Traditional Processing Can Enhance the Medicinal Effects of Polygonatum cyrtonema by Inducing Significant Chemical Changes in the Functional Components in Its Rhizomes
Abstract
1. Introduction
2. Results
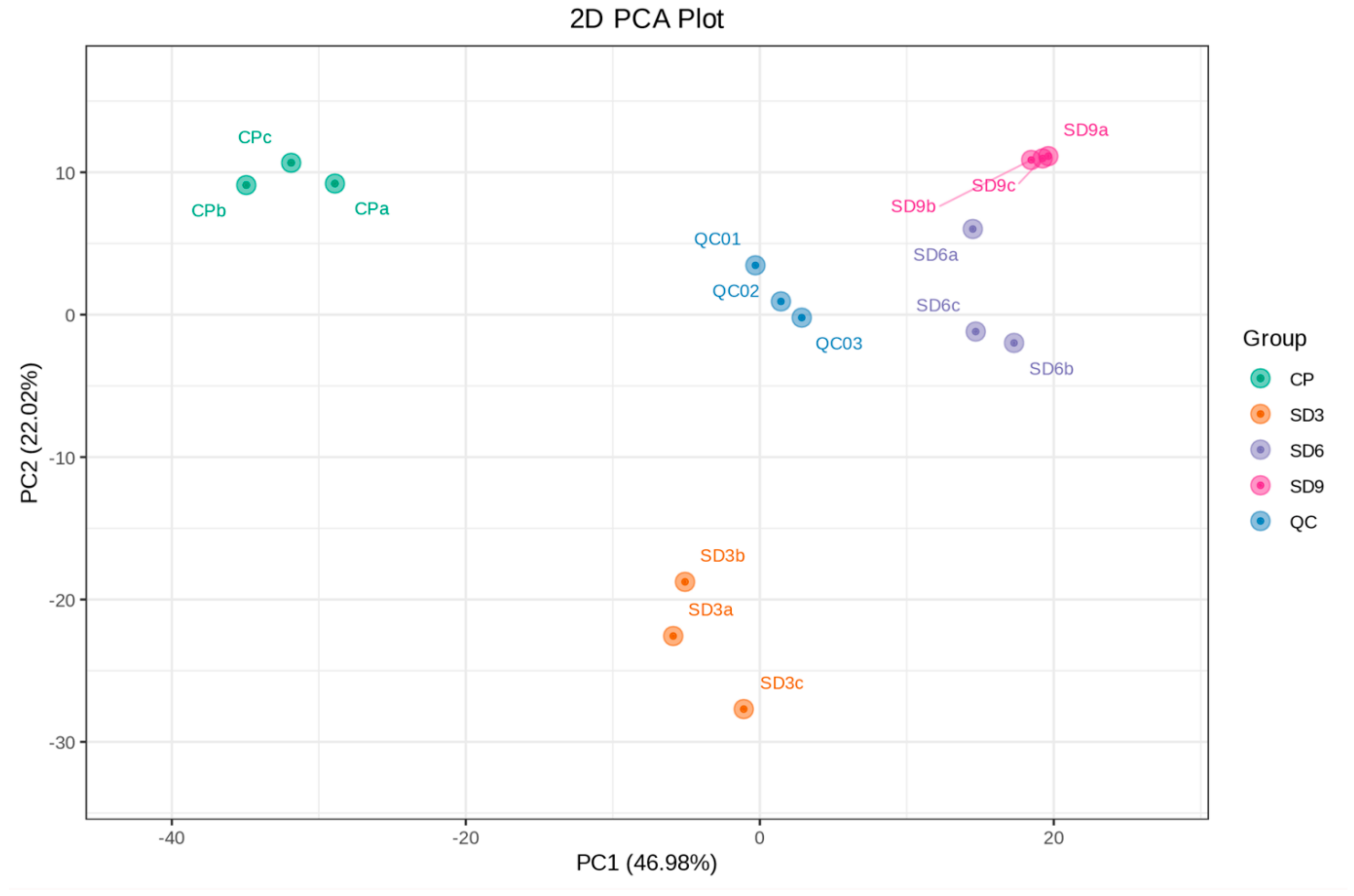
2.1. Global Analysis on Metabolomic Profile of P. cyrtonema Rhizome
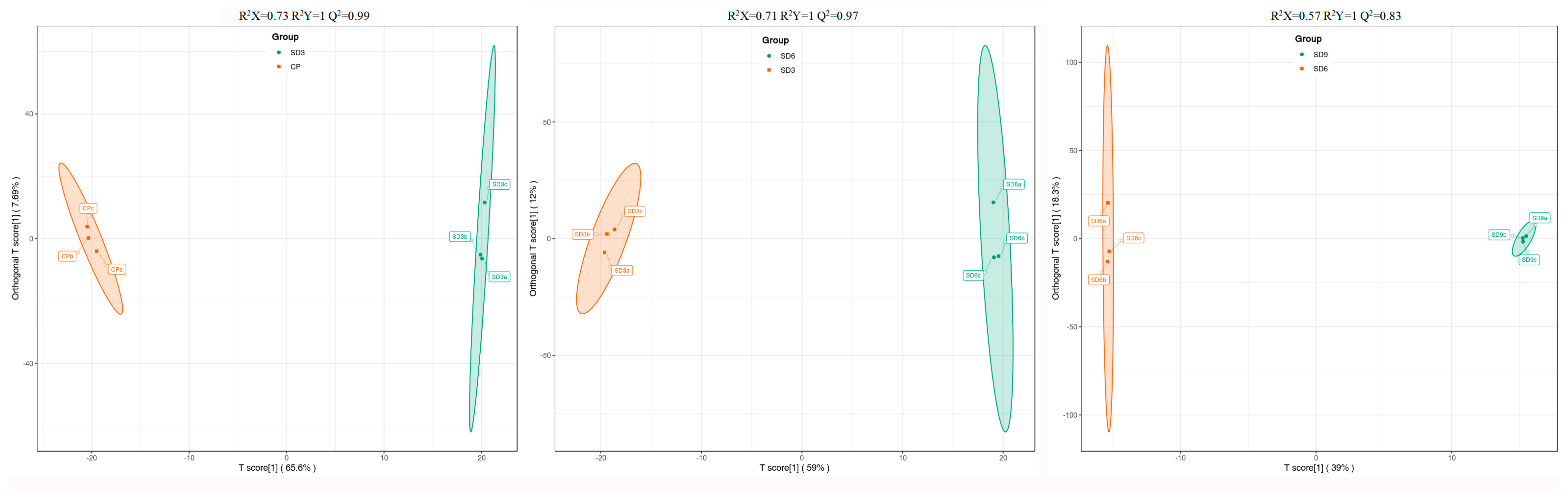
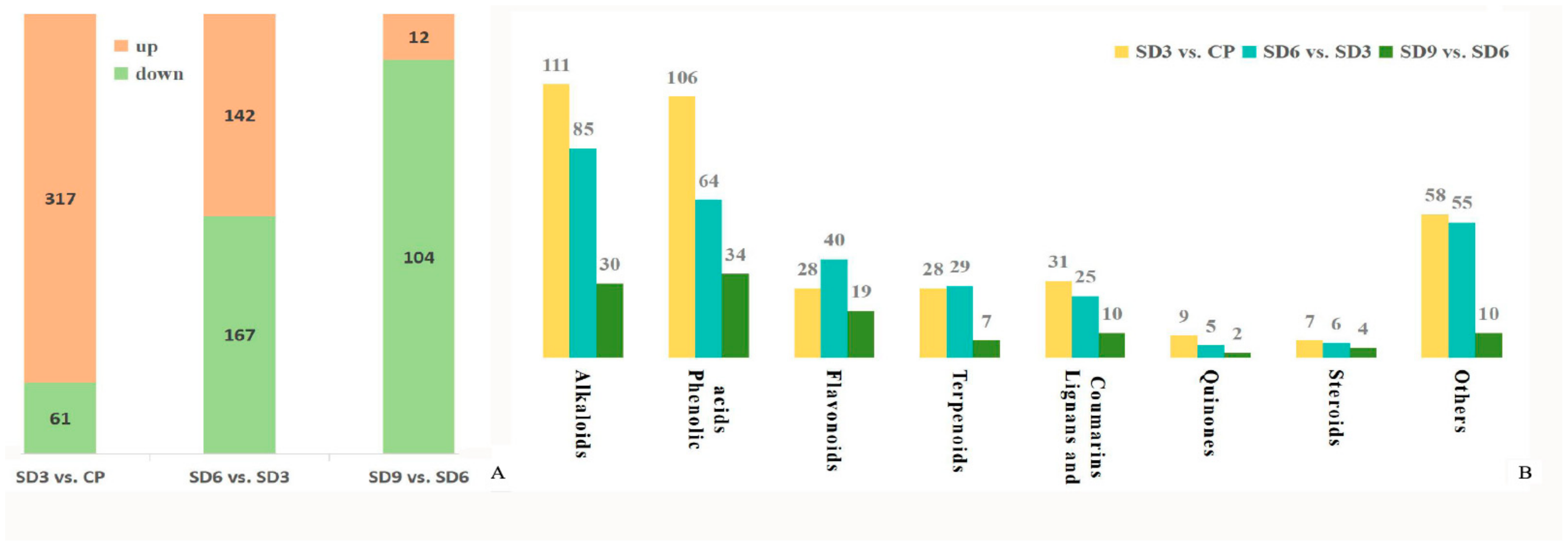
2.2. Differentially Accumulated Secondary Metabolite Profiling between Crude and Processed Rhizomes
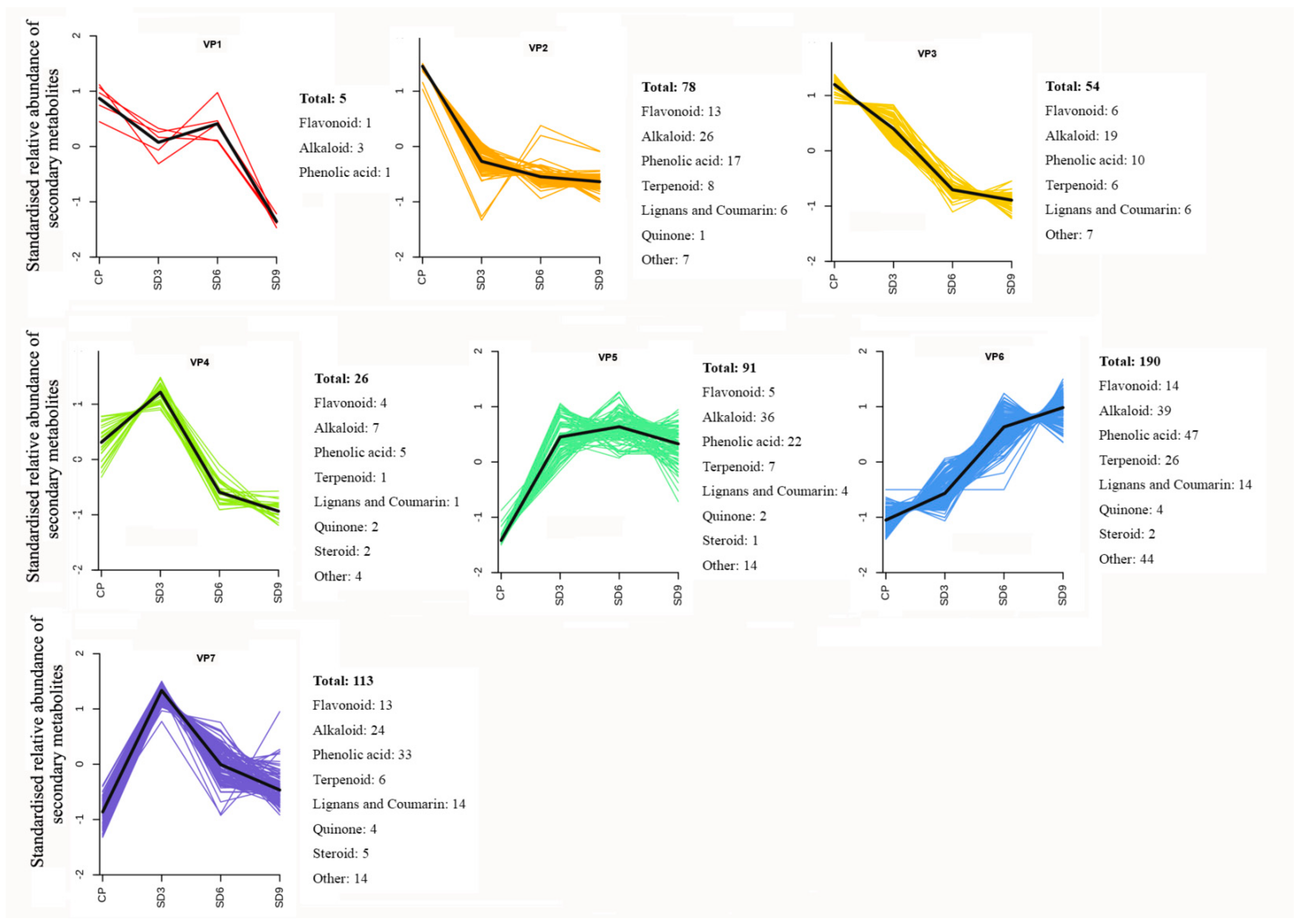
2.3. Variation Patterns in the Relative Abundance of Secondary Metabolites
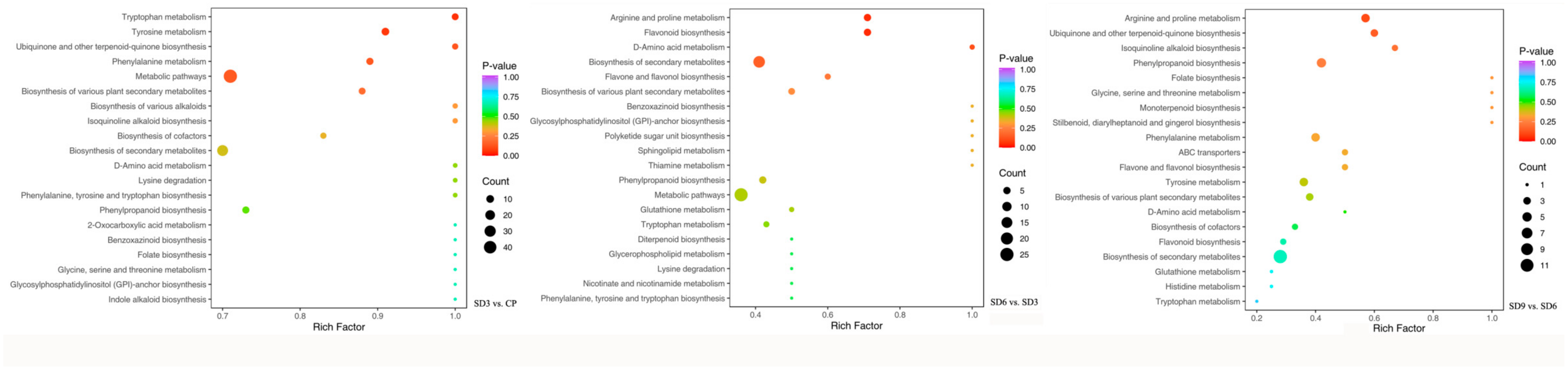
2.4. Annotation and Functional Classification of Secondary Metabolites
2.5. Processing-Induced Changes in the Functional Components of P. cyrtonema Rhizome
3. Discussion
4. Materials and Methods
4.1. Plant Material Selection and Pre-Treatment
4.2. Polygonatum Rhizome Processing
4.3. Sample Preparation for Metabolome Analysis
4.4. UPLC-MS/MS and ESI-Q TRAP-MS/MS
4.5. Metabolite Annotation
4.6. Multivariate Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yudaev, P.A.; Chistyakov, E.M. Progress in dental materials: Application of natural ingredients. Russ. Chem. Rev. 2024, 93, RCR5108. [Google Scholar] [CrossRef]
- Chen, X.; Tamura, M.N. Flora of China; Science Press: Beijing, China, 2000; Volume 24, pp. 223–232. [Google Scholar]
- National Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; China Medical Science & Technology Press: Beijing, China, 2020; Volume 4, pp. 387–388. [Google Scholar]
- Zhang, J.; Ma, B.; Yang, Y.; Sun, G. Development of steroidal saponins & pharmacologic activity from plants of Polygonatun genus. Chin. Pharm. J. 2006, 41, 330–332. (In Chinese) [Google Scholar]
- Zhang, J.; Wang, Y.; Yang, W.; Yang, M.; Zhang, J. Research progress in chemical constituents in plants of Polygonatum and their pharmacological effects. China J. Chin. Mater. Medica 2019, 44, 1989–2008. (In Chinese) [Google Scholar]
