Unlocking Cholesterol Metabolism in Metabolic-Associated Steatotic Liver Disease: Molecular Targets and Natural Product Interventions
Abstract
1. Introduction
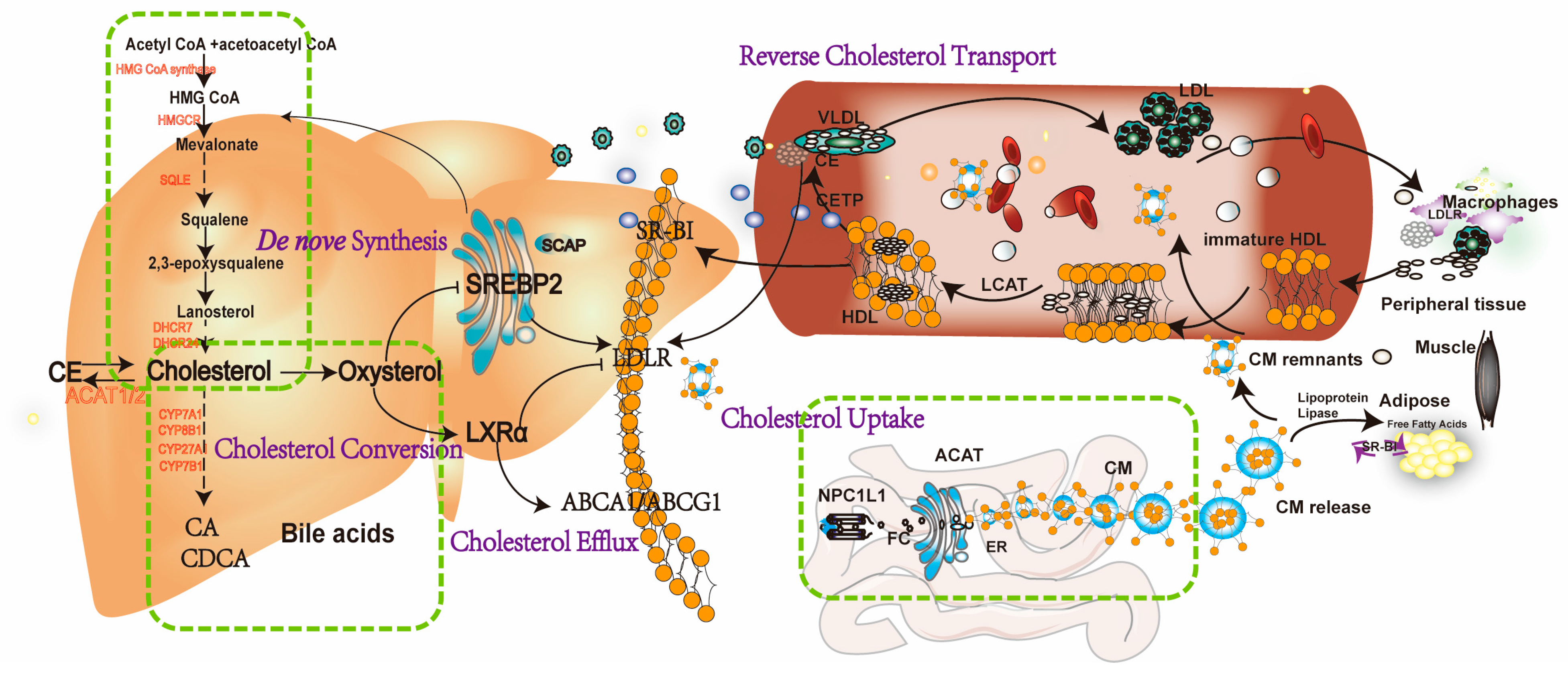
2. Cholesterol Homeostasis in Health
2.1. Intestinal Cholesterol Absorption and Blood Release
2.2. Reverse Cholesterol Transport (RCT)
2.3. Endogenous Cholesterol De Novo Synthesis
2.4. Cholesterol Conversion to Bile Acids (BAs)
3. The Role of Key Targets in Cholesterol Metabolism in MASLD
3.1. Cholesterol Absorption in MASLD
3.1.1. NPC1L1
3.1.2. LDLR
3.2. RCT in MASLD
3.2.1. LCAT
3.2.2. CETP
3.2.3. SB-RI
3.3. Cholesterol Biosynthesis in MASLD
HMGCR
3.4. Cholesterol Efflux in MASLD
LXRα
3.5. Cholesterol Conversion in MASLD
CYP7A1
4. Natural Product Targeting Cholesterol Metabolism for MASLD
4.1. Inhibiting Cholesterol Absorption
4.1.1. NPCL1L Inhibitors
4.1.2. LDLR Upregulation
4.2. Enhancing RCT
4.3. Inhibiting Cholesterol Biosynthesis
4.4. Modulating LXRα Pathways
4.5. Promoting BA Excretion via CYP7A1
5. Clinical Trials of Natural Products for the Treatment of MASLD
6. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACAT | Acyl-CoA acyltransferase |
| ALP | Alkaline phosphatase |
| ALT | Alanine aminotransferase |
| AMPK | AMP-activated protein kinase |
| APOA-I | Apolipoprotein A-I |
| AST | Aspartate aminotransferase |
| BMI | Body mass index |
| CA | Cholic acid |
| CDCA | Chenodeoxycholic acid |
| CE | Cholesteryl ester |
| CETP | Cholesteryl ester transfer protein |
| CM | Chylomicron |
| CYP7A1 | Cholesterol 7 alpha-hydroxylase |
| ER | Endoplasmic reticulum |
| ERK | Extracellular regulated protein kinase |
| Ev | Extracellular vesicle |
| FBG | Fasting blood glucose |
| FBI | Fasting blood insulin |
| FC | Free cholesterol |
| FH | Familial hypercholesterolemia |
| FFA | Free fatty acid |
| HCV | Hepatitis C virus |
| HCC | Hepatocellular carcinoma |
| HDL | High-density lipoprotein |
| HFD | High-fat diet |
| HOMA-IR | Homeostasis model assessment-insulin resistance |
| HMG-CoA | 3-hydroxy-3-methylglutarylcoenzyme A |
| Insig1/2 | Insulin-induced gene 1/2 |
| LCAT | Lecithin cholesterol acyltransferase |
| LDLR | Low-density lipoprotein receptor |
| LPL | Lipoprotein lipase |
| LXRα | Liver X receptor α |
| MASLD | Metabolic-associated steatotic liver disease |
| MASH | Metabolic dysfunction-associated steatohepatitis |
| MetS | Metabolic syndrome |
| RIG-I | Retinoic acid-inducible gene-I |
| PCSK9 | Proprotein convertase subtilisin/kexin type 9 |
| RCT | Reverse cholesterol transport |
| SCAP | SREBP cleavage-activating protein |
| SHP | Small heterodimer partner |
| SR-BI | Scavenger receptor class B type I |
| SREBP2 | Sterol regulatory element-binding protein 2 |
| TC | Total cholesterol |
| T2DM | Type 2 diabetes mellitus |
| VLDL | Very-low-density lipoprotein |
| WD | Western diet |
References
- Riazi, K.; Azhari, H.; Charette, J.H.; Underwood, F.E.; King, J.A.; Afshar, E.E.; Swain, M.G.; Congly, S.E.; Kaplan, G.G.; Shaheen, A.A. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2022, 7, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Bugianesi, E.; Petta, S. Nafld/Nash. J. Hepatol. 2022, 77, 549–550. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Zheng, L.; Jiang, S.; Yang, H.; Guo, J.; Jiang, L.Y.; Li, T.; Zhang, H.; Bai, Y.; Lou, Y.; et al. Exhaustion- associated cholesterol deficiency dampens the cytotoxic arm of antitumor immunity. Cancer Cell 2023, 41, 1276–1293. [Google Scholar] [CrossRef] [PubMed]
- Caballero, F.; Fernandez, A.; De Lacy, A.M.; Fernandez-Checa, J.C.; Caballeria, J.; Garcia-Ruiz, C. Enhanced free cholesterol, SREBP-2 and StAR expression in human NASH. J. Hepatol. 2009, 50, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Puri, P.; Baillie, R.A.; Wiest, M.M.; Mirshahi, F.; Choudhury, J.; Cheung, O.; Sargeant, C.; Contos, M.J.; Sanyal, A.J. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology 2007, 46, 1081–1090. [Google Scholar] [CrossRef]
- Liang, J.Q.; Teoh, N.; Xu, L.; Pok, S.; Li, X.; Chu, E.S.H.; Chiu, J.; Dong, L.; Arfianti, E.; Haigh, W.G.; et al. Dietary cholesterol promotes steatohepatitis related hepatocellular carcinoma through dysregulated metabolism and calcium signaling. Nat. Commun. 2018, 9, 4490. [Google Scholar] [CrossRef]
- Walenbergh, S.M.; Koek, G.H.; Bieghs, V.; Shiri-Sverdlov, R. Non-alcoholic steatohepatitis: The role of oxidized low-density lipoproteins. J. Hepatol. 2013, 58, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Duewell, P.; Kono, H.; Rayner, K.J.; Sirois, C.M.; Vladimer, G.; Bauernfeind, F.G.; Abela, G.S.; Franchi, L.; Nunez, G.; Schnurr, M.; et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010, 464, 1357–1361. [Google Scholar] [CrossRef]
- Clayton, Z.S.; Fusco, E.; Kern, M. Egg consumption and heart health: A review. Nutrition 2017, 37, 79–85. [Google Scholar] [CrossRef]
- Soliman, G.A. Dietary Cholesterol and the Lack of Evidence in Cardiovascular Disease. Nutrients 2018, 10, 780. [Google Scholar] [CrossRef]
- Luo, J.; Yang, H.; Song, B.L. Mechanisms and regulation of cholesterol homeostasis. Nat. Rev. Mol. Cell. Biol. 2020, 21, 225–245. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Stahel, P.; Lewis, G.F. Regulation of Chylomicron Secretion: Focus on Post-Assembly Mechanisms. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 487–501. [Google Scholar] [CrossRef]
- Donetti, E.; Hultin, M.; Soma, M.R.; Olivecrona, T. Barberi Conversion of chylomicrons into remnants. Atherosclerosis 1998, 141, S25–S29. [Google Scholar]
- Shrestha, S.; Wu, B.J.; Guiney, L.; Barter, P.J.; Rye, K.A. Cholesteryl ester transfer protein and its inhibitors. J. Lipid. Res. 2018, 59, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Howe, V.; Sharpe, L.J.; Prabhu, A.V.; Brown, A.J. New insights into cellular cholesterol acquisition: Promoter analysis of human HMGCR and SQLE, two key control enzymes in cholesterol synthesis. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 2017, 1862, 647–657. [Google Scholar] [CrossRef]
- Brown, M.S.; Radhakrishnan, A.; Goldstein, J.L. Retrospective on Cholesterol Homeostasis: The Central Role of Scap. Annu. Rev. Biochem. 2018, 87, 783–807. [Google Scholar] [CrossRef]
- Goldstein, J.L.; DeBose-Boyd, R.A.; Brown, M.S. Protein sensors for membrane sterols. Cell 2006, 124, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Gelissen, I.C.; Brown, A.J. An Overview of Cholesterol Homeostasis. Methods Mol. Biol. 2017, 1583, 1–6. [Google Scholar]
- Redinger, R.N. Nuclear receptors in cholesterol catabolism: Molecular biology of the enterohepatic circulation of bile salts and its role in cholesterol homeostasis. J. Lab. Clin. Med. 2003, 142, 7–20. [Google Scholar] [CrossRef]
