.S.; Kim, C.J. Inhibitory effects of bee venom and its components against viruses in vitro and in vivo. J. Microbiol. 2016, 54, 853–866. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, Y.M.; Kim, J.H.; Cho, C.W.; Jeon, J.W.; Park, J.K.; Lee, S.H.; Jung, B.G.; Lee, B.J. Nasal delivery of chitosan/alginate nanoparticle encapsulated bee (Apis mellifera) venom promotes antibody production and viral clearance during porcine reproductive and respiratory syndrome virus infection by modulating T cell related responses. Vet. Immunol. Immunop. 2018, 200, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Jang, A.Y.; Lin, S.; Lim, S.; Kim, D.; Park, K.; Han, S.M.; Yeo, J.H.; Seo, H.S. Melittin, a honeybee venom derived antimicrobial peptide, may target methicillin resistant Staphylococcus aureus. Mol. Med. Rep. 2015, 12, 6483–6490. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.M.; Imani, S.; Haghighi, S.; Mousavi, S.E.; Karimi, A. Effect of Iranian honey bee (Apis mellifera) venom on blood glucose and insulin in diabetic rats. J. Arthropod-Borne Dis. 2012, 6, 136–143. [Google Scholar] [PubMed]
- Denk, B.; Fidan, A.F. Effects of honeybee (Apis mellifera) venom on redox balance, biochemical and hematological profile in diabetic rats: A preliminary study. Turk. J. Vet. Anim. Sci. 2021, 45, 257–265. [Google Scholar]
- Denk, B. Exploring Apis mellifera L. venom’s antioxidant power in various solvents: Unveiling its in vitro potential. Kocatepe Vet. J. 2023, 16, 420–431. [Google Scholar] [CrossRef]
- Kostić, A.Ž.; Milinčić, D.D.; Barać, M.B.; Ali Shariati, M.; Tešić, Ž.L.; Pešić, M.B. The application of pollen as a functional food and feed ingredient—The present and perspectives. Biomolecules 2020, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Kročko, M.; Čanigová, M.; Bezeková, J.; Lavová, M.; Haščík, P.; Ducková, V. Effect of nutrition with propolis and bee pollen supplements on bacteria colonization pattern in gastrointestinal tract of broiler chicken. Sci. Pap. Anim. Sci. Biotechnol. 2012, 45, 63–67. [Google Scholar]
- Haščík, P.; Trembecká, L.; Tkáčová, J.; Kročko, M.; Čuboň, J.; Kačániová, M. Effect of bee pollen dietary supplementation on meat performance of Ross 308 broiler chickens. J. Microb. Biotec. Food 2015, 4, 55–58. [Google Scholar] [CrossRef]
- Fazayeli-Rad, A.R.; Afzali, N.; Farhangfar, S.H.; Asghari, M.R. Effect of bee pollen on growth performance, intestinal morphometry and immune status of broiler chicks. Eur. Poult. Sci. 2015, 79, 1–9. [Google Scholar] [CrossRef]
- Abood, S.S.; Ezzat, H.N. Effect of adding different levels from bee pollen in diet on productive performance of broiler chickens. Plant Arch. 2018, 18, 2435–2438. [Google Scholar]
- Petričević, V.; Lukić, M.; Škrbić, Z.; Rakonjac, S.; Stanojković, A.; Nikšić, D.; Živković, V. Production parameters, microbiological composition of intestines and slaughter performance of broilers fed with bee pollen. Züchtungskunde 2022, 94, 36–46. [Google Scholar]