- Bai, J.B.; Ge, J.C.; Zhang, W.J.; Liu, W.; Luo, J.P.; Xu, F.Q.; Wu, D.L.; Xie, S.Z. Physicochemical, morpho-structural, and biological characterization of polysaccharides from three Polygonatum spp. RSC Adv. 2021, 11, 37952–37965. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Zhao, C.; Li, X.; Gao, Q.; Huang, L.; Xiao, P.; Gao, W. The genus Polygonatum: A review of ethnopharmacology, phytochemistry and pharmacology. J. Ethnopharmacol. 2018, 214, 274–291. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.D.; Li, X.Y.; Deng, Y.Y.; Zha, X.Q.; Pan, L.H.; Li, Q.M.; Luo, J.P. Polygonatum cyrtonema Hua. polysaccharide exhibits anti-fatigue activity via regulating osteocalcin signaling. Int. J. Biol. Macromol. 2021, 175, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, H.; Kopp, B.; Krenn, L.; Guo, D.; Sendker, J. Traditional chinese herbal medicinal preparation: Invoking the butterfy efect. Science 2015, 350, S64–S66. [Google Scholar]
- Wu, X.; Wang, S.; Lu, J.; Jing, Y.; Li, M.; Cao, J.; Bian, B.; Hu, C. Seeing the unseen of Chinese herbal medicine processing (Paozhi): Advances in new perspectives. Chin. Med. 2018, 13, 4. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, L.; Zhao, Q.; Bao, K.; Jiang, C. Research progress on Polygonatum cyrtonema processed by nine times steaming and nine times shining. Chin. Tradit. Herb. Drugs 2020, 51, 5631–5637. (In Chinese) [Google Scholar]
- Zheng, X.; Xu, C.; Jin, C.; Liu, J.; Liu, C.; Li, L. Research on relationship between processing degree and internal and external quality of Polygonatum cyrtonema processed by “nine-steaming and nine-suncuring” based on color change. Chin. Tradit. Herb. Drugs 2022, 53, 1719–1729. (In Chinese) [Google Scholar]
- Xu, R.; Liang, J.; Yu, N.; Wu, H.; Wu, Z.; Zhou, A. Structural change of polysaccharide constituents from Polygonatum cyrtonema in Jiuhua Mountain after processing. J. Anhui Univ. Chin. Med. 2021, 40, 91–96. (In Chinese) [Google Scholar]
- Guo, T.; Wang, R.; Song, Y.; Ye, J.; Jiao, H.; Yu, D.; Chang, H. Chemical components dynamic variation of root of Polygonati rhizoma during a nine-time repeat of the steaming and sundrying process. J. Pharm. Res. 2022, 41, 220–229. (In Chinese) [Google Scholar]
- Yao, X.; Deng, Z.; Li, H.; Zhang, B. Effect of processing cycles on the composition of Polygonatum cyrtonema Hua during nine-steam-nine-bask processing. Food Biosci. 2022, 50, 102081. [Google Scholar] [CrossRef]
