Nanoparticle-Based Drug Delivery Systems in Inhaled Therapy: Improving Respiratory Medicine
Abstract
:1. Introduction
2. NP Inhaled Therapies: Key Advantages
3. Advances in Technology and Delivery Systems
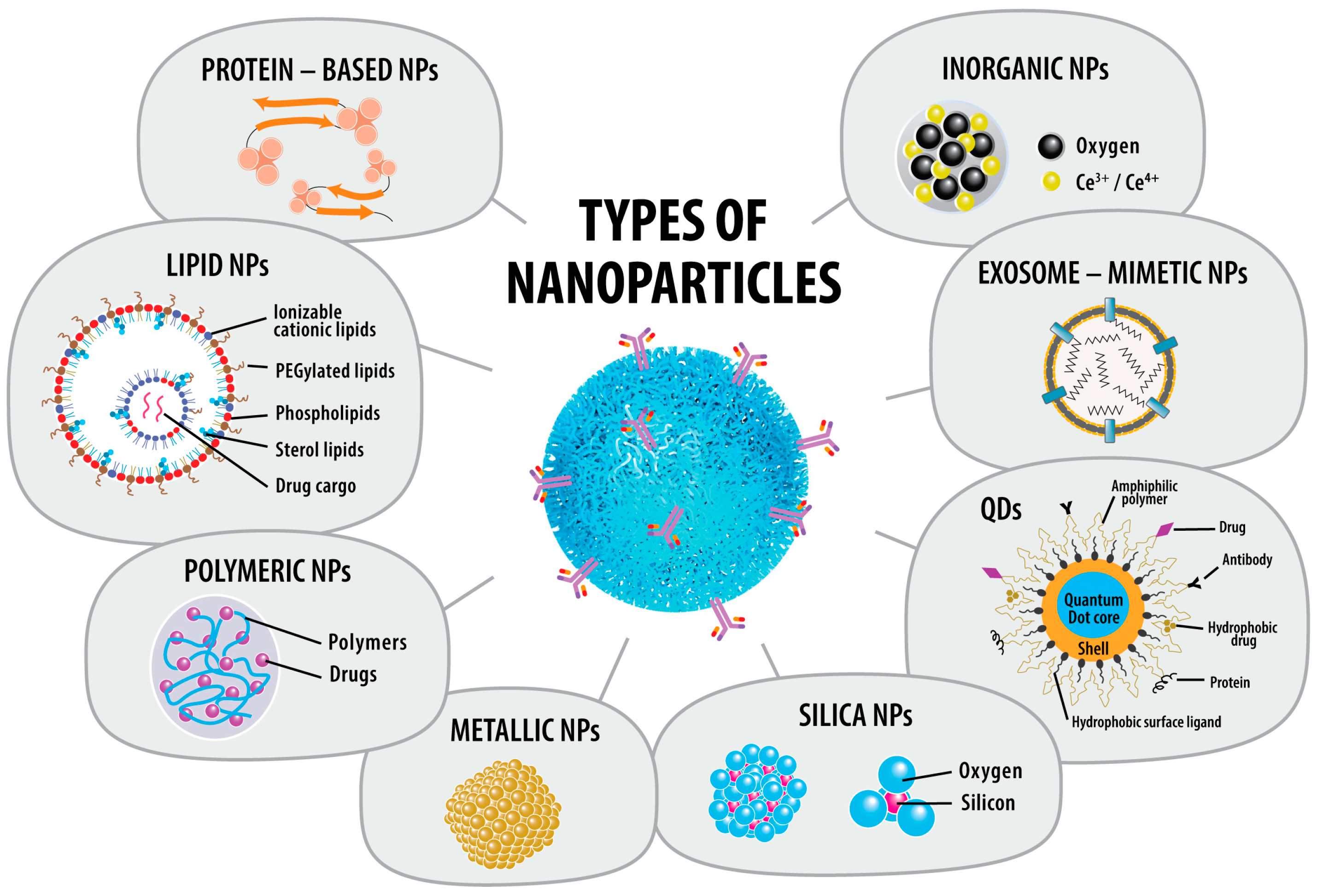
3.1. Advances in Types of NPs Used
3.1.1. Lipid-NPs (LNPs)
3.1.2. Polymeric NPs (PNPs)
3.1.3. Metallic NPs (MNPs)
3.1.4. Silica NPs
3.1.5. Quantum Dots (QDs)
3.1.6. Protein-Based NPs (PBNPs)
3.1.7. Inorganic NPs
3.1.8. Exosome-Mimetic NPs (EMNPs)
3.1.9. Nanocrystals/Nanosuspensions
3.2. Advances in Delivery Systems
3.2.1. DPIs
3.2.2. MDIs
3.2.3. Nebulizers
3.2.4. SMIs
3.2.5. pMDIs
3.2.6. Nasal Sprays
3.2.7. Nasal Nebulizers
4. Discussion
5. Conclusions
6. Challenges and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Momtazmanesh, S.; Moghaddam, S.S.; Ghamari, S.H.; Rad, E.M.; Rezaei, N.; Shobeiri, P.; Aali, A.; Abbasi-Kangevari, M.; Abbasi-Kangevari, Z.; Abdelmasseh, M.; et al. Global Burden of Chronic Respiratory Diseases and Risk Factors, 1990–2019: An Update from the Global Burden of Disease Study 2019. eClinicalMedicine 2023, 59, 101936. [Google Scholar] [CrossRef]
- The Nobel Prize in Chemistry 1925-Presentation Speech. Available online: https://www.nobelprize.org/prizes/chemistry/1925/summary/ (accessed on 24 June 2024).
- World Health Organization. Addressing the Impact of Nanotechnology on Health. Available online: https://www.who.int/europe/activities/addressing-the-impact-of-nanotechnology-on-health (accessed on 24 June 2024).
- US Food & Drug Administration. Nanotechnology Guidance Documents. Available online: https://www.fda.gov/science-research/nanotechnology-programs-fda/nanotechnology-guidance-documents (accessed on 24 June 2024).
- US Food & Drug Administration. Nanotechnology. Available online: https://www.fda.gov/about-fda/nctr-research-focus-areas/nanotechnology (accessed on 24 June 2024).
- Rasmussen, K.; Schoonjans, R.; Jantunen, P.; Rauscher, H. Chapter 19: European Union Legislation Addressing Environment, Health and Safety Aspects of Nanomaterials. In Environmental Nanopollutants: Sources, Occurrence, Analysis and Fate; 2022 ebook collection; The Royal Society of Chemistry: London, UK, 2022. [Google Scholar]
- Chan, H.W.; Chow, S.; Zhang, X.; Zhao, Y.; Tong, H.H.Y.; Chow, S.F. Inhalable Nanoparticle-Based Dry Powder Formulations for Respiratory Diseases: Challenges and Strategies for Translational Research. AAPS PharmSciTech 2023, 24, 98. [Google Scholar] [CrossRef]
- Joseph, T.M.; Kar Mahapatra, D.; Esmaeili, A.; Piszczyk, Ł.; Hasanin, M.S.; Kattali, M.; Haponiuk, J.; Thomas, S. Nanoparticles: Taking a Unique Position in Medicine. Nanomater 2023, 13, 574. [Google Scholar] [CrossRef]
- Loo, C.-Y.; Lee, W.-H.; Zhou, Q.T. Recent Advances in Inhaled Nanoformulations of Vaccines and Therapeutics Targeting Respiratory Viral Infections. Pharm. Res. 2023, 40, 1015–1036. [Google Scholar] [CrossRef]
- Barthold, S.; Kunschke, N.; Murgia, X.; Loretz, B.; Carvalho-Wodarz, C.d.S.; Lehr, C.-M. Overview of Inhaled Nanopharmaceuticals. J. Aerosol Med. Pulm. Drug Deliv. 2023, 36, 144–151. [Google Scholar] [CrossRef]
- Peng, S.; Wang, W.; Zhang, R.; Wu, C.; Pan, X.; Huang, Z. Nano-Formulations for Pulmonary Delivery: Past, Present, and Future Perspectives. Pharmaceutics 2024, 16, 161. [Google Scholar] [CrossRef]
- Jin, Z.; Gao, Q.; Wu, K.; Ouyang, J.; Guo, W.; Liang, X.-J. Harnessing Inhaled Nanoparticles to Overcome the Pulmonary Barrier for Respiratory Disease Therapy. Adv. Drug Deliv. Rev. 2023, 202, 115111. [Google Scholar] [CrossRef]
- Hami, Z. A Brief Review on Advantages of Nano-Based Drug Delivery Systems. Ann. Mil. Health Sci. Res. 2021, 19, e112274. [Google Scholar] [CrossRef]
- Forest, V.; Pourchez, J. Nano-Delivery to the Lung–by Inhalation or Other Routes and Why Nano When Micro Is Largely Sufficient? Adv. Drug Deliv. Rev. 2022, 183, 114173. [Google Scholar] [CrossRef]
- Pedersen, S. Inhalers and Nebulizers: Which to Choose and Why. Respir. Med. 1996, 90, 69–77. [Google Scholar] [CrossRef]
- Dolovich, M. New Propellant-Free Technologies under Investigation. J. Aerosol Med. Off. J. Int. Soc. Aerosols Med. 1999, 12 (Suppl. S1), S9–S17. [Google Scholar] [CrossRef]
- Newman, S.P.; Morén, F.; Trofast, E.; Talaee, N.; Clarke, S.W. Deposition and Clinical Efficacy of Terbutaline Sulphate from Turbuhaler, a New Multi-Dose Powder Inhaler. Eur. Respir. J. 1989, 2, 247–252. [Google Scholar] [CrossRef]
- Mehanna, M.M.; Mohyeldin, S.M.; Elgindy, N.A. Respirable Nanocarriers as a Promising Strategy for Antitubercular Drug Delivery. J. Control. Release Off. J. Control. Release Soc. 2014, 187, 183–197. [Google Scholar] [CrossRef]
- Mangal, S.; Gao, W.; Li, T.; Zhou, Q.T. Pulmonary Delivery of Nanoparticle Chemotherapy for the Treatment of Lung Cancers: Challenges and Opportunities. Acta Pharmacol. Sin. 2017, 38, 782–797. [Google Scholar] [CrossRef]
- Garcia-Mouton, C.; Hidalgo, A.; Cruz, A.; Pérez-Gil, J. The Lord of the Lungs: The Essential Role of Pulmonary Surfactant upon Inhalation of Nanoparticles. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgemeinschaft Pharm. Verfahrenstechnik 2019, 144, 230–243. [Google Scholar] [CrossRef]
- Borm, P.; Klaessig, F.C.; Landry, T.D.; Moudgil, B.; Pauluhn, J.; Thomas, K.; Trottier, R.; Wood, S. Research Strategies for Safety Evaluation of Nanomaterials, Part V: Role of Dissolution in Biological Fate and Effects of Nanoscale Particles. Toxicol. Sci. 2006, 90, 23–32. [Google Scholar] [CrossRef]
- Madl, A.K.; Pinkerton, K.E. Health Effects of Inhaled Engineered and Incidental Nanoparticles. Crit. Rev. Toxicol. 2009, 39, 629–658. [Google Scholar] [CrossRef]
- Kaler, L.; Iverson, E.; Bader, S.; Song, D.; Scull, M.A.; Duncan, G.A. Influenza A Virus Diffusion through Mucus Gel Networks. Commun. Biol. 2022, 5, 249. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Shoorei, H.; Noferesti, L.; Hussen, B.M.; Moghadam, M.H.B.; Taheri, M.; Rashnoo, F. Nanoparticle-Mediated Delivery of MicroRNAs-Based Therapies for Treatment of Disorders. Pathol. Res. Pract. 2023, 248, 154667. [Google Scholar] [CrossRef]
- Yan, X.; Sha, X. Nanoparticle-Mediated Strategies for Enhanced Drug Penetration and Retention in the Airway Mucosa. Pharmaceutics 2023, 15, 1457. [Google Scholar] [CrossRef]
- Deng, Z.; Kalin, G.T.; Shi, D.; Kalinichenko, V. V Nanoparticle Delivery Systems with Cell-Specific Targeting for Pulmonary Diseases. Am. J. Respir. Cell Mol. Biol. 2021, 64, 292–307. [Google Scholar] [CrossRef]
- Saadat, M.; Manshadi, M.K.D.; Mohammadi, M.; Zare, M.J.; Zarei, M.; Kamali, R.; Sanati-Nezhad, A. Magnetic Particle Targeting for Diagnosis and Therapy of Lung Cancers. J. Control. Release Off. J. Control. Release Soc. 2020, 328, 776–791. [Google Scholar] [CrossRef] [PubMed]
- Yoosefian, M.; Sabaghian, H. Silver Nanoparticle-Based Drug Delivery Systems in the Fight against COVID-19: Enhancing Efficacy, Reducing Toxicity and Improving Drug Bioavailability. J. Drug Target. 2024, 32, 794–806. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Anton, N.; Ta, T.M.C.; Zhao, M.; Messaddeq, N.; Vandamme, T.F. Microencapsulation of Nanoemulsions: Novel Trojan Particles for Bioactive Lipid Molecule Delivery. Int. J. Nanomed. 2011, 6, 1313–1325. [Google Scholar] [CrossRef]
- Paranjpe, M.; Müller-Goymann, C.C. Nanoparticle-Mediated Pulmonary Drug Delivery: A Review. Int. J. Mol. Sci. 2014, 15, 5852–5873. [Google Scholar] [CrossRef]
- Zachar, O. Nanomedicine Formulations for Respiratory Infections by Inhalation Delivery: Covid-19 and Beyond. Med. Hypotheses 2022, 159, 110753. [Google Scholar] [CrossRef] [PubMed]
- Eygeris, Y.; Gupta, M.; Kim, J.; Sahay, G. Chemistry of Lipid Nanoparticles for RNA Delivery. Acc. Chem. Res. 2022, 55, 2–12. [Google Scholar] [CrossRef]
- Yang, J.; Jia, L.; He, Z.; Wang, Y. Recent Advances in SN-38 Drug Delivery System. Int. J. Pharm. 2023, 637, 122886. [Google Scholar] [CrossRef]
- Eloy, J.O.; Petrilli, R.; Lee, R.J. Targeting of Drug Nanocarriers. In Nanocarriers for Drug Delivery. Nanomedicine and Nanotoxicology; Eloy, J.O., Abriata, J.P., Marchetti, J.M., Eds.; Springer: Cham, Switzerland, 2021; pp. 107–126. [Google Scholar]