- Locatelli, M.; Macchione, N.; Ferrante, C.; Chiavaroli, A.; Recinella, L.; Carradori, S.; Zengin, G.; Cesa, S.; Leporini, L.; Leone, S.; et al. Graminex pollen: Phenolic pattern, colorimetric analysis and protective effects in immortalized prostate cells (PC3) and rat prostate challenged with LPS. Molecules 2018, 23, 1145. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, R.Z.; Zhu, Y.Q.; Ren, Z.M.; Tong, Y.L.; Yang, F.; Dai, G.H. Study on the inhibition of Mfn1 by plant-derived miR5338 mediating the treatment of BPH with rape bee pollen. BMC Complement. Altern. Med. 2018, 18, 38. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, R.Z.; Ren, Z.M.; Tong, Y.L.; Chen, S.; Yang, F.; Dai, G.H. Regulation of microRNAs by rape bee pollen on benign prostate hyperplasia in rats. Andrologia 2020, 52, e13386. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Ahmed, O.M.; Hozayen, W.G.; Ahmed, M.A. Ameliorative effects of bee pollen and date palm pollen on the glycemic state and male sexual dysfunctions in streptozotocin-induced diabetic Wistar rats. Biomed. Pharmacother. 2018, 97, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Karadeniz, A.; Simsek, N.; Karakus, E.; Yildirim, S.; Kara, A.; Can, I.; Kisa, F.; Emre, H.; Turkeli, M. Royal jelly modulates oxidative stress and apoptosis in liver and kidneys of rats treated with cisplatin. Oxid. Med. Cell Longev. 2011, 2011, 981793. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Eldaim, M.A.; Abdel-Daim, M.M. Nephroprotective effect of bee honey and royal jelly against subchronic cisplatin toxicity in rats. Cytotechnology 2016, 68, 1039–1048. [Google Scholar] [CrossRef]
- Zargar, H.R.; Hemmati, A.A.; Ghafourian, M.; Arzi, A.; Rezaie, A.; Javad-Moosavi, S.A. Long-term treatment with royal jelly improves bleomycin-induced pulmonary fibrosis in rats. Can. J. Physiol. Pharm. 2017, 95, 23–31. [Google Scholar] [CrossRef]
- Albalawi, A.E.; Althobaiti, N.A.; Alrdahe, S.S.; Alhasani, R.H.; Alaryani, F.S.; BinMowyna, M.N. Antitumor activity of royal jelly and its cellular mechanisms against Ehrlich solid tumor in mice. BioMed Res. Int. 2022, 2022, 7233997. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Shao, Q.; Geng, H.; Su, S. The effect of royal jelly on the growth of breast cancer in mice. Oncol. Lett. 2017, 14, 7615–7621. [Google Scholar] [CrossRef]
- Okumura, N.; Toda, T.; Ozawa, Y.; Watanabe, K.; Ikuta, T.; Tatefuji, T.; Hashimoto, K.; Shimizu, T. Royal jelly delays motor functional impairment during aging in genetically heterogeneous male mice. Nutrients 2018, 10, 1191. [Google Scholar] [CrossRef]
- Ghanbari, E.; Khazaei, M.R.; Khazaei, M.; Nejati, V. Royal jelly promotes ovarian follicles growth and increases steroid hormones in immature rats. Int. J. Fertil. Steril. 2018, 11, 263–269. [Google Scholar] [PubMed]