- Jiang, T.; Wu, T.; Gao, P.; Wang, L.; Yang, X.; Chen, X.; Chen, Y.; Yue, C.; Liang, K.; Tang, L.; et al. Research on processing-induced chemical variations in Polygonatum cyrtonema rhizome by integrating metabolomics and glycomics. Molecules 2022, 27, 5869. [Google Scholar] [CrossRef] [PubMed]
- Keller, N.P.; Turner, G.; Bennett, J.W. Fungal secondary metabolism-from biochemistry to genomics. Nat. Rev. Microbiol. 2005, 3, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A. Natural products and plant disease resistance. Nature 2001, 411, 843–847. [Google Scholar] [CrossRef]
- Oksman-Caldentey, K.M.; Inze, D. Plant cell factories in the post-genomic era: New ways to produce designer secondary metabolites. Trends Plant Sci. 2004, 9, 433–440. [Google Scholar] [CrossRef]
- Kutchan, T.; Dixon, R.A. Physiology and metabolism-Secondary metabolism: Nature’s chemical reservoir under deconvolution. Curr. Opin. Plant Biol. 2005, 8, 227–229. [Google Scholar] [CrossRef]
- Seca, A.M.L.; Pinto, D.C.G.A. Biological potential and medical use of secondary. Metab. Med. 2019, 6, 66. [Google Scholar]
- Yang, D.; Du, X.; Yang, Z.; Liang, Z.; Guo, Z.; Liu, Y. Transcriptomics, proteomics, and metabolomics to reveal mechanisms underlying plant secondary metabolism. Eng. Life Sci. 2014, 14, 456–466. [Google Scholar] [CrossRef]
- Fiehn, O.; Kopka, J.; Dörmann, P.; Altmann, T.; Trethewey, R.N.; Willmitzer, L. Metabolite profiling for plant functional genomics. Nat. Biotechnol. 2000, 18, 1157–1161. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, O. Metabolomics-the link between genotypes and phenotypes. Plant Mol. Biol. 2002, 48, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.; Beale, M.; Fiehn, O.; Hardy, N.; Sumner, L.; Bino, R. Plant metabolomics: The missing link in functional genomics strategies. Plant Cell 2002, 14, 1437–1440. [Google Scholar] [CrossRef] [PubMed]
- Bino, R.J.; Hall, R.D.; Fiehn, O.; Kopka, J.; Saito, K.; Draper, J.; Nikolau, B.J.; Mendes, P.; Roessner-Tunali, U.; Beale, M.H.; et al. Potential of metabolomics as a functional genomics tool. Trends Plant Sci. 2004, 9, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Saito, K. Phytochemical genomics—A new trend. Curr. Opin. Plant Biol. 2013, 16, 373–380. [Google Scholar] [CrossRef]
- La, G.; Hao, X.; Li, X.; Ou, M.; Yang, T. Application of metabolomics in plant research. Bot. Res. 2016, 5, 26–33. (In Chinese) [Google Scholar]