- Kuehl, P.J.; Grimes, M.J.; Dubose, D.; Burke, M.; Revelli, D.A.; Gigliotti, A.P.; Belinsky, S.A.; Tessema, M. Inhalation Delivery of Topotecan Is Superior to Intravenous Exposure for Suppressing Lung Cancer in a Preclinical Model. Drug Deliv. 2018, 25, 1127–1136. [Google Scholar] [CrossRef]
- Amore, E.; Ferraro, M.; Manca, M.L.; Gjomarkaj, M.; Giammona, G.; Pace, E.; Bondì, M.L. Mucoadhesive Solid Lipid Microparticles for Controlled Release of a Corticosteroid in the Chronic Obstructive Pulmonary Disease Treatment. Nanomedicine 2017, 12, 2287–2302. [Google Scholar] [CrossRef]
- Sevinc, A.; Kalender, M.E.; Altinbas, M.; Ozkan, M.; Dikilitas, M.; Camci, C. Irinotecan as a Second-Line Monotherapy for Small Cell Lung Cancer. Asian Pac. J. Cancer Prev. 2011, 12, 1055–1059. [Google Scholar] [PubMed]
- Meng, Q.-F.; Tai, W.; Tian, M.; Zhuang, X.; Pan, Y.; Lai, J.; Xu, Y.; Xu, Z.; Li, M.; Zhao, G.; et al. Inhalation Delivery of Dexamethasone with ISEND Nanoparticles Attenuates the COVID-19 Cytokine Storm in Mice and Nonhuman Primates. Sci. Adv. 2023, 9, eadg3277. [Google Scholar] [CrossRef]
- Lokugamage, M.P.; Vanover, D.; Beyersdorf, J.; Hatit, M.Z.C.; Rotolo, L.; Echeverri, E.S.; Peck, H.E.; Ni, H.; Yoon, J.-K.; Kim, Y.; et al. Optimization of Lipid Nanoparticles for the Delivery of Nebulized Therapeutic MRNA to the Lungs. Nat. Biomed. Eng. 2021, 5, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Kuno, Y.; Sugimoto, S.; Takeuchi, H.; Kawashima, Y. Surface-Modified PLGA Nanosphere with Chitosan Improved Pulmonary Delivery of Calcitonin by Mucoadhesion and Opening of the Intercellular Tight Junctions. J. Control. Release Off. J. Control. Release Soc. 2005, 102, 373–381. [Google Scholar] [CrossRef]
- Andrée, L.; Oude Egberink, R.; Dodemont, J.; Hassani Besheli, N.; Yang, F.; Brock, R.; Leeuwenburgh, S.C.G. Gelatin Nanoparticles for Complexation and Enhanced Cellular Delivery of MRNA. Nanomater 2022, 12, 3423. [Google Scholar] [CrossRef]
- Tomoda, K.; Ohkoshi, T.; Hirota, K.; Sonavane, G.S.; Nakajima, T.; Terada, H.; Komuro, M.; Kitazato, K.; Makino, K. Preparation and Properties of Inhalable Nanocomposite Particles for Treatment of Lung Cancer. Colloids Surf. B. Biointerfaces 2009, 71, 177–182. [Google Scholar] [CrossRef]
- Jaulin, N.; Appel, M.; Passirani, C.; Barratt, G.; Labarre, D. Reduction of the Uptake by a Macrophagic Cell Line of Nanoparticles Bearing Heparin or Dextran Covalently Bound to Poly(Methyl Methacrylate). J. Drug Target. 2000, 8, 165–172. [Google Scholar] [CrossRef]
- Vij, N. Nano-Based Theranostics for Chronic Obstructive Lung Diseases: Challenges and Therapeutic Potential. Expert Opin. Drug Deliv. 2011, 8, 1105–1109. [Google Scholar] [CrossRef] [PubMed]
- Pirooznia, N.; Hasannia, S.; Lotfi, A.S.; Ghanei, M. Encapsulation of Alpha-1 Antitrypsin in PLGA Nanoparticles: In Vitro Characterization as an Effective Aerosol Formulation in Pulmonary Diseases. J. Nanobiotechnol. 2012, 10, 20. [Google Scholar] [CrossRef]
- Tseng, C.-L.; Wang, T.-W.; Dong, G.-C.; Yueh-Hsiu Wu, S.; Young, T.-H.; Shieh, M.-J.; Lou, P.-J.; Lin, F.-H. Development of Gelatin Nanoparticles with Biotinylated EGF Conjugation for Lung Cancer Targeting. Biomaterials 2007, 28, 3996–4005. [Google Scholar] [CrossRef]
- Hashimoto, T.; Yuba, E.; Harada, A.; Kono, K. Preparation of Photothermal-Chemotherapy Nanohybrids by Complexation of Gold Nanorods with Polyamidoamine Dendrimers Having Poly(Ethylene Glycol) and Hydrophobic Chains. J. Mater. Chem. B 2020, 8, 2826–2833. [Google Scholar] [CrossRef] [PubMed]
- Slama, Y.; Arcambal, A.; Septembre-Malaterre, A.; Morel, A.-L.; Pesnel, S.; Gasque, P. Evaluation of Core-Shell Fe(3)O(4)@Au Nanoparticles as Radioenhancer in A549 Cell Lung Cancer Model. Heliyon 2024, 10, e29297. [Google Scholar] [CrossRef] [PubMed]
- Sedighi, O.; Bednarke, B.; Sherriff, H.; Doiron, A.L. Nanoparticle-Based Strategies for Managing Biofilm Infections in Wounds: A Comprehensive Review. ACS Omega 2024, 9, 27853–27871. [Google Scholar] [CrossRef]
- Abdelkawi, A.; Slim, A.; Zinoune, Z.; Pathak, Y. Surface Modification of Metallic Nanoparticles for Targeting Drugs. Coatings 2023, 13, 1660. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular Uptake of Nanoparticles: Journey inside the Cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-González, B.; Rozalen, M.; Fernández-Perales, M.; Álvarez, M.A.; Sánchez-Polo, M. Methotrexate Gold Nanocarriers: Loading and Release Study: Its Activity in Colon and Lung Cancer Cells. Molecules 2020, 25, 6049. [Google Scholar] [CrossRef] [PubMed]
- Mandriota, G.; Di Corato, R.; Benedetti, M.; De Castro, F.; Fanizzi, F.P.; Rinaldi, R. Design and Application of Cisplatin-Loaded Magnetic Nanoparticle Clusters for Smart Chemotherapy. ACS Appl. Mater. Interfaces 2019, 11, 1864–1875. [Google Scholar] [CrossRef] [PubMed]
- Homayoonfal, M.; Aminianfar, A.; Asemi, Z.; Yousefi, B. Application of Nanoparticles for Efficient Delivery of Quercetin in Cancer Cells. Curr. Med. Chem. 2024, 31, 1107–1141. [Google Scholar] [CrossRef] [PubMed]
- Gulin-Sarfraz, T.; Jonasson, S.; Wigenstam, E.; von Haartman, E.; Bucht, A.; Rosenholm, J.M. Feasibility Study of Mesoporous Silica Particles for Pulmonary Drug Delivery: Therapeutic Treatment with Dexamethasone in a Mouse Model of Airway Inflammation. Pharmaceutics 2019, 11, 149. [Google Scholar] [CrossRef]
- Castillo, R.R.; Vallet-Regí, M. Recent Advances Toward the Use of Mesoporous Silica Nanoparticles for the Treatment of Bacterial Infections. Int. J. Nanomedicine 2021, 16, 4409–4430. [Google Scholar] [CrossRef]
- Ren, L.; Wang, L.; Rehberg, M.; Stoeger, T.; Zhang, J.; Chen, S. Applications and Immunological Effects of Quantum Dots on Respiratory System. Front. Immunol. 2021, 12, 795232. [Google Scholar] [CrossRef]
- Kaltbeitzel, J.; Wich, P.R. Protein-Based Nanoparticles: From Drug Delivery to Imaging, Nanocatalysis and Protein Therapy. Angew. Chem. Int. Ed. 2023, 62, e202216097. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Choi, D.W.; Kim, H.N.; Park, C.G.; Lee, W.; Park, H.H. Protein-Based Nanoparticles as Drug Delivery Systems. Pharmaceutics 2020, 12, 604. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, H.; Ma, Y.; Jiang, Y. Calcium Phosphate-Based Nanomaterials: Preparation, Multifunction, and Application for Bone Tissue Engineering. Molecules 2023, 28, 4790. [Google Scholar] [CrossRef] [PubMed]
- Hasanzadeh, L.; Kazemi Oskuee, R.; Sadri, K.; Nourmohammadi, E.; Mohajeri, M.; Mardani, Z.; Hashemzadeh, A.; Darroudi, M. Green Synthesis of Labeled CeO(2) Nanoparticles with (99m)Tc and Its Biodistribution Evaluation in Mice. Life Sci. 2018, 212, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Popowski, K.D.; López de Juan Abad, B.; George, A.; Silkstone, D.; Belcher, E.; Chung, J.; Ghodsi, A.; Lutz, H.; Davenport, J.; Flanagan, M.; et al. Inhalable Exosomes Outperform Liposomes as MRNA and Protein Drug Carriers to the Lung. Extracell. Vesicle 2022, 1, 100002. [Google Scholar] [CrossRef] [PubMed]
- Swetha, K.; Kotla, N.G.; Tunki, L.; Jayaraj, A.; Bhargava, S.K.; Hu, H.; Bonam, S.R.; Kurapati, R. Recent Advances in the Lipid Nanoparticle-Mediated Delivery of MRNA Vaccines. Vaccines 2023, 11, 658. [Google Scholar] [CrossRef]
- Kim, J.; Jozic, A.; Lin, Y.; Eygeris, Y.; Bloom, E.; Tan, X.; Acosta, C.; MacDonald, K.D.; Welsher, K.D.; Sahay, G. Engineering Lipid Nanoparticles for Enhanced Intracellular Delivery of MRNA through Inhalation. ACS Nano 2022, 16, 14792–14806. [Google Scholar] [CrossRef]
- Dinakar, Y.H.; Karole, A.; Parvez, S.; Jain, V.; Mudavath, S.L. Organ-Restricted Delivery through Stimuli-Responsive Nanocarriers for Lung Cancer Therapy. Life Sci. 2022, 310, 121133. [Google Scholar] [CrossRef]
- Leong, E.W.X.; Ge, R. Lipid Nanoparticles as Delivery Vehicles for Inhaled Therapeutics. Biomedicines 2022, 10, 2179. [Google Scholar] [CrossRef]