- Shimizu, S.; Matsushita, H.; Minami, A.; Kanazawa, H.; Suzuki, T.; Watanabe, K.; Wakatsuki, A. Royal jelly does not prevent bone loss but improves bone strength in ovariectomized rats. Climacteric 2018, 21, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Hattori, S.; Omi, N. The effects of royal jelly protein on bone mineral density and strength in ovariectomized female rats. Phys. Act. Nutr. 2021, 25, 33. [Google Scholar] [CrossRef]
- Pan, Y.; Xu, J.; Jin, P.; Yang, Q.; Zhu, K.; You, M.; Hu, F.; Chen, M. Royal jelly ameliorates behavioral deficits, cholinergic system deficiency, and autonomic nervous dysfunction in ovariectomized cholesterol-fed rabbits. Molecules 2019, 24, 1149. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.A. Effect of royal jelly on sexual efficiency in adult male rats. Iraqi J. Vet. Sci. 2009, 23, 155–160. [Google Scholar]
- Mahdivand, N.; Najafi, G.; Nejati, V.; Shalizar-Jalali, A.; Rahmani, F. Royal jelly protects male rats from heat stress-induced reproductive failure. Andrologia 2019, 51, e13213. [Google Scholar] [CrossRef]
- Asadi, N.; Kheradmand, A.; Gholami, M.; Saidi, S.H.; Mirhadi, S.A. Effect of royal jelly on testicular antioxidant enzymes activity, MDA level and spermatogenesis in rat experimental Varicocele model. Tissue Cell 2019, 57, 70–77. [Google Scholar] [CrossRef]
- Zahmatkesh, E.; Najafi, G.; Nejati, V.; Heidari, R. Protective effect of royal jelly on the sperm parameters and testosterone level and lipid peroxidation in adult mice treated with oxymetholone. Avicenna J. Phytomedi. 2014, 4, 43. [Google Scholar]
- Azad, F.; Nejati, V.; Shalizar-Jalali, A.; Najafi, G.; Rahmani, F. Royal jelly protects male mice against nicotine-induced reproductive failure. Vet. Res. Forum. 2018, 9, 231–238. [Google Scholar] [PubMed]
- El-Hanoun, A.M.; Elkomy, A.E.; Fares, W.A.; Shahien, E.H. Impact of royal jelly to improve reproductive performance of male rabbits under hot summer conditions. World Rabbit. Sci. 2014, 22, 241–248. [Google Scholar] [CrossRef]
- Khadr, A.H.; Abdou, A.; El-Sherbiny, A.M. Age of puberty and fertility of male New Zealand white rabbits orally administered with royal jelly or/and bee honey. J. Anim. Poult. Prod. Mansoura Univ. 2015, 6, 201–217. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Z.; Chen, Y.; Cao, J.; Tian, W.; Ma, B.; Dong, Y. Active components and biological functions of royal jelly. J. Funct. Foods 2021, 82, 104514. [Google Scholar] [CrossRef]
- Ghosh, S.; Jung, C.; Meyer-Rochow, V.B. Nutritional value and chemical composition of larvae, pupae, and adults of worker honey bee, Apis mellifera ligustica as a sustainable food source. J. Asia Pac. Entomol. 2016, 19, 487–495. [Google Scholar] [CrossRef]