- Cai, B.; Qin, K.; Hao, W.; Hao, C.; Lu, T.; Zhang, X. Chemical mechanism during Chinese medicine processing. Prog. Chem. 2012, 77, 637–649. [Google Scholar] [CrossRef]
- Liang, Z.; Pan, Y.; Qiu, L.; Wu, X.; Xu, X.; Shu, Y.; Yuan, Q. Analysis on chemical components changes of Polygonati rhizoma in processing of nine times steaming and nine times sunning by UPLC-Q-TOF-MS/MS. Chin. Tradit. Herb. Drugs 2022, 53, 4948–4957. (In Chinese) [Google Scholar]
- Matsuura, H.N.; Fett-Neto, A.G. Plant alkaloids: Main features, toxicity, and mechanisms of action. In Plant Toxins. Toxinology; Gopalakrishnakone, P., Carlini, C., Ligabue-Braun, R., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 1–15. [Google Scholar]
- Adibah, K.Z.M.; Azzreena, M.A. Plant toxins: Alkaloids and their toxicities. GSC Biol. Pharm. Sci. 2019, 6, 21–29. [Google Scholar]
- Molyneux, R.J.; Panter, K.E. Alkaloids toxic to livestock. Alkaloids 2009, 67, 143–216. [Google Scholar]
- Singh, M.; Kaur, M.; Silakari, O. Flavones, an important scaffold for medicinal chemistry. Eur. J. Med. Chem. 2014, 84, 206–239. [Google Scholar] [CrossRef] [PubMed]
- Chaabana, H.; Ioannoua, I.; Parisb, C.; Charbonnela, C.; Ghoula, M. The photostability of flavanones, flavonols and flavones and evolution of their antioxidant activity. J. Photochem. Photobiol. A Chem. 2017, 336, 131–139. [Google Scholar] [CrossRef]
- Tao, A.; Zhang, X.; Du, Z.; Zhao, F.; Xia, C.; Duan, B. Research progress on flavonoids in plants of Polygonatum Mill. and their pharmacological activities. Chin. Tradit. Herb. Drugs 2018, 49, 2163–2171. [Google Scholar]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- El Aziz, M.M.A.; Ashour, A.S.; Melad, A.S.G. A review on saponins from medicinal plants: Chemistry, isolation, and determination. J. Nanomed. Res. 2019, 7, 282–288. [Google Scholar]
- Kavya, N.M.; Adil, L.; Senthilkumar, P. A review on saponin biosynthesis and its transcriptomic resources in medicinal plants. Plant Mol. Biol. Report. 2021, 39, 833–840. [Google Scholar] [CrossRef]
- Shi, Y.; Si, D.; Chen, D.; Zhang, X.; Han, Z.; Yu, Q.; Liu, J.; Si, J. Bioactive compounds from Polygonatum genus as anti-diabetic agents with future perspectives. Food Chem. 2023, 408, 135183. [Google Scholar] [CrossRef]