- Luo, J.; Li, X.; Dong, S.; Zhu, P.; Liu, W.; Zhang, S.; Du, J. Layer-by-Layer Coated Hybrid Nanoparticles with PH-Sensitivity for Drug Delivery to Treat Acute Lung Infection. Drug Deliv. 2021, 28, 2460–2468. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Chen, Y.; Ge, Y.; Hu, Y.; Li, M.; Jin, Y. Inhalation Treatment of Primary Lung Cancer Using Liposomal Curcumin Dry Powder Inhalers. Acta Pharm. Sin. B 2018, 8, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Patlolla, R.R.; Chougule, M.; Patel, A.R.; Jackson, T.; Tata, P.N.V.; Singh, M. Formulation, Characterization and Pulmonary Deposition of Nebulized Celecoxib Encapsulated Nanostructured Lipid Carriers. J. Control. Release Off. J. Control. Release Soc. 2010, 144, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Couvreur, P.; Tulkens, P.; Roland, M.; Trouet, A.; Speiser, P. Nanocapsules: A New Type of Lysosomotropic Carrier. FEBS Lett. 1977, 84, 323–326. [Google Scholar] [CrossRef]
- Sung, J.C.; Pulliam, B.L.; Edwards, D.A. Nanoparticles for Drug Delivery to the Lungs. Trends Biotechnol. 2007, 25, 563–570. [Google Scholar] [CrossRef]
- Yu, J.; Chien, Y.W. Pulmonary Drug Delivery: Physiologic and Mechanistic Aspects. Crit. Rev. Ther. Drug Carrier Syst. 1997, 14, 395–453. [Google Scholar] [CrossRef] [PubMed]
- Pontes-Quero, G.M.; Benito-Garzón, L.; Pérez Cano, J.; Aguilar, M.R.; Vázquez-Lasa, B. Modulation of Inflammatory Mediators by Polymeric Nanoparticles Loaded with Anti-Inflammatory Drugs. Pharmaceutics 2021, 13, 290. [Google Scholar] [CrossRef]
- Jennings, L.K.; Dreifus, J.E.; Reichhardt, C.; Storek, K.M.; Secor, P.R.; Wozniak, D.J.; Hisert, K.B.; Parsek, M.R. Pseudomonas Aeruginosa Aggregates in Cystic Fibrosis Sputum Produce Exopolysaccharides That Likely Impede Current Therapies. Cell Rep. 2021, 34, 108782. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Shen, X.; Yu, H.; Tu, H.; Chittasupho, C.; Zhao, Y. Smart Polymeric Nanoparticles in Cancer Immunotherapy. Pharmaceutics 2023, 15, 775. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.; Alwani, S.; Badea, I. Polymeric Nanoparticles in Gene Therapy: New Avenues of Design and Optimization for Delivery Applications. Polymers 2019, 11, 745. [Google Scholar] [CrossRef]
- Garaiova, Z.; Strand, S.P.; Reitan, N.K.; Lélu, S.; Størset, S.Ø.; Berg, K.; Malmo, J.; Folasire, O.; Bjørkøy, A.; Davies, C.d.L. Cellular Uptake of DNA-Chitosan Nanoparticles: The Role of Clathrin- and Caveolae-Mediated Pathways. Int. J. Biol. Macromol. 2012, 51, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A. Pharmacological Strategies and Recent Advancement in Nano-Drug Delivery for Targeting Asthma. Life 2022, 12, 596. [Google Scholar] [CrossRef] [PubMed]
- Kazmi, I.; Shaikh, M.A.J.; Afzal, O.; Altamimi, A.S.A.; Almalki, W.H.; Alzarea, S.I.; Al-Abbasi, F.A.; Pandey, M.; Dureja, H.; Singh, S.K.; et al. Chitosan-Based Nano Drug Delivery System for Lung Cancer. J. Drug Deliv. Sci. Technol. 2023, 81, 104196. [Google Scholar] [CrossRef]
- Richard, I.; Thibault, M.; De Crescenzo, G.; Buschmann, M.D.; Lavertu, M. Ionization Behavior of Chitosan and Chitosan-DNA Polyplexes Indicate That Chitosan Has a Similar Capability to Induce a Proton-Sponge Effect as PEI. Biomacromolecules 2013, 14, 1732–1740. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, V.; Mehta, H.; Pharande, R.; Bannalikar, A.; Gupta, P.; Gupta, U.; Mukne, A. Mannosylated Gelatin Nanoparticles of Licorice for Use in Tuberculosis: Formulation, in Vitro Evaluation, in Vitro Cell Uptake, in Vivo Pharmacokinetics and in Vivo Anti-Tubercular Efficacy. J. Drug Deliv. Sci. Technol. 2018, 45, 255–263. [Google Scholar] [CrossRef]
- Jiang, X.; Du, Z.; Zhang, X.; Zaman, F.; Song, Z.; Guan, Y.; Yu, T.; Huang, Y. Gelatin-Based Anticancer Drug Delivery Nanosystems: A Mini Review. Front. Bioeng. Biotechnol. 2023, 11, 1158749. [Google Scholar] [CrossRef] [PubMed]
- Roointan, A.; Kianpour, S.; Memari, F.; Gandomani, M.; Hayat, S.M.G.; Mohammadi-Samani, S. Poly(Lactic-Co-Glycolic Acid): The Most Ardent and Flexible Candidate in Biomedicine! Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 1028–1049. [Google Scholar] [CrossRef]
- Kolte, A.; Patil, S.; Lesimple, P.; Hanrahan, J.W.; Misra, A. PEGylated Composite Nanoparticles of PLGA and Polyethylenimine for Safe and Efficient Delivery of PDNA to Lungs. Int. J. Pharm. 2017, 524, 382–396. [Google Scholar] [CrossRef]
- Pandey, R.; Sharma, A.; Zahoor, A.; Sharma, S.; Khuller, G.K.; Prasad, B. Poly (DL-Lactide-Co-Glycolide) Nanoparticle-Based Inhalable Sustained Drug Delivery System for Experimental Tuberculosis. J. Antimicrob. Chemother. 2003, 52, 981–986. [Google Scholar] [CrossRef]
- Saxena, J.; Bisen, M.; Misra, A.; Srivastava, V.K.; Kaushik, S.; Siddiqui, A.J.; Mishra, N.; Singh, A.; Jyoti, A. Targeting COPD with PLGA-Based Nanoparticles: Current Status and Prospects. BioMed Res. Int. 2022, 2022, 5058121. [Google Scholar] [CrossRef]
- Rezvantalab, S.; Drude, N.I.; Moraveji, M.K.; Güvener, N.; Koons, E.K.; Shi, Y.; Lammers, T.; Kiessling, F. PLGA-Based Nanoparticles in Cancer Treatment. Front. Pharmacol. 2018, 9, 1260. [Google Scholar] [CrossRef] [PubMed]
- Emami, F.; Yazdi, S.J.M.; Na, D.H. Poly(Lactic Acid)/Poly(Lactic-Co-Glycolic Acid) Particulate Carriers for Pulmonary Drug Delivery. J. Pharm. Investig. 2019, 49, 427–442. [Google Scholar] [CrossRef]
- Sahini, M.G. Polylactic Acid (PLA)-Based Materials: A Review on the Synthesis and Drug Delivery Applications. Emergent Mater. 2023, 6, 1461–1479. [Google Scholar] [CrossRef]
- Luxenhofer, R.; Sahay, G.; Schulz, A.; Alakhova, D.; Bronich, T.K.; Jordan, R.; Kabanov, A. V Structure-Property Relationship in Cytotoxicity and Cell Uptake of Poly(2-Oxazoline) Amphiphiles. J. Control. Release Off. J. Control. Release Soc. 2011, 153, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Luxenhofer, R.; Han, Y.; Schulz, A.; Tong, J.; He, Z.; Kabanov, A.V.; Jordan, R. Poly(2-Oxazoline)s as Polymer Therapeutics. Macromol. Rapid Commun. 2012, 33, 1613–1631. [Google Scholar] [CrossRef] [PubMed]
- Legros, C. Engineering of Poly (2-Oxazoline)s for Potential Use in Biomedical Applications. Ph.D. Thesis, Université de Bordeaux, Bordeaux, France, University of Waterloo, Waterloo, ON, Canada, 2014. [Google Scholar]
- Sabuj, M.Z.R.; Dargaville, T.R.; Nissen, L.; Islam, N. Inhaled Ciprofloxacin-Loaded Poly(2-Ethyl-2-Oxazoline) Nanoparticles from Dry Powder Inhaler Formulation for the Potential Treatment of Lower Respiratory Tract Infections. PLoS ONE 2021, 16, e0261720. [Google Scholar] [CrossRef] [PubMed]
- Łukasiewicz, S.; Mikołajczyk, A.; Błasiak, E.; Fic, E.; Dziedzicka-Wasylewska, M. Polycaprolactone Nanoparticles as Promising Candidates for Nanocarriers in Novel Nanomedicines. Pharmaceutics 2021, 13, 191. [Google Scholar] [CrossRef]
- Korang-Yeboah, M.; Gorantla, Y.; Paulos, S.A.; Sharma, P.; Chaudhary, J.; Palaniappan, R. Polycaprolactone/Maltodextrin Nanocarrier for Intracellular Drug Delivery: Formulation, Uptake Mechanism, Internalization Kinetics, and Subcellular Localization. Int. J. Nanomedicine 2015, 10, 4763–4781. [Google Scholar] [CrossRef]
- Chernenko, T.; Matthäus, C.; Milane, L.; Quintero, L.; Amiji, M.; Diem, M. Label-Free Raman Spectral Imaging of Intracellular Delivery and Degradation of Polymeric Nanoparticle Systems. ACS Nano 2009, 3, 3552–3559. [Google Scholar] [CrossRef]
- Mohammed, S.W.; El-Megrab, N.A.; Hasan, A.A.; Gomaa, E. A Remodeled Ivermectin Polycaprolactone-Based Nanoparticles for Inhalation as a Promising Treatment of Pulmonary Inflammatory Diseases. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2024, 195, 106714. [Google Scholar] [CrossRef]
- Nakamura, K.; Akagi, S.; Ejiri, K.; Yoshida, M.; Miyoshi, T.; Toh, N.; Nakagawa, K.; Takaya, Y.; Matsubara, H.; Ito, H. Current Treatment Strategies and Nanoparticle-Mediated Drug Delivery Systems for Pulmonary Arterial Hypertension. Int. J. Mol. Sci. 2019, 20, 5885. [Google Scholar] [CrossRef] [PubMed]
- Casper, J.; Schenk, S.H.; Parhizkar, E.; Detampel, P.; Dehshahri, A.; Huwyler, J. Polyethylenimine (PEI) in Gene Therapy: Current Status and Clinical Applications. J. Control. Release Off. J. Control. Release Soc. 2023, 362, 667–691. [Google Scholar] [CrossRef] [PubMed]