- Jia, W.; Xie, G.; Jia, W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 111–128. [Google Scholar] [CrossRef]
- Cai, J.; Rimal, B.; Jiang, C.; Chiang, J.Y.L.; Patterson, A.D. Bile acid metabolism and signaling, the microbiota, and metabolic disease. Pharmacol. Ther. 2022, 237, 108238. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Wang, J.; Qi, W.; Miao, H.H.; Cao, J.; Qu, Y.X.; Li, B.L.; Song, B.L. The cholesterol absorption inhibitor ezetimibe acts by blocking the sterol-induced internalization of NPC1L1. Cell Metab. 2008, 7, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.; Raman, G.; Vishwanathan, R.; Jacques, P.F.; Johnson, E.J. Dietary cholesterol and cardiovascular disease: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2015, 102, 276–294. [Google Scholar] [CrossRef] [PubMed]
- Di Rocco, M.; Pisciotta, L.; Madeo, A.; Bertamino, M.; Bertolini, S. Long term substrate reduction therapy with ezetimibe alone or associated with statins in three adult patients with lysosomal acid lipase deficiency. Orphanet J. Rare Dis. 2018, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.R., Jr.; Zhu, L.J.; Hoos, L.M.; Tetzloff, G.; Maguire, M.; Liu, J.; Yao, X.; Iyer, S.P.; Lam, M.H.; Lund, E.G.; et al. Niemann-Pick C1 Like 1 (NPC1L1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. J. Biol. Chem. 2004, 279, 33586–33592. [Google Scholar] [CrossRef] [PubMed]
- Temel, R.E.; Tang, W.; Ma, Y.; Rudel, L.L.; Willingham, M.C.; Ioannou, Y.A.; Davies, J.P.; Nilsson, L.M.; Yu, L. Hepatic Niemann-Pick C1-like 1 regulates biliary cholesterol concentration and is a target of ezetimibe. J. Clin. Investig. 2007, 117, 1968–1978. [Google Scholar] [CrossRef] [PubMed]
- Simonen, P.; Kotronen, A.; Hallikainen, M.; Sevastianova, K.; Makkonen, J.; Hakkarainen, A.; Lundbom, N.; Miettinen, T.A.; Gylling, H.; Yki-Jarvinen, H. Cholesterol synthesis is increased and absorption decreased in non-alcoholic fatty liver disease independent of obesity. J. Hepatol. 2011, 54, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.B.; Jang, K.; Jun, D.W.; Lee, B.H.; Shin, K.J. Expression of liver X receptor correlates with intrahepatic inflammation and fibrosis in patients with nonalcoholic fatty liver disease. Dig. Dis. Sci. 2014, 59, 2975–2982. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.B.; Jun, D.W.; Jang, K.; Lee, B.H.; Shin, K.J. Duodenal Niemann-Pick C1-like 1 expression was negatively correlated with liver X receptor expression in nonalcoholic fatty liver disease. Korean J. Intern. Med. 2019, 34, 777–784. [Google Scholar] [CrossRef]
- Toyoda, Y.; Takada, T.; Yamanashi, Y.; Suzuki, H. Pathophysiological importance of bile cholesterol reabsorption: Hepatic NPC1L1-exacerbated steatosis and decreasing VLDL-TG secretion in mice fed a high-fat diet. Lipids Health Dis. 2019, 18, 234. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, P.; Zhang, B.; Ding, Y.; Lei, S.; Hou, Y.; Guan, X.; Li, Q. Hepatic NPC1L1 overexpression attenuates alcoholic autophagy in mice. Mol. Med. Rep. 2019, 20, 3224–3232. [Google Scholar] [CrossRef]
- Yamanashi, Y.; Takada, T.; Tanaka, Y.; Ogata, Y.; Toyoda, Y.; Ito, S.M.; Kitani, M.; Oshida, N.; Okada, K.; Shoda, J.; et al. Hepatic Niemann-Pick C1-Like 1 exacerbates non-alcoholic fatty liver disease by re-absorbing specific biliary oxysterols. Biomed. Pharmacother. 2022, 156, 113877. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Han, D.H.; Nam, K.T.; Park, J.S.; Kim, S.H.; Lee, M.; Kim, G.; Min, B.S.; Cha, B.S.; Lee, Y.S.; et al. Ezetimibe, an NPC1L1 inhibitor, is a potent Nrf2 activator that protects mice from diet-induced nonalcoholic steatohepatitis. Free Radic. Biol. Med. 2016, 99, 520–532. [Google Scholar] [CrossRef]
- Fraunberger, P.; Gröne, E.; Gröne, H.J.; Drexel, H.; Walli, A.K. Ezetimibe reduces cholesterol content and NF-kappaB activation in liver but not in intestinal tissue in guinea pigs. J. Inflamm. 2017, 14, 3. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, H.X.; Zhang, M.; Long, S.Y.; Tuo, Q.H.; Tian, Y.; Chen, J.X.; Zhang, C.P.; Liao, D.F. Cholesterol in LDL receptor recycling and degradation. Clin. Chim. Acta 2020, 500, 81–86. [Google Scholar] [CrossRef]
- Yu, Q.; Zheng, H.; Zhang, Y. Inducible degrader of LDLR: A potential novel therapeutic target and emerging treatment for hyperlipidemia. Vascul. Pharmacol. 2021, 140, 106878. [Google Scholar] [CrossRef]
- Huang, Y.W.; Wang, L.T.; Zhang, M.; Nie, Y.; Yang, J.B.; Meng, W.L.; Wang, X.J.; Sheng, J. Caffeine can alleviate non-alcoholic fatty liver disease by augmenting LDLR expression via targeting EGFR. Food Funct. 2023, 14, 3269–3278. [Google Scholar] [CrossRef]
- Wang, F.; Yao, W.; Yu, D.; Hao, Y.; Wu, Y.; Zhang, X. Protective role of thymoquinone in hyperlipidemia-induced liver injury in LDL-R(−/−)mice. BMC Gastroenterol. 2023, 23, 276. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.; Mahmood, S.; Morrice, N.; Kamli-Salino, S.; Dekeryte, R.; Hoffmann, P.A.; Doherty, M.K.; Whitfield, P.D.; Delibegović, M.; Mody, N. Fenretinide inhibits obesity and fatty liver disease but induces Smpd3 to increase serum ceramides and worsen atherosclerosis in LDLR(−/−) mice. Sci. Rep. 2023, 13, 3937. [Google Scholar] [CrossRef]
- Singh, A.B.; Kan, C.F.; Shende, V.; Dong, B.; Liu, J. A novel posttranscriptional mechanism for dietary cholesterol-mediated suppression of liver LDL receptor expression. J. Lipid Res. 2014, 55, 1397–1407. [Google Scholar] [CrossRef]
- He, Y.; Rodrigues, R.M.; Wang, X.; Seo, W.; Ma, J.; Hwang, S.; Fu, Y.; Trojnár, E.; Mátyás, C.; Zhao, S.; et al. Neutrophil-to-hepatocyte communication via LDLR-dependent miR-223-enriched extracellular vesicle transfer ameliorates nonalcoholic steatohepatitis. J. Clin. Investig. 2021, 131, e141513. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Lee, S.P.; Linsley, P.S.; Gersuk, V.; Yeh, M.M.; Chen, Y.Y.; Peng, Y.J.; Dutta, M.; Mascarinas, G.; Molla, B.; et al. Pcsk9 Deletion Promotes Murine Nonalcoholic Steatohepatitis and Hepatic Carcinogenesis: Role of Cholesterol. Hepatol. Commun. 2022, 6, 780–794. [Google Scholar] [CrossRef] [PubMed]
- Calabresi, L.; Franceschini, G. Lecithin:cholesterol acyltransferase, high-density lipoproteins, and atheroprotection in humans. Trends Cardiovasc. Med. 2010, 20, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Tanigawa, H.; Billheimer, J.T.; Tohyama, J.; Fuki, I.V.; Ng, D.S.; Rothblat, G.H.; Rader, D.J. Lecithin: Cholesterol acyltransferase expression has minimal effects on macrophage reverse cholesterol transport in vivo. Circulation 2009, 120, 160–169. [Google Scholar] [CrossRef]