- Chen, M.; Huang, Z.; Zhou, D.; Lu, Y.; Tang, Q.; Zou, H.; Shi, X.; Xie, H.; Zeng, J.; Zheng, Y. Effect of processing on amino acid composition and nutritional value of protein from Polygonatum. Food Ferment. Ind. 2024, 50, 170–177. (In Chinese) [Google Scholar]
- Wu, W.; Huang, N.; Huang, J.; Wang, L.; Wu, L.; Wang, Q.; Zhao, H. Effects of the steaming process on the structural properties and immunological activities of polysaccharides from Polygonatum cyrtonema. J. Funct. Foods 2022, 88, 104866. [Google Scholar] [CrossRef]
- Song, Q.; Chen, Y.; Shao, Y.; Pu, W.; Ye, B.; Shi, X.; Shen, J.; Li, H. Investigation of the Functional Components in Health Beverages Made from Polygonatum cyrtonema Rhizomes Provides Primary Evidence to Support Their Claimed Health Benefits. Metabolites 2024, 14, 376. [Google Scholar] [CrossRef]
- Chen, W.; Gong, L.; Guo, Z.; Wang, W.; Zhang, H.; Liu, X.; Luo, J. A novel integrated method for large-scale detection, identification, and quantification of widely targeted metabolites: Application in the study of rice metabolomics. Mol. Plant 2013, 6, 1769–1780. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Wang, S.; Huang, Z.; Zhang, S.; Liao, Q.; Zhang, C.; Cao, X. Rewiring of the fruit metabolome in tomato breeding. Cell 2018, 172, 249–261.e12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qiu, X.; Tan, Q.; Xiao, Q.; Mei, S. A comparative metabolomics study of flavonoids in Radish with different skin and flesh colors (Raphanus sativus L.). J. Agric. Food Chem. 2020, 68, 14463–14470. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Jiang, Y.; Liu, C.; Shi, L.; Li, J.; Zeng, Y.; Guo, L.; Wang, S. Identification of medicinal compounds of Fagopyri dibotryis rhizome from different origins and its varieties using UPLC-MS/MS based metabolomics. Metabolites 2022, 12, 790. [Google Scholar] [CrossRef] [PubMed]
- Thévenot, E.A.; Roux, A.; Xu, Y.; Ezan, E.; Junot, C. Analysis of the human adult urinary metabolome variations with age, body mass index, and gender by implementing a comprehensive workflow for univariate and OPLS statistical analyses. J. Proteome Res. 2015, 14, 3322–3335. [Google Scholar] [CrossRef]





| Class | DASM//Log2 FC | ||
|---|---|---|---|