- Odemer, R.; Odemer, F.; Liebig, G.; de Craigher, D. Temporal increase of Varroa mites in trap frames used for drone brood removal during the honey bee season. J. Appl. Entomol. 2022, 146, 1207–1211. [Google Scholar] [CrossRef]
- Shoinbayeva, K.B.; Omirzak, T.; Bigara, T.; Abubakirova, A.; Dauylbay, A. Biologically active preparation and reproductive function of stud rams. Asian J. Pharm. 2017, 11, 184–191. [Google Scholar]
- Seres, A.B.; Ducza, E.; Báthori, M.; Hunyadi, A.; Béni, Z.; Dékány, M.; Hajagos-Tóth, J.; Verli, J.; Gáspár, R. Androgenic effect of honeybee drone milk in castrated rats: Roles of methyl palmitate and methyl oleate. J. Ethnopharmacol. 2014, 153, 446–453. [Google Scholar] [CrossRef]
- Yucel, B.; Acikgoz, Z.; Bayraktar, H.; Seremet, C. The effect of apilarnil (drone bee larvae) administration on growth performance and secondary sex characteristics of male broilers. J. Anim. Vet. Adv. 2011, 10, 2263–2266. [Google Scholar]
- Altan, O.; Yucel, B.; Açikgoz, Z.; Seremet, C.; Kosoglu, M.; Turgan, N.; Ozgonul, A.M. Apilarnil reduces fear and advances sexual development in male broilers but has no effect on growth. Brit. Poult. Sci. 2013, 54, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Zdorovjova, J.V.; Boryayev, G.I.; Nosov, A.V.; Katayev, O.G.; Meloyan, G.M.; Zemljanova, J.V.; Kistanova, J.K. Hormonal status and productive qualities of young pigs at inclusion in a diet feeding homogenate drone brood. Agrar. Sci. J. 2018, 2, 3–7. (In Russian) [Google Scholar]
- Kistanova, E.; Zdoroveva, E.; Nevitov, M.; Nosov, A.; Vysokikh, M.; Sukhanova, I.; Vishnyakova, P.; Abadjieva, D.; Ankova, D.; Rashev, P.; et al. Drone brood fed supplement impacts on the folliculogenesis in growing gilts. Vet. Arh. 2020, 90, 583–592. [Google Scholar] [CrossRef]
- Yemets, Y.M. Dietary effects of drone larves homogenate on the homeostatic constants and the reproductive capacity of Large White gilts. Transl. Res. Vet. Sci. 2020, 3, 27–39. [Google Scholar] [CrossRef]
- Hamamci, M.; Doganyigit, Z.; Silici, S.; Okan, A.; Kayma, E.; Yilmaz, S.; Tokpina, A.; Inan, L.E. Apilarnil: A novel neuroprotective candidate. Acta Neurol. Taiwan. 2020, 29, 33–45. [Google Scholar]
- Doğanyiğit, Z.; Okan, A.; Kaymak, E.; Pandır, D.; Silici, S. Investigation of protective effects of apilarnil against lipopolysaccharide induced liver injury in rats via TLR 4/HMGB-1/NF-κB pathway. Biomed. Pharmacother. 2020, 125, 109967. [Google Scholar] [CrossRef] [PubMed]
- Stevanovic, J.; Stanimirović, Z.; Aleksić, N.; Simeunović, P.; Vučićević, M. The influence of natural and synthetic substances applied in honey bee health care on the quality of bee products (invited paper). In Proceedings of the Apimondia Symposium “Apiecotech Serbia 2012”, Belgrade, Serbia, 18–19 February 2012; pp. 9–34. [Google Scholar]
- Kasiotis, K.M.; Zafeiraki, E.; Manea-Karga, E.; Anastasiadou, P.; Machera, K. Pesticide residues and metabolites in Greek honey and pollen: Bees and human health risk assessment. Foods 2023, 12, 706. [Google Scholar] [CrossRef] [PubMed]