- Zakeri, A.; Kouhbanani, M.A.J.; Beheshtkhoo, N.; Beigi, V.; Mousavi, S.M.; Hashemi, S.A.R.; Karimi Zade, A.; Amani, A.M.; Savardashtaki, A.; Mirzaei, E.; et al. Polyethylenimine-Based Nanocarriers in Co-Delivery of Drug and Gene: A Developing Horizon. Nano Rev. Exp. 2018, 9, 1488497. [Google Scholar] [CrossRef]
- Koshkina, N.V.; Agoulnik, I.Y.; Melton, S.L.; Densmore, C.L.; Knight, V. Biodistribution and Pharmacokinetics of Aerosol and Intravenously Administered DNA-Polyethyleneimine Complexes: Optimization of Pulmonary Delivery and Retention. Mol. Ther. 2003, 8, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, C.; Ortiz, A.; Schillinger, U.; Jauernig, J.; Plank, C.; Rosenecker, J. Methodological Optimization of Polyethylenimine (PEI)-Based Gene Delivery to the Lungs of Mice via Aerosol Application. J. Gene Med. 2005, 7, 59–66. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, T.; Varela, J.; Lynch, I.; Salvati, A.; Dawson, K.A. Effects of Transport Inhibitors on the Cellular Uptake of Carboxylated Polystyrene Nanoparticles in Different Cell Lines. PLoS ONE 2011, 6, e24438. [Google Scholar] [CrossRef] [PubMed]
- Gul, G.; Faller, R.; Ileri-Ercan, N. Polystyrene-Modified Carbon Nanotubes: Promising Carriers in Targeted Drug Delivery. Biophys. J. 2022, 121, 4271–4279. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.F.; Grimmett, M.E.; Domalewski, C.J.; Cui, H. Inhalable Nanotherapeutics to Improve Treatment Efficacy for Common Lung Diseases. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1586. [Google Scholar] [CrossRef] [PubMed]
- Sannidhi, A.; Zhou, C.; Choi, Y.S.; David, A.E.; Todd, P.W.; Hanley, T.R. Nanomaterial Endocytosis: Quantification of Adsorption and Ingestion Mechanisms. Magnetochemistry 2023, 9, 37. [Google Scholar] [CrossRef]
- Pereira, B.L.; Sampaio, V.S.; Goetten de Lima, G.; Lepienski, C.M.; Marins, M.; Chee, B.S.; Nugent, M.J.D. Biomedical Applications of Polyvinyl Alcohol-Based Bionanocomposites. In Polyvinyl Alcohol-Based Biocomposites and Bionanocomposites; Visakh, O.B.N., Ed.; Scrivener Publishing LLC: Beverly, MA, USA, 2023. [Google Scholar]
- Castro-Balado, A.; Mondelo-García, C.; Barbosa-Pereira, L.; Varela-Rey, I.; Novo-Veleiro, I.; Vázquez-Agra, N.; Antúnez-López, J.R.; Bandín-Vilar, E.J.; Sendón-García, R.; Busto-Iglesias, M.; et al. Development and Characterization of Inhaled Ethanol as a Novel Pharmacological Strategy Currently Evaluated in a Phase II Clinical Trial for Early-Stage SARS-CoV-2 Infection. Pharmaceutics 2021, 13, 342. [Google Scholar] [CrossRef]
- Kusumaatmaja, A.; Sukandaru, B.; Chotimah, C.; Triyana, K. Application of Polyvinyl Alcohol Nanofiber Membrane for Smoke Filtration. AIP Conf. Proc. 2016, 1755, 150006. [Google Scholar] [CrossRef]
- Ting, T.Y.; Gonda, I.; Gipps, E.M. Microparticles of Polyvinyl Alcohol for Nasal Delivery. I. Generation by Spray-Drying and Spray-Desolvation. Pharm. Res. 1992, 9, 1330–1335. [Google Scholar] [CrossRef] [PubMed]
- Sogias, I.A.; Williams, A.C.; Khutoryanskiy, V.V. Why Is Chitosan Mucoadhesive? Biomacromolecules 2008, 9, 1837–1842. [Google Scholar] [CrossRef] [PubMed]
- de la Rosa, V.R. Poly(2-Oxazoline)s as Materials for Biomedical Applications. J. Mater. Sci. Mater. Med. 2014, 25, 1211–1225. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Lautenschlaeger, C.; Kempe, K.; Tauhardt, L.; Schubert, U.S.; Fischer, D. Poly(2-Ethyl-2-Oxazoline) as Alternative for the Stealth Polymer Poly(Ethylene Glycol): Comparison of in Vitro Cytotoxicity and Hemocompatibility. Macromol. Biosci. 2012, 12, 986–998. [Google Scholar] [CrossRef]
- Cartiera, M.S.; Johnson, K.M.; Rajendran, V.; Caplan, M.J.; Saltzman, W.M. The Uptake and Intracellular Fate of PLGA Nanoparticles in Epithelial Cells. Biomaterials 2009, 30, 2790–2798. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yang, S.; Ge, Y.; Wan, X.; Zhu, Y.; Li, J.; Yin, L.; Pu, Y.; Liang, G. Polystyrene Nanoplastics Induce Lung Injury via Activating Oxidative Stress: Molecular Insights from Bioinformatics Analysis. Nanomaterials 2022, 12, 3507. [Google Scholar] [CrossRef] [PubMed]
- Gul, M.O.; Jones, S.A.; Dailey, L.A.; Nacer, H.; Ma, Y.; Sadouki, F.; Hider, R.; Araman, A.; Forbes, B. A Poly(Vinyl Alcohol) Nanoparticle Platform for Kinetic Studies of Inhaled Particles. Inhal. Toxicol. 2009, 21, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Penkina, Y.A.; Pavlovskaya, O.P.; Avramenko, G.V. Development of a Microencapsulated Medicinal Form of Vinpocetine for Administration by Inhalation. Pharm. Chem. J. 2017, 51, 60–64. [Google Scholar] [CrossRef]
- Party, P.; Klement, M.L.; Szabó-Révész, P.; Ambrus, R. Preparation and Characterization of Ibuprofen Containing Nano-Embedded-Microparticles for Pulmonary Delivery. Pharmaceutics 2023, 15, 545. [Google Scholar] [CrossRef]
- Joshi, N.; Shirsath, N.; Singh, A.; Joshi, K.S.; Banerjee, R. Endogenous Lung Surfactant Inspired PH Responsive Nanovesicle Aerosols: Pulmonary Compatible and Site-Specific Drug Delivery in Lung Metastases. Sci. Rep. 2014, 4, 7085. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Khullar, O.V.; Griset, A.P.; Wade, J.E.; Zubris, K.A.V.; Grinstaff, M.W.; Colson, Y.L. Paclitaxel-Loaded Expansile Nanoparticles Delay Local Recurrence in a Heterotopic Murine Non-Small Cell Lung Cancer Model. Ann. Thorac. Surg. 2011, 91, 1074–1077. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, A.; Nagarwal, R.C.; Pandit, J.K. Lomustine Loaded Chitosan Nanoparticles: Characterization and in-Vitro Cytotoxicity on Human Lung Cancer Cell Line L132. Chem. Pharm. Bull. 2011, 59, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.T.; Nakanishi, Y.; Kroll, D.J.; Griset, A.P.; Carnahan, M.A.; Wathier, M.; Oberlies, N.H.; Manikumar, G.; Wani, M.C.; Grinstaff, M.W. Dendrimer-Encapsulated Camptothecins: Increased Solubility, Cellular Uptake, and Cellular Retention Affords Enhanced Anticancer Activity in Vitro. Cancer Res. 2006, 66, 11913–11921. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, J.; Chu, L.; Wang, Y.; Duan, Y.; Feng, L.; Yang, C.; Wang, L.; Kong, D. Novel Peptide-Dendrimer Conjugates as Drug Carriers for Targeting Nonsmall Cell Lung Cancer. Int. J. Nanomed. 2010, 6, 59–69. [Google Scholar] [CrossRef]
- Babu, A.; Templeton, A.K.; Munshi, A.; Ramesh, R. Nanoparticle-Based Drug Delivery for Therapy of Lung Cancer: Progress and Challenges. J. Nanomater. 2013, 2013, 863951. [Google Scholar] [CrossRef]
- Davenne, T.; Percier, P.; Larbanoix, L.; Moser, M.; Leo, O.; Meylan, E.; Goriely, S.; Gérard, P.; Wauthoz, N.; Laurent, S.; et al. Inhaled Dry Powder Cisplatin Increases Antitumour Response to Anti-PD1 in a Murine Lung Cancer Model. J. Control. Release Off. J. Control. Release Soc. 2023, 353, 317–326. [Google Scholar] [CrossRef]
- Jung, J.; Park, S.-J.; Chung, H.K.; Kang, H.-W.; Lee, S.-W.; Seo, M.H.; Park, H.J.; Song, S.Y.; Jeong, S.-Y.; Choi, E.K. Polymeric Nanoparticles Containing Taxanes Enhance Chemoradiotherapeutic Efficacy in Non-Small Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, e77–e83. [Google Scholar] [CrossRef]
- Urso, A.; Meloni, F.; Malatesta, M.; Latorre, R.; Damoci, C.; Crapanzano, J.; Pandolfi, L.; Giustra, M.D.; Pearson, M.; Colombo, M.; et al. Endotracheal Nebulization of Gold Nanoparticles for Noninvasive Pulmonary Drug Delivery. Nanomedicine 2023, 18, 317–330. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, M. Research Progress on Toxicity, Function, and Mechanism of Metal Oxide Nanoparticles on Vascular Endothelial Cells. J. Appl. Toxicol. 2021, 41, 683–700. [Google Scholar] [CrossRef]
- Horie, M.; Stowe, M.; Tabei, M.; Kuroda, E. Metal Ion Release of Manufactured Metal Oxide Nanoparticles Is Involved in the Allergic Response to Inhaled Ovalbumin in Mice. Occup. Dis. Environ. Med. 2016, 4, 17–26. [Google Scholar] [CrossRef]
- Donaldson, K.; Hunter, A.; Poland, C.; Smith, S. Mechanism of Action of Combustion-Derived Nanoparticles. In Toxicology, Survival and Health Hazards of Combustion Products; Purser, D.A., Maynard, R.L., Wakefield, J.C., Eds.; Royal Society of Chemistry: London, UK, 2015; pp. 361–381. [Google Scholar]