| SD3 vs. CP | SD6 vs. SD3 | SD9 vs. SD6 | |
| Alkaloids | Cephalanthrin A//12.57 1-Acetyl-β-carboline//8.98 (R)-1,2,3,4-Tetrahydro-3-carboxy-2-carboline//8.52 N-benzoyl-2-aminoethyl-β-D-glucopyranoside//8.18 Valerine//7.27 (1R,3S)-1-Methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid//6.6 | 4′-O-Methylnorbelladine//4.14 Casuarine analogue//−3.2 Folicanthine//−3.66 | |
| Phenolic acids | Chlorogenic acid methyl ester//9.32 4-O-Caffeoylquinic acid methyl ester//7.92 3-hydroxyphenylacetic acid//7.38 methyl 5-caffeoylquinate//6.91 4-Hydroxybenzoic acid//6.87 1-O-Feruloylquinic acid//6.8 2,3-Dihydroxybenzoic acid//6.54 | Ethyl malto//4.14 Antiarol; 3,4,5-Trimethoxyphenol//3.64 methyl 5-caffeoylquinate//−3.33 1-O-Feruloylquinic acid//−3.41 3-O-Feruloylquinic acid//−3.48 4-O-Caffeoylquinic acid methyl ester//−3.64 p-Hydroxyphenyl 6-O-(E)-caffeoyl-β-D-allopyranoside//−4.7 | 1-O-p-Coumaroylquinic acid//−2.64 4-O-Caffeoylquinic acid methyl ester//−2.8 Chlorogenic acid methyl ester//−2.85 1-O-Feruloylquinic acid//−2.9 2-Hydroxycinnamic acid//−3.04 2-(Formylamino)benzoic acid//−3.13 α-Hydroxycinnamic Acid//−3.28 3-Hydroxycinnamic Acid//−3.33 Methyl 5-caffeoylquinate//−3.43 3,4,5-Trimethoxycinnamic acid//−3.73 |
| Flavonoids | Sesuvioside A//7.41 | 3-Hydroxy-4′,5,7-Trimethoxyflavanone//4.03 Butin; 3′,4′-Trihydroxyflavanone//3.41 Sesuvioside A//−3.25 Isorhamnetin-3-O-neohesperidoside//−3.73 3,5,7-Trihydroxy-6,8-dimethyl-3-(4′-hydroxybenzyl)-chroman-4-one (Polygonatone C)//−4.23 | 3-[(3,4-dihydroxyphenyl)methylidene]-5,7-dihydroxy-6-methoxy-2h-1-benzopyran-4-one glucosyl rhamnoside//−2.72 Apigenin-6-C-(2″-glucosyl)arabinoside//−2.78 Tricin (5,7,4′-Trihydroxy-3′,5′-dimethoxyflavone)//−2.95 |
| Steroids | Spirost-5-en-12-one-3-O-glucosyl(1→2)glucosyl(1→4)galactoside (Pratioside D1)//−4.0 | Spirost-5-ene-3,27-diol-27-O-glucoside-3-O-[rhamnosyl(1→4)]glucoside (Polygonatoside D)//−2.86 27-Hydroxyspirost-5-en-3-yl-O-rhamnosyl-(1→2)-O-[glucosyl-(1→6)]-glucoside//−3.17 Spirost-5-en-3-ol-3-O-glucosyl(1→2)glucosyl(l→4)galactoside (Neosibiricoside D)//−3.32 | |
| Lignans and coumarins | Phellodenol E//8.25 Guaiacylglycerol-β-guaiacyl ether//6.69 | 7,8-Dihydroxy-4-methylcoumarin//5.33 7-Hydroxycoumarin; Umbelliferone//3.38 5,7-dihydroxy-4-phenylcoumarin//3.22 | Phellodenol E//−3.12 |