- Kuroda, M.; Bujo, H.; Yokote, K.; Murano, T.; Yamaguchi, T.; Ogura, M.; Ikewaki, K.; Koseki, M.; Takeuchi, Y.; Nakatsuka, A.; et al. Current Status of Familial LCAT Deficiency in Japan. J. Atheroscler. Thromb. 2021, 28, 679–691. [Google Scholar] [CrossRef]
- Janac, J.; Zeljkovic, A.; Jelic-Ivanovic, Z.; Dimitrijevic-Sreckovic, V.; Miljkovic, M.; Stefanovic, A.; Munjas, J.; Vekic, J.; Kotur-Stevuljevic, J.; Spasojević-Kalimanovska, V. The association between lecithin-cholesterol acyltransferase activity and fatty liver index. Ann. Clin. Biochem. 2019, 56, 583–592. [Google Scholar] [CrossRef]
- Nass, K.J.; van den Berg, E.H.; Gruppen, E.G.; Dullaart, R.P.F. Plasma lecithin:cholesterol acyltransferase and phospholipid transfer protein activity independently associate with nonalcoholic fatty liver disease. Eur. J. Clin. Investig. 2018, 48, e12988. [Google Scholar] [CrossRef] [PubMed]
- Bril, F.; Pearce, R.W.; Collier, T.S.; McPhaul, M.J. Differences in HDL-Bound Apolipoproteins in Patients with Advanced Liver Fibrosis Due to Nonalcoholic Fatty Liver Disease. J. Clin. Endocrinol. Metab. 2022, 108, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; van der Tuin, S.; Tjeerdema, N.; van Dam, A.D.; Rensen, S.S.; Hendrikx, T.; Berbee, J.F.; Atanasovska, B.; Fu, J.; Hoekstra, M.; et al. Plasma cholesteryl ester transfer protein is predominantly derived from Kupffer cells. Hepatology 2015, 62, 1710–1722. [Google Scholar] [CrossRef] [PubMed]
- Barkowski, R.S.; Frishman, W.H. HDL metabolism and CETP inhibition. Cardiol. Rev. 2008, 16, 154–162. [Google Scholar] [CrossRef]
- Doggrell, S.A. The failure of torcetrapib: Is there a case for independent preclinical and clinical testing? Expert Opin. Pharmacother. 2008, 9, 875–878. [Google Scholar] [CrossRef] [PubMed]
- Rhainds, D.; Arsenault, B.J.; Brodeur, M.R.; Tardif, J.C. An update on the clinical development of dalcetrapib (RO4607381), a cholesteryl ester transfer protein modulator that increases HDL cholesterol levels. Future Cardiol. 2012, 8, 513–531. [Google Scholar] [CrossRef]
- Eyvazian, V.A.; Frishman, W.H. Evacetrapib: Another CETP Inhibitor for Dyslipidemia with No Clinical Benefit. Cardiol. Rev. 2017, 25, 43–52. [Google Scholar] [CrossRef]
- Nordestgaard, L.T.; Christoffersen, M.; Lauridsen, B.K.; Afzal, S.; Nordestgaard, B.G.; Frikke-Schmidt, R.; Tybjaerg-Hansen, A. Long-term Benefits and Harms Associated with Genetic Cholesteryl Ester Transfer Protein Deficiency in the General Population. JAMA Cardiol. 2022, 7, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.; Hemeda, S.A.; Albadrani, G.M.; Fadl, S.E.; Elgendey, F. Ameliorating effect of probiotic on nonalcoholic fatty liver disease and lipolytic gene expression in rabbits. Sci. Rep. 2023, 13, 6312. [Google Scholar] [CrossRef]
- Lucero, D.; Miksztowicz, V.; Gualano, G.; Longo, C.; Landeira, G.; Álvarez, E.; Zago, V.; Brites, F.; Berg, G.; Fassio, E.; et al. Nonalcoholic fatty liver disease associated with metabolic syndrome: Influence of liver fibrosis stages on characteristics of very low-density lipoproteins. Clin. Chim. Acta 2017, 473, 1–8. [Google Scholar] [CrossRef]
- Aller, R.; Izaola, O.; Primo, D.; de Luis, D. Cholesteryl Ester Transfer Protein Variant (RS1800777) with Liver Histology in Non-Alcoholic Fatty Liver Disease Patients. Ann. Nutr. Metab. 2018, 73, 265–270. [Google Scholar] [CrossRef]
- Perez-Robles, M.; Campos-Perez, W.; Rivera-Valdés, J.J.; Franco-Topete, R.A.; Navarrete-Medina, E.M.; Maldonado-González, M.; Ruíz-Madrigal, B.; Rodríguez-Reyes, S.C.; Martinez-Lopez, E. Elevated Serum Low-Density Lipoproteins-Cholesterol Levels and B1B2/B2B2 CETP Genotype Are Positively Associated with Nonalcoholic Fatty Liver Disease in Women with Gallstone Disease. Metab. Syndr. Relat. Disord. 2023, 21, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Liaw, Y.W.; Lin, C.Y.; Lai, Y.S.; Yang, T.C.; Wang, C.J.; Whang-Peng, J.; Liu, L.F.; Lin, C.P.; Nieh, S.; Lu, S.C.; et al. A vaccine targeted at CETP alleviates high fat and high cholesterol diet-induced atherosclerosis and non-alcoholic steatohepatitis in rabbit. PLoS ONE 2014, 9, e111529. [Google Scholar] [CrossRef] [PubMed]
- Rigotti, A.; Miettinen, H.E.; Krieger, M. The role of the high-density lipoprotein receptor SR-BI in the lipid metabolism of endocrine and other tissues. Endocr. Rev. 2003, 24, 357–387. [Google Scholar] [CrossRef]
- Knaack, D.A.; Chang, J.; Thomas, M.J.; Sorci-Thomas, M.G.; Chen, Y.; Sahoo, D. Scavenger receptor class B type I is required for efficient glucose uptake and metabolic homeostasis in adipocytes. bioRxiv 2023. [Google Scholar]
- Zago, V.H.S.; Scherrer, D.Z.; Parra, E.S.; Vieira, I.C.; Marson, F.A.L.; de Faria, E.C. Effects of SNVs in ABCA1, ABCG1, ABCG5, ABCG8, and SCARB1 Genes on Plasma Lipids, Lipoproteins, and Adiposity Markers in a Brazilian Population. Biochem. Genet. 2022, 60, 822–841. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, L.; Chen, J.; Song, H.; Xing, W.; Wang, Z.; Song, X.; Yang, H.; Zhao, W. Analysis of Intestinal Metabolites in SR-B1 Knockout Mice via Ultra-Performance Liquid Chromatography Quadrupole Time-of-Flight Mass Spectrometry. Molecules 2023, 28, 610. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Da Silva, J.R.; Reilly, M.; Billheimer, J.T.; Rothblat, G.H.; Rader, D.J. Hepatic expression of scavenger receptor class B type I (SR-BI) is a positive regulator of macrophage reverse cholesterol transport in vivo. J. Clin. Investig. 2005, 115, 2870–2874. [Google Scholar] [CrossRef] [PubMed]
- Xin, P.; Han, H.; Gao, D.; Cui, W.; Yang, X.; Ying, C.; Sun, X.; Hao, L. Alleviative effects of resveratrol on nonalcoholic fatty liver disease are associated with up regulation of hepatic low density lipoprotein receptor and scavenger receptor class B type I gene expressions in rats. Food Chem. Toxicol. 2013, 52, 12–18. [Google Scholar] [CrossRef]
- Qiu, Y.; Liu, S.; Chen, H.T.; Yu, C.H.; Teng, X.D.; Yao, H.T.; Xu, G.Q. Upregulation of caveolin-1 and SR-B1 in mice with non-alcoholic fatty liver disease. Hepatobiliary Pancreat. Dis. Int. 2013, 12, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Rein-Fischboeck, L.; Krautbauer, S.; Eisinger, K.; Pohl, R.; Meier, E.M.; Weiss, T.S.; Buechler, C. Hepatic scavenger receptor BI is associated with type 2 diabetes but unrelated to human and murine non-alcoholic fatty liver disease. Biochem. Biophys. Res. Commun. 2015, 467, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Soto-Acosta, R.; Bautista-Carbajal, P.; Cervantes-Salazar, M.; Angel-Ambrocio, A.H.; Del Angel, R.M. DENV up-regulates the HMG-CoA reductase activity through the impairment of AMPK phosphorylation: A potential antiviral target. PLoS Pathog. 2017, 13, e1006257. [Google Scholar] [CrossRef] [PubMed]