- Taghavizadeh Yazdi, M.E.; Qayoomian, M.; Beigoli, S.; Boskabady, M.H. Recent Advances in Nanoparticle Applications in Respiratory Disorders: A Review. Front. Pharmacol. 2023, 14, 1059343. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-S.; Wang, J.-T.; Tai, H.-M.; Chang, P.-C.; Huang, H.-C.; Yang, P.-C. Metal Nanoparticles and Nanoparticle Composites Are Effective against Haemophilus Influenzae, Streptococcus Pneumoniae, and Multidrug-Resistant Bacteria. J. Microbiol. Immunol. Infect. 2022, 55, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzad, M.; Hashemian, S.M.R.; Memarnejadian, A.; Akbarzadeh, I.; Hossein-Khannazer, N.; Vosough, M. The Nano-Based Theranostics for Respiratory Complications of COVID-19. Drug Dev. Ind. Pharm. 2021, 47, 1353–1361. [Google Scholar] [CrossRef]
- Li, K.; Chen, B.; Xu, L.; Feng, J.; Xia, G.; Cheng, J.; Wang, J.; Gao, F.; Wang, X. Reversal of Multidrug Resistance by Cisplatin-Loaded Magnetic Fe3O4 Nanoparticles in A549/DDP Lung Cancer Cells in Vitro and in Vivo. Int. J. Nanomed. 2013, 8, 1867–1877. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Tsai, C.-Y.; Huang, P.-Y.; Chang, M.-Y.; Cheng, P.-C.; Chou, C.-H.; Chen, D.-H.; Wang, C.-R.; Shiau, A.-L.; Wu, C.-L. Methotrexate Conjugated to Gold Nanoparticles Inhibits Tumor Growth in a Syngeneic Lung Tumor Model. Mol. Pharm. 2007, 4, 713–722. [Google Scholar] [CrossRef]
- Verma, N.K.; Crosbie-Staunton, K.; Satti, A.; Gallagher, S.; Ryan, K.B.; Doody, T.; McAtamney, C.; MacLoughlin, R.; Galvin, P.; Burke, C.S.; et al. Magnetic Core-Shell Nanoparticles for Drug Delivery by Nebulization. J. Nanobiotechnology 2013, 11, 1. [Google Scholar] [CrossRef]
- Huang, Y.; Li, P.; Zhao, R.; Zhao, L.; Liu, J.; Peng, S.; Fu, X.; Wang, X.; Luo, R.; Wang, R.; et al. Silica Nanoparticles: Biomedical Applications and Toxicity. Biomed. Pharmacother. 2022, 151, 113053. [Google Scholar] [CrossRef]
- Houshmand, F.; Schofield, J.; Moafi, Z. Electronic and Structural Properties of Functionalized Silica Nanoparticles: DFT and SCC DFTB Calculation. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Li, H.; Chen, X.; Shen, D.; Wu, F.; Pleixats, R.; Pan, J. Functionalized Silica Nanoparticles: Classification, Synthetic Approaches and Recent Advances in Adsorption Applications. Nanoscale 2021, 13, 15998–16016. [Google Scholar] [CrossRef]
- Yadav, D.; Wairagu, P.M.; Kwak, M.; Jin, J.-O. Nanoparticle-Based Inhalation Therapy for Pulmonary Diseases. Curr. Drug Metab. 2022, 23, 882–896. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, D.; Lavanya, S.J.; Girigoswami, A.; Girigoswami, K. Nanomedicine—A Boon for Respiratory Disease Management. Arch. Mater. Sci. Eng. 2023, 119, 71–85. [Google Scholar] [CrossRef]
- Chakraborty, A.; Selomulya, C. Formulation and Role of Polymeric and Inorganic Nanoparticles in Respiratory Diseases. In Targeting Chronic Inflammatory Lung Diseases Using Advanced Drug Delivery Systems; Dua, K., Hansbro, P.M., Wadhwa, R., Haghi, M., Pont, L.G., Williams, K.A., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 261–280. [Google Scholar]
- Khaliq, N.U.; Lee, J.; Kim, J.; Kim, Y.; Yu, S.; Kim, J.; Kim, S.; Sung, D.; Kim, H. Mesoporous Silica Nanoparticles as a Gene Delivery Platform for Cancer Therapy. Pharmaceutics 2023, 15, 1432. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.-Y.; Morris, R.; Cheng, F. Signaling Pathways Regulated by Silica Nanoparticles. Molecules 2021, 26, 1398. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Ho, W.; Chu, J.; Xiong, X.; Hu, B.; Boakye-Yiadom, K.O.; Xu, X.; Zhang, X.-Q. Inhalable SiRNA Nanoparticles for Enhanced Tumor-Targeting Treatment of KRAS-Mutant Non-Small-Cell Lung Cancer. ACS Appl. Mater. Interfaces 2023, 15, 31273–31284. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.; Winter, I.; Drumm, R.; Schneider, M. Cylindrical Microparticles Composed of Mesoporous Silica Nanoparticles for the Targeted Delivery of a Small Molecule and a Macromolecular Drug to the Lungs: Exemplified with Curcumin and SiRNA. Pharmaceutics 2021, 13, 844. [Google Scholar] [CrossRef] [PubMed]
- Sousa de Almeida, M.; Roshanfekr, A.; Balog, S.; Petri-Fink, A.; Rothen-Rutishauser, B. Cellular Uptake of Silica Particles Influences EGFR Signaling Pathway and Is Affected in Response to EGF. Int. J. Nanomed. 2023, 18, 1047–1061. [Google Scholar] [CrossRef] [PubMed]
- Madajewski, B.; Chen, F.; Yoo, B.; Turker, M.Z.; Ma, K.; Zhang, L.; Chen, P.-M.; Juthani, R.; Aragon-Sanabria, V.; Gonen, M.; et al. Molecular Engineering of Ultrasmall Silica Nanoparticle-Drug Conjugates as Lung Cancer Therapeutics. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 5424–5437. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ramadania, E.; Egap, E. Thiol Ligand Capped Quantum Dot as an Efficient and Oxygen Tolerance Photoinitiator for Aqueous Phase Radical Polymerization and 3D Printing under Visible Light. Polym. Chem. 2021, 12, 5106–5116. [Google Scholar] [CrossRef]
- Mehta, P.P.; Dhapte-Pawar, V. Resourceful Quantum Dots for Pulmonary Drug Delivery: Facts, Frontiers, and Future. In Pulmonary Drug Delivery Systems: Material and Technological Advances; Mehta, P.P., Dhapte-Pawar, V., Eds.; Springer: Singapore, 2023; pp. 345–368. [Google Scholar]
- Jassat, W.; Mudara, C.; Ozougwu, L.; Tempia, S.; Blumberg, L.; Davies, M.A.; Pillay, Y.; Carter, T.; Morewane, R.; Wolmarans, M.; et al. Difference in Mortality among Individuals Admitted to Hospital with COVID-19 during the First and Second Waves in South Africa: A Cohort Study. Lancet. Glob. Health 2021, 9, e1216. [Google Scholar] [CrossRef]
- Ruzycka-Ayoush, M.; Kowalik, P.; Kowalczyk, A.; Bujak, P.; Nowicka, A.M.; Wojewodzka, M.; Kruszewski, M.; Grudzinski, I.P. Quantum Dots as Targeted Doxorubicin Drug Delivery Nanosystems in Human Lung Cancer Cells. Cancer Nano 2021, 12, 1–27. [Google Scholar] [CrossRef]
- Panja, A.; Patra, P. A Review on Quantum Dots (QDs) and Their Biomedical Applications. 4Open 2023, 6, 1–11. [Google Scholar] [CrossRef]
- Singh, R.D.; Shandilya, R.; Bhargava, A.; Kumar, R.; Tiwari, R.; Chaudhury, K.; Srivastava, R.K.; Goryacheva, I.Y.; Mishra, P.K. Quantum Dot Based Nano-Biosensors for Detection of Circulating Cell Free MiRNAs in Lung Carcinogenesis: From Biology to Clinical Translation. Front. Genet. 2018, 9, 616. [Google Scholar] [CrossRef] [PubMed]
- Sadhukha, T.; Wiedmann, T.S.; Panyam, J. Inhalable Magnetic Nanoparticles for Targeted Hyperthermia in Lung Cancer Therapy. Biomaterials 2013, 34, 5163–5171. [Google Scholar] [CrossRef] [PubMed]
- Aljabali, A.A.; Rezigue, M.; Alsharedeh, R.H.; Obeid, M.A.; Mishra, V.; Serrano-Aroca, Á.; El-Tanani, M.; Tambuwala, M.M. Protein-Based Nanomaterials: A New Tool for Targeted Drug Delivery. Ther. Deliv. 2022, 13, 321–338. [Google Scholar] [CrossRef] [PubMed]
- Mauser, A.; Quevedo, D.F.; Zhang, B.; Bernandez, Y.; Berardi, A.; Brown, W.; Lee, S.; Miki, R.; Raymond, J.; Lahann, J.; et al. Enzyme-Based Synthetic Protein Nanoparticles as Colloidal Antioxidants. Adv. Ther. 2018, 6, 2300007. [Google Scholar] [CrossRef]
- Gagliardi, A.; Irache, J.M.; Cosco, D. Editorial: Protein Nanoparticles: Characterization and Pharmaceutical Application. Front. Pharmacol. 2023, 14, 1229068. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.K.; Garg, S. Current Status of Drug Delivery Technologies AndFuture Directions. Pharm. Technol. 2001, 25, 1–14. [Google Scholar]
- Gao, X.; Wang, M. Nanoparticles for Intracellular Protein Delivery. Encycl. Nanomater. 2023, 3, 590–603. [Google Scholar] [CrossRef]
- Suto, R.; Srivastava, P.K. A Mechanism for the Specific Immunogenicity of Heat Shock Protein-Chaperoned Peptides. Science 1995, 269, 1585–1588. [Google Scholar] [CrossRef]
- Boutureira, O.; Bernardes, G.J.L. Advances in Chemical Protein Modification. Chem. Rev. 2015, 115, 2174–2195. [Google Scholar] [CrossRef] [PubMed]
- Rani, P.; Singh, P.; Pandit, A.; Kumar, R. Nanoparticles Incorporated Soy Protein Isolate for Emerging Applications in Medical and Biomedical Sectors. In Emerging Nanomaterials and Their Impact on Society in the 21st Century; Materials Research Forum: Millersville, PA, USA, 2023; pp. 265–283. [Google Scholar]
- Administration, U.S.D. & F. PULMOZYME®. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/103532s5175lbl.pdf (accessed on 18 June 2024).