| Others | 2,5-Dihydroxybenzaldehyde//7.4 Protocatechualdehyde//6.96 4-Methyl-5-thiazoleethanol//6.75 4-Hydroxybenzaldehyde//6.46 | Squamocin K//3.56 | 4-hydroxyphenyl acrylaldehyde//−3.67 4-Methylbenzaldehyde//−3.92 3-Methylbenzaldehyde//−4.11 |
| Total | Up: 20; down: 0 | Up: 9; down: 11 | Up: 0; down: 20 |
| Secondary Metabolites | Comparison Group | Total Number | Class | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Alkaloids | Phenolic Acids | Flavonoids | Terpenoids | Lignans and Coumarins | Quinones | Steroids | Others | |||
| Newly formed | SD3 vs. CP | 164 | 45 | 42 | 23 | 13 | 15 | 8 | 5 | 13 |
| SD6 vs. SD3 | 16 | 6 | 3 | 4 | 1 | 2 | ||||
| SD9 vs. SD6 | 4 | 1 | 1 | 2 | ||||||
| Reduced | SD3 vs. CP | 3 | 1 | 1 | 1 | |||||
| SD6 vs. SD3 | 33 | 10 | 6 | 11 | 1 | 1 | 1 | 3 | ||
| SD9 vs. SD6 | 30 | 9 | 10 | 4 | 2 | 3 | 1 | 1 | ||
| Comparison Group | Biosynthesis of Secondary Metabolites | Number of DASMs | Amino acid Metabolism | Number of DASMs |
|---|---|---|---|---|
| SD3 vs. CP | Ubiquinone and other terpenoid-quinone (ko00130) | 4↑; 1↓ | Tryptophan (ko00380) | 4↑; 3↓ |
| Various plant secondary metabolites (ko00999) | 5↑; 2↓ | Tyrosine (ko00350) | 7↑; 3↓ | |
| Various alkaloids (ko00996) | 3↑ | Phenylalanine (ko00360) | 8↑ | |
| Isoquinoline alkaloid (ko00950) | 3↑ | D-Amino acid (ko00470) | 2↓ | |
| Secondary metabolites (ko01110) | 23↑; 5↓ | Lysine degradation (ko00310) | 1↑; 1↓ | |
| Indole alkaloid (ko00901) | 1↑ | Phenylalanine, tyrosine and tryptophan (ko00400) | 1↑; 1↓ | |
| Monoterpenoid (ko00902) | 1↑ | Glycine, serine and threonine (ko00260) | 1↑ | |
| Diterpenoid (ko00904) | 1↓ | Lysine (ko00300) | 1↑ | |
| Isoflavonoid (ko00943) | 1↑ | Biosynthesis of amino acids (ko01230) | 1↑ | |
| Flavonoid (ko00941) | 2↑ | Histidine (ko00340) | 2↑ | |
| Tropane, piperidine and pyridine alkaloid (ko00960) | 1↑; 2↓ | Arginine and proline (ko00330) | 2↓ | |
| SD6 vs. SD3 | Flavonoid biosynthesis (ko00941) | 5↑ | D-Amino acid (ko00470) | 2↓ |
| Secondary metabolites (ko01110) | 9↑; 9↓ | Tryptophan (ko00380) | 3↓ | |
| Flavone and flavonol (ko00944) | 3↓ | Phenylalanine, tyrosine and tryptophan (ko00400) | 1↓ | |
| Various plant secondary metabolites (ko00999) | 1↑; 3↓ | Histidine (ko00340) | 1↑ | |