- Clarke, P.R.; Hardie, D.G. Regulation of HMG-CoA reductase: Identification of the site phosphorylated by the AMP-activated protein kinase in vitro and in intact rat liver. EMBO J. 1990, 9, 2439–2446. [Google Scholar] [CrossRef]
- Song, B.L.; Javitt, N.B.; DeBose-Boyd, R.A. Insig-mediated degradation of HMG CoA reductase stimulated by lanosterol, an intermediate in the synthesis of cholesterol. Cell Metab. 2005, 1, 179–189. [Google Scholar] [CrossRef]
- Kerr, T.A.; Davidson, N.O. Cholesterol and nonalcoholic fatty liver disease: Renewed focus on an old villain. Hepatology 2012, 56, 1995–1998. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Yan, L.T.; Yao, Z.; Xiong, G.Y. Biochanin A Regulates Cholesterol Metabolism Further Delays the Progression of Nonalcoholic Fatty Liver Disease. Diabetes Metab. Syndr. Obes. 2021, 14, 3161–3172. [Google Scholar] [CrossRef] [PubMed]
- Shatoor, A.S.; Al Humayed, S.; Almohiy, H.M. Astaxanthin attenuates hepatic steatosis in high-fat diet-fed rats by suppressing microRNA-21 via transactivation of nuclear factor erythroid 2-related factor 2. J. Physiol. Biochem. 2022, 78, 151–168. [Google Scholar] [CrossRef] [PubMed]
- Cominguez, D.C.; Park, Y.J.; Kang, Y.M.; Nugroho, A.; Kim, S.; An, H.J. Clitorin ameliorates western diet-induced hepatic steatosis by regulating lipogenesis and fatty acid oxidation in vivo and in vitro. Sci. Rep. 2022, 12, 4154. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Li, Y.; Fu, Q.; Wang, J.; Lin, Y.; Qiu, L.; Ran, L.; Yang, J.; Yang, C. Bio-Assay-Guided Isolation of Fractions and Constituents with Antioxidant and Lipid-lowering Activity from Allium cepa. Antioxidants 2023, 12, 1448. [Google Scholar] [CrossRef] [PubMed]
- Poornima, M.S.; Sindhu, G.; Billu, A.; Sruthi, C.R.; Nisha, P.; Gogoi, P.; Baishya, G.; Raghu, K.G. Pretreatment of hydroethanolic extract of Dillenia indica L. attenuates oleic acid induced NAFLD in HepG2 cells via modulating SIRT-1/p-LKB-1/AMPK, HMGCR & PPAR-α signaling pathways. J. Ethnopharmacol. 2022, 292, 115237. [Google Scholar]
- Li, Z.; Zhou, Y.; Jia, K.; Yang, Y.; Zhang, L.; Wang, S.; Dong, Y.; Wang, M.; Li, Y.; Lu, S.; et al. JMJD4-demethylated RIG-I prevents hepatic steatosis and carcinogenesis. J. Hematol. Oncol. 2022, 15, 161. [Google Scholar] [CrossRef] [PubMed]
- Repa, J.J.; Berge, K.E.; Pomajzl, C.; Richardson, J.A.; Hobbs, H.; Mangelsdorf, D.J. Regulation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 by the liver X receptors alpha and beta. J. Biol. Chem. 2002, 277, 18793–18800. [Google Scholar] [CrossRef] [PubMed]
- Peet, D.J.; Turley, S.D.; Ma, W.; Janowski, B.A.; Lobaccaro, J.M.; Hammer, R.E.; Mangelsdorf, D.J. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell 1998, 93, 693–704. [Google Scholar] [CrossRef]
- Zhang, Y.; Breevoort, S.R.; Angdisen, J.; Fu, M.; Schmidt, D.R.; Holmstrom, S.R.; Kliewer, S.A.; Mangelsdorf, D.J.; Schulman, I.G. Liver LXRalpha expression is crucial for whole body cholesterol homeostasis and reverse cholesterol transport in mice. J. Clin. Investig. 2012, 122, 1688–1699. [Google Scholar] [CrossRef]
- Ai, Z.L.; Zhu, C.H.; Min, M.; Wang, J.; Lan, C.H.; Fan, L.L.; Sun, W.J.; Chen, D.F. The role of hepatic liver X receptor α- and sterol regulatory element binding protein-1c-mediated lipid disorder in the pathogenesis of non-alcoholic steatohepatitis in rats. .J. Int. Med. Res. 2011, 39, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Nakamuta, M.; Fujino, T.; Yada, R.; Yada, M.; Yasutake, K.; Yoshimoto, T.; Harada, N.; Higuchi, N.; Kato, M.; Kohjima, M.; et al. Impact of cholesterol metabolism and the LXRalpha-SREBP-1c pathway on nonalcoholic fatty liver disease. Int. J. Mol. Med. 2009, 23, 603–608. [Google Scholar] [PubMed]
- Salamone, F.; Li Volti, G.; Titta, L.; Puzzo, L.; Barbagallo, I.; La Delia, F.; Zelber-Sagi, S.; Malaguarnera, M.; Pelicci, P.G.; Giorgio, M.; et al. Moro orange juice prevents fatty liver in mice. World J. Gastroenterol. 2012, 18, 3862–3868. [Google Scholar] [CrossRef] [PubMed]
- Lima-Cabello, E.; García-Mediavilla, M.V.; Miquilena-Colina, M.E.; Vargas-Castrillón, J.; Lozano-Rodríguez, T.; Fernández-Bermejo, M.; Olcoz, J.L.; González-Gallego, J.; García-Monzón, C.; Sánchez-Campos, S. Enhanced expression of pro-inflammatory mediators and liver X-receptor-regulated lipogenic genes in non-alcoholic fatty liver disease and hepatitis C. Clin. Sci. 2011, 120, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Endo-Umeda, K.; Makishima, M. Liver X Receptors Regulate Cholesterol Metabolism and Immunity in Hepatic Nonparenchymal Cells. Int. J. Mol. Sci. 2019, 20, 5045. [Google Scholar] [CrossRef] [PubMed]
- Beigneux, A.; Hofmann, A.F.; Young, S.G. Human CYP7A1 deficiency: Progress and enigmas. J. Clin. Investig. 2002, 110, 29–31. [Google Scholar] [CrossRef]
- Jones, R.D.; Lopez, A.M.; Tong, E.Y.; Posey, K.S.; Chuang, J.C.; Repa, J.J.; Turley, S.D. Impact of physiological levels of chenodeoxycholic acid supplementation on intestinal and hepatic bile acid and cholesterol metabolism in Cyp7a1-deficient mice. Steroids 2015, 93, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Ratliff, E.P.; Gutierrez, A.; Davis, R.A. Transgenic expression of CYP7A1 in LDL receptor-deficient mice blocks diet-induced hypercholesterolemia. J. Lipid Res. 2006, 47, 1513–1520. [Google Scholar] [CrossRef]
- Mouzaki, M.; Wang, A.Y.; Bandsma, R.; Comelli, E.M.; Arendt, B.M.; Zhang, L.; Fung, S.; Fischer, S.E.; McGilvray, I.G.; Allard, J.P. Bile Acids and Dysbiosis in Non-Alcoholic Fatty Liver Disease. PLoS ONE 2016, 11, e0151829. [Google Scholar] [CrossRef]
- Luo, Y.; Decato, B.E.; Charles, E.D.; Shevell, D.E.; McNaney, C.; Shipkova, P.; Apfel, A.; Tirucherai, G.S.; Sanyal, A.J. Pegbelfermin selectively reduces secondary bile acid concentrations in patients with non-alcoholic steatohepatitis. JHEP Rep. 2021, 4, 100392. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Lin, Y.; Wang, Q.; Li, Y.; Zhao, Y.; Chen, L.; Wu, Q.; Xu, C.; Zhou, C.; Sun, Y.; et al. Integrated Multichip Analysis Identifies Potential Key Genes in the Pathogenesis of Nonalcoholic Steatohepatitis. Front. Endocrinol. 2020, 11, 601745. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Zhao, W.; Yu, L.; Hu, X.; Zhao, Y.; Guo, Q.; Wang, X.; Wu, X. Dihydroflavonoids as Bioactive Components of Penthorum chinense, a Miao Ethnomedicine, against NAFLD through Bile Acid Metabolism Pathway. Chem. Biodivers. 2022, 19, e202200146. [Google Scholar] [CrossRef] [PubMed]
- Kouno, T.; Liu, X.; Zhao, H.; Kisseleva, T.; Cable, E.E.; Schnabl, B. Selective PPARδ agonist seladelpar suppresses bile acid synthesis by reducing hepatocyte CYP7A1 via the fibroblast growth factor 21 signaling pathway. J. Biol. Chem. 2022, 298, 102056. [Google Scholar] [CrossRef]