- Végh, R.; Csóka, M.; Mednyánszky, Z.; Sipos, L. Pesticide residues in bee bread, propolis, beeswax and royal jelly–a review of the literature and dietary risk assessment. Food Chem. Toxicol. 2023, 176, 113806. [Google Scholar] [CrossRef]
- Simsek, I.; Kuzukiran, O.; Yurdakok-Dikmen, B.; Sireli, U.T.; Beykaya, M.; Filazi, A. Comparison of selected lipophilic compound residues in honey and propolis. J. Food Compos. Anal. 2021, 102, 104068. [Google Scholar] [CrossRef]
- Ilić, D.; Brkić, B.; Sekulić, M.T. Biomonitoring: Developing a beehive air volatiles profile as an indicator of environmental contamination using a sustainable in-field technique. Sustainability 2024, 16, 1713. [Google Scholar] [CrossRef]
- Stanimirović, Z.; Glavinić, U.; Lakić, N.; Radović, D.; Ristanić, M.; Tarić, E.; Stevanović, J. Efficacy of plant-derived formulation “Argus Ras” in control. Acta Vet. Beogr. 2017, 67, 191–200. [Google Scholar] [CrossRef]
- Stanimirovic, Z.; Glavinic, U.; Jovanovic, N.M.; Ristanic, M.; Milojković-Opsenica, D.; Mutic, J.; Stevanovic, J. Preliminary trials on effects of lithium salts on Varroa destructor, honey and wax matrices. J. Apicult. Res. 2022, 61, 375–391. [Google Scholar] [CrossRef]
- Stanimirović, Z.; Glavinić, U.; Ristanić, M.; Jelisić, S.; Vejnović, B.; Niketić, M.; Stevanović, J. Diet supplementation helps honey bee colonies in combat infections by enhancing their hygienic behaviour. Acta Vet. Beogr. 2022, 72, 145–166. [Google Scholar] [CrossRef]
- Jovanovic, N.M.; Glavinic, U.; Delic, B.; Vejnovic, B.; Aleksic, N.; Mladjan, V.; Stanimirovic, Z. Plant-based supplement containing B-complex vitamins can improve bee health and increase colony performance. Prev. Vet. Med. 2021, 190, 105322. [Google Scholar] [CrossRef]
- Jovanovic, N.M.; Glavinic, U.; Ristanic, M.; Vejnovic, B.; Stevanovic, J.; Cosic, M.; Stanimirovic, Z. Contact varroacidal efficacy of lithium citrate and its influence on viral loads, immune parameters and oxidative stress of honey bees in a field experiment. Front. Physiol. 2022, 13, 1000944. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, N.M.; Glavinic, U.; Ristanic, M.; Vejnovic, B.; Ilic, T.; Stevanovic, J.; Stanimirovic, Z. Effects of plant-based supplement on oxidative stress of honey bees (Apis mellifera) infected with Nosema ceranae. Animals 2023, 13, 3543. [Google Scholar] [CrossRef]
- Dolasevic, S.; Stevanovic, J.; Aleksic, N.; Glavinic, U.; Deletic, N.; Mladenovic, M.; Stanimirovic, Z. The effect of diet types on some quality characteristics of artificially reared Apis mellifera queens. J. Apicult. Res. 2020, 59, 115–123. [Google Scholar] [CrossRef]
- Glavinic, U.; Stankovic, B.; Draskovic, V.; Stevanovic, J.; Petrovic, T.; Lakic, N.; Stanimirovic, Z. Dietary amino acid and vitamin complex protects honey bee from immunosuppression caused by Nosema ceranae. PLoS ONE 2017, 12, e0187726. [Google Scholar]