- Domingo-Espín, J.; Unzueta, U.; Saccardo, P.; Rodríguez-Carmona, E.; Corchero, J.L.; Vázquez, E.; Ferrer-Miralles, N. Engineered Biological Entities for Drug Delivery and Gene Therapy Protein Nanoparticles. Prog. Mol. Biol. Transl. Sci. 2011, 104, 247–298. [Google Scholar] [CrossRef] [PubMed]
- Matthews, A.A.; Ee, P.L.R.; Ge, R. Developing Inhaled Protein Therapeutics for Lung Diseases. Mol. Biomed. 2020, 1, 11. [Google Scholar] [CrossRef] [PubMed]
- Chaurasiya, B.; Huang, L.; Du, Y.; Tang, B.; Qiu, Z.; Zhou, L.; Tu, J.; Sun, C. Size-Based Anti-Tumoral Effect of Paclitaxel Loaded Albumin Microparticle Dry Powders for Inhalation to Treat Metastatic Lung Cancer in a Mouse Model. Int. J. Pharm. 2018, 542, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Yang, S.; Liu, Y.; Liu, J.; Wang, Q.; Li, F.; Shang, X.; Teng, Y.; Guo, N.; Yu, P. Peptide Modified Albumin-Paclitaxel Nanoparticles for Improving Chemotherapy and Preventing Metastasis. Macromol. Biosci. 2022, 22, e2100404. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Qu, X. Cerium Oxide Nanoparticle: A Remarkably Versatile Rare Earth Nanomaterial for Biological Applications. NPG Asia Mater. 2014, 6, e90. [Google Scholar] [CrossRef]
- Qiu, C.; Wu, Y.; Guo, Q.; Shi, Q.; Zhang, J.; Meng, Y.; Xia, F.; Wang, J. Preparation and Application of Calcium Phosphate Nanocarriers in Drug Delivery. Mater. Today Bio 2022, 17, 100501. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Huang, Y.-W.; Zhou, X.-D.; Ma, Y. Toxicity of Cerium Oxide Nanoparticles in Human Lung Cancer Cells. Int. J. Toxicol. 2006, 25, 451–457. [Google Scholar] [CrossRef]
- Gass, S.; Cohen, J.M.; Pyrgiotakis, G.; Sotiriou, G.A.; Pratsinis, S.E.; Demokritou, P. A Safer Formulation Concept for Flame-Generated Engineered Nanomaterials. ACS Sustain. Chem. Eng. 2013, 1, 843–857. [Google Scholar] [CrossRef]
- Ma, J.; Mercer, R.R.; Barger, M.; Schwegler-Berry, D.; Cohen, J.M.; Demokritou, P.; Castranova, V. Effects of Amorphous Silica Coating on Cerium Oxide Nanoparticles Induced Pulmonary Responses. Toxicol. Appl. Pharmacol. 2015, 288, 63–73. [Google Scholar] [CrossRef]
- Graham, U.M.; Fernback, J.; Wang, C.; Dozier, A.K.; Drummy, L.; Mahalingam, K.; Molina, R.M.; Konduru, N.V.; Birch, M.E.; Oberdörster, G.; et al. Calcium Co-Localization with in Vivo Cerium Phosphate Nanoparticle Formation after Intratracheal Instillation Dosing with CeCl3 or CeO2 NPs. Microsc. Microanal. 2017, 23, 1344–1345. [Google Scholar] [CrossRef]
- Xu, X.; Xu, L.; Wen, C.; Xia, J.; Zhang, Y.; Liang, Y. Programming Assembly of Biomimetic Exosomes: An Emerging Theranostic Nanomedicine Platform. Mater. Today Bio 2023, 22, 100760. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, aau6977. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Dai, W.; Wei, Y.; Cao, S.; Liao, S.; Li, A.; Liu, P.; Lin, J.; Zeng, H. Unlocking the Potential of Exosomes: A Breakthrough in the Theranosis of Degenerative Orthopaedic Diseases. Front. Bioeng. Biotechnol. 2024, 12, 1377142. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhu, T.; Tang, L.; Li, Z.; Jiang, G.; Huang, X. Inhalable CAR-T Cell-Derived Exosomes as Paclitaxel Carriers for Treating Lung Cancer. J. Transl. Med. 2023, 21, 383. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Lee, Y.; Ha, J.; Han, S.; Lee, M. Engineering Exosomes for Pulmonary Delivery of Peptides and Drugs to Inflammatory Lung Cells by Inhalation. J. Control. Release Off. J. Control. Release Soc. 2021, 330, 684–695. [Google Scholar] [CrossRef] [PubMed]
- Dinh, P.-U.C.; Paudel, D.; Brochu, H.; Popowski, K.D.; Gracieux, M.C.; Cores, J.; Huang, K.; Hensley, M.T.; Harrell, E.; Vandergriff, A.C.; et al. Inhalation of Lung Spheroid Cell Secretome and Exosomes Promotes Lung Repair in Pulmonary Fibrosis. Nat. Commun. 2020, 11, 1064. [Google Scholar] [CrossRef] [PubMed]
- Aqil, F.; Munagala, R.; Jeyabalan, J.; Agrawal, A.K.; Gupta, R. Exosomes for the Enhanced Tissue Bioavailability and Efficacy of Curcumin. AAPS J. 2017, 19, 1691–1702. [Google Scholar] [CrossRef]
- Vázquez-Ríos, A.J.; Molina-Crespo, Á.; Bouzo, B.L.; López-López, R.; Moreno-Bueno, G.; de la Fuente, M. Exosome-Mimetic Nanoplatforms for Targeted Cancer Drug Delivery. J. Nanobiotechnology 2019, 17, 85. [Google Scholar] [CrossRef]
- Mehta, P.P.; Dhapte-Pawar, V. (Eds.) Pulmonary Drug Delivery Systems: Material and Technological Advances; Springer: Berlin/Heidelberg, Germany, 2023; ISBN 78-981-99-1923-9. [Google Scholar]
- Yue, P.; Zhou, W.; Huang, G.; Lei, F.; Chen, Y.; Ma, Z.; Chen, L.; Yang, M. Nanocrystals Based Pulmonary Inhalation Delivery System: Advance and Challenge. Drug Deliv. 2022, 29, 637–651. [Google Scholar] [CrossRef]
- Kraft, W.K.; Steiger, B.; Beussink, D.; Quiring, J.N.; Fitzgerald, N.; Greenberg, H.E.; Waldman, S.A. The Pharmacokinetics of Nebulized Nanocrystal Budesonide Suspension in Healthy Volunteers. J. Clin. Pharmacol. 2004, 44, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Rundfeldt, C.; Steckel, H.; Scherliess, H.; Wyska, E.; Wlaź, P. Inhalable Highly Concentrated Itraconazole Nanosuspension for the Treatment of Bronchopulmonary Aspergillosis. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgemeinschaft Pharm. Verfahrenstechnik 2013, 83, 44–53. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health Drug Delivery Systems. Available online: https://www.nibib.nih.gov/science-education/science-topics/drug-delivery-systems-getting-drugs-their-targets-controlled-manner (accessed on 24 June 2024).