- Yu, L.; Lu, H.; Yang, X.; Li, R.; Shi, J.; Yu, Y.; Ma, C.; Sun, F.; Zhang, S.; Zhang, F. Diosgenin alleviates hypercholesterolemia via SRB1/CES-1/CYP7A1/FXR pathway in high-fat diet-fed rats. Toxicol. Appl. Pharmacol. 2021, 412, 115388. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y.L.; Ferrell, J.M. Discovery of farnesoid X receptor and its role in bile acid metabolism. Mol. Cell. Endocrinol. 2022, 548, 111618. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; He, F.; Yan, X.; Xing, Y.; Lei, Y.; Gao, J.; He, M.; Li, D.; Bai, L.; Yuan, Z.; et al. Hepatic Reduction in Cholesterol 25-Hydroxylase Aggravates Diet-induced Steatosis. Cell. Mol. Gastroenterol. Hepatol. 2022, 13, 1161–1179. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Liu, W.; Liang, B.; Shi, L.; Yang, S.; Meng, J.; Chang, J.; Hu, X.; Zhang, R.; Xing, D. Inhibitory Effect of Isoliquiritigenin in Niemann-Pick C1-Like 1-Mediated Cholesterol Uptake. Molecules 2022, 27, 7494. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Chen, J.; Zhang, T.; Yuan, X.; Ge, A.; Wang, S.; Xu, H.; Zeng, L.; Ge, J. Efficacy and safety of dietary polyphenol supplementation in the treatment of non-alcoholic fatty liver disease: A systematic review and meta-analysis. Front. Immunol. 2022, 13, 949746. [Google Scholar]
- Feng, D.; Ohlsson, L.; Duan, R.D. Curcumin inhibits cholesterol uptake in Caco-2 cells by down-regulation of NPC1L1 expression. Lipids Health Dis. 2010, 9, 40. [Google Scholar] [CrossRef]
- Zou, J.; Zhang, S.; Li, P.; Zheng, X.; Feng, D. Supplementation with curcumin inhibits intestinal cholesterol absorption and prevents atherosclerosis in high-fat diet-fed apolipoprotein E knockout mice. Nutr. Res. 2018, 56, 32–40. [Google Scholar] [CrossRef]
- Meng, J.; Xu, J.; Yang, S.; Liu, W.; Zeng, J.; Shi, L.; Chang, J.; Zhang, R.; Xing, D. Emodin lows NPC1L1-mediated cholesterol absorption as an uncompetitive inhibitor. Bioorg. Med. Chem. Lett. 2022, 75, 128974. [Google Scholar] [CrossRef]
- Tavares, T.B.; Santos, I.B.; de Bem, G.F.; Ognibene, D.T.; da Rocha, A.P.M.; de Moura, R.S.; Resende, A.C.; Daleprane, J.B.; da Costa, C.A. Therapeutic effects of açaí seed extract on hepatic steatosis in high-fat diet-induced obesity in male mice: A comparative effect with rosuvastatin. J. Pharm. Pharmacol. 2020, 72, 1921–1932. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.S.; Park, J.M.; Lee, J.; Oh, B.C.; Jang, S.H.; Lee, Y.B.; Han, Y.M.; Ock, C.Y.; Cha, J.Y.; Hahm, K.B. Amelioration of non-alcoholic fatty liver disease with NPC1L1-targeted IgY or n-3 polyunsaturated fatty acids in mice. Metabolism 2017, 66, 32–44. [Google Scholar] [CrossRef]
- Wang, D.; Tan, K.S.; Zeng, W.; Li, S.; Wang, Y.; Xu, F.; Tan, W. Hepatocellular BChE as a therapeutic target to ameliorate hypercholesterolemia through PRMT5 selective degradation to restore LDL receptor transcription. Life Sci. 2022, 293, 120336. [Google Scholar] [CrossRef] [PubMed]
- Abbasi-Oshaghi, E.; Khodadadi, I.; Tavilani, H.; Mirzaei, F.; Goodarzi, M.T. Dill-normalized liver lipid accumulation, oxidative stress, and low-density lipoprotein receptor levels in high cholesterol fed hamsters. ARYA Atheroscler. 2018, 14, 218–224. [Google Scholar]
- Chen, P.; Li, Y.; Xiao, L. Berberine ameliorates nonalcoholic fatty liver disease by decreasing the liver lipid content via reversing the abnormal expression of MTTP and LDLR. Exp. Ther. Med. 2021, 22, 1109. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Wei, J.; Abidi, P.; Lin, M.; Inaba, S.; Li, C.; Wang, Y.; Wang, Z.; Si, S.; Pan, H.; et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat. Med. 2004, 10, 1344–1351. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, G.; Wang, Y.; Yin, J.; Wang, J.; Xia, B.; Li, T.; Yang, X.; Hou, P.; Hu, S.; et al. Fucoidan A2 from the Brown Seaweed Ascophyllum nodosum Lowers Lipid by Improving Reverse Cholesterol Transport in C57BL/6J Mice Fed a High-Fat Diet. J. Agric. Food. Chem. 2019, 67, 5782–5791. [Google Scholar] [CrossRef] [PubMed]
- Musolino, V.; Gliozzi, M.; Nucera, S.; Carresi, C.; Maiuolo, J.; Mollace, R.; Paone, S.; Bosco, F.; Scarano, F.; Scicchitano, M.; et al. The effect of bergamot polyphenolic fraction on lipid transfer protein system and vascular oxidative stress in a rat model of hyperlipemia. Lipids Health Dis. 2019, 18, 115. [Google Scholar] [CrossRef]
- Elseweidy, M.M.; Younis, N.N.; Elswefy, S.E.; Abdallah, F.R.; El-Dahmy, S.I.; Elnagar, G.; Kassem, H.M. Atheroprotective potentials of curcuminoids against ginger extract in hypercholesterolaemic rabbits. Nat. Prod. Res. 2015, 29, 961–965. [Google Scholar] [CrossRef]
- Indu, M.S.; Narayanankutty, A.; Ramavarma, S.K.; Manalil, J.J.; Padikkala, J.; Raghavamenon, A.C. Desmodium gyrans dc modulates lipid trafficking in cultured macrophages and improves functional high-density lipoprotein in male wistar rats. Indian J. Pharmacol. 2021, 53, 286–293. [Google Scholar] [PubMed]
- Lee, K.H.; Jeong, E.S.; Jang, G.; Na, J.R.; Park, S.; Kang, W.S.; Kim, E.; Choi, H.; Kim, J.S.; Kim, S. Unripe Rubus coreanus Miquel Extract Containing Ellagic Acid Regulates AMPK, SREBP-2, HMGCR, and INSIG-1 Signaling and Cholesterol Metabolism In Vitro and In Vivo. Nutrients 2020, 12, 610. [Google Scholar] [CrossRef]
- Tang, L.; Kuang, C.; Shan, D.; Shi, M.; Li, J.; Qiu, L.; Yu, J. The ethanol extract of Edgeworthia gardneri (Wall.) Meisn attenuates macrophage foam cell formation and atherogenesis in ApoE(−/−) mice. Front. Cardiovasc. Med. 2022, 9, 1023438. [Google Scholar] [CrossRef]
- Yi, S.; Chen, K.; Sakao, K.; Ikenaga, M.; Wang, Y.; Hou, D.X. Assessment of Areca Nut Bioactivities in Western Diet-Induced Mice NAFLD Model. Nutrients 2023, 15, 2403. [Google Scholar] [CrossRef]
- Tandrasasmita, O.M.; Berlian, G.; Tjandrawinata, R.R. Molecular mechanism of DLBS3733, a bioactive fraction of Lagerstroemia speciosa (L.) Pers., on ameliorating hepatic lipid accumulation in HepG2 cells. Biomed. Pharmacother. 2021, 141, 111937. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.Z.; Jiang, W.; Zhu, Y.Y.; Wang, C.Z.; Zhong, W.H.; Wu, G.; Chen, J.; Zhu, M.N.; Wu, Q.L.; Du, X.L.; et al. Highland barley Monascus purpureus Went extract ameliorates high-fat, high-fructose, high-cholesterol diet induced nonalcoholic fatty liver disease by regulating lipid metabolism in golden hamsters. J. Ethnopharmacol. 2022, 286, 114922. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Xu, J.; Chen, Q.; Liu, M.; Wang, S.; Yu, H.; Zhang, Y.; Wang, T. Regulation effects of total flavonoids in Morus alba L. on hepatic cholesterol disorders in orotic acid induced NAFLD rats. BMC Complement. Med. Ther. 2020, 20, 257. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.Y.; Chen, P.Y.; Hsu, H.J.; Lin, C.Y.; Wu, M.J.; Yen, J.H. Tanshinone IIA Downregulates Lipogenic Gene Expression and Attenuates Lipid Accumulation through the Modulation of LXRα/SREBP1 Pathway in HepG2 Cells. Biomedicines 2021, 9, 326. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Lee, J.Y.; Jhin, C.; Shin, J.M.; Kim, M.; Ahn, H.R.; Yoo, G.; Son, Y.J.; Jung, S.H.; Nho, C.W. Reduction of Hepatic Lipogenesis by Loliolide and Pinoresinol from Lysimachia vulgaris via Degrading Liver X Receptors. J. Agric. Food. Chem. 2019, 67, 12419–12427. [Google Scholar] [CrossRef]