- Glavinic, U.; Stevanovic, J.; Ristanic, M.; Rajkovic, M.; Davitkov, D.; Lakic, N.; Stanimirovic, Z. Potential of fumagillin and Agaricus blazei mushroom extract to reduce Nosema ceranae in honey bees. Insects 2021, 12, 282. [Google Scholar] [CrossRef]
- Glavinic, U.; Rajkovic, M.; Vunduk, J.; Vejnovic, B.; Stevanovic, J.; Milenkovic, I.; Stanimirovic, Z. Effects of Agaricus bisporus mushroom extract on honey bees infected with Nosema ceranae. Insects 2021, 12, 915. [Google Scholar] [CrossRef]
- Glavinic, U.; Blagojevic, J.; Ristanic, M.; Stevanovic, J.; Lakic, N.; Mirilovic, M.; Stanimirovic, Z. Use of thymol in Nosema ceranae control and health improvement of infected honey bees. Insects 2022, 13, 574. [Google Scholar] [CrossRef] [PubMed]
- Taric, E.; Glavinic, U.; Vejnovic, B.; Stanojkovic, A.; Aleksic, N.; Dimitrijevic, V.; Stanimirovic, Z. Oxidative stress, endoparasite prevalence and social immunity in bee colonies kept traditionally vs. those kept for commercial purposes. Insects 2020, 11, 266. [Google Scholar] [CrossRef]
- Taric, E.; Glavinic, U.; Stevanovic, J.; Vejnovic, B.; Aleksic, N.; Dimitrijevic, V.; Stanimirovic, Z. Occurrence of honey bee (Apis mellifera L.) pathogens in commercial and traditional hives. J. Apicult. Res. 2019, 58, 433–443. [Google Scholar] [CrossRef]
- Rostaher, A.; Mueller, R.S.; Meile, L.; Favrot, C.; Fischer, N.M. Venom immunotherapy for Hymenoptera allergy in a dog. Vet. Dermatol. 2021, 32, 206-e52. [Google Scholar] [CrossRef]
- Moore, A.; Burrows, A.K.; Rosenkrantz, W.S.; Ghubash, R.M.; Hosgood, G. Modified rush venom immunotherapy in dogs with Hymenoptera hypersensitivity. Vet. Dermatol. 2023, 34, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Revuelta, P.; Madrigal-Burgaleta, R. Death due to live bee acupuncture apitherapy. J. Investig. Allerg. Clin. 2018, 28, 45–46. [Google Scholar] [CrossRef]
- Chen, J.; Lariviere, W.R. The nociceptive and anti-nociceptive effects of bee venom injection and therapy: A double-edged sword. Prog. Neurobiol. 2010, 92, 151–183. [Google Scholar] [CrossRef]
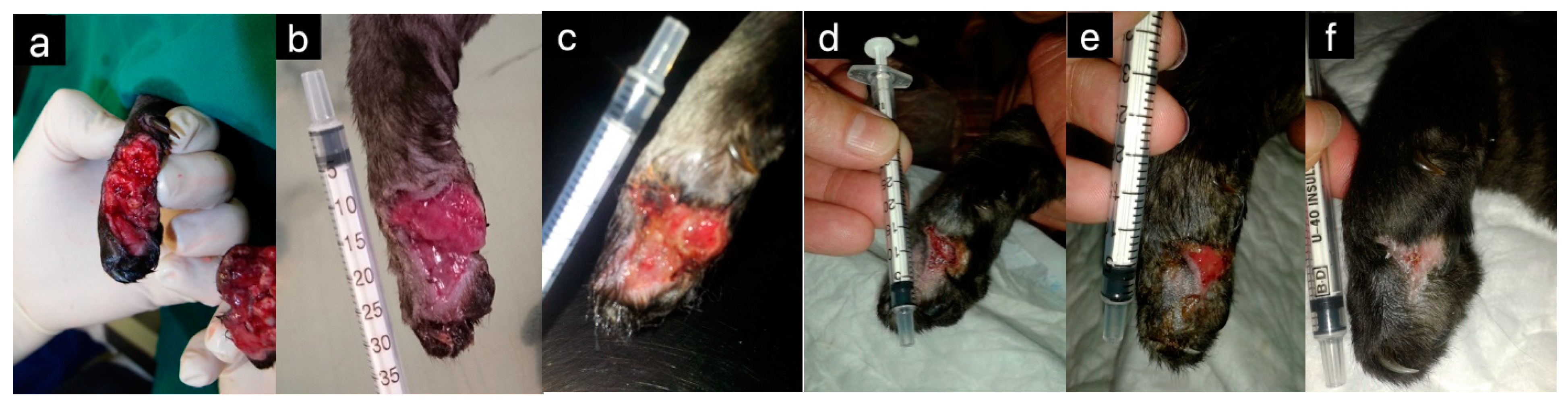
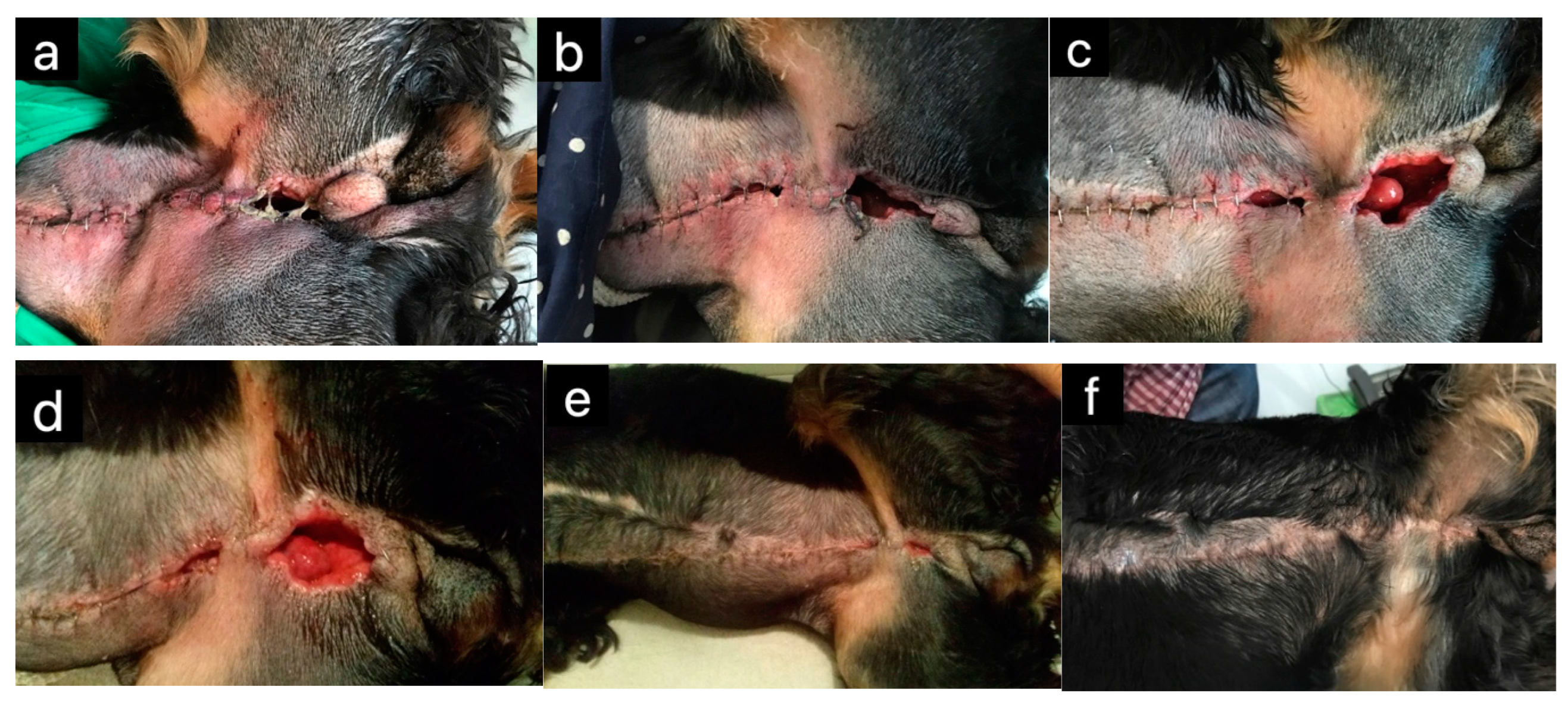
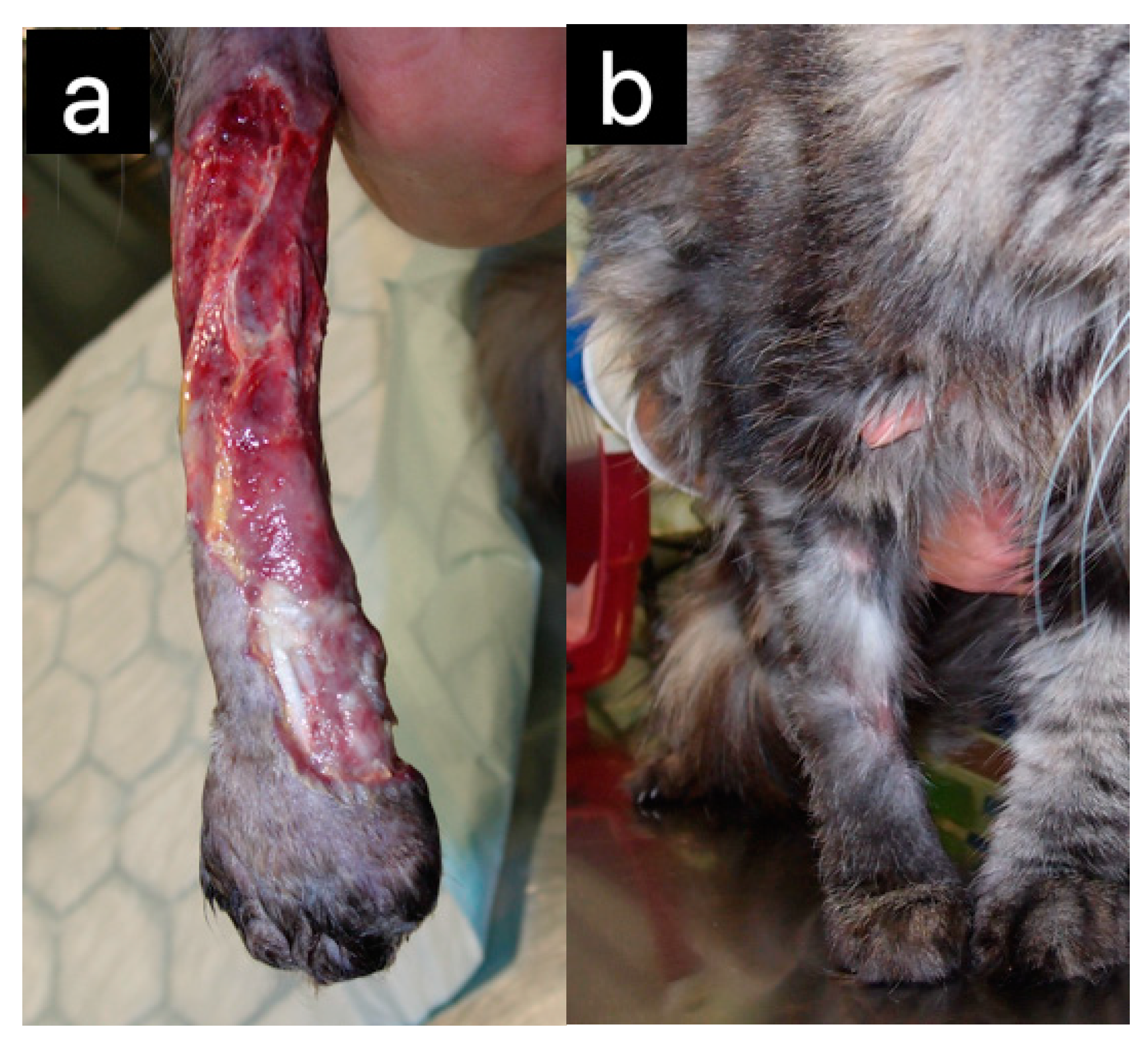
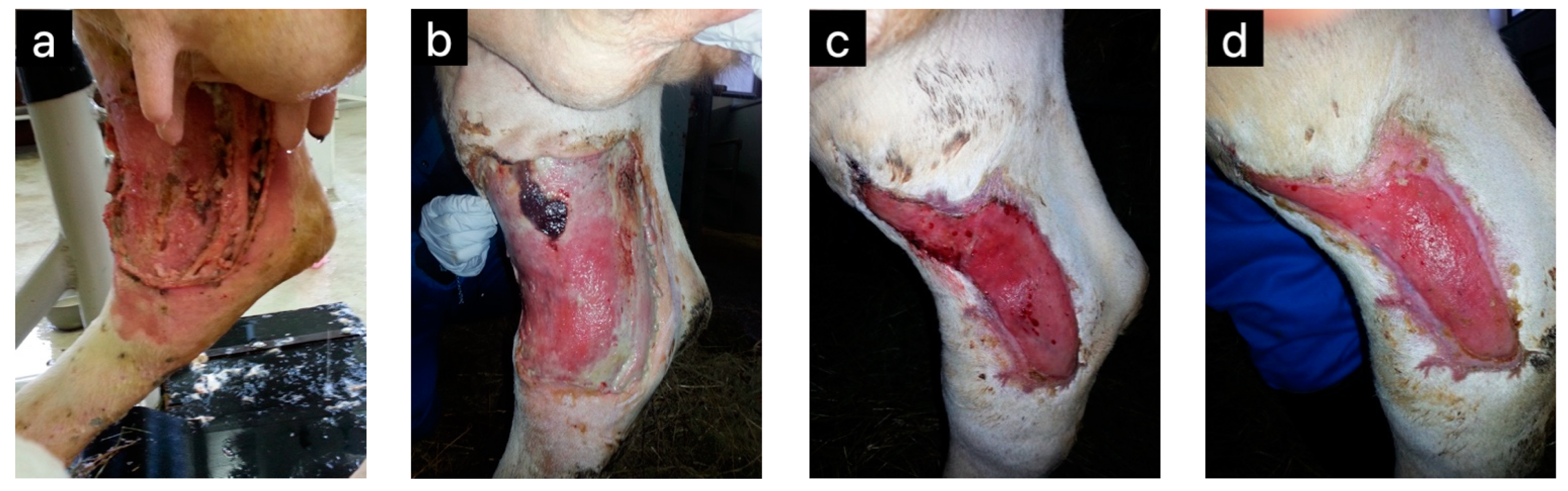
| Honey Bee Product | Animal | Effect | Reference(s) |
|---|---|---|---|
| Honey | Horses | Wound healing | [5] |
| Cats | Wound healing | [4,6,19] | |
| Dogs | Wound healing | [20,21] | |
| Mice | Wound healing | [7] | |
| Rats | Gastroprotective effect | [25] | |
| Gastric ulcer healing | [26,27,28] | ||
| Hypoglycemic and antioxidant effects | [29] | ||
| Protection of pancreas | [30] | ||
| Cardioprotective effects | [31] | ||
| Antidiabetic effect | [32] | ||
| Anti-atherogenic effect | [33] | ||