- Vega-Vásquez, P.; Mosier, N.S.; Irudayaraj, J. Nanoscale Drug Delivery Systems: From Medicine to Agriculture. Front. Bioeng. Biotechnol. 2020, 8, 79. [Google Scholar] [CrossRef] [PubMed]
- Party, P.; Ambrus, R. Investigation of Physico-Chemical Stability and Aerodynamic Properties of Novel “Nano-in-Micro” Structured Dry Powder Inhaler System. Micromachines 2023, 14, 1348. [Google Scholar] [CrossRef] [PubMed]
- Akdag, Y. Nanoparticle-Containing Lyophilized Dry Powder Inhaler Formulations Optimized Using Central Composite Design with Improved Aerodynamic Parameters and Redispersibility. Pharm. Dev. Technol. 2023, 28, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Kotkar, V.; Hingane, L.D.; Bagwan, L. Formulation and Evaluation of Dry Powder Inhaler. Ijraset J. Res. Appl. Sci. Eng. Technol. 2022, 10, 4625–4637. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, A.; Garg, N.; Siril, P.F. Solid Lipid Nanoparticles for the Controlled Delivery of Poorly Water Soluble Non-Steroidal Anti-Inflammatory Drugs. Ultrason. Sonochem. 2018, 40, 686–696. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Singh, A.; Sharma, K.; Dhasmana, D.; Garg, N.; Siril, P.F. Preparation, Characterization and in Vitro Cytotoxicity of Fenofibrate and Nabumetone Loaded Solid Lipid Nanoparticles. Mater. Sci. Eng. C. Mater. Biol. Appl. 2020, 106, 110184. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, C.; Kommineni, N.; Butreddy, A.; Kumar, R.; Bunekar, N.; Gugulothu, K. PLGA Nanoparticles in Drug Delivery. In Nanoengineering of Biomaterials; Sougata Jana, S.J., Ed.; WILEY-VCH GmbH: Hoboken, NJ, USA, 2022; pp. 217–260. [Google Scholar]
- Kumar, R.; Siril, P.F.; Javid, F. Unusual Anti-Leukemia Activity of Nanoformulated Naproxen and Other Non-Steroidal Anti-Inflammatory Drugs. Mater. Sci. Eng. C. Mater. Biol. Appl. 2016, 69, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Somavarapu, S.; Colombani, A.; Govind, N.; Taylor, K.M.G. Crosslinked Chitosan Nanoparticle Formulations for Delivery from Pressurized Metered Dose Inhalers. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgemeinschaft Pharm. Verfahrenstechnik 2012, 81, 74–81. [Google Scholar] [CrossRef]
- de Boer, A.; Thalberg, K. Metered Dose Inhalers (MDIs). In Inhaled Medicines: Optimizing Development through Integration of In Silico, In Vitro and In Vivo Approaches; Kassinos, S., Backman, P., Conway, J., Hickey, A.J.J., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2021; pp. 65–92. [Google Scholar]
- Barve, M.H.; Shardul, P.K.; Munne, S.S.; Bendale, A.R.; Naphade, V.; Pathan, V.T.; Borse, L.B. Metered Dose Inhalers (MDI’S) for High-Performance Pulmonary Drug Delivery in Assistance to Nanotechnology. Biosci. Biotech. Res. Asia 2023, 20, 433–447. [Google Scholar] [CrossRef]
- Huang, Z.; Wu, H.; Yang, B.; Chen, L.; Huang, Y.; Quan, G.; Zhu, C.; Li, X.; Pan, X.; Wu, C. Anhydrous Reverse Micelle Nanoparticles: New Strategy to Overcome Sedimentation Instability of Peptide-Containing Pressurized Metered-Dose Inhalers. Drug Deliv. 2017, 24, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Khairnar, S.V.; Jain, D.D.; Tambe, S.M.; Chavan, Y.R.; Amin, P.D. Nebulizer Systems: A New Frontier for Therapeutics and Targeted Delivery. Ther. Deliv. 2022, 13, 31–49. [Google Scholar] [CrossRef]
- Vu, T.-H.; Vu, H.-D.; Vu, N.-L.; Nguyen, H.T.; Dao, D.V.; Dau, V.T. Simultaneous Generation and Delivery of Neutral Polymeric Aerosol by Electro-Hydrodynamic Nebulizer. In Proceedings of the 2022 IEEE 17th International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), Taiwan, China, 14–17 April 2022; pp. 141–144. [Google Scholar]
- Komalla, V.; Wong, C.Y.J.; Sibum, I.; Muellinger, B.; Nijdam, W.; Chaugule, V.; Soria, J.; Ong, H.X.; Buchmann, N.A.; Traini, D. Advances in Soft Mist Inhalers. Expert Opin. Drug Deliv. 2023, 20, 1055–1070. [Google Scholar] [CrossRef] [PubMed]
- Jessamine, V.; Mehndiratta, S.; De Rubis, G.; Paudel, K.R.; Shetty, S.; Suares, D.; Chellappan, D.K.; Oliver, B.G.; Hansbro, P.M.; Dua, K. The Application of Nanoparticles as Advanced Drug Delivery Systems in Attenuating COPD. Heliyon 2024, 10, e25393. [Google Scholar] [CrossRef]
- Parray, H.A.; Shukla, S.; Perween, R.; Khatri, R.; Shrivastava, T.; Singh, V.; Murugavelu, P.; Ahmed, S.; Samal, S.; Sharma, C.; et al. Inhalation Monoclonal Antibody Therapy: A New Way to Treat and Manage Respiratory Infections. Appl. Microbiol. Biotechnol. 2021, 105, 6315–6332. [Google Scholar] [CrossRef]
- Leekha, A.; Saeedi, A.; Kumar, M.; Sefat, S.R.; Martinez-Paniagua, M.; Fathi, M.; Kulkarni, R.; Biswas, S.; Tsitoura, D.; Liu, X.; et al. An Intranasal Nanoparticle STING Agonist Has Broad Protective Immunity against Respiratory Viruses and Variants. bioRxiv 2022, bioRxiv:2022.04.18. [Google Scholar] [CrossRef]
- Cruz-Resendiz, A.; Acero, G.; Sampieri, A.; Gevorkian, G.; Salvador, C.; Escobar, L.; Rosendo-Pineda, M.J.; Medeiros, M.; Vaca, L. An Ambient-Temperature Stable Nanoparticle-Based Vaccine for Nasal Application That Confers Long-Lasting Immunogenicity to Carried Antigens. Front. Immunol. 2022, 13, 1057499. [Google Scholar] [CrossRef] [PubMed]
- Clementino, A.R.; Pellegrini, G.; Banella, S.; Colombo, G.; Cantù, L.; Sonvico, F.; Del Favero, E. Structure and Fate of Nanoparticles Designed for the Nasal Delivery of Poorly Soluble Drugs. Mol. Pharm. 2021, 18, 3132–3146. [Google Scholar] [CrossRef]
- Gujarathi, N.A.; Bakliwal, A.A.; Rane, B.R.; Pathan, V.; Keservani, R.K. Nanoencapsulated Nasal Drug Delivery System. In Topical and Transdermal Drug Delivery Systems; Apple Academic Press: Cambridge, MA, USA, 2023; p. 23. ISBN 9781003284017. [Google Scholar]
- Shim, S.; Yoo, H.S. The Application of Mucoadhesive Chitosan Nanoparticles in Nasal Drug Delivery. Mar. Drugs 2020, 18, 605. [Google Scholar] [CrossRef] [PubMed]
- Kanaki, K.; Pergantis, S.A. Using Nanoparticles to Determine the Transport Efficiency of Microflow and Nanoflow Nebulizers in Inductively Coupled Plasma-Mass Spectrometry. J. Anal. At. Spectrom. 2016, 31, 1041–1046. [Google Scholar] [CrossRef]
- Gabizon, A.; Martin, F. Polyethylene Glycol-Coated (Pegylated) Liposomal Doxorubicin. Rationale for Use in Solid Tumours. Drugs 1997, 54 (Suppl. S4), 15–21. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Aspitia, A.; Perez, E.A. Nanoparticle Albumin-Bound Paclitaxel (ABI-007): A Newer Taxane Alternative in Breast Cancer. Future Oncol. 2005, 1, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, M.K.; Lee, C.Y.; Tran, T.T.T.; Tan, R.; Chew, S.M.; Yeo, B.Z.J.; Loh, W.X.; Pirisinu, M.; Le, M.T.N. The Role of in Silico Research in Developing Nanoparticle-Based Therapeutics. Front. Digit. Health 2022, 4, 838590. [Google Scholar] [CrossRef] [PubMed]
- Ehrhardt, C.; Fiegel, J.; Fuchs, S.; Abu-Dahab, R.; Schaefer, U.F.; Hanes, J.; Lehr, C.-M. Drug Absorption by the Respiratory Mucosa: Cell Culture Models and Particulate Drug Carriers. J. Aerosol Med. Off. J. Int. Soc. Aerosols Med. 2002, 15, 131–139. [Google Scholar] [CrossRef]
- Riadh Boukef, H.U.S. Colloidal Silver, Treatment of COVID-19. Available online: https://www.clinicaltrials.gov/study/nct04978025?term=nanoparticlesinhalationtherapy&rank=5 (accessed on 24 June 2024).