- Yin, Y.; Gao, L.; Lin, H.; Wu, Y.; Han, X.; Zhu, Y.; Li, J. Luteolin improves non-alcoholic fatty liver disease in db/db mice by inhibition of liver X receptor activation to down-regulate expression of sterol regulatory element binding protein 1c. Biochem. Biophys. Res. Commun. 2017, 482, 720–726. [Google Scholar] [CrossRef]
- Wang, C.; Ma, C.; Gong, L.; Dai, S.; Li, Y. Preventive and therapeutic role of betaine in liver disease: A review on molecular mechanisms. Eur. J. Pharmacol. 2021, 912, 174604. [Google Scholar] [CrossRef] [PubMed]
- Shiragannavar, V.D.; Sannappa Gowda, N.G.; Puttahanumantharayappa, L.D.; Karunakara, S.H.; Bhat, S.; Prasad, S.K.; Kumar, D.P.; Santhekadur, P.K. The ameliorating effect of withaferin A on high-fat diet-induced non-alcoholic fatty liver disease by acting as an LXR/FXR dual receptor activator. Front. Pharmacol. 2023, 14, 1135952. [Google Scholar] [CrossRef]
- Munkong, N.; Somnuk, S.; Jantarach, N.; Ruxsanawet, K.; Nuntaboon, P.; Kanjoo, V.; Yoysungnoen, B. Red Rice Bran Extract Alleviates High-Fat Diet-Induced Non-Alcoholic Fatty Liver Disease and Dyslipidemia in Mice. Nutrients 2023, 15, 246. [Google Scholar] [CrossRef]
- Tai, T.S.; Tien, N.; Shen, H.Y.; Chu, F.Y.; Wang, C.C.N.; Lu, C.H.; Yu, H.I.; Kung, F.P.; Chuang, H.H.; Lee, Y.R.; et al. Sesamin, a Naturally Occurring Lignan, Inhibits Ligand-Induced Lipogenesis through Interaction with Liver X Receptor Alpha (LXRα) and Pregnane X Receptor (PXR). Evid. Based Complement. Altern. Med. 2019, 2019, 9401648. [Google Scholar] [CrossRef]
- Chen, Y.J.; Song, H.Y.; Zhang, Z.W.; Chen, Q.; Tang, Z.P.; Gu, M. Extracts of Vine Tea Improve Diet-Induced Non-Alcoholic Steatohepatitis Through AMPK-LXRα Signaling. Front. Pharmacol. 2021, 12, 711763. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Liang, S.; Liu, Q.; Deng, Z.; Zhang, Y.; Du, J.; Zhang, Y.; Li, S.; Cheng, B.; Ling, C. Diosgenin prevents high-fat diet-induced rat non-alcoholic fatty liver disease through the AMPK and LXR signaling pathways. Int. J. Mol. Med. 2018, 41, 1089–1095. [Google Scholar] [CrossRef]
- Vitaglione, P.; Mazzone, G.; Lembo, V.; D‘Argenio, G.; Rossi, A.; Guido, M.; Savoia, M.; Salomone, F.; Mennella, I.; De Filippis, F.; et al. Coffee prevents fatty liver disease induced by a high-fat diet by modulating pathways of the gut-liver axis. J. Nutr. Sci. 2019, 8, e15. [Google Scholar] [CrossRef]
- Xu, M.; Yang, L.; Zhu, Y.; Liao, M.; Chu, L.; Li, X.; Lin, L.; Zheng, G. Collaborative effects of chlorogenic acid and caffeine on lipid metabolism via the AMPKα-LXRα/SREBP-1c pathway in high-fat diet-induced obese mice. Food Funct. 2019, 10, 7489–7497. [Google Scholar] [CrossRef] [PubMed]
- Yarahmadi, S.; Farahmandian, N.; Fadaei, R.; Koushki, M.; Bahreini, E.; Karima, S.; Barzin Tond, S.; Rezaei, A.; Nourbakhsh, M.; Fallah, S. Therapeutic Potential of Resveratrol and Atorvastatin Following High-Fat Diet Uptake-Induced Nonalcoholic Fatty Liver Disease by Targeting Genes Involved in Cholesterol Metabolism and miR33. DNA Cell Biol. 2023, 42, 82–90. [Google Scholar] [CrossRef]
- Jeong, M.J.; Kim, S.R.; Jung, U.J. Schizandrin A supplementation improves nonalcoholic fatty liver disease in mice fed a high-fat and high-cholesterol diet. Nutr. Res. 2019, 64, 64–71. [Google Scholar] [CrossRef]
- Kang, M.; Kim, E.H.; Jeong, J.; Ha, H. Heukcha, naturally post-fermented green tea extract, ameliorates diet-induced hypercholesterolemia and NAFLD in hamster. J. Food Sci. 2021, 86, 5016–5025. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Wang, Y.; Cao, X.; Peng, Y.; Huang, J.; Chen, L.; Pang, J.; Jiang, Z.; Qian, S.; Liu, Y.; et al. Targeting mTOR/YY1 signaling pathway by quercetin through CYP7A1-mediated cholesterol-to-bile acids conversion alleviated type 2 diabetes mellitus induced hepatic lipid accumulation. Phytomedicine 2023, 113, 154703. [Google Scholar] [CrossRef]
- Yang, C.; Yang, L.; Yang, Y.; Wan, M.; Xu, D.; Pan, D.; Sun, G. Effects of flaxseed powder in improving non-alcoholic fatty liver by regulating gut microbiota-bile acids metabolic pathway through FXR/TGR5 mediating. Biomed. Pharmacother. 2023, 163, 114864. [Google Scholar] [CrossRef]
- Zhao, K.; Qiu, L.; He, Y.; Tao, X.; Zhang, Z.; Wei, H. Alleviation Syndrome of High-Cholesterol-Diet-Induced Hypercholesterolemia in Mice by Intervention with Lactiplantibacillus plantarum WLPL21 via Regulation of Cholesterol Metabolism and Transportation as Well as Gut Microbiota. Nutrients 2023, 15, 2600. [Google Scholar] [CrossRef]
- Mu, J.; Tan, F.; Zhou, X.; Zhao, X. Lactobacillus fermentum CQPC06 in naturally fermented pickles prevents non-alcoholic fatty liver disease by stabilizing the gut-liver axis in mice. Food Funct. 2020, 11, 8707–8723. [Google Scholar] [CrossRef]
- Vajdi, M.; Hassanizadeh, S.; Hassanizadeh, R.; Bagherniya, M. Curcumin supplementation effect on liver enzymes in patients with nonalcoholic fatty liver disease: A GRADE-assessed systematic review and dose-response meta-analysis of randomized controlled trials. Nutr. Rev. 2024. [Google Scholar] [CrossRef]
- Ebrahimzadeh, A.; Mohseni, S.; Safargar, M.; Mohtashamian, A.; Niknam, S.; Bakhoda, M.; Afshari, S.; Jafari, A.; Ebrahimzadeh, A.; Fooladshekan, S.; et al. Curcumin effects on glycaemic indices, lipid profile, blood pressure, inflammatory markers and anthropometric measurements of non-alcoholic fatty liver disease patients: A systematic review and meta-analysis of randomized clinical trials. Complement. Ther. Med. 2024, 80, 103025. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Malik, M. Effects of curcumin in patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis. Can. Liver J. 2024, 7, 299–315. [Google Scholar] [CrossRef]