| Propolis | Mice | Antifungal efficacy on vulvovaginal candidiasis | [34,35] |
| Antiparasitic (antimalarial activity) against Plasmodium chabaudi | [46] | ||
| Antiparasitic (antimalarial) effect against Plasmodium falciparum and P. berghei | [45] | ||
| Antiparasitic properties against Schistosoma mansoni | [47] | ||
| Anticarcinogenic potentials | [60] | ||
| Mitigating hepatotoxicity and nephrotoxicity by reducing oxidative stress | [68] | ||
| Honey bees | Antiparasitic effect against Nosema ceranae | [41,42,43] | |
| Rats | Antiparasitic effect against Trypanosoma brucei and T. congolense | [44] | |
| Wound healing | [49] | ||
| Anticarcinogenic potential | [58] | ||
| Gastroprotective properties due to anti-oxidant and anti-Helicobacter pylori activities | [56] | ||
| Protective role during the initial phases of lingual carcinogenesis | [61] | ||
|
Antimicrobial activity against mutans streptococci,
Caries prevention | [62] | ||
| Anticaries effects | [63] | ||
| Chemopreventive and gastroprotective effects | [64] | ||
| Corneal wound healing | [67] | ||
| Dogs | Wound healing | [50] | |
| Storage medium (on teeth replantation) | [65] | ||
| Antimicrobial effects in otitis externa | [66] | ||
| Pigs | Burn wounds healing | [51,52] | |
| Chickens | Protective effect on liver and kidney | [57] | |
| Bee venom | Dogs | Anti-inflammatory and analgesic effects | [77] |
| Healing effect on Malassezia-related otitis externa | [91] | ||
| Healing effect on facial paralysis | [84] | ||
| Mice | Neuroprotective effect in Parkinson’s disease | [78] | |
| Neuroprotective effect in multiple sclerosis | [79] | ||
| Antiviral efficacy against a broad panel of viruses | [93] | ||
| Neuroprotective effects | [80,81] | ||
| Rabbits | Positive impact on reproductive performance and immune response of male individuals | [85] | |
| Improvement of reproductive traits (sexual-stimulant), immune response, and health | [86] | ||
| Chickens | Growth promoter | [88,89] | |
| Immunoprophylactic effects | [92] | ||
| Pigs | Promotion of antibody production and viral clearance in PRRS virus infection | [94] | |
| Positive impact on growth, survival, and immunity | [90] | ||
| Rats | Gastroprotective effect | [87] | |
| Antidiabetic effect | [96,97] | ||
| Analgesic effect | [83] | ||
| Antibacterial activity against Staphylococcus aureus | [82] | ||
| Pollen | Rats | Anti-inflammatory and protective effects in prostatitis treatment | [105] |
| Healing effects in prostate hyperplasia and inflammation | [106,107] | ||
| Protective role in diabetes-related glycemic control problems and sexual dysfunctions in male individuals | [108] | ||
| Chickens | Positive effect on gut microflora colonization | [100] | |
| Growth promoter | [101] | ||
| Improvement of growth performance and immune status | [102] | ||
| Improvement in weight gain and food conversion rate | [103] | ||