- Rowe, S.M.; Zuckerman, J.B.; Dorgan, D.; Lascano, J.; McCoy, K.; Jain, M.; Schechter, M.S.; Lommatzsch, S.; Indihar, V.; Lechtzin, N.; et al. Inhaled MRNA Therapy for Treatment of Cystic Fibrosis: Interim Results of a Randomized, Double-Blind, Placebo-Controlled Phase 1/2 Clinical Study. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2023, 22, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, Y.; Ishihara, T.; Ishizaki, J.; Miyamoto, K.; Higaki, M.; Yamashita, N. Effect of Betamethasone Phosphate Loaded Polymeric Nanoparticles on a Murine Asthma Model. Cell. Immunol. 2009, 260, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, N.J.; Bratt, J.M.; Lee, J.; Luo, J.; Franzi, L.M.; Zeki, A.A.; Lam, K.S. Self-Assembling Nanoparticles Containing Dexamethasone as a Novel Therapy in Allergic Airways Inflammation. PLoS ONE 2013, 8, e77730. [Google Scholar] [CrossRef] [PubMed]
- Konduri, K.S.; Nandedkar, S.; Düzgünes, N.; Suzara, V.; Artwohl, J.; Bunte, R.; Gangadharam, P.R.J. Efficacy of Liposomal Budesonide in Experimental Asthma. J. Allergy Clin. Immunol. 2003, 111, 321–327. [Google Scholar] [CrossRef]
- Oh, Y.J.; Lee, J.; Seo, J.Y.; Rhim, T.; Kim, S.-H.; Yoon, H.J.; Lee, K.Y. Preparation of Budesonide-Loaded Porous PLGA Microparticles and Their Therapeutic Efficacy in a Murine Asthma Model. J. Control. Release Off. J. Control. Release Soc. 2011, 150, 56–62. [Google Scholar] [CrossRef]
- Chen, X.; Huang, W.; Wong, B.C.; Yin, L.; Wong, Y.F.; Xu, M.; Yang, Z. Liposomes Prolong the Therapeutic Effect of Anti-Asthmatic Medication via Pulmonary Delivery. Int. J. Nanomed. 2012, 7, 1139–1148. [Google Scholar] [CrossRef]
- Wang, L.; Feng, M.; Li, Q.; Qiu, C.; Chen, R. Advances in Nanotechnology and Asthma. Ann. Transl. Med. 2019, 7, 180. [Google Scholar] [CrossRef]
- De Rubis, G.; Paudel, K.R.; Manandhar, B.; Singh, S.K.; Gupta, G.; Malik, R.; Shen, J.; Chami, A.; MacLoughlin, R.; Chellappan, D.K.; et al. Agarwood Oil Nanoemulsion Attenuates Cigarette Smoke-Induced Inflammation and Oxidative Stress Markers in BCi-NS1.1 Airway Epithelial Cells. Nutrients 2023, 15, 1019. [Google Scholar] [CrossRef]
- Mohamed, A.; Kunda, N.K.; Ross, K.; Hutcheon, G.A.; Saleem, I.Y. Polymeric Nanoparticles for the Delivery of MiRNA to Treat Chronic Obstructive Pulmonary Disease (COPD). Eur. J. Pharm. Biopharm. Off. J. Arbeitsgemeinschaft Pharm. Verfahrenstechnik 2019, 136, 1–8. [Google Scholar] [CrossRef]
- Tang, J.; Ouyang, Q.; Li, Y.; Zhang, P.; Jin, W.; Qu, S.; Yang, F.; He, Z.; Qin, M. Nanomaterials for Delivering Antibiotics in the Therapy of Pneumonia. Int. J. Mol. Sci. 2022, 23, 15738. [Google Scholar] [CrossRef] [PubMed]
- Rathnayake, K.; Patel, U.; Pham, C.; McAlpin, A.; Budisalich, T.; Jayawardena, S.N. Targeted Delivery of Antibiotic Therapy to Inhibit Pseudomonas Aeruginosa Using Lipid-Coated Mesoporous Silica Core-Shell Nanoassembly. ACS Appl. Bio Mater. 2020, 3, 6708–6721. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.; Li, G.; De Backer, J.; Sadafi, H.; Wu, L.; Usmani, O.S. Small Airways Deposition of Two Fixed-Dose Triple Therapy Combinations Assessed With Functional Respiratory Imaging (FRI). In Proceedings of the American Thoracic Society 2023 International Conference, Washington, DC, USA, 19–24 May 2023; p. A1556. [Google Scholar]
- Xuan, L.; Ju, Z.; Skonieczna, M.; Zhou, P.-K.; Huang, R. Nanoparticles-Induced Potential Toxicity on Human Health: Applications, Toxicity Mechanisms, and Evaluation Models. Med. Comm. 2023, 4, e327. [Google Scholar] [CrossRef]
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Hsin, H.Y.; Ingber, D.E. Reconstituting Organ-Level Lung Functions on a Chip. Science 2010, 328, 1662–1668. [Google Scholar] [CrossRef]
- Morimoto, Y.; Horie, M.; Kobayashi, N.; Shinohara, N.; Shimada, M. Inhalation Toxicity Assessment of Carbon-Based Nanoparticles. Acc. Chem. Res. 2013, 46, 770–781. [Google Scholar] [CrossRef]
- Liu, H.; Yang, D.; Yang, H.; Zhang, H.; Zhang, W.; Fang, Y.; Lin, Z.; Tian, L.; Lin, B.; Yan, J.; et al. Comparative Study of Respiratory Tract Immune Toxicity Induced by Three Sterilisation Nanoparticles: Silver, Zinc Oxide and Titanium Dioxide. J. Hazard. Mater. 2013, 248–249, 478–486. [Google Scholar] [CrossRef]
- Halimu, G.; Zhang, Q.; Liu, L.; Zhang, Z.; Wang, X.; Gu, W.; Zhang, B.; Dai, Y.; Zhang, H.; Zhang, C.; et al. Toxic Effects of Nanoplastics with Different Sizes and Surface Charges on Epithelial-to-Mesenchymal Transition in A549 Cells and the Potential Toxicological Mechanism. J. Hazard. Mater. 2022, 430, 128485. [Google Scholar] [CrossRef] [PubMed]

| Key Criteria | Specifications |
|---|---|
| Biocompatibility | Compatibility with lipids/ proteins/polymers/metals |
| Biodegradability | Non-toxic |
| Cellular uptake | Efficiently taken up by target cells |
| Controlled release | Triggered release of drugs at the target site |
| Cost-effectiveness | The economic viability of producing nanoparticles for widespread clinical use |
| Dose response | Predictable therapeutic response |
| Dose uniformity | Consistent dosing in each inhalation |
| Drug loading capacity | High |
| Immune response | Minimal; reaction of the immune system to the nanoparticles |
| Immunogenicity | Engineering to avoid triggering a significant immune response |
| Optimal aerodynamic diameter | 1–5 μm for deep lung deposition |
| Pharmacokinetics | Prolonged retention |
| Production efficiency | Large-scale production |
| Safety | Low toxicity |
| Sustainable production | Environmentally friendly materials |
| Mucosal penetration | Able to cross mucosal barriers |
| Mass median Aerodynamic diameter | Important for deposition in Respiratory airways |
| Fine particle fraction | Nanoparticles small enough to reach deeper regions of the lungs |
| Protect drug entity in airflow | Able to ensure the stability and integrity of the drug during delivery |
| Ensure specific site targeting | Precision in nanoparticle delivery into the respiratory tract |
| Minimizing clearance | Reducing the rate at which the body removes the nanoparticles |
| Sustained release profile | The ability to release a drug over an extended period |
| Inhaled Nanoparticles | Material | Nanoparticle Functional Mechanisms | Nanoparticle and Nanoemulsion Formulations |
|---|---|---|---|
| LNPs | Liposomes solid lipid | Encapsulation [32] | - Liposomal SN-38 [33] - Curcumin [34] |
| - TOPOTECAN [35] - Cxb-NLCs [34] - Fluticasone propionate [36] | |||
| - Irinotecan [37] - NanoDEX [38] - NLD1 [39] - Budesonide [34] - LNPs-mRNA [32] | |||
| PNPs | - PLGA - Chitosan - PEG | - Mucoadhesion [40] - Enhanced cellular uptake via endocytosis [41] - Protection from enzymatic degradation [34] | TAS-103 [42] NanoDEX [43] Prednisolone [44] Theophylline [44] NLD1 [39] PLGA-α1AT [45] |
| Gel-BEGF [46] Paclitaxel [34] Lomustine [34] CPT-PGSA [34] Dox-Pep-Dend [47] PEG-PLA-taxanes NPs [34] | |||
| MNPs | - Gold - Silver - Fe3O4 | - Generation of ROS [48] - Disruption of microbial membranes [49] - Targeted delivery via surface conjugation [50] - Enhanced cellular uptake [51] | MTX-AuNPs [52] Cis-MagNPs [53] Q-Fe₃O₄-PLGA-MNPs [54] |
| Silica NPs | Mesoporous Silica SiO2 | - Enhanced cellular uptake [51] - Surface functionalization for targeted delivery [55] | Core-shell silica Nanocarriers [56] |
| QDs | Semiconductor materials (e.g., CdSe, CdTe) | - Fluorescent labeling [57] - Cellular uptake via endocytosis [51] | CdSe/CdTe NPs [57] |
| PBNPs | Albumin | - Receptor-mediated endocytosis [58] - Enzymatic degradation [58] | NDDS [59] PTX-BSA [59] |
| Inorganic NPs | - Calcium phosphate - Iron oxide - CeO2 | - Magnetic targeting [60] - Cellular uptake via endocytosis [51] | CeO2 NPs [61] |
| EMNPs | Lipid bilayers mimicking exosomes | - Drug encapsulation [61] - Fusion with target cell membranes [61] - Intercellular communication [61] | Curcumin [61] F-EMNs [62] |
| Polymeric NPs | Mechanism of Action (after Degradation following Inhaled Administration) | Respiratory Applications |
|---|---|---|
| Chitosan | - Mucoadhesion - Cellular uptake (clathrin-mediated endocytosis and caveolae-mediated endocytosis) [77] | Asthma [78] Lung cancer [79] |
| - Endosomal escape (“proton sponge effect”) [80] - Intracytoplasmic release of encapsulated therapeutic agents | ||
| Gelatin | - Cellular uptake - Endosomal escape through “the proton sponge effect” [41] | Tuberculosis [81] Protein and mRNA delivery [41] |
| Anti-tumoral [82] | ||
| PLGA | - Mucoadhesion (surface modifications; e.g., coating with Chitosan) - Cellular uptake (clathrin-mediated endocytosis, fluid-phase pinocytosis) [83] - Endosomal escape through “the proton sponge effect” | CF [84] Asthma [78] Tuberculosis [85] COPD [86] Anti-tumoral [87] Drug delivery [88] |
| PLA | - Hydrolysis [88] - Endocytosis - Endosomal Escape (“proton sponge effect” or direct fusion with the endosomal membrane) [88] | Drug delivery [88,89] |
| PEtOx | - Endocytosis [90,91] | Anti-inflammatory [92] |
| Respiratory infections [93] | ||
| PCL | - Cellular uptake (clathrin-mediated endocytosis and caveolae-mediated endocytosis) [94] - Micropinocytosis [95] - Late endosome [96] | Pulmonary inflammatory diseases [97] Pulmonary hypertension [98] |
| PEI | - Cellular uptake (endosomal and non-endosomal) - Endosomal escape- “proton sponge effect” [80] - Nuclear uptake organelle disturbance [99] | Gene therapy [99,100] Lung tumor inhibition [101,102] |
| PS-NPs | - Cellular uptake (clathrin-mediated endocytosis and caveolae-mediated endocytosis) [103] | Drug delivery [104,105] |
| PVA | - Cellular uptake (clathrin-mediated endocytosis and caveolae-mediated endocytosis) - Macropinocytosis [106] | Respiratory infections [107,108] Smoke filtration [109] Nasal drug delivery [110] |
| LNPs | PNPs | MNPs | Silica NPs | QDs | PBNPs | Inorganic NPs | EMNPs | |
|---|---|---|---|---|---|---|---|---|
| DPIs | + | + | + | + | ||||
| MDIs | + | + | + | |||||
| nebulizers | + | + | + | + | + | + | + | |
| SMIs | ||||||||
| pMDIs | + | + | + | |||||
| nasal sprays | + | |||||||
| nasal nebulizers | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cojocaru, E.; Petriș, O.R.; Cojocaru, C. Nanoparticle-Based Drug Delivery Systems in Inhaled Therapy: Improving Respiratory Medicine. Pharmaceuticals 2024, 17, 1059. https://doi.org/10.3390/ph17081059
Cojocaru E, Petriș OR, Cojocaru C. Nanoparticle-Based Drug Delivery Systems in Inhaled Therapy: Improving Respiratory Medicine. Pharmaceuticals. 2024; 17(8):1059. https://doi.org/10.3390/ph17081059
Chicago/Turabian StyleCojocaru, Elena, Ovidiu Rusalim Petriș, and Cristian Cojocaru. 2024. "Nanoparticle-Based Drug Delivery Systems in Inhaled Therapy: Improving Respiratory Medicine" Pharmaceuticals 17, no. 8: 1059. https://doi.org/10.3390/ph17081059