- Jazayeri-Tehrani, S.A.; Rezayat, S.M.; Mansouri, S.; Qorbani, M.; Alavian, S.M.; Daneshi-Maskooni, M.; Hosseinzadeh-Attar, M.J. Nano-curcumin improves glucose indices, lipids, inflammation, and Nesfatin in overweight and obese patients with non-alcoholic fatty liver disease (NAFLD): A double-blind randomized placebo-controlled clinical trial. Nutr. Metab. 2019, 16, 8. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, S.; Asgary, S.; Askari, G.; Keshvari, M.; Hatamipour, M.; Feizi, A.; Sahebkar, A. Treatment of Non-alcoholic Fatty Liver Disease with Curcumin: A Randomized Placebo-controlled Trial. Phytother. Res. 2016, 30, 1540–1548. [Google Scholar] [CrossRef]
- Nie, Q.; Li, M.; Huang, C.; Yuan, Y.; Liang, Q.; Ma, X.; Qiu, T.; Li, J. The clinical efficacy and safety of berberine in the treatment of non-alcoholic fatty liver disease: A meta-analysis and systematic review. J. Transl. Med. 2024, 22, 225. [Google Scholar] [CrossRef]
- Afsharinasab, M.; Mohammad-Sadeghipour, M.; Reza Hajizadeh, M.; Khoshdel, A.; Mirzaiey, V.; Mahmoodi, M. The effect of hydroalcoholic Berberis integerrima fruits extract on the lipid profile, antioxidant parameters and liver and kidney function tests in patients with nonalcoholic fatty liver disease. Saudi J. Biol. Sci. 2020, 27, 2031–2037. [Google Scholar] [CrossRef]
- Yan, H.M.; Xia, M.F.; Wang, Y.; Chang, X.X.; Yao, X.Z.; Rao, S.X.; Zeng, M.S.; Tu, Y.F.; Feng, R.; Jia, W.P.; et al. Efficacy of Berberine in Patients with Non-Alcoholic Fatty Liver Disease. PLoS ONE 2015, 10, e0134172. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Skonieczna-Żydecka, K.; Kałduńska, J.; Stachowska, E.; Gutowska, I.; Janda, K. Effects of Resveratrol Supplementation in Patients with Non-Alcoholic Fatty Liver Disease-A Meta-Analysis. Nutrients 2020, 12, 2435. [Google Scholar] [CrossRef]
- Wei, S.; Yu, X. Efficacy of resveratrol supplementation on liver enzymes in patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis. Complement. Ther. Med. 2021, 57, 102635. [Google Scholar] [CrossRef] [PubMed]
- Farzin, L.; Asghari, S.; Rafraf, M.; Asghari-Jafarabadi, M.; Shirmohammadi, M. No beneficial effects of resveratrol supplementation on atherogenic risk factors in patients with nonalcoholic fatty liver disease. Int. J. Vitam. Nutr. Res. 2020, 90, 279–289. [Google Scholar]
- Faghihzadeh, F.; Adibi, P.; Rafiei, R.; Hekmatdoost, A. Resveratrol supplementation improves inflammatory biomarkers in patients with nonalcoholic fatty liver disease. Nutr. Res. 2014, 34, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Kravchenko, L.; Unhurian, L.; Tiuzhinska, S.K.; Ivanova, Y.; Obrazenko, M.; Zahorodnya, L.; Yamilova, T. Increasing the efficiency of hypolipidemic therapy with the combined use of quercetin in patients with non-alcoholic fatty liver disease on the background of the metabolic syndrome. Ceska Slov. Farm. 2024, 72, 297–303. [Google Scholar] [PubMed]
- Yari, Z.; Cheraghpour, M.; Alavian, S.M.; Hedayati, M.; Eini-Zinab, H.; Hekmatdoost, A. The efficacy of flaxseed and hesperidin on non-alcoholic fatty liver disease: An open-labeled randomized controlled trial. Eur. J. Clin. Nutr. 2021, 75, 99–111. [Google Scholar] [CrossRef]
- Koperska, A.; Moszak, M.; Seraszek-Jaros, A.; Bogdanski, P.; Szulinska, M. Does berberine impact anthropometric, hepatic, and metabolic parameters in patients with metabolic dysfunction-associated fatty liver disease? Randomized, double-blind placebo-controlled trial. J. Physiol. Pharmacol. 2024, 75, 291–302. [Google Scholar]
- Safari, Z.; Bagherniya, M.; Khoram, Z.; Ebrahimi Varzaneh, A.; Heidari, Z.; Sahebkar, A.; Askari, G. The effect of curcumin on anthropometric indices, blood pressure, lipid profiles, fasting blood glucose, liver enzymes, fibrosis, and steatosis in non-alcoholic fatty livers. Front. Nutr. 2023, 10, 1163950. [Google Scholar] [CrossRef] [PubMed]
- Beheshti Namdar, A.; Ahadi, M.; Hoseini, S.M.; Vosoghinia, H.; Rajablou, H.; Farsi, S.; Zangouei, A.; Rahimi, H.R. Effect of nano-micelle curcumin on hepatic enzymes: A new treatment approach for non-alcoholic fatty liver disease (NAFLD). Avicenna J. Phytomed. 2023, 13, 615–625. [Google Scholar] [PubMed]
- Mirhafez, S.R.; Rezai, A.; Dehabeh, M.; Nobakht, M.G.B.F.; Bidkhori, M.; Sahebkar, A.; Hariri, M. Efficacy of phytosomal curcumin among patients with non-alcoholic fatty liver disease. Int. J. Vitam. Nutr. Res. 2021, 91, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Mirhafez, S.R.; Dehabeh, M.; Hariri, M.; Farimani, A.R.; Movahedi, A.; Naderan, R.D.; Jamialahmadi, T.; Simental-Mendía, L.E.; Sahebkar, A. Curcumin and Piperine Combination for the Treatment of Patients with Non-alcoholic Fatty Liver Disease: A Double-Blind Randomized Placebo-Controlled Trial. Adv. Exp. Med. Biol. 2021, 1328, 11–19. [Google Scholar]
- Saadati, S.; Hatami, B.; Yari, Z.; Shahrbaf, M.A.; Eghtesad, S.; Mansour, A.; Poustchi, H.; Hedayati, M.; Aghajanpoor-Pasha, M.; Sadeghi, A.; et al. The effects of curcumin supplementation on liver enzymes, lipid profile, glucose homeostasis, and hepatic steatosis and fibrosis in patients with non-alcoholic fatty liver disease. Eur. J. Clin. Nutr. 2019, 73, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Valizadegan, G.; Ahamdi, N.; Ganjali, S.; Majeed, M.; Sahebkar, A. Curcuminoids plus piperine improve nonalcoholic fatty liver disease: A clinical trial. J. Cell. Biochem. 2019, 120, 15989–15996. [Google Scholar] [CrossRef]
- Mansour, A.; Mohajeri-Tehrani, M.R.; Karimi, S.; Sanginabadi, M.; Poustchi, H.; Enayati, S.; Asgarbeik, S.; Nasrollahzadeh, J.; Hekmatdoost, A. Short term effects of coffee components consumption on gut microbiota in patients with non-alcoholic fatty liver and diabetes: A pilot randomized placebo-controlled, clinical trial. EXCLI J. 2020, 19, 241–250. [Google Scholar]
- Theodotou, M.; Fokianos, K.; Moniatis, D.; Kadlenic, R.; Chrysikou, A.; Aristotelous, A.; Mouzouridou, A.; Diakides, J.; Stavrou, E. Effect of resveratrol on non-alcoholic fatty liver disease. Exp. Ther. Med. 2019, 18, 559–565. [Google Scholar] [CrossRef] [PubMed]

| Natural Products | Source | Interfering Mechanism | Molecular Target | Models | Refs |
|---|---|---|---|---|---|
| Isoliquiritigenin | Glycyrrhiza glabra | Cholesterol absorption | NPC1L1 | HepG2 Caco-2 cells | [97] |
| Curcumin | Turmeric curcuminoids | Cholesterol absorption | NPC1L1 | Caco-2 ApoE−/− mice | [100] |
| Emodin | Rhubarb, Cassia, and Heshouwu | Cholesterol absorption | NPC1L1 | HepG2 Caco-2 cells | [101] |
| Hydroalcoholic extract | Euterpe oleracea Mart. | Cholesterol absorption Cholesterol excretion | NPC1L1 ABCG5 | C57BL/6 mice | [102] |
| N-IgY | A chicken egg yolk-derived IgY | Cholesterol uptake RCT Cholesterol conversion | NPC1L1 ABCG5 ABCG8 CYP7A1 LDLR | HepG2 Caco-2 cells C57BL/6 | [103] |
| Leaf extracts of Anethum graveolens | Anethum graveolens | Improve hypercholesterolemic | LDLR | Hamsters | [105] |
| Berberine | Coptidis Rhizoma | Improve lipid | LDLR | Sprague-Dawley rats | [106] |