| Diterpenoid (ko00904) | 1↑ | Tyrosine (ko00350) | 1↑; 1↓ | |
| Tropane, piperidine and pyridine alkaloid (ko00960) | 2↓ | Phenylalanine (ko00360) | 1↑ | |
| Isoflavonoid (ko00943) | 1↑ | |||
| Isoquinoline alkaloid (ko00950) | 1↓ | |||
| Ubiquinone and other terpenoid-quinone (ko00130) | 1↓ | |||
| SD9 vs. SD6 | Ubiquinone and other terpenoid-quinone (ko00130) | 3↓ | Arginine and proline (ko00330) | 4↓ |
| Isoquinoline alkaloid (ko00950) | 2↓ | Glycine, serine and threonine (ko00260) | 1↓ | |
| Monoterpenoid (ko00902) | 1↓ | Phenylalanine (ko00360) | 4↓ | |
| Flavone and flavonol (ko00944) | 2↓ | Tyrosine (ko00350) | 4↓ | |
| Various plant secondary metabolites (ko00999) | 1↑; 2↓ | D-Amino acid (ko00470) | 1↓ | |
| Flavonoid (ko00941) | 1↑; 1↓ | Histidine (ko00340) | 1↓ | |
| Secondary metabolites (ko01110) | 2↑; 10↓ | Tryptophan (ko00380) | 1↓ | |
| Tropane, piperidine and pyridine alkaloid (ko00960) | 1↓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, J.; Pu, W.; Song, Q.; Ye, B.; Shi, X.; Chen, Y.; Yu, Y.; Li, H. Traditional Processing Can Enhance the Medicinal Effects of Polygonatum cyrtonema by Inducing Significant Chemical Changes in the Functional Components in Its Rhizomes. Pharmaceuticals 2024, 17, 1074. https://doi.org/10.3390/ph17081074
Shen J, Pu W, Song Q, Ye B, Shi X, Chen Y, Yu Y, Li H. Traditional Processing Can Enhance the Medicinal Effects of Polygonatum cyrtonema by Inducing Significant Chemical Changes in the Functional Components in Its Rhizomes. Pharmaceuticals. 2024; 17(8):1074. https://doi.org/10.3390/ph17081074
Chicago/Turabian StyleShen, Jianjun, Weiting Pu, Qiyan Song, Bihuan Ye, Xiaoxiao Shi, Youwu Chen, Yefei Yu, and Haibo Li. 2024. "Traditional Processing Can Enhance the Medicinal Effects of Polygonatum cyrtonema by Inducing Significant Chemical Changes in the Functional Components in Its Rhizomes" Pharmaceuticals 17, no. 8: 1074. https://doi.org/10.3390/ph17081074
APA StyleShen, J., Pu, W., Song, Q., Ye, B., Shi, X., Chen, Y., Yu, Y., & Li, H. (2024). Traditional Processing Can Enhance the Medicinal Effects of Polygonatum cyrtonema by Inducing Significant Chemical Changes in the Functional Components in Its Rhizomes. Pharmaceuticals, 17(8), 1074. https://doi.org/10.3390/ph17081074