| Positive effect on daily gain, feed conversion, and microbiological composition of intestine | [104] | ||
| Royal jelly | Rats | Protection of liver and kidneys during chemotherapy | [109] |
| Nephroprotective effect | [110] | ||
| Anti-fibrotic effect against pulmonary fibrosis | [111] | ||
| Stimulation of folliculogenesis and secretion of steroid hormones | [115] | ||
| Improvement of bone strength after ovariectomy | [116] | ||
| Prevention of osteoporosis after ovariectomy | [117] | ||
| Improvement of fertility and reproductive success in males | [119,120,121] | ||
| Mice | Antioxidant, immunomodulatory and anticancer effects | [112] | |
| Apoptotic, antioxidant, anti-inflammatory and anticancer effects | [113] | ||
| Anti-aging effect | [114] | ||
| Improvement of fertility and reproductive success in males | [122,123] | ||
| Rabbits | Alleviation of neurological disorders after ovariectomy | [118] | |
| Improvement of fertility and reproductive success in males | [124,125] | ||
| Drone larvae | Sheep | Stimulation of reproductive function in rams | [129] |
| Rats | Androgenic effect in castrated males | [130] | |
| Liver-protective effects | [137] | ||
| Neuroprotective effect | [136] | ||
| Broilers | Androgenic effects | [131] | |
| Androgenic effects, Decrease in blood glucose and cholesterol levels | [132] | ||
| Pigs | Anabolic effects (increase in production parameters) in females | [133] | |
| Anabolic effect in females | [134] | ||
| Improvement of fertility; stimulation of reproductive function (by reducing the time to the first estrous cycle for artificial insemination) | [135] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stevanović, J.; Glavinić, U.; Ristanić, M.; Erjavec, V.; Denk, B.; Dolašević, S.; Stanimirović, Z. Bee-Inspired Healing: Apitherapy in Veterinary Medicine for Maintenance and Improvement Animal Health and Well-Being. Pharmaceuticals 2024, 17, 1050. https://doi.org/10.3390/ph17081050
Stevanović J, Glavinić U, Ristanić M, Erjavec V, Denk B, Dolašević S, Stanimirović Z. Bee-Inspired Healing: Apitherapy in Veterinary Medicine for Maintenance and Improvement Animal Health and Well-Being. Pharmaceuticals. 2024; 17(8):1050. https://doi.org/10.3390/ph17081050
Chicago/Turabian StyleStevanović, Jevrosima, Uroš Glavinić, Marko Ristanić, Vladimira Erjavec, Barış Denk, Slobodan Dolašević, and Zoran Stanimirović. 2024. "Bee-Inspired Healing: Apitherapy in Veterinary Medicine for Maintenance and Improvement Animal Health and Well-Being" Pharmaceuticals 17, no. 8: 1050. https://doi.org/10.3390/ph17081050
APA StyleStevanović, J., Glavinić, U., Ristanić, M., Erjavec, V., Denk, B., Dolašević, S., & Stanimirović, Z. (2024). Bee-Inspired Healing: Apitherapy in Veterinary Medicine for Maintenance and Improvement Animal Health and Well-Being. Pharmaceuticals, 17(8), 1050. https://doi.org/10.3390/ph17081050