| Biochanin A | Soybeans, Red clover, Alfalfa, peanuts, and chickpeas | Cholesterol uptake RCT Cholesterol conversion | CYP7A1 LDLR HMGCR | Sprague-Dawley rats | [72] |
| Fucoidan A2 | Brown seaweed Ascophyllum nodosum | RCT Cholesterol conversion Cholesterol efflux | ABCA1 ABCG8 LDLR SR-BI CYP7A1 | C57BL/6J mice | [108] |
| Bergamot polyphenolic fraction | Bergamot | RCT | LCAT CETP | Male Wistar rats | [109] |
| Lactobacillus acidophilus | Probiotics | RCT | CETP LDLR | Rabbits | [55] |
| Resveratrol | Polygonum cuspidatum | RCT Cholesterol efflux | LDLR SRBI LXRα ABCA1 ABCG1 | Wistar male rats | [65,129] |
| Schizandrin A | Schisandra genus | Cholesterol excretion Cholesterol efflux | ABCA1 CYP7A1 | C57BL/6J mice | [130] |
| Heukcha | Green tea extract | Bile acid synthesis | CYP7A1 | Golden Syrian hamsters | [131] |
| Quercetin | Flavonoid | Cholesterol conversion | CYP7A1 | HepG2 cells Mice | [132] |
| Flaxseed powder | Alpha linolenic acid | Cholesterol conversion | CYP7A1 | C57BL/6 mice | [133] |
| Lactiplantibacillus plantarum WLPL21 | Probiotics | Cholesterol transportation Cholesterol conversion | NPC1L1 CYP27A1 CYP7A1 ABCA1 ABCG5 ABCG8 | C57BL/6 mice | [134] |
| Lactobacillus fermentum CQPC06 | Fermented pickles | Cholesterol conversion | CYP7A1 | C57/BL6J mice | [135] |
| Coffee | Coffee beans | Cholesterol efflux | LXRα ABCA1 | C57BL/6J mice | [127] |
| Diosgenin | yams | Cholesterol absorption RCT Cholesterol conversion | SRBI CYP7A1 LXRα | Rat | [94,126] |
| Areca nut polyphenols | Areca nut | Cholesterol synthesis | SREBP2 HMGCR | C57BL/6N mice | [114] |
| DLBS3733 | Lagerstroemia speciosa (L.) Pers., | Cholesterol synthesis | HMGCR ACC SREBP | HepG2 cells | [115] |
| HBMPWE | Monascus purpureus | Cholesterol synthesis Cholesterol conversion | CYP7A1 HMGCR | Golden hamsters, | [116] |
| Morus alba L., flavonoids | Mulberry leaves | Cholesterol synthesis | CYP7A1 SREBP2 HMGCR | Rats | [117] |
| Hydroethanolic extract of Dillenia indica L., | Dillenia indica L. | Cholesterol synthesis | SREBP2 HMGCR | HepG2 cells | [76] |
| Natural Products | Administration and Dose | Outcomes | Clinical Study Type | Clinical Trial Registration | Adverse Effects (AE) | Refs |
|---|---|---|---|---|---|---|
| Berberine | 12 weeks, 1500 mg/day | Significant decrease in serum ALT, serum TC, and de Ritis ratio | Randomized, double-blind placebo-controlled trial | NCT05523024 | Mild and transient gastrointestinal reactions | [150] |
| Berberine | 16 weeks, 0.5 g three times/day | Improved body weight, HOMA-IR, and serum lipid profiles | Randomized parallel controlled open-label trial | NCT00633282 | Anorexia and upset stomach, diarrhea, and constipation | [143] |
| Berberis integerrima extract | 2 months, 750 mg twice/day | Decreased BMI, serum lipid profiles, FBG, liver enzymes, and renal parameters; increased serum HDL-c, glutathione peroxidase enzyme, and total antioxidant capacity | Randomized clinical trial | IR.RUMS.REC.1396.110. | None | [142] |
| Curcumin | 12 weeks, 250 mg/day | Reduced steatosis and fibrosis with FibroScan; reduced waist circumference and blood pressure | Double-blind parallel placebo-controlled trial | IRCT20121216011763N39 | No adverse events | [151] |
| Curcumin | 60 days, 160 mg/day | Decreased serum ALT and AST | Double-blind randomized clinical trial | IRCT2017012031423N1 | None | [152] |
| Curcumin | 8 weeks, 250 mg/day | Reduced ALT, AST, body weight, waist circumference, body fat percentage, and BMI | Randomized, double-blind controlled trial | IRCT2015052322381N1 | No severe adverse effects | [153] |
| Curcumin | 2 months, 500 mg/day | Reduced ALP concentrations and severity of MASLD | Double-blind placebo-controlled trial | IRCT2015052322381N1 | None | [154] |
| Curcumin | 12 weeks, 1500 mg/day | Reduced hepatic fibrosis, serum cholesterol, FBG, and ALT | Randomized placebo-controlled clinical trial | None | None | [155] |
| Curcumin | 12 weeks, 500 mg/day | Reduced serum ALT, ALP, TC, LDL-c, iron, and hemoglobin; increased total iron-binding capacity | Randomized controlled parallel-group trial | UMIN000033774 | None | [156] |
| Curcumin | 3 months, 80 mg/day | Improved fatty liver degree, liver transaminases, waist circumference, FBG, FBI, HbA1c, TG, TC, LDL, HOMA-IR, TNF-α, hs-CRP, and IL-6 | Double-blind randomized placebo-controlled trial | IRCT2016071915536N3 | Nausea | [139] |
| Curcumin | 8 weeks, 500 mg/day | Reduced liver fat content, BMI, TC, LDL-c, AST, ALT, FBG, and glycated hemoglobin | Randomized double-blind placebo-controlled trial | IRCT2014110511763N18 | Stomachache and nausea | [140] |
| Caffeine | 12 weeks, 200 mg/day | Reduced body weight and energy intake | Randomized, placebo-controlled trial | NCT02929901 | None | [157] |
| Flaxseed powder | 12 weeks, 30 g/day | Decreased BMI, glucose hemostasis parameters, and hepatic steatosis | Randomized parallel controlled open-label trial | NCT03734510 | No adverse effects reported | [149] |
| Resveratrol | 12 weeks, 600 mg/day | Reduced body weight, BMI, and waist circumference | Randomized double-blind placebo-controlled trial | IRCT201511233664N16 | No adverse events | [146] |
| Resveratrol | 6 months, 50 mg/day or 200 mg/day | Reduced liver fat, liver enzymes, serum glutamate pyruvic transaminase, gamma-glutamyl transpeptidase, and insulin resistance | Randomized clinical trial | ΕΕΒΚ/ΕΠ/2010/12 | None | [158] |
| Resveratrol | 12 weeks, 500 mg/day | Reduced serum ALT, inflammatory cytokines, nuclear factor κB activity, serum cytokeratin-18, and hepatic steatosis grade | Randomized double-blinded controlled trial | NCT0203097 | No significant adverse effects | [147] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Li, M. Unlocking Cholesterol Metabolism in Metabolic-Associated Steatotic Liver Disease: Molecular Targets and Natural Product Interventions. Pharmaceuticals 2024, 17, 1073. https://doi.org/10.3390/ph17081073
Li X, Li M. Unlocking Cholesterol Metabolism in Metabolic-Associated Steatotic Liver Disease: Molecular Targets and Natural Product Interventions. Pharmaceuticals. 2024; 17(8):1073. https://doi.org/10.3390/ph17081073
Chicago/Turabian StyleLi, Xiaoxiao, and Meng Li. 2024. "Unlocking Cholesterol Metabolism in Metabolic-Associated Steatotic Liver Disease: Molecular Targets and Natural Product Interventions" Pharmaceuticals 17, no. 8: 1073. https://doi.org/10.3390/ph17081073
APA StyleLi, X., & Li, M. (2024). Unlocking Cholesterol Metabolism in Metabolic-Associated Steatotic Liver Disease: Molecular Targets and Natural Product Interventions. Pharmaceuticals, 17(8), 1073. https://doi.org/10.3390/ph17081073







